Abstract
Purpose
The mechanism underlying curcumin’s protective effect on osteoarthritis (OA) has not been clarified. This study aimed to determine whether curcumin exerts a chondroprotective effect by inhibiting apoptosis via upregulation of E2F1/PITX1 and activation of autophagy via the Akt/mTOR pathway by targeting microRNA-34a (miR-34a).
Methods
Male Sprague–Dawley rats were fed a normal diet (ND) or high-fat diet (HFD) for 28 weeks. Five rats from each diet group were selected randomly for histological analysis of OA characteristics. Rats fed a HFD were given a single intra-stifle joint injection of the miR-34a mimic agomir-34a or negative control agomir (NC), followed by weekly low-dose (200 μg/kg body weight) or high-dose (400 μg/kg body weight) curcumin intra-joint injections from weeks 29 to 32. The rats’ stifle joints were submitted to histological analysis and to an apoptotic assay. Expression of miR-34a was detected using a real-time RT-PCR. E2F1 and PITX1 protein levels were determined by Western blot analysis, and the expressions of Beclin1, LC3B, p62, phosphorylated (p)-Akt, and p-mTOR were measured using immunofluorescence analysis.
Results
We found that rats fed a HFD had OA-like lesions in their articular cartilage and had increased apoptosis of chondrocytes and decreased autophagy compared to rats fed a ND. Curcumin treatment alleviated OA changes, inhibited apoptosis, and upregulated autophagy. Agomir-34a treatment reduced E2F1, PITX1, Beclin1, and LC3B expression and increased p62, p-Akt, and p-mTOR expression in HFD-fed rats given low- or high-dose curcumin. Greater numbers of apoptotic cells, lesser expression of p62, p-Akt, and p-mTOR, and greater expression of E2F1, PITX1, and LC3B were observed in the agomir-34a and high-dose curcumin-treated group than in agomir-34a and low-dose curcumin-treated group.
Conclusion
Curcumin’s chondroprotective effect was mediated by its suppression of miR-34a, apparently by reducing apoptosis, via upregulation of E2F1/PITX1, and by augmenting autophagy, likely via the Akt/mTOR pathway.
Keywords: curcumin, osteoarthritis, apoptosis, autophagy, microRNA-34a, high-fat diet
Introduction
Osteoarthritis (OA) is a degenerative inflammatory disease with specific manifestations that include articular cartilage loss, extracellular matrix degradation, subchondral bone ossification, and osteophyte formation.1,2 It is a major cause of disability in elderly populations.3 Although OA has been related to a variety of factors, including aging, obesity, genetic factors, and trauma,4,5 the underlying mechanisms of OA pathogenesis have not been elucidated. Intervention of OA in an early stage may be critical for delaying, or even reversing, OA progression.
Apoptosis and autophagy, which are critical processes for maintaining tissue homeostasis,6–8 have been shown to play an important role in the occurrence and development of OA. In OA, chondrocytes undergo programmed cell death via apoptosis.9 Meanwhile, autophagy, a process of cell degradation and recycling, has been shown to be closely related to the development of OA.10,11 Autophagy can inhibit the apoptosis of damaged cartilage cells and the two processes of apoptosis and autophagy are often regulated by similar factors.9,12
MicroRNAs (miRs) are short noncoding segments of RNA that regulate protein-encoding genes by interacting with elements in the 3ʹ untranslated regions of target genes.13,14 Studies have shown that some miRs regulate both cell apoptosis and autophagy, and that stable miR expression plays a vital role in maintaining chondrocyte quantities and inhibiting OA progression. Notably, miR-34a is expressed specifically in cartilage and upregulated in the articular cartilage and synovial fluid of OA patients.15 It has been reported that miR-34a regulates the processes of apoptosis and autophagy,16,17 but the mechanism of regulation remains to be clarified. In some diseases, miR-34a–stimulated apoptosis is closely related to the cell-cycle-related protein E2F1 (eukaryotic translation termination factor 1). Observations of miR-34a being able to promote cancer cell apoptosis directly by targeting E2F1 inhibition18 and of E2F1 being suppressed in OA chondrocytes19 indicate that miR-34a may be related to E2F1 expression. The promoter activity and mRNA transcription of PITX1 (paired-like homeodomain transcription factor 1) have been shown to be regulated by E2F1 in OA chondrocytes.20 However, the potential regulatory influences of miR-34a on E2F1, PITX1, and chondrocyte apoptosis remain to be explored.
Studies have shown that miR-34a can regulate the phosphoinositide 3-kinase/Akt/mammalian rapamycin (mTOR) signaling pathway,21,22 a classical autophagy pathway. Inflammatory responses in human and rat articular chondrocytes can be reduced by promoting autophagy through inhibition of Akt/mTOR pathway signaling.23,24 Thus, it is possible that miR-34a may inhibit chondrocyte autophagy via Akt/mTOR signaling in addition to promoting chondrocyte apoptosis.
Although there is no cure for OA, nutraceuticals are being considered as potential anti-OA treatments owing to their chondroprotective effects and minimal risk of adverse effects. The nutraceutical curcumin, which is used as a dietary spice and coloring agent, is a diketone compound derived from turmeric (the main component of curry powder). Turmeric and curcumin are relatively rare natural phytochemicals that can be obtained from the rhizomes of some plants in the Zingiberaceae and Araceae families.25 Curcumin has been reported to exert a chondroprotective influence through anti-inflammation and anti-apoptotic effects as well as through mechanisms involving anticatabolic and proteolytic enzymes.26–29 Additionally, curcumin has been shown to inhibit cancer cell proliferation and to alleviate disease processes via mechanisms that involve miR-34a regulation.30,31 However, it is not clear whether curcumin can inhibit miR-34a in articular cartilage. If so, it may be used to reduce chondrocyte apoptosis and activate autophagy with the goal of preventing or slowing the progression of OA.
A suitable animal model is essential for exploring the pathogenesis of OA. Recently, obesity has become a major cause of OA.32,33 Thus, in the present study, we focused on obesity-associated OA in rats fed a high-fat diet (HFD). We hypothesized that curcumin may exert a chondroprotective effect by suppressing miR-34a, and thereby inhibiting apoptosis via upregulation of E2F1 and PITX1 and activating autophagy via the Akt/mTOR pathway. To test this hypothesis, we injected miR-34a agomir (agomir-34a) into the stifle joint cavity of rats to increase miR-34a expression and then gave rats a series of curcumin injections.
Materials and Methods
Animals and Treatments
Sixty-six Sprague–Dawley male rats (6-week-old, 220–260 g) were acquired from China Medical University (Shenyang, China) and randomized into a HFD group (N = 54) and a normal diet (ND) group (N = 12). The HFD and ND foodstuffs consisted of 60% and 10% kcal from fat, respectively. All rats were housed at a controlled temperature (23–25 °C) and humidity (40–70%) with a 12-h light/dark cycle and weighed once a week for 28 weeks. Prior to starting curcumin treatments, 5 rats were selected randomly from each diet group and stifle joint samples were collected from them for histological analysis of OA changes.
Subsequently, the remaining seven rats in the ND group continued to be fed a ND and became a control (C) group in our curcumin treatment experiment. The remaining 49 rats in the HFD group were further divided into the following seven groups (N = 7 per group): HFD only (H), HFD plus low-dose curcumin (HL), HFD plus high-dose curcumin (HH), HFD plus low-dose curcumin and agomir negative control (HL-NC), HFD plus high-dose curcumin and agomir negative control (HH-NC), HFD plus low-dose curcumin and agomir-34a (HL-Ago), HFD plus high-dose curcumin and agomir-34a (HH-Ago). Curcumin was purchased from Xi’an Spring-chem Bio-tech (Xi’an, China) and agomirs were purchased from RiboBio (Guangzhou, China).
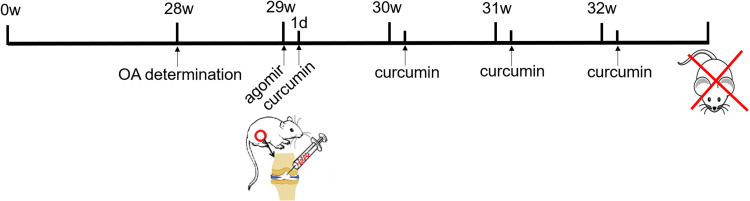
In preparation for treatment, agomir-34a (an in vivo activator of miRNA-34a, 5 nmol) and agomir negative control (5 nmol) were dissolved in 100 μL sterile phosphate buffered saline (PBS). Curcumin oil solution (12.5 mg/mL) was dissolved in 5% dimethyl sulfoxide. At the beginning of week 29, rats received one 100 μL intra-stifle joint injection of sterile PBS (C, H, HL and HH groups), agomir negative control (5 nmol) dissolved in sterile PBS (HL-NC and HH-NC groups), or agomir-34a (5 nmol) dissolved in sterile PBS (HL-Ago and HH-Ago groups). Subsequently, rats in the C and H groups were given an intra-stifle joint injection of 5% dimethyl sulfoxide oil solution. Rats in the other groups were given a weekly intra-stifle joint injection of curcumin solution from weeks 29 to 32 (a total of four injections). Low-dose (200 μg/kg body weight) and high-dose (400 μg/kg body weight) curcumin were administered according to the group designation. At the end of week 32, all rats were sacrificed (Figure 1). All procedures were approved by the local Institutional Animal Care Ethics Committee for animal studies at China Medical University (Approval number: 20180228-48). The welfare and treatment of the laboratory animals was followed with National Institute of Health Guide for the Care and Use of Laboratory Animals.
Figure 1.
Flowchart of mice treatment.
Gait Analysis
At the 28th and 32nd weeks, five randomly selected animals per group were subjected to gait assessment with the Catwalk XT system (Noldus Information Technology, Wageningen, NL).
Body Fat Determination
Immediately after rats were sacrificed, their subcutaneous, epididymis, perirenal, and mesentery adipose tissues were dissected and weighed for body fat determination.
Tissue Preparation
Whole stifle joints were fixed in 4% paraformaldehyde for 48 h and then decalcified for 3 months in 10% Na2EDTA at room temperature. After dehydration, the joints were embedded in paraffin and then sectioned serially at a thickness of 6 μm for histology, immunohistochemistry analysis, and TUNEL assay.
Histology
Sections were dyed with Safranin O/Fast Green (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) or hematoxylin and eosin (H&E) (Tianjin Guangfu Fine Chemical Research Institute, Tianjin, China). All sections were examined and evaluated by two researchers according to modified Mankin scores, with higher scores (range, 0–14) indicating more severe lesions.34
Immunofluorescence Analysis
Sections were deparaffinized into xylene, rehydrated through a series of decreased concentrations of ethanol, and washed with PBS. Antigen retrieval was achieved with 0.1% trypsin. Then, the slices were incubated with goat serum for 30 min at 37 °C, and then incubated overnight at 4 °C with primary antibodies targeting the following proteins: LC3B (1:50 dilution, Abcam, Cambridge, UK), Beclin1 (1:50, ABclonal Technology, Wuhan, China), p62 (1:100, Abcam), Ser473 phosphorylated (p)-Akt (1:50, Cell Signaling Technology, Boston, MA), or a mixture of LAMP2 (1:100, Abcam) and p-mTOR (1:50, Santa Cruz Biotechnology, Inc., Santa Cruz, CA), and matrix metalloproteinase (MMP)-2(1:500, Abcam), MMP-9 (1:100, Abcam). Fluorescent-conjugated secondary antibody (1:50, Zhongshan Goldenbridge Biotechnology Co., Ltd., Beijing, China) was applied for 2 h at room temperature. Images were taken with an Olympus microscope (Olympus, Tokyo, Japan). Six fields were selected from each slice for optical density analysis, conducted using ImageJ 2x software (National Institutes of Health, Bethesda, MD).
TUNEL Assay
TUNEL assays were carried out according to the instructions provided by the assay kit manufacturer (Roche Company, USA). Sections were treated with proteinase K, followed by TdT enzyme, and streptavidin-fluorescein, successively, and then counterstained with DAPI. Photomicrographs were obtained using an Olympus microscope. Six fields from each section were analyzed. The average number of positive cells was determined using ImageJ 2x software.
Real-Time Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
Total RNA was extracted from cartilage with RNAiso plus (TaKaRa, Dalian, China). According to the manufacturer’s instructions (TaKaRa), total RNA was reverse transcribed into cDNAs. Real-time qRT-PCR was conducted using SYBR Green PCR master mix (TaKaRa) in a PCR detection system (ABI, Carlsbad, CA). U6 was used as a reference for miR detection. Detected RNA levels were calculated using the 2−ΔΔCt method. A specific primer for miR-34a was synthesized by Sangon Biotech (Shanghai, China) with the following forward sequence: 5′-CTG GCA GTG TCT TAG CTG GTT GT-3′.
Western Blotting
Total proteins were extracted from cartilage with a protease inhibitors and phosphatase inhibitors in RIPA buffer (KeyGen Biotech, Nanjing, China). Protein concentrations were determined with a BCA kit (Thermo Fisher Scientific, San Jose, CA). The proteins were separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes. The membranes were blocked with 5% skimmed milk and incubated with primary antibodies targeting the following proteins: β-actin (1:1000, Proteintech Group, Wuhan, China), E2F1 (1:1000, Sangon Biotech), PITX1 (1:1000, Sangon Biotech). After incubation with horse radish peroxidase-conjugated secondary antibody (1:5000 dilution, ABclonal Technology), bands were detected with a chemiluminescent detection system (GelImage System Ver. 4.00, CA). Blot densities were measured with Image 4.0 software (Scion Corporation, Frederick, MD).
Statistical Analysis
Statistical analysis was performed with SPSS 20.0 software (IBM Corp., Armonk, NY). Data were expressed as means ± SD. One-way analyses of variance (ANOVA) and Fisher’s least significant difference multiple comparison tests were used with a significance criterion of p < 0.05.
Results
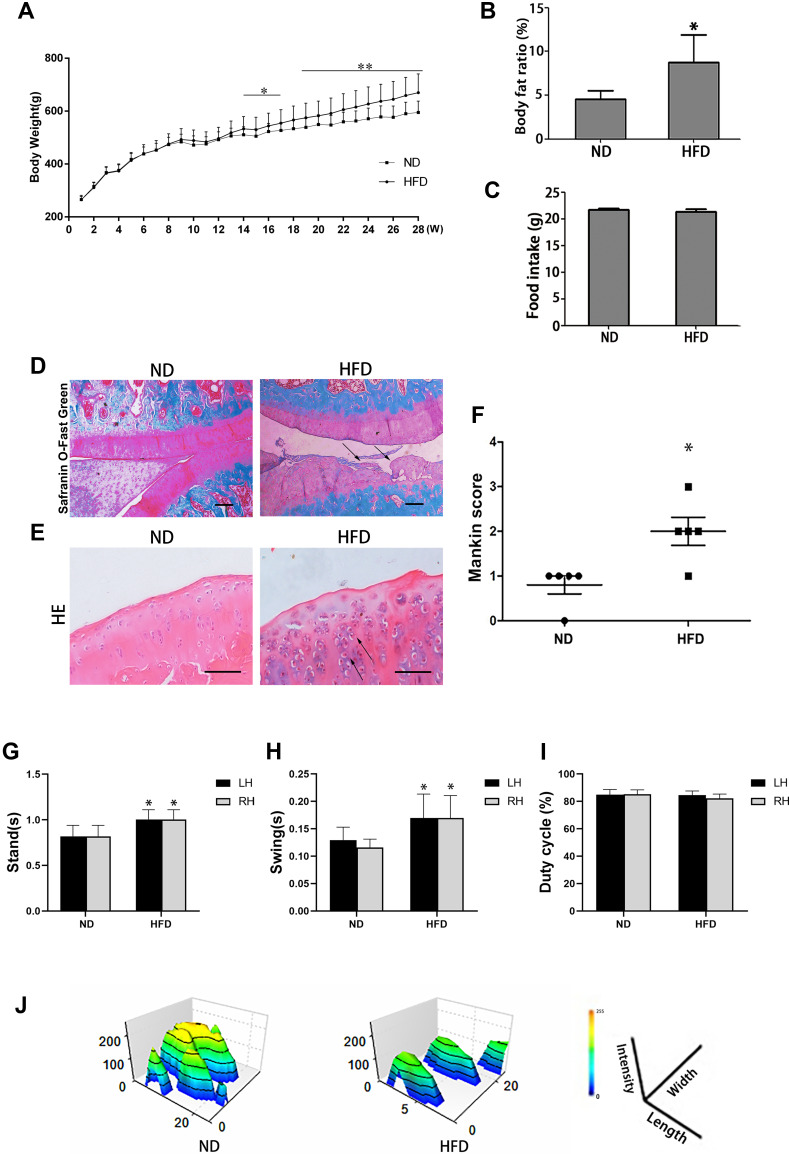
HFD Induced OA-Like Lesions in Rats
Rats fed a HFD had a higher bodyweight (Figure 2A) and body fat ratio (Figure 2B) than those in the ND group, while there was no difference in food intake (Figure 2C). Rats fed a HFD for 28 weeks presented OA-like characteristics, including a disordered arrangement of cells, decreased cartilage thickness, damage to the cartilage surface, and an unclear boundary between cartilage and bone (Figure 2D and E). The mean modified Mankin’s score of the HFD-fed rats was significantly higher than that of the ND-fed rats (p < 0.05) (Figure 2F). Gait analysis showed that the left-hind limb (LH) and right-hind limb (RH) in the HFD group had longer stand and swing times than those of the ND group (p < 0.05) (Figure 2G and H), but there was no difference in duty cycle between the two groups (Figure 2I). The footprint histograms of HFD group were lower than those of ND group (Figure 2J).
Figure 2.
Effects of a 28-week HFD on stifle-joint articular cartilage in rats. (A) Body weight, for ND group (N = 12) or HFD group (N = 54); (B) Body fat ratio; (C) food intake; (D) Safranin O/Fast Green staining (original magnification, 40 x, Scale bars = 500 µm); (E) H & E staining (original magnification, 200 x, Scale bars = 200 µm); (F) Modified Mankin scores; (G–J) Analysis of gait indices: Swing (G); Stand (H); Duty cycle (I) and footprint histograms (J). Arrows indicate sites of cartilage damage. Independent sample t-test was used to test for statistical significance. Data were expressed as the mean ± SD, N = 5, *p < 0.05, **p < 0.01 versus the ND group.
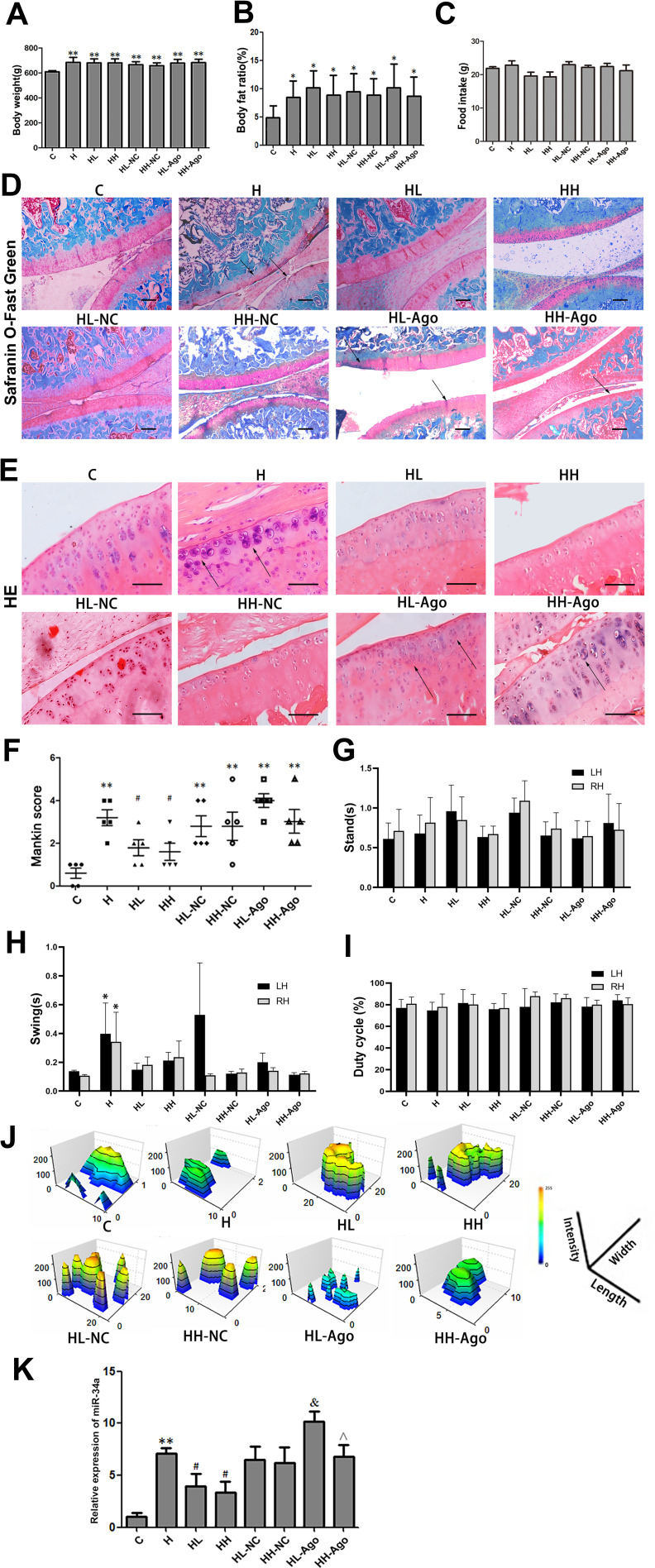
Curcumin Reduced miR-34a Levels and Alleviated OA-Like Lesions in HFD-Fed Rats
The mean body weights and body fat ratios of rats in the seven groups that were fed a HFD were all significantly higher than those of the C group (p < 0.01, p < 0.05), with no differences among the seven HFD groups (Figure 3A and B). As shown in Figure 3D–F, curcumin treatment ameliorated OA-like lesions, and the mean Mankin scores of the curcumin-treated groups were also lower than that of the H group (p < 0.05). Compared with the HL-NC group, histological changes in HL-Ago group appeared to be slightly worse, but there was no statistical difference in Mankin scores. Compared with the C group, the swing time of rats in the H group was increased (p < 0.05), and curcumin treatment decreased swing time (Figure 3H). Curcumin treatment also improved footprint histograms. After agomir-34a treatment, the footprint histograms in HL-Ago and HH-Ago groups were decreased compared to their respective NC control groups (Figure 3J). There was no difference in food intake (Figure 3C), stand time (Figure 3G) and duty cycle (Figure 3I) among all groups.
Figure 3.
HFD upregulated the expression of miR-34a in stifle articular cartilage, while curcumin reduced miR-34a and alleviated OA-like lesions. (A) Body weight; (B) Body fat ratio; (C) food intake; (D) Safranin O/Fast Green staining (original magnification, 40 x, Scale bars = 500 µm); Arrows indicated incomplete cartilage; (E) H & E staining (original magnification, 200 x, Scale bars = 200 µm); Arrows indicated chondrocytes are clustered or sparse; (F) Modified Mankin scores; (G–J) Analysis of gait indices: Stand (G); Swing (H); Duty cycle and (I) footprint histograms (J); (K) The expression of miR-34a in cartilage were measured by qRT-PCR. One-way ANOVA was used to test for statistical significance. Data were expressed as the mean ± SD, N = 7, *p < 0.05, **p < 0.01 versus the C group; #p < 0.05 versus the H group; &p < 0.05 versus the HL-NC group; ^p < 0.05 versus the HL-Ago group.
As shown in Figure 3K, compared with the C group, the expression of miR-34a in articular cartilage was significantly upregulated in the H group (p < 0.01), while curcumin treatment reduced its expression (p < 0.05). Expression of miR-34a in the HL-Ago group was significantly increased compared with the HL-NC group (p < 0.05), while being similar to that in the HH-NC group. The miR-34a expression in the HH-Ago group was significantly lower than that of the HL-Ago group (p < 0.05).
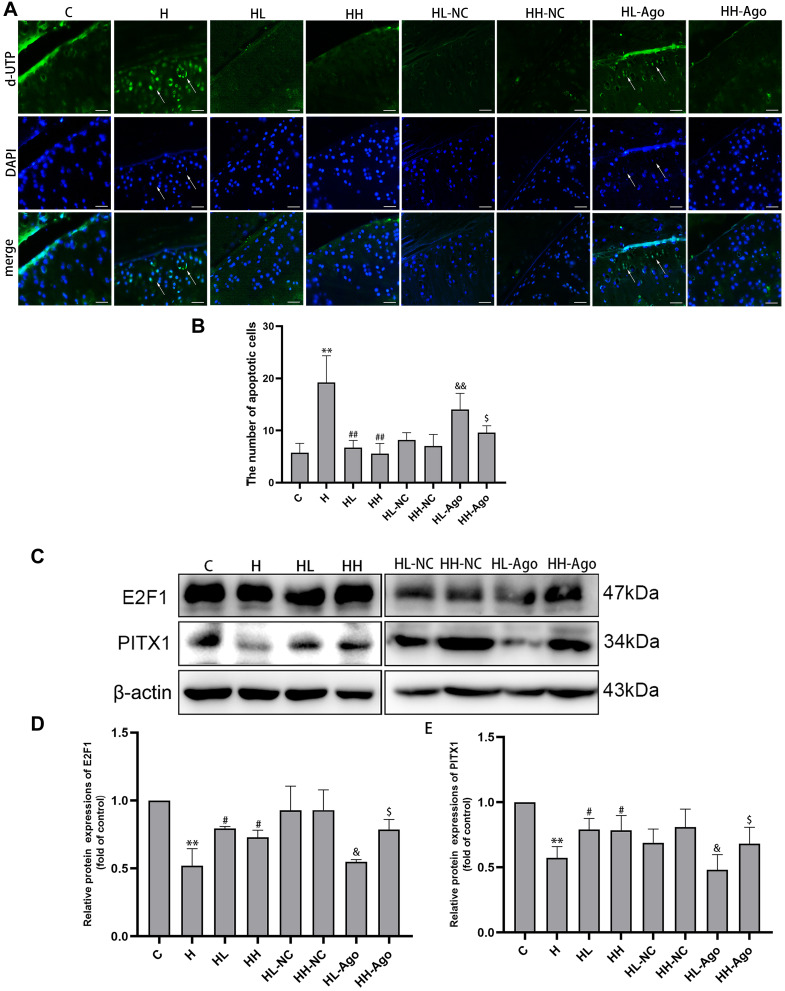
Curcumin Treatment Resulted in Suppressed miR-34a Levels, Upregulated E2F1 and PITX1, and Reduced Apoptosis in Cartilage in HFD-Fed Rats
TUNEL staining analysis showed that the number of apoptotic chondrocytes in rat articular cartilage was increased in the H group, compared to the C group, and decreased in curcumin-treated groups compared to the H group (p < 0.01). Agomir-34a treatment increased quantities of apoptotic cells in the low-dose curcumin group (p < 0.01 vs HL-NC group), but not in the high-dose group. Moreover, compared with the HL-Ago group, the number of apoptotic cells in the HH-Ago group was reduced (p < 0.05) (Figure 4A and B).
Figure 4.
Curcumin suppression of miR-34a was associated with inhibited apoptosis in cartilage and upregulation of E2F1/PITX1 in rats fed a HFD. (A) Apoptotic cells (green) were determined by TUNEL staining, and cell nuclei were counterstained with DAPI (blue) (original magnification, 200x, Scale bars = 200 µm); (B) Analysis of the numbers of apoptotic cells in cartilage; (C) The E2F1 and PITX1 protein expression were measured by Western blot; (D and E) The relative expression of E2F1 and PITX1, and β-actin was used as a reference standard. One-way ANOVA was used to test for statistical significance. Data were expressed as the mean ± SD, N = 7, **p < 0.01 versus the C group; #p < 0.05, ##p < 0.01 versus the H group; &p < 0.05, &&p < 0.01 versus the HL-NC group; $p < 0.05 versus the HL-Ago group.
As shown in Figure 4C–E, the expression of E2F1 and PITX1 proteins in the H group was significantly lower than that in the C group (p < 0.01), while curcumin treatment upregulated their expression (p < 0.05). Agomir-34a treatment reduced E2F1 and PITX1 expression in rats given low-dose, but not high-dose, curcumin (p < 0.05). The expression levels of E2F1 and PITX1 were greater in the HH-Ago group than in the HL-Ago group (p < 0.05).
Curcumin Treatment Resulted in Reduced miR-34a Levels, Reduced Akt/mTOR Signaling, and Increased Autophagy in Cartilage from HFD-Fed Rats
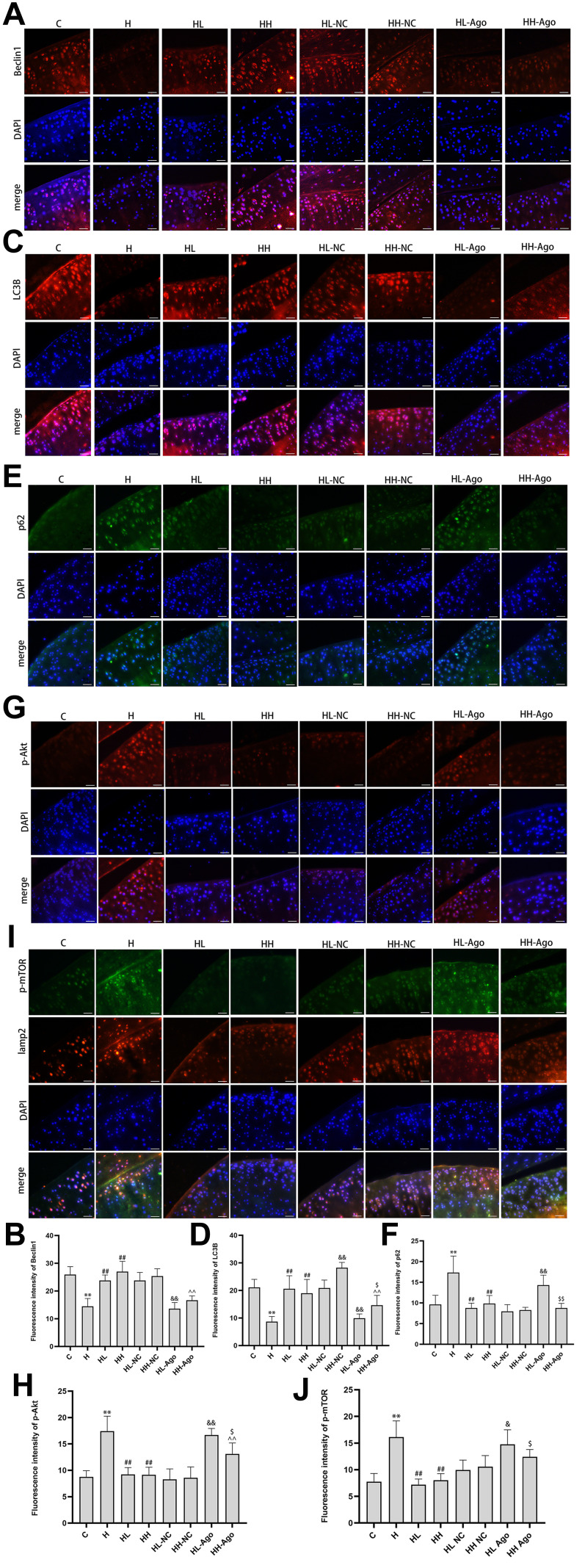
Compared with levels in rats fed a ND, HFD-fed rats had decreased protein expression of Beclin1 and LC3B (p < 0.01) (Figure 5A–D) and increased expression of p62 protein (p < 0.01) (Figure 5E and F). Compared to HFD-fed rats not given curcumin, rats given low- or high-dose curcumin treatment had upregulated Beclin1 (p < 0.01) and LC3B expression (p < 0.01) (Figure 5A–D), but down-regulated p62 expression (p < 0.01) (Figure 5E and F). There was no difference in these protein levels between the low- and high-dose curcumin groups. Administration of agomir-34a reduced Beclin1 and LC3B expression in both the low- and high-dose curcumin groups (p < 0.01 HH-Ago and HL-Ago groups vs HL-NC and HH-NC groups, respectively) (Figure 5A–D). LC3B expression was greater in the HH-Ago group than in the HL-Ago (p < 0.05) (Figure 5C and D). Agomir-34a treatment increased p62 expression in the HL-Ago group (p < 0.01), but not in the HH-Ago group (Figure 5E and F).
Figure 5.
Curcumin suppressed miR-34a, reduced Akt/mTOR signaling, and promoted autophagy in cartilage from rats fed an HFD. Immunofluorescence analysis was performed using Beclin1 (A), LC3B (C), p62 (E), p-Akt (G) and p-mTOR (I) antibody (red), and cell nuclei were counterstained with DAPI (blue) (original magnification, 200×), Scale bars=50μm. The fluorescence intensity of Beclin1 (B), LC3B (D), p62 (F), p-Akt (H) and p-mTOR (J). One-way ANOVA was used to test for statistical significance. Data were expressed as the mean ± SD, N = 7, *p < 0.05, **p < 0.01 versus the C group; ##p < 0.01 versus the H group; &p < 0.05, &&p < 0.01 versus the HL-NC group; ^^ p < 0.01 versus the HH-NC group; $p < 0.05, $$p < 0.01 versus the HL-Ago group.
As shown in Figure 5G–J, the expression of p-Akt and p-mTOR was significantly increased in the articular cartilage of H group rats compared to C group rats (p < 0.01), and curcumin treatment attenuated this increase (p < 0.01). Agomir-34a treatment upregulated p-Akt and p-mTOR expression in the HL-Ago group (p < 0.01, p < 0.05), but only upregulated p-Akt expression in the HH-Ago group (p < 0.01). Compared with levels in the HL-Ago group, p-Akt and p-mTOR expression were decreased in the HH-Ago group (p < 0.05).
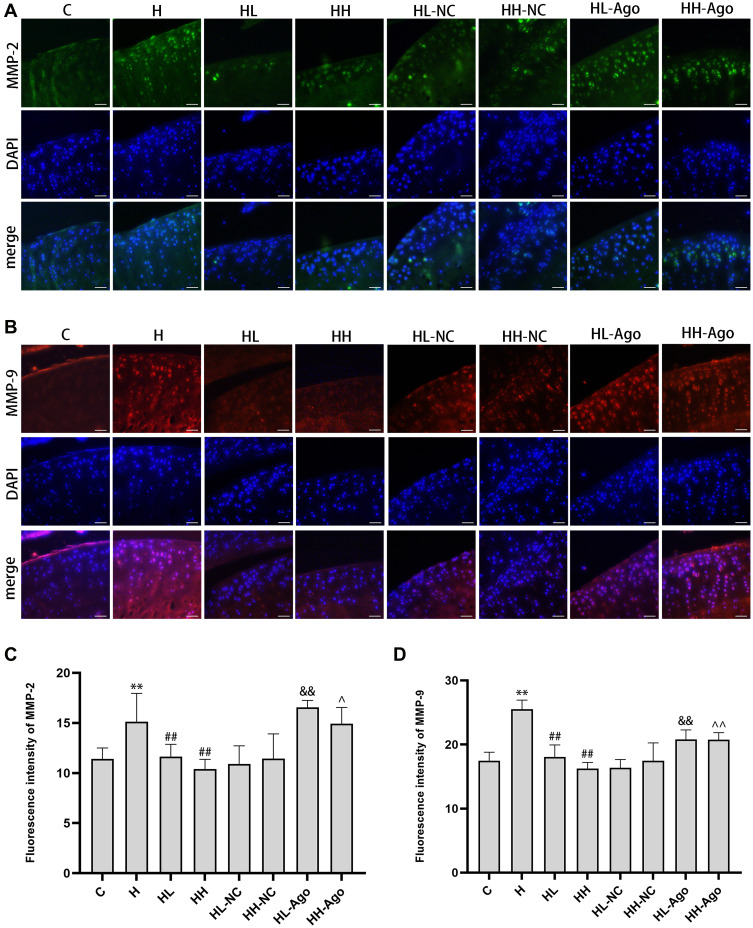
MiR-34a Treatment Upregulated MMP-2 and MMP-9 Expression, While Curcumin Treatment Reduced Their Expression in Cartilage from HFD-Fed Rats
As shown in Figure 6A–D, the expression of MMP-2 and MMP-9 was dramatically increased in the articular cartilage of H group rats compared to C group rats (p < 0.01), and low- or high-dose curcumin treatment attenuated this increase (p < 0.01). Agomir-34a treatment upregulated MMP-2 (Figure 6A and C) and MMP-9 (Figure 6B and D) expressions in both the HL-Ago and HH-Ago group (p < 0.01, p < 0.05). There was no difference in these protein levels between the HL-Ago and HH-Ago groups.
Figure 6.
MiR-34a treatment upregulated MMP-2 and MMP-9 expression, while curcumin treatment reduced their expression in cartilage from HFD-fed rats. Immunofluorescence analysis was performed using MMP-2 (A) antibody(green) and MMP-9 (B) antibody(red), and cell nuclei were counterstained with DAPI (blue) (original magnification, 200×), Scale bars=50μm. The fluorescence intensity of MMP-2 (C) and MMP-9 (D). One-way ANOVA was used to test for statistical significance. Data were expressed as the mean ± SD, N = 7, **p < 0.01 versus the C group; ##p < 0.01 versus the H group; &&p < 0.01 versus the HL-NC group; ^p < 0.05, ^^p < 0.01 versus the HH-NC group.
Discussion
Studies have suggested that curcumin may be an effective treatment for the prevention and treatment of OA.35–37 In the present study, we examined the effects of curcumin on signs in a common rat model of obesity-associated OA.38,39 Our histological and behavioral observations confirmed that rats fed a HFD presented OA-like lesions, consistent with our previous and others’ studies.40,41 Previously, oral administration of curcumin was shown to be effective in slowing the progression of OA in a post-traumatic OA mouse model.42 However, doubts have been raised regarding whether oral curcumin can reach pharmacologically active concentrations in synovial fluid or joint tissues43 and the oral bioavailability of curcumin is only 1%.44 Thus, in this study, we used an intra-articular injection to ensure that curcumin reached the joint capsule and to increase its bioavailability.
Our data showed that curcumin can alleviate OA progression in rats fed with HFD, affirming an anti-OA effect. Interestingly, miR-34a was increased in the articular cartilage of rats fed a HFD, while curcumin reduced the expression of miR-34a. MMP-2 and MMP-9 could degrade the extracellular matrix and related to OA development,45–47 and miR-34a was reported to influence their levels.48,49 Our results showed that curcumin treatment downregulated the expression of MMP-2 and MMP-9, and the effect was related to the inhibition of miR-34a. HFD-fed rats had upregulated apoptosis and down-regulated autophagy in their cartilage, and curcumin treatment alleviated these changes, suggesting that the regulation of apoptosis and autophagy in obesity-associated OA may be related to miR-34a, and that curcumin may exert its chondroprotective effects by decreasing apoptosis and promoting autophagy consequent to reduction of miR-34a expression. Consistent with this supposition, intra-joint agomir-34a increased the expression of miR-34a. Agomir increases miR-34a levels immediately after being injected,50,51 and miR-34a levels remain high in cartilage for 6 weeks after injection into the articular cavity, but then decline to baseline levels by 8 weeks post-injection.52 In the present study, our results demonstrated that weekly injections of curcumin for 4 weeks following agomir-34a administration may exert a chondroprotective effect by inhibiting apoptosis and activating autophagy consequent to downregulation of miR-34a.
MiR-34a expression is induced by p53, a protein that is related to apoptosis, during which the cell cycle ceases and cell death ensues through processes that involve E2Fs and cyclin-dependent protein kinase 6.53,54 E2F1 can affect cell cycle progression, regulate cell proliferation and metabolism, and thus regulate cell number and function.55 The E2F1-regulated protein PITX1 is an important regulatory factor whose inhibition can promote chondrocyte apoptosis. Pellicelli et al20 demonstrated that inhibition of E2F1 suppressed PITX1 promoter activity and mRNA transcription in normal and OA articular chondrocytes. Downregulation of E2F1 has been associated with increased apoptosis of articular chondrocytes and OA deterioration,56,57 while reduction of PITX1 synthesis and activity can lead to excessive lysis of chondrocytes, an important marker of articular cartilage degeneration.58,59 In the present study, we observed reduced E2F1 and PITX1 expression in the articular cartilage of rats fed a HFD, compared to that in ND rats. Conversely, E2F1 and PITX1 expression were increased after curcumin treatment, suggesting that upregulated E2F1 and PITX1 expression due to a HFD contributed to apoptosis, and that curcumin’s anti-apoptotic effect may involve downregulation of E2F1 and PITX1 expression. Notably, agomir-34a reduced the expression of E2F1 and PITX1 significantly in rats in the low-dose curcumin treatment (HL-Ago) group, but not rats in the high-dose curcumin treatment (HH-Ago) group. Meanwhile, our TUNEL staining analysis indicated that high-dose curcumin could exert a more pronounced inhibitory effect than low-dose curcumin on miR-34a over-expression in OA joint tissues. Furthermore, differential E2F1 and PITX1 expression between our low- and high-dose curcumin groups given agomir-34a suggests that curcumin-induced down-regulation of miR-34a may inhibit apoptosis via upregulation of E2F1 and PITX1.
There is evidence of crosstalk between autophagy and apoptosis,60,61 and a regulatory relationship between mTOR and E2F1 has been demonstrated.62,63 The starting point of autophagy is regulated by mTOR, a macromolecular signaling complex that has mTORC1 and mTORC2 constituents. Autophagy has been shown to be closely related to mTORC1, which receives signals from the (type I) phosphoinositide triphosphate kinase/Akt pathway. Akt phosphorylates mTORC1 at Ser2448, and hyperphosphorylated mTORC1 acts as a direct inhibitor of autophagy.64,65 Curcumin was reported to reduce p-Akt and p-mTOR levels in a rat model of rheumatoid arthritis.28 In the present study, we found that curcumin enhanced autophagy, as evidenced by the autophagy markers Beclin1, LC3B and p62,66 and inhibited the Akt/mTOR pathway in the articular cartilage of rats with obesity-associated OA-like degeneration. And these effects were associated with a reduction of miR-34a. Hence, the presently observed difference between high- and low-dose curcumin effects may be due to high-dose curcumin having a stronger miR-34a reducing effect than low-dose curcumin in rats treated with agomir-34a. Meanwhile, miR-34a levels were similar in rats given a high-dose curcumin treatment regimen, whether or not they received the agomir-34a treatment. These results are consistent with the apoptotic data above indicating that high-dose curcumin can suppress miR-34a expression when miR-34a is abnormally elevated, and thereby enhance autophagy.
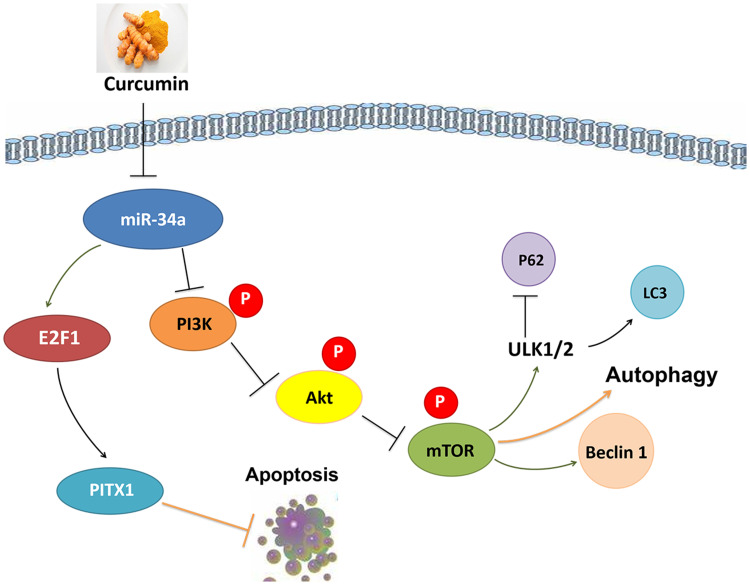
In summary, the present results support further exploration of the phytochemical curcumin as a potential strategy to prevent and treat OA. Our findings are consistent with the possibility that curcumin’s chondroprotective effects are mediated by its miR-34a–modulating influence leading to downregulation of apoptosis, via E2F1/PITX1 upregulation, and activation of autophagy, via the Akt/mTOR pathway (Figure 7). Although autophagy has been shown to be affected by E2F1 and mTORC2 is known to upregulate E2F1, there are few studies in the literature regarding the functions of mTORC2. Further research should explore the interactions between autophagy and apoptosis as well as the potential benefits of modulating miR-34a on OA.
Figure 7.
Potential chondroprotective mechanisms of curcumin on obesity-associated OA of rats induced by a HFD.
Conclusions
Curcumin’s chondroprotective effect was mediated by its suppression of miR-34a, apparently by reducing apoptosis, via upregulation of E2F1/PITX1, and by augmenting autophagy, likely via the Akt/mTOR pathway.
Acknowledgment
This study was supported by the Key R&D Program of Liaoning Province [No. 2020JH2/10300144] and Natural Science Foundation of Liaoning Province (2019JH3/10300415).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval for the version to be published; have agreed on the journal to which the article has been submitted; and agreed to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Tu C, He J, Wu B, et al. An extensive review regarding the adipokines in the pathogenesis and progression of osteoarthritis. Cytokine. 2019;113:1–12. doi: 10.1016/j.cyto.2018.06.019 [DOI] [PubMed] [Google Scholar]
- 2.Jiang Y, Tuan RS. Origin and function of cartilage stem/progenitor cells in osteoarthritis. Nat Rev Rheumatol. 2015;11(4):206–212. doi: 10.1038/nrrheum.2014.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silverwood V, Blagojevic-Bucknall M, Jinks C, et al. Current evidence on risk factors for knee osteoarthritis in older adults: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2015;23(4):507–515. doi: 10.1016/j.joca.2014.11.019 [DOI] [PubMed] [Google Scholar]
- 4.Musumeci G, Aiello FC, Szychlinska MA, et al. Osteoarthritis in the XXIst century: risk factors and behaviours that influence disease onset and progression. Int J Mol Sci. 2015;16(12):6093–6112. doi: 10.3390/ijms16036093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Neill TW, McCabe PS, McBeth J. Update on the epidemiology, risk factors and disease outcomes of osteoarthritis. Best Pract Res Clin Rheumatol. 2018;32(2):312–326. doi: 10.1016/j.berh.2018.10.007 [DOI] [PubMed] [Google Scholar]
- 6.Blanco FJ, Guitian R, Vazquez-Martul E, et al. Osteoarthritis chondrocytes die by apoptosis. A possible pathway for osteoarthritis pathology. Arthritis Rheum. 1998;41(2):284–289. doi: [DOI] [PubMed] [Google Scholar]
- 7.Kim HA, Lee YJ, Seong SC, et al. Apoptotic chondrocyte death in human osteoarthritis. J Rheumatol. 2000;27(2):455–462. [PubMed] [Google Scholar]
- 8.Wang Z, Hu J, Pan Y, et al. MiR-140-5p/miR-149 affects chondrocyte proliferation, apoptosis, and autophagy by targeting FUT1 in osteoarthritis. Inflammation. 2018;41(3):959–971. doi: 10.1007/s10753-018-0750-6 [DOI] [PubMed] [Google Scholar]
- 9.Hwang HS, Kim HA. Chondrocyte apoptosis in the pathogenesis of osteoarthritis. Int J Mol Sci. 2015;16(11):26035–26054. doi: 10.3390/ijms161125943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carames B, Taniguchi N, Otsuki S, et al. Autophagy is a protective mechanism in normal cartilage, and its aging-related loss is linked with cell death and osteoarthritis. Arthritis Rheum. 2010;62(3):791–801. doi: 10.1002/art.27305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sasaki H, Takayama K, Matsushita T, et al. Autophagy modulates osteoarthritis-related gene expression in human chondrocytes. Arthritis Rheum. 2012;64(6):1920–1928. doi: 10.1002/art.34323 [DOI] [PubMed] [Google Scholar]
- 12.Cooper KF. Till death do us part: the marriage of autophagy and apoptosis. Oxid Med Cell Longev. 2018;2018:4701275. doi: 10.1155/2018/4701275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malemud CJ. MicroRNAs and osteoarthritis. Cells. 2018;7(8):92. doi: 10.3390/cells7080092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Papanagnou P, Stivarou T, Tsironi M. The role of miRNAs in common inflammatory arthropathies: osteoarthritis and gouty arthritis. Biomolecules. 2016;6(4):44. doi: 10.3390/biom6040044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abouheif MM, Nakasa T, Shibuya H, et al. Silencing microRNA-34a inhibits chondrocyte apoptosis in a rat osteoarthritis model in vitro. Rheumatology (Oxford). 2010;49(11):2054–2060. doi: 10.1093/rheumatology/keq247 [DOI] [PubMed] [Google Scholar]
- 16.Chen P, Chen F, Lei J, et al. Activation of the miR-34a-mediated SIRT1/mTOR signaling pathway by urolithin A attenuates D-galactose-induced brain aging in mice. Neurotherapeutics. 2019;16(4):1269–1282. doi: 10.1007/s13311-019-00753-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang TC, Wentzel EA, Kent OA, et al. Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Mol Cell. 2007;26(5):745–752. doi: 10.1016/j.molcel.2007.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng SD, Mao Z, Liu C, et al. Simultaneous overexpression of miR-126 and miR-34a induces a superior antitumor efficacy in pancreatic adenocarcinoma. Onco Targets Ther. 2017;10:5591–5604. doi: 10.2147/OTT.S149632 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Dunn SL, Soul J, Anand S, et al. Gene expression changes in damaged osteoarthritic cartilage identify a signature of non-chondrogenic and mechanical responses. Osteoarthritis Cartilage. 2016;24(8):1431–1440. doi: 10.1016/j.joca.2016.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pellicelli M, Picard C, Wang D, et al. E2F1 and TFDP1 regulate PITX1 expression in normal and osteoarthritic articular chondrocytes. PLoS One. 2016;11(11):e0165951. doi: 10.1371/journal.pone.0165951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y, Zeng C, Hu J, et al. Long non-coding RNA-SNHG7 acts as a target of miR-34a to increase GALNT7 level and regulate PI3K/Akt/mTOR pathway in colorectal cancer progression. J Hematol Oncol. 2018;11(1):89. doi: 10.1186/s13045-018-0632-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu X, Zhang L, Yang L, et al. miR-34a/c induce caprine endometrial epithelial cell apoptosis by regulating circ-8073/CEP55 via the RAS/RAF/MEK/ERK and PI3K/AKT/mTOR pathways. J Cell Physiol. 2020;235(12):10051–10067. doi: 10.1002/jcp.29821 [DOI] [PubMed] [Google Scholar]
- 23.Khan NM, Ansari MY, Haqqi TM. Sucrose, but not glucose, blocks IL1-beta-induced inflammatory response in human chondrocytes by inducing autophagy via AKT/mTOR pathway. J Cell Biochem. 2017;118(3):629–639. doi: 10.1002/jcb.25750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xue JF, Shi ZM, Zou J, et al. Inhibition of PI3K/AKT/mTOR signaling pathway promotes autophagy of articular chondrocytes and attenuates inflammatory response in rats with osteoarthritis. Biomed Pharmacother. 2017;89:1252–1261. doi: 10.1016/j.biopha.2017.01.130 [DOI] [PubMed] [Google Scholar]
- 25.Marchiani A, Rozzo C, Fadda A, et al. Curcumin and curcumin-like molecules: from spice to drugs. Curr Med Chem. 2014;21(2):204–222. doi: 10.2174/092986732102131206115810 [DOI] [PubMed] [Google Scholar]
- 26.Wang J, Ma J, Gu JH, et al. Regulation of type II collagen, matrix metalloproteinase-13 and cell proliferation by interleukin-1beta is mediated by curcumin via inhibition of NF-kappaB signaling in rat chondrocytes. Mol Med Rep. 2017;16(2):1837–1845. doi: 10.3892/mmr.2017.6771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clutterbuck AL, Allaway D, Harris P, et al. Curcumin reduces prostaglandin E2, matrix metalloproteinase-3 and proteoglycan release in the secretome of interleukin 1beta-treated articular cartilage. F1000Res. 2013;2:147. doi: 10.12688/f1000research.2-147.v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dai Q, Zhou D, Xu L, et al. Curcumin alleviates rheumatoid arthritis-induced inflammation and synovial hyperplasia by targeting mTOR pathway in rats. Drug Des Devel Ther. 2018;12:4095–4105. doi: 10.2147/DDDT.S175763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang G, Cao J, Yang E, et al. Curcumin improves age-related and surgically induced osteoarthritis by promoting autophagy in mice. Biosci Rep. 2018;38. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Zhu M, Zheng Z, Huang J, et al. Modulation of miR-34a in curcumin-induced antiproliferation of prostate cancer cells. J Cell Biochem. 2019;120(9):15616–15624. doi: 10.1002/jcb.28828 [DOI] [PubMed] [Google Scholar]
- 31.Subramaniam D, Ponnurangam S, Ramamoorthy P, et al. Curcumin induces cell death in esophageal cancer cells through modulating notch signaling. PLoS One. 2012;7(2):e30590. doi: 10.1371/journal.pone.0030590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reyes C, Leyland KM, Peat G, et al. Association between overweight and obesity and risk of clinically diagnosed knee, hip, and hand osteoarthritis: a Population-Based Cohort Study. Arthritis Rheum. 2016;68(8):1869–1875. doi: 10.1002/art.39707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dickson BM, Roelofs AJ, Rochford JJ, et al. The burden of metabolic syndrome on osteoarthritic joints. Arthritis Res Ther. 2019;21(1):289. doi: 10.1186/s13075-019-2081-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuroki H, Nakagawa Y, Mori K, et al. Acoustic stiffness and change in plug cartilage over time after autologous osteochondral grafting: correlation between ultrasound signal intensity and histological score in a rabbit model. Arthritis Res Ther. 2004;6(6):R492–504. doi: 10.1186/ar1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feng K, Ge Y, Chen Z, et al. Curcumin inhibits the PERK-eIF2alpha-CHOP pathway through promoting SIRT1 expression in oxidative stress-induced rat chondrocytes and ameliorates osteoarthritis progression in a rat model. Oxid Med Cell Longev. 2019;2019:8574386. doi: 10.1155/2019/8574386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Henrotin Y, Malaise M, Wittoek R, et al. Bio-optimized Curcuma longa extract is efficient on knee osteoarthritis pain: a Double-Blind Multicenter Randomized Placebo Controlled Three-Arm Study. Arthritis Res Ther. 2019;21(1):179. doi: 10.1186/s13075-019-1960-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou Y, Ming J, Deng M, et al. Chemically modified curcumin (CMC2.24) alleviates osteoarthritis progression by restoring cartilage homeostasis and inhibiting chondrocyte apoptosis via the NF-kappaB/HIF-2alpha axis. J Mol Med (Berl). 2020;98(10):1479–1491. doi: 10.1007/s00109-020-01972-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Collins KH, Paul HA, Reimer RA, et al. Relationship between inflammation, the gut microbiota, and metabolic osteoarthritis development: studies in a rat model. Osteoarthritis Cartilage. 2015;23(11):1989–1998. doi: 10.1016/j.joca.2015.03.014 [DOI] [PubMed] [Google Scholar]
- 39.Rios JL, Bomhof MR, Reimer RA, et al. Protective effect of prebiotic and exercise intervention on knee health in a rat model of diet-induced obesity. Sci Rep. 2019;9(1):3893. doi: 10.1038/s41598-019-40601-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tan L, Harper L, McNulty MA, et al. High-fat diet induces endoplasmic reticulum stress to promote chondrocyte apoptosis in mouse knee joints. FASEB J. 2020;34(4):5818–5826. doi: 10.1096/fj.201902746R [DOI] [PubMed] [Google Scholar]
- 41.Jiang M, He J, Gu H, et al. Protective effect of resveratrol on obesity-related osteoarthritis via alleviating JAK2/STAT3 signaling pathway is independent of SOCS3. Toxicol Appl Pharmacol. 2020;388:114871. doi: 10.1016/j.taap.2019.114871 [DOI] [PubMed] [Google Scholar]
- 42.Zhang Z, Leong DJ, Xu L, et al. Curcumin slows osteoarthritis progression and relieves osteoarthritis-associated pain symptoms in a post-traumatic osteoarthritis mouse model. Arthritis Res Ther. 2016;18(1):128. doi: 10.1186/s13075-016-1025-y [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 43.Henrotin Y, Priem F, Mobasheri A. Curcumin: a new paradigm and therapeutic opportunity for the treatment of osteoarthritis: curcumin for osteoarthritis management. Springerplus. 2013;2(1):56. doi: 10.1186/2193-1801-2-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang KY, Lin LC, Tseng TY, et al. Oral bioavailability of curcumin in rat and the herbal analysis from Curcuma longa by LC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;853(1–2):183–189. doi: 10.1016/j.jchromb.2007.03.010 [DOI] [PubMed] [Google Scholar]
- 45.Malemud CJ. Inhibition of MMPs and ADAM/ADAMTS. Biochem Pharmacol. 2019;165:33–40. doi: 10.1016/j.bcp.2019.02.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bollmann M, Pinno K, Ehnold LI, et al. MMP-9 mediated Syndecan-4 shedding correlates with osteoarthritis severity. Osteoarthritis Cartilage. 2021;29(2):280–289. doi: 10.1016/j.joca.2020.10.009 [DOI] [PubMed] [Google Scholar]
- 47.Chang JC, Christiansen BA, Murugesh DK, et al. SOST/Sclerostin improves posttraumatic osteoarthritis and inhibits MMP2/3 expression after injury. J Bone Miner Res. 2018;33(6):1105–1113. doi: 10.1002/jbmr.3397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang L, Song X, Zhu J, et al. Tumor suppressor microRNA-34a inhibits cell migration and invasion by targeting MMP-2/MMP-9/FNDC3B in esophageal squamous cell carcinoma. Int J Oncol. 2017;51(1):378–388. doi: 10.3892/ijo.2017.4015 [DOI] [PubMed] [Google Scholar]
- 49.Hou Q, Han S, Yang L, et al. The interplay of microRNA-34a, LGR4, EMT-associated factors, and MMP2 in regulating uveal melanoma cells. Invest Ophthalmol Vis Sci. 2019;60(13):4503–4510. doi: 10.1167/iovs.18-26477 [DOI] [PubMed] [Google Scholar]
- 50.Liu H, Dong Y, Feng X, et al. MiR-34a promotes bone regeneration in irradiated bone defects by enhancing osteoblastic differentiation of mesenchymal stromal cells in rats. Stem Cell Res Ther. 2019;10(1):180. doi: 10.1186/s13287-019-1285-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wen F, An C, Wu X, et al. MiR-34a regulates mitochondrial content and fat ectopic deposition induced by resistin through the AMPK/PPARalpha pathway in HepG2 cells. Int J Biochem Cell Biol. 2018;94:133–145. doi: 10.1016/j.biocel.2017.11.008 [DOI] [PubMed] [Google Scholar]
- 52.Si HB, Zeng Y, Liu SY, et al. Intra-articular injection of microRNA-140 (miRNA-140) alleviates osteoarthritis (OA) progression by modulating extracellular matrix (ECM) homeostasis in rats. Osteoarthritis Cartilage. 2017;25(10):1698–1707. doi: 10.1016/j.joca.2017.06.002 [DOI] [PubMed] [Google Scholar]
- 53.Krajewska JB, Fichna J, Mosinska P. One step ahead: miRNA-34 in colon cancer-future diagnostic and therapeutic tool? Crit Rev Oncol Hematol. 2018;132:1–8. doi: 10.1016/j.critrevonc.2018.09.006 [DOI] [PubMed] [Google Scholar]
- 54.Chao J, Guo Y, Li P, et al. Role of kallistatin treatment in aging and cancer by modulating miR-34a and miR-21 expression. Oxid Med Cell Longev. 2017;2017:5025610. doi: 10.1155/2017/5025610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Denechaud PD, Fajas L, Giralt A. E2F1, a novel regulator of metabolism. Front Endocrinol (Lausanne). 2017;8:311. doi: 10.3389/fendo.2017.00311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ghorbani M, Themis M, Payne A. Genome wide classification and characterisation of CpG sites in cancer and normal cells. Comput Biol Med. 2016;68:57–66. doi: 10.1016/j.compbiomed.2015.09.023 [DOI] [PubMed] [Google Scholar]
- 57.Handle F, Prekovic S, Helsen C, et al. Drivers of AR indifferent anti-androgen resistance in prostate cancer cells. Sci Rep. 2019;9(1):13786. doi: 10.1038/s41598-019-50220-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kent LN, Leone G. The broken cycle: E2F dysfunction in cancer. Nat Rev Cancer. 2019;19(6):326–338. doi: 10.1038/s41568-019-0143-7 [DOI] [PubMed] [Google Scholar]
- 59.Wang L, Chen H, Wang C, et al. Negative regulator of E2F transcription factors links cell cycle checkpoint and DNA damage repair. Proc Natl Acad Sci U S A. 2018;115(16):E3837–E3845. doi: 10.1073/pnas.1720094115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maiuri MC, Zalckvar E, Kimchi A, et al. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol. 2007;8(9):741–752. doi: 10.1038/nrm2239 [DOI] [PubMed] [Google Scholar]
- 61.Rainey NE, Moustapha A, Petit PX. Curcumin, a multifaceted hormetic agent, mediates an intricate crosstalk between mitochondrial turnover, autophagy, and apoptosis. Oxid Med Cell Longev. 2020;2020:3656419. doi: 10.1155/2020/3656419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang CI, Chen YY, Wang CL, et al. mTOR regulates proteasomal degradation and Dp1/E2F1- mediated transcription of KPNA2 in lung cancer cells. Oncotarget. 2016;7(18):25432–25442. doi: 10.18632/oncotarget.8170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zou Z, Chen J, Liu A, et al. mTORC2 promotes cell survival through c-Myc-dependent up-regulation of E2F1. J Cell Biol. 2015;211(1):105–122. doi: 10.1083/jcb.201411128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wong P-M, Puente C, Ganley IG, et al. The ULK1 complex: sensing nutrient signals for autophagy activation. Autophagy. 2013;9(2):124–137. doi: 10.4161/auto.23323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim J, Kundu M, Viollet B, et al. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13(2):132–141. doi: 10.1038/ncb2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rabinowitz JD, White E. Autophagy and metabolism. Science. 2010;330(6009):1344–1348. doi: 10.1126/science.1193497 [DOI] [PMC free article] [PubMed] [Google Scholar]