Abstract
Objective.
Mechanical circulatory support (MCS) has emerged as lifesaving therapy for patients with advanced heart failure. However, MCS remains limited by a paradoxical coagulopathy accompanied by both thrombosis and bleeding. While mechanisms of MCS thrombosis are increasingly defined, MCS-related bleeding, as related to shear-mediated alteration of platelet function, remains poorly understood. We tested the hypothesis that platelet exposure to elevated shear stress, while a defined prothrombotic activator of platelets, coordinately induces downregulation of key platelet adhesion receptors GPIb-IX-V, αIIbβ3, and P-selectin, thus decreasing platelet functional responsiveness to physiological stimuli.
Approach and Results.
Human gel-filtered platelets were exposed to continuous or pulsatile shear stress in vitro. Surface expression of platelet receptors and platelet-derived microparticle (PDMP) generation were quantified by flow cytometry. Shedding of receptor soluble forms were assessed via ELISA, and platelet aggregation was measured by optical aggregometry. We demonstrate that platelet exposure to elevated shear stress led to a downregulation of GPIb and αIIbβ3 receptors on platelets with a progressive increase in the generation of PDMPs expressing elevated levels of αIIbβ3 and GPIb on their surface. No shear-mediated shedding of GPIb and β3 subunit soluble fragments was detected. Soluble P-selectin was extensively shed from platelets, while surface expression of P-selectin on platelets and microparticles was not significantly altered by shear. Shear-mediated downregulation of GPIb, αIIbβ3, and P-selectin on platelets was associated with an evident decrease of platelet aggregatory response induced by ADP and TRAP-6.
Conclusions.
Our data clearly indicate that accumulation of shear stress, consistent with supraphysiologic conditions characterizing device-supported circulation 1) induces adequate platelet degranulation, yet 2) causes downregulation of primary platelet adhesion receptors via ejection of receptor-enriched PDMPs, thus mechanistically limiting platelet activation and the aggregatory response.
Keywords: Shear Stress, Platelet Dysfunction, Receptor Downregulation, Platelet-Derived Microparticles, Mechanical Circulatory Support, Bleeding, Thrombosis
Graphical Abstract

INTRODUCTION
Mechanical circulatory support (MCS), via implantation of ventricular assist devices (VADs) or the total artificial heart, has emerged as primary therapy for patients with advanced and end-stage heart failure1. The significant rise in MCS utilization has been driven by recent advances in device technology, coupled with clinical guideline updates, and adoption of best practice protocols leading to improved patient survival2. Despite the growth, MCS remains limited by coagulation-related complications – involving both device-related thrombosis and thromboembolic phenomena3; and conversely, increasingly, by bleeding issues4,5. Central in this MCS-related coagulopathy is supraphysiologic shear stress exposure of blood cellular and protein constituents traversing these devices6,7. While mechanisms of shear-mediated platelet activation and thrombosis are progressively defined8,9, mechanisms of MCS-related bleeding, particularly as they relate to shear-mediated alteration of platelet function, remain poorly understood.
Platelets are small anucleate blood cells with a significant role in thrombosis, angiogenesis, inflammation, and immune response10. When stimulated by shear and biochemical means, platelets undergo rapid activation, shape change, release of secretory granule contents, with subsequent aggregation leading to the formation of a primary clot. Platelet adhesion and aggregation rely largely on glycoprotein (GP) receptors, constitutively expressed on the platelet surface, e.g. GPIb-V-IX, GPIIb-IIIa (integrin αIIbβ3), GPVI, or exposed as a result of platelet activation and granule secretion, e.g. P-selectin11. GPIb-V-IX primarily binds to von Willebrand factor (vWF), integrin αIIbβ3 integrin binds to fibrinogen and vWF as major ligands, GPVI interacts with collagen, and P-selectin is responsible for platelet binding to leukocytes and endothelial cells12. To limit untoward platelet interactions with plasma proteins and other cells, the surface expression and affinity of platelet adhesion receptors are tightly regulated. This control mechanism of alteration of surface receptor expression is particularly relevant in coagulation considerations related to MCS since exposed vascular sub-endothelium, a classic platelet adhesion substrate, does not exist within MCS devices. Further, MCS thrombosis often occurs in the “free flow” as thromboembolic events, untethered to the vascular wall8.
Among regulatory mechanisms of platelet receptor surface expression, enzymatic shedding of the ligand-binding ectodomain is the most well documented12,13. Shedding of the receptor ectodomain by members of a disintegrin and metalloprotease (ADAM) family (ADAM17 and ADAM10) released from platelet secretory granules has been reported for GPIb and GPVI12. Soluble fragments of GPIb (glycocalicin) and GPVI have been reported as being present in blood of healthy individuals suggesting continuous shedding of these receptors from the platelet surface14,15. P-selectin has also been shown to be shed following its exposure on platelet surface as a result of platelet degranulation16. Whether mechanical shear stress itself is capable of promoting shedding of platelet adhesion receptors remains to be resolved and is herein addressed.
An alternative mechanism of modulation of platelet surface receptor expression is restructuring of the entire platelet membrane as a result of microvesiculation, with the generation of microparticles17. Platelet-derived microparticles (PDMPs) are submicron membrane-enclosed vesicles released by platelets during their activation with strong agonists, aging, and apoptosis. Platelets and megakaryocytes are considered to be the primary sources of microvesicles in blood, with up to 90% carrying canonical platelet antigens – CD41, CD42b, GPVI, and phosphatidylserine18,19. Mechanisms of PDMP formation have been described 20–22 emphasizing the importance of high intracellular calcium, actin cytoskeleton rearrangement, and apoptotic machinery in this process. Studies of PDMP proteomics have revealed several types of microvesicles differing by size, protein composition, and their effect on clotting and endothelial cell phenotype23,24. PDMPs not only provide additional surface for activation of the coagulation cascade and thrombin generation, as initially believed, but also deliver platelet receptors, nucleic acids, signaling and pro-inflammatory molecules to other cells serving as “a specialized mechanism of intravascular and extravascular cell-cell communication.”25 Platelet exposure to device-related shear stress also promotes microparticle generation in vitro,26–28 and in vivo in patients supported with VADs29,30. Elevated levels of circulating procoagulant microparticles carrying phosphatidylserine are associated with an increased probability of adverse events in patients with continuous flow VADs31. Yet, the modulatory role of shear mediated PDMP generation in the regulation of surface expression of key platelet adhesion receptors and its implication for platelet function have not been investigated.
Here, we test the hypothesis that platelet exposure to elevated shear stress induces downregulation of key platelet adhesion receptors GPIb-IX-V, αIIbβ3, and P-selectin via redistribution between platelets and shear-generated microparticles. Herein, the accumulation of shear stress over an extended period of time is representative of damage incurred due to multiple platelet passages through transient supraphysiologic shear stress regions associated with MCS devices. Further, while we and others have demonstrated a defined relationship between shear exposure, platelet activation and thrombogenicity, we posit that this loss and redistribution of platelet receptors on shear-activated platelets underlies a decrease in overall platelet functional responsiveness. As a first step, we examined the effect of continuous and pulsatile shear on surface expression of GPIb-IX-V, integrin αIIbβ3, and P-selectin on gel-filtered platelets. Next, we examined the effect of shear on PDMP generation and surface expression of GPIb-IX-V, αIIbβ3, and P-selectin on PDMPs. Finally, we evaluated the ability of sheared platelets to undergo activation and aggregation in response to biochemical agonists compared to unsheared controls.
Overall, we demonstrate that platelet exposure to elevated shear stress accumulation led to a downregulation of αIIbβ3 and GPIb receptors on the platelet surface. Concurrently shear exposure was associated with a progressive increase in the generation of PDMPs with a coordinate increase in αIIbβ3 and GPIb being found on their surface. No shear-mediated shedding of GPIb and β3 subunit soluble fragments was detected. Alternatively, soluble P-selectin was extensively shed from the platelet surface as a result of shear exposure, while surface expression of P-selectin on platelets and microparticles was not significantly altered. Shear-mediated shedding and redistribution of GPIb, αIIbβ3, and P-selectin on PDMPs was associated with a contemporaneous shear-mediated platelet dysfunction, as evidenced by decreased platelet aggregation in response to stimulation with biochemical agonists.
MATERIALS AND METHODS
The data that support the findings of this study are available from the first author upon request (email: rokamoiia@arizona.edu). For details of reagents utilized, please see the Major Resources Tables in the Data Supplement.
Blood collection and platelet isolation
Blood was obtained from 30 healthy volunteers (17 men and 13 women), who provided written consent, and were free of medications affecting platelet function for two weeks prior to venipuncture. The study protocol was approved by the Institutional Review Board of the University of Arizona (protocol# 1810013264 “Optimization Cardiovascular & Mechanical Circulatory Support (MCS) Devices Thrombogenicity for Eliminating Anticoagulation”). Fresh blood samples were anticoagulated with acid citrate dextrose solution at blood : anticoagulant ratio – 9 : 1. Platelet-rich plasma (PRP) was obtained by centrifugation of blood at 400g for 15 min at room temperature. Residual blood was spun at 1430g for 20 min at room temperature to yield platelet-poor plasma. Gel-filtered platelets (GFP) were isolated from PRP by gel-chromatography through Sepharose-2B32, and platelet count was measured on Z1 Particle Counter (Beckman Coulter, Indianapolis, IN). Platelet-contained fractions were stored and handled at room temperature, to minimize storage-associated platelet function decline33. Similarly, to limit 37°C-stimulated platelet apoptosis34,35 and associated alterations of the surface expression of platelet receptors12 and microparticle generation36, most experiments were performed at room temperature if not otherwise specified.
Platelet exposure to shear stress and biochemical agonists.
Prior exposure to shear stress or biochemical agonists, PRP and GFP aliquots were diluted with modified Tyrode’s buffer to 100,000 and 20,000 platelets/μL, respectively. Additionally, GFP (but not PRP) were recalcified with 1 mM CaCl2.
Platelets were exposed to MCS-related either pulsatile or continuous shear stress in vitro. First, platelets were subjected to a repetitive short-term hypershear using a “platelet hammer,” a syringe-capillary viscometer developed by our group37,38. The platelet hammer allows accurate replication of MSC-related pulsatile shear patterns when platelets experience numerous repetitive hypershear passages (up to 1000 dynes/cm2) for an extremely short exposure time (milliseconds)38. For detailed device setup and experiment design refer to supplemental material (Supplemental Methods). Briefly, 2.5 mL of recalcified GFP (20,000 platelets/μL, 1 mM CaCl2) were subjected to repetitive hypershear of 100, 500, and 750 dyne/cm2 magnitude for 30 min, using the platelet hammer. Then, platelet samples were collected at 0 and 30 min and processed immediately for flow cytometric analysis.
Alternatively, 3.5 mL of diluted PRP or GFP were subjected to shear stress in a hemodynamic shearing device (HSD), a computer-controlled modified cone-plate-Couette viscometer allowing application of uniform continuous shear stress of defined magnitude (30, 50 and 70 dyne/cm2)39. For these conditions, the shear exposure dose or shear accumulation, defined as shear stress times exposure time, represents repeated platelet passages through high-shear regions of MCS devices, and was selected based on our previous numerical studies of hemodynamics of MCS devices as compared with physiological levels of shear stress present within the normal circulation37,40. For continuous shear exposure experiments reported herein, 10 minutes was standardized as shear exposure time based on our previous reports9,37,38.
To induce platelet activation with biochemical agonists, recalcified GFP (20,000 platelets/uL, 1 mM CaCl2) were incubated with 10 uM ADP, 32 uM TRAP-6, or 1 U/ml human thrombin for 10 minutes at room temperature, undisturbed. Activated platelets were then processed for flow cytometric detection of glycoprotein surface expression and microparticle generation.
In experiments aimed to study the potential costimulatory role of ADP and its receptor P2Y12 in shear-mediated effects on platelets, prior to shear exposure GFP were incubated with ticagrelor (10 uM), a selective inhibitor of P2Y12 receptor, for 10 min. and then subjected to shear stress or biochemical agonists. Chosen concentration was shown to inhibit platelet ADP-mediated platelet aggregation in GFP, PRP, and whole blood41,42.
Surface expression of platelet glycoprotein receptors GPIb, αIIbβ3, P-selectin, and microparticle generation
Flow cytometric detection of platelet surface glycoproteins was performed following published recommendations43,44. Briefly, recalcified GFP (20,000 platelets/uL, 1 mM CaCl2) or PRP (20,000 platelets/uL) previously exposed to shear stress and/or biochemical agonists, were stained with fluorescein-conjugated antibodies – anti-CD41- allophycocyanin, anti-CD42b-phycoerythrin, anti-CD41/CD61-fluorescein isothiocyanate (clone PAC-1), and anti-CD62P and incubated for 30 min at room temperature. Shear-stimulated samples were then fixed with 3.5% PFA (filtered via 0.22 μm syringe filter) for 20 min, to avoid dissipation of shear-mediated alterations of platelet morphology. Stained and fixed platelets were washed via centrifugation and resuspended in 0.05 M PBS (pH – 7.4, filtered via 0.22 μm syringe filter). Flow cytometry was conducted on FACSCanto II flow cytometer (BD Biosciences, San Jose, CA). Ten thousand events were captured within the stopping gate “Platelet + Microparticles”. FCS Express Version 3 software (DeNovo Software, San Jose, CA) was applied to analyze flow cytometry data. Single platelets were distinguished from microparticles within CD41+ or CD42b+ events based on forward versus side scatter characteristics (FSC-A/SSC-A), as compared with polystyrene beads of 880 nm and 1350 nm size from SPHERO™ Nano Fluorescent particle standard kit (Spherotech, Lake Forest, IL) as shown in Figure I. Platelet and microparticle number were expressed as % all events captured. Platelet and microparticle size were assessed as their median forward scatter (MFS)45,46. Arbitrary density of GPIb and αIIbβ3 on platelet and microparticle surfaces were calculated as median fluorescence intensity (MFI) of the population normalized to the median FSC indicating platelet or microparticle size.
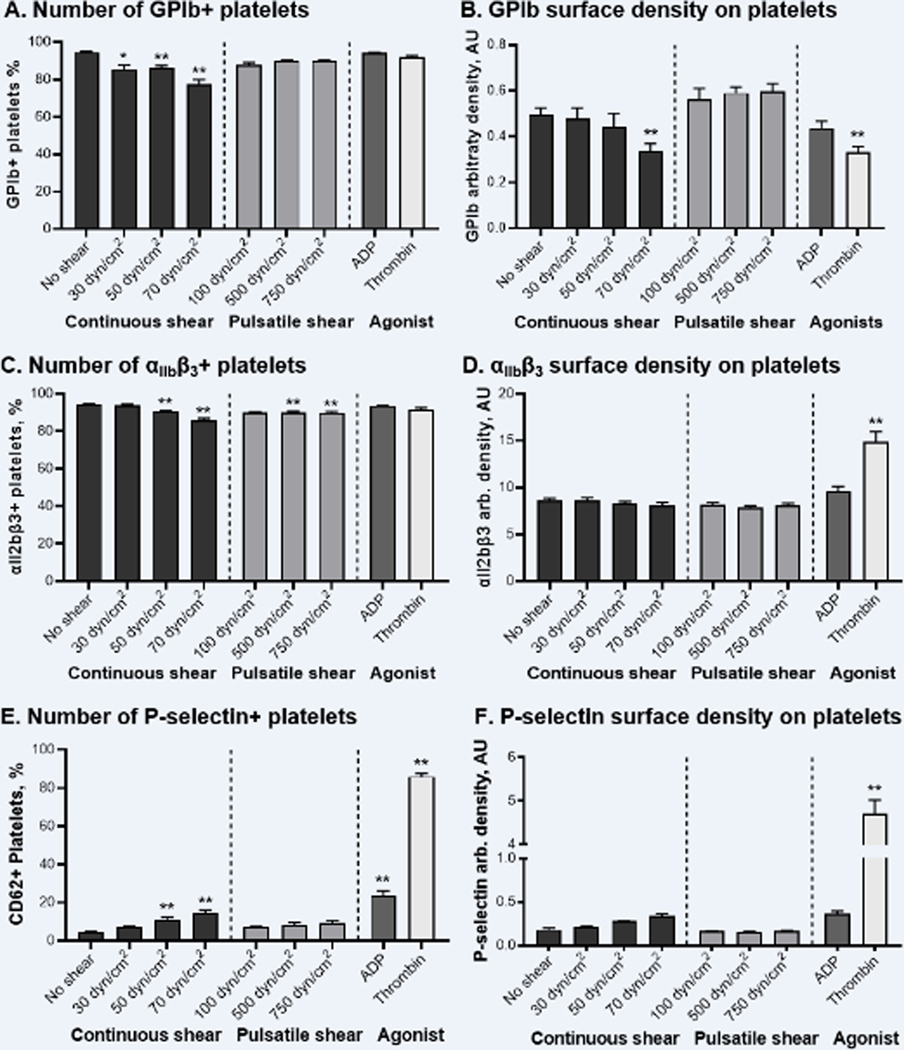
Figure 1. Shear stress induces downregulation of surface expression of GPIb, but not integrin αIIbβ3 and P-selectin, on platelets. Biochemical agonists also decrease GPIb surface expression and promote elevation of αIIbβ3 and P-selectin.
A, C, E – number of platelets expressing GPIb, αIIbβ3, and P-selectin; B, D, F – arbitrary density of GPIb, αIIbβ3, and P-selectin on platelets. N = 12–14, Mean ± SEM, one-way ANOVA followed by Dunnett’s multiple comparisons test: * - p < 0.05, ** - p < 0.01 vs. “No shear”.
ELISA detection of shedded fragments of platelet adhesion receptors and platelet secretory proteins
Recalcified GFP (100,000 platelets/uL, 1 mM CaCl2) were subjected to shear stress or biochemical activation as described above. Then, stimulated GFP were processed in sequential steps: centrifuged for 2000 × g for 15 min and 6000 × g for 20 min to sediment platelets, and 16000 × g for 30 min to sediment microparticles47. The resultant supernatant was snap-frozen and stored at −80°C until further analysis. Concentrations of soluble fragments of β3 subunit, GPIb (glycocalicin), and soluble P-selectin (sP-selectin) were quantified using commercial enzyme-linked immunosorbent assay (ELISA) kits: Human Integrin beta-3 ELISA Kit, Human Glycocalicin ELISA Kit (both from Biomatik Corporation, Ontario, Canada), and Human sP-selectin ELISA kit (eBioscience, San Diego, CA). As indicated by sample titration, no dilution of the samples was required. ELISAs were performed according to manufacturer instructions. Optical density (λ = 450 nm) was measured on Versa MAX microplate reader (Molecular Devices Corp., Sunnyvale, CA). Calibration curves and protein concentration calculations were made using SoftMax Pro6 software (Molecular Devices Corp., Sunnyvale, CA).
Platelet aggregation induced by shear stress or biochemical agonists.
Platelet aggregation was measured following PRP exposure to shear stress and/or biochemical agonists. The 300 μL aliquot of sheared PRP (200,000 platelets/μL) was transferred into the aggregation cuvette, and aggregation was initiated by recalcification with 1 mM CaCl2. The changes in relative light transmittance were recorded for 10 min by the optical aggregometer Platelet Aggregation Profiler PAP-8E (BioData Corporation, Horsham, PA). Maximal amplitude of aggregation was calculated by the PAP-8 software (BioData Corporation, Horsham, PA). To induce biochemical aggregation, 300 μL of non-sheared or sheared PRP (200,000 platelets/μL) were recalcified with 1 mM CaCl2, and increasing concentrations of biochemical agonists, ADP, 2-methylthio-ADP (MeS-ADP), or TRAP-6, were subsequently added. Platelet aggregation was then recorded.
In experiments aimed to study the potential role of ADP and P2Y12 receptor in shear-mediated effects on platelet aggregation, prior to shear exposure PRP were incubated with ADP-scavenger apyrase (1 U/ml) for 10 min48,49, then subjected to shear stress, and aggregation agonists as described above. Though, apyrase-resistant MeS-ADP (1–10 μM) was used as an ADP-mimetic to induce platelet aggregation in apyrase treated platelets50.
Statistical analysis
Results from 4 to 12 independent experiments with different donors were summarized in figures. All flow cytometry, ELISA, and aggregometry samples were run in duplicate. The data were tested for normality using Shapiro-Wilk normality test, and then statistically analyzed using one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparisons test, all from GraphPad Prism 8 software (GraphPad Software Inc., San Diego, CA). Averages are reported as the Mean ± SEM. The level of statistical significance is indicated on the figures as p ˂ 0.05 and p ˂ 0.01.
RESULTS
To evaluate the effect of MCS-related shear stress on platelet function in vitro, human platelets purified from other blood cells and plasma proteins were subjected to MCS-related continuous or pulsatile shear stress. The redistribution of key platelet adhesion receptors – αIIbβ3, GPIb, and P-selectin – on platelets and shear-generated PDMPs was examined. Additionally, shedding of soluble fragments of these receptors from the platelet surface was tracked, analyzing their content in the platelet media. The functional responsiveness of sheared platelets to physiologically relevant biochemical agonists, e.g. ADP, TRAP-6, and thrombin, was then tested to define retained platelet capability to undergo activation and aggregation following exposure to elevated shear stress. The potential contribution of ADP and P2Y12 signaling in shear-mediated effects on platelets was also tested.
Shear stress promotes downregulation of GPIb-IX-V, but not integrin αIIbβ3, on the platelet surface
First, we evaluated alterations of surface expression of GP Ib-IX-V and αIIbβ3 integrin on platelets under elevated shear stress conditions. Sheared platelets were stained with fluorescein-conjugated antibodies anti-CD42b binding GPIb and anti-CD41 binding αIIb subunit of integrin αIIbβ3. Then, the number and fluorescence intensity of stain-positive platelets were measured by flow cytometry. We found that platelet exposure to continuous but not pulsatile shear stress caused a significant decline in the number of platelets expressing GP Ib-IX-V (Figure 1.A). The fluorescence intensity of GPIb+ platelets exposed to shear stress was also decreased, as indicated by the left-side shift of the CD42b-fluorescence peak (Figure II.A). Both GPIb+ platelet number and their fluorescence gradually decreased with the magnitude of continuous shear stress applied, suggesting the downregulation of GP Ib-IX-V surface expression caused by shear exposure. Similarly, the number and fluorescence intensity of αIIbβ3+ platelets decreased when platelets were exposed to continuous and pulsatile hyper-shear (Figure 1C and II.C).
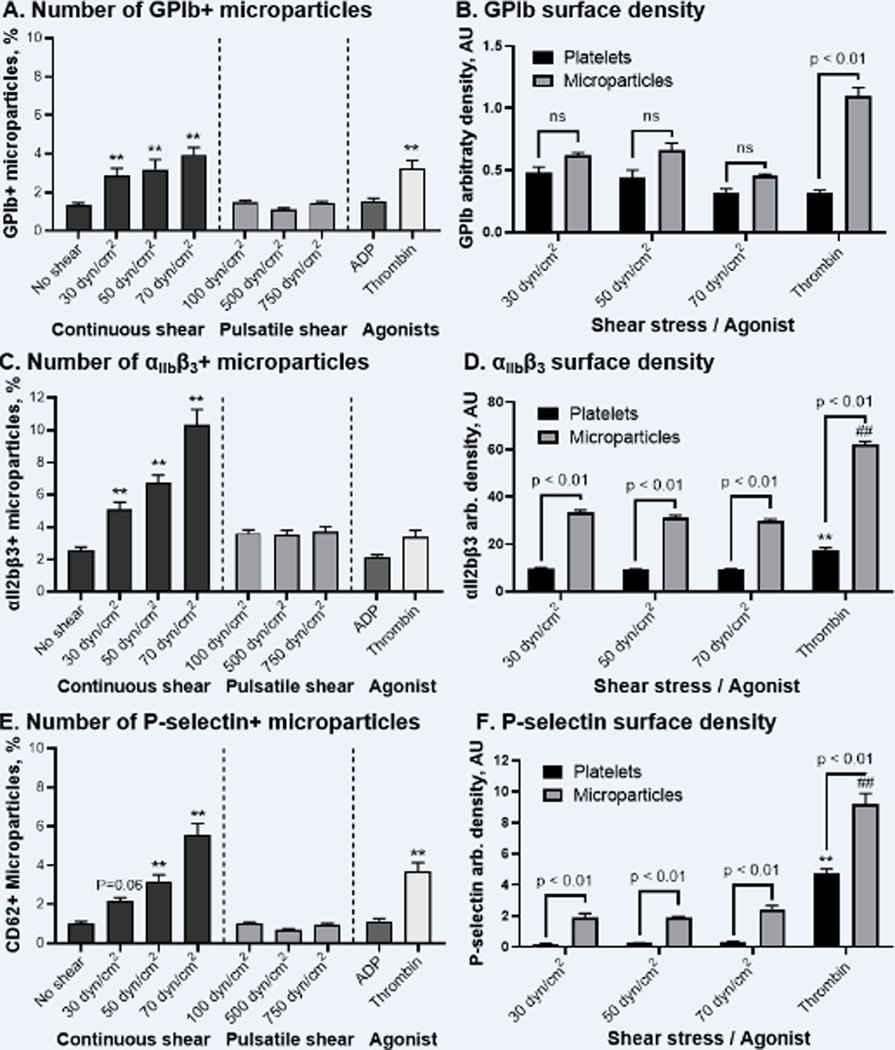
Figure 2. Elevated shear stress induces generation of GPIb-, αIIbβ3-, and P-selectin enriched microparticles. Thrombin stimulation also promoted ejection of GPIb- and P-selectin-, and to less extent αIIbβ3, positive microparticles.
A, C, E – number, of microparticles expressing GPIb, αIIbβ3, and P-selectin; B, D, F – arbitrary density of GPIb, αIIbβ3, and P-selectin on platelets as compared with microparticles. N = 12–14, Mean ± SEM, one-way ANOVA followed by Dunnett’s or Šidák’s multiple comparisons tests: **, ## - p < 0.01 vs. correspondent “No shear” group.
However, we also noticed that along with the shear exposure the size of GPIb+ and αIIbβ3+ platelets tended to shrink, as indicated by the decrease of their MFS (Figure II.B and II.D). Since the intensity of the fluorescence signal is proportional not only to the amount of fluorochrome on the particle surface but also to the particle diameter51, the shear-mediated decrease of GPIb and αIIbβ3 fluorescence signals may be a result of the decrease of platelet size rather than decrease of receptor density on the platelet surface. To test this hypothesis, we normalized CD42b and CD41 MFI to MFS indicating platelet size of GPIb+ and αIIbβ3+ platelets, thus calculating the arbitrary density of GP Ib-IX-V and αIIbβ3 on the platelet surface. We found that arbitrary density of GP Ib-IX-V significantly decreased following platelet exposure to 70 dyne/cm2 continuous shear stress (Figure 1B, ANOVA: p < 0.01), confirming that shear-mediated downregulation of GPIb surface expression did occur. However, the arbitrary density of αIIbβ3 integrin showed no significant change even following the highest continuous shear stress tested: 8.07 ± 0.35 AU vs. 8.65 ± 0.22 AU for 70 dyne/cm2 vs. non-sheared platelets (Figure 1D).
Platelet activation with biochemical agonists, ADP and thrombin, showed a differing effect on platelet receptor surface density. Stimulation with ADP, a well-known weak biochemical agonist, had no significant effect on GP Ib-IX-V and αIIbβ3 surface expression (Figure 1B & 1D). Thrombin however promoted prominent downregulation of GP Ib-IX-V, as indicated by 1.7-fold decline in the surface density (Figure 1B). In contrast, thrombin-stimulated platelets demonstrated significantly elevated density of αIIbβ3 integrin on their surface: 14.91 ± 1.05 AU vs. 8.65 ± 0.22 AU for intact platelets (Figure 1D). Similarly to sheared platelets, thrombin-treated platelets showed a decrease in size as indicated by the decrease of their MFS (Figure II.B and 2II.D).
Shear stress facilitates generation of platelet-derived microparticles enriched with αIIbβ3 and GPIb-IX-V receptors
We evaluated the effect of continuous and pulsatile shear stress on PDMP generation, and then characterized the distribution of platelet adhesion receptors GP Ib-IX-V and αIIbβ3 on the PDMP surface. We found that platelet exposure to continuous, and to a less extent pulsatile, shear stress induced generation of GPIb+ and αIIbβ3+ PDMPs, with the number of PDMPs increasing with the magnitude of shear stress applied (Figure III). Specifically, the number of GPIb+ microparticles increased with the magnitude of continuous shear yet was not altered by pulsatile shear regimens tested (Figure 2A). However, the percentage of αIIbβ3+ microparticles elevated more steeply, reaching 5.1 ± 0.4%, 6.7 ± 0.5%, and 10.3 ± 0.9% after 30, 50 and 70 dyne/cm2 continuous shear, respectively, and peaking at 3.5 ± 0.3% after 750 dyne/cm2 pulsatile shear (Figure 2C).
Figure 3. Shedding of soluble proteins from platelets following their exposure to shear stress or biochemical agonists.
A – soluble fragment of GPIb (glycocalicin); B – β3 subunit ectodomain (CD61), C – soluble P-selectin (sP-selectin). Red dash line (- - -) – the level of optical density of the lowest standard (detection limit of the ELISA kit): 7.5 pg/ml – for glycocalicin, 125 pg/ml - for soluble CD61. N = 4–6, Mean ± SEM, one-way ANOVA followed by Dunnett’s multiple comparisons test: ** - p < 0.01 vs. Intact platelets.
As indicated by MFS, the size of shear ejected GPIb+ and αIIbβ3+ microparticles remained unchanged across the populations (data not shown). Yet, GPIb fluorescence intensity and thus arbitrary density on microparticle surface tended to decrease with the increase of shear stress magnitude (Figures 2B, “Microparticle” group), suggesting a similar extent of receptor downregulation as was observed for platelets (Figure 1B). The GPIb surface density on shear-ejected microparticles was slightly higher than on sheared platelets (Figure 2B). In contrast, the surface density of αIIbβ3 integrin on microparticles remained unchanged regardless of the mechanical stimulus magnitude (Figures 2D). Yet, the surface density of αIIbβ3 integrin on shear-ejected PDMPs was nearly four-fold higher than on platelets (Figures 2D, one-way ANOVA: p < 0.01 for all sheared groups).
Among biochemical agonists, ADP did not promote generation of PDMPs, while thrombin stimulation resulted in minor generation of GPIb+ and αIIbβ3+ microparticles (Figure 2A and 2C). The surface density of GPIb-IX-V and integrin αIIbβ3 on thrombin-ejected microparticles appeared to be significantly higher as compared with platelets and shear-ejected PDMPs (Figure 2B and 2D, one-way ANOVA: p < 0.01 vs. all sheared groups).
P-selectin surface expression was barely elevated on platelets and microparticles following shear exposure
We also examined P-selectin surface expression on platelets and PDMPs under shear stress conditions. We showed that platelet exposure to pulsatile shear stress and low-magnitude continuous shear stress did not promote exposure of P-selectin on the platelet surface. Thus, the number and surface density of P-selectin+ platelets were not significantly elevated as compared with control group (Figure 1E and 1F, one-way ANOVA: p > 0.05). Platelet exposure to continuous elevated shear of 50 and 70 dyne/cm2 resulted in minor intensification of P-selectin externalization, with the number of P-selectin+ platelets increasing to 11.1 ± 1.1% and 14.8 ± 1.2%, respectively, as compared with 4.4 ± 0.4% in “No shear” group (one-way ANOVA: p < 0.01). In contrast, platelet activation with ADP and thrombin indeed facilitated P-selectin exposure on the platelet surface. Following ADP-mediated activation, the number of P-selectin+ platelets increased nearly 5-fold and P-selectin surface density tended to increase (Figure 1E and 1F). In the case of thrombin, a dramatic increase of P-selectin+ platelets up to 86.0 ± 1.6% was observed, as complemented by a 25-fold boost in fluorescence intensity.
Analyzing microparticle generation induced by shear and biochemical agonists, we found that no significant increase of P-selectin+ microparticles occurred following pulsatile and low-magnitude continuous shear exposure. Only elevated continuous shear stress of 50 and 70 dyne/cm2 led to an increase in the number of P-selectin+ PDMPs (Figure 2E). Platelet activation with ADP did not promote P-selectin+ microparticle generation, while thrombin resulted in a statistically significant increase of P-selectin+ microparticles up to 3.7 ± 0.5%. Interestingly, all populations of P-selectin+ microparticles ejected by shear stress and thrombin had 2–9 fold higher P-selectin surface density than activated platelets and shear-ejected microparticles (Figure 2F).
Platelet exposure to shear stress resulted in significant shedding of soluble P-selectin, yet neither glycocalicin nor β3 subunit ectodomain were shed
We examined whether platelet stimulation by mechanical shear stress alone could induce shedding of soluble fragments of GPIb, αIIbβ3 and P-selectin. Recalcified GFP was subjected to shear stress or the biochemical agonists, ADP and thrombin. Then, platelet media was purified from platelets and microparticles via sequential centrifugation47. Soluble fragments of platelet receptors were detected by ELISA using commercial kits. We found that shear stress did not promote shedding of GPIb fragment - glycocalicin. Glycocalicin was not detected in platelet media neither before nor after platelet exposure to shear stress for our experimental conditions (Figure 3A). Only following thrombin activation, a sharp increase of soluble GPIb was noted in the media, reaching detectable level of the protein (33.2 ± 5.6 pg/ml). Similarly, no soluble fragment of β3 subunit (CD61) of αII2bβ3 integrin was detected in the platelet media following exposure to shear stress and biochemical agonists. As indicated in Figure 3B, concentration of soluble CD61 in the media of sheared and biochemically activated platelets did not reach the detection limit of the kit (marked as a red dash line).
Yet, the concentration of soluble P-selectin in the media markedly increased with elevation of the shear stress magnitude, reaching 16.7 ± 2.0, 24.6 ± 2.2, and 40.7 ± 4.6 ng/ml after 30, 50, and 70 dyne/cm2 shear, respectively (Figure 3C). Platelet activation with thrombin, but not ADP, also resulted in more than a two-fold increase of soluble P-selectin concentration, as compared with intact platelet group (ANOVA: p < 0,01).
Exposure to shear stress did not promote αIIbβ3 integrin activation and platelet aggregation
To measure αIIbβ3 activation under shear conditions, sheared GFP were stained with fluorescein conjugated antibodies against inactive (anti-CD41) and active (anti-CD41/CD61, clone PAC-1) forms of the integrin. Then, CD41/CD61+ events were quantified within all CD41+ platelets as platelets exposing active integrin αIIbβ3. We observed that GFP exposure to continuous shear stress did not promote activation of αIIbβ3 integrin, as the number of CD41/CD61+ platelets remained equal to non-sheared control regardless of the shear stress magnitude applied (Figure 4A, one-way ANOVA: p > 0.05). Yet, platelet exposure to biochemical agonists, e.g. ADP, thrombin, or its mimetic TRAP-6, caused a steep increase in the number of platelets expressing active form of integrin αIIbβ3. Thus, platelet activation with ADP led to an increase of CD41/CD61+ platelet number to 66.0 ± 4.0%. Thrombin resulted in the highest extent of αIIbβ3 activation with the nearly 7-fold increase of CD41/CD61+ platelet number as compared with control (Figure 4A, ANOVA: p < 0.01).
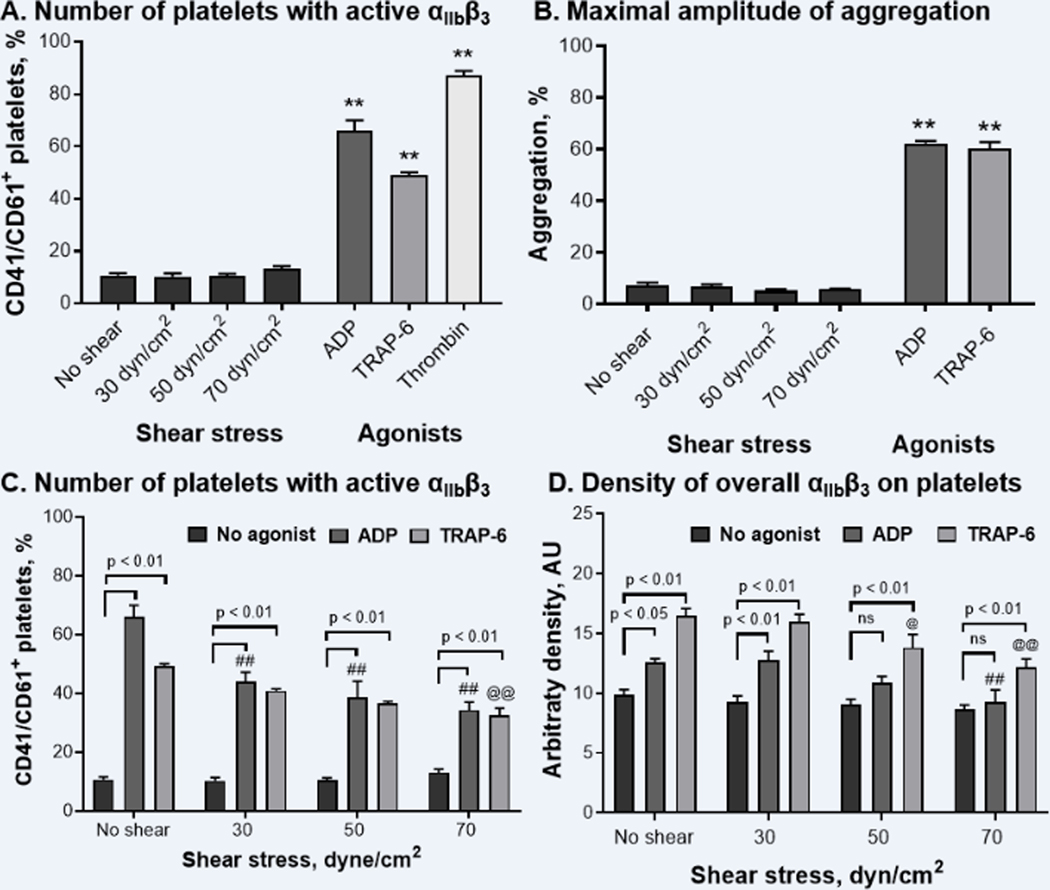
Figure 4. Shear stress alone does not induce αIIbβ3 activation nor platelet aggregation, while preventing activation and upregulation of integrin αIIbβ3 mediated by biochemical agonists.
A – number of platelets presenting activated αIIbβ3, B – maximal amplitude of platelet aggregation induced by shear and biochemical agonists in platelet rich plasma (n = 4), C – number of platelets presenting active αIIbβ3 on their surface, D – overall surface density of αIIbβ3 (inactive and active) on platelet surface. Platelets were double stained with anti-CD41/CD61 (PAC-1) and anti-CD41 antibodies to detect activated and non-activated forms of αIIbβ3, correspondingly. Platelets exposing activated αIIbβ3 (CD41/CD61+ platelets) were distinguished within CD41+ platelets, and their platelet number was expressed as % of all events. N = 6–8, Mean ± SEM, one-way ANOVA followed by Dunnett’s multiple comparisons test: ** - p < 0.01 vs. “No shear”; ##, @@ - p < 0.01 vs. “ADP: No shear” and “TRAP-6: No shear” groups, correspondingly.
To stimulate platelet aggregation, PRP was exposed to continuous shear stress in the HSD, recalcified, and platelet aggregation was recorded for 10 min. Alternatively, biochemical agonists, ADP and TRAP-6, were used as positive controls. We showed that continuous shear stress did not promote platelet aggregation in PRP, while biochemical agonists indeed induced high-amplitude platelet aggregation. Thus, following shear exposure the maximal amplitude of platelet aggregation was equal or even lower than spontaneous aggregation registered in recalcified PRP incubated for 10 min with stirring and no agonist added (Figure 4B). Maximal aggregation induced by ADP and TRAP-6 reached 62.2 ± 0.9% and 60.2 ± 2.5% respectively as compared with 7.2 ± 1.0% in unstimulated PRP (Figure 4B).
Flow cytometry of sheared PRP samples indicated that no shear-mediated platelet aggregation occurred directly in the HSD cuvette during exposure to shear stress. As reported in Figure IV.A–D, the number of CD41+ events in the joint gate “Platelets + Microparticles” located in quadrant 3 (Q3) was not significantly altered reaching 96.1%, 96.6% and 98.7% after 30, 50 and 70 dyne/cm2 shear exposure. At the same time, number of platelet aggregates captured in quadrants 2 and 4 (Q2 and Q4) did not exceed 2.3% of all CD41+ events. We also found that overall platelet size in sheared PRP, as indicated by FS distribution, significantly decreased following exposure to continuous shear of elevated magnitudes (Figure IV.E featuring left-side shift of FS distribution peak as compared with non-sheared control, and Figure IV.E summarizing alterations of MFS of sheared PRP in a bar graph).
Shear stress prevents activation of αIIbβ3 integrin and platelet aggregation induced by biochemical agonists
To further evaluate if shear stress exposure modulates the platelet functional response to biochemical stimuli, we analyzed the extent of αIIbβ3 integrin activation and upregulation on sheared platelets activated by ADP and TRAP-6. We found that shear stress greatly diminished αIIbβ3 activation induced by both biochemical agonists. Thus, even following 30 dyne/cm2 shear exposure the number of CD41/CD61+ platelets of ADP-stimulated platelets was significantly decreased as compared with non-sheared control (Figure 4C, one-way ANOVA: p < 0.01 vs. “No shear: ADP”). Following 70 dyne/cm2 shear, the number of platelets expressing active αIIbβ3 integrin reached only 34.3 ± 2.7%, as compared to 66.1 ± 4.0% in ADP stimulated non-sheared group. Similarly, the number of CD41/CD61+ platelets in the TRAP-stimulated group was significantly lower following 70 dyne/cm2 shear exposure: 32.4 ± 2.5% vs 49.2 ± 0.9% in non-sheared control (Figure 4C).
The upregulation of αIIbβ3 integrin expression on the platelet surface induced by biochemical agonists was also diminished by shear stress. We demonstrated that platelet activation with ADP and TRAP-6 alone induced a significant increase of αIIbβ3 surface density: 12.6 ± 0.3 AU and 16.5 ± 0.6 AU vs. 9.1 ± 0.4 AU in control. However, platelet exposure to shear stress of 70 dyne/cm2 resulted in significantly lower upregulation of αIIbβ3 surface expression following biochemical activation (Figure 4D). Thus, TRAP-6 stimulation promoted only a slight increase of αIIbβ3 arbitrary density, while ADP had no significant effect (Figure 4D, ANOVA: p < 0.01 for “70: TRAP-6” vs “70: No agonist”, p > 0.05 for “70: ADP” vs. “70: No agonist”).
To assess the effect of shear stress on platelet aggregation induced by biochemical agonists, PRP was exposed to shear stress in the HSD, recalcified, and stimulated with increasing concentrations of ADP or TRAP-6. We observed that both ADP (1–10 μM) and TRAP-6 (5–32 μM) induced platelet aggregation in the concentration range tested, while shear stress significantly diminished the platelet aggregatory response. Thus, following exposure to 30 and 50 dyne/cm2 shear, platelets responded to all ADP concentrations with the full extent aggregation (Figure 5A). Yet, the amplitude of ADP-induced aggregation was heavily affected by 70 dyne/cm2 shear even when 10 μM ADP concentration was applied (Figure 5A, “70 dyn/cm2: 10 uM” ADP vs. “No shear: 10 uM ADP”, one-way ANOVA: p > 0.01). With TRAP-6 stimulation, sheared platelets demonstrated no differential sensitivity to the increase of agonist concentration. Even after exposure to 50 dyne/cm2 continuous shear, platelets responded with high-amplitude platelet aggregation on all concentrations of TRAP-6 tested (Figure 5B). Yet, the amplitude of TRAP-6 induced aggregation was two-fold decreased when 70 dyne/cm2 shear stress was applied.
Figure 5. Shear stress inhibits platelet aggregation stimulated by increasing concentrations of biochemical agonists, ADP and TRAP-6, in platelet-rich plasma. Preservation of P2Y12 receptor from desensitization using apyrase did not prevent inhibition of platelet aggregation by shear stress (70 dynes/cm2).
Maximal amplitude of platelet aggregation induced by biochemical agonists ADP (A), TRAP-6 (B, D), and MeS-ADP (C) is reported in corresponded bar graphs: n = 4–5, Mean ± SEM, one-way ANOVA followed by Dunnett’s multiple comparisons test: *, # - p < 0.05, $ $, ##, @@, **, ## - p < 0.01 vs. correspondent “No shear” group.
We suggested that the observed inhibitory effect of shear stress on ADP-mediated platelet aggregation could be assigned to the desensitization of ADP-evoked P2Y12 receptor by pre-exposure to ADP released by platelets from their dense granules as a result of shear stimulation and/or mechanical damage. To prevent P2Y12 desensitization, PRP was pre-treated with 1 U/ml apyrase aimed at enzymatic cleavage of ADP/ATP released in platelet media during shear exposure48,49,52. First, we showed that MeS-ADP induced more potent platelet aggregation than its native counterpart at lower concentrations tested (Figure 5C, “No shear: No Apyrase” block vs. Figure 5A, “No shear”, 1 & 2 μM ADP). Nonetheless, 70 dyne/cm2 shear stress effectively inhibited MeS-ADP platelet aggregation across entire concentration range, and apyrase did not reverse the anti-aggregatory effect of shear stress on ADP-induced aggregation (Figure 5C). Similarly, no protective effect of apyrase was found towards shear-mediated inhibition of TRAP-6 platelet aggregation (Figure 5D). Interestingly, exogenous apyrase slightly potentiated the antiaggregatory effect of shear stress, so the maximum amplitude of aggregation in the apyrase-treated group was slightly decreased as compared with the untreated group after shear exposure (Figure 5D, Shear Stress: No apyrase vs. Shear stress: Apyrase).
Ticagrelor did not prevent shear-mediated downregulation of GPIb and generation of receptor-enriched microparticles
To evaluate the co-stimulatory role of ADP and P2Y12 signaling in shear-mediated effects on platelet receptors and microparticle generation, platelets were preincubated with 10 μM ticagrelor, a highly potent and specific inhibitor of P2Y12 receptor and ADP-mediated aggregation41,42. Ticagrelor-treated platelets were then subjected to continuous shear stress of 30 and 70 dyne/cm2 in the HSD or to biochemical agonists, ADP and thrombin. Surface expression of platelet adhesion receptors and generation of PDMPs were quantified by flow cytometry as described above. We found that the number of GPIb+ platelets and GPIb surface density significantly declined after exposure to shear stress of 30 and 70 dyne/cm2 in ticagrelor-treated GFP, similarly to the untreated control (Figure 6A and V.A). Similarly, shear- and thrombin-mediated generation of GPIb+ microparticles was not affected by P2Y12 inhibition (Figure 6B and V.B).
Figure 6.
P2Y12 receptor blockade with ticagrelor does not divert shear-mediated alteration of GPIb, αIIbβ3, and P-selectin surface expression on platelets, and generation of receptor-enriched microparticles.A, C, E, Number of platelets; B, D, F, number of microparticles expressing GPIb, αIIbβ3, and P-selectin. n=6, mean±SEM, 1-way ANOVA followed by Dunnett multiple comparisons test: *#P<0.05, **##P<0.01 vs correspondent no shear group.
Studying the effect of ticagrelor on shear-mediated alterations of αIIbβ3 surface expression, we noted that ticagrelor had no effect on shear-mediated decease of αIIbβ3+ platelet population and correspondent elevation of αIIbβ3+ microparticle number following platelet exposure to continuous shear stress (Figure 6C and 6D). In contrast, ticagrelor significantly inhibited upregulation of αIIbβ3 surface expression potentiated by ADP and thrombin, as indicated by a correspondent decrease of integrin αIIbβ3 arbitrary density in ticagrelor-treated platelets, as compared with “No drug” groups activated with ADP and thrombin (Figure V.B, one-way ANOVA: p < 0.01, P < 0.05, respectively). Lastly, ticagrelor did not alter shear-mediated upregulation of P-selectin surface expression as indicated by the minor increase of P-selectin+ platelets in both ticagrelor-treated and control groups following exposure to 70 dyne/cm2 shear stress (Figure 6E). Neither did ticagrelor affect shear-mediated ejection of P-selectin+ microparticles: the number of P-selectin+ microparticles equally increased in drug-treated and untreated groups (Figure 6F). Yet again, the inhibition of P2Y12 signaling resulted in dramatic decline of P-selectin exposure induced by ADP (Figure 6E, one-way ANOVA: p < 0.01 for “ADP: No Drug” vs. “ADP: Ticagrelor”), yet thrombin-mediated upregulation of P-selectin on platelets and microparticle generation were resistant to ticagrelor inhibition (Figure 6E and 6E).
DISCUSSION
Here, we report that platelet exposure to elevated shear stress, representative of repeated passages through supraphysiologic conditions in MCS devices, was associated with a decrease of both GPIb and integrin αIIbβ3 surface expression. Following shear exposure, platelet size was decreased with a corresponding increase in generation of PDMPs. Of note, correlating with this decrease of platelet surface expression of adhesion receptors, a corresponding increase in GPIb and αIIbβ3 detected on shear-ejected microparticles was observed. Yet, no shear-mediated shedding of soluble fragments of GPIb and β3 subunit was detected free in surrounding media. In contrast, platelet activation with thrombin, a strong biochemical agonist, promoted mild shedding of GPIb+ PDMPs and soluble GPIb fragments, also resulting in a significant decrease of GPIb surface density on platelets. In distinction to shear, the surface density of integrin αIIbβ3 increased two-fold after platelet activation by thrombin. While surface expression of P-selectin on platelets was not significantly elevated, a soluble form of P-selectin was extensively shed from the platelet surface due to shear exposure, suggesting ongoing shear-mediated release of platelet α-granules. Platelet activation with thrombin led to a dramatic increase of both P-selectin expression on the platelet surface and shedding of its soluble form, similarly indicating massive degranulation. Lastly, we showed that shear-mediated downregulation of GPIb and αIIbβ3 receptors on platelets was associated with decreased activation of integrin αIIbβ3 and consequently impaired platelet aggregation response to biochemical agonists, ADP and TRAP-6. The co-stimulatory effect of ADP and P2Y12 signaling was ruled out as potential contributor to the anti-platelet effect of elevated shear stress on platelet function, as 1) apyrase, an ADP-scavenger, did not restore platelet aggregatory response decreased by shear, and; 2) ticagrelor, a highly specific inhibitor of P2Y12, did not prevent shear-mediated downregulation of GPIb and generation of receptor-enriched microparticles.
Within the last two decades, MCS has emerged as an increasingly utilized lifesaving therapy for patients with advanced and end-stage heart failure1. Sadly, MCS therapy remains limited by a paradoxical coagulopathy accompanied by both thrombosis and bleeding4. In recent years, with improvement of devices designs, related management and therapeutic advances, device-related thrombosis has been reduced. Nevertheless, bleeding, in particular non-surgical bleeding, has increasingly been regarded as a major adverse event of concern, responsible for hospital readmissions and impaired quality of life for patients on both temporary and chronic MCS4. Our group, along with others, has recognized that platelet dysfunction caused by continuous exposure to elevated shear stress for extended durations of device-supported circulation, leads to stress accumulation in platelets and is a major contributor to device-related coagulopathy9,53,54. Platelet exposure to continuous shear stress in vitro induces pro-apoptotic alterations of the platelet phenotype, e.g. mitochondrial collapse, membrane depolarization, and promoting massive microvesiculation9,28. Recently, it has been shown that a persistent pro-apoptotic phenotype, accompanied by decreased levels of platelet adhesion receptors55,56 and elevated levels of circulating microparticles,29,30 were characteristic of VAD-implanted patients, being more pronounced in subjects experiencing post-implant non-surgical bleeding as opposed to non-bleeders54,57.
Platelet adhesion and aggregation rely largely on GP Ib-IX-V and αIIbβ3 – the two most abundant adhesion receptors constitutively expressed on the platelet surface. Interactions between GP Ib-IX-V and its ligand vWF are responsible for capture and arrest of circulating platelets at sites of vascular injury, and for initiation of platelet activation under shear conditions of normal circulation58. Integrin αIIbβ3 further reinforces platelet adhesion and aggregation, bridging platelets to one another via fibrinogen bonds59. Here, we aimed to define if mechanical shear stress, as a driving force of device-related coagulopathy, can modulate the surface expression of major platelet adhesion receptors, thus affecting platelet hemostatic function – activation and aggregation in response to biochemical stimuli. Human platelets were isolated from other blood cells and proteins, and then subjected to continuous or pulsatile shear stress of physiologic and pathologic magnitudes for extended durations - as representative patterns of the stress accumulation conferred on platelets due to repeated passages through transient supraphysiologic shear stress conditions in MCS devices. This previously-validated model allows for examination of the direct effect of mechanical shear stress on platelets, un-confounded by responses mediated by platelet interactions with shear-altered plasma proteins and cells9.
We found that continuous shear exposure resulted in a notable decrease of GPIb surface expression on platelets, as indicated by significant drop of GPIb+ platelet number and surface density of platelet GPIb (Figure 1A and 1B). GPIb downregulation and microparticle generation was not affected by ticagrelor, which indicates its independent from costimulatory signaling via ADP-evoked P2Y12 receptor. These observations are in agreement with previous reports showing that exposure of anticoagulated blood or PRP to supra-physiological shear stress in vitro also promotes a notable decrease of GPIb and GPVI surface expression on platelets via enzymatic shedding of their soluble fragments by platelet ADAMs60–62. Yet, other studies indicate that shear-mediated binding of plasma vWF to platelet GPIb, rather than shear itself, is required to cause enzymatic shedding of GPIb from platelet surface63. Moreover, inhibition of ADAM activity does not fully recover the levels of GPIb on sheared platelets, nor does it restore platelet vWF-binding and aggregation capabilities, thus suggesting that other mechanisms of shear-mediated downregulation of GPIb may be involved64. Here, we clearly demonstrate that shear stress alone does not promote shedding of GPIb soluble fragment glycocalicin (Fig. 3A), rather induces a modest increase of PDMPs which express GPIb on their surface (Fig. 2A). Interestingly, the surface density of GPIb on PDMPs was higher than on platelets exposed to shear stress (Fig. 2B).
We further compared shear-mediated effects on GPIb with those induced by biochemical agonists, ADP and thrombin. ADP showed no effect on GPIb surface expression, did not induce PDMP generation nor shedding of glycocalicin. Yet, platelet activation with thrombin resulted in a notable decrease of platelet GPIb surface expression, generation of GPIb+ PDMPs, and release of GPIb soluble fragment in platelet media. We speculate that thrombin-induced shedding of glycocalicin could be mediated by vWF and ADAMs released during platelet degranulation65. In our experiments, thrombin indeed promoted extensive degranulation as indicated by an increase of P-selectin on the platelet surface and release of its soluble form (Fig. 1E, 1F, and 3C). Therefore, our data suggest that shear-mediated microparticle generation serves as an alternative downregulation mechanism limiting GPIb surface expression on platelets via its redistribution to PDMPs. Consistently, no soluble GPIb fragment is shed in GFP due to shear exposure when vWF is in fact absent or limited by the endogenous platelet pool.
Evaluating the effect of shear stress and biochemical agonists on surface expression of platelet αIIbβ3 integrin, we found that platelet activation with thrombin and ADP resulted in a significant increase of αIIbβ3 surface expression on the platelet surface (Fig. 1D). Such upregulation is likely caused by platelet degranulation and fusion of granule membranes with the plasma membrane of platelets, leading to a quantitative transfer of additional αIIbβ3 integrin heterodimer complexes, with a resultant increase of its density on the platelet surface66. We indeed found an increase of P-selectin surface expression and release of soluble P-selectin following platelet activation with thrombin (Fig. 1E, 1F, and 3C). Observed upregulation αIIbβ3 and P-selectin surface expression induced by ADP and thrombin was partially inhibited by ticagrelor, a selective inhibitor of P2Y12 receptors, confirming the importance of ADP-mediated co-stimulatory signaling in platelet activation by biochemical agonists67. In contrast, platelet exposure to shear stress did not promote significant alterations of αIIbβ3 surface expression on platelets (Fig. 1D), rather αIIbβ3+ platelet was gradually increased after both continuous and pulsatile shear (Fig. 1C). Therefore, taking into consideration the notable increase of soluble P-selectin observed after platelet shear exposure (two-fold higher than the thrombin-mediated level), we suggest that shear-mediated upregulation of αIIbβ3 surface expression might have initially occurred transiently as a result of α-granule release, yet failed to be detected due to the following rapid integrin redistribution into the shear-ejected αIIbβ3+ microparticles. Our findings collectively support this statement. We found that, unlike biochemical agonists, shear stress induced a prominent increase of αIIbβ3+/P-selectin+ PDMPs, with the number of PDMPs increasing with the magnitude of shear applied (Fig. 2C & 2E). Furthermore, the surface density of αIIbβ3 on shear ejected PDMPs is four times (!) higher than on platelets (Fig. 2D). Simultaneously, no soluble fragment of the integrin β3 subunit was detected in the media of sheared GFP. It is noteworthy that shear-ejected PDMPs express increased levels of P-selectin (Fig. 2F), which further supports our hypothesis, clearly indicating that shear-forced separation of microparticles from the platelet body occurs after α-granule release.
While the underlying mechanism of shear-mediated receptor sorting remains unclear, several lines of evidence suggest that modulation of platelet membrane lipid distribution by shear could be involved of shear-mediated receptor sorting and selective shedding during microvesiculation. It is well documented that clusterization of platelet GP receptors in lipid rafts is essential for receptor signal transduction and further coordination of downstream events during platelet activation. On the other hand, it was recently reported that lipid rafts are required for release of microvesicles by platelets68, and PDMPs themselves are enriched with cholesterol rafts69. We previously showed that shear exposure drastically increased platelet membrane fluidity70, which may result from membrane cholesterol depletion via PDMP generation. These findings along with our data suggest that shear stress could promote clustering of GPIb and αIIbβ3 integrin complexes in lipid rafts and further induce their sorting and release within PDMP membranes. This mechanism is consistent with the well-recognized precedent in cell biology for lateral diffusion and mobility of plasma membrane constituents, induced via a range of stimuli71–73.
Unlike GP Ib-IX-V and αIIbβ3 receptors which are constitutively expressed on the platelet membrane, the surface expression of P-selectin is inducible. In resting platelets, P-selectin is stored in the membrane of α-granules. Upon platelet activation and hence degranulation, P-selectin is translocated on the platelet surface74. Via P-selectin, platelets interact with leukocytes and endothelial cells promoting their activation and expression of pro-inflammatory proteins. When underutilized, platelet P-selectin undergoes rapid “shut down” via shedding of soluble fragment (sP-selectin) from the platelet surface16. In a previous study we found that, unlike biochemical agonists, platelet activation mediated by shear stress does not result in massive P-selectin exposure9. Here, we elaborate on this finding and show that the low-level P-selectin exposure mediated by shear is associated with generation of P-selectin+ PDMPs and massive shedding of sP-selectin in the platelet media. Interestingly, thrombin promoted a massive “all at once” response, striking massive increase of P-selectin on platelets, release of P-selectin+ PDMPs, and shedding sP-selectin from the platelet surface. Cumulatively, our findings indicate that mechanical stimulation of platelets via shear stress indeed promotes α-granule secretion with the follow-on rapid shedding of sP-selectin, thereby rendering it been “undetectable” and deficient on the platelet surface after shear exposure. These findings are in agreement with an early proteomic study which documented the presence of P-selectin in the protein profile of PDMPs ejected by both shear stress and thrombin24, thus providing solid confirmation of shear-mediated platelet degranulation which is followed by microparticle release. Furthermore, recent in vivo studies examining platelet and leucocyte function in VAD-supported patients also report no significant increase of platelet surface P-selectin following device implantation75,76, yet reveal elevated levels of platelet-monocyte aggregates and ongoing monocyte activation76. It was previously shown that elevated levels of P-selectin and P-selectin+ PDMPs in plasma accelerated coagulation and intravascular fibrin deposition77, and promoted leukocyte aggregation under flow conditions78. Taken together, this evidence suggests that elevated levels of circulating P-selectin+ PDMPs, soluble P-selectin, and to less extent P-selectin carrying platelets promote prothrombotic and pro-inflammatory state accompanying MCS.
Platelet aggregation in blood is largely preconditioned by activation of integrin αIIbβ3. In resting platelets, αIIbβ3 adopts an inactive conformation with low affinity to its primary protein ligands, fibrinogen and vWF59. Following platelet exposure to biochemical stimuli, as ADP and thrombin, integrin αIIbβ3 undergoes a series of conformational changes leading to an increase of receptor adhesiveness. Activated integrin αIIbβ3 binds fibrinogen present in plasma or released from platelet α-granules bridging platelets to one another upon platelet aggregation. Here, we show that unlike biochemical agonists, mechanical shear stress does not cause αIIbβ3 activation nor platelet aggregation. All biochemical agonists tested (ADP, thrombin, and TRAP-6) promoted a notable increase of CD41/CD61+ platelets, while sheared GFP revealed no activated αIIbβ3 present on their surface. Yet, the following activation of sheared platelets with biochemical agonists resulted in diminished αIIbβ3 levels, as compared with non-sheared control. In PRP where a sufficient amount of fibrinogen is present, ADP and TRAP-6 induced high-extent platelet aggregation, while shear stress alone did not promote platelet aggregation. However, exposure of sheared and recalcified PRP to ADP and TRAP-6 resulted in a decreased platelet aggregation response. Interestingly, both ADP and TRAP-6 induced aggregation was compromised following 70 dyne/cm2 shear stress, while unaffected by physiological magnitudes of shear stress. Our findings are in alignment with the previous report by Mondal et al., demonstrating decreased levels of activated αIIbβ3 in ADP stimulated platelets subjected to short-term (0.05–1.5 s) supraphysiologic shear stress79.
We hypothesized that the anti-aggregatory effect of shear stress could be potentiated by desensitization of ADP-evoked P2Y12 receptors due to prolonged exposure to ADP/ATP supposedly released from platelet dense granules or platelets themselves as a result of shear-mediated damage. Yet, we showed that apyrase, added to PRP prior to shear exposure to protect P2Y12 from desensitization, did not revert inhibitory effect of shear on ADP- and TRAP-6 induced platelet aggregation. In case of TRAP-6 mediated platelet aggregation, apyrase even slightly promoted antiaggregatory effect of shear, thus suggesting that P2Y12 costimulatory signaling might contribute to thrombin mediated platelet aggregation67. While desensitization of P2Y12 does not contribute to anti-platelet effect of hyper-shear revealed in our study. Our findings are in agreement with observations by others. As such, Al-Tamimi et al. demonstrated that shear-mediated receptor shedding was not inhibited by apyrase, neither it required intracellular calcium flux49.
Alternatively, the decreased aggregability of sheared platelets could be caused by downregulation of platelet adhesion receptors and corrupted activation of integrin αIIbβ3. However, as can be seen in Fig. 4C, a significant amount of functional αIIbβ3 is present even after the highest shear magnitude applied (>50% of control level). In our previous study, we have shown that shear stress promotes pro-apoptotic platelet behavior, associated with the dissipation of platelet mitochondria membrane potential and membrane depolarisation9. It was complementarily reported that, unlike platelet activation, platelet apoptotic events, i.e. mitochondrial collapse, gelsolin cleavage, and membrane depolarization, are not affected by apyrase and PGE180,81. Thus, integrating together our findings and reports of others, we conclude that reduction of platelet mitochondria function, downregulation of platelet adhesion receptors, and reduced integrin αIIbβ3 activation – all collectively contribute to the observed diminished platelet aggregation response to biochemical agonists caused by shear stress. Shear-mediated decrease of platelet function could be considered as a “silencing or control mechanism” limiting excessive platelet activation in normal circulation. However, when out of control, shear-mediated platelet dysfunction contributes to the device-related coagulopathy of MCS patients.
We recognize a few methodological limitations which however do not diminish the significance of this study. The shear stress conditions applied in this study were chosen based on our previous numerical studies of hemodynamics of MCS devices as compared with physiological levels of shear existing within the circulation40. While we did not directly utilize MCS devices, our previous observations of a strong correlation between stress accumulation and platelet activation at physiological, sub-pathological, and supraphysiologic shear stress magnitudes support the selection of the shear stresses and exposure duration used in this study37,40. Yet, we note that continuous shear stress of shorter duration and lower magnitudes rendered a more significant effect on platelet receptor surface expression and microparticle generation than pulsatile hyper-shear. This observation dictates the need in a follow-up ex vivo study testing the anti-platelet effect of MCS devices in settings allowing application of higher magnitudes of pulsatile shear (1000× over 100× dyne/cm2) and longer exposure time (hours over minutes). While plasma protein-free GFP offers a good model for testing of “pure” effect of mechanical force on platelet phenotype and function, the presence of plasma proteins, i.e. vWF and fibrinogen, is ultimately required to integrate our observations with other elements of Virchow triad driving thrombosis and inflammation in vivo. Therefore, to extend on this work, we plan to examine platelet phenotype and circulating PDMPs in MCS-implanted patients to further define the translation potential of our observations.
Routine flow cytometry used to capture and characterize shear-ejected PDMPs is known to underestimate the low-size microparticle population (<300 nm) and exosomes (<100 nm). Therefore, the number of shear-mediated microvesicles detected in our study might also be underestimated. The human Integrin beta-3 ELISA kit used to detect shear-mediated αIIbβ3 shedding was selected based on the speculations of previous reports suggesting that only β3 subunit of the integrin complex is susceptible to shedding and enzymatic cleavage82. However, we do not exclude the possibility that alternative fragments of αIIbβ3, which might be detected by anti-CD41/CD61 antibodies targeting both integrin subunits, could be shed due to shear stress.
In summary, our findings indicate that mechanical shear stress promotes exocytosis of platelet α-granules, exposure of P-selectin on the platelet surface and follow-on extensive shedding of sP-selectin in the media. Subsequently, platelet exposure to shear stress causes notable downregulation of primary platelet adhesion receptors GPIb and αIIbβ3 via physical redistribution to PDMPs, rather than enzymatic shedding of their ectodomains. The illustrative “deck chair” model has been developed: it depicts adhesion receptors shedded from platelet surface by shear force as deck chairs shedded from the deck of the ship by strong wind (see graphic abstract). It is noteworthy that platelet microvesiculation occurs after the release of platelet α-granules, as indicated by the presence of P-selectin on PDMP surface and elevated surface density of integrin αIIbβ3. Thrombin, a powerful biochemical agonist of platelet activation, also promotes massive degranulation resulting in a striking increase of P-selectin and upregulation of αIIbβ3 surface expression on platelets. Thrombin-activated platelets show decreased levels of GPIb on their surface which may be attributed to modest shedding of soluble GPIb fragment and GPIb+ PDMPs. It appears that following shear exposure, platelets have diminished sensitivity to activation with biochemical agonists, showing reduced levels of activated integrin αIIbβ3 and lower aggregatory response than non-sheared platelets. Therefore, our data clearly indicate that high shear stress existing within device-supported circulation 1) induces adequate platelet degranulation, yet, 2) causes downregulation of primary platelet adhesion receptors via ejection of receptor-enriched PDMPs thus mechanistically limiting platelet activation and the aggregatory response.
Supplementary Material
HIGHLIGHTS.
Mechanical shear stress promotes exocytosis of platelet α-granules, exposure of P-selectin on platelets, and follow-on extensive shedding of sP-selectin
Shear stress causes downregulation of platelet adhesion receptors GPIb and αIIbβ3 via redistribution to receptor-enriched microparticles
Sheared platelets show reduced levels of activated integrin αIIbβ3 and lower aggregation stimulated by biochemical agonists
Acknowledgments
SOURCES OF FUNDING. This work is supported by grants from the National Institutes of Health (U01 HL131052 to Danny Bluestein and Marvin J. Slepian), the University of Arizona BIO5 Institute (Postdoctoral Fellowship to Yana Roka-Moiia), the University of Arizona Sarver Heart Center (Jack and Mildred Michelson Cardiovascular Research Award and John H. Midkiff Cardiovascular Research Award to Yana Roka-Moiia), and by the Arizona Center for Accelerated Biomedical Innovation of the University of Arizona.
ABBREVIATIONS
- ADAM
a disintegrin and metalloprotease
- ADP
adenosine diphosphate
- ANOVA
one-way analysis of variance
- AU
arbitrary unit
- CD
cluster of differentiation
- ELISA
enzyme-linked immunosorbent assay
- MFS
median forward scatter
- GFP
gel-filtered platelets
- GP
glycoprotein
- MCS
mechanical circulatory support
- MFI
median fluorescence intensity
- PDMP
platelet derived microparticle
- PRP
platelet-rich plasma
- SEM
standard error of mean
- SSC
side scatter
- TRAP-6
thrombin receptor activating peptide-6
- VAD
ventricular assist device
- vWF
von Willebrand factor
Footnotes
DISCLOSURE. None.
REFERENCES
- 1.Levine A, Gupta CA, Gass A. Advanced Heart Failure Management and Transplantation. Cardiology Clinics. 2019;37:105–111. [DOI] [PubMed] [Google Scholar]
- 2.Bartoli CR, Dowling RD. Next-generation mechanical circulatory support devices in different phases of development and clinical use are anticipated to improve outcomes and quality of life in patients with advanced heart failure. The Next Wave of Mechanical Circulatory Support Dev. Cardiac Interventions Today. 2019;13:53–59. [Google Scholar]
- 3.Doligalski CT, Jennings DL. Device-Related Thrombosis in Continuous-Flow Left Ventricular Assist Device Support. Journal of Pharmacy Practice. 2016;29:58–66. [DOI] [PubMed] [Google Scholar]
- 4.Baumann Kreuziger L, Massicotte MP. Mechanical circulatory support: balancing bleeding and clotting in high-risk patients. Hematology. 2016;2015:61–68. [DOI] [PubMed] [Google Scholar]
- 5.Teuteberg JJ, Cleveland JC, Cowger J, Higgins RS, Goldstein DJ, Keebler M, Kirklin JK, Myers SL, Salerno CT, Stehlik J, Fernandez F, Badhwar V, Pagani FD, Atluri P. The Society of Thoracic Surgeons Intermacs 2019 Annual Report: The Changing Landscape of Devices and Indications. Annals of Thoracic Surgery. 2020;109:649–660. [DOI] [PubMed] [Google Scholar]
- 6.Kroll MH, Hellums JD, McIntire L V, Schafer AI, Moake JL. Platelets and shear stress. Blood. 1996;88:1525 LP – 1541. [PubMed] [Google Scholar]
- 7.Chan CHH, Pieper IL, Robinson CR, Friedmann Y, Kanamarlapudi V, Thornton CA. Shear Stress-Induced Total Blood Trauma in Multiple Species. Artificial Organs. 2017;41:934–947. [DOI] [PubMed] [Google Scholar]
- 8.Slepian MJ, Sheriff J, Hutchinson M, Tran P, Bajaj N, Garcia JGN, Scott Saavedra S, Bluestein D. Shear-mediated platelet activation in the free flow: Perspectives on the emerging spectrum of cell mechanobiological mechanisms mediating cardiovascular implant thrombosis. Journal of biomechanics. 2017;50:20–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roka-Moiia Y, Walk R, Palomares DE, Ammann KR, Dimasi A, Italiano JE, Sheriff J, Bluestein D, Slepian MJ. Platelet Activation via Shear Stress Exposure Induces a Differing Pattern of Biomarkers of Activation versus Biochemical Agonists. Thrombosis and Haemostasis. 2020;120:776–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koupenova M, Kehrel BE, Corkrey HA, Freedman JE. Thrombosis and platelets: an update. European Heart Journal. 2016;38:785–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roka-Moya YM, Bilous VL, Zhernossekov DD, Grinenko T V. Novel aspects of platelet aggregation. Biopolymers and Cell. 2014;30:10–15. [Google Scholar]
- 12.Baaten CCFMJ, Swieringa F, Misztal T, Mastenbroek TG, Feijge MAH, Bock PE, Donners MMPC, Collins PW, Li R, van der Meijden PEJ, Heemskerk JWM Platelet heterogeneity in activation-induced glycoprotein shedding: functional effects. Blood advances. 2018;2:2320–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andrews RK, Gardiner EE. Metalloproteolytic receptor shedding…platelets “acting their age.” Platelets. 2016;27:512–518. [DOI] [PubMed] [Google Scholar]
- 14.Coller BS, Steinberg M, Scudder LE, Coller BS, Kalomirns E, Steinberg M, Scudder LE. Evidence that glycocalicin circulates in normal plasma. Evidence That Glycocalicin Circulates in Normal Plasma. J Clin Invest. 1984;73:794–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hosseini E, Ghasemzadeh M, Nassaji F, Jamaat ZP. GPVI modulation during platelet activation and storage: its expression levels and ectodomain shedding compared to markers of platelet storage lesion. Platelets. 2017;28:498–508. [DOI] [PubMed] [Google Scholar]
- 16.Dole VS, Bergmeier W, Patten IS, Hirahashi J, Mayadas TN, Wagner DD. PSGL-1 regulates platelet P-selectin-mediated endothelial activation and shedding of P-selectin from activated platelets. Thrombosis and Haemostasis. 2007;98:806–812. [PubMed] [Google Scholar]
- 17.Hosseini E, Mohtashami M, Ghasemzadeh M. Down-regulation of platelet adhesion receptors is a controlling mechanism of thrombosis, while also affecting post-transfusion efficacy of stored platelets. Thrombosis Journal. 2019;17(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boilard E, Duchez AC, Brisson A. The diversity of platelet microparticles. Current Opinion in Hematology. 2015;22:437–444. [DOI] [PubMed] [Google Scholar]
- 19.Flaumenhaft R, Mairuhu A, Italiano J. Platelet- and Megakaryocyte-Derived Microparticles. Seminars in Thrombosis and Hemostasis. 2010;36(08):881–887. [DOI] [PubMed] [Google Scholar]
- 20.Formation Flaumenhaft R. and fate of platelet microparticles. Blood Cells, Molecules, and Diseases. 2006;36:182–187. [DOI] [PubMed] [Google Scholar]
- 21.Morel O, Jesel L, Freyssinet JM, Toti F. Cellular mechanisms underlying the formation of circulating microparticles. Arteriosclerosis, Thrombosis, and Vascular Biology. 2011;31:15–26. [DOI] [PubMed] [Google Scholar]
- 22.Jackson SP, Mangin P, Yuan Y. "Pulling strings": shear, platelets, and microparticles. Blood. 2006;107:3418–3419. [Google Scholar]
- 23.Dean WL, Lee MJ, Cummins TD, Schultz DJ, Powell DW. Proteomic and functional characterisation of platelet microparticle size classes. Thrombosis and haemostasis. 2009;102:711–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shai E, Rosa I, Parguiña AF, Motahedeh S, Varon D, García Á. Comparative analysis of platelet-derived microparticles reveals differences in their amount and proteome depending on the platelet stimulus. Journal of Proteomics. 2012;76:287–296. [DOI] [PubMed] [Google Scholar]
- 25.Middleton EA, Weyrich AS, Zimmerman GA. Platelets in pulmonary immune responses and inflammatory lung diseases. Physiological Reviews. 2016;96:1211–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sakariassen KS, Holme PA, Ørvim U, Barstad RM, Solum NO, Brosstad FR. Shear-induced platelet activation and platelet microparticle formation in native human blood. Thrombosis Research. 1998;92:33–41. [DOI] [PubMed] [Google Scholar]
- 27.Miyazaki Y, Nomura S, Miyake T, Kagawa H, Kitada C, Taniguchi H, Komiyama Y, Fujimura Y, Ikeda Y, Fukuhara S. High shear stress can initiate both platelet aggregation and shedding of procoagulant containing microparticles. Blood. 1996;88:3456–3464. [PubMed] [Google Scholar]
- 28.Leytin V, Allen DJ, Mykhaylov S, Mis L, Lyubimov E V., Garvey B, Freedman J. Pathologic high shear stress induces apoptosis events in human platelets. Biochemical and Biophysical Research Communications. 2004;320:303–310. [DOI] [PubMed] [Google Scholar]
- 29.Ivak P, Pitha J, Netuka I. Circulating microparticles as a predictor of vascular properties in patients on mechanical circulatory support; hype or hope? Physiological research. 2016;65:727–735. [DOI] [PubMed] [Google Scholar]
- 30.McClane N, Jeske W, Walenga JM, Escalante V, Hoppensteadt D, Schwartz J, Bakhos M. Identification of Novel Hemostatic Biomarkers of Adverse Clinical Events in Patients Implanted With a Continuous-Flow Left Ventricular Assist Device. Clinical and Applied Thrombosis/Hemostasis. 2018;24:965–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nascimbene A, Hernandez R, George JK, Parker A, Bergeron AL, Pradhan S, Vijayan KV, Civitello A, Simpson L, Nawrot M, Lee VV, Mallidi HR, Delgado RM, Dong JF, Frazier OH. Association between cell-derived microparticles and adverse events in patients with nonpulsatile left ventricular assist devices. Journal of Heart and Lung Transplantation. 2014;33:470–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dimasi A, Roka-Moiia Y, Consolo F, Rasponi M, Fiore GB, Slepian MJ, Redaelli A. Microfluidic flow-based platforms for induction and analysis of dynamic shear-mediated platelet activation—Initial validation versus the standardized hemodynamic shearing device. Biomicrofluidics. 2018;12:042208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Silver WP, Keller MP, Ted R, Silver D. Effects of donor characteristics and in vitro time and temperature on platelet aggregometry platelet.; 1993;17:726–733 [PubMed] [Google Scholar]
- 34.Gyulkhandanyan AV., Mutlu A, Freedman J, Leytin V. Selective triggering of platelet apoptosis, platelet activation or both. British Journal of Haematology. 2013;161:245–254. [DOI] [PubMed] [Google Scholar]
- 35.Leytin V, Gyulkhandanyan AV., Freedman J. How to Avoid False-Negative and False-Positive Diagnoses of Platelet Apoptosis: Illustrative Experimental and Clinically Relevant Cases. Clinical and Applied Thrombosis/Hemostasis. 2018;24:1009–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shah MD, Bergeron AL, Dong JF, López JA. Flow cytometric measurement of microparticles: Pitfalls and protocol modifications. Platelets. 2008;19:365–372. [DOI] [PubMed] [Google Scholar]
- 37.Sheriff J, Tran PL, Hutchinson M, Decook T, Slepian MJ, Bluestein D, Jesty J. Repetitive Hypershear Activates and Sensitizes Platelets in a Dose-Dependent Manner. Artificial Organs. 2016;40:586–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sheriff J. Shear-Induced Platelet Sensitization and the Development of an Activation Model. Stony Brook University. 2010. [Google Scholar]
- 39.Nobili M, Sheriff J, Morbiducci U, Redaelli A, Bluestein D. Platelet activation due to hemodynamic shear stresses: damage accumulation model and comparison to in vitro measurements. ASAIO Journal. 2008;54:64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sheriff J, Soares JS, Xenos M, Jesty J, Bluestein D. Evaluation of Shear-Induced Platelet Activation Models Under Constant and Dynamic Shear Stress Loading Conditions Relevant to Devices. Annals of Biomedical Engineering. 2013;41:1279–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nylander S, Femia EA, Scavone M, Berntsson P, Asztély A-K, Nelander K, Löfgren L, Nilsson RG, Cattaneo M. Ticagrelor inhibits human platelet aggregation via adenosine in addition to P2Y 12 antagonism. Journal of Thrombosis and Haemostasis. 2013;11:1867–1876 [DOI] [PubMed] [Google Scholar]
- 42.van Giezen JJJ, Berntsson P, Zachrisson H, Björkman JA. Comparison of ticagrelor and thienopyridine P2Y12 binding characteristics and antithrombotic and bleeding effects in rat and dog models of thrombosis/hemostasis. Thrombosis Research. 2009;124:565–571. [DOI] [PubMed] [Google Scholar]
- 43.Schmitz G, Rothe G, Ruf A, Barlage S, Tschöpe D, Clemetson KJ, Goodall AH, Michelson AD, Nurden AT, Shankey TV. European Working Group on Clinical Cell Analysis: Consensus protocol for the flow cytometric characterisation of platelet function. Thrombosis and Haemostasis. 1998;79:885–896. [PubMed] [Google Scholar]
- 44.Saboor M, Moinuddin M, Ilyas S. New horizons in platelets flow cytometry. The Malaysian journal of medical sciences : MJMS. 2013;20:62–66. [PMC free article] [PubMed] [Google Scholar]
- 45.Javela K, Kekomäki R. Mean platelet size related to glycoprotein-specific autoantibodies and platelet-associated IgG. International Journal of Laboratory Hematology. 2007;29:433–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Araki R, Nishimura R, Kuroda R, Fujiki T, Mase S, Noguchi K, Ikawa Y, Maeba H, Yachie A. A characteristic flow cytometric pattern with broad forward scatter and narrowed side scatter helps diagnose immune thrombocytopenia (ITP). International Journal of Hematology. 2018;108:151–160. [DOI] [PubMed] [Google Scholar]
- 47.Beer BJ, Buchi L, Steiner B. Glycocalicin: A New Assay-The Normal Plasma Levels and Its Potential Usefulness in Selected Diseases. 1994;83:691–702. [PubMed] [Google Scholar]
- 48.Maloney SF, Brass LF, Diamond SL. P2Y12 or P2Y1 inhibitors reduce platelet deposition in a microfluidic model of thrombosis while apyrase lacks efficacy under flow conditions. Integrative Biology. 2010;2:183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Al-Tamimi M, Tan CW, Qiao J, Pennings GJ, Javadzadegan A, Yong ASC, Arthur JF, Davis AK, Jing J, Mu FT, Hamilton JR, Jackson SP, Ludwig A, Berndt MC, Ward CM, et al. Pathologic shear triggers shedding of vascular receptors: A novel mechanism for down-regulation of platelet glycoprotein VI in stenosed coronary vessels. Blood. 2012;119:4311–4320. [DOI] [PubMed] [Google Scholar]
- 50.Mills DCB. ADP receptors on platelets. Thrombosis and Haemostasis. 1996;76:835–856. [PubMed] [Google Scholar]
- 51.Wang L, Hoffman RA. Standardization, calibration, and control in flow cytometry. Curr. Protoc. Cytom. 2017;79:1–3. [DOI] [PubMed] [Google Scholar]
- 52.Kettlun AM, Uribe L, Calvo V, Silva S, Rivera J, Mancilla M, Antonieta M, Valenzuela, Traverso-Cori A. Properties of two apyrases from Solanum tuberosum. Phytochemistry. 1982;21:551–558. [Google Scholar]
- 53.Mondal NK, Sorensen EN, Hiivala NJ, Feller ED, Pham SM, Griffith BP, Wu ZJ. Intraplatelet reactive oxygen species, mitochondrial damage and platelet apoptosis augment non-surgical bleeding in heart failure patients supported by continuous-flow left ventricular assist device. Platelets. 2015;26:536–544. [DOI] [PubMed] [Google Scholar]
- 54.Mondal NK, Li T, Chen Z, Chen HH, Sorensen EN, Pham SM, Sobieski MA, Koenig SC, Slaughter MS, Griffith BP, Wu ZJ. Mechanistic insight of platelet apoptosis leading to non-surgical bleeding among heart failure patients supported by continuous-flow left ventricular assist devices. Molecular and Cellular Biochemistry. 2017;433:125–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lukito P, Wong A, Jing J, Arthur JF, Marasco SF, Murphy DA, Bergin PJ, Shaw JA, Collecutt M, Andrews RK, Gardiner EE, Davis AK. Mechanical circulatory support is associated with loss of platelet receptors glycoprotein Ibα and glycoprotein VI. Journal of Thrombosis and Haemostasis. 2016;14:2253–2260. [DOI] [PubMed] [Google Scholar]
- 56.Mazzeffi M, Hasan S, Abuelkasem E, Meyer M, Deatrick K, Taylor B, Kon Z, Herr D, Tanaka K. Von Willebrand Factor-GP1bα Interactions in Venoarterial Extracorporeal Membrane Oxygenation Patients. Journal of Cardiothoracic and Vascular Anesthesia. 2019;33:2125–2132. [DOI] [PubMed] [Google Scholar]
- 57.Mondal NK, Sorensen EN, Feller ED, Pham SM, Griffith BP, Wu ZJ. Comparison of intraplatelet reactive oxygen species, mitochondrial damage, and platelet apoptosis after implantation of three continuous flow left ventricular assist devices: HeartMate II, Jarvik 2000, and HeartWare. ASAIO Journal. 2015;61:244–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Feghhi S, Munday AD, Tooley WW, Rajsekar S, Fura AM, Kulman JD, Ló pez JA, Sniadecki NJ. Glycoprotein Ib-IX-V Complex Transmits Cytoskeletal Forces That Enhance Platelet Adhesion. Biophysj. 2016;111:601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huang J, Li X, Shi X, Zhu M, Wang J, Huang S, Huang X, Wang H, Li L, Deng H, Zhou Y, Mao J, Long Z, Ma Z, Ye W, et al. Platelet integrin αiIbβ3: Signal transduction, regulation, and its therapeutic targeting. Journal of Hematology and Oncology. 2019;12:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Al-Tamimi M, Tan CW, Qiao J, Pennings GJ, Javadzadegan A, Yong ASCC, Arthur JF, Davis AK, Jing J, Mu F-TT, Hamilton JR, Jackson SP, Ludwig A, Berndt MC, Ward CM, et al. Pathologic shear triggers shedding of vascular receptors: A novel mechanism for down-regulation of platelet glycoprotein VI in stenosed coronary vessels. Blood. 2012;119:4311–4320. [DOI] [PubMed] [Google Scholar]
- 61.Chen Z, Mondal NK, Ding J, Gao J, Griffith BP, Wu ZJ. Shear-induced platelet receptor shedding by non-physiological high shear stress with short exposure time: Glycoprotein Ibα and glycoprotein VI. Thrombosis Research. 2015;135:692–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen Z, Koenig SC, Slaughter MS, Griffith BP, Wu ZJ. Quantitative characterization of shear-induced platelet receptor shedding: Glycoprotein Ibα, Glycoprotein VI, and Glycoprotein IIb/IIIa. ASAIO Journal. 2018;64:773–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cheng H, Yan R, Li S, Yuan Y, Liu J, Ruan C, Dai K. Shear-induced interaction of platelets with von Willebrand factor results in glycoprotein Ibα shedding. American Journal of Physiology-Heart and Circulatory Physiology. 2009;297:H2128–H2135. [DOI] [PubMed] [Google Scholar]
- 64.Chen Z, Tran D, Li T, Arias K, Griffith BP, Wu ZJ. The Role of a Disintegrin and Metalloproteinase Proteolysis and Mechanical Damage in Nonphysiological Shear Stress-Induced Platelet Receptor Shedding. ASAIO Journal. 2020:524–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sehgal S, Storrie B. Evidence that differential packaging of the major platelet granule proteins von Willebrand factor and fibrinogen can support their differential release. Journal of Thrombosis and Haemostasis. 2007;5:2009–2016. [DOI] [PubMed] [Google Scholar]
- 66.Wencel-Drake JD, Plow EF, Kunicki TJ, Woods VL, Keller DM, Ginsberg MH. Localization of internal pools of membrane glycoproteins involved in platelet adhesive responses. American Journal of Pathology. 1986;124:324–334. [PMC free article] [PubMed] [Google Scholar]
- 67.Nylander S, Mattsson C, Ramström S, Lindahl TL. The relative importance of the ADP receptors, P2Y12 and P2Y 1, in thrombin-induced platelet activation. Thrombosis Research. 2003;111:65–73. [DOI] [PubMed] [Google Scholar]
- 68.Wei H, Malcor JDM, Harper MT. Lipid rafts are essential for release of phosphatidylserine-exposing extracellular vesicles from platelets. Scientific Reports. 2018;8:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Biró É, Akkerman JWN, Hoek FJ, Gorter G, Pronk LM, Sturk A, Nieuwland R. The phospholipid composition and cholesterol content of platelet-derived microparticles: A comparison with platelet membrane fractions. Journal of Thrombosis and Haemostasis. 2005;3:2754–2763. [DOI] [PubMed] [Google Scholar]
- 70.Sweedo A, Scott Saavedra S, Sheriff J, Bluestein D, Slepian MJ. Platelet Membrane Fluidity: A Mechanistic Component Of Shear- Mediated Platelet Activation. In: ASAIO Journal.Vol 64.; 2018:40. [Google Scholar]
- 71.Dragsten P, Henkart P, Blumenthal R, Weinstein J, Schlessinger J. Lateral diffusion of surface immunoglobulin, Thy-1 antigen, and a lipid probe in lymphocyte plasma membranes. Proceedings of the National Academy of Sciences of the United States of America. 1979;76:5163–5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.López-Ortega O, Santos-Argumedo L. Myosin 1g contributes to CD44 adhesion protein and lipid rafts recycling and controls CD44 capping and cell migration in B lymphocytes. Frontiers in Immunology. 2017;8:1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jacobson K, Liu P, Lagerholm BC. The Lateral Organization and Mobility of Plasma Membrane Components. Cell. 2019;177:806–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vandendries E R, Furie B, Furie B. Role of P-selectin and PSGL-1 in coagulation and thrombosis. Thrombosis and Haemostasis. 2004;92:459–466. [DOI] [PubMed] [Google Scholar]
- 75.Houël R, Mazoyer E, Boval B, Kirsch M, Vermès E, Drouet L, Loisance DY. Platelet activation and aggregation profile in prolonged external ventricular support. The Journal of Thoracic and Cardiovascular Surgery. 2004;128:197–202. [DOI] [PubMed] [Google Scholar]
- 76.Radovancevic R, Matijevic N, Bracey AW, Radovancevic B, Elayda M, Gregoric ID, Frazier OH. Increased leukocyte-platelet interactions during circulatory support with left ventricular assist devices. ASAIO Journal. 2009;55:459–464. [DOI] [PubMed] [Google Scholar]
- 77.André P, Hartwell D, Hrachovinová I, Saffaripour S, Wagner DD. Pro-coagulant state resulting from high levels of soluble P-selectin in blood. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:13835–13840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Forlow SB, McEver RP, Nollert MU. Leukocyte-leukocyte interactions mediated by platelet microparticles under flow. Blood. 2000;95:1317–1323. [PubMed] [Google Scholar]
- 79.Chen Z, Mondal NK, Ding J, Koenig SC, Slaughter MS, Griffith BP, Wu ZJ. Activation and shedding of platelet glycoprotein IIb/IIIa under non-physiological shear stress. Molecular and cellular biochemistry. 2015;409:93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li S, Wang Z, Liao Y, Zhang W, Shi Q, Yan R, Ruan C, Dai K. The glycoprotein Ibα-von Willebrand factor interaction induces platelet apoptosis. Journal of Thrombosis and Haemostasis. 2010;8:341–350. [DOI] [PubMed] [Google Scholar]
- 81.Shankaran H, Alexandridis P, Neelamegham S. Aspects of hydrodynamic shear regulating shear-induced platelet activation and self-association of von Willebrand factor in suspension. Blood. 2003;101:2637–2645. [DOI] [PubMed] [Google Scholar]
- 82.Bender M, Stegner D, Nieswandt B. Model systems for platelet receptor shedding. Platelets. 2017;28:325–332. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.