Abstract
Purpose
Ameloblastoma is a benign odontogenic neoplasm with a high local recurrence rate if the operation is not thorough. However, a useful clinical tool for the quantitative assessment of the prognosis and risk of postoperative recurrence of ameloblastoma has not yet been constructed. This study aims to develop a prognostic nomogram model for ameloblastoma of the jaw to assist surgeons in surgical decision-making.
Patients and Methods
Patients who underwent initial surgery for ameloblastoma in our department from October 2004 to March 2020 were enrolled and randomly divided into training and validation sets. Univariate and multivariate Cox proportional hazards regression analyses were performed to identify the independent prognostic factors, from which a nomogram for predicting 3-, 5- and 10-year recurrence-free survival (RFS) of ameloblastoma was constructed using the training set and internally validated using the validation set. The model performance was assessed by Harrell's concordance index (C-index) and calibration curves.
Results
A total of 302 eligible patients with ameloblastoma were enrolled, 54 of whom were confirmed to relapse during the follow-up period of 6 to 191 months. Four independent predictors, including cortical bone perforation, root(s) resorption, WHO classification, and treatment pattern, were identified and included in the construction of a nomogram for recurrence-free survival (RFS), which showed promising calibration performance and discrimination in the training set (C-index 0.790, 95% confidence interval [CI] 0.735–0.845) and the validation set (C-index 0.734, 95% CI 0.599–0.869).
Conclusion
A favorable nomogram was developed that accurately predicted the RFS of patients with ameloblastoma based on individual characteristics. Risk stratification using the nomogram could optimize tailored therapy and follow-up.
Keywords: ameloblastoma, nomogram, prognosis, recurrence, recurrence-free survival
Introduction
Ameloblastoma is a type of benign neoplasm originating from odontogenic epithelium but with local invasion.1,2 Characterized by its aggressive nature, occasional malignant transformation, and distant metastasis, ameloblastoma is also called a borderline tumor.3 Known as the most common odontogenic tumor in the oral cavity, ameloblastoma accounts for approximately 18% of odontogenic tumors.4 Ameloblastoma usually presents as a slow-growing and asymptomatic swelling neoplasm that causes expansive bone destruction or even cortical bone perforation.5 If neglected, ameloblastoma may grow to massive proportions over the course of months or years, eventually resulting in chewing dysfunction and facial asymmetry. To date, surgery, including conservative and radical surgery, is the standard and almost the only effective treatment for ameloblastoma.6,7 However, the recurrence rate and postoperative benefits of different treatment options are not the same. Although radical surgery may reduce the recurrence of ameloblastoma, it can also lead to severe facial deformities and chewing disorders, affecting a patient’s physical and mental health and quality of life. In contrast, conservative surgery is less invasive and can better preserve facial shape and function, but it could result in a more perceptible rate of recurrence. At present, making the choice of surgical methods lacks an effective and unified standard, and usually depends on the experience of the surgeon. Therefore, both surgeons and patients would benefit tremendously from an evaluation model to select a suitable and tailored treatment plan in order to reduce recurrence and maximize therapeutic benefits.
Previous studies have reported several possible risk factors associated with the recurrence of ameloblastoma, such as tumor size, radiographic pattern, and treatment modality.8,9 Nevertheless, there has not been an adequate and clinically available tool that quantitatively predicts the probability of recurrence of ameloblastoma. A nomogram is a statistical predictive tool that creates a simple graphical representation of a statistical model and generates a numerical probability of a clinical event.10,11 Nomograms can generate the individual probability of a clinical event through integrating various prognostic and decisive variables to meet the demand for biologically and clinically comprehensive models, and support our goal of more personalized medicine.12 Recently, nomograms have been widely developed and applied as a diagnostic and prognostic device in various diseases, including cardiovascular diseases,13 as well as in a variety of cancers, such as head and neck cancer,14,15 breast cancer,16 and lung cancer.17 However, we are unaware of the development of a nomogram for ameloblastoma.
Therefore, in this study, we sought to construct and validate a prognostic nomogram for predicting the recurrence-free survival (RFS) via a retrospective cohort to assist surgeons in tailoring surgical options for patients with ameloblastoma.
Patients and Methods
Patients and Study Design
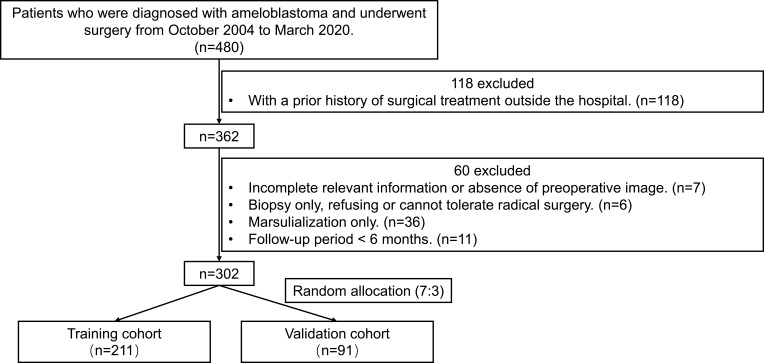
From October 2004 to March 2020, 480 patients with ameloblastoma who were diagnosed and underwent surgery at the Department of Oral and Maxillofacial Surgery, Hospital of Stomatology, Sun Yat-sen University were reviewed for inclusion in the study. Only patients that underwent the initial operation in our department with complete relevant information were included in the study. The detailed exclusion criteria and screening process are shown in Figure 1. After screening, 302 eligible consecutive ameloblastoma patients were enrolled in our study. This retrospective analysis was approved by the Ethics Board of the Hospital of Stomatology, Sun Yat-sen University (No. KQEC-2020-75-03), and is consistent with the Declaration of Helsinki.
Figure 1.
Study design flowchart. A total of 302 patients with ameloblastoma with complete relevant information were enrolled in this study and then randomly separated into training and validation sets with a ratio of 7:3.
The relevant demographic, clinical, radiographic, and pathological variables of all participants were obtained and derived from medical records. Briefly, tumor site characteristics were divided into two groups: anterior (premolar to premolar), and posterior (molar to tuberosity, or molar to ramus even up to condyle or coronoid process). Tumor size was recorded by measuring the maximum diameter on the preoperative panoramic radiograph. Preoperative radiographic features were separated into three types: unilocular radiolucency, multilocular radiolucency, and others (mixed radiolucent–radiopaque or radiopaque lesions). Additionally, the presence of cortical bone perforation (defined as “yes” if the radiography showed the cortical bone was not continuous), impacted tooth involvement, and root(s) resorption (defined as “yes” when the length of root resorption was more than 0.2 mm compared with the root of homonymous teeth) were also recorded by reviewing the radiographic reports and images. Histopathological types were grouped into plexiform, follicular, desmoplastic, unicystic (including luminal, intraluminal, and mural), and others (including granular cell, basal cell, and acanthomatous). All 302 cases in this cohort occurred in the jaw and there were no incidents of peripheral/extraosseous ameloblastoma or metastasizing ameloblastoma following the primary operation, which were confirmed via paraffin sectioning and H&E staining and X-ray. The final diagnosis of all patients was determined according to the histopathology report or review of slides, and grouped according to the fourth edition of the World Health Organization (WHO) Classification of Head and Neck Tumours (2017).5 Namely, there were two groups included in this study cohort: conventional and unicystic ameloblastoma. Treatment pattern was divided into two groups: curettage and radical treatment. Further, if patients underwent decompression and marsupialization initially, the second-stage surgical method (namely curettage or radical treatment) and date of tumor clearing were recorded as the beginning of follow-up. Recurrence was preliminarily judged by physical and radiographic examination during routine follow-up and pathologically confirmed. Only the first recurrence was investigated in the analysis. Additionally, local or distant metastasis (ie lung) was also considered before surgery and during follow-up. The follow-up period was defined as time between the date of first treatment to the date of confirmed recurrence or the last follow-up visit. RFS was defined as the period from the date of initial curettage or radical surgery to the date of the first confirmed recurrence. The survival information was obtained from medical records and clinical follow-up or telephone interviews.
Construction of the Nomogram
The included subjects were randomly divided into a training set and a validation set according to the ratio of 7:3 using R-generated random numbers (random seeds of 888). Based on the training set, univariate Cox analysis was applied to screen the clinical candidate predictive variables that achieved potential significance (p < 0.2). Thereafter, a multivariate Cox regression model was used to identify and select the significant prognostic factors of RFS using a backward step-down selection process with Akaike’s information criterion (AIC).18 Finally, a nomogram that incorporated the selected prognosis factors for predicting 3-, 5- and 10-year RFS was constructed.
Assessment and Internal Validation of the Nomogram
Both discrimination and calibration were applied to assess and validate the predictive accuracy of the model. Discrimination was quantified by the probability of Harrell's concordance index (C-index),19 whose values ranges from 0.5 (no discrimination) to 1.0 (perfect discrimination). The calibration curves were plotted to compare the nomogram-predicted RFS probability with the actual Kaplan-Meier estimates of the observed RFS probability. Subsequently, the two methods were applied to the validation set to conduct the internal validation for estimating the predictive accuracy of the model. Further, the total score for each patient was calculated according to the nomogram to reflect the probability of RFS. Then, the 302 patients were categorized into new risk groups (high-risk and low-risk) according to total nomogram scores, in which the threshold was identified using X-tile plots20 using the training set. The Log rank test was used to compare the RFS curves of the high-risk and low-risk groups in the training and validation sets, as well as in the patients overall.
Statistical Analysis
Statistical analysis and plotting were performed with R statistical software version 3.6.3 (http://www.r-project.org/) combined with the corresponding R packages. Continuous variables were expressed as mean ± standard deviation or median ± interquartile range (IQR). Categorical variables were described as frequencies and percentages, and analyzed using Pearson’s chi-squared test or Fisher’s exact test. Kaplan-Meier analysis with the Log rank test was used to draw the survival curves and calculate significance. Univariate and multivariate Cox proportional hazards regression analyses were performed to distinguish independent prognostic factors for RFS. Stepwise backward variable selection was performed to determine informative variables based on the AIC, and the model with the lowest AIC value was used to construct a graphical nomogram. The “survival”, “survminer”, and “MASS” packages were used to perform the survival, univariate and multivariate Cox analysis. The nomogram and calibration plots were generated using the “rms” package. Additionally, the X-tile plots were created to select the total optimum cutoff points using X-tile software version 3.6.1 (Yale University School of Medicine, New Haven, CT, USA). A two-tailed p-value of < 0.05 was considered statistically significant. This analysis was conducted according to the recommendations in the Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis statement (TRIPOD), and the completed checklist could be found in Supplementary Table S1.
Results
As shown in Figure 1, there were 480 patients who underwent surgery for ameloblastoma in our department between October 2004 and March 2020. After rigorous case review and screening, a total of 302 eligible patients were included in this study, and recurrence was confirmed in 54 patients (17.9%) during the follow-up period. The median follow-up time was 52 months (IQR, 20–96 months; range 6–191 months).
Basic Characteristics
The overall demographic and clinicopathological characteristics of the 302 included patients are shown in Supplementary Table S2. Briefly, the 302 enrolled patients comprised 127 females and 175 males (ratio of 1:1.38). The mean age at diagnosis was 31.64 ± 15.02 years old, and 62.6% of patients were aged between 20–49 years old. Of the 302 patients, 225 patients (74.5%) underwent curettage, and 77 patients (25.5%) underwent radical treatment, including 20 patients that underwent bone resection alone and 57 patients that underwent bone resection with free bone grafts (22 fibular flaps, 30 ilium flaps and 5 rib flaps). It is noteworthy that position, cortical bone perforation, root(s) resorption, and treatment pattern were significantly correlated with the recurrence of ameloblastoma (p < 0.05) through chi-square test. Based on clinical practice, the variable of “position” was excluded for further Cox analysis. In addition, none of the 302 subjects included in this study presented with metastasis during follow-up, which was confirmed by clinical and radiological examinations (eg chest X-ray or computed tomography (CT)).
Survival Analysis
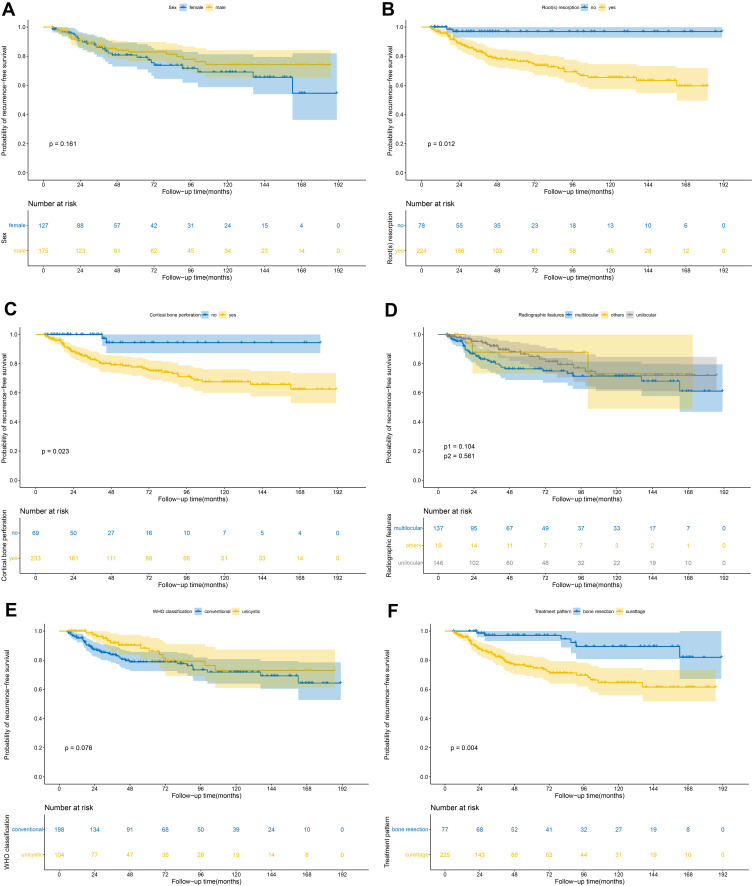
Kaplan-Meier survival analysis was applied to evaluate univariate prognostic factors of RFS in ameloblastoma (Figure 2). Among the 302 patients, 211 patients were randomly allocated to the training set, whereas the other 91 patients were allocated to the independent validation set as described in the methods (Table 1). The median follow-up time was 45 months (IQR, 22–92 months) for the training set and 36 months (IQR, 18–97 months) for the validation set. The 3-, 5- and 10-year RFS were 89.1%, 86.4% and 82.8%, respectively.
Figure 2.
Kaplan-Meier RFS curves in the 302 ameloblastoma patients according to: (A) sex; (B) root(s) resorption; (C) cortical bone perforation; (D) radiographic features; (E) WHO classification; (F) treatment pattern. (p-value was calculated by univariate Cox analysis. (D) p1 means unilocular vs multilocular, p2 means others vs multilocular).
Table 1.
Baseline Characteristics of Patients in the Training and Validation Sets
| Variables | Training Set (n=211) | Validation Set (n=91) | ||||
|---|---|---|---|---|---|---|
| Recurrence (n=41) (%) | Non-Recurrence (n=170) (%) | p | Recurrence (n=13) (%) | Non-Recurrence (n=78) (%) | p | |
| Sex | 0.180 | 0.554 | ||||
| Female | 22 (10.4) | 69 (32.7) | 4 (4.4) | 32 (35.2) | ||
| Male | 19 (9.0) | 101 (47.9) | 9 (9.9) | 46 (50.5) | ||
| Age, years | 0.548 | 0.586 | ||||
| <20 | 9 (4.3) | 47 (22.3) | 3 (3.3) | 14 (15.4) | ||
| 20–49 | 29 (13.7) | 103 (48.8) | 9 (9.9) | 47 (51.6) | ||
| ≥50 | 3 (1.4) | 20 (9.5) | 1 (1.1) | 17 (18.7) | ||
| Position | 0.005** | 0.588 | ||||
| Maxilla | 0 (0) | 25 (11.9) | 0 (0) | 7 (7.7) | ||
| Mandible | 41 (19.4) | 145 (68.7) | 13 (14.3) | 71 (78.0) | ||
| Laterality | 0.856 | 0.589 | ||||
| Left | 15 (7.1) | 58 (27.5) | 6 (6.6) | 24 (26.4) | ||
| Right | 20 (9.5) | 81 (38.4) | 5 (5.5) | 35 (38.4) | ||
| Bilateral | 6 (2.8) | 31 (14.7) | 2 (2.2) | 19 (20.9) | ||
| Site characteristics | 0.489 | 1.000 | ||||
| Anterior | 5 (2.4) | 31 (14.7) | 3 (3.3) | 19 (20.9) | ||
| Posterior | 36 (17.0) | 139 (65.9) | 10 (11.0) | 59 (64.8) | ||
| Size, cm | 0.307 | 0.898 | ||||
| <5 | 16 (7.5) | 84 (39.8) | 7 (7.7) | 37 (40.7) | ||
| ≥5 | 25 (11.9) | 86 (40.8) | 6 (6.6) | 41 (45.0) | ||
| Symptom | 0.479 | 0.206 | ||||
| Pain | 11 (5.2) | 58 (27.5) | 2 (2.2) | 29 (31.9) | ||
| Painless | 30 (14.2) | 112 (53.1) | 11 (12.1) | 49 (53.8) | ||
| Duration of symptom, months | 0.586 | 0.729 | ||||
| <12 | 32 (15.1) | 123 (58.3) | 11 (12.1) | 61 (67.0) | ||
| ≥12 | 9 (4.3) | 47 (22.3) | 2 (2.2) | 17 (18.7) | ||
| Numbness of lower lip | 1.000 | 1.000 | ||||
| Yes | 2 (0.9) | 10 (4.7) | 0 (0) | 2 (2.2) | ||
| No | 39 (18.5) | 160 (75.9) | 13 (14.3) | 76 (83.5) | ||
| Radiographic features | 0.226 | 0.611 | ||||
| Multilocular | 23 (10.9) | 71 (33.6) | 8 (8.8) | 35 (38.5) | ||
| Unilocular | 15 (7.1) | 86 (40.8) | 0 (0) | 3 (3.3) | ||
| Others | 3 (1.4) | 13 (6.2) | 5 (5.5) | 40 (43.9) | ||
| Cortical bone perforation | 0.002** | 0.033* | ||||
| Yes | 39 (18.5) | 125 (59.3) | 13 (14.3) | 56 (61.5) | ||
| No | 2 (0.99) | 45 (21.3) | 0 (0) | 22 (24.2) | ||
| Root (s) resorption | <0.001*** | 0.099 | ||||
| Yes | 40 (18.9) | 119 (56.4) | 12 (13.2) | 53 (58.2) | ||
| No | 1 (0.5) | 51 (21.2) | 1 (1.1) | 25 (27.5) | ||
| Impacted tooth involvement | 0.576 | 0.896 | ||||
| Yes | 18 (8.5) | 64 (30.3) | 6 (6.6) | 31 (34.1) | ||
| No | 23 (10.9) | 106 (50.3) | 7 (7.7) | 47 (51.6) | ||
| Pathological type | 0.562 | 0.187 | ||||
| Unicystic | 10 (4.7) | 62 (29.4) | 4 (4.4) | 28 (30.8) | ||
| Follicular | 5 (2.4) | 19 (9.0) | 3 (3.3) | 10 (11.0) | ||
| Plexiform | 22 (10.5) | 77 (36.5) | 4 (4.4) | 37 (40.6) | ||
| Desmoplastic | 3 (1.4) | 9 (4.3) | 1 (1.1) | 2 (2.2) | ||
| Others | 1 (0.4) | 3 (1.4) | 1 (1.1) | 1 (1.1) | ||
| WHO classification | 0.200 | 1.000 | ||||
| Unicystic | 10 (4.7) | 62 (29.4) | 4 (4.4) | 28 (30.8) | ||
| Conventional | 31 (14.7) | 108 (51.2) | 9 (9.9) | 50 (54.9) | ||
| Treatment pattern | 0.015* | 0.335 | ||||
| Curettage | 37 (17.5) | 123 (58.3) | 11 (12.1) | 54 (59.3) | ||
| Radical treatment | 4 (1.9) | 47 (22.3) | 2 (2.2) | 24 (26.4) | ||
Notes: *p < 0.05, **p < 0.01, ***p < 0.001. (chi-square test or Fisher’s exact test).
Construction of the Nomogram
According to the univariate Cox analysis using the training set, six candidate clinical variables, including sex, radiographic features, cortical bone perforation, root(s) resorption, WHO classification and treatment pattern (Table 2) were found to meet the p-value threshold of < 0.2 and were used for further multivariate Cox analysis. The variable set with the lowest AIC value of 360.2 was selected as the final model. Considering in combination with clinical experience, the variable of “sex” was excluded. Finally, four significant predictors, including cortical bone perforation, root(s) resorption, WHO classification and treatment pattern, were identified as independent prognostic factors associated with RFS (p < 0.05), and were incorporated in the construction of the nomogram model predicting 3-, 5- and 10-year probabilities for RFS (Figure 3). In general, patients suffering from unicystic ameloblastoma showing no cortical bone destruction and no root resorption had better outcomes. Additionally, patients who underwent radical bone resection exhibited improved RFS outcomes.
Table 2.
Univariate and Multivariate Cox Regression Analyses of Clinicopathologic Factors with Recurrence-Free Survival in the Training Set
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Sex | 0.161 | 0.144 | ||
| Female | Reference | Reference | ||
| Male | 0.644 (0.349–1.191) | 0.627 (0.335–1.174) | ||
| Age, years | ||||
| <20 | 1.363 (0.369–5.039) | 0.642 | – | – |
| 20–49 | 1.680 (0.511–5.519) | 0.393 | – | – |
| ≥50 | Reference | – | – | |
| Position | – | – | ||
| Maxilla | – | – | ||
| Mandible | – | – | ||
| Laterality | ||||
| Left | 1.694 (0.656–4.375) | 0.277 | – | – |
| Right | 1.367 (0.670–3.407) | 0.503 | – | – |
| Bilateral | Reference | – | – | |
| Site characteristics | 0.316 | – | ||
| Anterior | Reference | – | ||
| Posterior | 1.615 (0.633–4.118) | – | ||
| Size, cm | 0.862 | – | ||
| <5 | 0.945 (0.499–1.791) | – | ||
| ≥5 | Reference | – | ||
| Symptom | 0.434 | – | ||
| Pain | Reference | – | ||
| Painless | 1.318 (0.660–2.631) | – | ||
| Duration of symptom, months | 0.314 | – | ||
| <12 | 1.464 (0.698–3.074) | – | ||
| ≥12 | Reference | – | ||
| Numbness of lower lip | 0.787 | – | ||
| Yes | 0.822 (0.198–3.411) | – | ||
| No | Reference | – | ||
| Radiographic features | ||||
| Multilocular | Reference | Reference | ||
| Unilocular | 0.583 (0.304–1.118) | 0.104 | 0.791 (0.365–1.715) | 0.553 |
| Others | 0.700 (0.210–2.332) | 0.561 | 0.866 (0.257–2.919) | 0.816 |
| Cortical bone perforation | 0.023* | |||
| Yes | 5.242 (1.262–21.77) | 4.257 (1.007–18.008) | 0.049* | |
| No | Reference | Reference | ||
| Root(s) resorption | 0.012* | 0.044* | ||
| Yes | 12.74 (1.751–92.72) | 7.786 (1.053–57.582) | ||
| No | Reference | Reference | ||
| Impacted tooth involvement | 0.427 | – | ||
| Yes | 1.284 (0.693–2.38) | – | ||
| No | Reference | – | ||
| Pathological type | ||||
| Unicystic | 0.435 (0.119–1.593) | 0.209 | – | – |
| Follicular | 0.654 (0.155–2.762) | 0.564 | – | – |
| Plexiform | 0.870 (0.259–2.924) | 0.822 | – | – |
| Others | 0.911 (0.093–8.868) | 0.936 | – | – |
| Desmoplastic | Reference | – | – | |
| WHO classification | 0.076 | 0.041* | ||
| Unicystic | 0.520 (0.255–1.062) | 0.398 (0.164–0.964) | ||
| Conventional | Reference | Reference | ||
| Treatment pattern | 0.004** | <0.001*** | ||
| Curettage | 4.519 (1.603–12.74) | 7.526 (2.570–22.037) | ||
| Radical treatment | Reference | Reference | ||
Notes: *p < 0.05, **p < 0.01, ***p < 0.001.
Abbreviations: HR, hazards ratio; CI, confidence interval.
Figure 3.
Nomogram of prediction for 3-, 5- and 10-year RFS in patients with ameloblastoma. Each factor was given a score, and the total score for an individual patient could be obtained by summing up all scores. The predictive probabilities of RFS at 3-years, 5-years and 10-years were identified by the total score according to the bottom scale.
Assessment and Internal Validation of the Nomogram
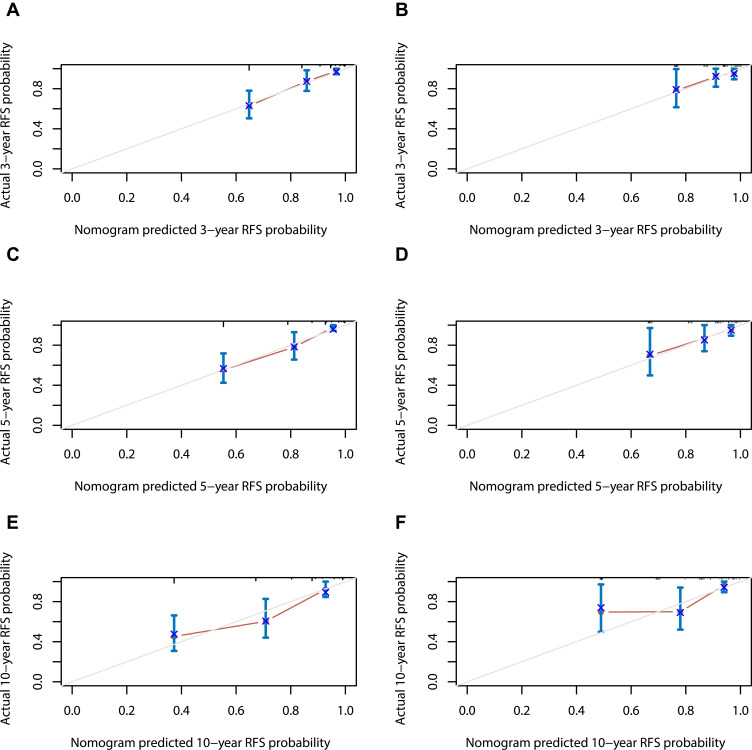
In the training set, the C-index for the nomogram was 0.790 (95% CI, 0.735–0.869), and the calibration curves (Figure 4) displayed favorably. Then, internal validation of the developed nomogram was performed using the validation set, and the calibration curves of the nomogram displayed a favorable agreement between the predicted and actual values of the 3-, 5- and 10-year probabilities for predicting RFS of ameloblastoma. Additionally, promising discrimination with a C-index of 0.734 (95% CI, 0.599–0.869) was observed for the nomogram predicting RFS in the validation set. These data demonstrate that our nomogram model was generally accurate after validation and performed well using both the training and validation sets.
Figure 4.
Calibration curves of the nomogram for predicting 3-, 5- and 10-year RFS in training set (A, C and E) and validation set (B, D and F). The dotted line represents the ideal match between the nomogram-predicted survival (X-axis) and actual survival (Y-axis). Vertical bars indicate 95% confidence intervals.
Survival Curves Based on Nomograms Scores
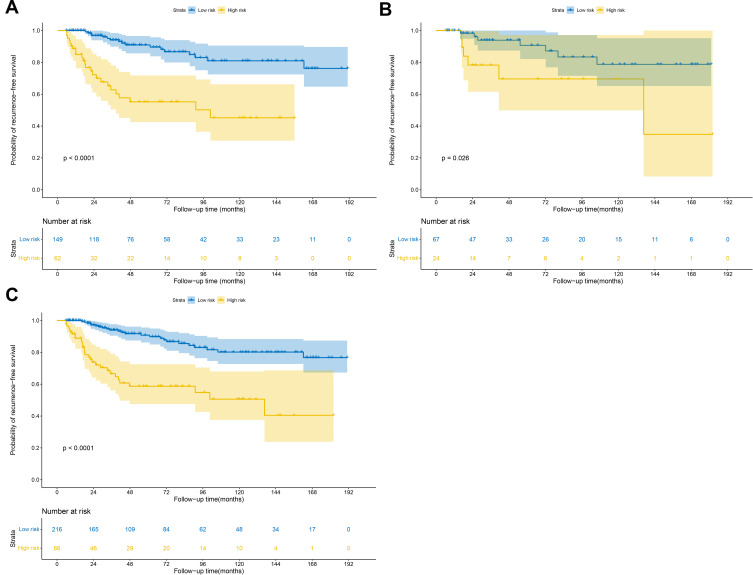
Total scores for each patient were calculated according to the nomogram constructed in the training set to reflect the risk of RFS (Supplementary Table S3). After obtaining the total score from the nomogram, the patients were classified into low- and high-risk groups according to the optimal cutoff value of 26.7 generated by the X-tile plots (Supplementary Figure S1).
Notably, significant discrimination between the RFS of the low-risk and high-risk patients was observed using the training set (Figure 5A), validation set (Figure 5B) and overall patients (Figure 5C). Therefore, our nomogram model can successfully distinguish and identify patients with a high risk of recurrence from those with low risk.
Figure 5.
Kaplan-Meier RFS curves of low- and high-risk patients in the (A) training set, (B) validation set, and (C) all patients. (Log rank test).
Discussion
The study results presented herein show that the 3-, 5- and 10-year RFS rates for ameloblastoma were 89.1%, 86.4% and 82.8%, respectively. The recurrence rate of ameloblastoma in this cohort was comparable to those reported in previous studies, which vary from 9.3% to 21.7%.8,21,22 Multivariate Cox analysis showed that root(s) resorption, cortical bone perforation, treatment pattern, and WHO classification were independent prognostic risk factors of ameloblastoma. Incorporating these four predictors, we constructed a nomogram with sufficient power in predicting the RFS of ameloblastoma.
To the best of our knowledge, this study is the first to construct a prognostic predictive nomogram model to quantitatively estimate the RFS for patients with ameloblastoma. As a favorable predictive tool based on easily identifiable risk factors, demographics, and comorbidities, nomograms have been developed for various cancers and non-cancerous diseases. For example, an updated QRISK3 algorithm was developed to quantify the absolute risk of cardiovascular disease and provide valid measures of absolute risk in the general population of patients.13 Pleijhuis et al16 developed a nomogram to estimate the preoperative risk of positive margins in breast-conserving surgery and to identify high-risk patients who might benefit from preoperative MRI and/or oncoplastic surgery. Studies have found some potential factors, such as age, tumor size, histopathological type, and treatment modality, that affect ameloblastoma prognosis.8,9,22,23 However, previous studies lack an integrated and quantitative analysis of these factors, and a nomogram had not been constructed specifically for ameloblastoma. In this study, cortical bone perforation, root(s) resorption, WHO classification, and treatment pattern were identified as important independent risk factors and were integrated into the construction of a nomogram for quantitatively predicting RFS of ameloblastoma. Furthermore, the C-index and calibration curves showed promising predictive power in both the training and validation sets. In addition, the nomogram was able to identify high risk patients, thereby assisting surgeons in developing a suitable surgical procedure for them, as well as serving as a counseling tool for surgeons to improve patients’ understanding of RFS. Therefore, our nomogram model may be applied clinically to create more tailored strategies to minimize and manage recurrence risk in patients with ameloblastoma. It should be noted that about 75.9% (41/54) of patients relapsed within 5 years of primary surgery, which was in line with previous studies,24 and 2 patients confirmed recurrence over 10 years. Therefore, the importance of long-term, regular follow-up cannot be stressed enough as a key for early detection of recurrence of ameloblastoma and timely intervention.
Ameloblastoma is an epithelial odontogenic benign neoplasm commonly found in the jaw.25 Tumors that occurred in the mandible accounted for 89.4% of cases, while only 10.6% of tumors occurred in the maxillary, most of which occur in the mandibular molar area and ramus or maxillary molar, consistent with previous reports.8,22 The prevalence according to sex and age at diagnosis was also similar to previous studies.22 In this cohort, 52.3% of the patients had tumors with a maximum diameter > 5cm on panorama, but we did not find a significant association between tumor size and recurrence. Slow growth and painless swelling are the dominant clinical features of ameloblastoma,25 and the latter is also the chief complaint of most patients, which was similar in our study. It may be for this reason that some patients have a long course of disease before seeing a doctor, and nearly one quarter of patients in this cohort had a course of greater than one year, and two of them as long as 10 years. Nonetheless, we did not observe an association between the demographic and clinical characteristics and RFS of ameloblastoma.
Our study revealed that the presence of cortical bone perforation and root(s) resorption on preoperative radiographies was associated with worse RFS of ameloblastoma, but there was no significant association between RFS and radiographical features or impacted tooth involvement. Ameloblastoma is characterized by an extensive and local aggressive nature, and the destruction of cortical bone and dental root(s) may indicate the aggressive capacity of ameloblastoma to some extent, which in turn affects the prognosis of ameloblastoma after surgery. Au et al8 reported that the radiographic pattern was a statistically significant factor associated with the recurrence of ameloblastoma, though not an independent predictor. Cortical bone invasion, root resorption, impacted tooth involvement, and pathological fracture were also analyzed, but none was an independent risk factor of the recurrence of ameloblastoma.8 Chrcanovic et al26 indicated that tooth displacement and root resorption were associated with the recurrence of central giant cell lesions of the jaws (CGCLJ), which also contributed to the division of CGCLJ lesions into aggressive and non-aggressive types. Thus, the destruction of dental roots may also indicate the invasiveness of the tumor. Our study was the first to reveal that cortical bone perforation and root(s) resorption may be independent predictors of RFS of ameloblastoma, implying that more attention should be paid to radiographic details, such as cortical bone destruction and root resorption, when devising treatment plans.
In terms of its histopathology, ameloblastoma presents in diverse types.27 In this cohort, the plexiform type accounted for 46.4% of cases, followed by unicystic (34.4%), follicular (12.3%), desmoplastic (5.0%) and others (2.0%, including 2 acanthomatous and 4 granular cell type). However, no significant difference between the histopathological type and recurrence of ameloblastoma was observed using univariate and multivariate Cox analyses, which is consistent with the findings of previous studies.8,9 According to the latest WHO Classification of Head and Neck Tumours, 198 (65.6%) patients were confirmed to have conventional ameloblastoma, and 104 (34.4%) patients were confirmed to have unicystic ameloblastoma in this cohort. Interestingly, our analysis showed that the WHO classification was associated with the RFS of ameloblastoma. In fact, compared with unicystic ameloblastoma, conventional ameloblastoma presents a higher rate of recurrence and poorer prognosis. Thus, the WHO classification may also play a role in the evaluation of prognosis of ameloblastoma, but a larger scale study sample is needed to confirm whether it is an independent factor predicting the recurrence of ameloblastoma.
Surgery is still the primary treatment modality for ameloblastoma today. A variety of treatment patterns, including marsupialization and decompression, enucleation, curettage, and radical treatment (such as maxillectomy and mandibulectomy with or without free bone auto-graft), have been applied for treating ameloblastoma.3,6,23 However, there is still no agreement on whether different treatment patterns are related to the relapse of ameloblastoma. Some researchers have proposed that the treatment modality is an independent prognostic factor for recurrence of ameloblastoma,7,8,28,29 while others have not.9 In the present study, 77 (25.5%) patients underwent radical bone resection and 225 (74.5%) patients underwent curettage, among which the proportion of recurrence was 7.8% (6/77) and 22.3% (48/225), respectively. Treatment pattern was a statistically significant factor in the overall cohort using univariate and multivariate Cox analyses. Undeniably, compared with the conventional curettage, radical treatment removes the lesions with a wider scope and more adequate surgical margin, which reduces the rate of recurrence of ameloblastoma. However, radical surgery also results in a greater burden to patients, as it may require a bone graft at a second operation site, affects patients’ facial appearance and chewing function, and results in a longer hospital stay and increased economic cost. Therefore, a majority of patients are inclined to a conservative surgical approach rather than radical bone resection. Considering the patient’s personal wishes combined with the benign nature of the tumor, surgeons typically attempt conservative therapies in some small-sized tumors or younger cases of conventional ameloblastoma. Nonetheless, patients should be fully informed of the high risk of recurrence and should maintain regular follow-up.
Admittedly, there are several limitations in our study. Due to the retrospective nature, missing variables and selection bias were inevitable Firstly, this study lacked an external dataset for validation of our nomogram model, although we validated our nomogram using an independent validation dataset that was extracted from the same institution. Secondly, the sample size was relatively limited, and the predictive ability of the model needs to be further verified in large-sample studies. Thirdly, the characteristics of some predictors, such as the specific location and extent of cortical bone destruction and the degree of root resorption, need to be measured more accurately by cone beam computed tomography (CBCT) and further refined to more effectively judge the relationship between such risk factors and RFS of ameloblastoma. Fourthly, the status of the BRAF mutation of this cohort was unknown and was not included in the model. Future studies should consider the status of BRAF mutations in patients with ameloblastoma, as adding BRAF mutations to the model may result in higher and more effective power for predicting the RFS of ameloblastoma.
In conclusion, the recurrence of ameloblastoma is significantly associated with cortical bone perforation, root(s) resorption, WHO classification, and treatment pattern. The study presented herein is the first to develop and use a nomogram to accurately predict the prognosis and RFS in patients with ameloblastoma following surgery. Additional large scale and multicenter studies are required to determine whether our model can be applied in a clinical setting.
Acknowledgments
The authors thank the staff of the Medical Record Room and the Department of Oral Radiology, Hospital of Stomatology, Sun Yat-sen University. The authors also thank AiMi Academic Services (www.aimieditor.com) for English language editing and review services.
Funding Statement
This work was supported by the Science and Technology Planning Project of Guangdong Province (No. 2017A020211025, No.2016A020220008).
Abbreviations
RFS, recurrence-free survival; WHO, world health organization; C-index, Harrell's concordance index; CI, confidence interval; IQR, interquartile range; AIC, Akaike’s information criterion; TRIPOD, Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis statement.
Data Sharing Statement
All data and code used and analyzed during the study are available from the corresponding author by reasonable request.
Ethic Approval and Informed Consent
The study was approved by the Ethic Board of Hospital of Stomatology, Sun Yat-sen University (No. KQEC-2020-75-03). This study was conducted in accordance with the Declaration of Helsinki.
Consent for Publication
All authors have reviewed the final version of the manuscript and are in agreement its content and submission.
Author Contributions
Clinical data collection was performed by Yao-Cheng Yang and Yun Huang; quality control of data and algorithms was performed by Yao-Cheng Yang, Jun-Jie Wang, and Wei-xin Cai; statistical analysis was performed by Yao-Cheng Yang and Jun-Jie Wang; the manuscript was prepared and edited by Yao-Cheng Yang, Jun-Jie Wang and Yun Huang; the study was designed by Qian Tao. All authors agreed to take responsibility and be accountable for the contents of the article. All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work. Yao-Cheng Yang and Jun-Jie Wang are co-first authors.
Disclosure
The authors declare no conflicts of interest in this work.
References
- 1.Shi HA, Ng CWB, Kwa CT, Sim QXC. Ameloblastoma: a succinct review of the classification, genetic understanding and novel molecular targeted therapies. Surgeon. 2020. doi: 10.1016/j.surge.2020.06.009 [DOI] [PubMed] [Google Scholar]
- 2.Mendenhall WM, Werning JW, Fernandes R, Malyapa RS, Mendenhall NP. Ameloblastoma. Am J Clin Oncol. 2007;30(6):645–648. doi: 10.1097/COC.0b013e3181573e59 [DOI] [PubMed] [Google Scholar]
- 3.Effiom OA, Ogundana OM, Akinshipo AO, Akintoye SO. Ameloblastoma: current etiopathological concepts and management. Oral Dis. 2018;24(3):307–316. doi: 10.1111/odi.12646 [DOI] [PubMed] [Google Scholar]
- 4.Lee SK, Kim YS. Current concepts and occurrence of epithelial odontogenic tumors: i. ameloblastoma and adenomatoid odontogenic tumor. Korean J Pathol. 2013;47(3):191–202. doi: 10.4132/KoreanJPathol.2013.47.3.191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ. WHO Classification of Head and Neck Tumours. Odontogenic and Maxillofacial Bone Tumors. Lyon, France: IARC Press; 2017. [Google Scholar]
- 6.Neagu D, Escuder-de la Torre O, Vázquez-Mahía I, et al. Surgical management of ameloblastoma. Review of literature. J Clin Exp Dent. 2019;11(1):e70–e75. doi: 10.4317/jced.55452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hendra FN, Kalla DSN, Van Cann EM, de Vet HCW, Helder MN, Forouzanfar T. Radical vs conservative treatment of intraosseous ameloblastoma: systematic review and meta-analysis. Oral Dis. 2019;25(7):1683–1696. doi: 10.1111/odi.13014 [DOI] [PubMed] [Google Scholar]
- 8.Au SW, Li KY, Choi WS, Su YX. Risk factors for recurrence of ameloblastoma: a long-term follow-up retrospective study. Int J Oral Maxillofac Surg. 2019;48(10):1300–1306. doi: 10.1016/j.ijom.2019.04.008 [DOI] [PubMed] [Google Scholar]
- 9.Yang R, Liu Z, Gokavarapu S, Peng C, Ji T, Cao W. Recurrence and cancerization of ameloblastoma: multivariate analysis of 87 recurrent craniofacial ameloblastoma to assess risk factors associated with early recurrence and secondary ameloblastic carcinoma. Chin J Cancer Res. 2017;29(3):189–195. doi: 10.21147/j.issn.1000-9604.2017.03.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moons KG, Altman DG, Vergouwe Y, Royston P. Prognosis and prognostic research: application and impact of prognostic models in clinical practice. BMJ. 2009;338:b606. doi: 10.1136/bmj.b606 [DOI] [PubMed] [Google Scholar]
- 11.Gu HQ, Liu C. Clinical prediction models: evaluation matters. Ann Transl Med. 2020;8(4):72. doi: 10.21037/atm.2019.11.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015;16(4):e173–e180. doi: 10.1016/S1470-2045(14)71116-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hippisley-Cox J, Coupland C, Brindle P. Development and validation of QRISK3 risk prediction algorithms to estimate future risk of cardiovascular disease: prospective cohort study. BMJ. 2017;357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang XR, Li YQ, Liang SB, et al. Development and validation of a gene expression-based signature to predict distant metastasis in locoregionally advanced nasopharyngeal carcinoma: a retrospective, multicentre, cohort study. Lancet Oncol. 2018;19(3):382–393. doi: 10.1016/S1470-2045(18)30080-9 [DOI] [PubMed] [Google Scholar]
- 15.Montero PH, Yu CH, Palmer FL, et al. Nomograms for preoperative prediction of prognosis in patients with oral cavity squamous cell carcinoma. Cancer. 2014;120(2):214–221. doi: 10.1002/cncr.28407 [DOI] [PubMed] [Google Scholar]
- 16.Pleijhuis RG, Kwast ABG, Jansen L, et al. A validated web-based nomogram for predicting positive surgical margins following breast-conserving surgery as a preoperative tool for clinical decision-making. Breast. 2013;22(5):773–779. doi: 10.1016/j.breast.2013.01.010 [DOI] [PubMed] [Google Scholar]
- 17.Liang W, Zhang L, Jiang G, et al. Development and validation of a nomogram for predicting survival in patients with resected non-small-cell lung cancer. J Clin Oncol. 2015;33(8):861–869. doi: 10.1200/JCO.2014.56.6661 [DOI] [PubMed] [Google Scholar]
- 18.Murtaugh PA. In defense of P values. Ecology. 2014;95(3):611–617. doi: 10.1890/13-0590.1 [DOI] [PubMed] [Google Scholar]
- 19.Harrell FE Jr, Califf RM, Pryor DB, Lee KL, Rosati RA. Evaluating the yield of medical tests. JAMA. 1982;247(18):2543–2546. doi: 10.1001/jama.1982.03320430047030 [DOI] [PubMed] [Google Scholar]
- 20.Camp RL, Dolled-Filhart M, Rimm DL. X-Tile. Clin Cancer Res. 2004;10(21):7252. doi: 10.1158/1078-0432.CCR-04-0713 [DOI] [PubMed] [Google Scholar]
- 21.Ledesma-Montes C, Mosqueda-Taylor A, Carlos-Bregni R, et al. Ameloblastomas: a regional Latin-American multicentric study. Oral Dis. 2007;13(3):303–307. doi: 10.1111/j.1601-0825.2006.01284.x [DOI] [PubMed] [Google Scholar]
- 22.Reichart PA, Philipsen HP, Sonner S. Ameloblastoma: biological profile of 3677 cases. Eur J Cancer B Oral Oncol. 1995;31b(2):86–99. [DOI] [PubMed] [Google Scholar]
- 23.McClary AC, West RB, McClary AC, et al. Ameloblastoma: a clinical review and trends in management. Eur Arch Otorhinolaryngol. 2016;273(7):1649–1661. doi: 10.1007/s00405-015-3631-8 [DOI] [PubMed] [Google Scholar]
- 24.Olaltan AA, Arole G, Adekeye EO. Recurrent ameloblastoma of the jaws: a follow-up study. Int J Oral Maxillofac Surg. 1998;27(6):456–460. doi: 10.1016/S0901-5027(98)80037-4 [DOI] [PubMed] [Google Scholar]
- 25.Barnes L, Eveson JW, Reichart P. World Health Organization Classification of Tumours. Pathology and Genetics of the Head and Neck Tumours. Lyon, France: IARC Press; 2005:283–327. [Google Scholar]
- 26.Chrcanovic BR, Gomes CC, Dos Santos TR, Abreu M, Gomez RS. Clinical factors associated with the recurrence of central giant cell lesions. J Oral Pathol Med. 2019;48(9):799–802. doi: 10.1111/jop.12937 [DOI] [PubMed] [Google Scholar]
- 27.Kramer IRH, Pindborg JJ. WHO Histological Typing ofOdontogenic Tumours. 2nd ed. Geneva: Springer-Verlag; 1992. [Google Scholar]
- 28.Hong J, Yun PY, Chung IH, et al. Long-term follow up on recurrence of 305 ameloblastoma cases. Int J Oral Maxillofac Surg. 2007;36(4):283–288. doi: 10.1016/j.ijom.2006.11.003 [DOI] [PubMed] [Google Scholar]
- 29.Milman T, Ying GS, Pan W, LiVolsi V. Ameloblastoma: 25 year experience at a single institution. Head Neck Pathol. 2016;10(4):513–520. doi: 10.1007/s12105-016-0734-5 [DOI] [PMC free article] [PubMed] [Google Scholar]