Abstract
Introduction
drug-resistant tuberculosis is a major global health problem and a threat to health security given the increase in the number of cases and the challenges associated with care. Besides, the relationship between poor nutritional status and tuberculosis is clearly established. For relevant and evidence-based public health decision-making regarding the management of malnutrition in patients with drug-resistant tuberculosis in the initial phase, it is essential to estimate the prevalence of malnutrition and understand the risk factors associated with it.
Methods
we performed a retrospective cohort study in drug-resistant tuberculosis patients aged 18 years and older, among which the nutritional status was assessed through BMI. All predictors were included in a prediction model using the multivariate logistic model according to the lowest Akaike criterion. Discrimination and model calibration was evaluated using receiver performance analysis, and the Hosmer and Lemeshow test.
Results
this study revealed a prevalence of malnutrition of 64.7% in drug-resistant tuberculosis patients in our 218-patient series. The factors associated with malnutrition were: unsuccessful treatment, the active presence of mycobacterium tuberculosis, increased bacteriological conversion time, increased serum creatinine, increased transaminase SGPT of the liver, and anaemia. Some of the factors not associated with malnutrition included the history of anti-tuberculosis treatment, vomiting, hepatic SGPT, initial AFB count, smear and culture conversion time, depression, and chest X-ray.
Conclusion
malnutrition remains a concern among drug-resistant tuberculosis patients in Guinea as it affects more than half of them with a negative impact on the outcome of treatment. Implementing specific interventions for these high-risk patients, including nutritional supplementation, psychosocial support, and treatment for tuberculosis, can improve management for better treatment outcomes.
Keywords: Drug-resistant tuberculosis, malnutrition, associated factors, Guinea
Introduction
Guinea is ranked among the countries with a high incidence of tuberculosis and TB/HIV coinfection, both of which are major causes of morbidity and mortality [1]. Even though incidence and mortality are steadily declining in Guinea, the tuberculosis epidemic remains a concern because of the increasing occurrence of drug-resistant forms and the poor control of comorbidities. The country is now experiencing the emergence of forms of resistance to major molecules for first-line treatment such as rifampicin and isoniazid. This is reflected in the increase in the number of cases of DR-TB detected, from 178 cases in 2016 to 265 cases in 2018 [2]. Among people infected with TB, 10-15% would develop the disease if they do not receive adequate antituberculosis treatment [3]. This risk is much higher for those with a compromised immune system, such as people living with HIV, who are malnourished or have diabetes [4]. Most DR-TB patients screened in Guinea have a longer therapeutic route (patients already treated for susceptible tuberculosis), as the Xpert® MTB/RIF test is not first-line for all patients.
Tuberculosis and malnutrition are linked in a complicated relationship that has been known for a long time. Infection can lead to undernutrition associated with increased metabolic demand and decreased intake, and nutritional deficiencies can aggravate the disease or delay healing by reducing essential immune functions [5]. Malnutrition is defined as the deficiency, excess, or imbalance in the energy and/or nutritional intake of a person [6]. This medical condition may support the disease in the direction of aggravation and lack of satisfactory therapeutic results, which is why it is essential to identify these comorbidities in people with tuberculosis to ensure early diagnosis and to improve management [7]. Most people with active TB experience weight loss that can be caused by several factors, including decreased dietary intake due to loss of appetite, nausea, and abdominal pain [8].
In 2015, according to the European Society of Clinical Nutrition, the diagnosis of malnutrition was based on a low body mass index (BMI), defined as less than <18.5 kg/m2, or a combination of weight loss and low mass free fat or low BMI (<20 kg/m2 or <22 kg/m2, depending on age). The same guideline is used by the WHO to identify malnutrition [9], although malnutrition has been documented in patients with high BMI [10, 11]. The body mass index at the beginning of tuberculosis treatment is a statistical nutritional risk factor frequently used in tuberculosis research [7]. However, a change in body weight after the start of TB treatment may be indicative of improvement or worsening of nutritional status during TB treatment [8].
The socioeconomic status of the patient is an element to be considered during the initiation of antituberculosis treatment. According to the 2012 DHS [12], concerning malnutrition in the population, significant disparities exist depending on the living environment and economic status. The analysis of the association between malnutrition and DR-TB could provide useful information for decision-making in the management and prevention of nutrition in patients. Our study aimed to determine the prevalence of malnutrition and to identify the associated factors in DR-TB patients during the initial phase of treatment. To our knowledge, no study has been conducted on this subject in Guinea.
Methods
Diagnosis of DR-TB, tuberculosis, and malnutrition: according to the technical guide of the tuberculosis control program, diagnosis of DR-TB cases is based on careful clinical examination, bacteriological examination (direct examination of sputum for AFB research), Xpert® MTB/RIF, culture and susceptibility testing of the bacillary strain against antituberculosis), these examinations are supplemented by radiography for the detection of lesions that could be associated and a pre-therapeutic assessment to examine the patient's ability to receive second-line antituberculosis treatment. The body mass index (BMI) was used to assess the nutritional status of patients. With the metric system, the formula for BMI is weight in kilograms divided by height in square meters. For adults, the BMI is interpreted using the standard weight status categories. These categories are the same for people of all types and ages [13]. Measurements of weight (in kilograms) and height (in centimeters) were made using a medical scale and an appropriate adult measuring rod, respectively. These devices are available at DR-TB treatment sites.
Study setting and population: we analyzed surveillance data from 218 patients with DR-TB enrolled between June 7, 2016, and June 22, 2018, in a multicenter longitudinal cohort study conducted in three large centres for DR-TB in Conakry (Ignace-Deen, Carrière, and Tombolia). All patients were initially seen and followed monthly for a period of 9 months, according to the WHO standardized treatment regimen guidelines of 9 to 12 months [14]. Patients younger than 18 years old were excluded from the analysis.
Data collection: the data were collected from March 2nd to July 12th, 2020, using a case report form (CRF) from the DR-TB register containing information on sociodemographic and clinical characteristics, laboratory test results (sputum smear or culture) and radiography for all registered patients in treatment centres. Additional information was collected through patient medical records and results reports. The following clinical, paraclinical, and demographic data were extracted: age, sex, residence, comorbidity, HIV status, history of previously treated tuberculosis, presence of caves on the chest X-ray as determined by the lead radiologist, baseline data on weight, height for calculation of BMI, sputum smear and culture, clinical symptoms (chest pain, cough, night sweats, nausea, vomiting) and biological data (haemoglobin, SGPT, SGOT, creatinine level, number of leukocytes, number of platelets, number of lymphocytes). Moreover, we extracted the depression status when the patient had to indicate whether he was depressed or anxious.
Outcome measures and selection of predictors: for the calculation of prevalence, a distinction was made between underweight (<18.5kg/m2), ideal weight (18.5-25kg/m2), excess weight (26-30kg/m2) and obesity (>30kg/m2). However, for reasons of focused analysis on malnutrition, we categorized the variable into two groups by grouping the ideal weight and obesity as a reference category, in addition to the underweight category. Potential predictors are contributed by sociodemographic predictors (gender, age), clinical (cough, dyspnea, chest pain, night sweats, nausea, vomiting, HIV status, history of TB treatment, depression, adherence, treatment outcome) and paraclinical (number of colonies by initial smear, initial number of colonies, initial culture, X-ray lung cavities, number of leukocytes, number of haemoglobin, number of platelets, number of lymphocytes, number of creatinine, liver SGOT, SGPT liver, smear conversion delay, culture conversion delay).
Statistical analysis: frequencies (per cent) or means (standard deviation; SD) were used to describe categorical and continuous variables. Univariate logistic regression was used to identify prognostic factors related to treatment with malnutrition, and then candidates with a p-value less than 0.10 were entered into the multivariate logistic regression. The independent predictors of malnutrition identified were selected using a backward procedure based on the lowest Akaike information criterion. Odds ratio (OR) with 95% confidence intervals (95% CI) were used as association parameters and the significance level was p less than 5%. The assumption of risk proportionality and log-linearity has been verified.
The Hosmer and Lemeshow fit validity test was used to measure the extent to which the probabilities predicted by the model correspond to the probabilities observed. Moreover, a calibration plot of observed and predicted probabilities was performed. An analysis of the operating characteristics of the receiver (ROC) by applying the Youden index method [15], to obtain the optimal score of the cutoff point was used to test the discriminating performances of our model. Then, at this optimal threshold, performance measures, including sensitivity, specificity, and positive and negative (PV) predictive values were estimated. The final model was validated internally using the 1000 bootstrap sample procedure. All analyses described above were performed with R version 3.5.1 software.
Ethics approval and consents to participate: the Cardio-pneumology Chair, attached to the Faculty of Medicine of the Gamal Abdel Nasser University in Conakry, approved the protocol for this study (023/CCP/18) which had also received administrative authorization from the Ministry of Health of Guinea via the National Tuberculosis Control Program (PNLAT). Patient data has been anonymized and confidentiality is guaranteed in accordance with the Declaration of Helsinki. The ethics approval process conforms to national regulations for such research.
Results
Description of demographic and clinical characteristics: our patients were predominantly male (68.3%), of whom 61% were malnourished, while women (31.6%) were women with a much higher proportion of malnourished women (72.5%). The average age of patients was 33.7, with 95% CI [32.2-35.2], most live in an urban setting (77.5%). HIV was positive in 50 patients, 36 (72%) of whom had a BMI <18.5. According to the category of patients, 38 patients (17.4%) were new DR-TB cases, and 180 patients (82.6%) had already received first-line anti-TB treatment. The most common clinical signs were cough (94%), night sweats (55%), chest pain (60.5%), and dyspnea (31.2%), and depression was seen in only (4.6%) patients. The cavity on chest radiograph was present in (16.9%) patients, and 72.9% of our patients had led their successful treatment at the end of follow-up (Table 1).
Table 1.
basic socio-demographic and clinical characteristics
| Variables | Gender | Total (%) | ||
|---|---|---|---|---|
| Female | Male | |||
| Age | Mean (SD) | 33.1 (10.3) | 34.0 (11.8) | 33.7 (11.3) |
| BMI | <=18.5 | 50 (72.5) | 91 (61.1) | 141 (64.7) |
| >18.5 | 19 (27.5) | 58 (38.9) | 77 (35.3) | |
| Residence | Urban | 53 (76.8) | 116 (77.9) | 169 (77.5) |
| Rural | 16 (23.2) | 33 (22.1) | 49 (22.5) | |
| Years of inclusion | 2016 | 5 (7.2) | 13 (8.7) | 18 (8.3) |
| 2017 | 40 (58.0) | 96 (64.4) | 136 (62.4) | |
| 2018 | 24 (34.8) | 40 (26.8) | 64 (29.4) | |
| HIV status | Negative | 41 (59.4) | 127 (85.2) | 168 (77.1) |
| Positive | 28 (40.6) | 22 (14.8) | 50 (22.9) | |
| History TB treatment | New DR-TB case | 22 (31.9) | 16 (10.7) | 38 (17.4) |
| Previous case treated | 47 (68.1) | 133 (89.3) | 180 (82.6) | |
| AFB count initial smear | <=3 | 43 (62.3) | 72 (48.3) | 115 (52.8) |
| >=3 | 26 (37.7) | 77 (51.7) | 103 (47.2) | |
| AFB count initial culture | <=3 | 30 (43.5) | 62 (41.6) | 92 (42.2) |
| >=3 | 39 (56.5) | 87 (58.4) | 126 (57.8) | |
| Lung Cavities X-Ray | No | 62 (89.9) | 129 (86.6) | 191 (87.6) |
| yes | 7 (10.1) | 20 (13.4) | 27 (12.4) | |
| Cough | No | 4 (5.8) | 9 (6.0) | 13 (6.0) |
| Yes | 65 (94.2) | 140 (94.0) | 205 (94.0) | |
| Dyspnea | No | 47 (68.1) | 103 (69.1) | 150 (68.8) |
| Yes | 22 (31.9) | 46 (30.9) | 68 (31.2) | |
| Chest Pain | No | 40 (58.0) | 92 (61.7) | 132 (60.6) |
| Yes | 29 (42.0) | 57 (38.3) | 86 (39.4) | |
| Night Sweat | No | 26 (37.7) | 72 (48.3) | 98 (45.0) |
| Yes | 43 (62.3) | 77 (51.7) | 120 (55.0) | |
| Nausea | No | 66 (95.7) | 137 (91.9) | 203 (93.1) |
| Yes | 3 (4.3) | 12 (8.1) | 15 (6.9) | |
| Vomiting | No | 65 (94.2) | 131 (87.9) | 196 (89.9) |
| Yes | 4 (5.8) | 18 (12.1) | 22 (10.1) | |
| Leukocytes count | Mean (SD) | 8.2 (5.5) | 7.9 (2.9) | 8.0 (3.9) |
| Haemoglobin count | Mean (SD) | 10.0 (2.1) | 10.9 (2.2) | 10.7 (2.2) |
| Platelets count | Mean (SD) | 71.9 (153.9) 3 | 92.5 (163.7) 3 | 86.0 (160.6) |
| Lymphocytes count | Mean (SD) | 1.8 (1.0) | 1.8 (1.4) | 1.8 (1.3) |
| Creatinine count | Mean (SD) | 75.0 (18.8) | 80.7 (19.3) | 78.9 (19.3) |
| Liver SGOT | Mean (SD) | 29.8 (5.7) | 29.1 (4.9) | 29.3 (5.2) |
| Liver SGPT | Mean (SD) | 30.9 (5.0) | 32.3 (5.1) | 31.9 (5.1) |
| Time to smear convert | Mean (SD) | 50.0 (26.8) | 58.7 (33.1) | 56.0 (31.5) |
| Time to culture convert | Mean (SD) | 46.6 (26.5) | 48.5 (22.6) | 47.9 (23.9) |
| Treatment outcome | Unsuccessful | 24 (34.8) | 35 (23.5) | 59 (27.1) |
| Successful | 45 (65.2) | 114 (76.5) | 159 (72.9) | |
| Depression | No | 63 (91.3) | 145 (97.3) | 208 (95.4) |
| Yes | 6 (8.7) | 4 (2.7) | 10 (4.6) | |
| SD = standard deviation; n = number; % = percentage; BMI = body mass index | ||||
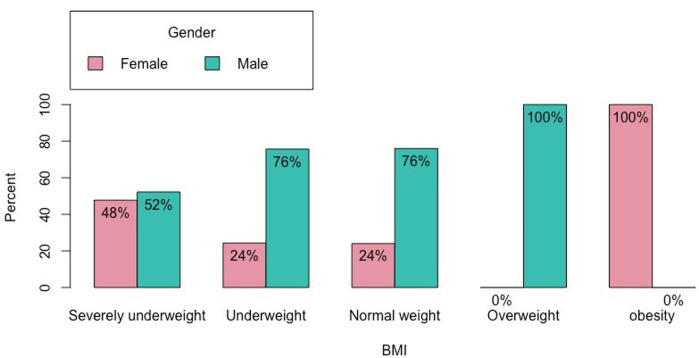
Prevalence of malnutrition: of the 218 participants in the study, 141 (64.7%) had a BMI of less than 18.5 and 77 (35.3%) had a BMI greater than 18.5. In classifying malnutrition according to the European Society of Clinical Nutrition and Metabolism, we note that 75 (34.4%) were of average weight, 74 (33.9%) were very underweight, 67 (30.7) had an underweight and one overweight patient (0.5%). Men were the most affected by severe underweight, with a proportion of 16% of the total against 14.7% for women and 25.7% against 8.3%, respectively, for the moderate weight (Figure 1).
Figure 1.
distribution of patients by weight status at initiation of second-line anti-TB treatment
Predictors of malnutrition: a univariate logistic regression (Table 2) found the factors potentially associated with malnutrition. After inclusion of all candidate variables in the univariate regression model, the decrease in hemoglobin (OR = 1.2[1.04-1.3, p = 0.01]), the presence of night sweats (OR = 0.5[0.3-0.9, p = 0.02]), the presence of cough (OR = 7.1[1.3-130.1, p = 0.06]) were the factors statistically associated with malnutrition. However, several other variables including sex, nausea, vomiting, leukocyte count, creatinine, and SGPT had p values <= 0.1. These candidate variables were included in the multivariate regression model (Table 2) and the predictors were selected through the backward procedure based on the lowest Akaike information criterion (AIC = 258.2). Thus, the final model (Table 3) identified as predictors of malnutrition: the unsuccessful treatment (OR = 3.8[1.7-8.9, p = 0.001]), the active presence of the mycobacterium tuberculosis characterized by the presence of cough (OR =32.8 [4.61-706.4, p = 0.003]), night sweats (OR =0.3[0.1-0.5, p <0.001]), the presence of cavity on chest X-ray (OR =3.7[1.4-10.6, p = 0.012]), and the increase in bacteriological conversion time (OR =0.99 [0.97-1.00, p = 0.014]). And, the increase in serum creatinine (OR = 1.02 [1.00-1.04, p = 0.036]), increase in liver SGPT transaminase (OR = 0.9[0.8-0.9, p = 0.02]) and anemia (OR = 1.1[0.9-1.3, p = 0.083]).
Table 2.
predictors of malnutrition among DR-TB patients in Guinea, uni, and multivariate logistic regression analysis
| Predictors | OR univariable (95% IC) | P-value | OR multivariable (95% IC) | P-value |
|---|---|---|---|---|
| Gender (Male vs Female) | 1.68 (0.91-3.18) | 0.103 | 1.76 (0.80-3.98) | 0.166 |
| Age | 0.99 (0.96-1.01) | 0.329 | 0.98 (0.95-1.01) | 0.228 |
| Residence (Urban vs rural) | 0.86 (0.43-1.67) | 0.657 | 0.62 (0.25-1.46) | 0.279 |
| Years of inclusion (2017 vs 2016) | 2.10 (0.71-7.72) | 0.212 | 1.79 (0.52-7.42) | 0.382 |
| Years of inclusion (2018 vs 2016) | 1.83 (0.58-7.07) | 0.332 | 2.08 (0.50-10.01) | 0.331 |
| HIV (Yes vs No) | 0.65 (0.32-1.27) | 0.219 | 0.74 (0.27-1.99) | 0.557 |
| History of TB treatment (Previously vs Newly) | 1.22 (0.59-2.67) | 0.596 | 0.70 (0.23-2.04) | 0.512 |
| AFB count initial smear (≥3+ vs <3 +) | 1.05 (0.60-1.83) | 0.860 | 1.10 (0.53-2.29) | 0.803 |
| AFB count initial culture (≥3+ vs <3 +) | 1.50 (0.85-2.67) | 0.166 | 1.63 (0.78-3.48) | 0.199 |
| Lung Cavities X Ray (Yes vs No) | 1.19 (0.53-2.57) | 0.670 | 3.06 (0.97-10.04) | 0.058 |
| Cough (Yes vs No) | 7.07 (1.35-130.06) | 0.063 | 35.70 (4.26-826.25) | 0.004 |
| Dyspnea (Yes vs No) | 0.56 (0.29-1.03) | 0.067 | 0.62 (0.27-1.41) | 0.262 |
| Chest Pain (Yes vs No) | 0.9 7 (0.55-1.71) | 0.913 | 1.30 (0.62-2.77) | 0.491 |
| Night sweat (Yes vs No) | 0.51 (0.29-0.89) | 0.018 | 0.27 (0.13-0.54) | P<0.001 |
| Nausea (Yes vs No) | 0.91 (0.27-2.66) | 0.867 | 1.90 (0.34-11.66) | 0.466 |
| Vomiting (Yes vs No) | 0.37 (0.11-1.05) | 0.086 | 0.27 (0.05-1.15) | 0.095 |
| Leukocytes count (m ± SD) | 0.91 (0.82-1.00) | 0.065 | 0.89 (0.77-1.00) | 0.080 |
| Haemoglobin count (m ± SD) | 1.21 (1.06-1.39) | 0.007 | 1.06 (0.89-1.28) | 0.513 |
| Platelets count (m ± SD) | 1.00 (1.00-1.00) | 0.970 | 1.00 (1.00-1.00) | 0.705 |
| Lymphocytes count (m ± SD) | 1.20 (0.99-1.53) | 0.092 | 1.21 (0.93-1.70) | 0.211 |
| Creatinine count (m ± SD) | 1.01 (1.00-1.03) | 0.112 | 1.02 (1.00-1.04) | 0.122 |
| Liver SGOT (m ± SD) | 0.99 (0.94-1.05) | 0.806 | 1.03 (0.95-1.12) | 0.426 |
| Liver SGPT (m ± SD) | 0.96 (0.91-1.02) | 0.186 | 0.92 (0.85-0.99) | 0.035 |
| Time to smear convert (m ± SD) | 0.99 (0.98-1.00) | 0.125 | 0.99 (0.97-1.00) | 0.049 |
| Time to culture convert (m ± SD) | 1.00 (0.99-1.01) | 0.952 | 1.00 (0.99-1.02) | 0.778 |
| Treatment outcome (Unsuccessful vs Successful) | 2.38 (1.22-4.92) | 0.014 | 3.20 (1.32-8.33) | 0.013 |
| Depression (Yes vs No) | 0.78 (0.16-2.88) | 0.719 | 1.73 (0.29-8.81) | 0.520 |
OR = odds ratio, CI = confidence interval, HIV = human immunodeficiency virus
Table 3.
predictors of malnutrition among DR-TB patients selected in the final model of the multivariate regression
| Predictors | Estimate | Std. Error | z value | Pr (>|z|) |
|---|---|---|---|---|
| (Intercept) | -4.311436 | 1.807937 | -2.385 | 0.017092 * |
| Treatmentoutcome (Unsuccessful vs. successful) | 1.234764 | 0.400429 | 3.084 | 0.002045 ** |
| Cough (Yes vs. No) | 3.560339 | 1.191524 | 2.988 | 0.002808 ** |
| Creatinine count | 0.019001 | 0.008806 | 2.158 | 0.030949 * |
| Liver SGPT | -0.075062 | 0.032983 | -2.276 | 0.022858 * |
| Lung cavities X-Ray (Yes vs. No) | 1.011918 | 0.523634 | 1.932 | 0.053299. |
| Night sweat (Yes vs No) | -1.258227 | 0.336029 | -3.744 | 0.000181 *** |
| Time to smear convert | -0.013739 | 0.005678 | -2.420 | 0.015527 * |
| Haemoglobin count | 0.140811 | 0.076279 | 1.846 | 0.064892 |
Log Likelihood= -120.102, Akaike Inf. Crit.=258.205
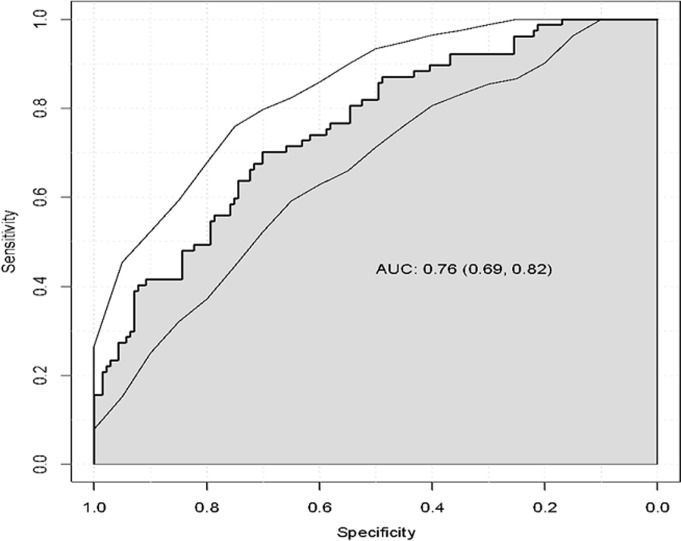
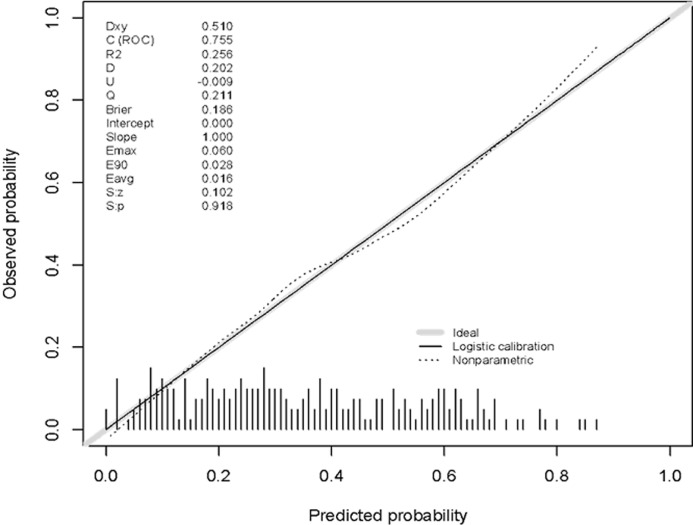
A characteristic operating curve of the receiver illustrates a good discriminatory capacity of our model (Figure 2), the index c adjusted to predict malnutrition was 0.76[95% CI, 0.69 - 0.82]. The optimism of 1000 samples was 0.018, which corresponds to a corrected value of the c-statistic with the optimism of 0.74. The p-value of 0.4 produced by the Hosmer and Lemeshow fit quality test confirms that the model is statistically adjusted with adequate discriminatory capacity (P> 0.05). Finally, the calibration curve (Figure 3) shows a good agreement between the risk of malnutrition estimated by the final model and the observed cases of malnutrition, across the predicted probability continuum.
Figure 2.
ROC-curve of the multivariate analyses; ROC-curve =receiver-operating characteristic; AUC = area under the curve; 95% CI = 95% confidence interval
Figure 3.
calibration plot of the predicted probability versus the observed probability
Discussion
This retrospective cohort study revealed a prevalence of malnutrition of 64.7% in tuberculosis patients on second-line treatment. We also note that 16% of men were severely malnourished, and 25.7% were underweight. A study in nine countries in West and Central Africa on the evaluation of the treatment results of patients on antituberculosis treatment regimens revealed a prevalence of 56 [16]. A similar prevalence (56%) has been reported by Elliot et al. in South Africa [17]. Another study by Nyaki FS et al. in Tanzania found a prevalence of 53% in Tanzania [18], unlike the research in Ethiopia, which reported a prevalence of only 10.2% [19]. At the same time, in East Asia, the Ho Park study in South Korea revealed 24.3% of malnutrition (BMI <18.5) [20] and Bei C 59.9% in China [21]. Our study population was relatively young and male 68.35%), with a prevalence of 64.7%, average age of patients 33.7, 95% CI [32.2, 35.2], resident most in urban areas. In Guinea, the same male predominance is also sawed in patients receiving first-line treatment.
The malnourished patients found in our study had active tuberculosis, which explained the persistence of night sweats from coughing and the presence of radiological lesions and was, for the most part, patients in retreatment. Our patients have the same profile as those described by other authors in Malawi [22], Tanzania [18], and South Africa [23]. Although several studies have shown that a low BMI is a risk factor for mortality in tuberculosis patients co-infected with HIV [23-25], this factor was not one of the identified predictors of our study. HIV was positive in 50 patients, 36 (72%) of whom had a BMI <18.5 but were not associated with malnutrition (P value> 0.05). The role of malnutrition, although recognized among the factors of non-therapeutic success, by several studies [17, 18, 26, 27], its management is still insufficient in several tuberculosis control programs due to lack of financial means. Our results are consistent with those of the literature according to which patients suffering from malnutrition were highly likely not to succeed in their antituberculosis treatment.
We found a hepatic transaminase (SGPT) disturbance during malnutrition (p = 0.02). Medicines used to treat tuberculosis can lead to high liver transaminases, which is a typical situation. The increases may be small, but hepatotoxicity can have serious consequences if left unattended [28-30]. Active bacillary tuberculosis is often associated with the most severe symptoms (fever, night sweats, cough, and chest pain); we have shown a link with malnutrition (p> 0.05). Infection can lead to undernutrition associated with increased metabolic demand and decreased intake, and nutritional deficiencies can worsen the disease or delay healing by reducing essential immune functions [5]. In a patient treated for active tuberculosis by second-line treatment, the occurrence of renal symptoms is common. Our study showed an increase in serum creatinine during malnutrition; this can be explained using second-line injectable antituberculosis drugs (aminoglycosides and capreomycin), known for their nephrotoxic and ototoxic effects [8, 31].
Conclusion
Our study highlighted the factors associated with malnutrition during the first phase of treatment in the context of cause and effect. The relevant factors found are the presence of active Tb, increased hepatic transaminases, increased bacteriological conversion time, and decreased serum creatinine. The study also proved the negative effect of malnutrition on the success of TB treatment. Taking these factors into account in the clinical practice of TB patients by taking steps to improve nutritional intake in patients would improve their management.
What is known about this topic
Tuberculosis can lead to malnutrition, and malnutrition can still make tuberculosis worse if left untreated. Nutritional support for tuberculosis patients is increasingly becoming a common practice;
Most people with active TB lose weight; weight gain during treatment is a sign of improvement in their condition;
Systematic screening for tuberculosis in malnourished patients is an initiative that has been tested in several countries and recognized as good practice by WHO.
What this study adds
We retain from this study that malnutrition is widespread among multidrug-resistant tuberculosis patients in Guinea. These patients generally have a long course because they benefit from a late diagnosis in most cases in our context, where the Xpert MTB/RIF test and other tests offered by the WHO is only reserved for a small group of targets (cases of retreatement, HIV +, children, and prisoners);
This study demonstrated a strong relationship between the deterioration of the nutritional status of multidrug-resistant tuberculosis patients and the deterioration of clinical characteristics (hemoglobin, active symptoms of tuberculosis, disturbance of renal workup and prolongation of the bacteriological conversion time). This justifies the legitimacy of the nutritional support that must be systematically provided to these patients to hope for compliance with treatment;
This study also shows the need to immediately apply the WHO recommendations concerning the systematization of the Xpert MTB/RIF test as a first-line diagnosis and to put in place a bold policy of nutritional support for patients to improve on the one hand the condition of the patient clinic and the outcome of the treatment at term.
Acknowledgments
We thank the National Tuberculosis Control Program for their collaboration, Action Damien Foundation for their patient's support and West African Network Against Tuberculosis (WARN-TB) for their technical support.
Footnotes
Cite this article: Aboubacar Sidiki Magassouba et al. Malnutrition prevalence and associated biochemical factors among drug-resistance tuberculosis (DR-TB) patients at key treatment sites in Conakry City, Republic of Guinea. Pan African Medical Journal. 2021;38(279). 10.11604/pamj.2021.38.279.27270
Competing interests
The authors declare no competing interests.
Authors' contributions
ASM, AAT and BDD conceived the study design, analyzed the data, and drafted the manuscript, LMC, and MD contributed to the design, AMB collected data and commented the manuscript, NC, DT contributed to the design, organization of the research project, and critical revision to the manuscript, TAK, AFT, GC and SNC commented on the manuscript. All authors read and approved the last version of the manuscript.
References
- 1.World Health Organization (WHO) Global tuberculosis reports. Accessed 10th September 2019.
- 2.NTP Annual report of tuberculosis control activities. Guinea. 2018.
- 3.Maher D. The natural history of Mycobacterium tuberculosis infection in adults. Tuberc E-Book Compr Clin Ref. 2009;129 [Google Scholar]
- 4.World Health Organization (WHO) Tuberculosis. Accessed 8th September 2019.
- 5.Sinclair D, Abba K, Grobler L, Sudarsanam TD. Nutritional supplements for people being treated for active tuberculosis. Cochrane Database Syst Rev. 2011 Nov 9;11:CD006086. doi: 10.1002/14651858.CD006086.pub3. [DOI] [PubMed] [Google Scholar]
- 6.Organisation mondiale de la santé Malnutrition. Accessed 26th August 2019.
- 7.Warmelink I, Hacken NH ten, Werf TS van der, Altena R van. Weight loss during tuberculosis is an important risk factor for drug-induced hepatotoxicity. Br J Nutr. 2011;105(3):400–408. doi: 10.1017/S0007114510003636. [DOI] [PubMed] [Google Scholar]
- 8.Gupta KB, Gupta R, Atreja A, Verma M, Vishvkarma S. Tuberculosis, and nutrition. Lung India. 2009 Jan;26(1):9–16. doi: 10.4103/0970-2113.45198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vest MT, Papas MA, Shapero M, McGraw P, Capizzi A, Jurkovitz C. Characteristics and Outcomes of Adult Inpatients with Malnutrition. JPEN J Parenter Enteral Nutr. 2018;42(6):1009–1016. doi: 10.1002/jpen.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaidar-Person O, Person B, Szomstein S, Rosenthal RJ. Nutritional deficiencies in morbidly obese patients: a new form of malnutrition? Part A: vitamins. Obes Surg. 2008;18(7):870–876. doi: 10.1007/s11695-007-9349-y. [DOI] [PubMed] [Google Scholar]
- 11.Via M. The Malnutrition of Obesity: Micronutrient Deficiencies That Promote Diabetes. ISRN Endocrinol. 2012;2012:103472. doi: 10.5402/2012/103472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Institute of Statistics (INS) Demographic and Health Survey and Multiple Indicators. Guinea.
- 13.Weir CB, Jan A. StatPearls. BMI Percentile Classification And Cut Off Points. Accessed August 25 2019. [PubMed] [Google Scholar]
- 14.Falzon D, Schünemann HJ, Harausz E, González-Angulo L, Lienhardt C, Jaramillo E, et al. World Health Organization treatment guidelines for drug-resistant tuberculosis, 2016 update. Eur Respir J. 2017 Mar 22;49(3):1602308. doi: 10.1183/13993003.02308-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Unal I. Defining an Optimal Cut-Point Value in ROC Analysis: An Alternative Approach. Comput Math Methods Med. 2017;2017:3762651. doi: 10.1155/2017/3762651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trébucq A, Schwoebel V, Kashongwe Z, Bakayoko A, Kuaban C, Noeske J, et al. Treatment outcome with a short multidrug-resistant tuberculosis in nine African countries. 2018;22(1):17–25. doi: 10.5588/ijtld.17.0498. [DOI] [PubMed] [Google Scholar]
- 17.Elliott E, Draper HR, Baitsiwe P, Claassens MM. Factors affecting the treatment of tuberculosis in Northern Cape, South Africa. Public Health Action. 2014;4(3):201–203. doi: 10.5588/pha.14.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nyaki FS, Taksdal M, Mbuya AW, Sariko M, Lekule IA, Kisonga RM, et al. Predictors of Nutritional Status in Treated Patients for Multidrug-Resistant Tuberculosis at a Referral Hospital in Tanzania. J Clin Infect Dis Pract. 2016;1(2):1–5. [Google Scholar]
- 19.Demile B, Zenebu A, Shewaye H, Xia S, Guadie A. Risk factors associated with multidrug-resistant tuberculosis (MDR-TB) in tertiary armed forces referral and teaching hospital, Ethiopia. BMC Infect Dis. 2018;18(1):249. doi: 10.1186/s12879-018-3167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park HO, Kim SH, SH Moon, Byun JH, JW Kim, Lee CE, et al. Association Between Body Mass Index and Sputum Culture Conversion among South Korean Patients with Multidrug-Resistant Tuberculosis in a Tuberculosis Referral Hospital. Infect Chemother. 2016;48(4):317–323. doi: 10.3947/ic.2016.48.4.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bei C, Fu M, Zhang Y, Xie H, Yin K, Liu Y, et al. Mortality and associated factors of patients with extensive drug-resistant tuberculosis: an emerging public health crisis in China. BMC Infect Dis. 2018 Jun 7;18(1):261. doi: 10.1186/s12879-018-3169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zachariah R, MP Spielmann, Harries AD, Salaniponi FML. Moderate to severe malnutrition in patients with tuberculosis is a risk factor associated with early death. Trans R Soc Trop Med Hyg. 2002;96(3):291–294. doi: 10.1016/s0035-9203(02)90103-3. [DOI] [PubMed] [Google Scholar]
- 23.Hanrahan CF, Golub JE, Mohapi L, Tshabangu N, Modisenyane T, Chaisson RE, et al. Body mass index and risk of tuberculosis and death. AIDS. 2010 Jun 19;24(10):1501–8. doi: 10.1097/QAD.0b013e32833a2a4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Podewils LJ, Holtz T, Riekstina V, Skripconoka V, Zarovska E, Kirvelaite G, et al. Impact of malnutrition on clinical presentation, clinical course, and mortality in MDR-TB patients. Epidemiol Infect. 2011;139(1):113–120. doi: 10.1017/S0950268810000907. [DOI] [PubMed] [Google Scholar]
- 25.Hicks RM, Padayatchi N, Shah NS, Wolf A, Werner L, Sunkari VB, et al. Malnutrition associated with unfavorable outcome and death among South African MDR-TB and HIV co-infected children. Int J Tuberc Lung Dis Off J Int Union Tuberc Lung Dis. 2014;18(9):1074–1083. doi: 10.5588/ijtld.14.0231. [DOI] [PubMed] [Google Scholar]
- 26.Mukati S, Julka A, Varudkar HG, Singapurwala M, Agrawat JC, Bhandari D, et al. A study of clinical profile of cases of MDR-TB and evaluation of challenges faced in initiation of second line Anti tuberculosis treatment for MDR-TB cases admitted in drug resistance tuberculosis centre. Indian J Tuberc. 2019;66(3):358–363. doi: 10.1016/j.ijtb.2016.11.031. [DOI] [PubMed] [Google Scholar]
- 27.Cegielski JP, McMurray DN. The relationship between malnutrition and tuberculosis: evidence from studies in humans and experimental animals. Int J Tuberc Lung Dis Off J Int Union Tuberc Lung Dis. 2004;8(3):286–298. [PubMed] [Google Scholar]
- 28.A World Health Organization resource . Safety Monitoring of Medicinal Products: Guidelines for Setting Up and Running a Pharmacovigilance Centre. Accessed 9th September 2019. [Google Scholar]
- 29.Bouazzi OE, Hammi S, Bourkadi JE, Tebaa A, Tanani DS, Soulaymani-Bencheikh R, et al. First line antituberculosis induced hepatotoxicity: incidence and risk factors. Pan Afr Med J. 2016 Nov 16;25:167. doi: 10.11604/pamj.2016.25.167.10060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Isa SE, Ebonyi AO, Shehu NY, Idoko P, Anejo-Okopi JA, Simji G, et al. Antituberculosis drugs and hepatotoxicity among hospitalized patients in Jos, Nigeria. Int J Mycobacteriology. 2016;5(1):21–26. doi: 10.1016/j.ijmyco.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 31.Shibeshi W, Sheth AN, Admasu A, Berha AB, Negash Z, Yimer G. Nephrotoxicity, and ototoxic symptoms of injectable second-line anti-tubercular drugs among patients treated for MDR-TB in Ethiopia: a retrospective cohort study. BMC Pharmacol Toxicol. 2019 May 23;20(1):31. doi: 10.1186/s40360-019-0313-y. [DOI] [PMC free article] [PubMed] [Google Scholar]