Graphical abstract
Keywords: Venous thromboembolism, COVID-19, Risk assessment models, Mortality
Abstract
Objectives
To investigate the association of risk of venous thromboembolism with 30-day mortality in COVID-19 patients.
Methods
A total of 1030 COVID-19 patients were retrospectively collected, with baseline data on demographics, sequential organ failure assessment (SOFA) score, and VTE risk assessment models (RAMs), including Padua prediction score (PPS), International Medical Prevention Registry (IMPROVE), and Caprini.
Results
Thirty-day mortality increased progressively from 2% in patients at low VTE risk to 63% in those at high risk defined by PPS. Similar findings were observed in IMPROVE and Caprini scores. Progressive increases in VTE risk were also associated with higher SOFA score. High risk of VTE was independently associated with mortality regardless of adjusted gender, smoking status and some comorbidities, with hazard ratios of 29.19, 37.37 and 20.60 for PPS, IMPROVE and Caprini RAM, respectively (P < 0.001 for all comparisons). The predictive accuracy of PPS (area under curve (AUC) 0.900), IMPROVE (AUC 0.917), or Caprini (AUC 0.861) RAM for risk of hospitalized mortality was unexpectedly strong.
Conclusions
We established that the presence of a high risk of VTE identifies a group of COVID-19 patients at higher risk for mortality. Furthermore, there is a high accuracy of VTE RAMs to predict mortality in these patients.
Introduction
Hospitalized patients with COVID-19 exhibit an increased risk of developing venous thromboembolism (VTE) (Moores et al., 2020a). For those patients, it is proposed to apply Padua prediction score (PPS) or International Medical Prevention Registry (IMPROVE) risk assessment models (RAM) to detect the risk stratification of VTE, guiding thromboprophylaxis (Zhai et al., 2020). Several VTE RAMs have been conducted with large cohorts of acutely ill hospitalized medical patients and introduced to individualize VTE risk of hospitalized patients (Kahn et al., 2012).
However, the VTE RAMs were found to have a novel predictive ability, demonstrating that higher PPS and IMPROVE scores were associated with patient mortality (Arpaia et al., 2020). Septic patients with higher PPS had a higher mortality risk (Vardi et al., 2013). A subsequent study indicated that a higher PPS and IMPROVE RAM score were associated with early mortality in infectious and other diseases (La Regina et al., 2016). These studies indicated that a high risk of VTE, defined by a higher VTE RAM score, was associated with mortality risk in sepsis or other medically ill patients, but the mechanism was not clarified.
A study Of 1026 COVID-19 patients shown that the mortality of patients at high risk of VTE (PPS ≥4) was increased compared with patients at low risk of VTE (PPS <4) (Wang et al., 2020). However, this study had some major limitations, with no age data (one of 11 parameters of PPS), a smaller proportion of critical patients than the present study, and no statistical analysis.
To date, there is little evidence on the relationship between the risk of VTE and mortality in COVID-19 patients. Therefore, in this study, we evaluated the association of VTE risk with mortality from any cause in COVID-19 patients.
Methods
Setting and study population
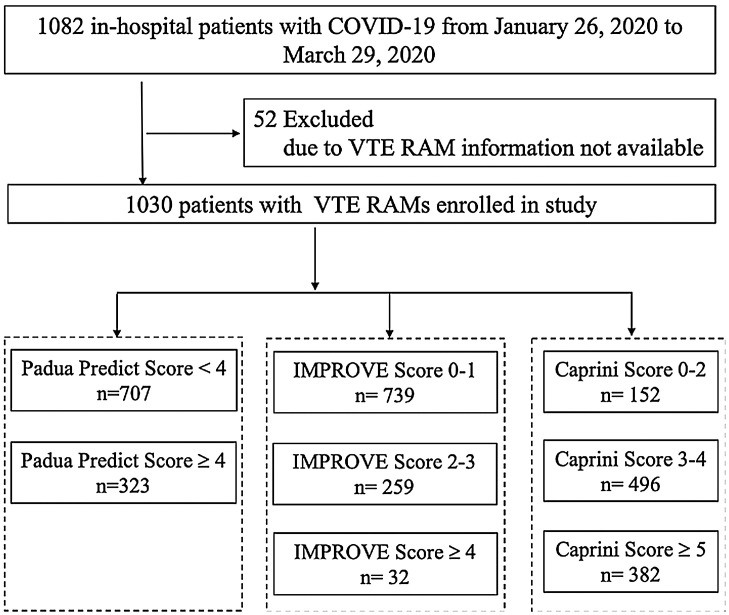
This multicenter, retrospective cohort study analyzed 1082 patients with COVID-19 in Jinyintan Hospital (n = 837) and Union Hospital, Tongji Medical College, Huazhong University of Science and Technology (n = 245) in Wuhan, China, from January 26 to March 29, 2020 (Figure 1 ). All patients met the diagnostic criteria according to the World Health Organization interim guidance (World Health Organization, 2021). The diagnosis of COVID-19 was defined as a positive result on polymerase chain reaction (PCR) assay of nasal and pharyngeal swab specimens. The study was approved by Jinyintan Hospital Ethics Committee (KY-2020-06.01) and Union Hospital Ethics Committee (2020-0039). Written informed consent was waived by the Ethics Commission.
Figure 1.
Flowchart of the study population.
Data collection
A trained team of physicians retrospectively reviewed clinical electronic medical records and laboratory findings for all the patients. We collected data on age, sex, body mass index (BMI), smoking status, the dates of hospital admission and discharge or death, the length of hospital stay, chronic disease history (including hypertension, coronary heart disease, diabetes, hematencephalon, cerebral infarction, malignancy, digestive system disease, respiratory system disease, and thyroid disease) and bleeding events. Charlson Comorbidity Index score was included, where higher scores indicate a greater burden of illness (de Groot et al., 2003). The forms of respiratory support were collected. Deep venous thrombosis was diagnosed by ultrasonography (LOGIQ e, GE). Pulmonary embolism was diagnosis by computer tomography pulmonary angiography. RAMs of VTE (PPS (Barbar et al., 2010), IMPROVE (Spyropoulos et al., 2011), and Caprini (Schunemann et al., 2018); details shown in Supplementary material), and sequential organ failure assessment (SOFA) score (Raith et al., 2017) at admission were calculated retrospectively according to the medical records by a blinded reviewer (critical care fellow). Due to VTE RAMs information missing, 52 patients were excluded (Figure 1). Of the remaining 1030 patients, 43.5% received pharmacologic thromboprophylaxis (low-molecular-weight heparin by subcutaneous injection) after admission in accordance with their VTE risk and bleeding risk assessments (Schunemann et al., 2018, Raith et al., 2017). In this study, we did not collect data on the incidence of VTE and bleeding events for 2 main reasons. First, our aim was to assess the relationship between VTE RAMs and mortality. Second, the incidence of VTE and bleeding events might bias restricted by medical conditions.
Risk assessment model and definitions
The RAMs adopted in this study were PPS, IMPROVE, and Caprini. The VTE risk profile calculated by PPS consists of 11 risk factors. A cumulative PPS ≥4 and <4 were defined as high risk and low risk of VTE, respectively. IMPROVE RAM consists of 7 risk factors; an overall score of ≥4 was considered to be high risk of VTE, a score of 2–3 and 0–1 was considered moderate and low VTE risk, respectively. Based on the 39 weighted risk factors of the Caprini RAM, patients were classified into 4 VTE risk grades: low risk (score 0–1), moderate risk (score 2), high risk (3–4), or highest risk (≥5) (Caprini, 2005). However, in this study, we merged low risk and moderate risk groups of patients (score 0–2) and re-classified the risk grades as: low risk (score 0–2), moderate risk (3–4), and high risk (≥5) for clinical characteristics analysis. Furthermore, we paid more attention to the highest risk group (score ≥5) for risk of mortality analysis based on data balancing.
Outcomes
The primary outcome of the study was 30-day mortality. The secondary outcome was the length of hospital stay.
Statistical analysis
Descriptive data were expressed as standard mean or median (interquartile range [IQR]) for continuous variables and number (%) for categorical variables. Comparison of continuous variables was examined by independent Student’s t-test and Mann–Whitney U test. Statistical analysis of categorical variables was performed using Pearson chi-square test and Fisher’s exact test as appropriate. Cox regression was used to estimate hazard ratio with 95% CI to evaluate the association between VTE risk and mortality with adjustment for sex, age, platelet, severity, smoking status, and comorbidity. Receiver operating characteristic (ROC) curve analysis was performed to estimate the sensitivity, specificity, positive predictive value, negative predictive value, and area under the ROC curve (AUC) of VTE RAMs to predict mortality. The optimal prediction threshold of VTE RAMs was defined as the cut-off point corresponding to the maximum difference between sensitivity and 1-specificity. Kaplan–Meier method was performed to estimate cumulative 30-day mortality after hospital admission. All statistical analyses were performed using SAS 9.4 and Stata 12.
Results
We retrospectively enrolled 1030 consecutively identified patients with COVID-19 in Union Hospital and Jinyintan Hospital of Wuhan (Figure 1, Table 1 ). On hospital admission, the mean SOFA score was 3 (IQR 2–5). Mechanical ventilation was required by 28.3% of patients; 17.3% and 44.9% had hypoxemia requiring high flow nasal cannula oxygen therapy and conventional oxygen therapy, respectively (Table 1). The median age of the patients was 47 years (IQR 44–65); 44.1% were female. The median length of hospital stay was 12 (IQR 8–16) days. All-cause 30-day mortality was 20.7% (95% CI, 14.1–30.0).
Table 1.
Comparison of clinical characteristics of COVID-19 patients between risk assessment models-based grouping.
| All patients | Padua prediction score <4 | Padua prediction score ≥4 | P value | IMPROVE score 0–1 | IMPROVE score 2–3 | IMPROVE score ≥4 | P value | Caprini score 0–2 | Caprini score 3–4 | Caprini score ≥5 | P value | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number, cases | 1030 | 707 | 323 | 739 | 259 | 32 | 152 | 496 | 382 | |||
| Age, years, median (IQR) | 55 (44, 65) | 51 (41, 62) | 63 (51, 72) | <0.001 | 51 (41, 62) | 63 (53, 72) | 68 (55, 75) | <0.001 | 35 (30, 40) | 52 (45, 60) | 65 (57, 74) | <0.001 |
| Sex | <0.001 | <0.001 | 0.007 | |||||||||
| Male, n (%) | 576 | 363 (51%) | 213 (66%) | 386 (52%) | 164 (63%) | 26 (81%) | 80 (53%) | 258 (52%) | 238 (62%) | |||
| Female, n (%) | 454 | 344 (49%) | 110 (34%) | 353 (48%) | 95 (37%) | 6 (19%) | 72 (47%) | 238 (48%) | 144 (38%) | |||
| BMI, kg/m2, median (IQR) | 1030 | 23 (22, 26) | 24 (23, 26) | 0.244 | 23 (22, 26) | 24 (23, 26) | 23 (21, 27) | 0.232 | 23 (21, 24) | 24 (22, 26) | 24 (23, 27) | <0.001 |
| SOFA score, median (IQR) | 3 (2, 5) | 2 (2, 3) | 7 (5, 8) | 0 | 3 (2, 3) | 7 (5, 8) | 6.5 (5, 8) | <0.001 | 2 (0, 3) | 3 (2, 3) | 6 (4, 8) | <0.001 |
| Required respiratory support, n (%) | 610 (86%) | 322 (99.7%) | <0.001 | 645 (87%) | 255 (98%) | 32 (100%) | <0.001 | 80 (52%) | 472 (95%) | 80 (99.5%) | <0.001 | |
| None | 98 | 97 (14%) | 1 (0.3%) | 94 (13%) | 4 (2%) | 0 (0%) | 72 (48%) | 24 (5%) | 2 (0.5%) | |||
| Conventional oxygen | 462 | 455 (64%) | 7 (2%) | 455 (62%) | 7 (3%) | 0 (0%) | 68 (45%) | 382 (77%) | 12 (3%) | |||
| HFNC | 178 | 155 (22%) | 23 (7%) | 156 (21%) | 20 (7%) | 2 (6%) | 12 (8%) | 76 (15%) | 90 (24%) | |||
| Noninvasive ventilation | 62 | 0 (0%) | 62 (19%) | 34 (5%) | 26 (10%) | 2 (6%) | 0 (0%) | 14 (3%) | 48 (13%) | |||
| Invasive mechanical ventilation | 230 | 0 (0%) | 230 (71%) | 0 (0%) | 202 (78%) | 28 (88%) | 0 (0%) | 0 (0%) | 230 (60%) | |||
| Smoking status, n | 0.026 | 0.003 | 0.029 | |||||||||
| Ex-smoked or smoking | 108 | 64 (9%) | 44 (14%) | 68 (9%) | 40 (15%) | 0 (0%) | 9 (6%) | 48 (10%) | 51 (13%) | |||
| Never-smoked | 922 | 643 (91%) | 279 (86%) | 671 (91%) | 219 (85%) | 32 (100%) | 143 (94%) | 448(90%) | 331(87%) | |||
| Charlson Comorbidity Index, median (IQR) | 1030 | 1 (0, 2) | 3 (2, 5) | <0.001 | 1 (0, 2) | 3 (2, 5) | 6 (4, 7) | <0.001 | 0 (0, 0) | 1 (0, 2) | 3 (2, 5) | <0.001 |
| Complications, n (%) | 692 | 408 (58%) | 284 (88%) | <0.001 | 423 (57%) | 237 (92%) | 32 (100%) | <0.001 | 55 (36%) | 307 (62%) | 330 (86%) | <0.001 |
| Medical history, n (%) | ||||||||||||
| Hypertension | 322 | 187 (26%) | 135 (42%) | <0.001 | 196 (27%) | 117 (45%) | 9 (28%) | <0.001 | 16 (11%) | 139 (28%) | 167 (44%) | <0.001 |
| Diabetes mellitus | 159 | 110 (16%) | 148 (46%) | <0.001 | 120 (16%) | 114 (44%) | 24 (75%) | <0.001 | 14 (9%) | 81 (16%) | 163 (43%) | <0.001 |
| Hematencephalon | 4 | 1 (0.1%) | 3 (1%) | 0.059 | 1 (0.1%) | 3 (1%) | 0 (0%) | 0.070 | 0 (0%) | 1 (0.2%) | 3 (1%) | 0.273 |
| Cerebral infarction | 84 | 39 (6%) | 45 (14%) | <0.001 | 38 (5%) | 44 (17%) | 2 (6%) | <0.001 | 152 (100%) | 30 (6%) | 54 (14%) | <0.001 |
| Malignancy | 62 | 4 (1%) | 58 (18%) | <0.001 | 1 (0.1%) | 29 (11%) | 32 (100%) | <0.001 | 1 (1%) | 7 (1%) | 54 (14%) | <0.001 |
| Thyroid diseases | 59 | 40 (6%) | 19 (6%) | 0.886 | 40 (5%) | 18 (7%) | 1 (3%) | 0.534 | 16 (11%) | 23 (5%) | 20 (5%) | 0.021 |
| Coronary heart disease | 101 | 50 (7%) | 51 (16%) | <0.001 | 51 (7%) | 44 (17%) | 6 (19%) | <0.001 | 1 (1%) | 35 (7%) | 65 (17%) | <0.001 |
| Digestive system disease | 103 | 63 (9%) | 40 (12%) | 0.085 | 65 (9%) | 31 (12%) | 7 (22%) | 0.026 | 6 (4%) | 51 (10%) | 46 (12%) | 0.018 |
| Hepatitis | 48 | 23 (3%) | 25 (8%) | 0.002 | 24 (3%) | 17 (7%) | 7 (22%) | <0.001 | 2 (1%) | 19 (4%) | 27 (7%) | 0.008 |
| Fatty liver | 6 | 5 (1%) | 1 (0.3%) | 0.437 | 5 (1%) | 1 (0.4%) | 0 (0%) | 0.789 | 3 (2%) | 2 (0.4%) | 1 (0.3%) | 0.049 |
| Respiratory system disease | 71 | 46 (7%) | 25 (8%) | 0.468 | 47 (6%) | 23 (9%) | 1 (3%) | 0.269 | 5 (3%) | 34 (7%) | 32 (8%) | 0.112 |
| COPD | 31 | 14 (2%) | 17 (5%) | 0.004 | 15 (2%) | 16 (6%) | 0 (0%) | 0.002 | 0 (0%) | 11 (2%) | 20 (5%) | 0.002 |
| Bronchiectasia | 16 | 11 (2%) | 5 (2%) | 0.992 | 11 (1%) | 5 (2%) | 0 (0%) | 0.682 | 2 (1%) | 0 (2%) | 5 (1%) | 0.808 |
| In-hospital length of stay, days, median (IQR) | 12 (8, 16) | 12 (8, 16) | 11 (7, 17) | 0.361 | 12 (8, 16) | 11 (7, 17) | 10 (8, 17.5) | 0.335 | 11 (7, 15.5) | 12 (9, 16) | 11 (7, 17) | 0.241 |
| Mortality, n (%) | 213 | 11 (2%) | 202 (63%) | <0.001 | 12 (2%) | 170 (66%) | 31 (97%) | <0.001 | 0 (0%) | 12 (2%) | 201 (53%) | <0.001 |
The criteria of PPS, IMPROVE or Caprini RAM for the VTE risk subgroup were stratified according to the details shown in the Supplementary materials. We detected statistically significant differences in baseline characteristics according to VTE risk defined by RAMs (Table 1). According to PPS, patients were stratified into high and low VTE risk subgroups. Patients at high VTE risk were older, more male, more ex-smokers or smoking, more mechanical ventilation, higher mortality, whereas those with low VTE risk were more likely to receive conventional oxygen or high flow nasal cannula oxygen therapy. Illness severity, as assessed by incremental SOFA scores and mechanical ventilation, correlated with increasing VTE risk. We failed to detect any correlation with BMI or length of hospital stay. In the high VTE risk subgroup, rates of all comorbidities except hematencephalon, thyroid diseases and digestive system disease appeared higher than in the low-risk subgroup. For example, patients at high VTE risk were significantly more likely to have an underlying malignancy, hypertension, coronary heart disease, cerebral infarction, diabetes mellitus, and chronic obstructive pulmonary disease compared with patients at low VTE risk.
According to IMPROVE or Caprini RAM, patients were stratified into high, moderate and low VTE risk subgroups. Their age and rates of some comorbidities, such as diabetes, coronary heart disease and hepatitis, increased with the severity of VTE risk. Furthermore, the 30-day mortality varied by VTE risk level. We found similar statistically significant differences in baseline characteristics between high and low risk subgroups according to IMPROVE or Caprini RAM, as categorized by PPS.
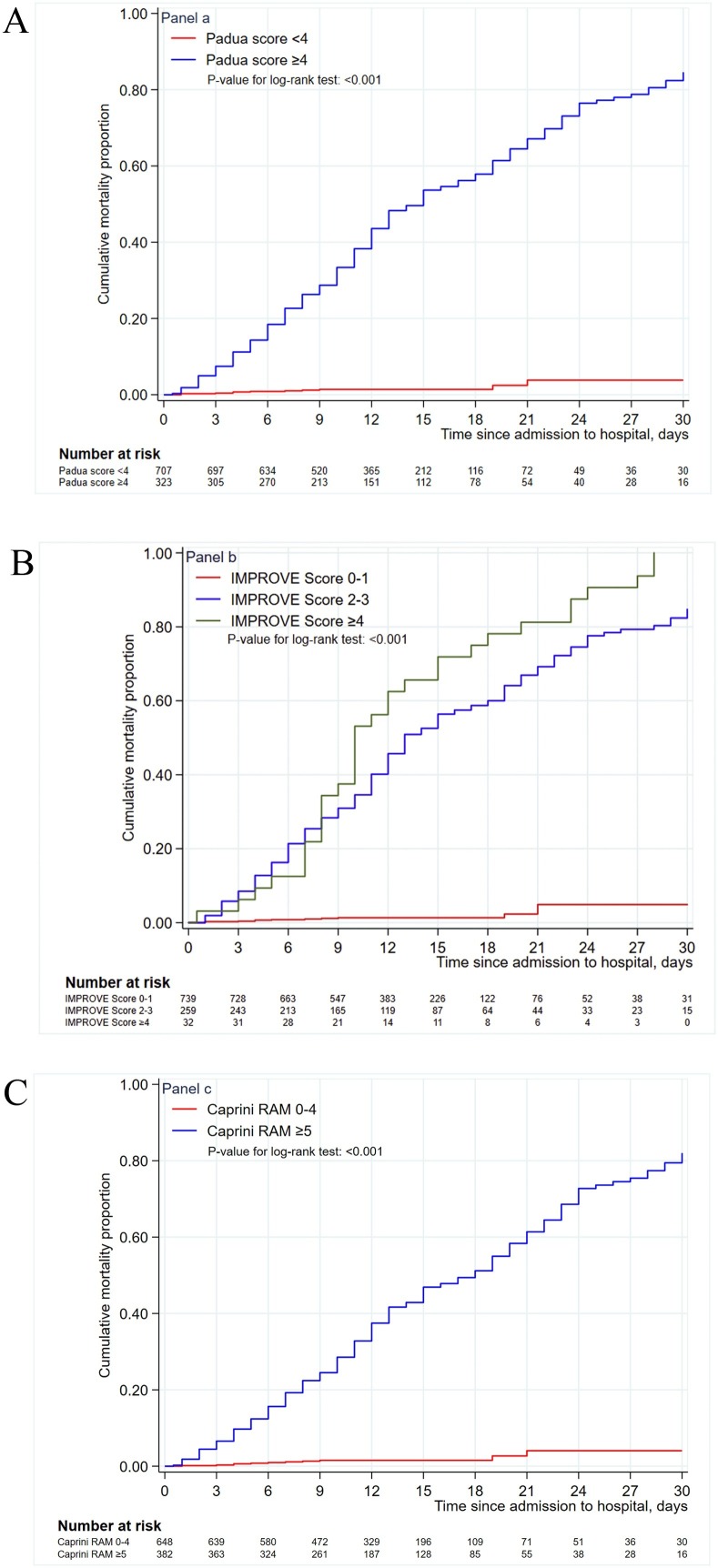
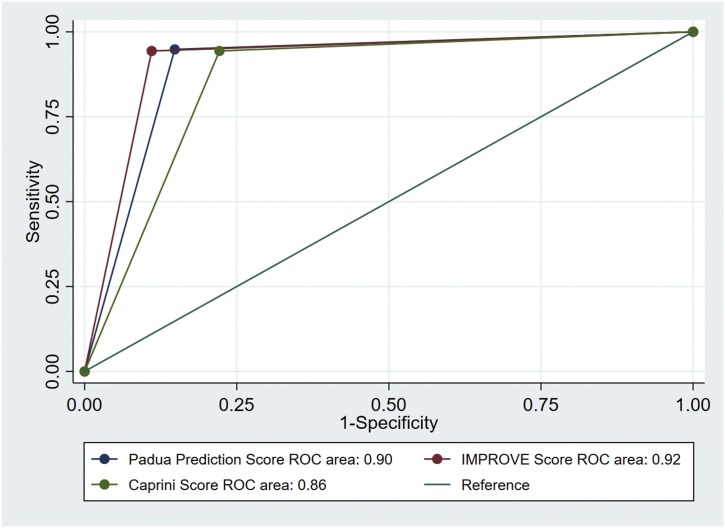
Kaplan–Meier curves confirmed the greater mortality among patients at high VTE risk compared with those at low VTE risk in all subgroups stratified by these 3 VTE RAMs (Figure 2 ). Multivariable analyses are shown in Table 2 . The presence or absence of high VTE risk (PPS or IMPROVE RAM score ≥4, Caprini RAM score ≥5), and the severity of VTE risk, was independently associated with 30-day mortality even when adjusted for gender, smoking status, and some comorbidities. We found higher hazard ratios in the presence of high VTE risk: 29.19 (95% CI, 15.76–54.05), 37.37 (95% CI, 18.43–75.78), and 20.60 (95% CI, 11.41–37.19) for PPS, IMPROVE and Caprini RAM, respectively. We computed the ROC curves to assess the accuracy of the VTE RAMs in predicting in-hospital mortality. The AUC were 0.90 (95% CI 0.88–0.92), 0.92 (95% CI 0.90–0.936), and 0.86 (95% CI 0.84–0.88) for PPS, IMPROVE, and Caprini RAM, respectively (Figure 2). At the cutoff point for high risk (PPS ≥4), the model had a negative predictive value of 98.4% (95% CI 97.2%–99.2%), a positive predictive value of 62.5% (95% CI, 57.0%–67.8%), a sensitivity of 94.8% (95% CI, 90.9%–97.4%) and a specificity of 85.2% (95% CI, 82.6%–87.6%) for 30-day mortality (Table 3 ). Similar findings were demonstrated according to IMPROVE and Caprini RAMs.
Figure 2.
Kaplan–Meier curves for cumulative 30-day mortality according to different venous thromboembolism risk. (A) Comparison of cumulative mortality rates between Padua prediction score <4 and ≥4 subgroups, P < 0.001. (B) Comparison of cumulative mortality rates between International Medical Prevention Registry score 0–1, 2–3, and ≥4 subgroups, P < 0.001. (C) Comparison of cumulative mortality rates between Caprini score 0–4 and ≥5 subgroups, P < 0.001.
Table 2.
Hazard ratio of mortality according to venous thromboembolism risk assessment models.
| Risk factors | Hazard ratio | 95% CI | P value |
|---|---|---|---|
| Padua prediction scorea | |||
| <4 | 1 (ref) | ||
| ≥4 | 29.19 | 15.76, 54.05 | <0.001 |
| IMPROVE scoreb | |||
| 0–1 | 1 (ref) | ||
| 2–3 | 30.79 | 17.12, 56.02 | <0.001 |
| ≥4 | 37.37 | 18.43, 75.78 | <0.001 |
| Caprini scorec | |||
| 0–4 | 1 (ref) | ||
| ≥5 | 20.60 | 11.41, 37.19 | <0.001 |
Adjusted gender, smoking status, comorbidities (hypertension, diabetes, hepatitis).
Adjusted gender, smoking status, comorbidities (hypertension, diabetes, hepatitis).
Adjusted gender, smoking status, comorbidities (hypertension, diabetes).
Table 3.
Diagnostic accuracy of venous thromboembolism risk assessment models for prediction of 30-day mortality.
| Padua prediction score ≥4 | IMPROVE score ≥2 | Caprini score ≥5 | |
|---|---|---|---|
| AUC (95%CI) | 0.900 (0.881, 0.919) | 0.917 (0.898, 0.936) | 0.861 (0.840, 0.882) |
| Sensitivity (95%CI) | 94.8% (90.9%, 97.4%) | 94.4% (90.4%, 97.1%) | 94.4% (90.4%, 97.1%) |
| Specificity (95%CI) | 85.2% (82.6%, 87.6%) | 89.0% (86.6%, 91.0%) | 77.8% (74.8%, 80.6%) |
| Positive predictive value (95%CI) | 62.5% (57.0%, 67.8%) | 69.1% (63.4%, 74.3%) | 52.6% (47.5%, 57.7%) |
| Negative predictive value (95%CI) | 98.4% (97.2%, 99.2%) | 98.4% (97.2%, 99.2%) | 98.1% (96.8%, 99.0%) |
Abbreviation: AUC, area under the ROC curve.
Discussion
To our knowledge, this is the first retrospective, multicenter study to investigate the association between VTE risk and the risk of 30-day mortality in patients admitted to hospital with COVID-19. Our findings identify that the presence of high VTE risk as defined by elevated scores of PPS, IMPROVE or Caprini RAM correlates with a group of patients with COVID-19 at elevated risk of mortality. In addition, there is an increasing risk of mortality as the scores of VTE RAM increase, especially from low VTE risk to moderate and high VTE risk. Incremental increases in VTE risk were also associated with higher SOFA score. Our findings also highlight that the presence of high VTE risk independently correlated with 30-day mortality after controlling for patient characteristics and comorbidities. Finally, the predictive accuracy of PPS, IMPROVE or Caprini RAM as hospitalized mortality predictors as reported here is, unexpectedly, strong (Figure 3 ).
Figure 3.
Receiver operating characteristic curve of the venous thromboembolism risk assessment models (Padua prediction, International Medical Prevention Registry, and Caprini scores) for mortality prediction.
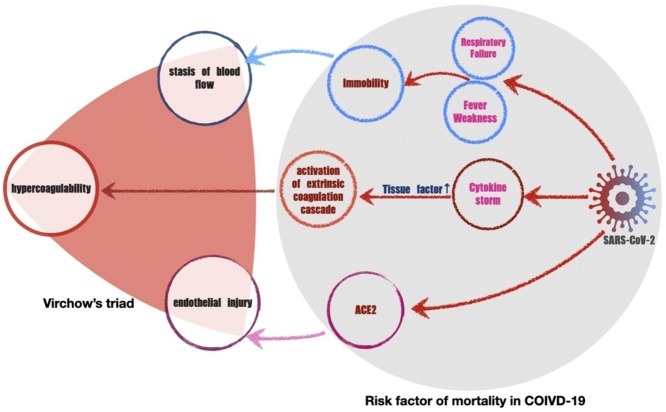
SARS-CoV-2 can induce a cytokine cascade, which could greatly increase tissue factor expression and activity in different cells related with VTE, such as endothelia, monocytes and pericytes (Moores et al., 2020b). Furthermore, almost all COVID-19 patients were immobilized in hospital, which is one risk factor of VTE. Some data indicate the need for higher prophylactic doses of low-molecular-weight heparin for VTE prevention in COVID-19 critical patients, compared with the doses used for non-COVID-19 critical patients (Spyropoulos et al., 2020). For the treatment of COVID-19 inpatients, our results have several possible implications. Firstly, all hospitalized patients with COVID-19 should be assessed for VTE risk by these RAMs to guide thromboprophylaxis. Although some guidelines recommended that all hospitalized patients receive pharmacologic prophylaxis (Moores et al., 2020b, Spyropoulos et al., 2020), we found that only 31.3% of inpatients were at high VTE risk, similar to the other study (Wang et al., 2020). Following this finding could avoid overusing anticoagulants, which may increase the risk of bleeding.
The implications of our findings are possibly more crucial for the conduct and justification of studies for new VTE management in COVID-19 and other patients. For example, there is controversy on the efficacy of pharmacologic prophylaxis on COVID-19 mortality. A study from China indicated that anticoagulant therapy did not decrease the mortality of unselected patients with COVID-19 (Tang et al., 2020). Another report from New York with 2773 patients similarly found that systemic anticoagulation did not change the mortality of the whole population of patients with COVID-19 (Paranjpe et al., 2020). Subgroup analysis indicated that the mortality benefit was limited to patients with severe illness or requiring mechanical ventilation (Tang et al., 2020, Paranjpe et al., 2020). Finally, a study from the UK failed to show any mortality benefit in intensive care unit patients receiving anticoagulants (Sivaloganathan et al., 2020). In these 3 studies, VTE RAMs were not used to stratify the VTE risk, and the mortality of the whole population failed to improve as patients at low VTE risk could not benefit from pharmacologic thromboprophylaxis compared with patients at high risk. Two large randomized clinical trials failed to identify that the use of heparin or low-molecular-weight heparin reduced the mortality of patients with sepsis or acutely ill medical patients (Gardlund, 1996, Kakkar et al., 2011). In these 2 studies, VTE risk was not assessed. According to our data, patients with low VTE risk had extremely low COVID-19 mortality. Therefore, a potential implication for pharmacologic thromboprophylaxis as a COVID-19 or sepsis therapeutic may be to study patients at high VTE risk. We detected that where these 3 VTE RAMs suggest VTE prophylaxis in hospitalized patients, this can at the same time be a potential tool for the prediction of mortality in patients with COVID-19. Higher PPS was significantly correlated with the risk of mortality in patients with sepsis and acute medical illness, besides reflecting the VTE risk (Arpaia et al., 2020, Vardi et al., 2013). However, in similar patients with sepsis and acute medical illness, a survival benefit with anticoagulation was not identified (Gardlund, 1996, Kakkar et al., 2011). These results seemed counterintuitive, as anticoagulant has been shown to decrease VTE risk by nearly half in hospitalized patients with acute medical illness. Multifarious comorbidities and other potential factors could make fatal pulmonary embolism a less crucial determinant of mortality in this population than in surgical patients, thereby decreasing the ability of pharmacologic thromboprophylaxis to improve overall survival. The parameters of PPS and other VTE RAMs, included age, immobility, comorbidities, BMI, and so on, may reflect a more general comorbidity and disease severity index, which probably affects survival. Furthermore, they function as a comorbid index rather than a specific VTE predictor and thus are associated with mortality rather than VTE risk.
The strengths of our study include its multicenter design, large and diverse sample, comprehensive data collection, and that our enrolled proportion of patients at high risk of VTE is relatively similar to proportions in another report (Wang et al., 2020) which could enhance the generalizability of our findings. Moreover, we have adjusted for numerous possible confounding variables, including patient characteristics, comorbidities, and smoking status. Finally, we calculate the predictive accuracy of VTE RAMs as mortality predictors to lend additional strength to our analysis.
There are several important limitations of our study. First, as VTE prevention in hospitalized patients with COVID-19 was not the primary objective of our study and patients at high risk of VTE received pharmacologic thromboprophylaxis, we did not screen for asymptomatic VTE. Second, these data are from 2 hospitals and may not be representative of other populations in different regions and countries. To our knowledge, there is no study with data that can be used to detect the presence of high VTE risk in a large population with COVID-19. Therefore, larger samples of COVID-19 patients are required to assess the true incidence of high VTE risk in the population setting. Third, we use the SOFA score to reflect the disease severity instead of APACHE II (acute physiology and chronic health evaluation scoring system), as detailed information was lacking for some patients. Finally, the causes of death in COVID-19 patients were judged according to clinical characteristics, not by autopsies which were rarely performed in these 2 hospitals.
Conclusion
In conclusion, COVID-19 patients at high VTE risk, as defined by PPS, IMPROVE or Caprini RAMs, appear to have a greater risk of death compared with those with low VTE risk. We also found that VTE RAMs had good accuracy as 30-day mortality predictors in COVID-19 inpatients. However, future research is required to verify our findings in larger populations. Importantly, future studies of COVID-19 related to VTE prophylaxis and mortality should take into account the risk stratification of VTE by RAMs when formulating potential study design.
Authors’ contributions
SC and JJ designed the study, SW, JJ and PW collected the data, TZ and YY analyzed the data, SC and JJ prepared drafted the paper. YS, QY, YX, SC and JJ revised the paper. All authors discussed the results and contributed to the final manuscript.
Compliance with ethical standards
The authors report no relationships that could be construed as a conflict of interest.
Human and animal rights
This article does not contain any study with humans and animals performed by any of the authors.
Informed consent
Written informed consent was waived by the Ethics Commission.
Funding
This study is funded by the National Natural Science Foundation of China (81870062 to Jinjun Jiang, 81900038 to Shujing Chen).
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.ijid.2021.06.005.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Arpaia G.G., Caleffi A., Marano G., Laregina M., Erba G., Orlandini F. Padua prediction score and IMPROVE score do predict in-hospital mortality in internal medicine patients. Intern Emerg Med. 2020;15(6):997–1003. doi: 10.1007/s11739-019-02264-4. PubMed PMID: WOS:000505364200001. [DOI] [PubMed] [Google Scholar]
- Barbar S., Noventa F., Rossetto V., Ferrari A., Brandolin B., Perlati M. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: the Padua Prediction Score. J Thromb Haemost. 2010;8(11):2450–2457. doi: 10.1111/j.1538-7836.2010.04044.x. PubMed PMID: 20738765. [Epub 27 August 2010] [DOI] [PubMed] [Google Scholar]
- Caprini J.A. Thrombosis risk assessment as a guide to quality patient care. Dis Mon. 2005;51(2–3):70–78. doi: 10.1016/j.disamonth.2005.02.003. PubMed PMID: WOS:000229391000003. [DOI] [PubMed] [Google Scholar]
- de Groot V., Beckerman H., Lankhorst G.J., Bouter L.M. How to measure comorbidity. A critical review of available methods. J Clin Epidemiol. 2003;56(3):221–229. doi: 10.1016/s0895-4356(02)00585-1. PubMed PMID: 12725876. [Epub 3 March 2003] [DOI] [PubMed] [Google Scholar]
- Gardlund B. Randomised, controlled trial of low-dose heparin for prevention of fatal pulmonary embolism in patients with infectious diseases. Lancet. 1996;347(9012):1357–1361. doi: 10.1016/s0140-6736(96)91009-0. PubMed PMID: WOS:A1996UL90400008. [DOI] [PubMed] [Google Scholar]
- Kahn S.R., Lim W., Dunn A.S., Cushman M., Dentali F., Akl E.A. Prevention of VTE in nonsurgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e195S–e226S. doi: 10.1378/chest.11-2296. PubMed PMID: 22315261; PubMed Central PMCID: PMCPMC3278052. [Epub 15 February 2012] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakkar A.K., Cimminiello C., Goldhaber S.Z., Parakh R., Wang C., Bergmann J.F. Low-molecular-weight heparin and mortality in acutely ill medical patients. New Engl J Med. 2011;365(26):2463–2472. doi: 10.1056/NEJMoa1111288. PubMed PMID: WOS:000298544600007. [DOI] [PubMed] [Google Scholar]
- La Regina M., Orlandini F., Marchini F., Marinaro A., Bonacci R., Bonanni P. Combined assessment of thrombotic and haemorrhagic risk in acute medical patients. Thromb Haemost. 2016;115(2):392–398. doi: 10.1160/TH14-12-1050. PubMed PMID: 26403152. [Epub 26 September 2015] [DOI] [PubMed] [Google Scholar]
- Moores L.K., Tritschler T., Brosnahan S., Carrier M., Collen J.F., Doerschug K. Prevention, diagnosis, and treatment of VTE in patients with COVID-19: CHEST guideline and expert panel report. Chest. 2020 doi: 10.1016/j.chest.2020.05.559. PubMed PMID: 32502594; PubMed Central PMCID: PMCPMC7265858. [Epub 6 June 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moores L.K., Tritschler T., Brosnahan S., Carrier M., Collen J.F., Doerschug K. Prevention, diagnosis, and treatment of vte in patients with coronavirus disease 2019: CHEST guideline and expert panel report. Chest. 2020 doi: 10.1016/j.chest.2020.05.559. PubMed PMID: 32502594; PubMed Central PMCID: PMCPMC7265858. [Epub 6 June 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paranjpe I., Fuster V., Lala A., Russak A.J., Glicksberg B.S., Levin M.A. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol. 2020;76(1):122–124. doi: 10.1016/j.jacc.2020.05.001. PubMed PMID: 32387623; PubMed Central PMCID: PMCPMC7202841. [Epub 11 May 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raith E.P., Udy A.A., Bailey M., McGloughlin S., MacIsaac C., Bellomo R. Prognostic accuracy of the SOFA score, SIRS criteria, and qSOFA score for in-hospital mortality among adults with suspected infection admitted to the intensive care unit. JAMA. 2017;317(3):290–300. doi: 10.1001/jama.2016.20328. PubMed PMID: 28114553. [Epub 24 January 2017] [DOI] [PubMed] [Google Scholar]
- Schunemann H.J., Cushman M., Burnett A.E., Kahn S.R., Beyer-Westendorf J., Spencer F.A. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized medical patients. Blood Adv. 2018;2(22):3198–3225. doi: 10.1182/bloodadvances.2018022954. PubMed PMID: 30482763; PubMed Central PMCID: PMCPMC6258910. [Epub 30 November 2018] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivaloganathan H., Ladikou E.E., Chevassut T. COVID-19 mortality in patients on anticoagulants and antiplatelet agents. Br J Haematol. 2020;190(4):e192–e195. doi: 10.1111/bjh.16968. PubMed PMID: WOS:000549789300001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spyropoulos A.C., Anderson F.A., Jr., FitzGerald G., Decousus H., Pini M., Chong B.H. Predictive and associative models to identify hospitalized medical patients at risk for VTE. Chest. 2011;140(3):706–714. doi: 10.1378/chest.10-1944. PubMed PMID: 21436241. [Epub 26 March 2011] [DOI] [PubMed] [Google Scholar]
- Spyropoulos A.C., Levy J.H., Ageno W., Connors J.M., Hunt B.J., Iba T. Scientific and Standardization Committee Communication: clinical guidance on the diagnosis, prevention and treatment of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18(8):1859–1865. doi: 10.1111/jth.14929. PubMed PMID: 32459046; PubMed Central PMCID: PMCPMC7283841. [Epub 28 May 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang N., Bai H., Chen X., Gong J.L., Li D.J., Sun Z.Y. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094–1099. doi: 10.1111/jth.14817. PubMed PMID: WOS:000528704600001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardi M., Ghanem-Zoubi N.O., Zidan R., Yurin V., Bitterman H. Venous thromboembolism and the utility of the Padua Prediction Score in patients with sepsis admitted to internal medicine departments. J Thromb Haemost. 2013;11(3):467–473. doi: 10.1111/jth.12108. PubMed PMID: 23279085. [Epub 3 January 2013] [DOI] [PubMed] [Google Scholar]
- Wang T., Chen R.C., Liu C.L., Liang W.H., Guan W.J., Tang R.D. Attention should be paid to venous thromboembolism prophylaxis in the management of COVID-19. Lancet Haematol. 2020;7(5):E362–E363. doi: 10.1016/S2352-3026(20)30109-5. PubMed PMID: WOS:000530901900006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . 2021. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected. [Google Scholar]
- Zhai Z., Li C., Chen Y., Gerotziafas G., Zhang Z., Wan J. Prevention and treatment of venous thromboembolism associated with coronavirus disease 2019 infection: a consensus statement before guidelines. Thromb Haemost. 2020;120(6):937–948. doi: 10.1055/s-0040-1710019. PubMed PMID: 32316065. [Epub 22 April 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.