Abstract
A new variant of SARS-CoV-2 (Lineage B.1.1.7) was identified in the UK in December 2020 which was associated with higher transmissibility of COVID-19. The AusDiagnostics SARS-CoV-2, Influenza and RSV 8-well assay is used at sixteen UK hospitals and detects part of the ORF8 gene (together with a segment from the ORF1a gene). The objective of this study was to determine if the recently identified mutation in ORF8 (G28048T) in the B.1.1.7. lineage could be used to identify the new variant quickly in clinical cases with PCR positive results. The melt data from SARS-CoV-2 positives from two hospitals (October through December 2020) were reviewed, and distribution over time and location was evaluated. A low melt variant of the ORF8 amplicon started to appear in samples from Guy's and St. Thomas’ NHS Trust, London, at the start of November, and grew as a proportion of the total positives during the subsequent two months. These low melt variants were very rare during the same period at the Northern Care Alliance, Greater Manchester, North West of UK. It was confirmed that these carried the G28048T mutation. The geographic and temporal distribution of the low melt amplicons makes it very likely that these are lineage B.1.1.7 strains. The melt temperature of this amplicon could be used to discriminate between the original and new variants in advance of the full sequencing of the isolate. However, the appearance of other mutations in the same amplicon means that this approach would be of diminishing value over time.
Keywords: SARS-Cov-2 Lineage B.1.1.7, Variant detection, Molecular diagnostics, MT-PCR
1. Background
Genomes belonging to a newly defined SARS-CoV-2 B.1.1.7 lineage were first identified in London and the South East of the UK towards the end of September 2020 [5] and by mid-December approximately two thirds of the cases in London were caused by the new variant [3] .
2. Objectives
The AusDiagnostics MT-PCR methodology [6] uses a two-step PCR process to amplify segments from pathogen nucleic acid. These amplicons are then further characterised using melt analysis to provide an additional layer of specificity and to discriminate primer dimers or non-specific background amplification. The melt analysis can discriminate as little as 0.2 °C difference in amplicons, particularly within the same run and on the same Real-Time Analyser. Depending on the assay design it is therefore possible to observe a single nucleotide polymorphism (SNP) within an amplicon if the substitution is a ‘G’ or ‘C’ is replaced with an ‘A’ or ‘T’ as such a substitution results in a 0.3 °C to 0.5 °C change in amplicon melt temperature using this methodology.
The B.1.1.7 lineage [5]of the SARS-CoV-2 virus which emerged around October in the UK, carries seventeen non- synonymous and six synonymous differences from the original strain including eight in the S gene region which are believed to be contributing to the increased transmissibility of the virus in the community. One of the other SNPs is in the ORF8 gene (G28048T) and this appears to be confined to this lineage, Fig. 1 . This SNP sits within the amplicon used in the AusDiagnostics assay, and so it was predicted that the ORF8 amplicons from the new variant strains would have a melt temperature that was 0.3 °C to 0.5 °C lower than the original strain. The objective of this study was to confirm if this was the case. If so, the lower melt variant could provide an early warning of new variant circulation at the time of the initial assay and prior to sequencing result becoming available.
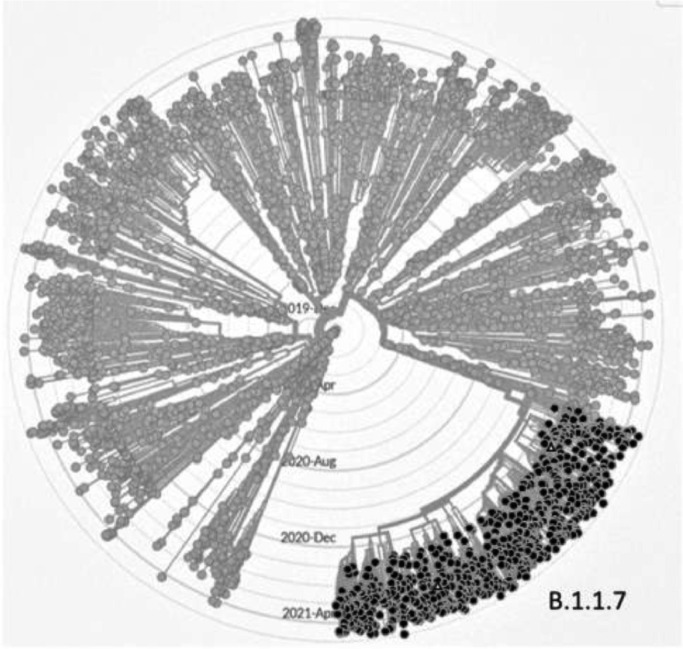
Fig. 1.
Phylogenetic tree (radial view) of 3913 of 3913 genomes sampled between Dec 2019 and Apr 2021. These were analysed using GISAID CoVsurver mutations App [1] to highlight G28048T mutations (black dots). These are observed in all the newly emerged B.1.1.7 lineage with the exception of two isolates (white triangles). G28048T mutations were not observed in any other lineages.
3. Study design
Guy's and St. Thomas’ NHS Trust (Viapath, sited in Central London) and Northern Care Alliance (Royal Oldham Hospital, Greater Manchester, sited in North West of UK) are currently using the automated SARS-CoV-2, Influenza and RSV 8-well multiplex assay as part of their testing approach. The ORF8 amplicon melt temperatures from all the positives detected using the assay between October 1st and December 22nd were analysed using R Studio and plotted against time. The melt temperature of a second assay (from a region of the ORF1a gene) was used as a control to determine the temperature variation in a region where no changes were expected between the original and new variants. The concentrations of the viral genes in the sample (broadly equivalent to the Ct) were also analysed to determine if there was any relationship between the variant and the quantity of virus detected. Ten individual samples collected during December at St. Thomas’ Hospital were also subjected to DNA sequence analysis (Eurofins) to determine if the G28048T SNP was present.
4. Results
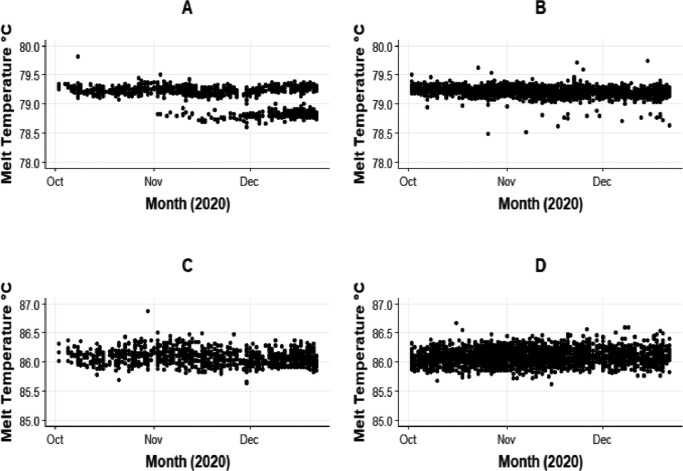
Amplicon melt and concentration data from 4979 samples which had been tested between October 1st and December 22nd 2020 were analysed. This dataset comprised 3675 SARS-CoV-2 PCR positives from Royal Oldham Hospital (tested using six different High-Plex analytical systems) and 1304 SARS-CoV-2 positives from Viapath (tested using four separate High-Plex analytical systems). Fig. 2 shows the ORF8 amplicon melts over time for the two locations and for comparison the ORF1a amplicon melts for the same samples. A lower temperature amplicon can be seen emerging in the Viapath (London) samples from the start of November, while there were are only a handful of these observed at Oldham (despite the higher number of positive tests using this assay). By the 22nd of December, the proportion of low melt variants from Viapath was approximately 75%, while at Oldham this reached 7%. These proportions are consistent with the know geographical distribution during this period [2]. Over the same time there was no change observed in the ORF1a melt profile.
Fig. 2.
Appearance of a lower melt ORF8 amplicon during November at Viapath (London). Panels: A) ORF 8 amplicon from Viapath (London). B) ORF 8 amplicon from Oldham. C) ORF1a amplicon from Viapath (London). D) ORF1a from Oldham.
The MT-PCR assay uses an internal control of known relative concentration, so it is possible to assess the concentration of the pathogen in the starting extract. These concentration values (equivalent to Ct values, and very approximately ‘gene copies per 10µl’) for the higher melt ORF8 amplicons were compared to those of the ‘lower melt’ amplicons (Fig. 3 .). The peak density for the higher melt amplicon (assumed to be the original variant) was about 1000 units, whereas the peak density for the lower melt amplicon (assumed to be the new variant) was much higher at 100,000 units. This may indicate that the new variant is generally detected at higher concentrations than the original.
Fig. 3.
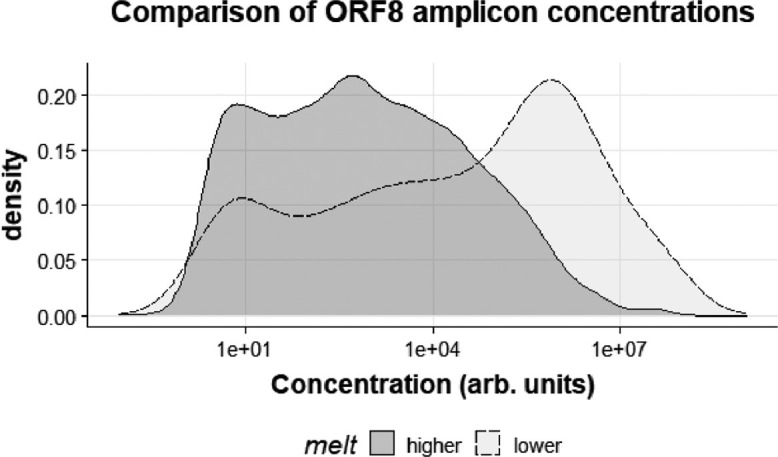
Kernel Density Plots of the concentrations of the higher melt amplicion versus the lower melt amplicon.
Ten SARS-CoV-2 PCR positive samples previously confirmed as positives from Viapath were reamplified and the amplicons were submitted for sequence analysis using the ORF8 primers (Table 1 ). Nine out of the ten samples had sequences as predicted, but one sample (#9, high melt), which was predicted to be an ‘original variant’ also had the G28048T mutation. In this case, a second (A28058G) mutation (synonymous) was also present which raised the melt temperature back up to the same as the original.
Table 1.
Characteristics of a set of ten clinical samples from Viapath (London), December 2020.
| Sample | Conc. (Arb. Units) | Melt Temp. ( °C) | Calculated Ct | Predicted Variant | Sequence at 28,048 | Sequence at 28,058 |
|---|---|---|---|---|---|---|
| 1 | 2472 | 78.77 | 23.10 | New | T | A |
| 2 | 654,770 | 79.19 | 15.00 | Original | G | A |
| 3 | 18,954 | 78.75 | 20.53 | New | T | A |
| 4 | 1672,579 | 79.19 | 13.45 | Original | G | A |
| 5 | 414,054 | 78.75 | 15.65 | New | T | A |
| 6 | 869,026 | 78.77 | 14.41 | New | T | A |
| 7 | 6297 | 78.73 | 22.14 | New | T | A |
| 8 | 614,949 | 78.76 | 14.94 | New | T | A |
| 9 | 602,697 | 79.23 | 14.73 | Original | T | G |
| 10 | 782,032 | 78.71 | 14.87 | New | T | A |
Finally, two samples (one high melt and one lower melt) which tested positive at Norwich Hospital in December were also evaluated. These had been sent to the COVID-19 Genomics UK (COG-UK Consortium) for full genome sequencing, and this confirmed that the low melt sample belonged in the new variant clade, while the high melt variant belonged in the ‘Spanish lineage’.
5. Discussion
The use of melt temperature can provide some additional information about the composition of the amplicon. In the data reported here, a clear correlation was seen between a 0.4 °C lower melt and a G to T mutation associated with the new B.1.1.7 lineage. This information is available as soon as the assay is reported as positive by the testing laboratory and could help to guide decision making during the period before definitive, full sequence information is available. In the case of the new variant, it is thought [4] that the key mutations related to transmissibility are within the spike gene and are not related to this SNP in the ORF8 gene. The G28048T mutation (R52I) has not been identified (GSAID; www.gisaid.org) other SARS-CoV-2 lineages so far (April 2021). The appearance of additional mutations that could reverse the amplicon melt change, such as A28058G represents a limitation to this approach, although this particular SNP has not been detected in any of the full SARS-CoV-2 genomes deposited to date.
The method described is specific to the detection of B.1.1.7 and in this case the melt difference was noted by chance in a particular set of samples. Although it appears robust, its accuracy is likely to diminish over time as other mutations appear in the viral population. In the future it may be possible to develop a broader multiplex panel measuring amplicon melt temperatures across a number of different SARS-2 regions to provide a useful tool for surveillance of emerging variants in primary testing labs.
Declaration of Competing Interest
RH and AG are full-time paid employees of AusDiagnostics UK Limited. EC and JP have no conflicts of interest.
Acknowledgements
We gratefully acknowledge the Authors from the originating laboratories responsible for obtaining the specimens and the submitting laboratories where genetic sequence data were generated and shared via the GISAID Initiative, on which some of this research is based. We also thank Dr Lindsay Coupland for providing the sequence data from Norwich.
References
- 1.Elbe S., Buckland-Merrett G. Data, disease and diplomacy: GISAID's innovative contribution to global health. Glob. Challenges. 2017;1(1):33–46. doi: 10.1002/gch2.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Investigation of novel SARS-CoV-2 variant Variant of Concern 202012/01 Technical briefing 5 . 2021. [Google Scholar]
- 3.Kirby T. Vol. 0. 2021. New variant of SARS-CoV-2 in UK causes surge of COVID-19. (The Lancet Respiratory). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leung K. Early transmissibility assessment of the N501Y mutant strains of SARS-CoV-2 in the United Kingdom. Eurosurveillance. 2021;26(1) doi: 10.2807/1560-7917.ES.2020.26.1.2002106. October to November 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rambaut A. 2020. Preliminary Genomic Characterisation of an Emergent SARS-CoV-2 Lineage in the UK Defined By a Novel Set of Spike Mutations - SARS-CoV-2 Coronavirus /nCoV-2019 Genomic Epidemiology - Virological, COVID-19 Genomics Consortium UK (CoG-UK)https://virological.org/t/preliminary-genomic-characterisation-of-an-emergent-sars-cov-2-lineage-in-the-uk-defined-by-a-novel-set-of-spike-mutations/563 Available at. (Accessed: January 6, 2021) [Google Scholar]
- 6.Stanley K.K., Szewczuk E. Multiplexed tandem PCR: gene profiling from small amounts of RNA using SYBR Green detection. Nucl. Acids Res. 2005;33(20):e180. doi: 10.1093/nar/gni182. [DOI] [PMC free article] [PubMed] [Google Scholar]