Abstract
Introduction
Although many vaccines are in development and clinical trials, and at least seven vaccines have been distributed worldwide, the world has faced a huge challenge in line with the willingness to accept a COVID-19 vaccine in a different country including Ethiopia. However, no study has been conducted on the knowledge, attitudes, acceptance, and determinates of COVID-19 vaccine acceptance in Ethiopia. Therefore, this study was aimed to investigate the knowledge, attitudes, acceptance, and determinants of acceptance of the COVID-19 vaccine among the adult population in Ethiopia.
Methods
A community-based cross-sectional study was conducted among 492 study participants from March 1 to March 15, 2021. A multistage sampling technique was used to recruit study participants. Six skilled and qualified data collectors had participated to gather the data using a pretested structured-administered questionnaire. A multivariable logistic regression model was used to identify factors associated with the acceptance of the COVID-19 vaccine. P-value <0.05 was considered to indicate statistically significant association.
Results
This study revealed that the level of good knowledge, positive attitude and intention to accept the COVID-19 vaccine were 74%, 44.7%, and 62.6%, respectively. Moreover, having an age ≥46 years with an adjusted odds ratio of 2.36 [95% CI, 1.09–5.39], attended secondary and above education adjusted odds ratio 2.59 [95% CI, 1.52–4.39], having a chronic disease adjusted odds ratio of 3.14 [95% CI, 1.21–8.14], and having good knowledge about COVID-19 vaccine adjusted odds ratio 2.59 [95% CI, 1.67–4.02] were significantly associated with COVID-19 vaccine acceptance.
Conclusion
In this study, the level of good knowledge, positive attitude, and intention to accept the COVID-19 vaccine were 74%, 44.7%, and 62.6%, respectively. Thus, health education and communication from government sources are very crucial methods to alleviate the negative attitude of the COVID-19 vaccine.
Keywords: knowledge, attitudes, acceptance, vaccine, Ethiopia
Introduction
As the number of cases of coronavirus disease (COVID-19) is increasing worldwide, promising COVID-19 vaccine candidates are being produced, necessitating consideration of their potential demand, distribution, and adoption to optimize their desired effects. COVID-19 was first identified in December 2019 in Wuhan, Hubei Province, China, and quickly spread to other countries before being declared a pandemic on March 11, 2020.1
The COVID-19 pandemic has disrupted not only the world economy but also health systems including routine childhood vaccination in many countries.2 COVID-19 also causes persistent symptoms that can impact the quality of life of survivors.3
This pandemic has affected 223 countries, with over 118, 278, 711 reported cases and 2, 624, 426 deaths worldwide.4 Of these, North America (34,228,219 cases and 781,745 deaths), Europe (35,456,449 cases and 843,039 deaths), Asia (25,748,391 cases and 406,251 deaths), and South America (18,779,624 cases and 485,659 deaths) have higher rates than Africa (4,013,352 cases and 106,623 deaths) and Oceania (4,013,352 cases and 106,623 deaths) (51,955 cases and 1094 deaths).4
On March 13, 2020, the first COVID-19 case in Ethiopia was identified.5,6 Since then, the number of new cases in the country has been rapidly increasing. As of March 10, 2021, the country had 168,335 COVID-19 positive cases and 2451 deaths domestically.4 As a result, Ethiopia became one of the five African countries with the largest COVID-19 case burden.7 Ethiopia’s government, on the other hand, has been working hard to disseminate information about COVID-19 prevention measures through television, radio, and social media, and has declared a state of emergency.8
To fight the coronavirus disease (COVID-19) pandemic, researchers from all over the world have made remarkable efforts to create vaccines against the disease.9 At least seven vaccines across three platforms have been carried out in countries. Vaccination is prioritized for vulnerable groups in all countries. Simultaneously, more than 200 additional vaccine candidates are being produced, with more than 60 of them in clinical trials.10
Seventy percent of available COVID vaccines have been pre-ordered by 16% of the global population in 2021,11 and 312.25 million COVID19 vaccines have been distributed worldwide by March 8/2021.12 COVID-19 infection will continue to advance, according to a group of virus experts, unless low-income countries have access to vaccines.11 As a result, we have been eagerly waiting for the launch of safe and successful COVID-19 vaccines as the COVID-19 pandemic continues while any vaccination program’s effectiveness is dependent on participants’ awareness, behaviors, and willingness to accept the COVID-19 vaccine.13–24
Previous studies showed that the general population’s knowledge and attitudes toward the COVID-19 vaccine ranged from 31.6% to 86.2% in Africa, Europe, India, England, and France, respectively.19–21,25,26 Despite this, more than 200 COVID-19 vaccines were being produced around the world, with numerous governments negotiating agreements to gain access to advance doses. However, access is just one issue.
Throughout the pandemic, countries’ aspirations to use a COVID-19 vaccine as it becomes available to have differed significantly. In the United States, the United Kingdom, New Zealand, France, and Austria, it varies from 58% to 86%.27–31 The intent to use the COVID-19 vaccine was found to be 54.6% in Chain.17 In addition, COVID-19 vaccine intentions in Africa ranged from 43.55% in Egypt to 82.765% in Mauritius.18
Accordingly, studies conducted in different countries of the world, the most determinate of intention to use COVID-19 vaccine are age, parity, occupational status, gender, marital status, educational status, income, perceived risk of COVID-19 infection, a healthcare worker, attitude towards, knowledge of COVID-19, being sick with COVID-19, the pre-existence of chronic disease.17,23–26,32 Moreover, multiple myths and conspiracy theories on vaccines and COVID-19 would also potentially affect the COVID-19 vaccine acceptance in a population,33 perception of government performance, health care status, recovery status from COVID-19, efficacy, side-effects, and speed of development of a COVID-19 vaccine are also the determinant of COVID-19 vaccine acceptance.17,34–36
These low levels of knowledge, attitude, and low level of intention to accept vaccine for COVID-19 may be a concern of the globe. Since the most efficient means of stopping the virus from spreading is by protecting oneself from being infected to COVID-19, it is also important to vaccinate the most vulnerable people as soon as possible.37
We need to know the general public’s awareness, behavior, expectation, hesitancy, and determinates of the intention to use the COVID-19 vaccine and to introduce the most successful vaccination strategy in Ethiopia. People’s awareness, behaviors, desire to use, expectations, hesitancy, and ability to pay for the COVID-19 vaccine are critical for government and policymakers to overcome all obstacles to vaccine delivery in such a scenario. To date, no prior study has been conducted among the adult population to investigate the mentioned issues of COVID-19 vaccine. Therefore, this study was aimed to determine the level of knowledge, attitudes, acceptance, and determinates of COVID-19 vaccine acceptance among the adult population in Ethiopia.
Methods and Materials
Study Setting and Period
This research was carried out in Gurage Zone which is one of administrative areas in the south region, Ethiopia. Gurage Zone is divided into 13 districts and two local administrations. It is located 156 kilometers southwest of Addis Ababa capital, Ethiopia. According to the 2007 national household census, the zone has a total population of 1,279,646, with 657,568 women.22 All peoples found in Gurage Zone are served by eight hospitals (five public and two private). All hospitals offer comprehensive emergency care, including COVID-19 treatment. In addition to these, the Zone hs 74 health centers that offer basic emergency care. This study was taken place from March 1 to March 15, 2021.
Study Design
A community-based cross-sectional study was conducted among the adult population in Gurage Zone.
Populations
All adult populations over the age of 18 years old were included in the source population. The research participants were all adult populations over the age of 18 who had lived in the Gurage Zone for at least 6 months.
Eligibility Criteria
Adult populations over the age of 18 years old who had lived in the study area for at least 6 months were included in the study. Respondents who were mentally or seriously ill at the time of the study were not eligible.
Sample Size Determination
The research sample size was estimated using the Epi InfoTM version 7 StatCalc function of the population survey, with the assumption of a 95% confidence interval (CI), a 5% margin of error, and 27% of the adult population intention to accept COVID-19 vaccine from a similar study was done in France.30 By considering a 10% non-response rate, a total of 334 research participants were estimated for this study. Finally, because of the 1.5 design effects, the minimum sample size for this study was 501.
Sampling Procedure
The study participants were recruited using a multi-stage sampling technique. Using a simple random sampling technique, six districts and one town administration were selected from the woreda of the zone. Then, three kebeles from each district and two kebeles from Wolkite town were chosen at random. Households with adult populations were identified in the family folders of health extension staff (HEWs), and study participants were chosen using a systematic random sampling process. Hence, a systematic random sampling technique was done by determining the K value via dividing the source population of each kebeles (small administrative unit Ethiopia) by the calculated sample size. Finally, after determining the K value we had taken a single number between 0 and the K value using the lottery method then by accustoming this selected number in each K value we recruited the study participants.
Data Collection Tools and Procedure
A pre-tested, standardized interviewer-administered questionnaire was used to collect the data. The instrument was created after a detailed analysis of several related types of literature.24–31 Socioeconomic variables, attitude-related variables, knowledge-related variables, individual health-related variables, and intention to accept related variables were all included in the questionnaire. The reliability of variables such as awareness, attitude, and acceptance toward COVID-19 were determined using Cronbach’s alpha coefficient. The Cronbach’s alpha value for questions about knowledge and attitude toward COVID-19 were found to be 0.761 and 0.832 respectively. According to the rule of Griethuijsen criteria, the range of Cronbach’s alpha from 0.6 to 0.7 is considered sufficient and accurate measurement. As a result, the item used to evaluate the knowledge and attitude towards the COVID-19 vaccine was scientifically acceptable. The data were collected using six diploma nurses with the supervision of three BSc nurses who spoke the local language. The data were obtained at the household level through face-to-face interviews. The household head or anyone over the age of 18 was told of all the details of the study before giving their consent to participate.
Adult populations were encouraged to feel free and guaranteed that their responses would be kept private and no information would be exchanged with third parties, except the researcher. The participants who were willing to integrate and sign the informed consent document were then interviewed in a quiet and spacious space. The investigator had also taken corrective efforts to boost the validity of the conclusion by reviewing and cross-checking the questionnaires or data for completeness, accuracy, and consistency.
Operational Definitions/Study Variables
Knowledge of Respondents Towards COVID-19 Vaccine
Comprehensive knowledge of COVID-19 vaccine was computed from summing up all relevant five knowledge related “Yes” and “No” questions, respondents were asked “Does COVID-19 vaccination increase allergic reactions”; respondents who respond “yes” will score 1 and “No” response will earn zero scores. The correct answer for each item was scored “1” and the incorrect answer was scored “0.” The same pattern of questioning and scoring was made for the rest of the four knowledge-related items. Accordingly, respondents who scored greater than or equal to the mean value of the sum of knowledge assessment questions were thought as having good knowledge, and respondents who answered less than the mean value of the sum of knowledge assessment questions were thought as having poor knowledge.
Attitude of Respondents Towards COVID-19 Vaccine
Attitude of COVID-19 vaccine was computed from summing up all relevant six attitude related ‘Agree, “Undecided and disagree” questions, respondents were asked “Does the newly discovered COVID-19 vaccine is safe”; respondents who respond “agree” will score 1 and “Undecided and disagree” response will earn zero scores. The correct answer for each item was scored “1” and the incorrect answer was scored “0.” The same pattern of questioning and scoring was made for the rest of the five attitude-related items. Accordingly, respondents who scored greater than or equal to the mean value of the sum of attitude-related questions were thought of as having a positive attitude, and respondents who answered less than the mean value of the sum of knowledge assessment questions were thought as having a negative attitude.
Intention of Respondents Towards COVID-19 Vaccine Acceptance
COVID-19 vaccine acceptance was measured using “Yes” and “No” questions, respondents were asked “Did you have an intention to accept COVID-19 vaccine if it is available in the future”; respondents who respond “yes” will score 1 and “No” response will earn zero scores. Accordingly, respondents who scored 1 were thought of as having the intention to accept the COVID-19 vaccine, and respondents who scored 0 were thought of as having no intention to accept the COVID-19 vaccine.
Data Quality Control
Data collectors and supervisors received a two-day intensive training on data collection methods, the research aim, data collection techniques, and the template for data abstraction. In addition, the principal investigator and supervisors performed frequent reviews of the data collected for accuracy, quality, and completeness, and any necessary changes were made on the spot.
Data Analysis and Processing
The data were coded, cleaned, modified and entered into Epi data version 4.0.0 before being exported to SPSS version 24 for analysis. The results of the Univariate analysis were provided in the form of texts, graphs, and figures. Binary logistic regression was used to determine the relationship between each independent variable and the outcome variable.
Hosmer–Lemeshow test was used to determine model fitness because it was insignificant (p-value = 0.790) and the Omnibus test was significant (P-value 0.001). All variables with a P≤0.25 in the bivariate analysis were included in the multivariate analysis to adjust for all potential confounders. Furthermore, even if the above parameters were not met, variables from the context point of view and that were significant in previous studies were included in the final model.
A standard error of more than two and a variance inflation factor of more than ten was used to infer the presence of multi co-linearity. An odds ratio with a 95% confidence interval was used to assess the direction and strength of statistical association between the response and covariate variables. A p-value of less than 0.05 was used to assert statistical significance in this analysis.
Results
Socio-Demographic Attributes of Participants
A total of four hundred ninety-two adult populations were successfully interviewed, giving a 98.2% response rate. The mean age of the study participants was 27.3 with a standard deviation of 4.8. Nearly two-thirds, 310 (63.0%) of the respondents were Orthodox followers by religion. Nearly three-fourths, 359 (73.0%) of the participants were Gurage by ethnicity. Nearly two-fifths, 194 (39.4%) of the respondents had attended primary education. Almost one-fourth, 128 (26.0%) of the study participants were merchants by their occupation. Nearly half, 241 (49.0%) of the respondents had 2000–3999 Ethiopian birr average monthly income (See Table 1).
Table 1.
Sociodemographic Characteristics of Respondents in the Gurage Zone, Ethiopia, 2021
| Variable | Frequency | Percent |
|---|---|---|
| Age (Year) | ||
| 18–25 | 181 | 36.8 |
| 26–35 | 106 | 21.5 |
| 36–45 | 150 | 30.5 |
| >46 | 55 | 11.2 |
| Residence | ||
| Urban | 390 | 79.3 |
| Rural | 102 | 20.7 |
| Education | ||
| No formal Education | 116 | 23.6 |
| Primary Education | 194 | 39.4 |
| Secondary Education | 182 | 37.0 |
| Occupation | ||
| Housewife | 20 | 4.1 |
| Merchant | 128 | 26.0 |
| Government Employee | 105 | 21.3 |
| Student | 113 | 23.0 |
| Farmer | 126 | 25.6 |
| Sex of respondents | ||
| Male | 274 | 55.7 |
| Female | 218 | 44.3 |
| Religion | ||
| Orthodox | 310 | 63.0 |
| Muslim | 146 | 29.7 |
| Protestant | 36 | 7.3 |
| Ethnicity | ||
| Gurage | 359 | 73.0 |
| Amhara | 103 | 20.9 |
| Oromo | 30 | 6.1 |
| Average monthly income | ||
| < 1999 | 143 | 29.1 |
| 2000–3999 | 241 | 49.0 |
| > 4000 | 108 | 22.0 |
Knowledge of Respondents Towards COVID-19 Vaccine
Three hundred sixty-two, (73.6%) of the participants had been aware of the development of the COVID-19 vaccine. More than four-fifth, 410 (83.3%) of the respondents knew about the effectiveness of the developed COVID-19 vaccine. Nearly three-fourths, 347 (70.5%) of the study participants had responded the overdose of COVID-19 vaccine would become dangerous for humans. Three-fourths, 369 (75.0%) of the respondents explained that COVID-19 vaccination did not increase allergic reactions. Two-hundred eight six, (58.1%) of the participants explained that COVID-19 vaccination would not increase autoimmune diseases. Nearly three-fourths, 364 (74.0%) of the participants had good comprehensive knowledge about the COVID-19 vaccine (See Table 2).
Table 2.
Respondents Knowledge Towards COVID-19 Vaccine in Gurage Zone, Ethiopia, 2021
| Variable | Category | Frequency | Percent |
|---|---|---|---|
| Do you know about the COVID-19 vaccine development | Yes | 362 | 73.6 |
| No | 130 | 26.4 | |
| Do you know about the effectiveness of the COVID-19 vaccine | Yes | 410 | 83.3 |
| No | 82 | 16.7 | |
| Is it dangerous to use an overdose of COVID-19 vaccines | Yes | 347 | 70.5 |
| No | 145 | 29.5 | |
| Does COVID-19 vaccination increase allergic reactions | Yes | 123 | 25.0 |
| No | 369 | 75.0 | |
| Does vaccination increase autoimmune diseases | Yes | 206 | 41.9 |
| No | 286 | 58.1 | |
| Knowledge toward COVID-19 | Good | 364 | 74.0 |
| Poor | 128 | 26.0 |
Attitude of Respondents Towards the COVID-19 Vaccine
Nearly one-fourth, 108 (22.0%) of the participants had agreed that the newly discovered COVID-19 vaccine was safe. Almost one-fourth, 126 (25.6%) of the participants had agreed that the COVID-19 vaccine was essential for us. One hundred thirteen, (23.0%) of the participants had agreed that the COVID-19 vaccine developed in Europe and America is safer than those made in other countries. Almost one-tenth, 56 (11.4%) of the respondents had agreed to encourage family/friends/relatives to get vaccinated. Nearly one-fifth, 84 (17.1%) of the respondents had agreed that it is not possible to reduce the incidence of COVID-19 without vaccination. Almost one-fourth, 124 (25.2%) of the participants agreed that the COVID-19 vaccine should be distributed fairly to all of us. Almost two-fifth, 229 (44.7%) of the respondents had a positive attitude toward the newly developed COVID-19 vaccine (See Table 3).
Table 3.
Respondents Attitude Towards COVID-19 Vaccine in Gurage Zone, Ethiopia, 2021
| Variable | Category | Frequency | Percent |
|---|---|---|---|
| Does the newly discovered COVID-19 vaccine is safe | Agree | 108 | 22.0 |
| Undecided | 214 | 49.0 | |
| Disagree | 143 | 29.0 | |
| Does the COVID-19 vaccine is essential for us | Agree | 126 | 25.6 |
| Undecided | 193 | 39.2 | |
| Disagree | 173 | 35.2 | |
| COVID vaccine developed in Europe and America are safer than those made in other world countries | Agree | 113 | 23.0 |
| Undecided | 299 | 60.8 | |
| Disagree | 80 | 16.2 | |
| May you encourage your family/friends/relatives to get vaccinated. | Agree | 56 | 11.4 |
| Undecided | 301 | 61.2 | |
| Disagree | 135 | 27.4 | |
| It is not possible to reduce the incidence of COVID-19 without vaccination | Agree | 84 | 17.1 |
| Undecided | 255 | 51.8 | |
| Disagree | 153 | 31.1 | |
| The COVID-19 vaccine should be distributed fairly to all of us | Agree | 124 | 25.2 |
| Undecided | 253 | 51.4 | |
| Disagree | 115 | 23.4 | |
| Respondents comprehensive attitude toward COVID-19 vaccine | Positive | 229 | 44.7 |
| Negative | 272 | 53.3 |
Respondent’s Intention to Accept Towards COVID-19 Vaccine
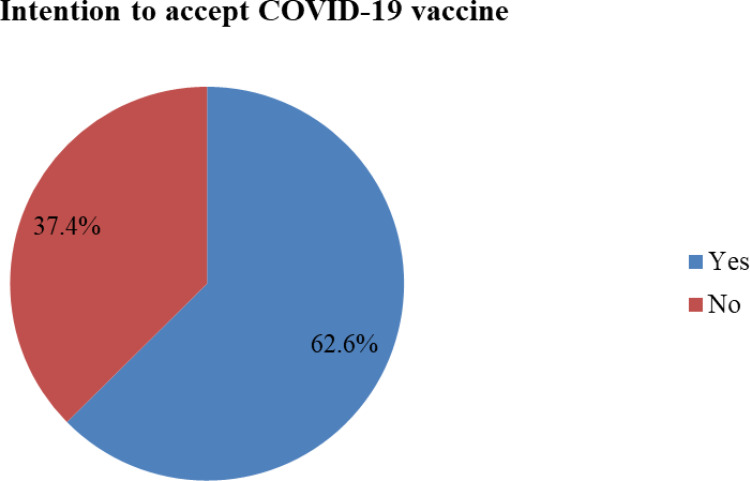
Regarding respondents’ intention to accept the COVID-19 vaccine, 308 (62.6%) of the respondents had the intention to use the COVID-19 vaccine if the vaccine will be available in Ethiopia. While the remaining, 184 (37.4%) of the respondents were hesitant to receive any COVID-19 vaccine if available in Ethiopia vaccine (See Figure 1).
Figure 1.
Respondents intention and hesitancy towards COVID-19 vaccine in Ethiopia, 2021.
Factor Associated to Accept Towards COVID-19 Vaccine
To determine the factors associated to accept with the COVID-19 vaccine, bi-variable and multivariable logistic regression analysis was carried out. Multivariable logistic regression was performed for variables with a p-value≤ of 0.25 on bi-variable logistic regression. Accordingly, variables including the age of respondents, educational status, residence, chronic disease, health insurance, perceived severity of the disease, received all the necessary vaccines, and knowledge towards COVID-19 vaccine have a p≤0.25 on bi-variable logistic regression; thereby further analyzed on multivariable logistic regression.
The independent predictors of the outcome variable include age ≥ 46 years with AOR 2.36 [95% CI, 1.09–5.39], attending secondary education and above, AOR 2.59 [95% CI, 1.52–4.39], having a chronic disease AOR 3.14 [95% CI, 1.21–8.14], and having good knowledge 2.59 [95% CI, 1.67–4.02].
COVID-19 vaccine is nearly three times more likely to be accepted by adult population with age greater than 46 years old as compared to adult population with age in between 18 and 25 years old.
Attending secondary education and above is also one of the factors associated to accept the COVID-19 vaccine. Hence, adult populations who had attended secondary education and above were nearly three times more likely to accept the COVID-19 vaccine compared to the counterpart. Having chronic disease would increase the acceptance of the COVID-19 vaccine by almost three times on adult populations compared to adult populations with no chronic disease. Good knowledge about the COVID-19 vaccine was also another main factor for the intention to accept the COVID-19 vaccine. Adult populations who had good knowledge about COVID-19 were nearly three times more likely to accept the COVID-19 vaccine as compared to the counterpart (See Table 4).
Table 4.
Factors Associated with Intention to Accept COVID-19 Vaccine Among Adult Population in Ethiopia, 2021 (N=492)
| Variables | Intention to Accept COVID-19 Vaccine | COR (95%) | P-value | AOR (95%) | P-value | |
|---|---|---|---|---|---|---|
| Yes (%) | No (%) | |||||
| Educational status | ||||||
| Secondary education and above | 138(44.8%) | 44(23.9%) | 2.83(1.72–4.65) | < 0.001 | 2.59(1.52–4.39) | < 0.001 |
| Primary education | 109(35.4% | 85(46.2%) | 1.16(0.73–1.84) | 0.23 | 1.16(0.71–1.89) | 0.563 |
| No formal education | 61(19.8%) | 55(29.9%) | 1.00 | 1.00 | ||
| Residences | ||||||
| Urban | 250(81.2) | 140(76.1%) | 1.35(0.87–2.11) | 0.179 | 1.32(0.81–2.17) | 0.270 |
| Rural | 58(18.8%) | 44(23.9%) | 1.00 | 1.00 | ||
| Age | ||||||
| ≥46 | 44(14.3%) | 11(6.0%) | 2.62(1.34–5.71) | 0.013 | 2.36(1.09–5.39) | 0.028 |
| 36–45 | 100(32.5%) | 50(27.2%) | 1.31(0.88–2.17) | 0.213 | 1.39(0.86–2.27) | 0.172 |
| 26–35 | 57(18.5%) | 49(26.6%) | 0. 76(0.49–1.30) | 0.361 | 0.94(0.56–1.58) | 0.812 |
| 18–25 | 107(34.7%) | 70(40.2%) | 1.00 | 1.00 | ||
| Health insurance | ||||||
| Yes | 183(59.4) | 98(53.3%) | 1.28(0.89–1.86) | 0.233 | 1.11(0.71–1.89) | 0.600 |
| No | 125(40.6%) | 86(46.7%) | 1.00 | 1.00 | ||
| Chronic Disease | ||||||
| Yes | 30(9.7%) | 6(3.3%) | 3.20(1.31–7.85) | 0.011 | 3.14(1.21–8.14) | 0.018 |
| No | 278(90.3%) | 178(96.7%) | 1.00 | 1.00 | ||
| Severity Perception | ||||||
| Perceived severe at all | 48(15.6%) | 20(10.9%) | 1.51(0.87–2.64) | 0.138 | 1.56(0.85–2.98) | 0.146 |
| Perceived not severe at all | 260(84.4%) | 164(89.1%) | 1.00 | 1.00 | ||
| Received all the necessary vaccines in your lifetime | ||||||
| Yes | 254(82.5%) | 143(77.7%) | 1.35(0.86–2.13) | 0.168 | 1.41(0.84–2.35) | 0.191 |
| No | 54(17.5%) | 41(22.3%) | 1.00 | 1.00 | ||
| Knowledge of COVID-19 vaccine | ||||||
| Good | 250(81.2%) | 114(62.0%) | 2.65(1.75–3.99) | < 0.001 | 2.59(1.67–4.02) | < 0.001 |
| Poor | 58(18.8%) | 70(38.0%) | 1.00 | 1.00 | ||
Source of Information About COVID-19 Vaccine
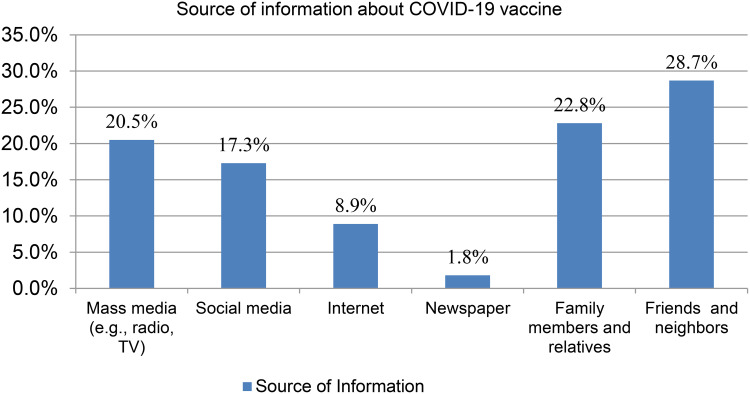
Concerning the source of information about the development of the COVID-19 vaccine, almost one-fourth, 112 (22.8%) and 141 (28.7%) of the respondents had heard about the COVID-19 vaccine from their family members and relative, and friends and neighbors respectively (See Figure 2).
Figure 2.
Respondents source of information about COVID-19 vaccine in Ethiopia, 2021.
Discussion
This study revealed that the level of good knowledge, positive attitude, and intention to accept towards COVID-19 vaccine was found to be 74%, 44.7%, and 62.6% respectively. Besides, attending secondary education and above, age ≥ 46 years, having a chronic disease and good knowledge about COVID-19 are the determinate factors of intention to accept towards COVID-19 vaccine. For healthcare planners, the finding is important. This information can then be used to create relevant programs, channeling limited resources to teach what is necessary, as opposed to distributing already established messages.
The level of good knowledge towards the COVID-19 vaccine is found to be 74% in this study. This study was higher than a study conducted in West India (35.5) and Bangladeshi (62.1%).26,38 As compared to a study conducted in France (81.2%) and England (83.0%) the finding is low. The disparity in the methodology used and research setting, socio-demographic features of the participants in the study and the availability and accessibility of health service infrastructures may be the possible reason.
In this study, the level of positive attitude towards the COVID-19 vaccine is also found to be 44.7%. Hence, this is higher than the study conducted in England (36.9%) and Egypt (34.3%).16,23 However, this study is lower than a study done in Belgium (72.4%) and Italy (66%).23,39 The discrepancy of these findings might be explained by the difference in socio-demographic characteristics of the study participants, method used and study settings and the availability and accessibility of the health services infrastructures. Based on the finding it is inferred that there is a lack of balance that the zone health office and regional health bureau could work in collaboration with the local health care provider to enhance a positive attitude towards the COVID-19 vaccine among adult populations in Ethiopia.
Similarly, the level of intention to accept the COVID-19 vaccine is found to be (62.6%). This means the finding is lower than a study reported in Australia (89.88%), UK (71%), and the USA (69%).18,25,39 This finding is higher than the study conducted in Libya, Morocco, Russia, and France.25,39 This difference can be explained by the difference in the respondent socio-demographic attribute and material and method used, and the availability and accessibility of the infrastructures. In addition to this, the health system-related factors might be contributing to this difference. This study inferred that there is a created platform for COVID-19 prevention task forces in Ethiopia which could help them to be aware of local norms, cultures, and traditions to enhance and fit the demands of adult populations and work with them to mitigate the pandemic of COVID-19.
In this finding, attending secondary education and above can increase the intention to use the COVID-19 vaccine compared to no formal education. The adult population who had attended secondary and above education were almost three times more likely to accept the COVID-19 vaccine compared to adult populations who did not attend formal education. This might be, adult populations attending secondary and above education can easily understand the benefit of taking the COVID-19 vaccine on the health of the adult population and the society at large. Moreover, adult populations who had attended secondary and above education will have better awareness about the benefits of the preventive health-related problem including the pandemic of COVID-19 and higher receptivity to new health-related information. The same finding is reported in Saudi Arabia.13 This finding implies that improving educational status could be one of the strategies for achieving a full immunization of the adult population in alleviating any disease influencing the globe.
Adult populations who had aged ≥ 46 years old were almost two times more likely to accept the COVID-19 vaccine than those adults who had ages between 18 and 25 years old. This report is in line with a study done by Saudi Arabia, China, and South Africa.13,24,32 Since the world health organization has declared the COVID-19 pandemic is a killer for the elder population and more prevalent in the aged human population, and as age increases adult population will prone to infection and chronic inflammation. This leads to adult populations who had an age greater than 46 years old will have a fear for COVID-19 related morbidities and mortality this in turn the adult population age group need to have a vaccine for COVID-19 infection.
Intention to accept the COVID vaccine had significantly correlated with chronic disease. Adult populations who had a chronic disease were almost three times more likely to accept the COVID-19 vaccine than those adult populations who had no chronic disease. This result is consistent with a report from WHO and Australian studies.10,21 This finding showed us there should be a created platform that could enable adult populations whose age greater than 46 years old is a first age group to be vaccinated if a COVID-19 vaccine is available in Ethiopia.
Adult populations who had good knowledge about the COVID-19 vaccine are significantly associated to accept the COVID-19 vaccine. This result is in line with a survey carried out in Southeast Asia and England.28,30 This study indicates that creating awareness towards the COVID-19 vaccine will increase the utilization of the COVID-19 vaccine among the adult population so that the government of Ethiopia has to work on enhancing the knowledge of the population about the COVID-19 vaccine. This finding is also explained by having good knowledge about the COVID-19 vaccine will help the adult population to know the benefit of the COVID-19 vaccination program.
The main importance of this study for any organization working in COVID-19 prevention is: identifying the determinate of intention to accept the COVID-19 vaccine to mitigate the pandemic of COVID-19 and to provide immediate intervention. The finding of this study is also motivated the different organizations to lay out suitable techniques and objectives for the intervention to give attention to the determinate of COVID-19 vaccine acceptance, both in the health care organization as well as in the society at large. This study becomes one tool for health policy planners for focusing on COVID-19 in the health care system.
Conclusions
In this study, the level of good knowledge, positive attitude, and intention to accept towards COVID-19 vaccine was found to be 74%, 44.7%, and 62.6% respectively. Besides, having attended secondary education and above, having age ≥ 46 years old, having a chronic disease, and good knowledge about the COVID-19 vaccine were significantly associated to accept the COVID-19 vaccine. Therefore, all COVID-19 vaccine taskforce which were established by the World Health Organization and Ethiopia’s Ministry of Health including the public health institute in collaboration with regional and zonal health offices should create awareness about the efficacy and safety of the COVID-19 vaccine. Besides, health planners and policymakers should encourage COVID-19 vaccine uptake behaviors in all regions of Ethiopia by providing trusted information about the COVID-19 vaccine and aiming to provide COVID-19 vaccine for the most vulnerable group for the COVID-19 pandemic including the elder population, who have a chronic disease, who have no formal education and who have poor knowledge toward COVID-19 vaccines are recommended.
Acknowledgment
We would like to extend our thanks to Wolkite University College of Medicine and Health Science for accepting our research project. Furthermore, we express our deepest gratitude to data collectors for their sincere efforts to provide reliable information. Finally, we would like to express our appreciation to all study participants without them this research work would not have been possible.
Funding Statement
The authors had not been provided with a particular grant for this research project from any public, private, or non-profit funding agency.
Abbreviations
AOR, adjusted odds ratio; CI, confidence interval; COR, crude odds ratio; COVID-19, corona virus disease; UK, United Kingdom; US, United States.
Data Sharing Statement
On reasonable requests, the full data set and other materials relevant to this study can be obtained from the corresponding author.
Ethical Approval and Consent to Participate
Wolkite University College of Medicine and Health Science research ethical review board had approved the study procedure, and Ethical clearance had been provided to the research group. The Gurage Zone health office and the respective district health office had given a permission letter. Before the interview, each respondent had given their informed consent after receiving clear and relevant details about the study’s aim. Adult populations who could read and understand the Amharic language were used to give information which was obtained a one-page written summary of the research. The adult populations who met the criteria were voluntarily included in the study while the adult populations who were unable to participate were withdrawn from the study since this research was carried out under the Helsinki Declaration. The data were collected anonymously, and the participants’ information was kept private.
Consent for Publication
Not applicable.
Disclosure
The authors reported no conflicts of interest for this work.
References
- 1.World Health Organization. Coronavirus disease 2019 (COVID-19): situation report; March. 2021. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports. Accessed May19, 2021.
- 2.Fahriani M, Anwar S, Yufika A, et al. Disruption of childhood vaccination during the COVID-19 pandemic in Indonesia. Narra J. 2021;1(1):e7. doi: 10.52225/narraj.v1i1.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yusuf F, Fahriani M, Mamada SS, et al. Global prevalence of prolonged gastrointestinal symptoms in COVID-19 survivors and potential pathogenesis: a systematic review and meta-analysis. F1000Research. 2021;10:301. doi: 10.12688/f1000research.52216.1;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. COVID-19 weekly epidemiological update. Geneva. Available from: https://apps.who.int/iris/bitstream/handle/10665/340087/nCoV-weekly-sitrep9Mar21-eng.pdf?sequence=1. Accessed March 10, 2021. [Google Scholar]
- 5.The Federal Ministry of Health (FMOH) - Ethiopia. COVID-19; Ethiopia COVID-19 monitoring platform. Available from: https://www.moh.gov.et/ejcc/en/node/196. Accessed March 27, 2021
- 6.World Health Organization (WHO). The first case of covid-19 is confirmed in Ethiopia. Available from: https://www.afro.who.int/news/first-case-covid-19-confirmed-ethiopia. Accessed May19, 2021.
- 7.World meter. Coronavirus Update (Live): cases and Deaths from COVID-19 Virus Pandemic; 2021. Available from: https://www.worldometers.info/coronavirus/?utm_referrer=mirtesen.ru. Accessed March10, 2021.
- 8.Zikargae MH. COVID-19 in Ethiopia: assessment of How the Ethiopian Government 329 has executed administrative actions and managed risk communications and community 330 engagement. Risk Manag Healthc Policy. 2020;13:2803. doi: 10.2147/RMHP.S278234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.European Centre for Disease Prevention and Control (ECDC). First COVID-19 vaccine authorized for use in the European Union. Stockholm: ECDC; 2020. Available from: https://www.ecdc.europa.eu/en/news-events/first-covid-19-vaccine-authorised-use-european-union. Accessed May19, 2021. [Google Scholar]
- 10.World Health Organization (WHO). Status of COVID-19 Vaccines within WHO EUL/PQ evaluation process. Available from. Available from: https://extranet.who.int/pqweb/sites/default/files/documents/Status_COVID_VAX_04May2021.pdf. Accessed May19, 2021.
- 11.World open access government news. Available COVID vaccines in 2021. Available from: https://www.oecd.org/coronavirus/policy-responses/access-to-covid-19-vaccines-global-approaches-in-a-global-crisis-c6a18370/. Accessed March10, 2021.
- 12.Wikipedia (free encyclopedia): deployment of COVID-19 vaccines. Available from: https://en.wikipedia.org/wiki/Deployment_of_COVID-19_vaccines. Accessed May19, 2021.
- 13.Al-Mohaithef M, Padhi BK. Determinants of COVID-19 Vaccine Acceptance in Saudi Arabia: a Web-Based National Survey. J Multidiscip Healthc. 2020;13:1657–1663. doi: 10.2147/JMDH.S276771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grech V, Gauci C, Agius S, et al. 2020; Withdrawn: vaccine hesitancy among Maltese Healthcare workers toward influenza and novel COVID-19 vaccination. Early Hum Dev. 105213. doi: 10.1016/j.earlhumdev.2020.105213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hager E, Odetokun IA, Bolarinwa O, et al. Knowledge, attitude, and perceptions towards the 2019 Coronavirus Pandemic: a bi-national survey in Africa. PLoS One. 2020;15(7):e0236918 doi: 10.1371/journal.pone.0236918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harapan H, Wagner AL, Yufika A, et al. Acceptance of a COVID-19 Vaccine in Southeast Asia: a Cross-Sectional Study in Indonesia. Front Public Health. 2020;8:381. doi: 10.3389/fpubh.2020.00381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin Y, Hu Z, Zhao Q, et al. Understanding COVID-19 vaccine demand and hesitancy: a nationwide online survey in China. PLoS Negl Trop Dis. 2020;14(12):e0008961. doi: 10.1371/journal.pntd.0008961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mannan KA, Farhana KM. Knowledge, Attitude and Acceptance of a COVID-19 Vaccine: a Global Cross-Sectional Study. SSRN Electronic J. 2020. doi: 10.2139/ssrn.3763373 [DOI] [Google Scholar]
- 19.Paul E, Steptoe A, Fancourt D, et al. Attitudes towards vaccines and intention to vaccinate against COVID-19: implications for public health communications. The Lancet Regional Health - Europe. 2021;1:100012. doi: 10.1016/j.lanepe.2020.100012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Popa GL. Knowledge and Attitudes on Vaccination in Southern Romanians: a Cross-Sectional Questionnaire.”. Vaccines (Basel). 2020;8(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rhodes A, Hoq M, Measey M-A, et al. Intention to vaccinate against COVID-19 in Australia. Lancet Infect Dis. 2020;21. doi: 10.1016/S1473-3099(20)30724-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verger P, Dube E. Restoring confidence in vaccines in the COVID-19 era. Expert Rev Vaccines. 2020;19(11):991–993. doi: 10.1080/14760584.2020.1825945. [DOI] [PubMed] [Google Scholar]
- 23.Verger P, Scronias D, Dauby N, et al. Attitudes of healthcare workers towards COVID-19 vaccination: a survey in France and French-speaking parts of Belgium and Canada, 2020. Eurosurveillance. 2021;26(3). doi: 10.2807/1560-7917.ES.2021.26.3.2002047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang J. Acceptance of COVID-19 Vaccination during the COVID-19 Pandemic in China. Vaccines (Basel). 2020;8(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Freeman D. Covid-19-vaccine-hesitancy-in-the-uk-the-oxford-coronavirus-explanations-attitudes-and-narratives-survey-oceans. Psychol Med. 2021;12(25):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhartiya S, Kumar N, Singh T, et al. Knowledge, attitude and practice towards COVID-19 vaccination acceptance in West India. Int J Commun Med Public Health. 2021;8(3):1170–1176 doi: 10.18203/2394-6040.ijcmph20210481 [DOI] [Google Scholar]
- 27.Dodd RH, Cvejic E, Bonner C, Pickles K, McCaffery K. Willingness to vaccinate against COVID-19 in Australia. Lancet Infect Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peretti-Watel P, Seror V, Cortaredona S, The COCONEL Group. A future vaccination campaign against COVID-19 at risk of vaccine hesitancy and politicization. Lancet Infect Dis. 2020; 20:769–770. doi: 10.1016/S1473-3099(20)30426-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fisher KA, Bloomstone SJ, Walder J, Crawford S, Fouayzi H, Mazor KM. Attitudes toward a potential SARS-CoV-2 vaccine: a survey of US adults. Ann Intern Med; 2020;173(12):964–973. doi: 10.7326/M20-3569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sherman S, Smith L, Sim J, et al. COVID-19 vaccination intention in the UK: results from the COVID-19 Vaccination Acceptability Study (CoVAccS), a nationally representative cross-sectional. Available from: https://pubmed.ncbi.nlm.nih.gov/33242386/. Accessed May19, 2021. [DOI] [PMC free article] [PubMed]
- 31.Menon RGV, Thaker J. Aotearoa-New Zealand public attitudes to COVID-19 vaccine; 2020. https://mro.massey.ac.nz/handle/10179/15567. Accessed May19, 2021.
- 32.Hoque AM, Buckus S, Hoque M, et al. COVID-19 vaccine acceptability among pregnant women at a primary health care facility in Durban, South Africa. Eur J Med Health Sci. 2020;2(5). doi: 10.24018/ejmed.2020.2.5.493. [DOI] [Google Scholar]
- 33.Ullah I, Khan KS, Tahir MJ, Ahmed A, Harapan H. Myths and conspiracy theories on vaccines and COVID-19: potential effect on global vaccine refusals. Vacunas. 2021. doi: 10.1016/j.vacun.2021.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Covid-19: global attitudes towards a COVID-19 vaccine. Institute of Global health Innovation. Imperial College London. Available from: https://www.imperial.ac.uk/media/. Accessed May19, 2021. [Google Scholar]
- 35.García LY, Cerda AA. Contingent assessment of the COVID-19 vaccine. Elsevier Ltd J Vaccine. 2020;10:1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oscar SA, Carlos E, Carpio A, Darren HA, Patricia A. The demand for a COVID-19 vaccine in Ecuador. Elsevier Ltd J Vaccine. 2020;10:1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiao Y, Torok ME. Taking the right measures to control COVID-19. Lancet Infect Dis. 2020; 20:523–524. doi: 10.1016/S1473-3099(20)30152-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Islama MS. Knowledge, attitudes and perceptions towards COVID-19 vaccinations: a cross-sectional community survey in Bangladesh. medRxiv. 2021;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Biasio LR. Assessing COVID-19 vaccine literacy: a preliminary online survey. Hum Vaccin Immunother. 2020;10:108. [DOI] [PMC free article] [PubMed] [Google Scholar]