ABSTRACT
Objective
To analyse the susceptibility to ceftolozane-tazobactam and comparators in Enterobacterales and Pseudomonas aeruginosa isolates recovered from intraabdominal (IAI), urinary (UTI), respiratory (RTI) and bloodstream infection (BSI) in the SMART (Study for Monitoring Antimicrobial Resistance Trends) study.
Methods
. The susceptibility of 5,351 isolates collected in 11 Spanish hospitals (2016-2018) were analysed (EUCAST-2020 criteria) by broth microdilution and were phenotypically studied for the presence of extended-spectrum beta-lactamases (ESBL). Ceftolozane-tazobactam and/or carbapenem resistant isolates were genetically characterized for ESBL and carbapenemases.
Results
Escherichia coli was the most frequent pathogen (49.3% IAI, 54.9% UTI, 16.7% RTI and 50% BSI), followed by Klebsiella pneumoniae (11.9%, 19.1%, 13.1% and 15.4%, respectively). P. aeruginosa was isolated in 9.3%, 5.6%, 32% and 9%, respectively. The frequency of isolates with ESBLs (2016-2017) was: 30.5% K. pneumoniae, 8.6% E. coli, 2.3% Klebsiella oxytoca and 0.7% Proteus mirabilis. Ceftolozane-tazobactam was very active against non-ESBL-(99.3% susceptible) and ESBL-(95.2%) producing E. coli being less active against K. pneumoniae (98% and 43.1%, respectively) isolates. CTX-M-15 was the most prevalent ESBL in E. coli (27.5%) and K. pneumoniae (51.9%) frequently associated with OXA-48-like carbapenemase. Overall, 93% of P. aeruginosa isolates were susceptible to ceftolozane-tazobactam, preserving this activity (>75%) in isolates resistant to other beta-lactams except in those resistant to meropenen or ceftazidime-avibactam. GES-5, PER-1, VIM-1/2 were the most prevalent enzymes in isolates resistant to ceftolozane-tazobactam.
Conclusions
Ceftolozane-tazobactam showed high activity rates against isolates recovered in the SMART study although it was affected in K. pneumoniae and P. aeruginosa isolates with ESBL and/or carbapenemases.
Key-words: Ceftolozane-tazobactam, Enterobacterales, Pseudomonas aeruginosa, surveillance study
RESUMEN
Objetivo
Analizar la sensibilidad a ceftolozano-tazobactam y antimicrobianos comparadores en Enterobacterales y Pseudomonas aeruginosa procedentes de infecciones intraabdominales (IIA), urinarias (ITU) y respiratorias (ITR) y bacteriemias del estudio SMART (Study for Monitoring Antimicrobial Resistance Trends).
Métodos
Se analizó (EUCAST-2020) la sensibilidad de 5.351 aislados recogidos en 11 hospitales españoles (2016-2018) mediante microdilución en caldo y se estudió fenotípicamente la presencia de betalactamasas de espectro extendido (BLEE). En aislados resistentes a ceftolozano-tazobactam y/o carbapenémicos se caracterizaron las BLEE y carbapenemasas.
Resultados
. Escherichia coli fue el patógeno más frecuente (49,3% IIA, 54,9% ITU, 16,7% ITR y 50% bacteriemia), seguido de Klebsiella pneumoniae (11,9%, 19,1%, 13,1% y 15,4%, respectivamente). P. aeruginosa se aisló en el 9,3%, 5,6%, 32% y 9%, respectivamente. La frecuencia de aislados con BLEE (2016-2017) fue: 30,5% K. pneumoniae, 8,6% E. coli, 2,3% Klebsiella oxytoca y 0,7% Proteus mirabilis. Ceftolozanotazobactam fue muy activo en E. coli no productor (sensibilidad 99,3%) y productor de BLEE (95,2%) y menos activo en K. pneumoniae (98% y 43,1%, respectivamente). CTX-M-15 fue la BLEE más prevalente en E. coli (27,5%) y K. pneumoniae (51,9%) frecuentemente asociada con OXA-48-like. Un 93% de los aislados de P. aeruginosa fueron sensibles a ceftolozanotazobactam, que mantuvo su actividad (>75%) en aislados resistentes a otros betalactámicos excepto en los resistentes a meropenen o ceftazidima-avibactam. GES-5, PER-1, VIM-1/2 fueron las enzimas más prevalentes en aislados resistentes a ceftolozano-tazobactam.
Conclusiones
Ceftolozano-tazobactam mostró elevada sensibilidad frente a los aislados del estudio SMART, aunque disminuyó en K. pneumoniae y P. aeruginosa con BLEE y/o carbapenemasas.
Palabras clave: Ceftolozano-tazobactam, Enterobacterales, Pseudomonas aeruginosa, estudio de vigilancia
INTRODUCTION
Over the last years health authorities and professionals have alerted of the worrisome increase of antimicrobial resistance as well as its consequences in term of sanitary costs, management of the patients and mortality [1-3]. Different documents, including, among others, the Global Action Plan on Antimicrobial Resistance from the World Health Organization (WHO) [4], the Interagency Coordination Group on Antimicrobial Resistance of the United Nations [5] and the European One Health Action Plan against Antimicrobial Resistance from the European Commission [6], have delineated the strategies to address this problem. All of them agree not only to establish or to improve antimicrobial stewardship programs, hygiene, sanitation and infection control practices, to reduce the antimicrobial use and to increase the research on new antimicrobial drugs but also to intensify our efforts on surveillance. Surveillance programs are delineated to monitor antimicrobial use and resistance trends, to detect the emergence of new resistance mechanisms, to measure the impact of the introduction of new drugs into the antimicrobial armamentarium and to better use these compounds empirically.
The Study for Monitoring Antimicrobial Resistance Trends (SMART) is a world-wide surveillance program created in 2002 to monitor trends of antimicrobial susceptibility of aerobic and facultative Gram-negative bacilli from intra-abdominal infections (IAIs) with special attention on ertapenem and extended-spectrum beta-lactamases (ESBLs) producing Enterobacterales [7]. The objectives were expanded to include isolates recovered from urinary tract infections (UTIs) in 2009, lower respiratory tract infections (RTI) in 2015 and bloodstream infections (BSI) in 2018. Moreover, it is now also focused on carbapenem resistance and carbapenemases in Enterobacterales and multidrug resistance in Pseudomonas aeruginosa and to monitor newly approved antimicrobial agents, including ceftolozane-tazobactam and imipenem-relebactam [8,9].
Spain has participated in the SMART program since its initiation and several publications have summarized resistance in Enterobacterales and trends in ESBL producers [10-14]. In this article we present data on the activity of ceftolozane-tazobactam, a new beta-lactam-beta-lactamase inhibitor combination [15], in comparison with other antimicrobial drugs used in IAI, UTI, RTI, and BSI in SMART isolates recovered in the 2016-2018 period in Spain.
MATERIAL AND METHODS
Microorganisms and participating centres. Consecutive unselected aerobic and facultative Gram-negative bacilli were obtained from 2016 to 2018 in 11 participating Spanish Hospitals (2016: Clínico de San Carlos, Madrid; Virgen Macarena, Seville; Marqués de Valdecilla, Santander; Basurto, Bilbao; Clínico Lozano Blesa, Zaragoza; 2016 to 2018: Ramón y Cajal, Madrid; Bellvitge, Barcelona; Gregorio Marañón, Madrid; La Fe, Valencia; Virgen del Rocío, Seville; and Son Espases, Palma de Mallorca). Each site collected up to the indicated number of isolates (one isolate per species and patient to avoid duplicates): 100 from IAI, 100 from RTI and 50 from UTI in 2016; 75 from IAI, 100 from RTI, and 75 from UTI in 2017; and 50 from IAI, 100 from RTI, 50 from UTI and 50 from blood in 2018.
Peritoneal fluid (23%) was the most frequent intra-abdominal sample followed by gall bladder (20%) samples. Almost 100% of the samples were from urine in patients with UTI. Regarding RTI, sputum (26%) was the most frequent sample. Isolates were identified at the species level in each hospital and sent to a central laboratory [IHMA (International Health Management Associates, Inc., Schaumburg, IL, USA)] to confirm the identification and determine the susceptibility to ceftolozane-tazobactam and comparator antimicrobial agents. All the results were included in a centralized database. Patient’s age was also included. Moreover, following the standard criteria of the Centers for Disease Control and Prevention (CDC), the organisms were also rated as associated with a community-acquired infection (isolates obtained within 48 h after hospitalization) or with a nosocomial-infection (isolates obtained after 48 h of hospital stay) [16].
Antimicrobial susceptibility and interpretive criteria. Broth microdilution following the standard ISO recommendations was performed at the central laboratory (IHMA) using MicroScan microdilution panels (Beckman, West Sacramento, CA, USA). The antimicrobials and range of concentrations (mg/L) tested were: piperacillin-tazobactam (2/4-64/4), ceftriaxone (1-8), ceftazidime (1-16), cefepime (1-16), ceftolozane-tazobactam (0.125/4-1/4), ceftazidime-avibactam (0.125/4-0.5/4), aztreonam (1-16), imipenem (0.125-4), meropenem (0.125-0.5), ertapenem (0.06-4), amikacin (4-16), colistin (1-2), ciprofloxacin (0.25-2) and levofloxacin (0.5-4). Susceptibility to amoxicillin-clavulanic acid (2/2-256/2) was performed with a MIC gradient strip (Etest®, bioMérieux, Lyon, France). Escherichia coli ATCC 25922, E. coli ATCC 35218, Klebsiella pneumoniae ATCC 700603 (positive control for ESBL) and P. aeruginosa ATCC 27853 were used as quality control strains. All MIC results were interpreted using EUCAST recommendations [17]. When isolates were categorized as “I” (formerly “intermediate” and now indicating “susceptible, increased exposure”), the percentage of “I” isolates were collated with “S” isolates (“susceptible, standard dose”) and presented as susceptible.
Phenotypic and molecular characterization of ESBL and carbapenemases. Production of ESBL in 2016 and 2017 was phenotypically inferred in E. coli, Klebsiella spp. and Proteus mirabilis following CLSI criteria [19]. In 2018, ESBLs were not phenotypically investigated. Additionally, the presence of ESBL and carbapenemase genes were characterized molecularly in all E. coli and Klebsiella spp. isolates that were non-susceptible to ertapenem (MIC >0.5 mg/L) and/or ceftolozane-tazobactam (MIC >2/4 mg/L) as well as in approximately 50% of isolates susceptible to ertapenem, imipenem and/or ceftolozane-tazobactam that were non-susceptible to one or more of the following antibiotics: ceftriaxone (MIC >1 mg/L), cefepime (MIC >2 mg/L), ceftazidime (MIC >4 mg/L), and aztreonam (MIC >4 mg/L). These criteria were slightly modified in 2017 and 2018 and ESBL and carbapenemase characterization was restricted to isolates from these species non-susceptible to ertapenem (MIC >0.5 mg/L), imipenem (MIC >1 mg/L) and/ or imipenem-relebactam (MIC >1/4 mg/L) and/or ceftolozane-tazobactam (MIC >2/4 mg/L). In P. aeruginosa isolates, ESBL and carbapenemase genes were characterized in isolates non-susceptible to ceftolozane-tazobactam (MIC >4/4 mg/L) and/or imipenem (MIC >2 mg/l) and/or imipenem-relebactam (MIC >2/4 mg/L) (data of imipenem-relebactam not shown).
Screening of the resistance genes was performed as described previously by multiplex PCR and sequencing and include the following β-lactamase genes: class A ESBLs (TEM, SHV, CTX-M, VEB, PER, and GES); class C plasmid AmpC (ACC, ACT, CMY, DHA, FOX, MIR, MOX), and carbapenemases [KPC, GES (class A), NDM, IMP, VIM GIM, SPM (class B, MBLs), OXA-48-like (Enterobacterales only) and OXA-24-like (P. aeruginosa only) (class D)] [19,20]. Rates of carbapenemase-positive isolates were calculated based on data available for molecularly characterized isolates.
Statistical analysis. Comparison of different frequencies were performed using the chi-squared test (χ2) taking P<0.05 as statistically significant.
RESULTS
Bacterial distribution. Over the three-year period (2016-2018), a total of 5,351 isolates were collected in the Spanish participating centers in the SMART study. Breakdown by type of infection is shown for 5,334 in table S1 (supplementary material) as 0.3% of the isolates were excluded in this analysis but not in the susceptibility and molecular studies as their specimen source was not provided. Enterobacterales (n=4,151) constituted 77.8% of the total isolates, with E. coli in this group being the most frequently isolated microorganism (48.8%), followed by Klebsiella spp. (26.4%). The other Gram-negative bacilli (1,183, 22.2%) were mostly non-fermenters, being P. aeruginosa the most frequent (77.9%) followed by Stenotrophomonas maltophilia (12.4%). As expected, relevant differences regarding the infection site were observed, being E. coli isolates more relevant in UTI (54.9%) than in other infection sites (range 16.7-50.0%) and P. aeruginosa isolates in RTI (32.0%) than in the others (range 5.6-9.3%).
Antimicrobial activity and phenotypic ESBL production in Enterobacterales. Table 1 shows the susceptibility profile of different antimicrobials tested against the most common Enterobacterales species. Overall, ceftolozane-tazobactam susceptibility (isolates categorized as S plus I) ranged from 79.4% to 100%. Of note, susceptibility was higher for ceftolozane-tazobactam than for piperacillin-tazobactam (range 72.7-99.5%) or amoxicillin-clavulanate (range 66.5-85.5%) and similar or slightly inferior than that of ceftazidime-avibactam (97.7-100.0%). Antibiotics for which all different frequent species demonstrated susceptibilities higher than 90% were colistin, amikacin, imipenem and meropenem (Table 1).
Table 1.
Activity of different antimicrobial agents against most common Enterobacterales species collected in Spain in the SMART study (2016-2018)
| Microorganisms | Percentage of susceptible (S+I)a isolates | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AMC (≤8/4) | PTZ (≤16/4) | CTX (≤2) | CAZ (≤4) | FEP (≤4) | ATM (≤4) | CTZ (≤2/4) | CZA (≤8/4) | IMP (≤4) | MEM (≤8) | ETP (≤0.5) | CIP (≤0.5) | LVX (≤1) | AMK (≤8) | COL (≤2) | |
| Escherichia coli | 79.4 | 92.6 | 89.9 | 91.4 | 92.7 | 90.3 | 99.2 | 100.0 | 100.0 | 100.0 | 99.2 | 67.7 | 68.5 | 98.4 | 99.5 |
| Klebsiella pneumoniae | 66.5 | 72.7 | 67.6 | 71.1 | 71.9 | 71.6 | 83.7 | 100.0 | 97.1 | 97.6 | 85.6 | 65.0 | 70.2 | 99.0 | 94.9 |
| Klebsiella oxytoca | 83.0 | 89.4 | 97.7 | 97.2 | 97.8 | 91.1 | 97.2 | 97.8 | 99.4 | 99.4 | 98.3 | 95.5 | 96.1 | 100.0 | 100.0 |
| Proteus mirabilis | 85.5 | 99.5 | 96.6 | 99.1 | 100.0 | 100.0 | 99.1 | 100.0 | 98.6 | 100.0 | 100.0 | 59.2 | 64.2 | 99.1 | --b |
| Enterobacter cloacae | --b | 78.8 | 70.8 | 75.5 | 89.2 | 78.4 | 81.3 | 95.7 | 97.5 | 97.9 | 85.1 | 86.3 | 87.1 | 100.0 | 90.9 |
| Citrobacter freundii | --b | 77.9 | 66.1 | 67.6 | 97.1 | 73.5 | 79.4 | 100.0 | 97.1 | 97.1 | 97.1 | 92.6 | 91.2 | 100.0 | 100.0 |
| Morganella morganii | --b | 98.9 | 83.1 | 86.8 | 97.8 | 96.7 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 63.7 | 75.8 | 100.0 | --b |
| Serratia marcescens | --b | 95.9 | 92.1 | 98.8 | 98.8 | 98.8 | 97.6 | 97.7 | 95.9 | 99.4 | 97.1 | 91.8 | 93.5 | 98.8 | --b |
S = susceptible, standard dose, I = susceptible, increased exposure, EUCAST criteria except AMC in which CLSI criteria were considered; bThis species is considered intrinsically resistant to this antimicrobial; AMC: amoxicillin-clavulanic acid, PTZ: piperacillin-tazobactam, CTX: cefotaxime, CAZ: ceftazidime, FEP: cefepime, ATM: aztreonam, CTZ: ceftolozane-tazobactam, CZA: ceftazidime-avibactam, IMP: imipenem, MEM: meropenem, ETP: ertapenem, CIP: ciprofloxacin, LVX: levofloxacin, AMK: amikacin, COL: colistin
In the 2016 and 2017 years, the presence of ESBLs was studied phenotypically in 2,298 Enterobacterales (1,447 E. coli, 704 Klebsiella spp. and 147 Proteus mirabilis), of which 303 (13.1%) presented this phenotype. Figure S1 (supplementary material) shows the distribution of ESBL producers, also including information by infection site. The highest frequency was found in K. pneumoniae (30.5%, 174/571), followed by E. coli (8.6%, 125/1447) and Klebsiella oxytoca (2.3%, 3/130), being irrelevant in P. mirabilis (0.7%, 1/147). The percentage of ESBL-producing K. pneumoniae was higher in UTI (32.7%, 67/205) than in the other locations. In E. coli the highest rate was present in RTI (12.2%, 29/237).
Overall, ESBL production was significantly associated with nosocomial infections when compared with those acquired in the community in isolates with this information for K. pneumoniae (33.7%, 142/421 vs. 21.3%, 32/150, P=0.004) but not for E. coli (9.7%, 74/690 vs. 7.5%, 51/678, P=0.144). Moreover, an increase of ESBL production was observed both in K. pneumoniae (<30 years: 21.9%, 9/41; 30-60 years: 25.4%, 43/169; >60 years: 51.0%, 122/361. P<0.001) and E. coli (<30 years: 4.2%, 7/171; 30-60 years: 7.3%, 29/425; >60 years: 11.6%,89/850. P=0.07) with the increasing of age in patients.
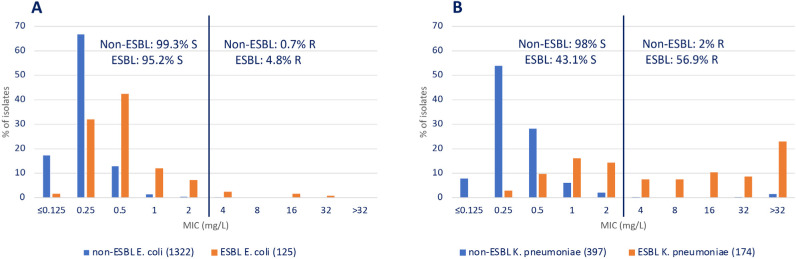
The activity of ceftolozane-tazobactam and other antimicrobials against E. coli and K. pneumoniae non-ESBL and ESBL producers recovered in 2016 and 2017 is shown in Figure S2 (supplementary material). Figure 1 shows ceftolozane-tazobactam MIC distribution of these organisms. This combination was very active in both E. coli non-ESBL (99.3% susceptible) and ESBL (95.2% susceptible) producers. The activity of ceftolozane-tazobactam was similar or close to that of carbapenems, amikacin, and colistin. On the contrary, the presence of ESBL in K. pneumoniae was associated with a reduction in the activity of ceftolozane-tazobactam (98% vs. 43.1% susceptible); nevertheless, it was less affected than piperacillin-tazobactam (90.2% vs. 25.9% susceptible) or amoxicillin-clavula-nate (84.1 vs. 25.1% susceptible). As expected, the presence of ESBL dramatically affected the activity of extended spectrum cephalosporins and was associated with a decreased susceptibility of ciprofloxacin both in E. coli and K. pneumoniae isolates (Figure S2).
Figure 1.
MIC distribution of ceftolozane-tazobactam in non-ESBL and ESBL producing Escherichia coli (A) and Klebsiella pneumoniae (B) isolates recovered in Spain in the SMART study (2016 and 2017). Ceftolozane-tazobactam susceptibility was calculated using EUCAST-2020 breakpoints [18]. Breakpoints are indicated with a line.
Table 2 analyses the activity of ceftolozane-tazobactam in E. coli and K. pneumoniae non-ESBL and ESBL producers that are resistant to different antibiotics. In E. coli, ceftolozane-tazobactam was active against more than 80% of isolates that were resistant to amoxicillin-clavulanate, piperacillin-tazobactam, meropenem or ciprofloxacin both in non-ESBL and ESBL producers. This activity was lower in ESBL-producing K. pneumoniae isolates that were resistant to these antimicrobial agents. Resistance to meropenem in these isolates denotes coproduction of carbapenemases in which ceftolozane-tazobactam is not active and all of them were categorized as resistant.
Table 2.
Activity of ceftolozane-tazobactam in non-ESBL and ESBL producing Escherichia coli and Klebsiella pneumoniae isolates resistant to amoxicillin/ clavulanate (AMC), piperacillin/tazobactam (P/T), meropenem (MER) and ciprofloxacin (CIP) in the SMART study (2016 and 2017) in Spain.
| Microorganisms | ESBL (No.) | Antimicrobial | No. (% of resistant isolates) | Ceftolozane-tazobactam | |
|---|---|---|---|---|---|
| Susceptible No. (%) |
Resistant No. (%) |
||||
| Escherichia coli | Negative (318)a | AMCa | 52 (16.3) | 50 (96.1) | 2 (3.9) |
| Positive (29)a | 16 (55.1) | 14 (87.5) | 2 (12.5) | ||
| Negative (1322) | P/T | 85 (6.4) | 78 (91.7) | 7 (8.3) | |
| Positive (125) | 23 (18.4) | 19 (82.6) | 4 (17.4) | ||
| Negative (1322) | MER | 0 (0) | -- | -- | |
| Positive (125) | 0 (0) | -- | -- | ||
| Negative (1322) | CIP | 331 (25) | 325 (98.2) | 6 (1.8) | |
| Positive (125) | 110 (88) | 106 (96.4) | 4 (3.6) | ||
| Klebsiella pneumoniae | Negative (62)a | AMCa | 5 (8.0) | 5 (100) | 0 (0) |
| Positive (19)a | 9 (47.3) | 3 (33.3) | 6 (66.6) | ||
| Negative (397) | P/T | 39 (9.8) | 31 (79.5) | 8 (20.5) | |
| Positive (174) | 129 (74.1) | 32 (24.8) | 97 (75.2) | ||
| Negative (397) | MER | 5 (1.2) | 0 (0) | 5 (100) | |
| Positive (174) | 11 (6.3) | 0 (0) | 11 (100) | ||
| Negative (397) | CIP | 48 (12.0) | 41 (85.4) | 7 (14.6) | |
| Positive (174) | 164 (94.2) | 69 (42.1) | 95 (57.9) | ||
AMC: data are only from 2017
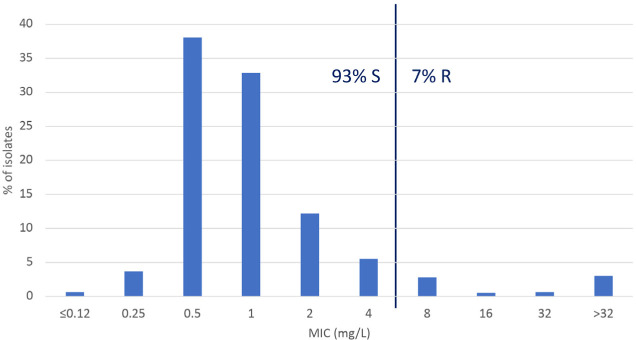
Antimicrobial activity in P. aeruginosa. The antibiotic susceptibility profile of P. aeruginosa is shown in Table 3. The behaviour of ceftolozane-tazobactam was excellent; only 7.0% of the 925 isolates studied were resistant to this antibiotic. Regarding the percentage of susceptible isolates (S+I), the most active antimicrobials were colistin (99.3%), followed by amikacin (94.6%), ceftazidime-avibactam (94.0%) and ceftolozane-tazobactam (93.0%). The least active compounds were the fluoroquinolones with susceptible figures of 63.5% for ciprofloxacin and 56.0% for levofloxacin. The activity of ceftolozane-tazobactam was not compromised by infection site with 93.1%, 93.0%, 92.9% and 92.6% of the IAI, UTI, RTI and BSI isolates susceptible to this antibiotic. Overall MIC distribution for this antibiotic can be observed in figure 2.
Table 3.
Activity of different antimicrobials against Pseudomonas aeruginosa collected in Spain in the SMART study (2016-2018)
| Antimicrobial | No. of isolates tested | Range | MIC50 | MIC90 | S (susceptible, standard dose) |
I (susceptible, increased exposure) |
R (resistant) |
|---|---|---|---|---|---|---|---|
| Piperacillin-tazobactam | 925 | ≤2/4 - >64/4 | 8/4 | >64/4 | -- | 66.8 | 33.2 |
| Ceftazidime | 925 | ≤1 - >32 | 4 | >32 | -- | 71.8 | 28.2 |
| Cefepime | 925 | ≤1 - >32 | 4 | 32 | -- | 72.3 | 27.7 |
| Ceftolozane-tazobactam | 925 | ≤0.12/4 - >32/4 | 1/4 | 4/4 | 93.0 | -- | 7.0 |
| Ceftazidime-avibactama | 217 | ≤0.12/4 - >32/4 | 2/4 | 8/4 | 94.0 | -- | 6.0 |
| Aztreonam | 925 | ≤1 - >16 | 8 | >16 | -- | 79.7 | 20.3 |
| Imipenem | 925 | ≤0.12 - >32 | 1 | 16 | -- | 75.5 | 24.5 |
| Meropenem | 925 | ≤0.12 - >32 | 0.5 | 16 | 74.7 | 14.0% | 11.3 |
| Tobramycina | 217 | <= 0.5 - >8 | ≤0.5 | >8 | 77.4 | -- | 22.6 |
| Amikacin | 925 | ≤4 - >32 | ≤4 | 8 | 94.6 | -- | 5.4 |
| Ciprofloxacin | 925 | ≤0.25 - >2 | ≤0.25 | >2 | -- | 63.5 | 36.5 |
| Levofloxacin | 925 | ≤0.5 - >4 | ≤1 | >4 | -- | 56.0 | 44.0 |
| Colistin | 925 | ≤1 - >4 | ≤1 | ≤1 | 99.3 | -- | 0.7 |
Data are only for 2018
Figure 2.
MIC distribution of ceftolozane-tazobactam in Pseudomonas aeruginosa isolates recovered in Spain in the SMART study (2016-2018). Ceftolozane-tazobactam susceptibility was calculated using EUCAST-2020 breakpoints [18]. Breakpoints are indicated with a line.
Table 4 shows the activity of ceftolozane-tazobactam in P. aeruginosa isolates resistant to different beta-lactams, including multidrug resistant ones. The activity of ceftolozane-tazobactam ranged from 75.4% to 80.8%, except for isolates that were resistant either alone or combined to meropenem and ceftazidime-avibactam, denoting the potential involvement of carbapenemase associated resistance mechanisms [21].
Table 4.
Ceftolozane-tazobactam activity in P. aeruginosa isolates resistant to different antimicrobials, including multidrug resistant ones.
| Antimicrobial resistance | No. of isolates | No. of resistant isolates (%) | No. of ceftolozane susceptible isolates (%) |
|---|---|---|---|
| Piperacillin-tazobactam | 925 | 307 (33.2) | 248 (80.8) |
| Ceftazidime | 925 | 261 (28.2) | 199 (76.3) |
| Cefepime | 925 | 256 (27.7) | 193 (75.4) |
| Imipenem | 925 | 227 (24.5) | 174 (76.7) |
| Meropenem | 925 | 105 (11.3) | 58 (55.3) |
| Ceftazidime-avibactama | 217 | 13 (5.9) | 4 (30.8) |
| Piperacillin-tazobactam, ceftazidime | 925 | 243 (26.2) | 187 (77.0) |
| Imipenem, meropenem | 925 | 99 (10.7) | 55 (55.5) |
| Piperacillin-tazobactam, ceftazidime, meropenem | 925 | 75 (8.1) | 33 (44.0) |
| Piperacillin-tazobactam, ceftazidime, imipenem, meropenem | 925 | 72 (7.7) | 33 (45.9) |
| Piperacillin-tazobactam, ceftazidime, imipenem, meropenem, ceftazidime-avibactam | 217 | 9 (4.1) | 2 (22.3) |
Data are only for 2018
Molecular characterization of ESBLs and carbapenemases. Molecular studies for ESBLs and carbapenemases were performed in 80 E. coli, 160 K. pneumoniae and 267 P. aeruginosa isolates (Table S2 and S3 supplementary material). In E. coli, CTX-M-15 (27.5%, 22/80), either alone or with another enzyme, including carbapenemases, was the most prevalent ESBL, followed by CTX-M-27 (8.7%, 7/80), SHV-12 (7.5%, 6/80), and CTX-M-14 (6.2%, 5/80). The most prevalent carbapenemase in this species was OXA-48-like (11.2%, 9/80) mainly present in ceftolozane-tazobactam susceptible isolates. Highly resistant isolates (MIC >8/4 mg/L) frequently presented combinations of different enzymes being two of them a VIM-1 and a KPC-type carbapenemase. To note that one ceftolozane-tazobactam susceptible E. coli isolate (CMI=0.25/4 mg/L) presented a KPC-3 enzyme.
In K. pneumoniae, the situation was more complex than in E. coli (Table S2) The most prevalent ESBL was also CTX-M-15 (51.9%, 83/160), in most cases associated with OXA-48-like (n=61). SHV-12 was also prevalent (18.1%, 29/160), also associated with OXA-48-like (n=15). Ceftolozane-tazobactam resistant isolates normally presented with 2 or 3 enzymes. KPC-3 and metallo-beta-lactamases (VIM-1 or NDM-1) were only present in highly resistant ceftolozane-tazobactam resistant isolates (MIC >8/4 mg/L).
Finally, in P. aeruginosa isolates (Table S3), PER-1 (0.7%, 2/267) and GES-5 (0.7%, 2/267) enzymes were scarcely found. Metallo-beta-lactamases (VIM-1, VIM-2, VIM-20 and IMP-13) were present in 8.6% (23/267) of the isolates. KPC were not found, but one isolate had a CTX-M-2 ESBL.
DISCUSSION
Surveillance studies have been highlighted as an important tool to address the problem of antimicrobial resistance both at local and global levels [4-6,22]. The SMART study had monitored the activity of ertapenem in IAI since 2002 but now it is also including ceftolozane-tazobactam susceptibility, expanding the focus to UTI, RTI and BSI [8]. In this publication we present, for the first time in the SMART program, specific data of ceftolozane-tazobactam susceptibility in isolates recovered in Spain from 2016 to 2018. Other publications of this international surveillance program have evaluated the activity of this antibiotic in isolates recovered from Asia Pacific region, including Taiwan [23,24], Brazil [25] and the US [8,9,26]. To note, we analysed the Spanish data using the new EUCAST criteria for ceftolozane-tazobactam published in 2020 (Enterobacterales, susceptible ≤2/4 mg/L and resistant >2/4 mg/L; P. aeruginosa, susceptible ≤4/4 mg/L and resistant >4/4 mg/L) [17].
E. coli and K. pneumoniae susceptibility to ceftolozane-tazobactam in Spain (99.2% and 83.7%, respectively) was slightly higher than that in Taiwan (96.5% and 80.7%, respectively) [24] and the US (94.0% and 78.3% respectively [26]. In Europe, the activity of ceftolozane-tazobactam has been also monitored in UTI and IAI in the PACTS surveillance study (Program to Assess Ceftolozane/Tazobactam Susceptibility) but had used the previous EUCAST breakpoint for Enterobacterales [27]. Nevertheless, whole MIC distributions were presented in this publication allowing comparison with our results. In the PACTS study ceftolozane-tazobactam susceptibility was slightly lower (98.8% E. coli and 82.1% K. pneumoniae) than that obtained in the analysis of the SMART Spain database. We also performed the SUPERIOR study in Spain in which we evaluated the activity of ceftolozane-tazobactam in isolates recovered from UTI and IAI in ICU patients. Reanalysing the SUPERIOR data using 2020 EUCAST criteria, the susceptibility to this antibiotic was 96.2% in E. coli and 72.6% in Klebsiella spp. [28].
The SMART study also monitors ESBL trends and, more recently, carbapenemases. We confirm in the present analysis an increase in the prevalence of ESBLs in Spain in K. pneumoniae and maintenance in E. coli when compared with previous SMART analyses [13,14], with CTX-Ms being the most important ESBLs. Regarding carbapenemases, their presence in E. coli and K. pneumoniae isolates was also relevant, particularly in K. pneumoniae isolates. OXA-48-like was the most prevalent carbapenemases in E. coli (11.2%, 9/80) and K. pneumoniae (55%, 88/160) isolates, followed by metallo-beta-lactamases (1.2%, 1/80 and 4.4%, 7/160, respectively) and KPCs (1.2%, 1/80 and 3,1%, 5/160, respectively). This situation is also reflected in other studies performed in Spain in which carbapenemases have been characterized [29-31].
In our study, the reduction of ceftolozane-tazobactam susceptibility was small in ESBL-producing E. coli isolates (95.3% susceptible) but was higher in ESBL-producing K. pneumoniae (43.1% susceptible) (Figures S1, S2 and 1). This fact in the latter species might be related to the coproduction of carbapenemases in ESBL producers, a frequent situation in molecularly characterized isolates with ceftolozane-tazobactam MICs >2/4 mg/L (74.2%, 92/124 isolates) and it was also reflected in the SUPERIOR study [32]. Coproduction of both type of enzymes was less prevalent in E. coli (23.5%, 4/17 isolates). Interestingly, the activity of ceftolozane-tazobactam was less affected than that of amoxicillin-clavulanate and piperacillin-tazobactam in ESBL producers (Figure S1). In fact, more than 80% of ESBL-producing E. coli isolates that were resistant to amoxicillin-clavulanate or piperacillin-tazobactam were susceptible to ceftolozane-tazobactam. This figure was nearly 25% in ESBL-producing K. pneumoniae isolates (Table 3). In other Enterobacterales species, ceftolozane-tazobactam presented susceptibility percentages higher than 97%, unlike E. cloacae and C. freundii. Reduction of ceftolozane-tazobactam activity in these AmpC producers has been also highlighted in other studies [27,28].
For P. aeruginosa isolates, colistin (99.3%) and amikacin (94.6%) were the most active agents tested with similar susceptibility values of ceftazidime-avibactam (94%) and ceftolozane-tazobactam (93%). Values for ceftolozane-tazobactam were similar in the SMART study in the US (94.7%) but slightly lower in the PACTS (91.7%) and SUPERIOR (91.3%) surveillance studies [8,27,28]. Interestingly, the activity of ceftolozane-tazobactam was higher than 75% in isolates resistant to other anti-pseudomonal agents such as piperacillin-tazobactam, ceftazidime, cefepime and imipenem and was only compromised (range of susceptible isolates 22.3%-55.3%) when isolates were resistant to meropenem or ceftazidime-avibactam (alone or simultaneously affected with other antimicrobials). This could be associated with the presence of GES, PER or metallo-beta-lactamase enzymes, a fact previously identified in this microorganism [21,33,34]. Nevertheless, other resistance mechanisms including amino acid replacements in chromosomal AmpC might be present as recently demonstrated in a Spanish multicentre study [35].
Despite the fact that our study has some limitations such as not addressing clonality, discontinuation of ESBL phenotypic detection in the SMART study in 2018 or the use of different criteria in different years over the study period to select isolates for molecular characterization of ESBLs and carbapenemases, it has valuable information on Enterobacterales and P. aeruginosa susceptibility to ceftolozane-tazobactam and other antimicrobial agents recovered from different infections in Spain. Ceftolozanetazobactam showed high susceptibility rates against all microorganisms tested but was affected in K. pneumoniae and P. aeruginosa isolates with ESBL and/or carbapenemases.
ACKNOWLEDGEMENTS
We thank MSD, Spain and IHMA (Inter national Health Management Associates, S.A., Schaumburg, Illinois, U.S.) for providing access to the database of the SMART epidemiological surveillance study and molecular data.
Acknowledgments
The SMART Spain working group is represented by the following investigators who have participated in the study: E. Loza, M. García-Castillo, P. Ruiz-Garbajosa, and R. Cantón (Hospi tal Universitario Ramón y Cajal-IRYCIS, Madrid); R.M. Arcay, X. Mulet and A. Oliver (Hospital Universitario Son Espases, Mallorca); E. Cercenado (Hospital Universitario Gregorio Marañón, Madrid); F.J. Castillo and C. Seral (Hospital Clínico Universitario Lozano Blesa, Zarago za); R. Figueroa and R. Cisterna (Hospital Basurto, Bilbao); L. Gálvez-Benítez, I. Pupo-Ledo and A. Rodríguez-Villodres (Hospital Universitario Virgen del Rocío, Sevilla); F. González Romo and A. Delgado Iribarren (Hospital Clínico San Carlos, Madrid); A. HernándezCabezas, M. Bosch Alepuz and J.L. López-Hontangas (Hospital Universitario y Politécnico La Fe, Valencia); J. Rodriguez-Lozano and J. Calvo (Hospital Universitario Marqués de Valdecilla, Santander); A.I. Suárez-Barrenechea and A. Pascual and (Hospi tal Universitario Virgen Macarena, Sevilla); F. Tubau, D. Berbel-Palau and M.A. Domínguez (Hos pital Universitari Bellvitge-IDIBELL, Hospitalet de Llobregat. Barcelona).
FUNDING
SMART surveillance program is sponsored by MSD. Writing of this manuscript has been performed through a contract of services between MSD Spain and Fundación para la Investigación Biomédica del Hospital Universitario Ramón y Cajal, Madrid, Spain.
CONFLICTS OF INTEREST
RC and EC have collaborated in educational meetings sponsored by MSD, Spain and Pfizer. He has also research grants from MSD. JDR and DLM are employees of MSD, Spain. All other authors declare that they have no conflicts of interest regarding this publication.
References
- 1.Friedman ND, Temkin E, Carmeli Y. The negative impact of antibiotic resistance. Clin Microbiol Infect 2016; 22:416–22. doi: 10.1016/j.cmi.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Cassini A, Högberg LD, Plachouras D, Quattrocchi A, Hoxha A, Simonsen GS, et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: a population-level modelling analysis. Lancet Infect Dis. 2019; 19:56-66. doi: 10.1016/S1473-3099(18)30605-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cantón R, Huarte R, Morata L, Trillo-Mata JL, Muñoz R, González J, et al. Determining the burden of infectious diseases caused by carbapenem-resistant gram-negative bacteria in Spain. Enferm Infecc Microbiol Clin. 2020: S0213-005X(20)30191-9. doi: 10.1016/j.eimc.2020.04.009. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization Global . Action Plan on Antimicrobial Resistance. 2015. https://www.who.int/antimicrobial-resistance/publications/global-action-plan/en/) [DOI] [PubMed]
- 5.Interagency Coordination Group on Antimicrobial Resistance . No time to wait: securing the future from drug-resistant infections. Report of the Secretary-General of the United Nations. April 2019. (https://www.who.int/antimicrobial-resistance/interagency-coordination-group/final-report/en/)
- 6.European Commission . A European One Health Action Plan against Antimicrobial Resistance (AMR). 2017. (https://ec.europa.eu/health/amr/action_eu_en)
- 7.Paterson DL, Rossi F, Baquero F, Hsueh PR, Woods GL, Satishchandran V, et al. In vitro susceptibilities of aerobic and facultative Gram-negative bacilli isolated from patients with intra-abdominal infections worldwide: the 2003 Study for Monitoring Antimicrobial Resistance Trends (SMART). J Antimicrob Chemother. 2005; 55:965-73. doi: 10.1093/jac/dki117. [DOI] [PubMed] [Google Scholar]
- 8.Lob SH, Hoban DJ, Young K, Motyl MR, Sahm DF. Activity of ceftolozane-tazobactam and Comparators against Pseudomonas aeruginosa from patients in different risk strata--SMART United States 2016-2017. J Glob Antimicrob Resist. 2019. pii: . doi: 10.1016/j.jgar.2019.07.017. [DOI] [PubMed] [Google Scholar]
- 9.Karlowsky JA, Lob SH, Raddatz J, DePestel DD, Young K, Motyl MR, et al. In vitro activity of imipenem/relebactam and ceftolozane/ tazobactam against clinical isolates of Gram-negative bacilli with difficult-to-treat resistance and multidrug-resistant phenotypes-SMART United States 2015-2017. Clin Infect Dis. 2020. April 3; ciaa381. doi: 10.1093/cid/ciaa381. [DOI] [PubMed] [Google Scholar]
- 10.Baquero F, Cercenado E, Cisterna R, de la Rosa M, García-Rodríguez JA, Gobernado M, et al. Patrones de sensibilidad a antimicrobianos de Enterobacteriaceae causantes de infecciones intraabdominales en España: resultados del estudio SMART 2003. Rev Esp Quimioter. 2006; 19:51-9. PMid: . [PubMed] [Google Scholar]
- 11.Guembe M, Cercenado E, Alcalá L, Marín M, Insa R, Bouza E. Evolution of antimicrobial susceptibility patterns of aerobic and facultative gram-negative bacilli causing intra-abdominal infections: results from the SMART studies 2003-2007. Rev Esp Quimioter 2008; 21:166-73. PMid: . [PubMed] [Google Scholar]
- 12.Cantón R, Loza E, Aznar J, Calvo J, Cercenado E, Cisterna R, et al. Antimicrobial susceptibility of Gram-negative organisms from intraabdominal infections and evolution of isolates with extended spectrum β-lactamases in the SMART study in Spain (2002-2010). Rev Esp Quimioter. 2011; 24:223-32. PMid: . [PubMed] [Google Scholar]
- 13.Cantón R, Loza E, Aznar J, Barrón-Adúriz R, Calvo J, Castillo FJ, et al. Antimicrobial susceptibility trends and evolution of isolates with extended spectrum β-lactamases among Gram-negative organisms recovered during the SMART study in Spain (2011-2015). Rev Esp Quimioter 2018; 31:136-45. PMid: . [PMC free article] [PubMed] [Google Scholar]
- 14.Cantón R, Loza E, Aznar J, Castillo FJ, Cercenado E, Fraile-Ribot PA, et al. Monitoring the antimicrobial susceptibility of Gram-negative organisms involved in intraabdominal and urinary tract infections recovered during the SMART study (Spain, 2016 and 2017). Rev Esp Quimioter 2019; 32:145-55. PMid: . [PMC free article] [PubMed] [Google Scholar]
- 15.Giacobbe DR, Bassetti M, De Rosa FG, Del Bono V, Grossi PA, Menichetti F, et al. Ceftolozane/tazobactam: place in therapy. Expert Rev Anti Infect Ther. 2018; 16:307-20. doi: 10.1080/14787210.2018.1447381. [DOI] [PubMed] [Google Scholar]
- 16.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008; 36:309-32. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 17.The European Committee on Antimicrobial Susceptibility Testing . Breakpoint tables for interpretation of MICs and zone diameters. Version 10.0, 2020. http://www.eucast.org/clinical_breakpoints/ [Google Scholar]
- 18.Clinical and Laboratory Standards Institute . Performance standards for antimicrobial susceptibility testing. Document M100-S26. Wayne, PA: CLSI, 2016. [Google Scholar]
- 19.Lob SH, Kazmierczak KM, Badal RE, Hackel MA, Bouchillon SK, Biedenbach DJ, et al. Trends in susceptibility of Escherichia coli from intra-abdominal infections to ertapenem and comparators in the United States according to data from the SMART program, 2009 to 2013. Antimicrob Agents Chemother. 2015; 59:3606-10. doi: 10.1128/AAC.05186-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karlowsky JA, Lob SH, Kazmierczak KM, Hawser SP, Magnet S, Young K, et al. In vitro activity of imipenem/relebactam against Gram-negative ESKAPE pathogens isolated in 17 European countries: 2015 SMART surveillance programme. J Antimicrob Chemother. 2018; 73:1872-9. doi: 10.1093/jac/dky107. [DOI] [PubMed] [Google Scholar]
- 21.Hernández-García M, García-Castillo M, García-Fernández S, Melo-Cristino J, Pinto MF, Gonçalves E, et al. Distinct epidemiology and resistance mechanisms affecting ceftolozane/tazobactam in Pseudomonas aeruginosa isolates recovered from ICU patients in Spain and Portugal depicted by WGS. J Antimicrob Chemother. 2021; 76:370-9. doi: 10.1093/jac/dkaa430. [DOI] [PubMed] [Google Scholar]
- 22.Cantón R, Morosini MI. Surveillance studies on antimicrobial susceptibility, from international to local studies. Enferm Infecc Microbiol Clin. 2020; 38:147-149. English, Spanish. doi: 10.1016/j.eimc.2020.02.012. [DOI] [PubMed] [Google Scholar]
- 23.Kuo SC, Liu CE, Lu PL, Chen YS, Lu MC, Ko WC, et al. Activity of ceftolozane-tazobactam against Gram-negative pathogens isolated from lower respiratory tract infections in the Asia-Pacific region: SMART 2015-2016. Int J Antimicrob Agents. 2020; 55:105883. doi: 10.1016/j.ijantimicag.2020.105883. [DOI] [PubMed] [Google Scholar]
- 24.Jean SS, Lu MC, Shi ZY, Tseng SH, Wu TS, Lu PL, et al. In vitro activity of ceftazidime-avibactam, ceftolozane-tazobactam, and other comparable agents against clinically important Gram-negative bacilli: results from the 2017 Surveillance of Multicenter Antimicrobial Resistance in Taiwan (SMART). Infect Drug Resist. 2018; 11:1983-92. doi: 10.2147/IDR.S175679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beirão EM, Rodrigues SDS, Andrade TK, Serra FB, Paula MDN, Polis TJB, et al. Activity of ceftolozane-tazobactam and comparators against gram-negative bacilli: Results from the study for monitoring antimicrobial resistance trends (SMART-Brazil; 2016-2017). Braz J Infect Dis. 2020; 24:310-321. doi: 10.1016/j.bjid.2020.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karlowsky JA, Kazmierczak KM, Young K, Motyl MR, Sahm DF. In vitro activity of ceftolozane/tazobactam against phenotypically defined extended-spectrum β-lactamase (ESBL)-positive isolates of Escherichia coli and Klebsiella pneumoniae isolated from hospitalized patients (SMART 2016). Diagn Microbiol Infect Dis. 2020; 96(4):114925. doi: 10.1016/j.diagmicrobio.2019.114925. [DOI] [PubMed] [Google Scholar]
- 27.Pfaller MA, Bassetti M, Duncan LR, Castanheira M. Ceftolozane/tazobactam activity against drug-resistant Enterobacteriaceae and Pseudomonas aeruginosa causing urinary tract and intraabdominal infections in Europe: report from an antimicrobial surveillance programme (2012-15). J Antimicrob Chemother. 2017; 72:1386-95. doi: 10.1093/jac/dkx009. [DOI] [PubMed] [Google Scholar]
- 28.García-Fernández S, García-Castillo M, Bou G, Calvo J, Cercenado E, Delgado M, et al. Activity of ceftolozane/tazobactam against Pseudomonas aeruginosa and Enterobacterales isolates recovered from intensive care unit patients in Spain: The SUPERIOR multicentre study. Int J Antimicrob Agents. 2019; 53:682-88. doi: 10.1016/j.ijantimicag.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 29.Grundmann H, Glasner C, Albiger B, Aanensen DM, Tomlinson CT, Andrasević AT, et al. Occurrence of carbapenemase-producing Klebsiella pneumoniae and Escherichia coli in the European survey of carbapenemase-producing Enterobacteriaceae (EuSCAPE): a prospective, multinational study. Lancet Infect Dis. 2017; 17:153-63. doi: 10.1016/S1473-3099(16)30257-2. [DOI] [PubMed] [Google Scholar]
- 30.García-Castillo M, García-Fernández S, Gómez-Gil R, Pitart C, Oviaño M, Gracia-Ahufinger I, et al. Activity of ceftazidime-avibactam against carbapenemase-producing Enterobacteriaceae from urine specimens obtained during the infection-carbapenem resistance evaluation surveillance trial (iCREST) in Spain. Int J Antimicrob Agents. 2018; 51:511-5. doi: 10.1016/j.ijantimicag.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 31.Hernández-García M, Pérez-Viso B, Turrientes C, Díaz-Agero C, LópezFresneña N, Bonten M, et al. Characterization of carbapenemase-producing Enterobacteriaceae from colonized patients in a university hospital in Madrid, Spain, during the R-GNOSIS project depicts increased clonal diversity over time with maintenance of high-risk clones. J Antimicrob Chemother. 2018; 73:3039-43. doi: 10.1093/jac/dky284. [DOI] [PubMed] [Google Scholar]
- 32.Hernández-García M, García-Fernández S, García-Castillo M. Bou G, Cercenado E, Delgado-Valverde M, et al. WGS characterization of MDR Enterobacterales with different ceftolozane/tazobactam susceptibility profiles during the SUPERIOR surveillance study in Spain. JAC Antimicrob Resist 2020; 2(4), December 20. dlaa084. doi: 10.1093/jacamr/dlaa084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Del Barrio-Tofiño E, López-Causapé C, Cabot G, Rivera A, Benito N, Segura C, et al. Genomics and susceptibility profiles of extensively drug-resistant Pseudomonas aeruginosa isolates from Spain. Antimicrob Agents Chemother. 2017; 61(11). pii: e01589-17. doi: 10.1128/AAC.01589-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ortiz de la Rosa JM, Nordmann P, Poirel L. ESBLs and resistance to ceftazidime/avibactam and ceftolozane/tazobactam combinations in Escherichia coli and Pseudomonas aeruginosa. J Antimicrob Chemother. 2019; 74:1934-9. doi: 10.1093/jac/dkz149. [DOI] [PubMed] [Google Scholar]
- 35.Del Barrio-Tofiño E, Zamorano L, Cortes-Lara S, López-Causapé C, Sánchez-Diener I, Cabot G, et al. Spanish nationwide survey on Pseudomonas aeruginosa antimicrobial resistance mechanisms and epidemiology. J Antimicrob Chemother. 2019; 74:1825-35. doi: 10.1093/jac/dkz147. [DOI] [PubMed] [Google Scholar]