Abstract
Safety and efficacy of allogeneic anti-CD19 chimeric antigen receptor T cells (CAR-T cells) in persons with CD19-positive B-cell acute lymphoblastic leukemia (B-ALL) relapsing after an allotransplant remain unclear. Forty-three subjects with B-ALL relapsing post allotransplant received CAR-T cells were analyzed. 34 (79%; 95% confidence interval [CI]: 66, 92%) achieved complete histological remission (CR). Cytokine release syndrome (CRS) occurred in 38 (88%; 78, 98%) and was ≥grade-3 in 7. Two subjects died from multiorgan failure and CRS. Nine subjects (21%; 8, 34%) developed ≤grade-2 immune effector cell-associated neurotoxicity syndrome (ICANS). Two subjects developed ≤grade-2 acute graft-versus-host disease (GvHD). 1-year event-free survival (EFS) and survival was 43% (25, 62%). In 32 subjects with a complete histological remission without a second transplant, 1-year cumulative incidence of relapse was 41% (25, 62%) and 1-year EFS and survival, 59% (37, 81%). Therapy of B-ALL subjects relapsing post transplant with donor-derived CAR-T cells is safe and effective but associated with a high rate of CRS. Outcomes seem comparable to those achieved with alternative therapies but data from a randomized trial are lacking.
Subject terms: Cancer immunotherapy, Acute lymphocytic leukaemia
Introduction
Persons experiencing B-cell acute lymphoblastic leukemia (B-ALL) relapse after an allogeneic hematopoietic cell transplant are typically treated by stopping immune suppression, receiving a donor lymphocyte infusion (DLI) and/or receiving a second transplant from the same or a different donor. The outcomes of these interventions are unsatisfactory [1–3].
Autologous anti-CD19 chimeric antigen receptor T cells (CAR-T cells) are an effective therapy for advanced CD19-positive B-ALL, often followed by an allotransplant [4–8]. Cumulative incidence of relapse (CIR) and event-free survival (EFS) of persons receiving CAR-T cells without an allotransplant are typically short [9]. Allogeneic anti-CD19 CAR-T cells receive activation signals from T-cell receptors (TCRs) to target cell alloantigens and from CD19 on leukemia cells. This dual signaling may increase the anti-leukemia efficacy compared with autologous CAR-T cells. Allogeneic anti-CD19 CAR-T cells can be developed from donor T cells in allotransplant recipients who relapse. However, the safety and efficacy of this approach are unknown.
We determined the safety and efficacy of donor-derived anti-CD19 CAR-T cells in 43 subjects with CD19-positive B-ALL relapsing after an allotransplant. Outcomes from data reported in this setting with DLI and with a second allotransplant were then compared. Results of donor-derived anti-CD19 CAR-T cells seem at least comparable if not better than these alternatives. However, these results can be tested only in a randomized trial.
Methods
Subjects and data collection
Forty-three subjects with CD19-positive B-ALL who received an allotransplant, had a bone marrow relapse and received donor-derived anti-CD19 CAR-T cells from July 2015 to March 2019 were enrolled. Major inclusion criteria included (1) Eastern Cooperative Oncology Group performance score ≤grade-2; (2) estimated survival >3 months; (3) no prior acute graft-versus-host disease; and (4) refusal to receive a second allotransplant. Posttransplant relapse was defined as >5% bone marrow blasts or a positive measurable residual disease (MRD) test after ≥2 prior posttransplant MRD tests. Details of MRD testing are reported [6, 8]. Data were extracted from the electronic medical records of subjects enrolled in ChiCTR-OOC-16008447, protocol number: ChIECRCT-20160022 and ChiCTR-OIC-17012374, protocol number: XYFY2017-KL033-01. The study was approved by the Ethics Committee of the Army Medical University.
CD19-targeting CAR-T manufacturing
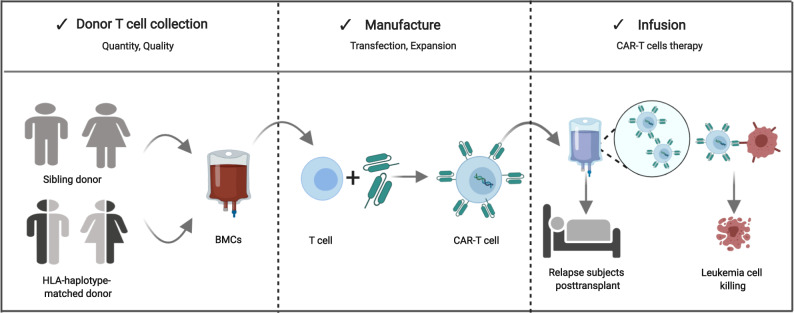
The method for obtaining anti-CD19 CAR-T cells is previously described [7]. Briefly, blood mononuclear cells were obtained from transplant donors by leukapheresis, T cells were purified and transfected with lentivirus containing sequence expressing chimeric antigen receptors (CARs) with the 4-1BB or CD28 intracellular domain as co-stimulation signal and expanded in vitro. Quality control was based on the Chinese Pharmacopoeia (2015 Version), which includes viability >70% (Fig. 1).
Fig. 1. The procedure of donor-derived CD19 CAR-T cells.
The BMCs were apheresised from sibling donor or HLA-haplotype-matched donor, then the T cells were selected and transfected with lentivirus to generate CARs. At last, the qualified CAR-T cells were infused into relapse subjects to kill the leukemia cells. CAR chimeric antigen receptor, BMC blood mononuclear cells.
CD19-targeting CAR-T cell therapy
Three preinfusion immune suppressive regimens were: (1) regimen 1: fludarabine, 30 mg/mE + 2/day for 2–4 days and cyclophosphamide, 200 mg/mE + 2/day for 2 days; (2) regimen 2: fludarabine, 30 mg/mE + 2/day for 3 days, cyclophosphamide, 350 mg/mE + 2/day for 2 days and cytarabine, and 100 mg/mE+2/day for 4 days; and (3) regimen 3: cyclophosphamide, 500 mg/mE + 2/day for 3 days. Overall, 34 subjects received regimen 1. Regimen 2 was given to five younger subjects with many leukemia cells. Regimen 3 was given to four older subjects with few leukemia cells. Expansion and persistence of CAR-T cells were analyzed by flow cytometry or by CAR-T cell DNA copy number [7]. Median dose of infused CAR-T cells was 1.76 × 10E + 6/kg (range, 0.4–12 × 10E + 6/kg).
Response assessment
Complete remission was defined by histology (<5% bone marrow blasts) and a negative MRD test (<0.01% bone marrow blasts) assessed by multiparameter flow cytometry to detect the leukemia-associated profiles [8, 10]. It also included normal maturation of all cell components in the bone marrow, no extramedullary leukemia (e.g. central nervous system, testes or soft tissue), blood neutrophil concentration ≥1 × 10E + 9/L, blood platelet concentration ≥100 × 10E + 9/L and RBC and platelet transfusion independence.
Relapse included histologic and a positive MRD test. Histologic relapse was defined as blasts ≥5% in blood or bone marrow and/or in an extramedullary site after achieving a complete histologic remission post transplant. Molecular relapse was defined as a positive MRD test (>0.01% and <5%) without evidence of histologic relapse post transplant [11].
Adverse events
Grading of acute GvHD was based on published criteria [12, 13], as was grading of cytokine release syndrome (CRS) [14]. Immune effector cell-associated neurotoxicity syndrome (ICANS) was graded using the Common Terminology Criteria for Adverse Events [15]. CRS, ICANS, and acute GvHD were managed as previously described [7, 14, 15].
Statistics
The time of CAR-T cell transfusion was used as the origin in all the time-to-event analyses. Analysis of CIR used relapse as the event. For analysis of EFS, no response, relapse or death, whichever occurred first, was regarded as the event. In survival analyses, death was the event. Subjects without an event were censored at the date they were last known to be alive. Two subjects receiving a second allotransplant were censored at the time of second transplant. The primary study endpoints were safety and efficacy. Secondary endpoints were covariates associated with safety and efficacy. Data were analyzed as of September 30, 2019 with a median follow-up of survivors of 17 months (range, 6–47 months).
The chi-square statistic or Fisher exact test was used for comparisons between categorical variables, and the Mann–Whitney U test was used for continuous variables. The Kaplan–Meier method was used to calculate the probability of EFS and survival. P values were two-sided, and P < 0.05 was considered significant. SPSS statistical software for Windows, version 24.0 (SPSS, Chicago, IL, USA) was used for statistical analyses.
Results
Subjects
Forty-three subjects were enrolled and analyzed (Table 1). Twenty-nine were male. Median age was 24 years (range, 4–60 years). Pretransplant conditioning regimens are displayed in Table 1. Donors were HLA-identical siblings (N = 17) or HLA-haplotype-matched relatives (N = 26). Bone marrow blasts at relapse were 0.01–5% in 13 subjects, 5–50% in 20 and >50% in 10 subjects. The median interval from relapse to CAR-T cell infusion was 42 days (range, 35–59 days). Postrelapse therapies included stopping immune suppression (N = 12), DLI (N = 7), chemotherapy (N = 19), and DLI and chemotherapy (N = 5; Supplementery Table 1).
Table 1.
Patient covariates (N = 43).
| Male | 29 |
| Age (Median; range) | 24 (4–60) |
| Donor | |
| HLA-identical sibling | 17 |
| HLA-haplotype-matched | 26 |
| Graft | |
| Blood | 17 |
| Blood and bone marrow | 26 |
| BCR/ABL1 positive | |
| Yes | 5 |
| No | 38 |
| Cytogenetics | |
| Abnormal | 14 |
| Normal | 29 |
| Mutation | |
| Yes | 23 |
| No | 20 |
| Transplant conditioning regimen | |
| BU/CY | 17 |
| BU/CY/Ara-C/CCNU | 26 |
| Posttransplant immune suppression | |
| CSA/MMF/MTX | 17 |
| Tacrolimus/MMF/ATG/MTX | 26 |
| Interval from transplant to relapse (mo; median; range) | 8 (1–25) |
| Interval from relapse to CAR-T (d; median; range) | 42 (35–59) |
| Therapy for relapse | |
| Stop immune suppression | 12 |
| DLI | 7 |
| Chemotherapy | 19 |
| Chemotherapy/DLI | 5 |
| Bone marrow blasts pre infusion | |
| 0.01-(MRD-positive) | 13 |
| 5–50% | 20 |
| >50% | 10 |
| Co-stimulatory molecular | |
| CD28 | 18 |
| 4–1BB | 25 |
| Preinfusion therapy | |
| Regimen 1 | 34 |
| Regimen 2 | 5 |
| Regimen 3 | 4 |
| CAR-T cell dose (×10E + 6/kg; median; range) | 1.76 (0.4–12) |
| <1 | 4 |
| 1~2 | 26 |
| >2 | 13 |
HLA human leukocyte antigen, BU busulfan, CY cyclophosphamide, Ara-C cytarabine, CCNU lomustine, CSA cyclosporine, MMF mycophenolate mofetil, MTX methotrexate, ATG anti-thymocyte globulin, MRD measurable residual disease, DLI donor lymphocyte infusion.
Overall, 18 subjects received CD19–28z CAR-T cells and 25, CD19-BBz CAR-T cells. Pre infusion, 34 subjects (79%) received regimen 1, 5 regimen 2, and 4, regimen 3. Median numbers of infused CAR-T cells were 1.76 × 10E + 6/kg (range, 0.4–12 × 10E + 6/kg; Table 1).
Safety
All subjects had a decreased concentration of hemoglobin, WBCs, neutrophils, lymphocytes, and platelets. One subject had an increased activated partial thromboplastin time. Two subjects had an increased alkaline phosphatase, alanine aminotransferase, aspartate aminotransferase, and lactic dehydrogenase levels.
CRS of any grade developed in 38 subjects (88%; 95% confidence interval (CI). 78, 98%) and was ≥grade-3 in 7 subjects. Six of seven subjects with ≥grade-3 CRS had bone marrow blasts >5% including four with bone marrow blasts >50% and two, 20–50%. There was no significant correlation between risk of severe CRS or incidence or severity of ICANS and percentage bone marrow blasts. Overall, 16 of 18 subjects receiving CD19–28z CAR-T cells developed CRS, severe in 5. 22 of 25 subjects receiving CD19-BBz CAR-T cells developed CRS, severe in 4. 3 of 18 subjects receiving CD19-28z CAR-T cells developed ICANS compared with 6 of 25 receiving CD19-BBz CAR-T cells (P = 0.79). Two subjects receiving CAR-T cells developed ≤grade-2 acute GvHD. Nine subjects (21% [8, 34%]) developed grade-1/-2 ICANS with none ≥grade-3.
Efficacy
41 subjects survived ≥21 days and were evaluable for response. Two died in <21 days from CRS and multiorgan failure on days 14 and 21 and were included in the intent-to-treat analysis. 34 subjects (79% [66, 92%]) achieved a complete histological remission including 12 of 13 with 0.01–5% bone marrow blasts and a positive MRD test, 14 of 20 with 5–50% bone marrow blasts and 8 of 10 with ≥50% bone marrow blasts. Although there are no significant differences in rates between the cohorts, the comparison is not adjusted for other covariates such as the preinfusion regimen or type of CAR-T cells. 14 of 18 subjects receiving CD19–28z CAR-T cells achieved a complete histological remission compared with 20 of 25 receiving CD19-BBz CAR-T cells. Because therapy assignment was not random and not adjusted for other covariates, we did not compare these rates statistically.
The 34 subjects achieving a complete histological remission received different CAR-T doses, including two of four receiving <1 × 10E + 6, 23 of 26 receiving 1–2 × 10E + 6, and 9 of 13 receiving >2 × 10E + 6. Because therapy assignment was not random, we did not compare these rates statistically. These 34 received different preinfusion regimens, including 26 receiving regimen 1, five of five receiving regimen 2, and three of four receiving regimen 3.
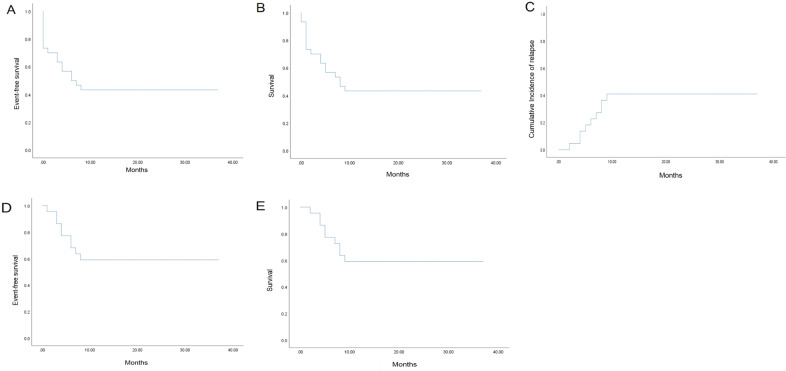
1-year probabilities of EFS and survival were 43% (25, 62%; Fig. 2a, b). 1-year probability of CIR was 41% (25, 62%) in subjects achieving a complete histological remission not receiving a second transplant (Fig. 2c). In the 32 subjects achieving a complete histological remission not receiving a second transplant 1-year probabilities of EFS and survival were 59% (37, 81%; Fig. 2d, e). Two of nine subjects not achieving a complete histological remission lost CD19 expression. Three other subjects who relapsed lost CD19 expression.
Fig. 2. Survival after CD19 donor-derived CAR-T cell treatment of relapsed B-ALL after an allotransplant.
a 1-year EFS; b 1-year survival; c 1-year CIR in subjects with a complete histological remission without a second transplant; d, e 1-year EFS and survival in subjects with a complete histological remission without a second transplant. EFS event-free survival, CIR cumulative incidence of relapse.
CAR-T cell dynamics
CAR-T cell numbers peaked on day 9 (range, day 3–61) after CAR-T cell infusion in the 36 subjects with complete data. Median interval of detectable blood CAR-T cells post infusion was 89 days (range, 10–1230 days). Postinfusion CAR-T cells peaked on day 10 (range, days 3–61) and 9 (range, days 7–21) in subjects with and without a complete histological remission, respectively. Corresponding median postinfusion intervals of detectable blood CAR-T cells were 118 days (range, 10–1230 days) and 21 days (range, 14–28 days). Median peak concentration was 4.85 × 10E + 5/L (range, 0.14–1.18 × 10 + 5/L). Median peak percentage of all cells was 23% (range, 2–65%).
Discussion
Our data indicate that donor-derived anti-CD19 CAR-T cells are a safe and effective therapy for B-ALL recurrence after allotransplantation. Adverse effects of CAR-T cell therapy are mainly CRS and ICANS, severity of which reportedly correlates with cancer volume, preinfusion regimen and CAR-T cell dose in some but not all studies [16, 17]. We found no such correlations but we had relatively few subjects and many confounding covariates. Others have reported similar data [18, 19]. Although the incidence of CRS in our study is highly compared with studies of autologous CAR-T cells, most cases were low-grade and controllable. Absent a randomized comparison any conclusion is tentative. Nine subjects developed grade-1/-2 ICANS with none ≥grade-3. This rate is like that reported after autologous CAR-T cell therapy [20].
Donor-derived CAR-T cell treatment can potentially cause acute GvHD. In a mouse model, donor-derived CAR-T cells had little GvHD but a potent graft-versus-leukemia (GvL) effect [21]. A systematic review reported a low risk of acute GvHD after allogeneic CAR-T cells [22]. Two subjects in our study developed mild acute GvHD. These data imply an ability to separate GvHD from GvL, albeit in an artificial setting.
There are a few reports of using allogeneic CAR-T cells to treat B-cell cancers after an allogeneic hematopoietic stem cell transplant (Table 2) [18, 23–29]. No study had >30 subjects. Moreover, most subjects in these reports received a second transplant making critical analyses of safety and efficacy of the CAR-T cell infusion impossible. We censored data from subjects who received a second transplant, making it possible to ascertain the safety and efficacy of CAR-T cell therapy alone.
Table 2.
Reports of donor CAR-T cells given for relapse of B-cell cancers after an allotransplant.
| Subjects (N) | Agea | B-ALL | T-ALL (CD19+) | B-CLL | Lymphoma | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ph1+ or BCRABL1+ | Ph1− or BCRABL1− | MCL | DLBCL | Follicular | Nodular HL | |||||
| 8 | 32 (9–59) | 2 | 2 | 4 | [23] | |||||
| 10 | 52 (44–66) | 4 | 4 | 2 | [24] | |||||
| 16 | 50 (23–74) | 4 | 12 | [25] | ||||||
| 9 | 39 (15–64) | 5 | 4 | [26] | ||||||
| 20 | 46 (20–68) | 1 | 4 | 5 | 5 | 5 | [27] | |||
| 26 | 40 (21–61) | 17 | 1 | 4 | 3 | 1 | [28] | |||
| 30 | 14 (5–60) | 2 | 27 | 1 | [29] | |||||
| 6 | 27 (8–44) | 1 | 5 | [18] | ||||||
Ph1 Philadelphia1-chromosome, BCRABL1 qRT-PCR result, Ref reference.
aYears (y); median; range.
Other potential therapies of relapse after an allotransplant include stopping immune suppression, receiving DLI and/or receiving a second transplant from the same or a different donor. We summarize the data using these strategies in Table 3. It was difficult to compare our outcomes with these other strategies. There are only four studies of DLI, three of which had ≤10 subjects. There were only five studies of a second transplant only two of which had many subjects and none indicated consecutive subjects. Studies of stopping posttransplant immune suppression were typically confounded by combination with other therapies, often given concurrently. These limited data and confounding factors make it impossible to critical compare our data with those from other studies. A randomized trial is needed to resolve this question but is highly unlikely.
Table 3.
Therapies of relapse of ALL post allotransplant.
| N | ALL | Agea | CR | Stop immune suppressionb | CIR | EFS | Survival | aGvHD ≥ grade 2 | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| B | T | ||||||||||
| This study | 43 | 43 | ─ | 24 (4–60) | 79% | ─ | 57% | 43% | 43% | 0 | |
| DLI | 10 | ─ | ─ | 11 (< 1–25) | ─ | N/A | N/A | N/A | ─ | N/A | [30] |
| DLI | 30 | ─ | ─ | 21 (10–52) | 25% | N/A | N/A | N/A | 5% | N/A | [31] |
| DLI | 8 | ─ | ─ | 24 (18–39) | ─ | 5 (2–14) | N/A | ─ | ─ | 5 | [32] |
| DLI | 10 | ─ | ─ | 33 (18–40) | 70% | N/A | N/A | N/A | ─ | 6 | [33] |
| Second transplant | 245 | 186 | 59 | 35 (18–74) | N/A | N/A | 56% | 20% | 30% | 127 | [34] |
| Second transplant | 11 | ─ | ─ | 41 (18–65) | N/A | Tapered at 3 mo | N/A | ─ | ─ | N/A | [35] |
| Second transplant | 214 | ─ | ─ | 8 (1–18) | 78% | N/A | 44% | 34% | 43% | 53 | [36] |
| Second transplant | 27 | 22 | 5 | 37 (7–60) | N/A | N/A | 56% | 30% | 41% | 15 | [37] |
| Second transplant | 31 | ─ | ─ | 26 (7–49) | 75% | N/A | N/A | N/A | 23% | 13 | [38] |
Estimates for studies with <30 subjects are unreliable with wide 95% confidence intervals so complete histological remission rates, CIR, EFS, and survival are not indicated.
CR complete histological remission, DLI donor lymphocyte infusion, EFS event-free survival, GvHD graft-versus-host disease, Ref reference, N/A not available.
aYear; median range.
bMonth; median; range.
Our study has important limitations. We had relatively few subjects who with different diagnoses received diverse postrelapse interventions before CAR-T cell therapy. They also received different preinfusion regimens, different CAR-T cell constructs and different doses which preclude us from making definitive conclusions regarding subject-, disease- and therapy-related covariates correlated with outcomes. Consequently, we refrained from comparing outcomes of covariates such as preinfusion regimen and type and dose of CAR-T cells. Results of such comparisons are likely to be confounded by known and latent (unknown) covariates and small sample sizes, are unreliable and are not statically justifiable. Also, because our subjects are not consecutive cases of everyone relapsing at the study centers there are important selection biases. Our study was retrospective, and participating center admission records were not independently audited.
It should also be noted that, CAR-T cell manufacturing in different medical centers may cause heterogeneity, but every procedure complies with a rigorous quality control, which is consistent. The outcomes of our study with the largest number of relapsed ALL subjects are satisfactory, donor-derived CAR-T is a good choice for those who are not eligible for receiving a second transplant.
In summary, we show therapy of recurrent B-ALL after an allotransplant with donor-derived anti-CD19 CAR-T cells is safe and effective. Outcomes seem comparable to those achieved with alternative therapies. However, the relative safety and efficacy of these alternatives can be accurately determined only in a randomized trial.
Supplementary information
Acknowledgements
Funded by the Clinical Key Foundation of Xinqiao Hospital of the Army Medical University (No. 2018JSLC020), the Key Technical Innovation Projects in Clinical Fields of Army Medical University (No. CX2019LC111), Clinical Fields of Army Medical University (No. 2018XLC3028), and Chongqing Science and Technology Innovation Leading Talents Project (CSTCCXLJRC201718). RPG acknowledges support from the National Institute of Health Research Biomedical Research Centre funding scheme.
Author contributions
All authors contributed to the study design, data collection, analyses, interpretation, and preparation of the typescript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Cheng Zhang, Xiao-Qi Wang
Contributor Information
Ming-Zhe Han, Email: mzhan@ihcams.ac.cn.
Xi Zhang, Email: zhangxxi@sina.com.
Supplementary information
The online version of this article (10.1038/s41375-020-01056-6) contains supplementary material, which is available to authorized users.
References
- 1.Wolach O, Amitai I, DeAngelo DJ. Current challenges and opportunities in treating adult subjects with Philadelphia-negative acute lymphoblastic leukaemia. Br J Haematol. 2017;179:705–23. doi: 10.1111/bjh.14916. [DOI] [PubMed] [Google Scholar]
- 2.Spyridonidis A, Labopin M, Schmid C, Volin L, Yakoub-Agha I, Stadler M, et al. Outcomes and prognostic factors of adults with acute lymphoblastic leukemia who relapse after allogeneic hematopoietic cell transplantation. An analysis on behalf of the Acute Leukemia Working Party of EBMT. Leukemia. 2012;26:1211–7. doi: 10.1038/leu.2011.351. [DOI] [PubMed] [Google Scholar]
- 3.Wang YX, Li YH. Efficacy of donor lymphocyte infusion for treating relapsed high-risk leukemia subjects after allogeneic hematopoietic stem cell transplantation. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2015;23:982–8. doi: 10.7534/j.issn.1009-2137.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 4.Zhang C, Liu J, Zhong JF, Zhang X. Engineering CAR-T cells. Biomark Res. 2017;5:22. doi: 10.1186/s40364-017-0102-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu J, Zhong JF, Zhang X, Zhang C. Allogeneic CD19-CAR-T cell infusion after allogeneic hematopoietic stem cell transplantation in B cell malignancies. J Hematol Oncol. 2017;10:35. doi: 10.1186/s13045-017-0405-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu J, Zhang X, Zhong JF, Zhang C. Use of chimeric antigen receptor T cells in allogeneic hematopoietic stem cell transplantation. Immunotherapy. 2018;1:37–44. doi: 10.2217/imt-2018-0089. [DOI] [PubMed] [Google Scholar]
- 7.Zhang C, Kong PY, Li S, Chen T, Ni X, Li YE, et al. Donor-derived CAR-T Cells serve as a reduced-intensity conditioning regimen for haploidentical stem cell transplantation in treatment of relapsed/refractory acute lymphoblastic leukemia: case report and review of the literature. J Immunother. 2018;41:306–11. doi: 10.1097/CJI.0000000000000233. [DOI] [PubMed] [Google Scholar]
- 8.Park JH, Rivière I, Gonen M, Wang X, Sénéchal B, Curran KJ, et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med. 2018;378:449–59. doi: 10.1056/NEJMoa1709919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu J, Zhang X, Zhong JF, Zhang C. CAR-T cells and allogeneic hematopoietic stem cell transplantation for relapsed/refractory B-cell acute lymphoblastic leukemia. Immunotherapy. 2017;9:1115–25. doi: 10.2217/imt-2017-0072. [DOI] [PubMed] [Google Scholar]
- 10.Hourigan CS, Gale RP, Gormley NJ, Ossenkoppele GJ, Walter RB. Measurable residual disease testing in acute myeloid leukaemia. Leukemia. 2017;31:1482–90. doi: 10.1038/leu.2017.113. [DOI] [PubMed] [Google Scholar]
- 11.Rautenberg C, Germing U, Haas R, Kobbe G, Schroeder T. Relapse of acute myeloid leukemia after allogeneic stem cell transplantation: prevention, detection, and treatment. Int J Mol Sci. 2019;20:228. doi: 10.3390/ijms20010228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 consensus conference on acute GVHD grading. Bone Marrow Transpl. 1995;15:825–8. [PubMed] [Google Scholar]
- 13.Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National Institutes of Health Consensus Development Project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transpl. 2015;21:389–401. doi: 10.1016/j.bbmt.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Porter DL, Hwang WT, Frey NV, Lacey SF, Shaw PA, Loren AW, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med. 2015;7:303ra139. doi: 10.1126/scitranslmed.aac5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle C, Brudno JN, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transpl. 2019;25:625–38. doi: 10.1016/j.bbmt.2018.12.758. [DOI] [PubMed] [Google Scholar]
- 16.Brudno JN, Kochenderfer JN. Recent advances in CAR T-cell toxicity: mechanisms, manifestations and management. Blood Rev. 2019;34:45–55. doi: 10.1016/j.blre.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hay KA. Cytokine release syndrome and neurotoxicity after CD19 chimeric antigen receptor-modified (CAR-) T cell therapy. Br J Haematol. 2018;183:364–74. doi: 10.1111/bjh.15644. [DOI] [PubMed] [Google Scholar]
- 18.Chen Y, Cheng Y, Suo P, Yan C, Wang Y, Chen Y, et al. Donor-derived CD19-targeted T cell infusion induces minimal residual disease-negative remission in relapsed B-cell acute lymphoblastic leukaemia with no response to donor lymphocyte infusions after haploidentical haematopoietic stem cell transplantation. Br J Haematol. 2017;179:598–605. doi: 10.1111/bjh.14923. [DOI] [PubMed] [Google Scholar]
- 19.Smith M, Zakrzewski J, James S, Aplenc R, Barrett DM, Bunin NJ, et al. Posttransplant chimeric antigen receptor therapy. Blood. 2018;131:1045–52. doi: 10.1182/blood-2017-08-752121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378:439–48. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghosh A, Smith M, James SE, Davila ML, Velardi E, Argyropoulos KV, et al. Donor CD19 CAR T cells exert potent graft-versus-lymphoma activity with diminished graft-versus-host activity. Nat Med. 2017;23:242–9. doi: 10.1038/nm.4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anwer F, Shaukat AA, Zahid U, Husnain M, McBride A, Persky D, et al. Donor origin CAR T cells: graft versus malignancy effect without GVHD, a systematic review. Immunotherapy. 2017;9:123–30. doi: 10.2217/imt-2016-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cruz CR, Micklethwaite KP, Savoldo B, Ramos CA, Lam S, Ku S, et al. Infusion of donor-derived CD19-redirected virus-specific T cells for B-cell malignancies relapsed after allogeneic stem cell transplant: a Phase 1 study. Blood. 2013;122:2965–73. doi: 10.1182/blood-2013-06-506741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kochenderfer JN, Dudley ME, Carpenter RO, Kassim SH, Rose JJ, Telford WG, et al. Donor-derived CD19-targeted T cells cause regression of malignancy persisting after allogeneic hematopoietic stem cell transplantation. Blood. 2013;122:4129–39. doi: 10.1182/blood-2013-08-519413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014;6:224ra25. doi: 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dai H, Zhang W, Li X, Han Q, Guo Y, Zhang Y, et al. Tolerance and efficacy of autologous or donor-derived T cells expressing CD19 chimeric antigen receptors in adult B-ALL with extramedullary leukemia. Oncoimmunology. 2015;4:e1027469. doi: 10.1080/2162402X.2015.1027469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brudno JN, Somerville RP, Shi V, Rose JJ, Halverson DC, Fowler DH, et al. Allogeneic T cells that express an anti-CD19 chimeric antigen receptor induce remissions of B-cell malignancies that progress after allogeneic hematopoietic stem-cell transplantation without causing graft-versus-host disease. J Clin Oncol. 2016;34:1112–21. doi: 10.1200/JCO.2015.64.5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kebriaei P, Singh H, Huls MH, Figliola MJ, Bassett R, Olivares S, et al. Phase I trials using sleeping beauty to generate CD19-specific CAR T cells. J Clin Investig. 2016;126:3363–76. doi: 10.1172/JCI86721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371:1507–17. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Willasch AM, Salzmann-Manrique E, Krenn T, Duerken M, Faber J, Opper J, et al. Treatment of relapse after allogeneic stem cell transplantation in children and adolescents with ALL: the Frankfurt experience. Bone Marrow Transpl. 2017;52:201–8. doi: 10.1038/bmt.2016.224. [DOI] [PubMed] [Google Scholar]
- 31.Shiobara S, Nakao S, Ueda M, Yamazaki H, Takahashi S, Asano S, et al. Donor leukocyte infusion for Japanese subjects with relapsed leukemia after allogeneic bone marrow transplantation: indications and dose escalation. Ther Apher. 2001;5:40–5. doi: 10.1046/j.1526-0968.2001.005001040.x. [DOI] [PubMed] [Google Scholar]
- 32.Yegin ZA, Ozkurt ZN, Aki SZ, Sucak GT. Donor lymphocyte infusion for leukemia relapse after hematopoietic stem cell transplantation. Transfus Apher Sci. 2010;42:239–45. doi: 10.1016/j.transci.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 33.Choi SJ, Lee JH, Lee JH, Kim S, Lee YS, Seol M, et al. Treatment of relapsed acute lymphoblastic leukemia after allogeneic bone marrow transplantation with chemotherapy followed by G-CSF-primed donor leukocyte infusion: a prospective study. Bone Marrow Transpl. 2005;36:163–9. doi: 10.1038/sj.bmt.1705024. [DOI] [PubMed] [Google Scholar]
- 34.Nagler A, Labopin M, Dholaria B, Finke J, Brecht A, Schanz U, et al. Second allogeneic stem cell transplantation in subjects with acute lymphoblastic leukaemia: a study on behalf of the Acute Leukaemia Working Party of the European Society for Blood and Marrow Transplantation. Br J Haematol. 2019;186:767–76. doi: 10.1111/bjh.15973. [DOI] [PubMed] [Google Scholar]
- 35.Schneidawind C, Hagmaier V, Faul C, Kanz L, Bethge W, Schneidawind D. Second allogeneic hematopoietic cell transplantation enables long-term disease-free survival in relapsed acute leukemia. Ann Hematol. 2018;97:2491–500. doi: 10.1007/s00277-018-3454-y. [DOI] [PubMed] [Google Scholar]
- 36.Yaniv I, Krauss AC, Beohou E, Dalissier A, Corbacioglu S, Zecca M, et al. Second hematopoietic stem cell transplantation for post-transplantation relapsed acute leukemia in children: a retrospective EBMT-PDWP study. Biol Blood Marrow Transpl. 2018;24:1629–42. doi: 10.1016/j.bbmt.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 37.Al Malki MM, Aldoss I, Stiller T, Nakamura R, Snyder DS, Forman SJ, et al. Outcome of second allogeneic hematopoietic cell transplantation in subjects with acute lymphoblastic leukemia. Clin Lymphoma Myeloma Leuk. 2016;16:519–22. doi: 10.1016/j.clml.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 38.Poon LM, Bassett R, Jr, Rondon G, Hamdi A, Qazilbash M, Hosing C, et al. Outcomes of second allogeneic hematopoietic stem cell transplantation for subjects with acute lymphoblastic leukemia. Bone Marrow Transpl. 2013;48:666–70. doi: 10.1038/bmt.2012.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.