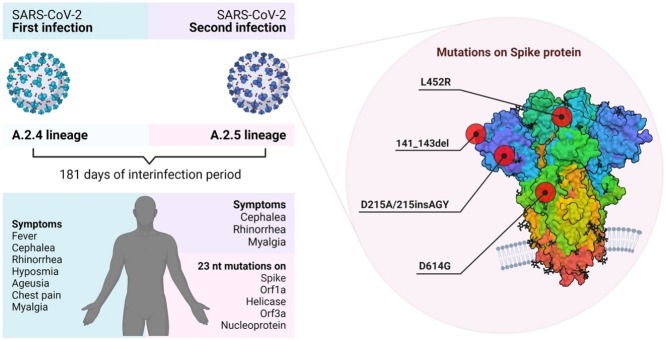
Graphical abstract
Keywords: SARS-CoV-2, COVID-19, Panama, Reinfection, Mutation
Abstract
We report a case of reinfection by SARS-CoV-2 with the second virus harboring amino acid changes in the Spike protein (141-143del, D215A, ins215AGY, L452R, D614G), orf1a, helicase, orf3a, and Nucleocapside. The virus associated with the reinfection, from an endemic lineage containing the S:L452R immune escape mutation, was circulating in Panama at the time.
Introduction
In March 2020, the World Health Organization declared the COVID-19 pandemic. As it is evolving, more cases of reinfection by SARS-CoV-2 are being reported (Gupta et al., 2020, To et al., 2020), even if it is considered a rare event. New variants with the potential to spread more efficiently have been described (Naveca et al., 2019, Tegally et al., 2021). Some mutations in the Spike (S) protein could increase viral infectivity (Korber et al., 2020; Tegally et al., 2021; Volz et al., 2021) or decrease sensitivity to neutralizing antibodies (Hoffmann et al., 2020, Li et al., 2020a, Li et al., 2020b, Naveca et al., 2019, Resende et al., 2021, Tchesnokova et al., 2021) and have been associated with secondary SARS-CoV-2 infections. Here, we report a case of reinfection with SARS-CoV-2 in Panama, confirmed by complete genome sequencing, in which the second virus contained new and previously described mutations implicated in immune escape and higher transmission.
Methodology
SARS-CoV-2 diagnosis
Viral RNA was extracted from the first nasopharyngeal swab sample using Qiagen viral RNA extraction kit and from the second using Chemagic360 kit. SARS-CoV-2 was detected using the RT-qPCR Powercheck kits version 1.0 and version 2.0 for the first and second samples, respectively. The cut-off value for both was cycle threshold (Ct) < 35.
Serology
Antibodies evaluation was performed using Virclia IgM and IgG monotest. The cut-off index for positive samples was calculated as >0.6 for immunoglobulin(Ig)M and >1.6 for IgG.
Viral isolation
Samples were used for inoculation on Vero cells (ATCC CCL81). Once cytopathic effect was observed in 75% of cells, virus isolation was confirmed by reverse transcription-quantitative polymerase chain reaction (RT-qPCR).
Genome characterization and phylogenetic analysis
SARS-CoV-2 genome sequencing was performed using ARTIC network protocol. New mutations were confirmed at depth of 250× and allele frequency higher than 15%. Lineage classification was performed using pangoLEARN version 2021-01-30. A maximum-likelihood tree was drawn using a GTR substitution model (Supplementary methods).
3. D protein modeling
RNAs were translated into proteins using MEGA-7 software for 3D modeling (Supplementary methods).
Results
Case description
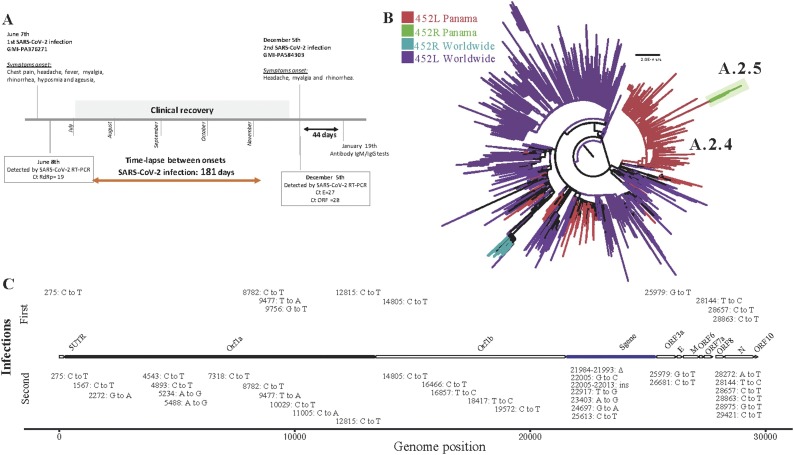
On June 8, 2020, a 36-year-old man with no reported comorbidities exhibited chest pain since the day before, myalgia, fever, cephalea, and rhinorrhea (Figure 1 A). SARS-CoV-2 RNA (GMI-PA376271) was detected by RT-PCR with a Ct = 19 for RdRp gene. The patient lives with 4 persons; one of them had COVID-19 symptoms and was RT-qPCR positive at the same time. During the 14 days of home quarantine isolation, the patient developed hyposmia and ageusia. On December 5, 2020, 181 days after the first symptoms onset, he developed cephalea, myalgia and rhinorrhea. SARS-CoV-2 (GMI-PA584303) was detected with a Ct = 27 for gene E and a Ct = 28 for RdRp. On both infections, the patient had total recovery with no sequelae. SARS-CoV-2 (GMI-PA584303) was isolated and induced a cytopathic effect on cells. Serology tests between the 2 infections were not done. Forty-four days after the second infection, serology tests IgM and IgG positive index results were 0.695 and 11.04, respectively.
Figure 1.
Timeline of SARS-CoV-2 reinfection case and genomic findings. (A) Time-lapse of SARS-CoV-2 reinfection case with the description of symptoms and laboratory findings. (B) Phylogenetic tree of SARS-CoV-2 complete genome from the viruses related to the first and second infections. (C) Mutations identified in the SARS-CoV-2 viruses from the first and second infections compared with the sequence of reference MN908947.3.
The patient signed the Gorgas informed consent for clinical cases, approved by the ICGES IRB (N.1079/CNBI/ICGES/17).
SARS-CoV-2 genome characterization
Whole-genome sequencing showed that both viruses belong to 2 endemic lineages (Figure 1B, Supplementary Figure S1): GMI-PA376271 (EPI_ISL_1001457) being A.2.4, previously named A.2.1. (Franco et al., 2020). The second genome GMI-PA584303 accumulated 33 nucleotide mutations compared with reference MN908947.3 and 23 (21 nucleotide substitutions, 1 deletion, 1 insertion) compared to the first sample GMI-PA376271 (Figure 1C, Supplementary Table S1). Of these nucleotide substitutions, 15 induced amino acid changes: 3 in the S protein (D215A, L452R, D614G), 5 in ORF1a (1 in NSP1, 2 in NSP3, 1 in NSP4, 1 in NSP6), 4 in the Nucleocapsid (N), 2 in helicase and 1 in ORF3a. Additionally, we detected a nucleotide deletion (21984-21993del) inducing a loss of 3 amino acids (L141del, G142del, V143del) and a nucleotide insertion (22005-22013 in.) associated with the insertion of 3 amino acids (A215, G216, Y217), balancing the reading frame of the translated S protein.
Mutations in the Spike and Nucleotide proteins
The amino acid mutations identified in the S protein are located in the subunit S1 (Supplementary Table S1, Supplementary Figure S2A, Li et al., 2020b, Vilar and Isom, 2021), responsible for receptor binding. The deletion of amino acid LGV (Leucine, Glycine, Valine) at positions 141–143 and the insertion of AGY (Alanine, Glycine, Tyrosine) inducing the substitution D215A are in the N-terminal domain (NTD). In contrast, the L452R mutation is located in the region binding domain (RBD) for ACE2 receptor, D614G being between the RBD and the amino acid bridge between the S1 and S2 subunits.
The mutations S197L, M234I are located in the link region between the RNA-binding domain and the dimerization domain, whereas the P383L is in the C-terminal domain of the N protein (Supplementary Table S1, Supplementary Figure S2B, Cubuk et al., 2021).
Discussion
We describe the first confirmed SARS-CoV-2 reinfection in Panama in an immunocompetent patient who presented mild symptomatic disease during both infections. Reinfections of seasonal coronaviruses have been described in settings where a high rate of community infection and/or incomplete immunologic protection took place (Edridge et al., 2020). Previous SARS-CoV-2 reinfection reported cases showed that an induced neutralizing antibody response generated by the first infection is not sufficient for protection (Tohidinia and Sefid, 2020). Several variables could be implicated in reinfection including the absence of a proper immune response, low amount of neutralizing antibodies or viruses with mechanisms to evade the immune response. A caveat of this clinical case is that there is no serological sample between both infections to determine if the patient developed neutralizing antibodies after the first exposure. However, the mild symptoms after the reinfection could indicate some partial protection, and the genetic characterization of the viruses showed that the second virus harbored some mutations already described as implicated in escape from neutralizing antibodies. The fact that the second virus was isolated could suggest no or low neutralizing antibodies in the sample that could have inhibited the infection of cells. The specific reasons for this reinfection remain an open question.
SARS-CoV-2 genome analysis showed that both infections were induced by viruses from Panamanian endemic lineages, A.2.4 (Franco et al., 2020) for the first, and A.2.5, containing Spike mutations D614G and L452R, for the second. Most of the 15 induced amino acid changes in the second virus, as with those in ORF1a, have not been previously described. Most mutations detected in S and N, whose coding genes have the highest degree of variability in the SARS-CoV-2 genome (Vilar and Isom, 2021), have been previously associated with features that could facilitate reinfection. D614G mutation in S, with a higher affinity for ACE2 receptor, results in increased infectivity and higher virus transmission (Korber et al., 2020; Li et al., 2020b); probably explaining why this mutation, detected in most dominant variants, has overtaken the entire globe (Vilar and Isom, 2021). Spike mutation L452R, decreasing sensitivity to neutralizing antibodies, has been associated with immune escape and reinfection and is present in the variant of interest (VOI) B.1.526.1, and the variants of concern (VOC) B.1.427 and B.1.429 (Hoffmann et al., 2020, Li et al., 2020a, Li et al., 2020b, Naveca et al., 2019, Resende et al., 2021, Tchesnokova et al., 2021). Currently, lineage A.2.5 is the only A lineage with this mutation which is widely spread in B lineages. The observed 3 amino acid deletion (L141del, G142del, V143del) in S was located in an epitope involved in the generation of neutralizing antibodies (Tohidinia and Sefid, 2020) and Y144del detected in the VOC B.1.1.7. D215A in the NTD of Spike has not been described; however, D215G has been detected in the VOC B.1.351. As NTD has a role in interaction with the receptor, future studies are needed to analyze the role of these Spike mutations. In the N protein, residues S194 and M234 are some of the most mutated (Vilar and Isom, 2021), suggesting a purifying selection, and mutation P365S was associated with B cell epitopes (Tohidinia and Sefid, 2020), suggesting that it could also let to an immune escape phenotype. These changes may reflect the ability of the second virus to escape neutralizing antibodies that could have been produced during the previous infection.
Future studies associated with the genomic surveillance of the virus should be done to determine the prevalence of the mutations described, their role in SARS-CoV-2 transmission and immune escape, their contribution to the reinfection cases in the country and whether they should be considered VOI.
Authors’ contribution
Study design: YD, JMP, AAM, SL-V; Data Collection: YD, AO, AW, DC, CG, BM, MM-M, MC, GV, LL, DF, YP, OC, JG, AM, LA, DB, IG, JC, ZC, IG, AV, MG, DA, EM, MC-G, EV, RR, RC, RC-C, AAM, SL-V, Gorgas COVID19 Team; Patient follow-up: AO, AW; Data analysis: YD, AAM, SL-V; Writing: YD, AO, AW, JMP, AAM, SL-V
Ethical approval
The patient signed the Gorgas informed consent for clinical cases, approved by the ICGES IRB (N.1079/CNBI/ICGES/17).
Funding
This work was supported by the COVID-19 Panama’s response funds, grant [SINIP:0199.001] of the Ministry of Economy (YD), the S istema Nacional de Investigación SNI grants 21-2020 (S.L-V.) and 191-2017 (A.A.M.), as well as funds from Sus Buenos Vecinos Foundation.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgments
S.L-V., A.A.M., J.M.P., L.E.A., A.M.M., are members of Sistema Nacional de Investigación (SNI) from SENACYT, Panama. We gratefully acknowledge the authors, originating and submitting laboratories of the sequences from GISAID’s EpiFlu Database on which this research is based (Supplementary Table S2).
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.ijid.2021.06.004.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- Cubuk J., Alston J.J., Incicco J.J., Singh S., Stuchell-Brereton M.D., Ward M.D., et al. The SARS-CoV-2 nucleocapsid protein is dynamic, disordered, and phase separates with RNA. Nat Commun. 2021;12:1–17. doi: 10.1038/s41467-021-21953-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edridge A.W.D., Kaczorowska J., Hoste A.C.R., Bakker M., Klein M., Loens K., et al. Seasonal coronavirus protective immunity is short-lasting. Nat Med. 2020;26:1691–1693. doi: 10.1038/s41591-020-1083-1. [DOI] [PubMed] [Google Scholar]
- Franco D., Gonzalez C., Abrego L.E., Carrera J.P., Diaz Y., Caisedo Y., et al. Early transmission dynamics, spread, and genomic characterization of SARS-CoV-2 in Panama. Emerg Infect Dis. 2020;27 doi: 10.1101/2020.07.31.20160929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta V., Bhoyar R.C., Jain A., Srivastava S., Upadhayay R., Imran M., et al. Asymptomatic reinfection in 2 healthcare workers from india with genetically distinct severe acute respiratory syndrome coronavirus 2. Clin Infect Dis. 2020:ciaa1451. doi: 10.1093/cid/ciaa1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann C.A., Sarturi J.O., Weindorf D.C., Darren D., Ramirez H.A.R., Jackson S., et al. Confirmed reinfection with SARS-CoV-2 variant VOC-202012/01. Clin Infect Dis. 2020:1–27. doi: 10.1093/cid/ciab014/6076528. [DOI] [Google Scholar]
- Korber B., Fisher W.M., Gnanakaran S., Yoon H., Theiler J., Abfalterer W., et al. Tracking Changes in SARS-CoV-2 Spike: Evidence that D614G Increases Infectivity of the COVID-19 Virus. Cell. 2020;182(4):812–827. doi: 10.1016/j.cell.2020.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Liao C., Wang Q., Tan Y., Luo R., Qiu Y., et al. The ORF6, ORF8 and nucleocapsid proteins of SARS-CoV-2 inhibit type I interferon signaling pathway. Virus Res. 2020;286 doi: 10.1016/j.virusres.2020.198074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Wu J., Nie J., Li Zhang, Hao H., Liu S., et al. The impact of mutations in SARS-CoV-2 spike on viral infectivity and antigenicity. Cell. 2020;182:1284–1294.e9. doi: 10.1016/j.cell.2020.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naveca F., Costa C., Nascimento V., Souza V., Nascimento F., Costa Á, et al. virological.org; 2019. SARS-CoV-2 reinfection by the new variant of concern (VOC) P.1 in Amazonas. [Google Scholar]
- Resende P.C., Bezerra J.F., Teixeira de Vasconcelos R.H., Arantes I., Appolinario L., Mendonça A.C., et al. Spike E484K mutation in the first SARS-CoV-2 reinfection case confirmed in Brazil. Oxford J. 2021;33 [Google Scholar]
- Tchesnokova V., Kulakesara H., Larson L., Bowers V., Rechkina E., Kisiela D., et al. Acquisition of the L452R mutation in the ACE2-binding interface of Spike protein triggers recent massive expansion of SARS-Cov-2 variants. bioRxiv. 2021 doi: 10.1101/2021.02.22.432189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegally H., Wilkinson E., Lessells R.J., Giandhari J., Pillay S., Msomi N., et al. Sixteen novel lineages of SARS-CoV-2 in South Africa. Nat Med. 2021;27(3):440–446. doi: 10.1038/s41591-021-01255-3. [DOI] [PubMed] [Google Scholar]
- To K.K.-W., Hung I.F.-N., Ip J.D., Chu A.W.-H., Chan W.-M.-M., Tam A.R., et al. Coronavirus disease 2019 (COVID-19) re-infection by a phylogenetically distinct severe acute respiratory syndrome coronavirus 2 strain confirmed by whole genome sequencing. Clin Infect Dis. 2020:ciaa1275. doi: 10.1093/cid/ciaa1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohidinia M., Sefid F. Identification B and T-Cell epitopes and functional exposed amino acids of S protein as a potential vaccine candidate against SARS-CoV-2/COVID-19. Microb Pathogen. 2020;148 doi: 10.1016/j.micpath.2020.104459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilar S., Isom D.G. One year of SARS-CoV-2: how much has the virus changed? Biology (Basel) 2021;10(2):91. doi: 10.3390/biology10020091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volz E., Mishra S., Chand M., Barrett J.C., Johnson R., Geindelberg L., et al. Assessing transmissibility of SARS-CoV-2 lineage B.1.1.7 in England. Nature. 2021;593(7858):266–269. doi: 10.1038/s41586-021-03470-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.