Abstract
Macro- and microalgae-based foods are becoming popular due to their high nutritious value. The algal biomass is enriched with polysaccharides, protein, polyunsaturated fatty acids, carotenoids, vitamins and minerals. However, the most promising fraction is polysaccharides (PS) or their derivatives (as dietary fibers) which are not entirely fermented by colonic bacteria hence act as potential prebiotic. Primarily, algae become famous as prominent protein sources. Recently, these are widely adopted as functional food (e.g., desserts, dairy products, oil-derivatives, pastas etc.) or animal feed (for poultry, cattle, fish etc.). Besides prebiotic and balanced amino acids source, algae derived compounds implied as therapeutics due to comprising bioactive properties to elicit immunomodulatory, antioxidative, anticancerous, anticoagulant, hepato-protective, and antihypertensive responses. Despite the above potentials, broader research determinations are inevitable to explore these algal compounds until microalgae become a business reality for broader and specific applications in all health domains. However, scale up of algal bioprocess remains a major challenge until commercial affordability is accomplished which can be possible by discovering their hidden potentials and increasing their value and application prospects. This review provides an overview of the significance of algae consumption for several health benefits in humans and animals mainly as prebiotics, however their functional food and animal feed potential are briefly covered. Moreover, their potential to develop an algal-based food industry to meet the people's requirements not only as a sustainable food solution with several health benefits but also as therapeutics is inevitable.
Keywords: Microalgae, Macroalgae, Seaweeds, Dietary fibre, Prebiotics, Polysaccharides
Background
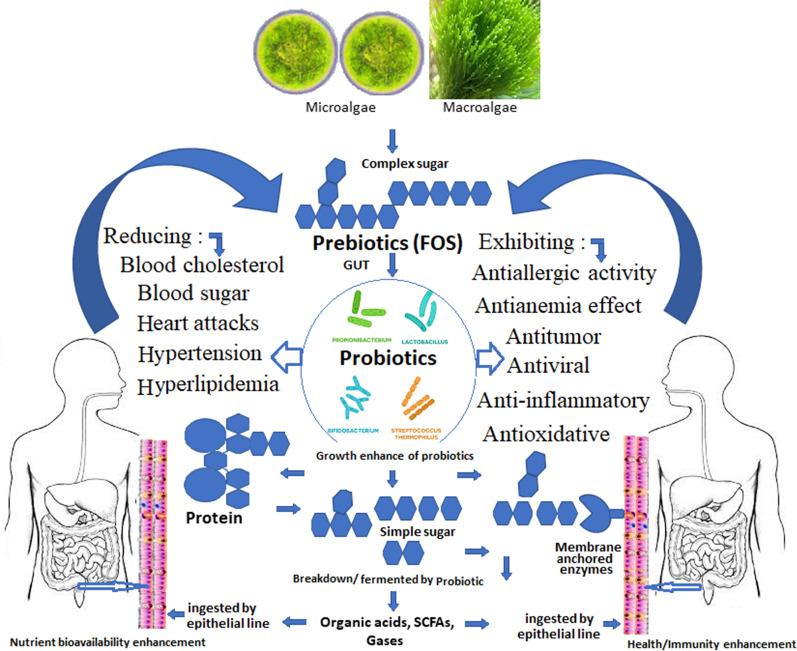
Recently, there has been a growing interest in functional foods as well as the prebiotic potential of foods for numerous health benefits [1–3]. Functional food can provide not only the nutrition but also the positive health effects against numerous conjoint diseases appearing in recent times. It can be proactive against those diseases and must carry properties like anti-inflammatory, antioxidant, antimicrobial, and antiviral, moreover be preventive for constipation, gastric ulcers, diabetes, anaemia, and hypertension. However, prebiotic potential is such a unique characteristic of certain foods which hardly get digested in the host’s gastrointestinal tract or fermented by the host’s gut microbiota. Therefore, it helps to enhance growth of health beneficial organisms called probiotics in the lower gastrointestinal tract or colon.
Apart from functional foods, several foods with and without prebiotic potentials are also blended with probiotics for improving their positive health effects. There are a large number of probiotics existing in numerous dairy products to improve gut health for example yoghurt, curd, cheese, and ice-cream. They are comprising a diverse group of health-boosting microorganisms. In which some are usual dwellers of the gut and some as fermentative bacteria. The latter are utilised in the food industries for improving processes and product quality, e.g., texture, flavour and stability. They have specialized enzymes and mechanisms to perform such effects precisely in adverse gut conditions [4, 5]. Several group of probiotic bacteria such as Bifidobacterium, Lactobacillus, Bacillus, Streptococcus, Saccharomyces and Lactococcus have been investigated and most of them are certified by health organizations in food products due to their specific positive health effects [4, 6–9]. These probiotics are recognized for numerous health effects including immunity enhancement, diarrhoea prevention, constipation inhibition, [10, 11], lactose intolerance, blood cholesterol reduction and cancer prevention [12] and associated side effects [1]. Moreover, probiotics also protects against several opportunistic pathogens [13].
In recent years, evidence has appeared for the positive health effects of foods, food ingredients or biochemical compounds derived from certain macro- and microalgae. These algae potentially show the widest range of products of the microbial world owing to their nutritional quality [14], in which some are important sources of human and animal foods [10, 15]. Some compounds exhibit the prebiotic potential to support probiotic growth in the host gut upon consumption [16]. Microalgal biomass comprised of a wide range of bioactive compounds such as protein, polysaccharides, pigments, vitamins, polyunsaturated fatty acids (PUFAs), and minerals as intracellular compounds and oligosaccharides as extracellular compounds [17–19]. Among them, the most promising found to be polysaccharides (PS) and their derivatives (as soluble fibres). Some of these PS (e.g., exopolysaccharides, fucoidans, alginates, and carrageenans) are not fermented completely by colonic microbiota and act as prebiotic. However, growth promotion and performance of probiotic by prebiotic microalgae is not limiting by these compounds directly, such enhancements are also reported indirectly such as suppression of pathogens, removing toxic substances, improving gut adsorption, improving disease resistance and immunity, enhancing their viability and storage etc. which are summarized in Table 1. Researchers were also investigating health improving bioactive compounds as well as whole dried biomass of macro- and microalgae. Their attributes greatly depend on composition of the biomass as well as on the species and growth condition provided. Scientific evidence is still lacking about probiotic roles of microalgae in humans, though intermittent studies have exhibited the probiotic role in marine animals.
Table 1.
Prebiotic role of various algae strains on growth promotion of probiotics and related health improvements
| Microalgae sp. | Probiotic | Microalgae conc (mg. ml−1) |
Main focus Of study |
Other remarks | Reference |
|---|---|---|---|---|---|
| Chlorella vulgaris | Lactobacillus brevis | 0.1–1.5% | Improving the probiotic growth, health, product yield and other desirable properties | Algae shortening the log phase, improving lactic acid yield, enzyme activity and acidifying activity of probiotics | [20] |
| Euglena gracilis | Streptococcus iniae | ND | Development as animal feed, paramylon activity was tested | Immunostimulant activity offered to the animal host | [21] |
| Pavlova pinguis | Phaeobacter inhibens | ND | Disease management in bivalve V. coralliilyticus | Vibrio sp. infection reduction for reducing the mortality of larval shellfish | [22] |
| Chlorella vulgaris and Spirulina platensis | Lactic acid bacteria | 3 | Supplementing microalgae in milk products for improving its storage and self-life | Increasing the viability of probiotics in final product but also the sensory attributes | [23] |
| Euglena gracilis |
Bacillus licheniformis or B. subtilis |
ND | Development as animal feed, β-glucan was tested in poultry, cow, horses, dogs, cats, birds and reptiles | Improved the health and immune system of animal hosts | [24] |
| Spirulina platensis | Lactococcus lactis sp. | 1 | Supplementing microalgae in yogurt to improve health benefits due to probiotic enrichment | Increasing the viability of probiotics and lactic acid bacteria | [25] |
| Spirulina platensis | Lactococcus lactis sp. | 1 | Supplementing microalgae in yogurt to improve health benefits due to healthy bacterial enrichment | Increasing the viability of probiotics and lactic acid bacteria | [26] |
| Phaeodactylum triconutum, Tetraselmis chuii | Bacillus subtilis | ND | Developed as animal feed, effect of protein fraction was examined | Immune system was improved and intestinal adsorption was increased | [27] |
| Spirulina platensis | Bifidobacterium bifidum and other | 1–2 | Feed for animals suffering from disease due to imbalance of insulin and adipose distributions | It helped to adsorb metal ions in animal gut to restore gut disorders | [28] |
| Spirulina platensis | Lactobacillus acidophilus, L. Casei, S. thermophilus | 5–10 | Stimulating growth of lactic acid bacteria | Three LAB have been improved in their viability and activity, and suppressed the growth of pathogenic bacteria, improved intestinal adsorption of host | [29] |
| Dunaliella tertiolecta | Bacillus sp. | ND | Development as animal feed, β carotene effect was tested in shrimp | Improved immune system and disease resistance | [30] |
| Spirulina platensis, Chlorococcum, D. salina, S. magnus, Chlorella | Lactobacillus lactis, Lactobacillus bulgaricus and Bifidobacterium longum | Stimulating growth of lactic acid bacteria | Xylose and galactose in algal extract stimulate the growth of probiotics | [31] | |
| Navicula sp. | Lactobacillus sakei | ND | Developed as animal feed, Oligosaccharide effect was tested | Immune system was improved and antioxidant property was enhanced | [32] |
ND Not determined
Algae are multicellular, eukaryotic, non-flowering, photosynthetic aquatic plants which include microalgae, macroalgae (seaweeds) and sometimes unicellular cyanobacteria. They are constituting the base of aquatic food chains. Phylogenetically they are distinct and encompassing different phyla and classes [10, 33, 34]. These algae grow well in all types of aquatic environments, for example freshwater, marine, and hypersaline, also moist soils and rocks [35]. They are recognised for several potential applications such as functional food [16, 36, 37], animal feed [16, 21], biomedicals [38, 39], prebiotics [33, 40, 41], cosmetics [42], and organic manures [43], wastewater treatment [43–45] high value [46] and biofuel production [15, 47]. Furthermore, several studies have addressed health benefits of such microalgal compounds comprising antioxidant, anti-inflammatory, antimicrobial, antiobesity, and anticancer properties, besides hypocholesterolemic characteristics. Thus, it serves as nutraceuticals [38, 39]. The demand in algae-based food and feed ingredients in the food market is expected to grow soon; however, steady applications exist mainly in the aquaculture and dairy industry [48]. Moreover, an existing trend has been marked to blend microalgal biomasses into fermented milks to improve the medicinal and nourishing attributes via promoting the probiotics stability [49, 50]. Table 2 summarizing the challenges and their possible solutions for microalgae probiotic formulations in milk products to enhance their commercial attributes and applications. Nevertheless, before seeking application of algal-based products, it is important that microalgae cultivation and related facilities must be cost-effective.
Table 2.
A summary of prebiotic microalgae formulation in milk products for technological improvements
| Technological attributes and remunerations of microalgae supplementation | |
|---|---|
| Challenges | Probable solutions |
| Increasing cost of final product | Cost-effective production of microalgae added healthy fermented milks |
| It leads to sensory flaws due to oxidation of unsaturated fatty acids | Add fruit flavors (kiwi, strawberry) to suppress off flavor of microalgae addition |
| Lower product texture and color options due to non-solubility of microalgal powders | Improving product texture and color range by external green sources by homogenizing them effectively |
| Product property | |
| Lower viability of healthy bacteria in milk products due to lower prebiotic effects and high active oxygen sp. | Improving their viability by microalgal prebiotic effects: altering redox potential, improving O2 scavengers (vit. C, β-carotene, carotenoids) and nutritional level (amino acids, minerals, peptides, B-vit etc.) |
In the recent development on algae cultivation, they are not limited to only photoautotrophic cultivation mode, under which they only can utilize inorganic carbon (CO2) and not to organic carbon to enhance their growth using dual pathway photosynthesis and oxidative phosphorylation. Thus, a new cultivation strategy of microalgae to grow them mixotrophically is very important to remove the economic constraints and their effective exploitation for obtaining higher biomass [51–53]. Moreover, another advantage as in the CCU technology, algae platform is most promising among others specially for increased CO2 mitigation rate mainly due to their higher productivity than any other plants [15, 54]. These attributes along with mixotrophic cultivation mode can greatly reduce challenges associated with their biomass harvesting, shelf life extension and constrained industrial viability. Recent advances in microalgae research could be a breakthrough towards exploiting high throughput screening techniques to sort out potential strains, especially high yielding desired products for health applications [55].
The main aim of this short review is to highlight recent research developments on widening applications of algae-based products in functional foods, animal feed, nutraceutics and/or therapeutics, encompassing products of macro- microalgae/cyanobacteria, which independently or with some formulations exhibit potential to improve human and/or animal health. The knowledge gaps between research and development as well as stage of commercialization of these products are also discussed briefly.
Prebiotic research advancements
Prebiotic concept improvements
Usually, prebiotics are assumed to offer a selective effect on the host microbiota which leads to their improved health. When prebiotics are not well fermented, they often exert an osmotic response in the host GIT, whereas once they are effectively fermented by GIT flora shows higher metabolic gas production and exert its prebiotic effect [56].
Prebiotics works as growth stimulators to commensal bacilli such as Lactobacillus sp. These are known bacteria for improving GIT barrier function during external stress by protecting the tight epithelial junction [57]. By observation, approved prebiotics mainly augment the count of Bifidobacteria in the human GIT [58]. The general finding suggests that the above benefit in the human health offered by pathogens removal as well as immune system modulation [59]. Bifidobacteria can metabolize carbohydrates having shorter chain lengths and known as oligosaccharides [60]. Study shows that these prebiotics can modulate the gut microbiota especially promoting Bifidobacterium group [61]. For this, prebiotics not only alter the mucosal lining of the colon but also the transportation of the SCFAs across trans-epithelium. In which, transportation of cationic minerals is induced by the reduced abdomen pH.
Prebiotics can be served as a substitute to probiotics or as a supplementary boost for them. longer stability of prebiotic, durability during processing, and their physicochemical characteristics can encourage prebiotics compared to probiotics [62, 63]. Also, high tolerance to gastric acids, bile salts, and hydrolytic proteases occurring in GIT could be other desirable attributes of prebiotics. Moreover, prebiotics are leading to lower intestinal pH and promote osmotic water retention in the bowel [64]. However, it was recorded that excessive prebiotics intake can cause diarrhea and abdominal gas. Instead, prebiotics at an optimum amount exert several positive health effects and override all adverse effects. Prebiotics are not allergenic compounds, also not proliferating the genes involved in antibiotic resistance. Although the impact of pathogens removal by prebiotics could be less than antibiotics, their desirable attributes discussed above to support them as a natural potential alternative for antibiotics [64].
Primary definitions were revised with times as per the development appeared on novel prebiotics and understanding up on their structure and metabolic mechanisms with respect to gut flora (Fig. 1). Prospects of the specified prebiotics effect was extended by International Scientific Association for Probiotics and Prebiotics in 2003, and defined that prebiotic effects were not limited to the colon, it also reaches to the skin, mouth, abdomen, intestine, and vagina [65]. In this, during the prebiotic convention in the year 2008, the most important modifications in the prebiotic’s phenomenon taken place by the Food and Agriculture Organization [66], where prebiotics were characterized “a nonviable edible which exert several health benefits to the host via alteration of the microflora.” Such description deleted the measures of specificity and limitation to the GIT. Moreover, extended the lists of prebiotics beyond FOS, inulin, HMO, GOS, and lactulose. Hence, novel prebiotics have been included for example resistant starch, sugar alcohols, XOS, SOS, lactosucrose, IMO, and POS. Accordingly, the necessity of only GIT flora has been removed to metabolize the prebiotics, authors also recommended the exclusion of selectivity obligations. Moreover, this classification underlines the prebiotics-based ecological and operational characteristics of the GIT flora, for example ecosystem diversity, also a mixed microbiota and the SCFAs production [67].
Fig. 1.
Overview of prebiotic digestion process via probiotic in human gut and associated health benefits
Regardless of the above refinements, experts, strongly demanded for specificity correlative with taxonomic groups or positive metabolic functionalities must remain the main criteria for prebiotic selection and classification [68, 69]. This amendment shows, prebiotics may not be completely metabolized, instead digested by precise microbes in a way promoting the health of the host. However, the selectivity perseverance would not ignore the impacts on species which are not dominant like Bifidobacterium and Lactobacillus. For example, some prebiotics found to encourage the growth of butyrate producing Firmicutes sp. They are advantageous to colonic health [60]. Whereas Bifidobacterium sp. are not a producer of butyrate.
Towards a new amendment in prebiotic development, Clostridium leptum, Faecalibacterium prausnitzi, Akkermansia muciniphila, and Bacteroides fragilis are known probiotics exerting positive effect against obesity and colitis, In which, Clostridium and Bacteroides groups are also involved to produce some health detrimental toxic metabolites, In this context, the recent ISAPP agreement panel now recommends new prebiotic definition: “any substrate which specifically uptakes by the host to exert a health effect” [60].
Role of prebiotics in intestinal microflora
The impacts of ingested prebiotics on human GIT microbiota are well addressed. These prebiotics have a major role to alter the abundance of certain microorganisms after a few weeks of their consumption based on their compositions and structures [16, 18]. In previous studies, the incidence of Bifidobacterium augmented in two weeks period with 15 g.d−1 oligofructose or inulin ingestion, and reduced the density of Clostridium, Bacteroides, and Fusobacterium from oligofructose and gram-positive cocci from inulin [70]. Other classical prebiotics such as FOS and GOS, have exhibited the great abundance of actinobacteria improved substantially with prebiotics dosing, which are primarily known to induce Bifidobacterium population. Majority of the studies discovered the growth augmentation of Bifidobacterium followed by Lactobacillus by these prebiotics consumption, other studies also described increase the numbers of Faecalibacterium and Atopobium sp [71]. The count of GIT bacteria also reported to reduce after the ingestion of these prebiotics, maybe due to competition with other species which specially ferment the same prebiotics in the human intestine. Nevertheless, fatty acids, mainly SCFAs, which are intermediate of prebiotic metabolism, found to encourage variations in the GIT microbiome, includes colonic pH reduction which also inhibits many bacteria such as Clostridium and Bacteroides [71].
Overall, these studies revealed that prebiotics certainly have potential to modify gut environments for advantageous members while reducing chances to proliferate harmful bacteria in the GIT environment and progressing the composition of colonic microbiota of the host towards healthier. However, still the consensus has not been set about which microbes are positive or negative members of the gut [67]. These shortcomings suggest more studies for establishing a comprehensive association between prebiotics and GIT microbiota.
Recently, with the progress in prebiotics research and associated GIT microbiome range e.g., Eubacterium, Bacteroides, Roseburia, Faecalibacterium, Akkermansia and Ruminococcus have been main targets of prebiotics [72]. A human trial showed, the FOS intake stimulated the abundance of butyrate-synthesizing microorganisms such as Ruminococcus, Faecalibacterium and Oscillospira which are detected in the feces [73]. Previous studies [74, 75] addressed that seaweed dietary polysaccharides could augment the count of Bacteroides in mice feces and their fundamental mechanism attributed the specific PULs expedite its absolute metabolic niche. Similarly, count of Faecalibacterium in the healthy adult feces significantly rose in the 16-d period after 10 g d−1 inulin ingestion [76]. A 3-month treatment of obese women with 16 g d−1 dietary inulin-type fructans resulted an enhancement in Faecalibacterium [77]. An 8-week in vivo study demonstrated a 10-time rise in the count of Faecalibacterium in the feces of an adult with the consumption of 1-kestose at 5 g d−1 [72]. Oligosaccharides obtained from lemon waste augmented Faecalibacterium, Roseburia and Enterobacter recorded by in vitro study carried out with feces inocula [78]. The Akkermansia count in mice feces was enhanced over 100-fold with the FOS ingestion [79]. Likewise, uptake of polyphenols rich fruits, mainly grapes, also enhanced the Akkermansia count [80].
Prebiotic mechanism of action in GIT condition
Prebiotics are partly metabolized in the higher sections of the gastrointestinal tract as the human genes do not transcribe certain carbohydrate hydrolysing enzymes called CAZymes [81]. When prebiotics compounds (FOS, GOS, inulin, and lactulose) and dietary carbohydrates (XOS, PDX, SOS, resilient starch, gluco-oligosaccharides, lactosucrose, etc.) with recognized prebiotic effects arrive in the colon then they are specifically fermented by hydrolytic microbes [82]. This process produces several metabolites such as organic acids (lactate, succinate and pyruvate) short chain fatty acids (C1–C4), and gases (CO2, H2, CH4, and H2S) which help in the intestinal metabolic balance, leading to the reduction in nitrogen-based final products, colonic pH, and faecal enzymes [83]. The above prebiotic specificity for intestinal bacteria is separated into two groups (I) lactate and acetate fermentative (Bifidobacterium and Lactobacillus sp) and lactate and acetate consumers (Eubacterium, Faecalibacterium and Roseburia sp) for improving butyrate formation. It can be concluded that there are two routes, one is direct growth stimulation of these intestinal bacteria by consuming prebiotics and second is growth stimulation of other gut microbiota from their metabolites such as acetate.
Several studies have demonstrated how these prebiotics exhibit precise health effects upon its consumption which have been recorded case by case. For example, to improve bowel condition and colon condition in patients of IBD, IBS, Ulcerative colitis, Crohn’s disease etc. Prebiotic dosing reduces the pro-inflammatory immune markers and improves the calprotectin performance. It also enhances the cytokine production. Prebiotic effectively reduces the IBD symptoms by modulating the Bifidobacterium counts upon is appropriate dosing and much enhancement was observed in butyrate supplemented systems [69, 84–86]. For improving GIT condition from colon cancer, prebiotics usually show substantial reduction in the number of putrefactive compounds generation by colonic microflora from butyrate, especially Bifidobacteria play a major role to down regulate the carcinogenic promoters as well as reduce the genotoxins level on biomarkers which is leading to cell proliferation with reduced cancer features [86, 87]. Prebiotic found to improve bone mass and density by enhancing calcium absorption and through reducing GIT pH due to production of SCFAs [69, 85]. Mechanisms to regulate the gut metabolism and digestate transit with the reduction in onset of constipation, dysentery and diarrhea. To improve the host heath from antibiotic-linked and traveller-diarrhoea, prebiotics exhibit functionality to reduce the fever and vomiting in children through inducing the growth of Bifidobacteria. It was also observed that probiotics can reduce the prevalence of diarrhoea upon regular optimized intake [86, 88]. The mechanisms of prebiotics, for improvement in the host immune system has been described through the production of pro-inflammatory cytokines (TNF-α) and by stimulating overexpression of receptors on macrophages and lymphocytes B and T cells [69, 89].
From several recent studies, carried out in vitro and in vivo revealed that the gut flora apparently plays a much additional key role for the host’s health than it was formerly apprehended, and this microbiota can be selectively modified by various important groups of prebiotics. Among all, a various polysaccharide groups can elicit their effect through various noticeable mechanisms such as (a) specific fermentation (b) the pH of the GIT (iii) bulking of fecal matter (iv) pathogens inhibition for gut colonization (v) prevention of putrefactive bacteria to avoid toxic metabolites production for the host. Algal Oligo- and polysaccharides could exhibit health effects similar or more effective than the products derived from other sources. This is obvious through biochemical characterizations, especially for some oligo- and polysaccharides from marine macro- and microalgae which are undigested by human enzymes in the upper region of the GIT. Thus, these algal Oligo- and polysaccharides offer a great potential as an emerging prebiotic for health application, especially for microalgae, it is more opportunities to develop a cost-effective biorefining process for extracting these products from harvested wet algal biomass or dried biomass as such or as nutraceuticals [90]. They can be encompassed as human food, animal feed, and/or administered as liquid drinks and solid/semisolid pills Moreover, the advances of novel enzyme technologies especially from marine, algae, bacteria and molluscs will enable us to explore these marine PS towards developing novel prebiotics regimen. Table 3 summarizing the name of prebiotic microalgae with their specific bioactive compounds responsible for various health benefits upon precise applications.
Table 3.
Prebiotic algae with specific bioactive compounds exhibiting various health benefits upon precise applications
| Macro/microalgae | Commercial biomass form | Products | Bioactive compounds | Positive health effects | Reference |
|---|---|---|---|---|---|
| Chlorella sp., Arthrospira platensis | Powder | Cheese | Carbohydrates, protein, ω3-FA | Anticancer; lowering gastric ulcers, neurosis, hypertension, anemia, constipation, diabetes, infant malnutrition, | [91] |
| Spirulina sp. | Powder and extract | Non-alcohol beverage | Protein, chlorophylls, phycocyanin | Enhanced immunity and lymphatic performance, anticancer and antiulcer property | [92] |
| Tetraselmis suecica | Food supplement | Extract | – | Prevention from diabetes and obesity | [93] |
|
Hematococcus pluvialis Phaeodactylum tricornutum |
Powder or flour | Biscuits | Protein, ω3-FA, DHA, EPA, astaxanthin | Antioxidative response | [94, 95] |
|
Chlorella sp. Schizochytrium sp. Thraustochytrium sp. |
Food supplement |
Powder, flour, tablet or liquid |
Proteins, ω3-FA |
Prevent from constipation, satiety induction | [96] |
| Ulva, Porphyra, Laminaria/saccharina, Enteromorpha, Undaria, Rhodella, Fucus, Ascophyllum, Sargassum | Food supplements | Powder | Polysaccharide | Immunomodulatory, Antilipidaemic and hypocholesterolaemic | [39, 97] |
| Dunaliella sp. Spirulina sp. | Powder | Miso | Protein, vitamins, minerals | Antioxidative response | [98] |
| Arthrospira platensis | Oil | – | Carotenoids | Antimicrobial and antiviral properties | [99] |
| Dunaliella salina |
Culinary condiment with sea salt |
Powder | Carotenoids | Antioxidative response | [100] |
| Porphyridium | Food supplements | Powder | Polysaccharide | Immunomodulatory, Antilipidaemic and hypocholesterolaemic | [39, 97] |
| Arthrospira platensis, Chlorella sp. | Powder or flour | Bread and cookies | Protein, vitamins, minerals | Reduction in cholesterol and fat levels, satiety induction | [101] |
| Gracilaria, Cladosiphon, Monostroma, Capsosiphon, Kappaphycus, Furcellaria, Soliera | Food supplements | Powder | Polysaccharide | Immunomodulatory | [39, 97] |
| Haematococcus pluvialis | Food supplement | Capsules | Astaxanthin | UV protection, anticoagulatory & anti-inflammatory effects, immunity modulation, improve cardiovascular health | [102] |
| Chlorella sp. and Spirulina sp. | Powder and extract | Milk | Proteins, ω-3FA, EPA, DHA | Reduced onset of anemia | [103] |
| Chlorella, Phaedactlylum, Gyrodinium, | Food supplements | Powder | Polysaccharide | Immunomodulatory | [39, 97] |
Prebiotic potential of algal compounds
Prebiotics potential were observed in some compounds of seaweeds and marine microalgae, mainly native as well as modified forms of polysaccharides (PS) were recognized as prebiotics such as XOS, GOS, AGAROS, ALGOS, NAOS, galactans, arabinoxylans, β-glucans. These algal PS are usually not digested by metabolic enzymes in the upper gut. Therefore, they can be used as dietary prebiotics and able to augment the growth of probiotics [104]. Specific PS found in certain algal biomass having probiotic potential have been described with their monosaccharide compositions and the linkage types, moreover some di- and oligosaccharides which are part of the PS of some microalgae are also described as fibers. Fucoidans: Brown seaweeds are rich in fucoidans, a soluble homo- or heteropolymeric PS, in which L-fucose are the main sugar residue. It is an irregularly branched and sulphated high molecular weight polysaccharide (HMW-PS), whose monomers are linked by alternating (1,3)- and (1,4)-α bonds. Galactofucans are another PS found in Laminaria and Undaria brown macroalgae [39].
Alginates are major approx. 20–29% DW carbohydrates in Fucus, Ascophyllum and Sargassum. These species also contain fucoidans in lower amounts (10–11% DW) [105]. It is an anionic-acidic, water soluble, non-branched PS, being used in the food industry (E400–E407), it comprises L-guluronic acid and D-mannuronic acid monomers. Alginates mainly occur in both Laminaria and Macrocystis. A β-glucan for example Laminaran, (1,3)-and (1,6)-β-linkages with some other laterally linked sugar residues found in Laminaria, Ascophyllum, Undaria and Fucus. [106]. Carrageenans are broadly used as gelling agents in the food industry. Moreover, polysaccharides reported from green seaweeds includes: ulvan as main PS in Enteromorpha and Ulva species, Capsosiphon (1,3-β-mannan) in Codium fragile, Rhamnans in Enteromorpha, galactans in Caulerpa species and other PS have also reported [39].
On the other hand, there are not many reports over complex PS from microalgae, except β-glucan and homogalactan respectively in C. vulgaris and Gyrodinium, other PS are usually heteropolymers comprising numerous different monosaccharides. The glycosidic linkages of these PSs were poorly described for limited PS, for example PS from Phaeodactylum tricornutum and Aphanothece halophytica. But the simple polymeric structures especially for replicating mono-, di- and oligosaccharides were well explained for several PSs from Porphyrium, Arthrospira and Rhodella [39]. Hemicelluloses (HC) are most common soluble PS in the algal biomass, HC are branched polymers found in the cell as well as produced/released into the culture medium. HC are heteropolymers and can be simply hydrolysed by hemicellulases as well as by acid and basic solutions. Moreover, PS, which are non-soluble fibers for example cellulose found in seaweeds, is a non-branched linear polymer composed of mainly anhydrous glucose residues which are linked together by β-(1,4) linkages. Lignin is also a non-soluble fiber, which is resistant to microbial enzymes [85]. Table 4 summarizing the dietary fibers from macro- and microalgae sources reported for promotion of specific probiotics and suppression of other harmful gut bacteria.
Table 4.
Various prebiotics recorded for affecting probiotic abundance in GIT environment
| Prebiotic components | Induced bacteria | Suppressed bacteria | Reference |
|---|---|---|---|
| FOS |
Lactobacillus, Bifidobacteria, Ruminococcus, Faecalibacterium, Oscillospira |
– | [73] |
| Fractan* | Bifidobacteria, Anaerostipes | Bilophila | [107] |
| GOS | Bifidobacteria |
Holdemania, Synergistes Dehalobacterium, Ruminococcus, |
[108] |
| Inulin | Actinobacteria | Clostridia | [109] |
| GOS |
Bifidobacteria, Bacteroides, Atopobium |
– | [110] |
| Inulin (long chain) |
Lactobacillus, Bifidobacteria, Atopobium, |
Bacteroides-Prevotella | [111] |
| Fractan* | Bifidobacteria, Lactobacillus | – | [112] |
| FOS | Bifidobacterium | Salmonella, Phascolarctobacterium Enterobacter, Coprococcus, Turicibacter | [108] |
| -NAOS | Bifidobacteria, Lactobacillus |
Bacteroides, Enterococci, Putrefactive bacteria |
[113] |
| Alginate | Bifidobacteria, Lactobacillus | [114] | |
| Fucoidan | Lactobacteria | – | [115] |
| Fractan* | Bifidobacteria, Faecalibacterium prausnitzii | Bacteroides, Propionibacterium | [77] |
| Oligos/PS in Ascophyllum biomass | Lactobacillus, E. coli | – | [116] |
| GOS | Bifidobacteria, Actinobaceria | Bacteroides | [117] |
| Oligo/PS in Gelidium extract | Bifidobacteria | – | [118] |
| Agave inulin | Actinobacteria, Bifidobacterium |
Lachnobacterium, Desulfovibrio Ruminococcus |
[119] |
| Resistant starch Type 4 | Bifidobacteria, Parabacteroides distasonis, Clostridia | – | [120] |
| Oligo/PS in Spirulina biomass | Bifidobacterium, L. casei, L. acidophilus, S. thermophillus | P. vulgaris, B. subtilis, B. pumulis | [29, 121] |
| Oligo/PS in Isochrysis biomass | Lactic acid bacteria | – | [122] |
*Fractan: matching Inulin structure
In addition to PS, several other important bioactive compounds produced by algae which are showing comparatively fewer prebiotic properties and have reported several health benefits. As mentioned in the previous section, microalgae are a promising source of these compounds like proteins, steroids, carotenoids, fatty acids, lectins, minerals, vitamins, amino acids, halogenated compounds, and polyketides [123]. Microalgae produce essential amino acids, minerals, unsaturated fatty acids, and several vitamins (A, B, E, and K) and serve as functional foods for therapeutic and nutraceutical applications [95, 124, 125] which are well described in Table 5. Prebiotics commonly oblige as substrate to be biologically degraded by the colonic microflora with the help of enzymes. These prebiotics can be oligosaccharides, dietary fibers (mainly PS having DP > 10), resistant starches, sugar alcohols, non-absorbable sugars, proteins, amino acids, and also could be other biomaterials, such as mucins, microbial metabolites and products obtained from cell lysis. From recent studies it was understood that both macro- and microalgae are promising sources of the majority of above compounds, few of them are already verified to possess prebiotic attributes [69].
Table 5.
Use of microalgae products and their specific applications in various health sectors
| Category | Microalgae used | Products | Nutrient source/Health effects |
|---|---|---|---|
| Nutraceutics | Spirulina, Nannochloropsis, Dunaliella, Schizochytrium etc |
Fatty acids and sterols, fibres, carbohydrates (EPA, DHA, GLA, SDA, Poriferasterol, Clinosterols, agar, alginates etc.) Carotenoids (β-carotene, astaxanthin, lutein, fucoxanthin etc.) Protein and amino acids (Single Cell Protein-spirulina, phycocyanin) Vitamins and minerals |
High Saturated/unsaturated fatty acids, high fibers, high carbohydrates for nutrition Antioxidative effects Enriched with essential amino acids and good protein source Source of high vitamins (A, B2, B6, B8, B12, E, K), high minerals (Fe & Ca) |
| Therapeutics and/or Pharmaceutics | Genetically modified microalgae strain such as C. reinhardtii, Schizochytrium, Spirulina, Chlorococcum, Haematococcus, Chlorella etc |
Specific microalgal extract, lotions (enriching either bioactive compounds, Tyrosine inhibitors or hydrolytic enzymes, Phytases, etc.) |
Reducing blood cholesterol, antiallergic activity, decreasing blood sugar, antianemia effect, reducing heart attacks, antitumor activity, antiviral activity, reducing hypertension, reducing hyperlipidemia, improving immunity, stress reducing action, protecting from harmful chemicals, anti-inflammatory and antioxidative activities against neurodegenerative disorders, atherosclerosis disorders, T2DM, Cancer etc |
| Cosmeceutics | Spirulina, Haematococcus, Dunaliella, Chlorella etc |
Moisturizers and lotions: Antiaging and UV-protection Polysaccharides Antioxidant enzymes Microsporin like amino acid Skin whitening and haircare |
Poly unsaturated fatty acids (PUFAs) Carotenoids (astaxanthin, fucoxanthin) Fucoidan, alginates, galactans, agar, ulvans etc Superoxide dismutase, catalase, peroxidases, and glutathione Fucoxanthin, microalgal extract |
Nevertheless, the health benefits determined in vitro or in vivo studies, more human trials must be completed to establish the optimized doses and the health effects in hosts for attesting positively confirmed prebiotic candidates to be approved finally for human use. For launching prebiotics from macro- and microalgae, such more trials are yet to be done especially for most of those PS obtained largely from them and to be recognized as safe prebiotics. Moreover, already established oligo- and polysaccharides (XOS, GOS, galactans, xyloarabinans, β-glucans), as prebiotics from these algae must be effectively outlined [126]. According to a past review, specific characters were not well-focused for screening of the potential prebiotics, therefore recent past PS from macro- and microalgae have already been subjected to human trials [39].
Microalgae models and their products in use as nutraceutics/therapeutics
Various microalgal strains and their derived compounds have been already approved which have to be used either as food or food additives in various countries. For example, microalgae Tetraselmis chuii and Ulkenia sp. have been approved in Europe [127, 128]. Arthrospira sp. approved in the United States with the GRAS Notice [129], whereas its product phycocyanin is approved as a food additive only in Japan [130]. Euglena gracilis is approved as food in the United States recently [131]. DHA rich oil from Schizochytrium sp. has been approved as food in Europe through three Commission implementing decisions [132–134] and in Australia, New Zealand and Japan with a GRAS Notice and Schedule 25 respectively [135, 136]. However, DHA rich oil from Ulkenia sp. is approved to use in the United States, Australia and New Zealand with a GRAS Notice and Schedule 25 respectively [136, 137]. DHA rich oil from other microalgae species are approved only in the United States such as Dunaliella salina, Auxenochlorella protothecoides and Chlorella vulgaris with independent GRAS Notices [138–140]. DHA rich oil from Chlorella sp. are approved for human consumption as well as Carotene from Dunaliella sp. and Haematococcus algae color are also approved to be used as food additives in Japan [130].
The PS discussed in the above section for probiotic growth promotion, however its biodegradability and bioconversion are also tied to the huge variability of activities they encompass, which make them a promising material as pharmaceutics, therapeutics, and regenerative medicine [90]. Number of desirable activities were confirmed in PS and their derivatives both in vitro and in vivo, such as immunomodulatory, anticoagulant, antithrombotic, antitumor and anticancer activities. Moreover, they also found promising antilipidemic and hypoglycemic, antioxidants, anti-inflammatory and antibiotics agents. Other medicinal characteristics of PS are angiogenic, antinociceptive, gastroprotective, cardioprotective, etc. Their most common biomedical scope in medicines are wound healing, mucobioadhesion of bone and tissue, biolubrication in stiff joints, immunotherapy cancer vaccines, or as new versions of biotextiles and therapeutic fibres especially in drug delivery as well as promising platforms for regenerative remedy. For instance, Porphyridium and Enteromorpha PS have been confirmed as potent candidate for immunomodulation and antitumor possessions [141, 142]; PS from Dyctiota menstrualis and Caulerpa cupressoides are decent antinociceptive mediators [143, 144], whereas Cladosiphon okaramanus PS showed angiogenic, gastro- and cardioprotective properties [145, 146]. Table 5 demonstrating various specific microalgae prebiotic under nutraceutics, therapeutics and cosmeceutics applications. In which, the cosmeceutics is not a major focus of this article as it is a non-ingested for health application in GIT but implies on host skin surface.
Challenges for commercialization and research advancement
With numerous bioactive compounds in algal biomass in which some are already arrived in commercial forms and playing an important role for human and animal health as functional food and animal feed. Consumption of these commercial forms already proved for positive health effects for various minor and major health issues. Still, scale up remains a major challenge for new compounds having prebiotic potential but yet to be tested at all stages before attaining commercial affordability [147]. Nevertheless, these obstacles, some probiotic companies have already overcome existing market constraints, and they are magnificently trading extracts and powder of microalgae as food supplement, colorant, and animal feeds. Solid technical evidence for probiotic roles of macro- and microalgae in humans and animals is awaited, while rare studies have addressed about delivering probiotic efficacy in marine animals upon these prebiotics ingestion [16].
Majority of the technological advancement in algal research failed to reach commercial stage mainly due to number of constraints. Primary is small market size; then production at higher cost than fossil materials. Others reasons are from chemical and biological routes covering fungal and bacterial process. Moreover, stringent regulations for safety assurance, quality specifications, and environmental impact reduction are also accountable [148]. Moreover, limitations in algal biomass harvesting and short viability are indeed constrained commercial success. Still, there is dispersed evidence about prebiotic potential of microalgae owing to their abundance in oligosaccharides which are barely fermented by GIT microbiota. Though, reliable applications occur only in the aquaculture and dairy industries. Commercial microalgal production facilities are scattered globally (Table 6) However, the majority of the facilities are dominated from North America and Asia, and rather less contribution by Europe, Africa, and the rest of the world.
Table 6.
Commercial algal prebiotics products, their forms globally available for human health benefits upon consumption
| Product name | Form of the product and application | Microalgae | Company | Production size (ton/year) | Reference |
|---|---|---|---|---|---|
|
Spirulina Natural, Spirulina Gold |
Tablets, powders, extracts | Spirulina (world largest farm) | Earthrise Nutritionals, California, USA | 2000 | [149] |
| Hawaiian Spirulina | Tablets, powders | Spirulina pacifica | Nutrex-Hawaii, USA | 3000 | [150] |
|
Chlorella premium, Green gems, Chlorella Plus Chlorella Spirulina Tablets etc |
Tablets, powders, nectar, noodles |
Chlorella sp. Spirulina sp. |
Taiwan Chlorella Manufacturing Company (TCMC) 1964 | 2000 | [151, 152], |
| Chlorella supplements | Powders, tablets, extracts, drinks | Chlorella sp. | Hainan Simai Pharmacy Co. (China) | 2000 | [153] |
| Vitamineral Green | Powders, tablets, extracts | Spirulina Azteca | Health Force Nutritionals, Chile | – | [154] |
| FEBICO SOROKINA® | Powders (vitamins, proteins, dietary fibre, growth factors, phytochemicals etc.) | Chlorella sorokiniana, Schizochytrium sp, | Far East Bio-Tec Co., Ltd. FEBICO (ALGAPHARMA BIOTECH CORP.) Taiwan 1976 | 2000 | [155] |
| JUNE Spirulina, SPIRUJU, Spilova wine, Juno fried chips etc |
Tablets, extract, liquid chips, noodles and pasta |
Spirulina sp | Myanmar Spirulina Factory | 3000 | [156] |
| ALGOMED® Chlorella natürlich | Powders | Chlorella sp. | Klotze (Germany) | 2000 | [156] |
| Hawaiian BioAstin | Tablets, powders, | Haematococcus | Nutrex-Hawaii, USA | 3000 | [150] |
|
Hawaiian Spirulina and astaxanthin |
Tablets, powders, beverages, extracts | Spirulina sp., Haematococcus | Cyanotech Corp. (USA) | 3000 | [150] |
| Blue green foods, Stem naturals, AFA organic dietary supplements | Capsules, crystal Powders, capsules | Aphanizomenon flos-aquae | Blue Green Foods (USA), Vision (USA) | 500 | [156] |
| Betatene® | Powders of β-carotene | Dunaliella salina | Cognis Nutrition and health (Australia) | 1200 | [156] |
| Astapure® | astaxanthin Powder in cosmetics | Haematococcus pluvialis | Algatech (Israel) | 2000 | [157] |
| AlgaVia™ | Powder for flour supplement | Chlorella sp. | Solazyme | – | [158] |
Regardless of the fundamental development in properties and functional food and animal feed formulation, wider research and development are prerequisite before macro- and microalgae are developed as a commercial realism in prebiotic formulation for several health applications. Table 6 summarizes various commercial microalgal products, compound forms, their brand names and manufacturing companies along with the production scales.
Conclusion
Algae showed a marked potential to accomplish the people's alimentary and remedial needs, hence offer sustainable diet solutions. Coming years, the possibility of the potential use of algal prebiotics to regulate the gut microbiome specially to prevent several host diseases is anticipated. Besides being a rich source of amino acid, it’s potential for several bioactive compounds offers great promise for broader health applications. The opulence of nutritive as well as therapeutic compounds in microalgae provide a platform to raise an industry aimed to provide algae-based innovative functional foods which can boost not only nutrition scope of the host but also prophylactic effects. Currently, algal products are not affordable due to technological non-readiness as well as budget of scale in up- and downstream processes. Some obstacles need to be removed to launch the algae as a sustainable food solution for the rising population. Moreover, the prebiotic applications benefits offered by marine seaweeds and microalgae must not be limited to their PSs and lignin, but it must be rather wide up to other fractions such as PUFAs), monosaccharides, polyphenols, enzymes, alcohols, and peptides as these have been proved in analogous fractions of other sources. In the coming years, the likelihood of marine seaweeds PS as prebiotics, to modify the microbiome, and to get numerous health benefits is anticipated.
Acknowledgements
AKP and RRS would like to acknowledge the Taiwan MOST for funding support (Ref. No. 109-2222-E-992-002).
Abbreviations
- PS
Polysaccharide
- HC
Hemicellulose
- LAB
Lactic acid bacteria
- DHA
Docosahexaenoic acid
- EPA
Eicosapentaenoic acid
- FOS
Fructo-oligosaccharides
- XOS
Xylo-oligosaccharides
- HMO
Human milk oligosaccharides
- GOS
Galacto-oligosaccharides
- SOS
Soya-oligosaccharides
- LS
Lactosucrose
- IMO
Isomalto-oligosaccharides
- POS
Pectic-oligosaccharides
- SCFAs
Short chain fatty acids
- PDX
Polydextrose
- IBS
Inflammatory bowel disease
- IBD
Inflammatory bowel syndrome
- ALGOS
Alginate-oligosaccharides
- NAOS
Neoagaros-oligosaccharides
- GLA
γ-Linolenic acid
- PUFAS
Polyunsaturated fatty acids
Authors’ contributions
Conceptualization- RRS, MA, and AKP; Background and data collection- SV, SKB, MA and RRS; Formal correction- AKP, CWC and CDD; Validation- CWC, AKP and CDD; Data curation- AKP, RRS and CDD; Writing-original draft preparation- RRS, SKB, SLH, and MLT; Writing review and editing, MLT, SLH and RRS; Visualization- MLT, SLH and AKP; Supervision- CWC and CDD; All authors have read and agreed to the published version of the manuscript.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
AnilKumar Patel and ReetaRani Singhania Equally contributed
References
- 1.Markowiak P, Śliżewska K. The role of probiotics, prebiotics and synbiotics in animal nutrition. Gut Pathog. 2018;10:21. doi: 10.1186/s13099-018-0250-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Terpou A, Papadaki A, Lappa IK, Kachrimanidou V, Bosnea LA, Kopsahelis N. Probiotics in food systems: Significance and emerging strategies towards improved viability and delivery of enhanced beneficial value. Nutrients. 2019;11(7):1591. doi: 10.3390/nu11071591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adefegha SA. Functional foods and nutraceuticals as dietary intervention in chronic diseases, novel perspectives for health promotion and disease prevention. J Diet Supple. 2018;15(6):977–1009. doi: 10.1080/19390211.2017.1401573. [DOI] [PubMed] [Google Scholar]
- 4.Patel AK, Deshattiwar MK, Chaudhari BL, Chincholkar SB. Production, purification, and chemical characterization of the catecholate siderophore from potent probiotic strains of Bacillus sp. Bioresour Technol. 2009;100:368–373. doi: 10.1016/j.biortech.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 5.Patel AK, Singhania RR, Michaud P, Pandey A. Polysaccharides from probiotics: New developments as food additives. Food Technol Biotechnol. 2010;48(4):451–463. [Google Scholar]
- 6.Puupponen-Pimia R, Aura AM, Oksman-Caldentey KM, Myllarinen P, Saarela M, Mattila-Sandholm T, et al. Development of functional ingredients for gut health. Trend Food Sci Technol. 2002;13:3–11. doi: 10.1016/S0924-2244(02)00020-1. [DOI] [Google Scholar]
- 7.Patel AK, Ahire JJ, Pawar SP, Chaudhari BL, Souche YS, Chincholkar SB. Comparative accounts of probiotic characteristics of Bacillus spp isolated from food waste. Food Res Int. 2009;42(4):505–510. doi: 10.1016/j.foodres.2009.01.013. [DOI] [Google Scholar]
- 8.Patel AK, Ahire JJ, Pawar SP, Chaudhari BL, Shouche YS, Chincholkar SB. Evaluation of probiotic characteristics of siderophoregenic Bacillusspp. isolated from dairy waste. Appl Biochem Biotechnol. 2010;160:140–155. doi: 10.1007/s12010-009-8583-2. [DOI] [PubMed] [Google Scholar]
- 9.Patel AK, Singhania RR, Pandey A, Chincholkar SB. Probiotic bile salt hydrolase: current advances and perspectives. Appl Biochem Biotechnol. 2010;162(1):166–180. doi: 10.1007/s12010-009-8738-1. [DOI] [PubMed] [Google Scholar]
- 10.Omar HH, Dighriri KA, Gashgary RM. The benefit roles of micro- and macro-algae in probiotics. Nat Sci. 2019;17(11):258–279. [Google Scholar]
- 11.Caporgno MP, Mathys A. Trends in microalgae incorporation into innovative food products with potential health benefits. Front Nutr. 2018;5:58. doi: 10.3389/fnut.2018.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee NK, Park JS, Park E, Paik HD. Adherence anticarcinogenic effects of Bacillus polyfermenticus SCD in the large intestine. Lett Appl Microbiol. 2007;44(3):274–278. doi: 10.1111/j.1472-765X.2006.02078.x. [DOI] [PubMed] [Google Scholar]
- 13.Collado MC, Jalonen L, Meriluoto J, Salminen S. Protection mechanism of probiotic combination against human pathogens: in vitro adhesion to human intestinal mucus. Asia Pac J Clin Nutr. 2006;15(4):570–575. [PubMed] [Google Scholar]
- 14.Batista AP, Niccolai A, Fradinho P, Fragoso S, Bursic I, Rodolfi L, Biondi N, Tredici MR, Sousa I, Raymundo A. Microalgae biomass as an alternative ingredient in cookies: sensory, physical and chemical properties, antioxidant activity and in vitro digestibility. Algal Res. 2017;26:161–171. doi: 10.1016/j.algal.2017.07.017. [DOI] [Google Scholar]
- 15.Choi YY, Patel AK, Hong ME, Chang WS, Sim SJ. Microalgae bioenergy carbon capture utilization and storage (BECCS) technology: an emerging sustainable bioprocess for reduced CO2 emission and biofuel production. Bioresour Technol Rep. 2019;7:100270. doi: 10.1016/j.biteb.2019.100270. [DOI] [Google Scholar]
- 16.Camacho F, Macedo A, Malcata F. Potential industrial applications and commercialization of microalgae in the functional food and feed industries: a short review. Mar Drugs. 2019;17:312. doi: 10.3390/md17060312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gouveia L, Batista AP, Sousa I, Raymundo A, Bandarra NM. Microalgae in novel food products. In: Papadoupoulos K, editor. Food chemistry research developments. Nova Science Publishers; 2008. pp. 75–112. [Google Scholar]
- 18.Raposo MFJ, De Morais RMSC, De Morais AMMB. Bioactivity and applications of sulphated polysaccharides from marine microalgae. Mar Drugs. 2013;11:233–252. doi: 10.3390/md11010233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vidanarachchi JK, Kurukulasuriya MS, Malshani Samaraweera A, Silva KFST. Applications of marine nutraceuticals in dairy products. Adv Food Nutr Res. 2012;65:457–478. doi: 10.1016/B978-0-12-416003-3.00030-5. [DOI] [PubMed] [Google Scholar]
- 20.Ścieszka S, Klewicka E. Influence of the microalga Chlorella vulgaris on the growth and metabolic activity of Lactobacillusspp. bacteria. Foods. 2020;9(7):959. doi: 10.3390/foods9070959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamamoto FY, Yin F, Rossi W, Hume M, Gatlin DM. β-1,3 glucan derived from Euglena gracilis and AlgamuneTM enhances innate immune responses of red drum (Sciaenops ocellatus L.) Fish Shellfish Immunol. 2018;77:273–279. doi: 10.1016/j.fsi.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 22.Samuel H. Coculture of probiotic bacteria in algal feedstocks for disease management in bivalve hatcheries, Master's Theses. University of Rhode Island Paper 2019; p. 1448.
- 23.Beheshtipour H, Mortazavian AM, Mohammadi R, Sohrabvandi S, Khosravi‐Darani K. Supplementation of Spirulina platensis and Chlorella vulgaris algae into probiotic fermented milks. 2013; 12 (2): 144-154
- 24.Lebrun JR, Levine R, Horst GP. Animal feed compositions and methods of using the same. E.P. Patent 2817012, 31 December 2014.
- 25.Molnar N, Sipos-Kozma ZS, Toth A, Asvanyi B, Varga L. Development of a functional dairy food enriched with Spirulina (Arthrospira platensis) Tejgazdasag. 2009;69(2):15–22. [Google Scholar]
- 26.Narayana R, Kale A. 2019. Functional probiotic yoghurt with Spirulina, Asian J. Dairy Food Res. 2013; 38(4): 311-314
- 27.Cerezuela R, Meseguer J, Esteban MA. Effects of dietary inulin, Bacillus subtilis and microalgae on intestinal gene expression in gilthead seabream (Sparus aurata L.) Fish Shellfish Immunol. 2013;34:843–848. doi: 10.1016/j.fsi.2012.12.026. [DOI] [PubMed] [Google Scholar]
- 28.Michalak I, Mironiuk M, Godlewska K, Trynda J, Marycz K. Arthrospira (Spirulina) platensis: an effective biosorbent for nutrients. Proc Biochem. 2020;88:129–137. doi: 10.1016/j.procbio.2019.10.004. [DOI] [Google Scholar]
- 29.Bhowmik D, Dubey J, Mehra S. Probiotic efficiency of Spirulina platensis—stimulating growth of lactic acid bacteria. Am Euras J Agric Environ Sci. 2009;6(5):546–549. [Google Scholar]
- 30.Marques A, Thanh TH, Sorgeloos P, Bossier P. Use of microalgae and bacteria to enhance protection of gnotobiotic Artemia against different pathogens. Aquaculture. 2006;258:116–126. doi: 10.1016/j.aquaculture.2006.04.021. [DOI] [Google Scholar]
- 31.Nontando H. Isolation and characterization of prebiotic oligosaccharides from algal extracts and their effect on gut microflora. Thesis, Durban University of Technology, Durban, South Africa.
- 32.Reyes-Becerril M, Angulo C, Estrada N, Murillo Y, Ascencio-Valle F. Dietary administration of microalgae alone or supplemented with Lactobacillus sakei affects immune response and intestinal morphology of Pacific red snapper (Lutjanus peru) Fish Shellfish Immunol. 2014;40:208–216. doi: 10.1016/j.fsi.2014.06.032. [DOI] [PubMed] [Google Scholar]
- 33.Chandrarathna HPSU, Liyanage TD, Edirisinghe SL, Dananjaya SHS, Thulshan EHT, Nikapitiya C, Oh C, Kang DH, Zoysa MD. Marine microalgae, Spirulina maxima-Derived modified pectin and modified pectin nanoparticles modulate the gut microbiota and trigger immune responses in mice. Mar Drugs. 2020;18:175. doi: 10.3390/md18030175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hayes M, Skomedal H, Skjånes K, Mazur-Marzec H, Torúnska-Sitarz A, Catala M, Isleten Hosoglu M, García-Vaquero M. Microalgal proteins for feed, food and health. In: Gonzalez-Fernandez C, Muñoz R, editors. Microalgae-based biofuels and bioproducts: from feedstock cultivation to end-products. Duxford: Elsevier-Woodhead Publishing Series in Energy; 2017. pp. 347–368. [Google Scholar]
- 35.Dittami SM, Heesch S, Olsen JL, Collén J. Transitions between marine and freshwater environments provide new clues about the origins of multicellular plants and algae. J Phycol. 2017;53:731–745. doi: 10.1111/jpy.12547. [DOI] [PubMed] [Google Scholar]
- 36.Gouveia L, Raymundo A, Batista AP, Sousa I, Empis J. Chlorella vulgaris and Haematococcus pluvialis biomass as colouring and antioxidant in food emulsions. Eur Food Res Technol. 2006;222:362–367. doi: 10.1007/s00217-005-0105-z. [DOI] [Google Scholar]
- 37.Villarruel-López A, Ascencio F, Nuño K. Microalgae, a potential natural functional food source—a review. Pol J Food Nutr Sci. 2017;67(4):251–263. doi: 10.1515/pjfns-2017-0017. [DOI] [Google Scholar]
- 38.Hamed I, Özogul F, Özogul Y, Regenstein JM. Marine bioactive compounds and their health benefits: a review. Compr Rev Food Sci Food Saf. 2015;14:446–465. doi: 10.1111/1541-4337.12136. [DOI] [Google Scholar]
- 39.Raposo MFJ, Morais AMMB, Morais RMSC. Marine polysaccharides from algae with potential biomedical applications. Mar Drugs. 2015;13:2967–3028. doi: 10.3390/md13052967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raposo MFDJ, Morais AMMBD, Morais RMSCD. Emergent sources of prebiotics: seaweeds and microalgae. Mar Drugs 2016; 14: 27. [DOI] [PMC free article] [PubMed]
- 41.Gupta S, Gupta C, Gard AP, Prakash D. Prebiotic efficiency of blue green algae on probiotics microorganisms. J Microbiol Exp. 2017;4(4):00120. [Google Scholar]
- 42.Wang HMD, Chen CC, Huynh P, Chang JS. Exploring the potential of using algae in cosmetics. Bioresour Technol. 2015;184:355–362. doi: 10.1016/j.biortech.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 43.Uysal O, Uysal O, Ek K. Determination of fertilizing characteristics of three different microalgae cultivated in raceways in greenhouse conditions could increase soil fertility and product yield. Agron Ser Sci Res. 2016;59:15–19. [Google Scholar]
- 44.Rashid N, Park WK, Selvaratnam T. Binary culture of microalgae as an integrated approach for enhanced biomass and metabolites productivity, wastewater treatment, and bioflocculation. Chemosphere. 2018;194:67–75. doi: 10.1016/j.chemosphere.2017.11.108. [DOI] [PubMed] [Google Scholar]
- 45.Patel AK, Choi YY, Sim SJ. Emerging prospects of mixotrophic microalgae: Way forward to bioprocess sustainability, environmental remediation and cost-effective biofuels. Bioresour Technol. 2020;300:122741. doi: 10.1016/j.biortech.2020.122741. [DOI] [PubMed] [Google Scholar]
- 46.Hong ME, Chang WS, Patel AK, Oh MS, Lee JJ, Sim SJ. Microalgae based carbon sequestration by converting LNG-fired waste CO2 into red gold astaxanthin: the potential applicability. Energies. 2019;12:1718. doi: 10.3390/en12091718. [DOI] [Google Scholar]
- 47.Lum KK, Kim J, Lei XG. Dual potential of microalgae as a sustainable biofuel feedstock and animal feed. J Anim Sci Biotechnol. 2013;4:1–7. doi: 10.1186/2049-1891-4-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Priyadarshani I, Rath B. Commercial and industrial applications of micro algae—a review. J Algal Biomass Util. 2012;3:89–100. [Google Scholar]
- 49.Varga L, Szigeti J. Microbial changes in natural and algal yoghurts during storage. Acta Aliment Hung. 1998;27(2):127–135. [Google Scholar]
- 50.Varga L, Szigeti J, Kovacs R, Foldes T, Buti S. Influence of a Spirulina platensis biomass on the microflora of fermented ABT milks during storage. J Dairy Sci. 2002;85:1031–1038. doi: 10.3168/jds.S0022-0302(02)74163-5. [DOI] [PubMed] [Google Scholar]
- 51.Patel AK, Joun JM, Hong ME, Sim SJ. Effect of light conditions on mixotrophic cultivation of green microalgae. Bioresour Technol. 2019;282:245–253. doi: 10.1016/j.biortech.2019.03.024. [DOI] [PubMed] [Google Scholar]
- 52.Sim SJ, Joun JM, Hong ME, Patel AK. Split mixotrophy: a novel mixotrophic cultivation strategy to improve mixotrophic effects in microalgae cultivation. Bioresour Technol. 2019;291:121820. doi: 10.1016/j.biortech.2019.121820. [DOI] [PubMed] [Google Scholar]
- 53.Patel AK, Joun JM, Hong ME, Sim SJ. A sustainable mixotrophic microalgae cultivation from dairy wastes for carbon credit, bioremediation and lucrative biofuels. Bioresour Technol. 2020;313:123681. doi: 10.1016/j.biortech.2020.123681. [DOI] [PubMed] [Google Scholar]
- 54.Chisti Y. Biodiesel from microalgae. Biotechnol Adv. 2007;25:294–306. doi: 10.1016/j.biotechadv.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 55.Sung YJ, Patel AK, Yu BS, Kim J, Choi HI, Sim SJ. Sedimentation rate-based screening of oleaginous microalgal for fuel production. Bioresour Technol. 2019;293:122045. doi: 10.1016/j.biortech.2019.122045. [DOI] [PubMed] [Google Scholar]
- 56.Roberfoid M, Slavin J. Non-digestible oligosaccharides. Crit Rev Food Sci Nutr. 2000;40:461–480. doi: 10.1080/10408690091189239. [DOI] [PubMed] [Google Scholar]
- 57.Seth A, Yan F, Polk DB, Rao RK. Probiotics ameliorate the hydrogen peroxide-induced epithelial barrier disruption by a PKC- and MAP kinase-dependent mechanism. Am J Physiol Gastrointest Liver Physiol. 2008;294(4):G1060–G1069. doi: 10.1152/ajpgi.00202.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.So D, Whelan K, Rossi M, Morrison M, Holtmann G, Kelly JT, Shanahan ER, Staudacher HM, Campbell KL. Dietary fiber intervention on gut microbiota composition in healthy adults: a systematic review and meta-analysis. Am J Clin Nutr. 2018;107(6):965–983. doi: 10.1093/ajcn/nqy041. [DOI] [PubMed] [Google Scholar]
- 59.Wallace IM, Urbanus ML, Luciani GM, Burns AR, Han MK, Wang H, Arora K, Heisler LE, Proctor M, St Onge RP, Roemer T, Roy PJ, Cummins CL, Bader GD, Nislow C, Giaever G. Compound prioritization methods increase rates of chemical probe discovery in model organisms. Chem Biol. 2011;18(10):1273–1283. doi: 10.1016/j.chembiol.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, Reid G. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017;14(8):491–502. doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- 61.Roberfroid M, Gibson GR, Hoyles L, McCartney AL, Rastall R, Rowland I, et al. Prebiotic effects: metabolic and health benefits. Br J Nutr. 2010;104(2):S1–S63. doi: 10.1017/S0007114510003363. [DOI] [PubMed] [Google Scholar]
- 62.Van Den Abbeele P, Venema K, van de Wiele T, Verstraete W, Possemiers S. Different human gut models reveal the distinct fermentation patterns of arabinoxylan versus inulin. J Agr Food Chem. 2013;61:9819–9827. doi: 10.1021/jf4021784. [DOI] [PubMed] [Google Scholar]
- 63.Sivieri K, Morales MLV, Saad SMI, Adorno MAT, Sakamoto IK, Rossi EA. Prebiotic effect of fructooligosaccharide in the simulator of the human intestinal microbial ecosystem (SHIME (R) Model) J Med Food. 2014;17:894–901. doi: 10.1089/jmf.2013.0092. [DOI] [PubMed] [Google Scholar]
- 64.Crittenden R, Playne MJ. Prebiotics. In: Lee YK, Salminen S, editors. Handbook of probiotics and prebiotics. Hoboken, NJ: Wiley; 2009. pp. 535–561. [Google Scholar]
- 65.Reid G, Sanders ME, Gaskins HR, Gibson GR, Mercenier A, Rastall R, et al. New scientific paradigms for probiotics and prebiotics. J Clin Gastroenterol. 2003;37(2):105–118. doi: 10.1097/00004836-200308000-00004. [DOI] [PubMed] [Google Scholar]
- 66.Pineiro M, Asp NG, Reid G, Macfarlane S, Morelli L, Brunser O, Tuohy K. FAO technical meeting on prebiotics. J Clin Gastroenterol. 2008;42:S156–S159. doi: 10.1097/MCG.0b013e31817f184e. [DOI] [PubMed] [Google Scholar]
- 67.Bindels LB, Delzenne NM, Cani PD, Walter J. Towards a more comprehensive concept for prebiotics. Nat Rev Gastroenterol Hepatol. 2015;12(5):303–310. doi: 10.1038/nrgastro.2015.47. [DOI] [PubMed] [Google Scholar]
- 68.Gibson GR, Probert HM, Loo JV, Rastall RA, Roberfroid MB. Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutr Res Rev. 2004;17(2):259–275. doi: 10.1079/NRR200479. [DOI] [PubMed] [Google Scholar]
- 69.Gibson GR, Scott KP, Rastall RA, Tuohy KM, Hotchkiss A, Dubert-Ferrandon A, et al. Dietary prebiotics: current status and new definition. Food Sci Technol Bull Funct Foods. 2010;7(1):1–19. doi: 10.1616/1476-2137.15880. [DOI] [Google Scholar]
- 70.Gibson GR, Beatty ER, Wang X, Cummings JH. Selective stimulation of Bifidobacteria in the human colon by oligofructose and inulin. Gastroenterology. 1995;108(4):975–982. doi: 10.1016/0016-5085(95)90192-2. [DOI] [PubMed] [Google Scholar]
- 71.Wang S, Xiao Y, Tian F, Zhao J, Zhang H, Zhai Q, Chen W. Rational use of prebiotics for gut microbiota alterations: Specific bacterial phylotypes and related mechanisms. J Funt Food. 2020;66:103838. doi: 10.1016/j.jff.2020.103838. [DOI] [Google Scholar]
- 72.Takumi T, Yoshihiro K, Toshio T, Yasuhiro K. 1-Kestose, the smallest fructooligosaccharide component, which efficiently stimulates Faecalibacterium prausnitzii as well as Bifidobacteria in humans. Foods. 2018;7(9):140. doi: 10.3390/foods7090140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tandon D, Haque MM, Gote M, Jain M, Bhaduri A, Dubey AK, Mande SS. A prospective randomized, double-blind, placebo-controlled, dose-response relationship study to investigate efficacy of fructo-oligosaccharides (FOS) on human gut microflora. Sci Rep. 2019;9(1):5473. doi: 10.1038/s41598-019-41837-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kearney SM, Gibbons SM, Erdman SE, Alm EJ. Orthogonal dietary niche enables reversible engraftment of a gut bacterial commensal. Cell. 2018;24(7):1842–1852. doi: 10.1016/j.celrep.2018.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shepherd ES, DeLoache WC, Pruss KM, Whitaker WR, Sonnenburg JL. An exclusive metabolic niche enables strain engraftment in the gut microbiota. Nature. 2018;557(7705):434–438. doi: 10.1038/s41586-018-0092-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ramirez-Farias C, Slezak K, Fuller Z, Duncan A, Holtrop G, Louis P. Effect of inulin on the human gut microbiota: stimulation of Bifidobacterium adolescentis and Faecalibacterium prausnitzii. Br J Nutr. 2009;101(4):541–550. doi: 10.1017/S0007114508019880. [DOI] [PubMed] [Google Scholar]
- 77.Dewulf EM, Cani PD, Claus SP, Fuentes S, Puylaert PG, Neyrinck AM, et al. Insight into the prebiotic concept: lessons from an exploratory, double blind intervention study with inulin-type fructans in obese women. Gut. 2013;62(8):1112–1121. doi: 10.1136/gutjnl-2012-303304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gómez B, Gullón B, Yáñez R, Schols H, Alonso JL. Prebiotic potential of pectins and pectic oligosaccharides derived from lemon peel wastes and sugar beet pulp: a comparative evaluation. J Func Food. 2016;20:108–121. doi: 10.1016/j.jff.2015.10.029. [DOI] [Google Scholar]
- 79.Everard V, Lazarevic N, Gaia M, Johansson M, Stahlman F, Backhed F, et al. Microbiome of prebiotic-treated mice reveals novel targets involved in host response during obesity. ISME J. 2014;8(10):2116–2130. doi: 10.1038/ismej.2014.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Baldwin J, Collins B, Wolf PG, Martinez K, Shen W, Chuang CC, et al. Table grape consumption reduces adiposity and markers of hepatic lipogenesis and alters gut microbiota in butter fat-fed mice. J Nutr Biochem. 2016;27:123–135. doi: 10.1016/j.jnutbio.2015.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Flint HJ, Bayer EA, Rincon MT, Lamed R, White BA. Polysaccharide utilization by gut bacteria: Potential for new insights from genomic analysis. Nat Rev Microbiol. 2008;6(2):121–131. doi: 10.1038/nrmicro1817. [DOI] [PubMed] [Google Scholar]
- 82.Macfarlane GT, Steed H, Macfarlane S. Bacterial metabolism and health-related effects of galacto-oligosaccharides and other prebiotics. J Appl Microbiol. 2008;104:305–344. doi: 10.1111/j.1365-2672.2007.03520.x. [DOI] [PubMed] [Google Scholar]
- 83.Lee YK. Handbook of probiotics and prebiotics. New Jersey: Wiley; 2009. [Google Scholar]
- 84.Uchida M, Mogami O, Matsueda K. Characteristic of milk whey culture with Propionibacterium freudenreichii ET-3 and its application to the inflammatory bowel disease therapy. Inflamm-opharmacology. 2007;15:105–108. doi: 10.1007/s10787-007-1557-5. [DOI] [PubMed] [Google Scholar]
- 85.Praznik W, Loeppert R, Viernstein H, Haslberger AG, Unger FM. Dietary fiber and prebiotics. In: Ramawat KG, Mérillon JM, editors. Polysaccharides: bioactivity and biotechnology. Cham: Springer International Publishing; 2015. pp. 891–925. [Google Scholar]
- 86.Kasubuchi M, Hasegawa S, Hiramatsu T, Ichimura A, Kimura I. Dietary gut microbial metabolites, short-chain fatty acids, and host metabolic regulation. Nutrients. 2015;7:2839–2849. doi: 10.3390/nu7042839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rafter J, Bennett M, Caderni G, Clune Y, Hughes R, Karlson PC, Klinder A, O’Riordan M, O’Sullivan GC, Pool-Zobel B, et al. Dietary synbiotics reduce cancer risk factors in polypectomized and colon cancer patients. Am J Clin Nutr. 2007;85:488–496. doi: 10.1093/ajcn/85.2.488. [DOI] [PubMed] [Google Scholar]
- 88.Waligora-Dupriet AJ, Campeotto F, Nicolis I, Bonet A, Soulaines P, Dupont C, Butel MJ. Effect of oligofructose supplementation on gut microflora and well-being in young children attending a day care centre. Int J Food Microbiol. 2007;113:108–113. doi: 10.1016/j.ijfoodmicro.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 89.Sotnikova N, Antsiferova I, Malyshkina A. Cytokine network of eutopic and ectopic endometrium in women with adenomyosis. Am J Reprod Immunol. 2002;47:251–255. doi: 10.1034/j.1600-0897.2002.01040.x. [DOI] [PubMed] [Google Scholar]
- 90.Cotas J, Leandro A, Pacheco D, Concalves AMM, Pereira L. A Comprehensive review of the nutraceutical and therapeutic applications of red seaweeds (Rhodophyta) Life. 2020;10:19. doi: 10.3390/life10030019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Changhai W, Jie D, Meilin H, Jie J, Shanmei Z. Plant essential oil compound preservative and application thereof in preservation of living Spirulina beverage. C.N. Patent 105454976, 6 April 2016.
- 92.Paulsen S, Klamczynska B, Plasse K, Bowman C. High-protein gelled food products made using high-protein microalgae. U.S. Patent 0021923, 18 February 2016.
- 93.Kyung M. Composition comprising fraction of Tetraselmis suecica for preventing or treating obesity or diabetes. K.R. Patent 102016000797, 6 January 2017.
- 94.Hossain AKMM, Brennan MA, Mason SL, Guo X, Zeng XA, Brennan CS. The effect of astaxanthin-rich microalgae “Haematococcus pluvialis” and wholemeal flours incorporation in improving the physical and functional properties of cookies. Foods. 2017;6:57. doi: 10.3390/foods6080057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rafael JF. Algae-based food formulation, bread-making, bakery and confectionery products containing it, method for obtaining thereof and its use. E.P. Patent 3243520A1, 15 November 2017.
- 96.Moshitzky S, Eisenstadt D, Levi G, Chen O. Transgenic microalgae and use thereof for oral delivery of proteins. U.S. Patent 9827280, 28 November 2017.
- 97.Raposo MFJ, Morais AMMB, Morais RMSC. Polysaccharides from marine microalgae. In: Ramawat KG, Mérillon JM, editors. Polysaccharides: bioactivity and biotechnology. Cham: Springer International Publishing; 2015. pp. 1683–1727. [Google Scholar]
- 98.El-Baz FK, Abdo SM, Hussein AMS. Microalgae Dunaliella salina for use as food supplement to improve pasta quality. Int J Pharm Sci Rev Res. 2017;46:45–51. [Google Scholar]
- 99.Deremaux L, Wils D. Composition of soluble indigestible fibers and of microalgae, used in the well-being field. U.S. Patent 0369681, 12 September 2017.
- 100.Lei X. Compositions comprising defatted microalgae, and treatment methods. U.S. Patent 0119018, 4 May 2017.
- 101.Brooks G, Franklin S, Avila J, Decker SM, Baliu E, Rakitsky W, Piechocki J, Zdanis D, Norris LM. Microalgal food compositions. U.S. Patent 0139994, 24 May 2018.
- 102.Chan K, Chen S, Chen P. Astaxanthin attenuated thrombotic risk factors in type 2 diabetic patients. J Funct Foods. 2019;53:22–27. doi: 10.1016/j.jff.2018.12.012. [DOI] [Google Scholar]
- 103.Golmakani MT, Soleimanian-Zad S, Alavi N, Nazari E, Eskandari MH. Effect of Spirulina (Arthrospira platensis) powder on probiotic bacteriologically acidified feta-type cheese. J Appl Phycol. 2019;31:1085–1094. doi: 10.1007/s10811-018-1611-2. [DOI] [Google Scholar]
- 104.Zaporozhets TS, Besednova NN, Kusnetsova TA, Zvyagintseva TN, Makarenkova ID, Kryzhanovsky SP, Melnikov VG. The prebiotic potential of polysaccharides and extracts of seaweeds. Russ J Mar Bot. 2014;40:1–9. doi: 10.1134/S1063074014010106. [DOI] [Google Scholar]
- 105.Doty MS, Caddy JF, Santelices B (editors) Case studies of seven commercial seaweed resources. In FAO fisheries technical paper-281, Food and Agriculture Organization of the United Nations, FAO: Rome, Italy, 1987. ISBN: 92–5–102540–1.
- 106.Ramsden L. Plant and algal gums and mucilages. In: Tomasik P, editor. Chemical and functional properties of food saccharides. Boca Raton, FL : CRC Press; 2004. pp. 231–254. [Google Scholar]
- 107.Vandeputte D, Falony G, Vieira-Silva S, Wang J, Sailer M, Theis S, et al. Prebiotic inulin-type fructans induce specific changes in the human gut microbiota. Gut. 2017;66(11):1968–1974. doi: 10.1136/gutjnl-2016-313271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu F, Li P, Chen M, Luo Y, Prabhakar M, Zheng H, et al. Fructooligosaccharide (FOS) and galactooligosaccharide (GOS) increase Bifidobacterium but reduce butyrate producing bacteria with adverse glycemic metabolism in healthy young population. Sci Rep. 2017;7(1):11789. doi: 10.1038/s41598-017-10722-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chambers ES, Byrne CS, Morrison DJ, Murphy KG, Preston T, Tedford C, et al. Dietary supplementation with inulin-propionate ester or inulin improves insulin sensitivity in adults with overweight and obesity with distinct effects on the gut microbiota, plasma metabolome and systemic inflammatory responses: a randomised cross-over trial. Gut. 2019;21(2):372–376. doi: 10.1136/gutjnl-2019-318424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vulevic J, Juric A, Walton GE, Claus SP, Tzortzis G, Toward RE, Gibson GR. Influence of galacto-oligosaccharide mixture (B-GOS) on gut microbiota, immune parameters and metabonomics in elderly persons. Br J Nutr. 2015;114(4):586–595. doi: 10.1017/S0007114515001889. [DOI] [PubMed] [Google Scholar]
- 111.Costabile A, Kolida S, Klinder A, Gietl E, Bauerlein M, Frohberg C, et al. A double-blind, placebo-controlled, cross-over study to establish the bifidogenic effect of a very-long-chain inulin extracted from globe artichoke (Cynara scolymus) in healthy human subjects. Br J Nutr. 2010;104(7):1007–1017. doi: 10.1017/S0007114510001571. [DOI] [PubMed] [Google Scholar]
- 112.Lohner S, Jakobik V, Mihalyi K, Soldi S, Vasileiadis S, Theis S, et al. Inulin-type fructan supplementation of 3- to 6-year-old children is associated with higher fecal Bifidobacterium concentrations and fewer febrile episodes requiring medical attention. J Nutr. 2018;148(8):1300–1308. doi: 10.1093/jn/nxy120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hu B, Gong QN, Wang Y, Ma Y, Li J, Yu W. Prebiotic effects of neoagaro-oligosaccharides prepared by enzymatic hydrolysis of agarose. Anaerobe. 2006;12:260–266. doi: 10.1016/j.anaerobe.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 114.Wang Y, Han F, Hu B, Li J, Yu W. In vivo prebiotic properties of alginate oligosaccharides prepared through enzymatic hydrolysis of alginate. Nutr Res. 2006;26:597–603. doi: 10.1016/j.nutres.2006.09.015. [DOI] [Google Scholar]
- 115.Lynch MB, Sweeney T, Callan JJ, O’Sullivan JT, O’Doherty JV. The effects of dietary Laminaria-derived laminarin and fucoidan on nutrient digestibility, nitrogen utilization, intestinal microflora and volatile fatty acid concentration in pigs. J Sci Food Agric. 2010;90:430–437. doi: 10.1002/jsfa.3834. [DOI] [PubMed] [Google Scholar]
- 116.Dierick N, Ovyn A, de Smet S. Effect of feeding intact brown seaweed Ascophyllum nodosum on some digestive parameters and on iodine content in edible tissues in pigs. J Sci Food Agric. 2009;89:584–594. doi: 10.1002/jsfa.3480. [DOI] [Google Scholar]
- 117.Davis LM, Martinez I, Walter J, Goin C, Hutkins RW. Barcoded pyrosequencing reveals that consumption of galactooligosaccharides results in a highly specific bifidogenic response in humans. PLoS ONE. 2011;6(9):e25200. doi: 10.1371/journal.pone.0025200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ramnani P, Chitarrari R, Tuohy K, Grant J, Hotchkiss S, Philp K, Campbell R, Gill C, Rowland I. In vitro fermentation and prebiotic potential of novel low molecular weight polysaccharides derived from agar and alginate seaweeds. Anaerobe. 2012;18:1–16. doi: 10.1016/j.anaerobe.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 119.Holscher HD, Bauer LL, Gourineni V, Pelkman CL, Fahey GC, Jr, Swanson KS. Agave inulin supplementation affects the faecal microbiota of healthy adults participating in a randomized, double-blind, placebo-controlled, crossover trial. J Nutr. 2015;145(9):2025–2032. doi: 10.3945/jn.115.217331. [DOI] [PubMed] [Google Scholar]
- 120.Martinez I, Kim J, Duffy PR, Schlegel VL, Walter J. Resistant starches types 2 and 4 have differential effects on the composition of the fecal microbiota in human subjects. PLoS ONE. 2010;5(11):e15046. doi: 10.1371/journal.pone.0015046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Beheshtipour H, Mortazavian AM, Haratian P, Darani KK. Effects of Chlorella vulgaris and Arthrospira platensis addition on viability of probiotic bacteria in yogurt and its biochemical properties. Eur J Food Res Technol. 2012;235:719–728. doi: 10.1007/s00217-012-1798-4. [DOI] [Google Scholar]
- 122.Nuño K, Villaruel-López A, Puella-Pérez AM, Romero-Velarde E, Puela-Mora AG, Ascencio F. Effects of the marine microalgae Isochrysis galbana and Nannochloropsis oculata in diabetic rats. J Funct Foods. 2013;5:106–115. doi: 10.1016/j.jff.2012.08.011. [DOI] [Google Scholar]
- 123.Kumar A, Deshane JS, Crossman DK, Bolisetty S, Yan BS, Kramnik I, Agarwal A, Steyn AJ. Heme oxygenase-1-derived carbon monoxide induces the Mycobacterium tuberculosis dormancy regulon. J Biol Chem. 2008;283:18032–18039. doi: 10.1074/jbc.M802274200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pal A, Kamthania MC, Kumar A. Bioactive compounds and properties of seaweeds—a review. OALib. 2014;1:1–17. doi: 10.4236/oalib.1100752. [DOI] [Google Scholar]
- 125.Sidari R, Tofalo R. Comprehensive Overview on microalgal-fortified/based food and beverages. Food Rev Int. 2019;35(8):778–805. doi: 10.1080/87559129.2019.1608557. [DOI] [Google Scholar]
- 126.Binns N. Probiotics, prebiotics and the gut microbiota; ILSI Europe Concise Monograph. 2013; 1–32.
- 127.Regulation (EC) N° 258/97 of the European Parliament and of the 27 January 1991 concerning novel foods and novel food ingredients.
- 128.Commission Decision 2009/777/EC of 21 October 2009 concerning the extension of uses of algal oil from the micro-algae Ulkenia sp. as a novel food ingredient under Regulation (EC) N° 258/97 of the European Parliament and of the Council (notified under document C (2009) 7932), Brussels, Belgium
- 129.GRAS Notice N° 000101—GRAS Notification for Spirulina microalgae, Maryland, USA; 2002
- 130.Australia New Zealand Food Standards Code—Schedule 25—Permitted novel foods. Authorised Version F2017C00413 registered 25/05/2017, Canberra (Australia), Wellington (New Zealand). p. 1–3.
- 131.GRAS Notice N° 000396—GRAS exemption claim for Chlorella vulgaris as an ingredient in foods, Maryland, USA; 2011
- 132.Commission Implementing Decision (EU) 2015/545 of 31 March 2015 authorising the placing on the market of oil from the micro-algae Schizochytrium sp. (ATCC PTA-9695) as a novel food ingredient under Regulation (EC) N° 258/97 of the European Parliament and of the Council (notified under document C (2015) 2082), Brussels, Belgium
- 133.Commission Implementing Decision (EU) 2015/546 of 31 March 2015 authorising an extension of use of DHA and EPA-rich oil from the micro-algae Schizochytrium sp. as a novel food ingredient under Regulation (EC) N° 258/97 of the European Parliament and of the Council (notified under document C (2015) 2083), Brussels, Belgium
- 134.Commission Implementing Decision 2014/463/EU: on authorising the placing on the market of oil from the micro-algae Schizochytrium sp. as a novel food ingredient under Regulation (EC) N° 258/97 of the European Parliament and of the Council and repealing Decisions 2003/ 427/EC and 2009/778/EC, Brussels, Belgium.
- 135.JETRO Specifications and Standards for Foods, Food Additives, etc. Under the Food Sanitation Act, Japan External Trade Organization (JETRO): Tokyo, Japan; 2011. p. 1–180.
- 136.GRAS Notice N° 697—GRAS Notice for dried Euglena gracilis (ATCC PTA-123017), Maryland, USA; 2017
- 137.GRAS Notice N° 000137—GRAS Exemption claim for DHA algal oil derived from Schizochytriurm sp. as a source of DHA for use in foods, Maryland, USA; 2003.
- 138.GRAS Notice N° 000160—Expert panel consensus oil under the conditions of intended use in traditional foods, Maryland, USA; 2004
- 139.GRAS Notice N° 000330—GRAS Notice for an Algal flour (Chlorella) ingredient, Maryland, USA; 2010
- 140.GRAS Notice N° 000351—GRAS Assessment for Nikken Sohonsha Corporation Dunaliella bardawil powder, Maryland, USA; 2010
- 141.Matsui SM, Muizzudin N, Arad SM, Marenus K. Sulfated polysaccharides from red microalgae anti-inflammatory properties in vitro and in vivo. Appl Biochem Biotechnol. 2003;104:13–22. doi: 10.1385/ABAB:104:1:13. [DOI] [PubMed] [Google Scholar]
- 142.Jiao L, Jiang P, Zhang L, Wu M. Antitumor and immunomodulating activity of polysaccharides from Enteromorpha intestinalis. Biotechnol Biopro Eng. 2010;15:421–428. doi: 10.1007/s12257-008-0269-z. [DOI] [Google Scholar]
- 143.Rodrigues JAG, Oliveira Vanderlei EDS, Silva LM, De Araujo IW, De Queiroz IN, De Paula GA, Abreu TM, Ribeiro NA, Bezerra MM, Chaves HV, et al. Antinociceptive and anti-inflammatory activities of a sulfated polysaccharide isolated from the green seaweed Caulerpa cupressoides. Pharmacol Rep. 2012;64:282–292. doi: 10.1016/S1734-1140(12)70766-1. [DOI] [PubMed] [Google Scholar]
- 144.Albuquerque IRL, Cordeiro SL, Gomes DL, Dreyfuss JL, Filgueira LGA, Leite EL, Nader HB, Rocha HAO. Evaluation of anti-nociceptive and anti-inflammatory activities of a heterofucan from Dictyota menstrualis. Mar Drugs. 2013;11:2722–2740. doi: 10.3390/md11082722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cumashi A, Ushakova NA, Preobrazhenskaya ME, D’Incecco A, Piccoli A, Totani L, Tinari N, Morozevich GE, Berman AE, Bilan MI, et al. A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology. 2007;5:541–552. doi: 10.1093/glycob/cwm014. [DOI] [PubMed] [Google Scholar]
- 146.Thomes P, Rajendran M, Pasanban B, Rengasamy R. Cardioprotective activity of Cladosiphon okamuranus against isoproterenol induced myocardial infraction in rats. Phytomedicine. 2010;18:52–57. doi: 10.1016/j.phymed.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 147.Khatoon N, Pal R (2015) Microalgae in biotechnological application: a commercial approach. In: Bahadur B et al, editors. Plant biology and biotechnology, vol. II: Plant genomics and biotechnology. USA: Springer. p. 27–47
- 148.Xiong JQ, Kurade MB, Jeon BH. Can microalgae remove pharmaceutical contaminants from water? Trends Biotechnol. 2018;36:30–44. doi: 10.1016/j.tibtech.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 149.Shop California spirulina. https://www.earthrise.com/shop. Accessed 28 Apr 2021
- 150.Nutrex Hawaii. https://www.nutrex-hawaii.com/collections/hawaiian-spirulina. Accessed 28 Apr 2021
- 151.Taiwan Chlorella. https://www.earthrise.com/shop. Accessed 28 Apr 2021.
- 152.Sharma P, Sharma N. Industrial and biotechnological applications of algae: a review. J Adv Plant Biol. 2017;1(1):01–25. doi: 10.14302/issn.2638-4469.japb-17-1534. [DOI] [Google Scholar]
- 153.Andrade LM, Andrade CJ, Dias M, et al. Chlorella and spirulina microalgae as sources of functional foods, nutraceuticals, and food supplements, an overview. MOJ Food Process Technol. 2018;6(1):45–58. doi: 10.15406/mojfpt.2018.06.00144. [DOI] [Google Scholar]
- 154.Bulksupplements. https://www.bulksupplements.com/products/chlorella-blue-green-algae. Accessed 28 Apr 2021
- 155.Febico Far East Bio-Tec. Co., Ltd. https://www.febico.com/en/product/Sorokina-PPAR-tablets.html. Accessed 22 Apr 2021
- 156.Handayania NA, Ariyantib D, Hadiyanto Potential production of polyunsaturated fatty acids from microalgae. 2012;1: 180. Doi:10.4172/scientificreports.180 Algatech https://www.algatech.com/. Accesses 28 Apr 2021
- 157.Nethravathy MU, Mehar JG, Mudliar SN, Shekh AY. Recent advances in microalgal bioactives for food, feed, and healthcare products: Commercial potential, market space, and sustainability. Compr Rev Food Sci Food Saf. 2019 doi: 10.1111/1541-4337.12500. [DOI] [PubMed] [Google Scholar]
- 158.Algavia microalgae food ingredients. http://algavia.com/wp-content/uploads/2015/04/AlgaVia-Discover-Whole-Algal-Flour-White-Paper.pdf. Accessed 22 Apr 2021
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.