Abstract
Background and purpose
Research suggests comparable long-term psychosocial outcomes following mild traumatic brain injury (mTBI) and minor stroke, but no direct comparison has been made. This study aimed to directly compare psychosocial outcome over time in persons with mTBI and minor stroke.
Methods
In this multicenter, prospective longitudinal cohort study, community-dwelling persons with mTBI (n = 182) and minor stroke (n = 48) were assessed at 6 weeks, 3, 6 and 12 months post-injury. Outcome measures included anxiety and depression symptoms (Hospital Anxiety and Depression Scale—HADS), cognitive problems in daily life (Checklist for Cognitive and Emotional Consequences of Stroke—CLCE-24) and quality of life (EuroQol-5D-5L—EQ-5D-5L). Multilevel growth curve modeling, controlled for demographic variables, was used to determine outcomes over time between groups. Proportions of persons reporting persistent psychosocial symptoms at 6 months post-injury were compared using Pearson’s Chi-squared tests.
Results
Improvements in outcomes were observed in the first 6 months and effects stabilized to 12 months post-injury in both groups. Minor stroke cases reported significantly higher levels of HADS anxiety and a significantly reduced increase in EQ-5D-5L utility scores than mTBI cases, but differences were small in absolute numbers. No significant differences were observed between groups regarding HADS depression and CLCE-24 cognition scores. Proportions of persons reporting persistent psychosocial symptoms were equal between groups.
Conclusions
Psychosocial outcome is largely comparable following mTBI and minor stroke. Specific attention should be paid to anxiety symptoms and cognitive problems in daily life for which uniform aftercare seems appropriate.
Keywords: Stroke, Brain injuries, traumatic, Longitudinal studies, Social participation, Emotional adjustment, Primary health care
Introduction
Brain injuries are among the most disabling chronic conditions worldwide [1–3], of which the most frequently occurring are of a traumatic and cerebrovascular nature (stroke) [2, 3]. Numbers of both conditions keep increasing worldwide because of the aging population [2, 3]. Overall, the majority of traumatic brain injuries (TBI) (80–90%) [1] and strokes (57%) [4] are of mild severity. Although we acknowledge the differences in pathophysiological processes underlying the forms of brain injury, in the longer term the two groups become more similar regarding deficits and course of treatment. Persons with mild brain injury are usually community residing, having minor motor and communication deficits, being discharged home without rehabilitation or aftercare because of expected full functional recovery [5, 6]. However, it is estimated that 20–25% of these community-residing, ‘walking and talking’ persons with mild brain injury do not recover and report persisting psychosocial symptoms including mostly emotional and cognitive problems [6–9].
Recovery following brain injury stabilizes typically after 6 months which can cause symptoms to persist into the long term if left unattended [6, 10, 11]. Anxiety and depressive symptoms persist in 23 and 16% of mild TBI cases (mTBI), respectively [12]. It is estimated from stroke registries that 29% of community-dwelling persons report long-term anxiety symptoms and 24% report depressive symptoms [13]. Estimates of cognitive problems vary depending on assessment methods, but persist into the long term in around 50% of mTBI cases [14] and approximately 35% of minor stroke cases [9]. Quality of life (QoL) is negatively affected by these persisting symptoms, because of confrontation with difficulties on a daily basis and trouble managing daily activities [15]. Despite these long-term consequences, coordinated attention for psychosocial symptoms in aftercare is currently missing for both (m)TBI and (minor) strokes.
Research suggests that long-term psychosocial outcomes following mTBI and minor stroke are comparable, but a direct and longitudinal comparison between injuries has not been performed to the best of our knowledge. Studies have included either mixed samples of brain injuries [16–18], mixed severities [19] or made a direct comparison cross-sectionally [20]. A direct longitudinal comparison could support the seemingly comparable psychosocial outcome over time between injuries which would justify a transdiagnostic design of uniform aftercare.
This study primarily aimed to enable a direct longitudinal comparison of emotional functioning, cognitive problems in daily life, and QoL in the first year after mTBI and minor stroke. Secondary, we compared proportions of persons experiencing persistent symptoms at 6 months between groups. We hypothesized comparable long-term psychosocial outcome between groups, regarding recovery over time and persistent psychosocial symptomatology at 6 months.
Methods
Design
The current study is part of the COmplaints, HEalthcaRE Needs and cosTs (COHERENT) cohort study which is a multicenter, prospective, longitudinal observational study following persons with mild to moderate acquired brain injury from onset to 1 year post-injury. The aim of the COHERENT study was to obtain a comprehensive overview and investigate the psychosocial well-being, healthcare consumption and healthcare needs of community-dwelling persons over the course of 1 year following brain injury.
Participants
Persons with mild or moderate brain injury were included in the COHERENT cohort study between April 2017 and October 2018 by five participating hospitals in the Southern part of the Netherlands. Persons eligible for participation were adults (> 18 years of age) who suffered from TBI, stroke or other forms of acquired brain injury of mild to moderate severity as diagnosed by a clinician, within the past 6 weeks and were discharged home after visiting the emergency department (ED) or hospital admission. Persons were excluded if they were legally incompetent, insufficiently capable to follow up for 1 year or had insufficient command of the Dutch language to understand and complete the questionnaires, as judged by the clinician. Severity of TBI and stroke were assessed with the Glasgow Coma Scale (GCS) and the National Institutes of Health Stroke Scale (NIHSS) score at admission, respectively. The current study selected persons with mTBI and minor stroke from the COHERENT cohort. Identification of mTBI was performed according to the clinical criteria of the World Health Organization (WHO) Collaborating Center Task Force. These criteria include a GCS of 13–15 and one of the following symptoms: confusion or disorientation, post-traumatic amnesia (< 24 h), loss of consciousness (< 30 min) or other transient neurological symptoms such as focal signs, seizures or intracranial lesions not requiring surgery [21]. Cases not meeting the criteria of mTBI, (GCS score of 15 and no additional symptoms) were not selected for this study and considered head injuries. Minor stroke was defined according to a NIHSS score of 1–4 [22].
Procedure
Eligible persons were informed of the study by the treating physician at the emergency department or neurology outpatient clinic. If interested, they received an information leaflet and their contact details were sent to the researcher who provided more study information by telephone. If willing to participate, written study information, an informed consent form and the first questionnaire were sent to the person’s home. This first assessment took place within the first 6 weeks after brain injury (T0). Follow-up assessments took place at 3 (T1), 6 (T2) and 12 months post-injury (T3). If the signed informed consent form and the first questionnaire were not returned by the 6-week deadline, they could not participate in follow-up assessments.
Measures
Demographic and medical information
Demographic information was collected from T0 questionnaires and included age, sex, educational level, pre-injury working status, relationship status, and living situation. Injury-related information was collected from medical files upon receiving signed informed consent. TBI-related information concerned cause of injury, loss of consciousness (in minutes), post-traumatic amnesia (PTA; in hours), other transient neurological symptoms and substance use at time of injury. TBI severity was assessed with the GCS in which lower scores indicate more severe TBI (range 3–15). Stroke-related information concerned stroke type, hemisphere, location, severity and acute treatment (such as intravenous thrombolysis; yes/no). Stroke severity was assessed with the NIHSS in which higher scores are indicative of more severe stroke symptoms (range 0–30) [22]. Neurological history was collected for both injuries.
Anxiety and depression
The Hospital Anxiety and Depression Scale (HADS) consists of 14 items in which higher scores indicate more severe symptoms, for either subscale anxiety or depression (range 0–21) [23]. A score of ≥ 8 is indicative of clinically relevant symptoms on the given domain [24].
Cognitive problems
The cognition domain of the Checklist for Cognitive and Emotional Consequences of Stroke (CLCE-24) consists of 13 items scored by absence or presence of symptoms. Higher scores indicate more cognitive problems experienced in daily life (range 0–13) [25]. The CLCE-24 was adapted to the study population by replacing ‘stroke’ with ‘brain injury.’
Quality of life
Quality of life was measured by the EuroQol-5D-5L (EQ-5D-5L) which assesses five domains: mobility, self-care, usual activities, pain/discomfort and anxiety/depression. The domains are scored on five levels, from ‘no problems’ to ‘extreme problems’ [26]. Dutch tariffs were used to calculate utility scores which range from − 0.446 (worst health state) to 1 (full health) [27].
Analyses
The mTBI and minor stroke groups were compared on demographic information using independent sample t tests and Pearson Chi-square tests. Injury-related information was described per group.
The course of psychosocial outcomes in the first-year post-injury were modeled by multilevel growth curve analyses with the HADS anxiety and depression, CLCE-24 cognition and EQ-5D-5L utility scores as dependent variables in separate models. All available data could be used with this statistical technique. As a first step, an unconditional means model (without any predictors) was fitted, with random intercepts across persons to account for the fact that repeated measures are correlated within individuals. Second, covariates were added to the model as fixed effects: sex, age, educational level, neurological history, along with the linear function of time as a continuous variable (6 weeks, 3, 6 and 12 months). These were added regardless of improved fit. Third, the quadratic function of time was added if overall fit improved. Fourth, group (type of injury: mTBI/minor stroke) was added as a main effect, along with time × group effects (group × linear time and group × quadratic time) to describe possible distinct courses of complaints over time per injury type. Likelihood ratio tests were used to assess model fit. Random slopes were allowed if the model was significantly better compared to a model with only random intercepts based on likelihood ratio testing. Likewise, covariance structures were specified according to the best fit.
Outcomes at T2 were dichotomized to compare proportions of persons experiencing persistent psychosocial symptoms as recovery after 6 months typically stabilizes. Persistent mood symptoms were defined by individual HADS anxiety and depression score of ≥ 8. The CLCE-24 cognition scores were dichotomized with a cutoff ≥ 2 based on mean of 1.9 (standard deviation = 1.9) in healthy controls [28], and the five most frequently CLCE-24 items scored as present were described. Persistent problems regarding QoL were described by the EQ-5D-5L in case problems were reported on ≥ 2 of the five domains. Pearson Chi-square tests were used to statistically compare the proportions of persons reporting persisting symptoms on the HADS, CLCE-24 and EQ-5D-5L between injury groups.
An alpha of 5% (two-sided) was set for significance testing. All statistical analyses were performed with IBM SPSS Statistics for Macintosh, Version 25.0 (New York).
Results
A total of 316 persons were included in the COHERENT cohort. Seventy cases of head injury (not meeting criteria of mTBI) (22%) were excluded from analyses, as well as seven cases with moderate brain injuries (2%), two cases of other brain injuries (1%) and seven cases who did not complete baseline measurements (2%). A total of 230 cases were included for analyses of whom 182 (79%) suffered from mTBI and 48 (21%) from minor stroke. Twenty-two cases did not complete all follow-up assessments (lost to follow-up: nine at T1, ten at T2 and three at T3). Demographic and injury-related information is displayed in Table 1. Significant differences were observed between mTBI and minor stroke on the variables sex, age at brain injury, hospital admission rates, neurological history and psychological consultation at any point during the study period (p < 0.05).
Table 1.
Demographic and injury-related characteristics of the samples with mild traumatic brain injury and minor stroke
| mTBI (n = 182) | Minor stroke (n = 48) | |||
|---|---|---|---|---|
| n | n (%), mean (SD) or median (IQR) | n | n (%), mean (SD) or median (IQR) | |
| Sex (men) | 182 | 91 (50%) | 48 | 36 (75%)* |
| Age at brain injury (years) | 182 | 56.0 (18.7) | 48 | 66.7 (9.5)* |
| Educational level (high) | 182 | 52 (29%) | 48 | 11 (23%) |
| In relationship (yes) | 181 | 123 (68%) | 48 | 39 (81%) |
| Pre-injury working status (working) | 182 | 82 (45%) | 48 | 15 (31%) |
| Living situation (with others) | 181 | 142 (78%) | 48 | 40 (83%) |
| Hospital admission (yes) | 181 | 44 (24%) | 48 | 42 (88%)* |
| Multidisciplinary outpatient rehabilitation (yes) | 182 | 2 (1%) | 51 | 0 (0%) |
| Neurological history (yes) | 182 | 37 (20%) | 51 | 19 (40%)* |
| Psychological consultationa (yes) | 182 | 20 (11%) | 51 | 15 (31%)* |
| mTBI | ||||
| Cause of injury | 180 | |||
| Fall | 87 (48%) | |||
| Traffic | 70 (39%) | |||
| Violence | 4 (2%) | |||
| Sports | 10 (6%) | |||
| Hit by object | 7 (4%) | |||
| Other | 2 (1%) | |||
| Severity (GCS)a | 182 | 15.0 (15–15) | ||
| Loss of consciousness (yes) | 173 | 109 (63%) | ||
| Duration in minutes | 91 | 4.8 (6.1) | ||
| PTA (yes) | 181 | 108 (59%) | ||
| Duration in hours | 98 | 2.2 (4.4) | ||
| Other transient neurological symptoms (yes) | 182 | 83 (46%) | ||
| CT abnormality (yes) | 181 | 29 (16%) | ||
| Substance use at brain injury (yes) | 182 | 38 (21%) | ||
| Minor stroke | ||||
| Severity (NIHSS)a | 48 | 2.0 (1–3) | ||
| Type of stroke (ischemic) | 48 | 46 (96%) | ||
| Hemisphere | 47 | |||
| Left | 18 (38%) | |||
| Right | 18 (38%) | |||
| Other | 11 (24%) | |||
| Location (territory of middle cerebral artery) | 46 | 33 (69%) | ||
| Intravenous thrombolysis (yes) | 48 | 8 (17%) | ||
Medians (IQR) are displayed for the GCS and NIHSS, being more informative than means (SD).
GCS Glasgow Coma Scale, IQR interquartile range, NIHSS National Institutes of Health Stroke Scale, PTA post-traumatic amnesia, mTBI mild traumatic brain injury
*p < 0.05
aAt any point during the study period
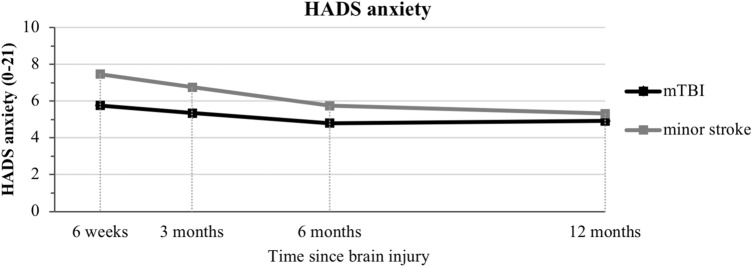
Table 2 shows the results of the multilevel growth models of outcomes over time. Regarding HADS anxiety, no significant interaction (time × group) effect, but a significant main effect was observed, indicating a significant higher HADS anxiety score over time in minor stroke compared to mTBI. Overall, HADS anxiety showed a significant negative linear time effect and a positive quadratic time effect, indicative of a decrease in severity of anxiety symptoms which levels off over time (Fig. 1).
Table 2.
Multilevel growth modeling of outcomes over time and interaction effects with injury type
| Time effects | Group effect | Interaction effects | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Linear | Quadratic | Main (stroke = 1) | Linear × group | Quadratic × group | |||||||||||
| Estimate | SE | 95% CI | Estimate | SE | 95% CI | Estimate | SE | 95% CI | Estimate | SE | 95% CI | Estimate | SE | 95% CI | |
| HADS anxiety | − 0.31 | 0.11 | − 0.52 to − 0.11 | 0.02 | 0.01 | 0.01 to 0.04 | 10.70 | 0.77 | 0.18 to 30.21 | − 0.20 | 0.23 | − 0.64 to 0.25 | 0.01 | 0.02 | − 0.02 to 0.04 |
| HADS depression | − 0.37 | 0.11 | − 59 to − 0.15 | 0.03 | 0.01 | 0.01 to 0.04 | 0.10 | 0.81 | − 10.51 to 10.70 | 0.20 | 0.24 | − 0.28 to 0.68 | − 0.02 | 0.02 | − 0.05 to 0.02 |
| CLCE-24 cognition | − 0.30 | 0.10 | − 0.49 to − 0.10 | 0.02 | 0.01 | 0.01 to 0.03 | 0.12 | 0.69 | − 10.24 to 10.48 | 0.20 | 0.21 | − 0.22 to 0.62 | − 0.01 | 0.02 | − 0.04 to 0.01 |
| EQ5D5L | 0.04 | 0.01 | 0.03 to 0.06 | − 0.003 | 0.0004 | − 0.003 to − 0.002 | 0.06 | 0.04 | − 0.01 to 0.13 | − 0.03 | 0.01 | − 0.05 to − 0.007 | 0.002 | 0.001 | 0.0002 to 0.003 |
Model covariates: age, sex, level of education, relationship status and neurological history. Significant estimates are displayed by 95% CI in bold (zero not included)
CI confidence interval, CLCE-24 Checklist for Cognitive and Emotional Consequences of Stroke, EQ-5D-5L EuroQol 5 domains 5 levels, HADS Hospital Anxiety and Depression Scale, SE standard error
Fig. 1.
Hospital Anxiety and Depression Scale (HADS) anxiety score displayed over time through multilevel growth curve model estimates
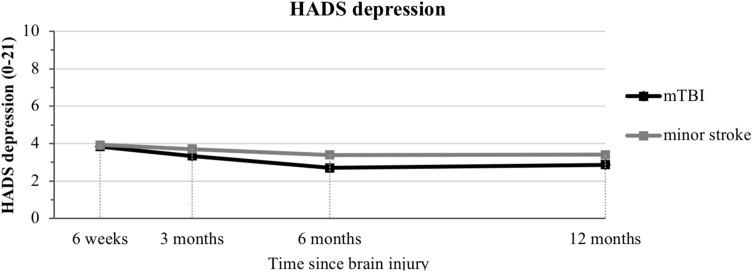
HADS depression showed no significant (time × group) interaction or main effects, indicative of no significant differences between mTBI and minor stroke over time. Overall, HADS depression showed a significant negative linear time effect and a positive quadratic time effect, indicative of a decrease in severity of depressive symptoms which levels off over time (Fig. 2).
Fig. 2.
Hospital Anxiety and Depression Scale (HADS) depression score displayed over time through multilevel growth curve model estimates
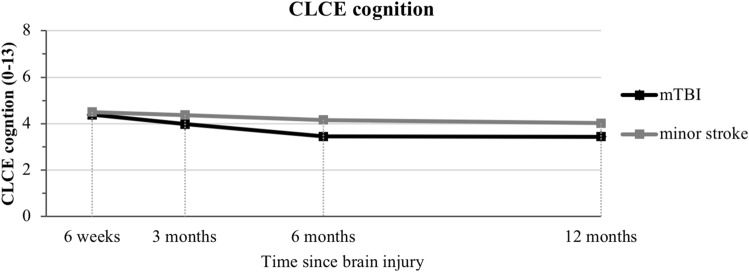
CLCE-24 cognition showed no significant (time × group) interaction or main effects, indicative of no significant differences between mTBI and minor stroke over time. Overall, CLCE-24 cognition score showed a significant negative linear time effect and a positive quadratic time effect, indicative of a decrease in cognitive problems over time, which levels off over time (Fig. 3).
Fig. 3.
Checklist for cognitive and emotional consequences following stroke (CLCE-24) cognition score displayed over time through multilevel growth curve model estimates
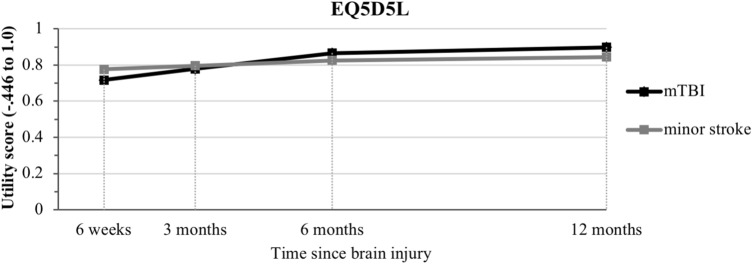
EQ-5D-5L showed a significant negative interaction term of the linear time effect with group, indicative of significantly less improvement over time in minor stroke compared to mTBI. The significant positive interaction term of quadratic time effect with group is indicative of stable levels in minor stroke minor stroke remaining on stable levels, whereas effects in mTBI leveled off over time on the EQ-5D-5L (Fig. 4).
Fig. 4.
EuroQol 5 dimensions 5 levels (EQ-5D-5L) utility score displayed over time through multilevel growth curve model estimates
No significant differences were observed between mTBI and minor stroke in proportions of persons reporting persistent symptoms at T2 on the HADS anxiety (χ2 (1) = 0.15, p = 0.70), HADS depression (χ2 (1) = 0.33, p = 0.57), CLCE-cognition (χ2 (1) = 3.61, p = 0.06), and EQ-5D-5L (χ2 (1) = 2.23, p = 0.14). Persistent symptoms on the HADS anxiety were reported in 17% of mTBI compared to 14% of minor stroke and on HADS depression by 15 and 12%, respectively. Persistent symptoms on the CLCE-24 cognition were reported by 57% of mTBI and 73% of minor stroke. The top five of experienced cognitive problems were equal for mTBI and minor stroke, despite varying percentages: doing two things at once (42% in mTBI vs. 49% in minor stroke), attending to things (43 vs. 46%), keeping up (mental slowness) (46 vs. 59%), remembering new information (43 vs. 56%) and forgetfulness (46 vs 39%). Persistent symptoms on the EQ-5D-5L regarding QoL was experienced by 38% in mTBI and 51% in minor stroke.
Discussion
This study provides a first direct comparison of the long-term psychosocial outcome between mTBI and minor stroke. Psychosocial functioning generally improved up to 6 months, after which stabilization took place up to 12 months post-injury in both groups. On average, the mTBI and minor stroke cases seemed to recover well over time, especially for depressive symptoms and QoL. On average, significantly more severe anxiety symptoms and less improvement in QoL was observed over time in minor stroke compared to mTBI. No differences over time were observed between groups in severity of depressive symptoms and number of cognitive problems. Proportions of persons experiencing persistent psychosocial symptoms were similar between mTBI and minor stroke.
Compared with the general population aged 57–65 years, levels of anxiety symptoms were higher in both mTBI and minor strokes and remained higher up to 12 months post-injury [29]. Also, significant percentages of persons with minor stroke reported persistent anxiety symptoms, which could presumably be due a disproportional fear of stroke recurrence. Fear of stroke recurrence is an illness cognition, most commonly provoking anxiety in minor stroke, and which could lead to maladaptive avoidant behavior and phobic anxiety [30]. Illness cognitions are of interest in the mTBI population as well. Cognitions concerning the impact and duration of symptoms were shown to be highly predictive of persistent psychosocial symptoms following mTBI [31].
The number of cognitive problems in daily life was shown to be above general population levels, which is consistent with previous findings [28]. More than half of the samples experienced persistent cognitive problems in daily life despite the mild severity of the injuries. Moreover, mTBI and stroke experienced problems in similar cognitive domains: attention and memory. Persistent symptoms in daily life can impact QoL, as observed in significant percentages reporting problems regarding the QoL domains at 6 months post-injury, and which continue impacting QoL up to 5 years post-injury [15].
Clinical implications
The observed similarities in progression of psychosocial outcome outweigh the differences, justifying a transdiagnostic design of uniform aftercare for mild brain injury, providing continued attention to long-term psychosocial outcome and persisting symptomatology in both mTBI and minor strokes. It is critical to actively monitor psychosocial functioning because psychosocial symptoms are not easily recognized and known to impact daily functioning and QoL [6, 32, 33]. Current stroke healthcare programs primarily focus on secondary prevention and functional outcome [34] and usually do not focus on long-term psychosocial symptoms. In mTBI, coordinated systems of care are deemed crucial, but deficiencies exist in the continuum of care and are suboptimal in reality [2]. Appropriate timing for providing uniform aftercare is considered to be at 6 months post-injury. At 6 months, functioning typically stabilizes and persons start encountering problems in daily life [6] which potentially persist into the long term if left unattended [6, 10, 11]. Uniform aftercare could be incorporated in primary health care which has been put forward by the WHO to ensure availability of quality and effective health care while containing healthcare costs [35]. The WHO also describes the essence of adopting a holistic and person-centered approach in supporting well-being [35]. Besides, support from specialized health care is only needed for the minority, according to the stepped-care model, while the majority is in need of low-intensity interventions [36] which fit the primary healthcare setting.
Primary care-based uniform aftercare can include low-intensity interventions such as monitoring, counseling through motivational interviewing, and psychoeducation, which have been shown to have beneficial psychosocial effects [37, 38]. It is essential to ensure specialists knowledge of brain injury in uniform aftercare. Despite significant proportions receiving psychological consultation, cognitive symptoms and anxiety remained above levels observed in the general population. Moreover, practitioners of uniform aftercare should be aware of, and pay specific attention to individual and brain injury-specific differences related to the underlying causes. For instance, the necessary attention for secondary prevention in the stroke population should be given in any case. Importantly, the heightened levels of anxiety in the stroke population should receive specific attention from the psychosocial perspective. Exposure techniques could be introduced through promotion of resuming activities of daily living [30]. Exposure is known to decrease anxiety [39] and promote self-management [40]. Psychoeducation could help persons in dealing with cognitive problems in daily life. Moreover, improving emotional well-being has also been associated with reduced cognitive symptoms experienced in daily life [41].
Accurate prognostic models could assist clinical management by identifying persons most in need of uniform aftercare. Prognostic models should take into account the complex nature of persistent psychosocial symptoms and look beyond hospital variables such as injury-related information, frequently shown to be unrelated to psychosocial outcome [31]. Instead, models should consider the biopsychosocial model and incorporate for example, illness perceptions.
Strengths and limitations
This study concerned a multicenter, prospective, longitudinal cohort following persons from the ED up to 1-year post-injury. With multilevel growth modeling, we attempted to make best use of the available data and provide the most comprehensive overview of outcomes over time. Validated self-report measures of psychosocial functioning were used. It must be noted that the CLCE-24 is not validated in the TBI population but was selected to enable the comparison of common cognitive and emotional symptoms across conditions. Furthermore, the sample size of minor stroke, which was rather small compared to the mTBI group, potentially reduces statistical power. Further, our study was hospital based which may lead to missing large numbers of mTBI cases who do not visit the hospital. Ideally, population-based cohort studies are performed. Finally, in order to strengthen the direct comparison and in line, recommendations for uniform aftercare, future studies should extend covariates to, for example, premorbid psychological conditions and residual neurological deficits across conditions.
Conclusion
This study showed that psychosocial outcome is largely comparable following mTBI and minor stroke. Uniform aftercare for mild brain injury seems appropriate, and when implemented, specific attention should be paid to anxiety symptoms and cognitive problems in daily life. Accurate prognostic models could help in identifying persons most in need of uniform aftercare and keep numbers of persons visiting feasible by offering stepped-care models.
Acknowledgements
We would like to thank all the participants of this study and the staff of all participating hospitals: Maastricht University Medical Center (MUMC+), Zuyderland Medical Center Heerlen and Sittard, VieCuri Medical Center Venlo and Sint Jans Gasthuis Weert. Furthermore, we want to thank Charlotte Southcombe for reviewing the manuscript as native speaker.
Authors’ contributions
DV, RP, MK and CH designed the study. DV analyzed and interpreted the data. DV drafted the manuscript. RP, MK, MW, DB, RP, JS and CH advised on the preparation of the manuscript. All authors read, edited and approved the final version of the manuscript.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Compliance with ethical standards
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethics approval
The medical ethics committee of the Maastricht University Medical Center (MUMC+; 16-4-181) as well as the medical committees of all other participating hospitals approved the COHERENT study (Zuyderland Medical Center Heerlen and Sittard, VieCuri Medical Center Venlo, Sint Jans Gasthuis Weert). This study was performed according to the Declaration of Helsinki’s principles.
Consent to participate
Written informed consent was obtained from all participants.
Consent for publication
Not applicable.
References
- 1.Levin HS, Diaz-Arrastia RR. Diagnosis, prognosis, and clinical management of mild traumatic brain injury. Lancet Neurol. 2015;14(5):506–517. doi: 10.1016/S1474-4422(15)00002-2. [DOI] [PubMed] [Google Scholar]
- 2.Maas AI, Menon DK, Adelson PD, et al. Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017;16(12):987–1048. doi: 10.1016/S1474-4422(17)30371-X. [DOI] [PubMed] [Google Scholar]
- 3.Johnson CO, Nguyen M, Roth GA, Nichols E, Alam T, Abate D, Adebayo OM, et al. Global, regional, and national burden of stroke, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(5):439–458. doi: 10.1016/S1474-4422(19)30034-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strambo D, Zambon AA, Roveri L, et al. Defining minor symptoms in acute ischemic stroke. Cerebrovasc Dis. 2015;39(3–4):209–215. doi: 10.1159/000375151. [DOI] [PubMed] [Google Scholar]
- 5.Fischer U, Baumgartner A, Arnold M, et al. What is a minor stroke? Stroke. 2010;41(4):66–66. doi: 10.1161/STROKEAHA.109.572883. [DOI] [PubMed] [Google Scholar]
- 6.Barker-Collo S, Jones K, Theadom A, et al. Neuropsychological outcome and its correlates in the first year after adult mild traumatic brain injury: a population-based New Zealand study. Brain Inj. 2015;29(13–14):1604–1616. doi: 10.3109/02699052.2015.1075143. [DOI] [PubMed] [Google Scholar]
- 7.Coutts SB, Modi J, Patel SK, et al. What causes disability after transient ischemic attack and minor stroke? Results from the CT and MRI in the Triage of TIA and minor Cerebrovascular Events to Identify High Risk Patients (CATCH) study. Stroke. 2012;43(11):3018–3022. doi: 10.1161/STROKEAHA.112.665141. [DOI] [PubMed] [Google Scholar]
- 8.Nedeltchev K, Schwegler B, Haefeli T, et al. Outcome of stroke with mild or rapidly improving symptoms. Stroke. 2007;38(9):2531–2535. doi: 10.1161/STROKEAHA.107.482554. [DOI] [PubMed] [Google Scholar]
- 9.Morsund ÅH, Ellekjær H, Gramstad A, et al. The development of cognitive and emotional impairment after a minor stroke: a longitudinal study. Acta Neurol Scand. 2019;140(4):281–289. doi: 10.1111/ane.13143. [DOI] [PubMed] [Google Scholar]
- 10.van Mierlo ML, van Heugten CM, Post MW, et al. Quality of life during the first two year post stroke: the Restore4Stroke cohort study. Cerebrovasc Dis. 2016;41(1–2):19–26. doi: 10.1159/000441197. [DOI] [PubMed] [Google Scholar]
- 11.Cassidy JD, Cancelliere C, Carroll LJ, et al. Systematic review of self-reported prognosis in adults after mild traumatic brain injury: results of the International Collaboration on Mild Traumatic Brain Injury Prognosis. Arch Phys Med Rehabil. 2014;95(3):S132–S151. doi: 10.1016/j.apmr.2013.08.299. [DOI] [PubMed] [Google Scholar]
- 12.Moore EL, Terryberry-Spohr L, Hope DA. Mild traumatic brain injury and anxiety sequelae: a review of the literature. Brain Inj. 2006;20(2):117–132. doi: 10.1080/02699050500443558. [DOI] [PubMed] [Google Scholar]
- 13.Broomfield NM, Quinn TJ, Abdul-Rahim AH, et al. Depression and anxiety symptoms post-stroke/TIA: prevalence and associations in cross-sectional data from a regional stroke registry. BMC Neurol. 2014;14(1):198. doi: 10.1186/s12883-014-0198-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McInnes K, Friesen CL, MacKenzie DE, et al. Mild traumatic brain injury (mTBI) and chronic cognitive impairment: a scoping review. PLoS ONE. 2017;12(4):e0174847. doi: 10.1371/journal.pone.0174847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skoglund E, Westerlind E, Persson HC, et al. Self-perceived impact of stroke: a longitudinal comparison between one and five years post-stroke. J Rehabil Med. 2019;51(9):660–664. doi: 10.2340/16501977-2595. [DOI] [PubMed] [Google Scholar]
- 16.Cieza A, Bostan C, Ayuso-Mateos JL, et al. The psychosocial difficulties in brain disorders that explain short term changes in health outcomes. BMC Psychiatry. 2013;13(1):78. doi: 10.1186/1471-244X-13-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jonasson A, Levin C, Renfors M, et al. Mental fatigue and impaired cognitive function after an acquired brain injury. Brain Behav. 2018;8(8):e01056. doi: 10.1002/brb3.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matérne M, Strandberg T, Lundqvist L-O. Change in quality of life in relation to returning to work after acquired brain injury: a population-based register study. Brain Inj. 2018;32(13–14):1731–1739. doi: 10.1080/02699052.2018.1517224. [DOI] [PubMed] [Google Scholar]
- 19.Castor N, El Massioui F. Traumatic brain injury and stroke: does recovery differ? Brain Inj. 2018;32(13–14):1803–1810. doi: 10.1080/02699052.2018.1508748. [DOI] [PubMed] [Google Scholar]
- 20.Bulloch AG, Fiest KM, Williams JV, et al. Depression—a common disorder across a broad spectrum of neurological conditions: a cross-sectional nationally representative survey. Gen Hosp Psychiatry. 2015;37(6):507–512. doi: 10.1016/j.genhosppsych.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 21.Carroll LJ, Caddidy JD, Holm L, et al. Methodological issues and research recommendations for mild traumatic brain injury: the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med. 2004;43:113–125. doi: 10.1080/16501960410023877. [DOI] [PubMed] [Google Scholar]
- 22.Brott T, Adams HP, Jr, Olinger CP, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20(7):864–870. doi: 10.1161/01.STR.20.7.864. [DOI] [PubMed] [Google Scholar]
- 23.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 24.Bjelland I, Dahl AA, Haug TT, et al. The validity of the Hospital Anxiety and Depression Scale: an updated literature review. J Psychosom Res. 2002;52(2):69–77. doi: 10.1016/S0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 25.van Heugten C, Rasquin S, Winkens I, et al. Checklist for cognitive and emotional consequences following stroke (CLCE-24): development, usability and quality of the self-report version. Clin Neurol Neurosurg. 2007;109(3):257–262. doi: 10.1016/j.clineuro.2006.10.002[publishedOnlineFirst:2006/11/28]. [DOI] [PubMed] [Google Scholar]
- 26.Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L) Qual Life Res. 2011;20(10):1727–1736. doi: 10.1007/s11136-011-9903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Versteegh MM, Vermeulen KM, Evers SM, et al. Dutch tariff for the five-level version of EQ-5D. Value Health. 2016;19(4):343–352. doi: 10.1016/j.jval.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 28.van Rijsbergen MW, Mark RE, de Kort PL, et al. Prevalence and profile of poststroke subjective cognitive complaints. J Stroke Cerebrovasc Dis. 2015;24(8):1823–1831. doi: 10.1016/j.jstrokecerebrovasdis.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 29.Spinhoven P, Ormel J, Sloekers PP, et al. A validation study of the Hospital Anxiety and Depression Scale (HADS) in different groups of Dutch subjects. Psychol Med. 1997;27(2):363–370. doi: 10.1017/S0033291796004382. [DOI] [PubMed] [Google Scholar]
- 30.Chun H-YY, Whiteley WN, Dennis MS, et al. Anxiety after stroke: the importance of subtyping. Stroke. 2018;49(3):556–564. doi: 10.1161/STROKEAHA.117.020078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whittaker R, Kemp S, House A. Illness perceptions and outcome in mild head injury: a longitudinal study. J Neurol Neurosurg Psychiatry. 2007;78(6):644–646. doi: 10.1136/jnnp.2006.101105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.White J, Magin P, Attia J, et al. Predictors of health-related quality of life in community-dwelling stroke survivors: a cohort study. Fam Pract. 2016;33(4):382–387. doi: 10.1093/fampra/cmw011. [DOI] [PubMed] [Google Scholar]
- 33.Carlsson G, Möller A, Blomstrand C. A qualitative study of the consequences of 'hidden dysfunctions' one year after a mild stroke in persons < 75 years. Disabil Rehabil. 2004;26(23):1373–1380. doi: 10.1080/09638280400000211. [DOI] [PubMed] [Google Scholar]
- 34.Turner GM, McMullan C, Atkins L, et al. TIA and minor stroke: a qualitative study of long-term impact and experiences of follow-up care. BMC Fam Pract. 2019;20(1):176. doi: 10.1186/s12875-019-1057-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.World Health Organization . WHO global strategy on people-centred and integrated health services: interim report. Geneva: World Health Organization; 2015. [Google Scholar]
- 36.Kneebone II. Stepped psychological care after stroke. Disabil Rehabil. 2016;38(18):1836–1843. doi: 10.3109/09638288.2015.1107764. [DOI] [PubMed] [Google Scholar]
- 37.Watkins CL, Wathan JV, Leathley MJ, et al. The 12-month effects of early motivational interviewing after acute stroke: a randomized controlled trial. Stroke. 2011;42(7):1956–1961. doi: 10.1161/STROKEAHA.110.602227. [DOI] [PubMed] [Google Scholar]
- 38.Forster A, Brown L, Smith J et al (2012) Information provision for stroke patients and their caregivers. Cochrane Database Syst Rev. 10.1002/14651858.CD001919.pub3 [DOI] [PMC free article] [PubMed]
- 39.Wolitzky-Taylor KB, Horowitz JD, Powers MB, et al. Psychological approaches in the treatment of specific phobias: a meta-analysis. Clin Psychol Rev. 2008;28(6):1021–1037. doi: 10.1016/j.cpr.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 40.Satink T, Josephsson S, Zajec J, et al. Self-management develops through doing of everyday activities—a longitudinal qualitative study of stroke survivors during two years post-stroke. BMC Neurol. 2016;16(1):221. doi: 10.1186/s12883-016-0739-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duits A, Munnecom T, van Heugten C, et al. Cognitive complaints in the early phase after stroke are not indicative of cognitive impairment. J Neurol Neurosurg Psychiatry. 2008;79(2):143–146. doi: 10.1136/jnnp.2007.114595. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.