Abstract
It is estimated that one in ten people in the USA have diabetes. Approximately 40% of those with diabetes also develop chronic kidney disease (CKD), which in turn increases their risk of developing cardiovascular disease. Evidence-based recommendations for the treatment of patients with type 2 diabetes (T2D) and concomitant CKD are provided by several medical societies, including the American Diabetes Association (ADA), but in real life are only carried out in fewer than 50% of individuals for whom they are recommended. Screening for CKD is recommended using the spot urine albumin-to-creatinine ratio and estimated glomerular filtration rate in all patients with T2D at the time of diagnosis, and at least annually thereafter. Screening enables early CKD diagnosis, counseling, pharmacologic intervention and, when appropriate, referral to a nephrologist. The ADA guidelines recommend good glycemic and blood pressure control and the use of medications that are kidney protective. Medications shown to slow progression of CKD include renin–angiotensin system inhibitors, sodium–glucose cotransporter-2 inhibitors, glucagon-like peptide 1 receptor agonists and, more recently, non-steroidal mineralocorticoid receptor antagonists. Novel agents with different mechanisms of action are also in development that have the potential to further slow or prevent disease progression when used with currently recommended therapies.
Keywords: Albuminuria; Diabetes mellitus, Type 2; Glomerular filtration rate; Glucagon-like peptide 1 receptor agonists; Renal insufficiency, Chronic; Renin–angiotensin system; Sodium–glucose cotransporter-2 inhibitors; UACR
Key Summary Points
| Evidence-based recommendations for the treatment of patients with chronic kidney disease (CKD) and type 2 diabetes (T2D) are currently followed in fewer than 50% of individuals. |
| Screening for CKD is recommended using spot urine albumin-to-creatinine ratio and estimated glomerular filtration rate in all patients with T2D at diagnosis, and at least annually thereafter. |
| Screening enables early CKD diagnosis, counseling, pharmacologic intervention and, when appropriate, referral to a nephrologist. |
| Medications shown to slow progression of CKD include renin–angiotensin system inhibitors, sodium–glucose cotransporter-2 inhibitors, glucagon-like peptide 1 receptor agonists, and, more recently, non-steroidal mineralocorticoid receptor antagonists. |
Digital Features
This article is published with digital features, including a summary slide to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.14208032.
Introduction
Patients with diabetes mellitus are at increased risk for developing chronic kidney disease (CKD) [1, 2]. In the USA, diabetic nephropathy is the leading cause of kidney failure [3]. The presence of concomitant CKD in patients with type 2 diabetes (T2D) is associated with significantly increased risks of cardiovascular disease (CVD), including myocardial infarction, ischemic stroke/transient ischemic attacks, and all-cause mortality, when compared with the risk in patients with T2D without CKD [4, 5]. Therapies exist that can slow the progression of CKD in persons with diabetes; therefore, early detection and intervention for CKD is important.
In this review, we assess the burden of CKD in US patients with T2D and describe how clinicians can use early screening and diagnosis, together with guideline-recommended management strategies provided by the American Diabetes Association (ADA), to optimize patient outcomes. This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Burden of CKD
Current estimates suggest that approximately 463 million people globally have diabetes mellitus, and this number is projected to rise to an estimated 700 million by 2045 [6]. In 2018, approximately 10.5% of the US population had diabetes [7]. Up to 40% of patients with diabetes also develop CKD, which is associated with significant morbidity, deficits in quality of life, and increased healthcare burden [8–12]. Progression of CKD can lead to kidney failure (end-stage kidney disease [ESKD]), with patients requiring either dialysis or a kidney transplant for survival. In 2015, 661,000 Americans had ESKD [10]; approximately 71% were receiving hemodialysis or peritoneal dialysis, and 29% were living with a functioning kidney transplant. Of the individuals with ESKD, approximately 44–45% had diabetes listed as the primary cause of ESKD [11, 12]. Per-person costs for patients aged > 65 years with CKD and diabetes are 51% higher than for patients with diabetes who do not have CKD [2]. Furthermore, health-related quality of life decreases as CKD progresses [9].
Screening Enables Early Diagnosis OF CKD
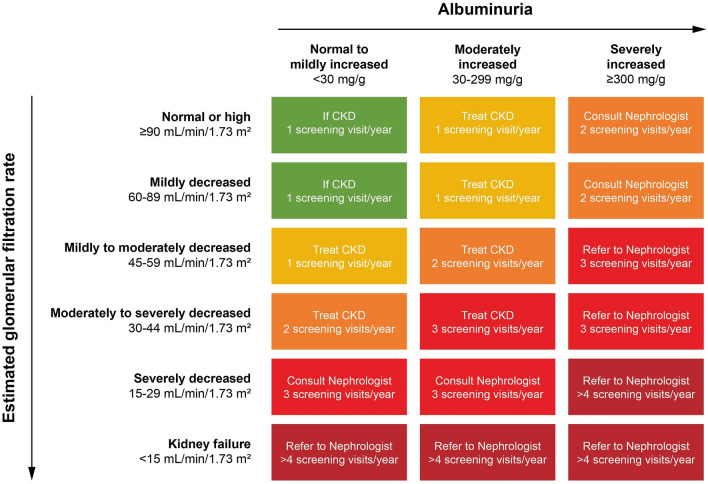
Chronic kidney disease is asymptomatic in its early stages and is diagnosed via estimation of the glomerular filtration rate (eGFR), which assesses kidney function, and detection of albuminuria, a marker for kidney damage [13]. The ADA 2020 Standards of Care recommend that patients with newly diagnosed T2D should be screened for CKD with urinary albumin (e.g., spot urine albumin-to-creatinine ratio [UACR]) testing and eGFR [14]. They should then be retested annually thereafter, with the frequency of retesting driven by the level of kidney damage and function (Fig. 1) [14]. For patients with a UACR > 30 mg/g albumin/creatinine, and/or an eGFR < 60 mL/min/1.73 m2, monitoring should be performed twice annually to guide therapy [14]. Of note, in 2013 the American College of Physicians made the recommendation that patients with T2D and CKD (stage 1–3) receiving an angiotensin-converting enzyme inhibitor (ACEi) or angiotensin II receptor blocker (ARB) did not need to undergo annual testing for proteinuria [15]. This recommendation has been retired, and therefore it is important that primary care providers (PCPs) take note of this change and adjust their practice accordingly. Based on the ADA guidelines, patients with stage 1–3 CKD should be screened between one and three times annually based on their level of albuminuria (Fig. 1) [14].
Fig. 1.
Risk of CKD progression, frequency of visits, and referral to a nephrologist according to glomerular filtration rate (GFR) and albuminuria. The GFR and albuminuria grid depicts the risk of progression, morbidity, and mortality by color, from best (green) through yellow, orange, and red to worst (dark red). CKD Chronic kidney disease.
Modified from Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group [61], copyright 2013, with permission from Elsevier
The preferred method for assessment of albuminuria is the UACR, measured in a random spot urine collection [14]. This method of assessment is easy to perform compared with a 24-h urine collection, which is inconvenient for patients and subject to incomplete collection [16]. Concurrent measurement of urinary creatinine ensures that variations in albumin concentration due to hydration levels do not confound the result. A positive diagnosis of increased urinary albumin excretion, defined as ≥ 30 mg/g albumin/creatinine, should only be made when two of three samples collected over a 3- to 6-month period exceed that threshold [14].
It is important to assess both the UACR and the eGFR because albuminuria can predict CKD risk earlier than a decrease in GFR; for example, the UACR can be moderately increased when the eGFR is still normal (Fig. 1) [14, 17]. The eGFR can also be high (eGFR > 90 mL/min/1.73 m2) due to hyperfiltration, which is common early in diabetes [8] and may mask the degree of kidney damage if eGFR is measured in isolation.
Although the importance of albuminuria screening in patients with T2D has been understood for many years, rates of testing remain suboptimal. The US Renal Data System has reported testing trends from 2016 in two populations of patients with T2D and without CKD: a Medicare 5% sample aged ≥ 65 years and an Optum Clinformatics sample aged 22–64 years [1]. During that year, less than half of these patients in both the Medicare (42%) and Optum Clinformatics (49%) populations had undergone any urine albumin testing. Furthermore, different methods of assessing albuminuria were used, such as measurement of urinary protein, which has lower sensitivity for predicting kidney events compared with the UACR [18].
Management OF CKD In Patients With T2D
Glycemic Control
Intensive glycemic control in patients with T2D results in significant reductions in the development of microvascular complications, with the evidence of prevention of incident CKD being stronger than that for decreasing progression of established CKD [19, 20]. Therefore, the ADA guidelines provide treatment goals for glucose control (target levels of glycated hemoglobin [A1C], described in Table 1), and recommend that A1C is assessed at least twice per year in patients meeting these goals, and quarterly in patients who have switched therapy or who are not meeting treatment goals [14]. This approach is endorsed in the Kidney Disease Improving Global Outcomes (KDIGO®) 2020 Clinical Practice Guidelines [17]. It is important to note that in patients with advanced CKD, A1C levels may be falsely low, and therefore patients with T2D and CKD should be encouraged to self-monitor their blood glucose levels more frequently [21]. This is due to patients commonly having anemia of chronic disease, and the decreased survival time of erythrocytes in these patients leads to falsely low A1C results [22]. The incidence of adverse effects associated with intensive glycemic control is increased in patients with concomitant CKD, so careful individualization of glycemic goals is recommended (Table 1) [14].
Table 1.
American Diabetes Association treatment goals and guidance [14]
| Treatment targets | ADA Guidance |
|---|---|
| A1C |
• A1C goal for many nonpregnant adults is < 7% (53 mmol/mol) • More stringent A1C goals (such as < 6.5% [48 mmol/mol]) for some patients, but care should be taken to avoid significant hypoglycemia or polypharmacy (e.g., in patients with a short duration of diabetes, T2D treated with lifestyle or metformin only, long life expectancy, or no significant CVD) • Less stringent A1C goals (e.g., < 8% [64 mmol/mol]) may be appropriate for some patients (e.g., those with a history of severe hypoglycemia, limited life expectancy, advanced micro- or macrovascular complications, extensive comorbid conditions, or long-standing diabetes in whom the goal is difficult to achieve) • Reassess glycemic targets over time |
| BP |
• BP control should be optimized to reduce the risk or slow the progression of CKD • BP targets should be individualized through a shared decision-making process • Patients with hypertension should, at a minimum, be treated to BP targets of < 140/90 mmHg to reduce CVD mortality and slow CKD progression • Lower BP targets (e.g., < 130/80 mmHg) should be considered for some patients based on individual anticipated benefits and risks (e.g., those with ≥ 300 mg/day albuminuria) |
A1C Glycated hemoglobin, ADA American Diabetes Association, BP blood pressure, CKD chronic kidney disease, CVD cardiovascular disease, T2D type 2 diabetes
Dietary Intervention
Dietary interventions can help to improve blood pressure (BP) and glucose control, as well as slow the progression of CKD [14]. Recommended nutritional interventions include protein intake of approximately 0.8 g/kg/day and sodium intake < 2300 mg/day (ADA) or < 2000 mg/day (KDIGO) [14, 17] Dietary protein intake less than the recommended daily allowance of 0.8 g/kg/day does not improve outcomes. For patients on dialysis, higher levels of dietary protein intake should be considered, as malnutrition is a problem in some dialysis patients [14, 17]. It is helpful to have the input of a registered dietician or a certified diabetes educator to help operationalize these goals for patients.
BP Control
Patients with CKD are at increased risk of developing both kidney failure and CVD [1]. The prevalence of CVD in US persons aged ≥ 66 years is twofold higher in those with CKD (69.6%) than in those without (34.7%) [10]. CVD is a leading cause of death in patients with CKD regardless of disease stage, accounting for over a quarter of deaths in patients with early (stage 1 or 2) CKD and more than half of deaths in patients with ESKD [23].
Patients with T2D and hypertension should receive appropriate treatment to reduce their risk of atherosclerotic CVD events, heart failure, and microvascular complications [14]. BP levels < 140/90 mmHg are recommended by the ADA to reduce CVD mortality and slow CKD progression among all people with diabetes. Patients with CKD are at increased risk of CKD progression (particularly those with albuminuria) and CVD and, therefore, may be suitable in some cases for lower BP targets [14]. The ADA states that lower BP targets (< 130/80 mmHg) should be considered for patients based on individual anticipated benefits and risks. It should be noted that there are varying recommendations for BP control between different governing bodies. The 2020 International Society of Hypertension Global Hypertension Practice Guidelines recommends that for patients with CKD, “Blood pressure should be lowered if ≥ 140/90 mmHg and treated to a target < 130/80 mmHg (< 140/80 in elderly patients)” [24]. Patients with hypertension and albuminuria (UACR ≥ 30 mg/g albumin/creatinine) should initially receive treatment with the maximum tolerated dose of either an ACEi or an ARB, as agents targeting the renin–angiotensin system have demonstrated kidney protective effects in clinical trials (Table 2) [14, 17, 25–27]. If a patient cannot tolerate treatment with an ACEi, treatment should be switched to an ARB, or vice-versa [14]. ACEis and ARBs should not be used concomitantly due to an increased risk of adverse events, including hyperkalemia and acute kidney injury [14].
Table 2.
ADA-recommended pharmacologic interventions and treatment goals for the management of CKD in T2D, together with relevant renal and cardiovascular outcomes findings from clinical studies
| Intervention | ADA comments on intervention [14] | Example agent | Positive effects on renal outcomes reported in clinical studies | Positive effects on CV outcomes reported in clinical studies |
|---|---|---|---|---|
| ACEi or ARB |
• Strongly recommended in patients with hypertension and UACR ≥ 300 mg/g albumin/creatinine and/or eGFR < 60 ml/min/1.73 m2 • Recommended in patients with hypertension and UACR 30–299 mg/g albumin/creatinine |
Losartan (ARB) |
RENAAL study [25] Median follow-up: 3.4 years Patients with T2D and renal impairment (urinary albumin ≥ 300 g/L) |
|
|
• Reduced the risk of doubling of SCr, ESKD, or death by 16% vs. placebo • Reduced UACR by 35% vs. placebo (p <0.001) • Reduced decline in eGFR by 15.2% vs. placebo |
• Reduced the risk of first hospitalization with heart failure by 32% vs. placebo | |||
| Irbesartan (ARB) |
IDNT [27] Median follow-up: 2.6 years Patients with T2D and renal impairment (urinary protein ≥ 900 mg/24 h) |
|||
|
• 20% reduced the risk doubling of SCr, ESKD, or death vs. placebo • Reduced rate of increase in SCr by 24% vs. placebo • Reduced proteinuria by 33% vs. 10% in the placebo group |
• Rate of CHF requiring hospitalization was 23% lower with irbesartan vs. placebo | |||
| SGLT-2i | • Consider in patients with an eGFR ≥ 30 mL/min/1.73 m2 and UACR > 30 mg/g albumin/creatinine, particularly in those with UACR > 300 mg/g albumin/creatinine, to reduce risk of CKD progression, CVD, or both | Canagliflozin |
CREDENCE trial [34] (+ ACEi/ARB) Median follow-up: 2.6 years Patients with T2D and renal impairment (eGFR 30–90 mL/min/1.73 m2 and albumin:creatinine > 300–5000) |
|
|
• Reduced the relative risk of ESKD, doubling of SCr, or death from renal or CV disease vs. placebo • Geometric mean UACR was 31% lower for patients receiving canagliflozin vs. placebo during follow-up • Decline in eGFR was slower for patients receiving canagliflozin vs. placebo after the first 3 weeks |
• Reduced the relative risk of CV death or hospitalization for heart failure vs. placebo • Reduced the relative risk of CV death, MI, or stroke vs. placebo |
|||
|
CANVAS program [46] Median follow-up: 2.2 years Patients with T2D and high CV risk (eGFR > 30 mL/min/1.73 m2) | ||||
|
• Reduced the relative risk of sustained 40% reduction in eGFR, need for renal replacement therapy, or death from renal causes by 40% vs. placebo (not significant) • Reduced the relative risk of progression of albuminuria by 27% vs. placebo (not significant) |
• Reduced the relative risk of CV death, MI, or stroke by 14% vs. placebo • Reduced the relative risk of hospitalization for heart failure by 33% vs. placebo |
|||
| Empagliflozin |
EMPA-REG OUTCOME trial [47, 48] Median follow-up: 3.1 years Patients with T2D with renal impairment (eGFR > 90 mL/min/1.73 m2) and established CV disease |
|||
|
• Reduced the relative risk of very high albuminuria, doubling of SCr with eGFR ≤ 45 ml/min/1.73 m2, need for renal replacement therapy, or death from renal causes by 39% vs. placebo • Reduced the relative risk of very high albuminuria by 38% vs. placebo • Reduced the relative risk of doubling of SCr with eGFR ≤ 45 mL/min/1.73 m2 by 44% vs. placebo • Reduced the relative risk of need for renal replacement therapy by 55% vs. placebo |
• Reduced the relative risk of CV death, MI, or stroke by 14% vs. placebo • Reduced the relative risk of CV death by 38% vs. placebo • Reduced the relative risk of hospitalization for heart failure by 35% vs. placebo |
|||
| Dapagliflozin |
DECLARE-TIMI 58 trial [49] Median follow-up: 4.2 years Patients with T2D and renal impairment (creatinine clearance > 60 mL/min) |
|||
|
• Reduced the relative risk of ≥ 40% reduction in eGFR, ESKD, or death from renal or CV causes by 24% vs. placebo • Reduced the relative risk of ≥ 40% reduction in eGFR, ESKD, or death from renal causes by 47% vs. placebo |
• Reduced the relative risk of CV death or hospitalization for heart failure by 17% vs. placebo • Reduced the relative risk of hospitalization for heart failure by 27% vs. placebo |
|||
|
DAPA-CKD Trial Median follow-up: 2.4 years Patients with T2D and renal impairment (eGFR ≥ 25 to 75 mL/min/1.73 m2 and albumin:creatinine 200–5000 | ||||
|
• Reduced the risk of sustained decline in eGFR ≥ 50%, ESKD, or death from CV or kidney causes by 37% vs. placebo • Reduced the risk of sustained decline in eGFR ≥ 50%, ESKD, or death from kidney causes by 42% vs. placebo |
• Reduced the relative risk of CV death or hospitalization for heart failure by 28% vs. placebo | |||
| Ertugliflozin |
VERTIS trial [50] Study duration: 1 year Patients with T2D and renal impairment (eGFR ≥ 30 to < 60 mL/min/1.73 m2) Primary endpoint was glycemic control (change from baseline in HbA1c at Week 26) |
|||
| • Modest reductions in eGFR at Week 6, which were maintained until Week 52 | • No CV outcomes assessed | |||
| GLP-1 RA | • May reduce risk of progression of albuminuria, CVD, or both in patients with CKD and increased CV risk | Liraglutide |
LEADER trial [51, 52] (+ ACEi/ARB) Median follow-up: 3.8 years Patients with T2D and ≥ 1 CV comorbidity |
|
|
• Reduced the risk of new onset persistent very high albuminuria, persistent doubling of SCr, ESKD, or renal death vs. placebo • Decline in eGFR at 36 months was slower with liraglutide (2% less decrease) vs. placebo • Increase in UACR at 36 months was 17% lower for liraglutide vs. placebo |
• Reduced the risk of first occurrence of nonfatal MI, nonfatal stroke, or death from CV causes vs. placebo | |||
| Semaglutide (injectable) |
SUSTAIN-6 trial [53] Median follow-up: 2.1 years Patients with T2D and established CVD or CKD stage > 3 |
|||
| • Reduced the relative risk of very high albuminuria, doubling of SCr with eGFR < 45 mL/min/1.73m2, or need for renal replacement therapy by 36% vs. placebo |
• Reduced the relative risk of CV death, MI, or stroke by 26% vs. placebo • Reduced the relative risk of CV death, MI, stroke, revascularization, or hospitalization for heart failure or unstable angina by 26% vs. placebo |
|||
| Semaglutide (oral) |
PIONEER-6 trial [54] Median follow-up: 1.3 years Patients with T2D and established CV disease or CKD |
|||
| • Not reported |
• Did not reduce the relative risk of CV death, MI, or stroke vs. placebo • Reduced the relative risk of CV death by 51% vs. placebo |
|||
| Dulaglutide |
Median follow-up: 5.4 years Patients with T2D and CV event or risk factors |
|||
|
• Reduced relative risk of new-onset UACR > 33.9 mg/mmol, sustained ≥ 30% reduction in eGFR, or need for renal replacement therapy by 15% vs. placebo • Reduced relative risk of new-onset UACR > 33.9 mg/mmol by 23% vs. placebo |
• Reduced the relative risk of CV death, MI, or stroke by 12% vs. placebo | |||
|
AWARD-7 trial [57] Median follow-up: 1-year treatment Patients with T2D and moderate-to-severe chronic kidney disease (stage 3–4) | ||||
|
• Reduced eGFR decline vs. insulin glargine • Greater reduction in UACR vs. insulin glargine in patients with very high baseline albuminuria |
• Not reported | |||
| Exenatide |
EXSCEL trial [58] Median follow-up: > 3 years Patients with T2D with or without CVD |
|||
|
• Reduced relative risk of 40% reduction in eGFR, need for renal replacement therapy, or death from renal causes by 12% vs. placebo • Did not reduce relative risk of new very high albuminuria vs. placebo |
• Did not reduce the relative risk of CV death, MI, or stroke vs. placebo • Reduced the relative risk of death from any cause by 14% vs. placebo • CV outcomes did not differ from placebo in patients with eGFR <60 mL/min/1.73 m2 |
|||
| Lixisenatide |
ELIXA trial (patients with T2D and ACS) [59, 60] Median follow-up: 2.1 years Patients with T2D with recent acute coronary event |
|||
|
• Greater reduction in UACR vs. placebo (34% vs. 24%) • Greater reduction in UACR vs. placebo in patients with very high albuminuria at baseline (42% vs. 2%) |
• Did not reduce the relative risk of CV death, MI, or stroke vs. placebo | |||
ACEi angiotensin-converting enzyme inhibitor, ACS acute coronary syndrome, ARB angiotensin II receptor blocker, CHF congestive heart failure, CREDENCE Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation; CV, cardiovascular; eGFR estimated glomerular filtration rate, ESKD end-stage kidney disease, GLP-1 RA glucagon-like peptide-1 receptor agonist, IDNT Irbesartan Diabetic Nephropathy Trial, LEADER liraglutide effect and action in diabetes: evaluation of cardiovascular outcome results, MI myocardial infarction, RENAAL Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan, SCr serum creatinine concentration, SGLT-2i sodium–glucose cotransporter-2 inhibitor, UACR urine albumin-to-creatinine ratio
Despite the guideline recommendations advocating the use of ACEis/ARBs in patients with T2D and CKD, reported rates of the use of these agents remain low. Although specific data regarding the use of ACEis/ARBs in patients with T2D and CKD are lacking, rates of use in patients with CKD and hypertension are low (only 36% of patients received an ACEi/ARB between 2010 and 2014) and have decreased since 2006 [28]. There is evidence to suggest that patients with CKD who are comanaged by a PCP and a nephrologist have higher ACEi/ARB prescription rates [29] and that patients with more severe renal impairment are more likely to receive an ACEi/ARB when they have received care from both a PCP and a nephrologist compared with a PCP alone [30]. Notably, PCPs may have reservations about prescribing ACEis/ARBs given the potential risk for developing hyperkalemia. To mitigate the risk of hyperkalemia, the Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines recommend starting ACEi/ARBs at a lower dose and titrating upwards in patients with an eGFR > 45 mL/min/1.73 m2 [31]. Importantly, eGFR and serum potassium should be monitored within a few weeks of a patient starting an ACEi/ARB [31]. If hyperkalemia develops, consideration for a nephrology referral is appropriate. Additional strategies that can be considered include identification and restriction of dietary potassium, treatment of metabolic acidosis where required, initiation/up titration of thiazide or loop diuretic(s) to increase potassium excretion, and treatment with a potassium-binding exchange resin [31].
Choice of Glucose-Lowering Agent
Sodium-glucose cotransporter-2 inhibitors (SGLT-2is) and glucagon-like peptide 1 receptor agonists (GLP-1 RAs) have demonstrated beneficial effects on kidney and cardiovascular outcomes. GLP-1 RAs have been shown to decrease the progression of microalbuminuria, while SGLT-2is have been shown to both decrease progression of microalbuminuria and slow the decline in eGFR [32, 33]. Evidence supporting the kidney protective benefits of SGLT-2is comes from the Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) and Dapagliflozin and Prevention of Adverse Outcomes in CKD (DAPA-CKD) studies [34, 35], which showed that canagliflozin and dapagliflozin on a background of ACEi/ARBs reduced the incidence of the composite primary (Table 2), as well as post hoc analyses of placebo-controlled cardiovascular outcomes trials of empagliflozin, dapagliflozin, and ertugliflozin (Table 2).
Both SGLT-2is and GLP-1 RAs have been shown to reduce major cardiac adverse events. The SGLT-2is dapagliflozin, canagliflozin, and empagliflozin reduce the risk of hospitalization for heart failure in patients with T2D and CKD, whereas ertugliflozin does not (Table 2) [33]. The GLP-1 RAs liraglutide, semaglutide (injection), and dulaglutide reduce the risk of cardiovascular death, myocardial infarction, and/or stroke relative to placebo, whereas semaglutide (oral), exenatide, and lixisenatide do not (Table 2).
The ADA recommends that independent of baseline A1C or individualized A1C target, if a patient has heart failure or CKD, then an SGLT-2i with evidence of reducing heart failure and/or CKD progression should be a part of a patient’s glucose-lowering regimen as long as the eGFR is adequate for the use of an SGLT-2i. If an SGLT-2i is not tolerated or if the eGFR is less than can be used with an SGLT-2i, then a GLP-1 RA should be used [14]. SGLT-2is and GLP-1 RAs can also be used for the treatment of patients who are unable to use or tolerate metformin. In the KDIGO guidelines, an SGLT2i is recommended for the treatment of patients with T2D, CKD, and an eGFR ≥ 30 mL/min/1.73 m2, and if metformin and SGLT2is are not effective or unusable, a long-acting GP-1 RA is recommended [17].
Reducing Cardiorenal Outcomes: Agents in Development
Tight glycemic and BP control, along with the use of recommended agents, can slow CKD onset and progression. However, progression of CKD still does occur for many patients. Agents that may provide additive beneficial effects on slowing progression of CKD and decreasing the incidence of CVD are currently being evaluated. These include the selective endothelin-A receptor antagonist atrasentan [36] and the mineralocorticoid receptor antagonist (MRA) finerenone [37, 38]
Endothelin receptor antagonists reduce BP and proteinuria in patients with T2D and CKD but have been associated with fluid retention, which can be life-threatening [39]. The recent Study Of diabetic Nephropathy with AtRasentan (SONAR) study was carried out to evaluate the effects of atrasentan in patients with T2D and CKD [36]. Enrolled patients who responded to 6 weeks of atrasentan 0.75 mg daily with ≥ 30% decrease in UACR without fluid retention were randomized to continued atrasentan or placebo. After a median follow-up of 2.2 years, the risk of increased kidney disease (composite kidney endpoint of ESKD or doubling of serum creatinine for ≥ 30 days) was reduced by 35%. The risk for cardiorenal events (composite endpoint comprising: doubling serum creatinine, ESKD, cardiovascular death, non-fatal myocardial infarction, or non-fatal stroke) was also reduced, although atrasentan had no effect on a composite CVD measure (cardiovascular death, non-fatal myocardial infarction, or non-fatal stroke) [36]. However, the rates of anemia and hypervolemia were higher in the atrasentan group than in the placebo group. The rate of hospitalization for heart failure was numerically, but not statistically significantly, higher in the atrasentan group [36].
Non-steroidal MRAs have also been shown to reduce albuminuria in clinical trials in patients with CKD in T2D. Following encouraging results from a phase II trial, ARTS-DN, finerenone, a novel non-steroidal MRA is now being evaluated for the reduction of kidney and cardiovascular outcomes in patients with T2D and CKD in a large clinical trial program [37, 38]. The results of the FIDELIO-DKD study (n = 5734) have been recently reported, while the FIGARO-DKD study (n = 7437) is ongoing and study completion is expected in July 2021 [38].
FIDELIO-DKD recruited patients aged ≥ 18 years with T2D and CKD with eGFR (≥ 25 to < 60 mL/min/1.73 m2), UACR (30 to < 300 mg/g), and a history of diabetic retinopathy; or eGFR (≥ 25 to < 75 mL/min/1.73 m2), UACR (≥ 300 mg/g), and a serum potassium ≤ 4.8 mmol/L. Patents with heart failure with reduced ejection fraction were excluded from study, ACEis/ARBs were maximized in a 16-week run-in, and doses were stable for ≥ 4 weeks prior to randomization.
The primary composite endpoint was time to first occurrence of kidney failure (ESKD or eGFR < 15 mL/min/1.73 m2), a sustained decrease of eGFR ≥ 40% from baseline over at least 4 weeks, or renal death [37]. The incidence of the primary composite outcome was significantly lower in the finerenone group than in the placebo group (504 patients [17.8%] vs. 600 patients [21.1%]; hazard ratio (HR) 0.82; 95% confidence interval (CI) 0.73–0.93; p = 0.001) with a number needed to treat (NNT) of 29 at 3 years [40]. Furthermore, the incidence of the secondary outcome (death from cardiovascular causes, non-fatal myocardial infarction, non-fatal stroke, or hospitalization for heart failure) was also lower with finerenone treatment (367 patients [13.0%] vs. 420 patients [14.8%]; HR 0.86; 95% CI 0.75–0.99; p = 0.03) with an NNT of 42 [40]. The reduction in UACR from baseline to month 4 was greater with finerenone compared with placebo (31% reduction), and the incidence of the secondary composite kidney outcome (kidney failure, sustained decrease of ≥ 57% in the eGFR from baseline [consistent with a doubling of serum creatinine], or death from kidney causes) occurred in fewer patients in the finerenone group (252 patients [8.9%] vs. 326 patients [11.5%]; HR 0.76; 95% CI 0.65–0.90) [40]. Finerenone was generally well tolerated with a similar incidence of adverse events observed between the finerenone and placebo groups. Although finerenone was associated with a higher overall risk of hyperkalemia compared with placebo, discontinuation due to hyperkalemia was low in patients receiving finerenone compared with those receiving placebo (2.3 vs. 0.9%). Notably, these rates of discontinuations were lower than in trials of dual RAS blockade (combination of direct renin inhibitor + ACEi or ARB 4.8%; combination therapy with ACEi + ARB 9.2%) [41, 42].
Studies of the steroidal MRAs spironolactone or eplerenone in combination with an ACEi/ARB have demonstrated reductions in BP and in albuminuria in patients with diabetes and kidney disease [43]. However, this combination is associated with an increased incidence of hyperkalemia, resulting in high rates of treatment discontinuation [43, 44].
Nephrologist Referral
Appropriate referral of patients to a nephrologist for specialist assessment is encouraged as it is associated with improved quality of care, delayed dialysis, and reduced costs [14, 45]. Comanagement of patients with T2D and CKD by their PCP and a nephrologist have also been associated with significantly increased rates of eGFR testing and prescription of an ACEi/ARB [29]. Guidance for referral (based on GFR and albuminuria) is shown in Fig. 1. Referrals should be considered if the cause of CKD is unclear, or if there are issues with disease management, such as for patients with anemia, secondary hyperparathyroidism, metabolic bone disease, resistant hypertension, or electrolyte disturbances. Patients should also be referred if they have stage 4 CKD (eGFR < 30 mL/min/1.73 m2) to plan for kidney replacement therapy [14].
Conclusions
Despite guideline recommendations to screen for CKD in patients with T2D, many patients remain underdiagnosed and undertreated. Evidence from recently completed clinical trials indicate that progressive kidney disease and cardiovascular outcomes can be slowed or prevented by treatment including ACEis/ARBs, SGLT2-is, GLP-1 RAs, and non-steroidal MRAs. We now have the opportunity to target multiple pathways in the treatment of CKD in patients with T2D, which lends itself to optimizing therapy based on the individual patients’ clinical needs. New treatments are currently under investigation and results from large cardiorenal studies, specifically in patients with CKD and T2D, are anticipated in 2021.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published. Both authors participated in the drafting, critical revision, and approval of the final version of the manuscript.
Medical Writing, Editorial, and Other Assistance
Medical writing assistance was provided by Tania Dickson, PhD, CMPP, of Envision Pharma Group, and was funded by Bayer Corporation. ProScribe’s services complied with international guidelines for Good Publication Practice (GPP3).
Disclosures
Dr. Alyssa Style has no relevant conflicts to disclose. Dr. Neil Skolnik reports “personal fees” and “other” for activities outside the submitted work from AstraZeneca, Boehringer Ingelheim, GSK, Sanofi, and Sanofi Pasteur. Additionally, Dr. Skolnik reports “non-financial support” for activities outside the submitted work from Eli Lilly and Company, Intarcia Therapeutics, Merck and Company, Mylan, and Teva Pharmaceuticals.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1.United States Renal Data System. 2018 USRDS annual data report. Executive summary. Bethesda: National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health; 2018. https://www.usrds.org/annual-data-report/previous-adrs/. Accessed 18 Jan 2021.
- 2.United States Renal Data System. 2019 USRDS annual data report. Executive summary. Bethesda: National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health; 2019. https://www.usrds.org/annual-data-report/previous-adrs/. Accessed 18th January 2021
- 3.Umanath K, Lewis JB. Update on diabetic nephropathy: core curriculum 2018. Am J Kidney Dis. 2018;71(6):884–895. doi: 10.1053/j.ajkd.2017.10.026. [DOI] [PubMed] [Google Scholar]
- 4.Afkarian M, Sachs MC, Kestenbaum B, et al. Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol. 2013;24(2):302–308. doi: 10.1681/ASN.2012070718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cea Soriano L, Johansson S, Stefansson B, Rodriguez LA. Cardiovascular events and all-cause mortality in a cohort of 57,946 patients with type 2 diabetes: associations with renal function and cardiovascular risk factors. Cardiovasc Diabetol. 2015;14:38. doi: 10.1186/s12933-015-0204-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.International Diabetes Federation . IDF diabetes atlas. 9. Brussels, Belgium: International Diabetes Federation; 2019. [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. National diabetes statistics report, 2020. Atlanta: Centers for Disease Control and Prevention, U.S. Dept of Health and Human Services; 2020.
- 8.Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol. 2017;12(12):2032–2045. doi: 10.2215/CJN.11491116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zimbudzi E, Lo C, Ranasinha S, Gallagher M, Fulcher G, Kerr PG, et al. Predictors of health-related quality of life in patients with co-morbid diabetes and chronic kidney disease. PLoS ONE. 2016;11(12):e0168491. doi: 10.1371/journal.pone.0168491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Institute of Diabetes and Digestive and Kidney Diseases. Kidney disease statistics for the United States 2020. https://www.niddk.nih.gov/health-information/health-statistics/kidney-disease. Accessed Jan 2021.
- 11.United States Renal Data System. 2017 USRDS annual data report. Epidemiology of kidney disease in the United States. Bethesda: US Department of Health and Human Services, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2016. https://www.usrds.org/adr.aspx. Accessed Jan 2021.
- 12.Burrows NR, Hora I, Geiss LS, Gregg EW, Albright A. Incidence of end-stage renal disease attributed to diabetes among persons with diagnosed diabetes—United States and Puerto Rico, 2000–2014. MMWR Morb Mortal Wkly Rep. 2017;66(43):1165–1170. doi: 10.15585/mmwr.mm6643a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levey AS, Becker C, Inker LA. Glomerular filtration rate and albuminuria for detection and staging of acute and chronic kidney disease in adults: a systematic review. JAMA. 2015;313(8):837–846. doi: 10.1001/jama.2015.0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.American Diabetes Association Standards of medical care in diabetes. Diabetes Care. 2020;43(Suppl 1):S1–S204. doi: 10.2337/dc20-Sint. [DOI] [PubMed] [Google Scholar]
- 15.Qaseem A, Hopkins RH Jr, Sweet DE, Starkey M, Shekelle P, Clinical Guidelines Committee of the American College of Physicians. Screening, monitoring, and treatment of stage 1 to 3 chronic kidney disease: A clinical practice guideline from the American College of Physicians. Ann Intern Med. 2013;159(12):835–47. [DOI] [PubMed]
- 16.Gaitonde DY, Cook DL, Rivera IM. Chronic kidney disease: detection and evaluation. Am Fam Physician. 2017;96(12):776–783. [PubMed] [Google Scholar]
- 17.Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group. KDIGO 2020 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int. 2020;98(4S):S1–S115. [DOI] [PubMed]
- 18.Lambers Heerspink HJ, Gansevoort RT, et al. Comparison of different measures of urinary protein excretion for prediction of renal events. J Am Soc Nephrol. 2010;21(8):1355–1360. doi: 10.1681/ASN.2010010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 20.Ohkubo Y, Kishikawa H, Araki E, et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract. 1995;28(2):103–117. doi: 10.1016/0168-8227(95)01064-k. [DOI] [PubMed] [Google Scholar]
- 21.Bloomgarden Z, Handelsman Y. How does CKD affect HbA1c? J Diabetes. 2018;10(4):270. doi: 10.1111/1753-0407.12624. [DOI] [PubMed] [Google Scholar]
- 22.Radin MS. Pitfalls in hemoglobin A1c measurement: when results may be misleading. J Gen Intern Med. 2014;29(2):388–394. doi: 10.1007/s11606-013-2595-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet. 2013;382(9889):339–352. doi: 10.1016/S0140-6736(13)60595-4. [DOI] [PubMed] [Google Scholar]
- 24.Unger T, Borghi C, Charchar F, et al. 2020 International society of hypertension global hypertension practice guidelines. Hypertension. 2020;75(6):1334–1357. doi: 10.1161/HYPERTENSIONAHA.120.15026. [DOI] [PubMed] [Google Scholar]
- 25.Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345(12):861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 26.Emdin CA, Rahimi K, Neal B, Callender T, Perkovic V, Patel A. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015;313(6):603–615. doi: 10.1001/jama.2014.18574. [DOI] [PubMed] [Google Scholar]
- 27.Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345(12):851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 28.Tummalapalli SL, Powe NR, Keyhani S. Trends in quality of care for patients with CKD in the United States. Clin J Am Soc Nephrol. 2019;14(8):1142–1150. doi: 10.2215/CJN.00060119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Samal L, Wright A, Waikar SS, Linder JA. Nephrology co-management versus primary care solo management for early chronic kidney disease: a retrospective cross-sectional analysis. BMC Nephrol. 2015;16:162. doi: 10.1186/s12882-015-0154-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ricardo AC, Roy JA, Tao K, et al. Influence of nephrologist care on management and outcomes in adults with chronic kidney disease. J Gen Intern Med. 2016;31(1):22–29. doi: 10.1007/s11606-015-3452-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vassalotti JA, Centor R, Turner BJ, et al. Practical approach to detection and management of chronic kidney disease for the primary care clinician. Am J Med. 2016;129(2):153–162.e7. [DOI] [PubMed]
- 32.Toyama T, Neuen BL, Jun M, et al. Effect of SGLT2 inhibitors on cardiovascular, renal and safety outcomes in patients with type 2 diabetes mellitus and chronic kidney disease: A systematic review and meta-analysis. Diabetes Obes Metab. 2019;21(5):1237–1250. doi: 10.1111/dom.13648. [DOI] [PubMed] [Google Scholar]
- 33.Zelniker TA, Wiviott SD, Raz I, et al. Comparison of the effects of glucagon-like peptide receptor agonists and sodium-glucose cotransporter 2 inhibitors for prevention of major adverse cardiovascular and renal outcomes in type 2 diabetes mellitus. Circulation. 2019;139(17):2022–2031. doi: 10.1161/CIRCULATIONAHA.118.038868. [DOI] [PubMed] [Google Scholar]
- 34.Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295–2306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 35.Heerspink HJL, Stefansson BV, Correa-Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436–1446. doi: 10.1056/NEJMoa2024816. [DOI] [PubMed] [Google Scholar]
- 36.Heerspink HJL, Parving HH, Andress DL, et al. Atrasentan and renal events in patients with type 2 diabetes and chronic kidney disease (SONAR): a double-blind, randomised, placebo-controlled trial. Lancet. 2019;393(10184):1937–1947. doi: 10.1016/S0140-6736(19)30772-X. [DOI] [PubMed] [Google Scholar]
- 37.Bakris GL, Agarwal R, Anker SD, et al. Design and baseline characteristics of the Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease trial. Am J Nephrol. 2019;50(5):333–344. doi: 10.1159/000503713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruilope LM, Agarwal R, Anker SD, et al. Design and baseline characteristics of the finerenone in reducing cardiovascular mortality and morbidity in diabetic kidney disease trial. Am J Nephrol. 2019;50(5):345–356. doi: 10.1159/000503712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mann JF, Green D, Jamerson K, et al. Avosentan for overt diabetic nephropathy. J Am Soc Nephrol. 2010;21(3):527–535. doi: 10.1681/ASN.2009060593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bakris GL, Agarwal R, Anker SD, et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med. 2020;383(23):2219–2229. doi: 10.1056/NEJMoa2025845. [DOI] [PubMed] [Google Scholar]
- 41.Parving HH, Brenner BM, McMurray JJ, et al. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med. 2012;367(23):2204–2213. doi: 10.1056/NEJMoa1208799. [DOI] [PubMed] [Google Scholar]
- 42.Imai E, Chan JC, Ito S, et al. Effects of olmesartan on renal and cardiovascular outcomes in type 2 diabetes with overt nephropathy: a multicentre, randomised, placebo-controlled study. Diabetologia. 2011;54(12):2978–2986. doi: 10.1007/s00125-011-2325-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mavrakanas TA, Gariani K, Martin PY. Mineralocorticoid receptor blockade in addition to angiotensin converting enzyme inhibitor or angiotensin II receptor blocker treatment: an emerging paradigm in diabetic nephropathy: a systematic review. Eur J Intern Med. 2014;25(2):173–176. doi: 10.1016/j.ejim.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 44.Trevisan M, de Deco P, Xu H, et al. Incidence, predictors and clinical management of hyperkalaemia in new users of mineralocorticoid receptor antagonists. Eur J Heart Fail. 2018;20(8):1217–1226. doi: 10.1002/ejhf.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smart NA, Dieberg G, Ladhani M, Titus T. Early referral to specialist nephrology services for preventing the progression to end-stage kidney disease. Cochrane Database Syst Rev. 2014(6):CD007333. [DOI] [PubMed]
- 46.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin andcardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 47.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 48.Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375(4):323–334. doi: 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]
- 49.Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2018;380(4):347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 50.Grunberger G, Camp S, Johnson J, et al. Ertugliflozin in patients with stage 3 chronic kidney disease and Type 2 diabetes mellitus: The VERTIS renal randomized Study. Diabetes Ther. 2018;9(1):49–66. doi: 10.1007/s13300-017-0337-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mann JFE, Orsted DD, Brown-Frandsen K, et al. Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med. 2017;377(9):839–848. doi: 10.1056/NEJMoa1616011. [DOI] [PubMed] [Google Scholar]
- 52.Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834–1844. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 54.Husain M, Birkenfeld AL, Donsmark M, et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019;381(9):841–851. doi: 10.1056/NEJMoa1901118. [DOI] [PubMed] [Google Scholar]
- 55.Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomised, placebo-controlled trial. Lancet. 2019;394(10193):131–138. doi: 10.1016/S0140-6736(19)31150-X. [DOI] [PubMed] [Google Scholar]
- 56.Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394(10193):121–130. doi: 10.1016/S0140-6736(19)31149-3. [DOI] [PubMed] [Google Scholar]
- 57.Tuttle KR, Lakshmanan MC, Rayner B, et al. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): a multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol. 2018;6(8):605–617. doi: 10.1016/S2213-8587(18)30104-9. [DOI] [PubMed] [Google Scholar]
- 58.Bethel MA, Mentz RJ, Merrill P, et al. Microvascular and cardiovascular outcomes according to renal function in patients treated with once-weekly exenatide: Insights from the EXSCEL trial. Diabetes Care. 2020;43(2):446–452. doi: 10.2337/dc19-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Muskiet MHA, Tonneijck L, Huang Y, et al. Lixisenatide and renal outcomes in patients with type 2 diabetes and acute coronary syndrome: an exploratory analysis of the ELIXA randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2018;6(11):859–869. doi: 10.1016/S2213-8587(18)30268-7. [DOI] [PubMed] [Google Scholar]
- 60.Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373(23):2247–2257. doi: 10.1056/NEJMoa1509225. [DOI] [PubMed] [Google Scholar]
- 61.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.