Abstract
Background
The E-Cadherin gene (CDH1, Cadherin 1), located at 16q22.1 encodes for a calcium-dependent membranous glycoprotein with an important role in cellular adhesion and polarity maintenance.
Methods
To systematically determine E-Cadherin protein expression in normal and cancerous tissues, 14,637 tumor samples from 112 different tumor types and subtypes as well as 608 samples of 76 different normal tissue types were analyzed by immunohistochemistry in a tissue microarray format.
Results
E-Cadherin was strongly expressed in normal epithelial cells of most organs. From 77 tumor entities derived from cell types normally positive for E-Cadherin, 35 (45.5%) retained at least a weak E-Cadherin immunostaining in ≥99% of cases and 61 (79.2%) in ≥90% of cases. Tumors with the highest rates of E-Cadherin loss included Merkel cell carcinoma, anaplastic thyroid carcinoma, lobular carcinoma of the breast, and sarcomatoid and small cell neuroendocrine carcinomas of the urinary bladder. Reduced E-Cadherin expression was linked to higher grade (p = 0.0009), triple negative receptor status (p = 0.0336), and poor prognosis (p = 0.0466) in invasive breast carcinoma of no special type, triple negative receptor status in lobular carcinoma of the breast (p = 0.0454), advanced pT stage (p = 0.0047) and lymph node metastasis in colorectal cancer (p < 0.0001), and was more common in recurrent than in primary prostate cancer (p < 0.0001). Of 29 tumor entities derived from E-Cadherin negative normal tissues, a weak to strong E-Cadherin staining could be detected in at least 10% of cases in 15 different tumor entities (51.7%). Tumors with the highest frequency of E-Cadherin upregulation included various subtypes of testicular germ cell tumors and renal cell carcinomas (RCC). E-Cadherin upregulation was more commonly seen in malignant than in benign soft tissue tumors (p = 0.0104) and was associated with advanced tumor stage (p = 0.0276) and higher grade (p = 0.0035) in clear cell RCC, and linked to advanced tumor stage (p = 0.0424) and poor prognosis in papillary RCC (p ≤ 0.05).
Conclusion
E-Cadherin is consistently expressed in various epithelial cancers. Down-regulation or loss of E-Cadherin expression in cancers arising from E-Cadherin positive tissues as well as E-Cadherin neo-expression in cancers arising from E-Cadherin negative tissues is linked to cancer progression and may reflect tumor dedifferentiation.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40364-021-00299-4.
Keywords: E-cadherin, Neoplastic tissue, Renal cell cancer, Lobular breast cancer, TMA, Immunohistochemistry
Background
The E-Cadherin gene (CDH1, Cadherin 1), located at 16q22.1 encodes for a calcium-dependent membrane glycoprotein with an important role in cellular adhesion and polarity maintenance. It consists of 5 cadherin repeats in the extracellular domain, one transmembrane domain, and an intracellular domain that binds p120-catenin (p120-ctn) and beta-catenin. The intracellular domain contains a highly-phosphorylated beta-catenin binding site which is essential for E-Cadherin function. In epithelial cells, E-Cadherin-containing intracellular junctions are often adjacent to actin-containing filaments of the cytoskeleton [1–5]. The pivotal role of E-Cadherin is highlighted by its expression starting at the 2-cell stage of mammalian embryonic development [5, 6]. In adult tissues, E-Cadherin – also called epithelial cadherin - is expressed in virtually all epithelial tissues, where it is constantly regenerated with a 5 to 10 h half-life on the cell surface [4, 5]. Loss of E-Cadherin function or expression plays a relevant role in cancer progression [7]. E-Cadherin downregulation diminishes cellular adhesion in epithelial tissues. As a result, increased cell motility may facilitate invasive growth and metastasis [7]. Heterozygous germline alterations of the CDH1 gene are associated with hereditary diffuse gastric cancer syndrome and invasive lobular carcinoma of the breast [8, 9].
More than 1000 studies have analyzed the role of E-Cadherin expression in cancer using immunhistochemistry. The data show that E-Cadherin expression occurs in a wide range of human tumors and that not only reduced [10–16] but also elevated E-Cadherin protein levels [17–19] can be associated with unfavorable tumor parameters. However, the accumulated data on the prevalence of E-Cadherin expression is controversial in the literature. For example, E-Cadherin positivity has been described in 25 to 100% of invasive breast carcinoma of no special type [20–22], 32 to 100% of oral squamous cell carcinomas [23–25], 31 to 100% of intestinal gastric carcinomas [26–28], 26 to 100% of colorectal adenocarcinomas [29–31], and 5 to 54% of clear cell renal cell carcinomas [17, 32, 33].
These conflicting data make it virtually impossible to compare different tumor types with respect to their E-Cadherin expression levels. Because highly variable results have been reported even from the same histological tumor subtype in different studies using different experimental conditions and scoring criteria, it appears likely that many of the controversial data in the literature are due likely due to the use of different antibodies, immunostaining protocols, and criteria to categorize E-Cadherin in these studies.
Knowledge on the relative expression levels of E-Cadherin in different tumor types would substantially add to the understanding of the role of this protein in these cancers. In addition, data on rare tumor types or subtypes are lacking. To better understand the prevalence and significance of E-Cadherin expression in across human cancers, a comprehensive study analyzing a large number of neoplastic and non-neoplastic tissues under highly standardized conditions is needed. Therefore, E-Cadherin expression was analyzed in more than 14,000 tumor tissue samples from 112 different tumor types and subtypes as well as 76 non-neoplastic tissue categories by immunohistochemistry (IHC) in a tissue microarray (TMA) format in this study.
Methods
Aim, design and setting of the study
This study aimed at the comprehensive analysis of E-Cadherin expression across all human types of normal tissue as well as more than 100 different human tumor types and subtypes. The tissue microarray format was employed to allow for a highly standardized analysis using immunohistochemistry with identical experimental conditions and identical amount of analyzed tissue for all > 14,000 tissue samples included in the study. The result is a ranking list of human tumor types according to the level of E-Cadherin expression.
Tissue microarrays (TMAs)
Our normal tissue TMA was composed of 8 samples from 8 different donors for each of 76 different normal tissue types (608 samples on one slide). The cancer TMAs contained a total of 14,637 primary tumors from 112 tumor types and subtypes. Detailed histopathological data on grade, tumor stage (pT), and lymph node status (pN) status were available from 4478 cancers (breast, colorectal, and kidney carcinoma). Clinical follow up data were available from 1183 breast cancer and 1174 renal cell cancer (RCC) patients with a median follow-up time of 49 and 48 months, respectively (range 1–88/1–250). The composition of both normal and cancer TMAs is described in detail in the results section. All samples come from the archives of the Institute of Pathology, University Hospital of Hamburg, Germany, the Institute of Pathology, Clinical Center Osnabrueck, Germany, and Department of Pathology, Academic Hospital Fuerth, Germany. Tissues were fixed in 4% buffered formalin and then embedded in paraffin. TMA tissue spot diameter was 0.6 mm.
Immunohistochemistry (IHC)
Freshly cut TMA sections were all immunostained on the same day and in a single run. Slides were deparaffinized with xylol, rehydrated through a graded alcohol series and exposed to heat-induced antigen retrieval for 5 min in an autoclave at 121 °C in pH 9 DakoTarget Retrieval Solution (Agilent; #S2367). Endogenous peroxidase activity was blocked with Dako peroxidase blocking solution (Agilent; #52023) for 10 min. Primary antibody specific for E-Cadherin (mouse monoclonal, MSVA-035, MS Validated Antibodies, Hamburg, Germany) was applied at 37 °C for 60 min at a dilution of 1:300. Bound antibody was then visualized using the EnVision Kit (Agilent, CA, USA; #K5007) according to the manufacturer’s directions. The sections were counterstained with haemalaun.
One trained pathologist evaluated all stainings. Normal tissue spots were scored as negative (no detectable staining) or positive (detectable staining of any intensity). For tumor tissue spots, the staining was scored semiquantitatively. Four staining categories were identified based on the staining intensity (0, 1+, 2+, 3+) of the tumor cells and the fraction of stained tumor cells in each tissue spot. These categories included “negative” (no detectable staining), “weak” (1+ staining intensity in ≤70% of tumor cells or 2+ intensity in ≤30% of tumor cells), “moderate” (1+ staining intensity in > 70% of tumor cells, or 2+ intensity in 31–70% of tumor cells, or 3+ intensity in ≤30% of tumor cells), and “strong” (2+ intensity in > 70% of tumor cells or 3+ intensity in > 30% of of tumor cells).
Statistics
Statistical calculations were performed with JMP 14 software (SAS Institute Inc., NC, USA). Contingency tables and the chi2-test were performed to search for associations between E-Cadherin and tumor phenotype. Survival curves were calculated according to Kaplan-Meier. The Log-Rank test was applied to detect significant differences between groups. A p value of ≤0.05 was considered as statistically significant.
Results
Technical issues
A total of 10,851 (74.1%) of 14,637 tumor samples were interpretable in our TMA analysis. The remaining 3786 (25.9%) samples were not interpretable due to the lack of unequivocal tumor cells or loss of the tissue spot during the technical procedures. On the normal tissue TMA, a sufficient number of samples were always analyzable for each tissue type to determine its E-cadherin expression status.
E-Cadherin in normal tissue
A moderate to strong (2+/3+) membranous E-Cadherin staining was found in most epithelial cells of various organs (skin, lip, oral cavity, tonsils, salivary glands, esophagus, stomach, duodenum, ileum, appendix, colon, rectum, anal canal, gall bladder, liver, pancreas, ectocervix, endocervix, endometrium, fallopian tube, breast, thyroid gland, kidney pelvis, urinary bladder, prostate gland, seminal vesicle, epididymis, respiratory epithelium of bronchus and sinus paranasales, and lung (Fig. 1a). A distinct distribution of E-Cadherin expression were seen in the kidney, where only distal tubuli showed an E-Cadherin expression (Fig. 1b) and in the placenta, where only the cytotrophoblastic layer showed a positive staining (Fig. 1c). The anterior and posterior lobe of the pituitary gland showed a moderate to strong positive staining (Fig. 1d). Lymphatic tissue sometimes showed weak staining and some small vessel-like structures were also positive. In the thymus, positive staining was seen in Hassall’s corpuscles. E-Cadherin immunostaining was absent in endothelium and media of the aorta, the heart, striated muscle, tongue muscle, myometrium of the uterus, muscularis of the gastrointestinal tract, kidney pelvis and urinary bladder, corpus spongiosum of the penis, testis, ovarian stroma, corpus luteum of the ovary, adrenal gland, fat, cerebellum and cerebrum.
Fig. 1.
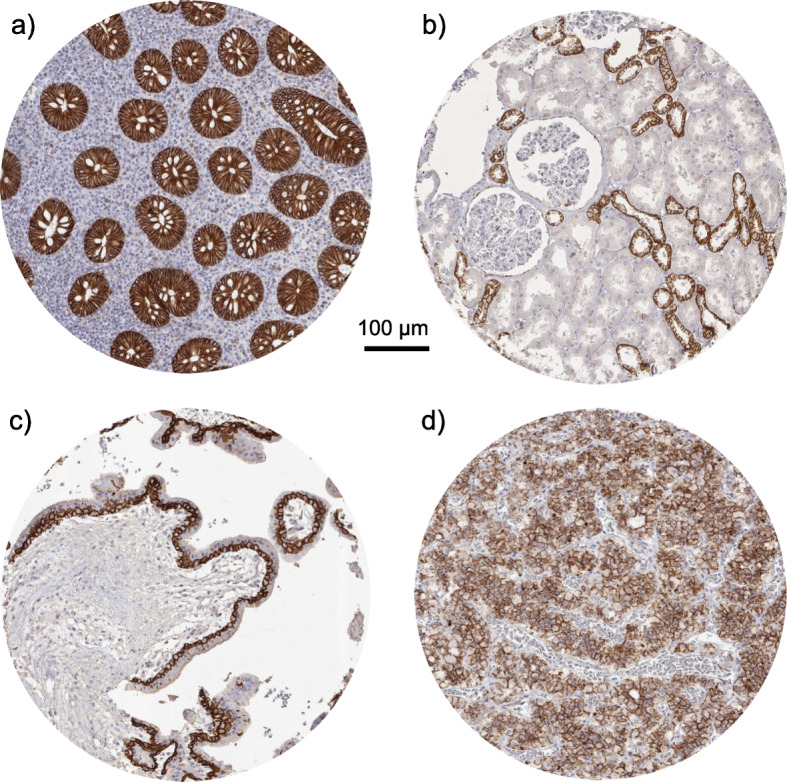
E-Cadherin expression in normal tissues. Moderate to strong E-Cadherin immunostaining is seen in epithelial cell of the appendix (a), in distal tubuli of the kidney (b), in the cytotrophoblastic layer of the placenta (c), in the pituitary gland (d)
E-Cadherin in neoplastic tissues
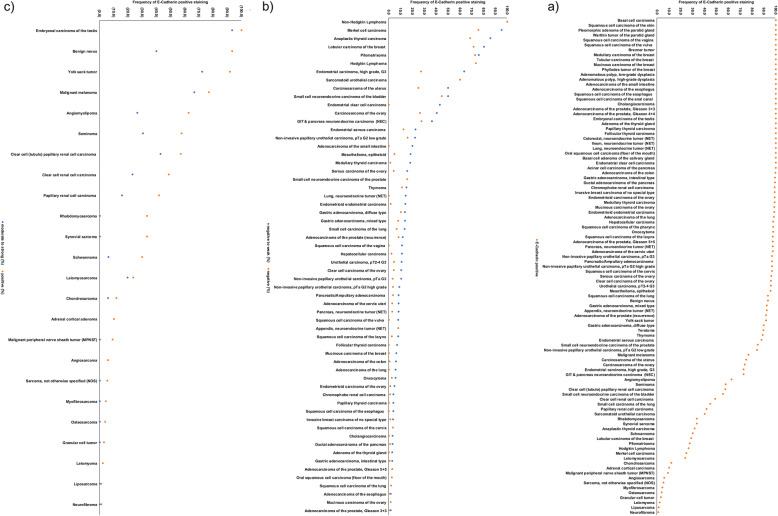
Membranous E-Cadherin immunostaining was observed in 8819 (81.3%) of 10,851 interpretable tumors, including 7013 (64.6%) with strong, 927 (8.5%) with moderate, and 879 (8.1%) with weak staining. Overall, at least focal weak E-Cadherin immunostaining could be detected in 101 (90.2%) of 112 different tumor types and tumor subtypes (Table 1, Fig. 2a). In tumor entities derived from normally E-Cadherin positive cells types (Fig. 2b), at least a weak E-Cadherin positivity was preserved in 35 (45.5%) of 77 tumor entities in ≥99% of all interpretable examples and in 61 (79.2%) tumor entities in ≥90% of all analyzable cases. Tumors with the highest rates of E-Cadherin loss included Merkel cell carcinoma (75.6% negative), anaplastic thyroid carcinoma (68.6% negative), lobular carcinoma of the breast (71.5% negative), and sarcomatoid (60.0% negative) and small cell neuroendocrine carcinoma (44.4% negative) of the urinary bladder. In tumor entities derived from cell types normally negative for E-Cadherin (n = 29; Fig. 2c), a weak to strong E-Cadherin positivity could be detected in at least 10% of cases in 15 different tumor entities (51.7%). Tumors with the highest frequency of E-Cadherin upregulation included various subtypes of testicular germ cell tumors (positive 57 to 100%), melanocytic tumors (40 to 67%), and RCC (positive 42 to 57%). Representative images of E-Cadherin immunostaining in tumors are given in Fig. 3.
Table 1.
E-Cadherin immunostaining in human tumors
| Tumor type | n on TMA | E-Cadherin expression | ||||||
|---|---|---|---|---|---|---|---|---|
| analyzable (n) | negative (%) | weak (%) | moderate (%) | strong (%) | positive (%) | |||
| Tumors of the skin | Pilomatrixoma | 35 | 33 | 72.7 | 3.0 | 15.2 | 9.1 | 27.3 |
| Basal cell carcinoma | 48 | 33 | 0.0 | 0.0 | 3.0 | 97.0 | 100.0 | |
| Benign nevus | 29 | 15 | 6.7 | 53.3 | 33.3 | 6.7 | 93.3 | |
| Squamous cell carcinoma of the skin | 50 | 29 | 0.0 | 0.0 | 6.9 | 93.1 | 100.0 | |
| Malignant melanoma | 48 | 39 | 23.1 | 10.3 | 5.1 | 61.5 | 76.9 | |
| Merkel cell carcinoma | 46 | 41 | 75.6 | 19.5 | 2.4 | 2.4 | 24.4 | |
| Tumors of the head and neck | Squamous cell carcinoma of the larynx | 50 | 39 | 2.6 | 5.1 | 0.0 | 92.3 | 97.4 |
| Squamous cell carcinoma of the pharynx | 50 | 43 | 2.3 | 0.0 | 9.3 | 88.4 | 97.7 | |
| Oral squamous cell carcinoma (floor of the mouth) | 50 | 36 | 0.0 | 2.8 | 5.6 | 91.7 | 100.0 | |
| Pleomorphic adenoma of the parotid gland | 49 | 45 | 0.0 | 0.0 | 0.0 | 100.0 | 100.0 | |
| Warthin tumor of the parotid gland | 15 | 14 | 0.0 | 0.0 | 7.1 | 92.9 | 100.0 | |
| Tumors of the lung, pleura and thymus | Basal cell adenoma of the salivary gland | 250 | 123 | 0.0 | 5.7 | 3.3 | 91.1 | 100.0 |
| Adenocarcinoma of the lung | 127 | 54 | 1.9 | 0.0 | 9.3 | 88.9 | 98.1 | |
| Squamous cell carcinoma of the lung | 20 | 15 | 6.7 | 6.7 | 0.0 | 86.7 | 93.3 | |
| Small cell carcinoma of the lung | 76 | 56 | 55.4 | 3.6 | 16.1 | 25.0 | 44.6 | |
| Mesothelioma, epitheloid | 39 | 22 | 4.5 | 13.6 | 36.4 | 45.5 | 95.5 | |
| Thymoma | 29 | 27 | 11.1 | 3.7 | 14.8 | 70.4 | 88.9 | |
| Tumors of the female genital tract | Squamous cell carcinoma of the vagina | 48 | 18 | 0.0 | 11.1 | 5.6 | 83.3 | 100.0 |
| Squamous cell carcinoma of the vulva | 50 | 26 | 0.0 | 7.7 | 11.5 | 80.8 | 100.0 | |
| Squamous cell carcinoma of the cervix | 50 | 29 | 3.4 | 0.0 | 3.4 | 93.1 | 96.6 | |
| Adenocarcinoma of the cervix uteri | 50 | 37 | 2.7 | 5.4 | 5.4 | 86.5 | 97.3 | |
| Endometrioid endometrial carcinoma | 236 | 200 | 1.5 | 12.5 | 15.5 | 70.5 | 98.5 | |
| Endometrial serous carcinoma | 82 | 49 | 12.2 | 10.2 | 16.3 | 61.2 | 87.8 | |
| Carcinosarcoma of the uterus | 48 | 32 | 25.0 | 25.0 | 15.6 | 34.4 | 75.0 | |
| Endometrial clear cell carcinoma | 8 | 7 | 0.0 | 42.9 | 28.6 | 28.6 | 100.0 | |
| Endometrial carcinoma, high grade, G3 | 13 | 11 | 27.3 | 36.4 | 0.0 | 36.4 | 72.7 | |
| Endometrial stromal sarcoma | 12 | 11 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Endometrioid carcinoma of the ovary | 115 | 84 | 1.2 | 3.6 | 6.0 | 89.3 | 98.8 | |
| Serous carcinoma of the ovary | 567 | 480 | 4.0 | 11.5 | 21.3 | 63.3 | 96.0 | |
| Mucinous carcinoma of the ovary | 97 | 69 | 1.4 | 0.0 | 4.3 | 94.2 | 98.6 | |
| Clear cell carcinoma of the ovary | 54 | 48 | 4.2 | 6.3 | 10.4 | 79.2 | 95.8 | |
| Carcinosarcoma of the ovary | 47 | 42 | 26.2 | 14.3 | 11.9 | 47.6 | 73.8 | |
| Brenner tumor | 9 | 6 | 0.0 | 0.0 | 0.0 | 100.0 | 100.0 | |
| Tumors of the breast | Invasive breast carcinoma of no special type | 1391 | 820 | 1.1 | 2.4 | 3.7 | 92.8 | 98.9 |
| Lobular carcinoma of the breast | 294 | 158 | 71.5 | 8.9 | 6.3 | 13.3 | 28.5 | |
| Medullary carcinoma of the breast | 26 | 8 | 0.0 | 0.0 | 25.0 | 75.0 | 100.0 | |
| Tubular carcinoma of the breast | 27 | 6 | 0.0 | 0.0 | 0.0 | 100.0 | 100.0 | |
| Mucinous carcinoma of the breast | 58 | 16 | 0.0 | 6.3 | 6.3 | 87.5 | 100.0 | |
| Phyllodes tumor of the breast | 50 | 29 | 0.0 | 0.0 | 0.0 | 100.0 | 100.0 | |
| Tumors of the digestive system | Adenomatous polyp, low-grade dysplasia | 50 | 39 | 0.0 | 0.0 | 0.0 | 100.0 | 100.0 |
| Adenomatous polyp, high-grade dysplasia | 50 | 37 | 0.0 | 0.0 | 0.0 | 100.0 | 100.0 | |
| Adenocarcinoma of the colon | 1882 | 1644 | 0.5 | 5.5 | 9.7 | 84.3 | 99.5 | |
| Adenocarcinoma of the small intestine | 10 | 5 | 0.0 | 20.0 | 0.0 | 80.0 | 100.0 | |
| Gastric adenocarcinoma, diffuse type | 176 | 147 | 10.2 | 3.4 | 2.0 | 84.4 | 89.8 | |
| Gastric adenocarcinoma, intestinal type | 174 | 144 | 0.7 | 2.1 | 3.5 | 93.8 | 99.3 | |
| Gastric adenocarcinoma, mixed type | 62 | 52 | 7.7 | 5.8 | 1.9 | 84.6 | 92.3 | |
| Adenocarcinoma of the esophagus | 133 | 62 | 0.0 | 1.6 | 1.6 | 96.8 | 100.0 | |
| Squamous cell carcinoma of the esophagus | 124 | 55 | 0.0 | 3.6 | 0.0 | 96.4 | 100.0 | |
| Squamous cell carcinoma of the anal canal | 50 | 30 | 0.0 | 0.0 | 3.3 | 96.7 | 100.0 | |
| Cholangiocarcinoma | 114 | 93 | 0.0 | 3.2 | 7.5 | 89.2 | 100.0 | |
| Hepatocellular carcinoma | 50 | 45 | 2.2 | 8.9 | 11.1 | 77.8 | 97.8 | |
| Ductal adenocarcinoma of the pancreas | 612 | 437 | 0.9 | 2.3 | 9.6 | 87.2 | 99.1 | |
| Pancreatic/Ampullary adenocarcinoma | 89 | 61 | 3.3 | 4.9 | 3.3 | 88.5 | 96.7 | |
| Acinar cell carcinoma of the pancreas | 13 | 12 | 0.0 | 0.0 | 8.3 | 91.7 | 100.0 | |
| Gastrointestinal stromal tumor (GIST) | 50 | 40 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Tumors of the urinary system | Non-invasive papillary urothelial carcinoma, pTa G2 low grade | 177 | 95 | 15.8 | 6.3 | 11.6 | 66.3 | 84.2 |
| Non-invasive papillary urothelial carcinoma, pTa G2 high grade | 141 | 91 | 3.3 | 5.5 | 6.6 | 84.6 | 96.7 | |
| Non-invasive papillary urothelial carcinoma, pTa G3 | 187 | 125 | 3.2 | 7.2 | 8.8 | 80.8 | 96.8 | |
| Urothelial carcinoma, pT2–4 G3 | 940 | 755 | 4.4 | 6.4 | 8.5 | 80.8 | 95.6 | |
| Small cell neuroendocrine carcinoma of the bladder | 18 | 18 | 44.4 | 5.6 | 11.1 | 38.9 | 55.6 | |
| Sarcomatoid urothelial carcinoma | 25 | 15 | 60.0 | 0.0 | 6.7 | 33.3 | 40.0 | |
| Clear cell renal cell carcinoma | 858 | 614 | 51.3 | 25.7 | 13.8 | 9.1 | 48.7 | |
| Papillary renal cell carcinoma | 255 | 180 | 58.3 | 26.1 | 6.1 | 9.4 | 41.7 | |
| Chromophobe renal cell carcinoma | 131 | 103 | 1.0 | 2.9 | 3.9 | 92.2 | 99.0 | |
| Oncocytoma | 177 | 127 | 2.4 | 3.1 | 12.6 | 81.9 | 97.6 | |
| Clear cell (tubulo) papillary renal cell carcinoma | 21 | 14 | 42.9 | 14.3 | 14.3 | 28.6 | 57.1 | |
| Tumors of the male genital organs | Adenocarcinoma of the prostate, Gleason 3 + 3 | 83 | 73 | 0.0 | 1.4 | 1.4 | 97.3 | 100.0 |
| Adenocarcinoma of the prostate, Gleason 4 + 4 | 80 | 70 | 0.0 | 0.0 | 0.0 | 100.0 | 100.0 | |
| Adenocarcinoma of the prostate, Gleason 5 + 5 | 85 | 76 | 2.6 | 0.0 | 0.0 | 97.4 | 97.4 | |
| Adenocarcinoma of the prostate (recurrence) | 330 | 217 | 8.3 | 3.2 | 8.3 | 80.2 | 91.7 | |
| Small cell neuroendocrine carcinoma of the prostate | 17 | 13 | 15.4 | 0.0 | 0.0 | 84.6 | 84.6 | |
| Seminoma | 624 | 522 | 42.3 | 27.2 | 14.6 | 15.9 | 57.7 | |
| Embryonal carcinoma of the testis | 50 | 44 | 0.0 | 6.8 | 18.2 | 75.0 | 100.0 | |
| Yolk sack tumor | 50 | 36 | 8.3 | 19.4 | 5.6 | 66.7 | 91.7 | |
| Teratoma | 50 | 19 | 10.5 | 5.3 | 0.0 | 84.2 | 89.5 | |
| Tumors of endocrine organs | Adenoma of the thyroid gland | 114 | 104 | 0.0 | 2.9 | 9.6 | 87.5 | 100.0 |
| Papillary thyroid carcinoma | 392 | 354 | 0.0 | 3.7 | 7.6 | 88.7 | 100.0 | |
| Follicular thyroid carcinoma | 158 | 147 | 0.0 | 6.8 | 10.9 | 82.3 | 100.0 | |
| Medullary thyroid carcinoma | 107 | 84 | 1.2 | 16.7 | 39.3 | 42.9 | 98.8 | |
| Anaplastic thyroid carcinoma | 45 | 35 | 68.6 | 17.1 | 2.9 | 11.4 | 31.4 | |
| Adrenal cortical adenoma | 50 | 43 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Adrenal cortical carcinoma | 26 | 20 | 90.0 | 0.0 | 0.0 | 10.0 | 10.0 | |
| Phaeochromocytoma | 50 | 42 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Appendix, neuroendocrine tumor (NET) | 22 | 13 | 7.7 | 0.0 | 7.7 | 84.6 | 92.3 | |
| Colorectal, neuroendocrine tumor (NET) | 10 | 10 | 0.0 | 0.0 | 0.0 | 100.0 | 100.0 | |
| Ileum, neuroendocrine tumor (NET) | 49 | 43 | 0.0 | 0.0 | 0.0 | 100.0 | 100.0 | |
| Lung, neuroendocrine tumor (NET) | 19 | 14 | 0.0 | 14.3 | 7.1 | 78.6 | 100.0 | |
| Pancreas, neuroendocrine tumor (NET) | 102 | 75 | 2.7 | 5.3 | 4.0 | 88.0 | 97.3 | |
| GIT & pancreas neuroendocrine carcinoma (NEC) | 28 | 11 | 27.3 | 9.1 | 9.1 | 54.5 | 72.7 | |
| Tumors of haemotopoetic and lymphoid tissues | Hodgkin Lymphoma | 45 | 38 | 73.7 | 0.0 | 5.3 | 21.1 | 26.3 |
| Non-Hodgkin Lymphoma | 48 | 39 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Tumors of soft tissue and bone | Tenosynovial giant cell tumor | 45 | 37 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Granular cell tumor | 53 | 34 | 97.1 | 2.9 | 0.0 | 0.0 | 2.9 | |
| Leiomyoma | 50 | 45 | 97.8 | 0.0 | 0.0 | 2.2 | 2.2 | |
| Angiomyolipoma | 91 | 64 | 37.5 | 35.9 | 9.4 | 17.2 | 62.5 | |
| Angiosarcoma | 73 | 51 | 94.1 | 0.0 | 0.0 | 5.9 | 5.9 | |
| Dermatofibrosarcoma protuberans | 21 | 12 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Ganglioneuroma | 14 | 9 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Kaposi sarcoma | 8 | 5 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Leiomyosarcoma | 87 | 72 | 76.4 | 4.2 | 9.7 | 9.7 | 23.6 | |
| Liposarcoma | 132 | 89 | 98.9 | 1.1 | 0.0 | 0.0 | 1.1 | |
| Malignant peripheral nerve sheath tumor (MPNST) | 13 | 11 | 90.9 | 9.1 | 0.0 | 0.0 | 9.1 | |
| Myofibrosarcoma | 26 | 23 | 95.7 | 4.3 | 0.0 | 0.0 | 4.3 | |
| Neurofibroma | 117 | 99 | 99.0 | 1.0 | 0.0 | 0.0 | 1.0 | |
| Sarcoma, not otherwise specified (NOS) | 75 | 58 | 94.8 | 5.2 | 0.0 | 0.0 | 5.2 | |
| Paraganglioma | 41 | 38 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Primitive neuroectodermal tumor (PNET) | 23 | 13 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Rhabdomyosarcoma | 7 | 6 | 66.7 | 33.3 | 0.0 | 0.0 | 33.3 | |
| Schwannoma | 121 | 100 | 70.0 | 24.0 | 4.0 | 2.0 | 30.0 | |
| Synovial sarcoma | 12 | 9 | 66.7 | 33.3 | 0.0 | 0.0 | 33.3 | |
| Osteosarcoma | 43 | 27 | 96.3 | 3.7 | 0.0 | 0.0 | 3.7 | |
| Chondrosarcoma | 39 | 17 | 88.2 | 5.9 | 5.9 | 0.0 | 11.8 | |
Fig. 2.
Ranking order of E-Cadherin immunostaining in tumors. a Tumor types and subtypes with positive (orange dots) staining (n = 101), b tumor types and subtypes from E-Cadherin positive normal cells with negative (orange dots) and negative to weak (blue dots) immunostaining (n = 60; not shown: 17 tumor types without E-Cadherin loss), and c tumor types and subtypes from E-Cadherin negative normal cells with positive (orange dots) and moderate to strong (blue dots) immunostaining (n = 24, not shown: 5 tumor types without E-Cadherin staining)
Fig. 3.
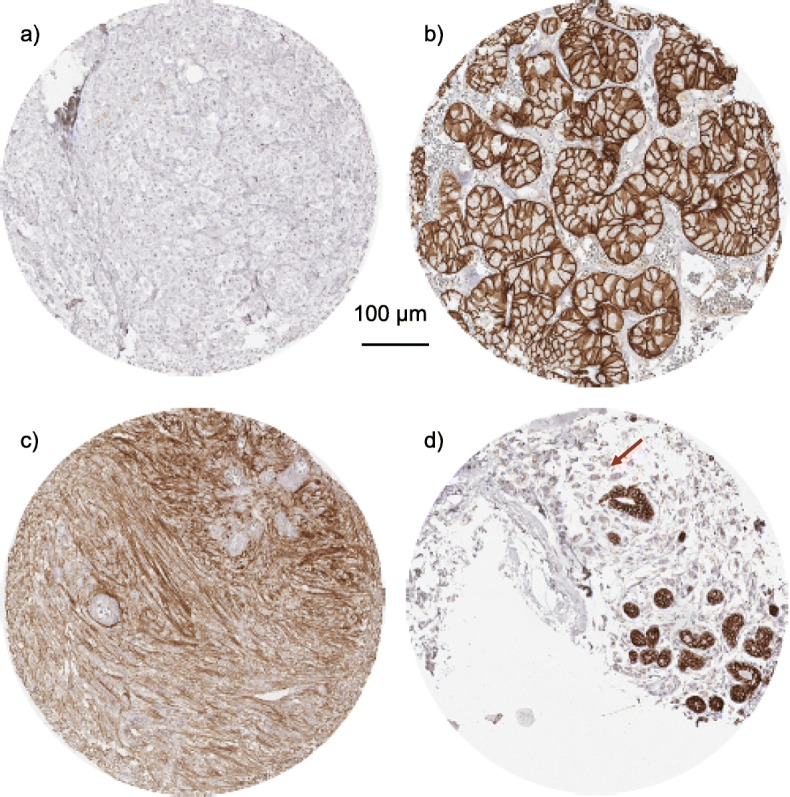
E-Cadherin expression in cancer tissue. a Absent E-Cadherin immunostaining in a castration resistent prostate cancer with discoherent growth pattern, b strong membranous E-Cadherin staining in a chromophobe renal cell carcinoma, c moderate intensity E-Cadherin staining in a Schwannoma, and d absence of E-Cadherin staining in the cells of an invasive lobular carcinoma (red arrow) in the vicinity of positively stained normal breast glands
E-Cadherin expression, tumor phenotype, and prognosis
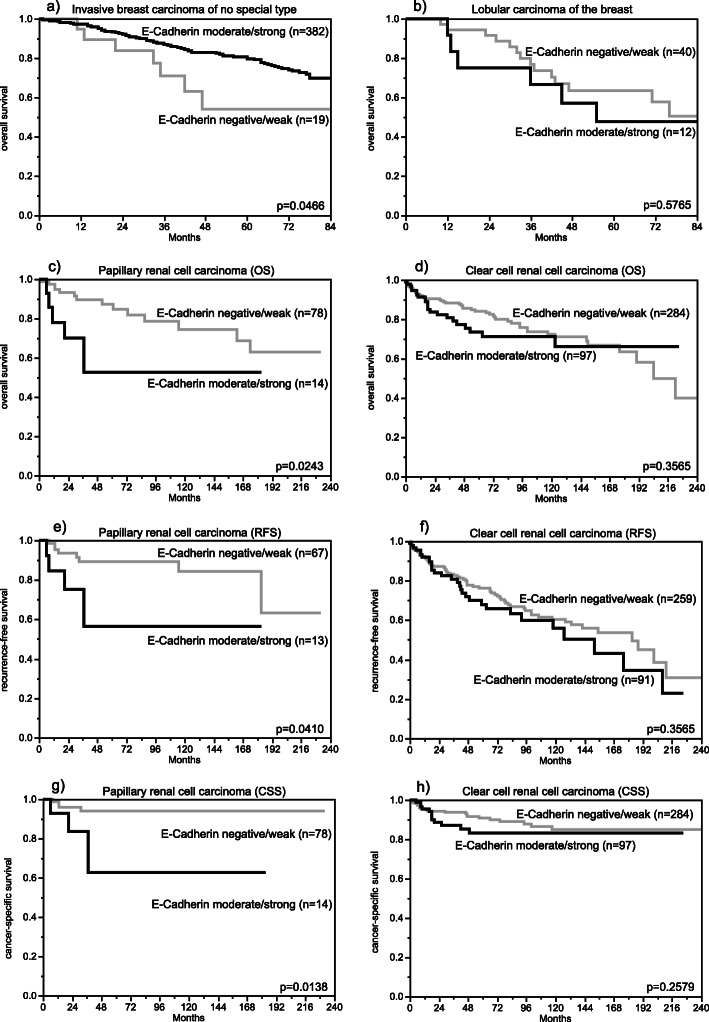
The relationship between E-Cadherin expression and clinico-pathological parameters or prognosis could be analyzed in three cancer types (breast, colorectal, and prostate cancer) derived from normally E-Cadherin positive cells and in two cancer types (papillary and clear cell RCC) derived from normally E-Cadherin negative cells. Reduced E-Cadherin expression was associated with high grade (p = 0.0009), triple negative receptor status (p = 0.0336), and reduced overall survival (p = 0.0466) in invasive breast carcinoma of no special type, triple negative receptor status (p = 0.0454) – but not with patient outcome - in lobular breast cancer, and with advanced pT stage (p = 0.0047) and nodal metastasis in colorectal cancer (p < 0.0001; Table 2, Fig. 4). In prostate cancer, E-Cadherin downregulation was more common in recurrent than in primary cancer. Negative to weak immunostaining was observed in 25 (11.5%) of 217 prostate cancer recurrences, and only in 3 (1.4%) of 219 primary prostate cancers (p < 0.0001). Increased E-Cadherin expression was related to advanced tumor stage (p = 0.0424), reduced overall survival (p = 0.0243), higher risk of recurrence (p = 0.0410) and cancer specific survival (p = 0.0138) in papillary RCC, and to advanced tumor stage (p = 0.0276) and high Thoenes grade (p = 0.0035) – but not patient prognosis – in clear cell RCC (Table 2, Fig. 4). E-Cadherin upregulation was also more commonly seen in malignant (43/331; 13.0%) than in benign (23/327; 7.0%) soft tissue tumors (p = 0.0104, Supplementary Figure 1).
Table 2.
E-Cadherin immunostaining and tumor phenotype
| E-Cadherin immunostaining | |||||||
|---|---|---|---|---|---|---|---|
| n | negative (%) | weak (%) | moderate (%) | strong (%) | P | ||
| Invasive breast carcinoma of no special type | all cancers | 757 | 1.1 | 2.6 | 3.6 | 92.7 | |
| pT1 | 392 | 1.3 | 2.6 | 2.8 | 93.4 | 0.6066 | |
| pT2 | 276 | 0.7 | 2.9 | 3.6 | 92.8 | ||
| pT3–4 | 60 | 1.7 | 3.3 | 8.3 | 86.7 | ||
| Grade 1 | 111 | 0.0 | 0.0 | 0.0 | 100.0 | 0.0009 | |
| Grade 2 | 393 | 1.8 | 3.6 | 3.6 | 91.1 | ||
| Grade 3 | 252 | 0.4 | 2.4 | 5.2 | 92.1 | ||
| pN0 | 360 | 1.1 | 1.9 | 3.3 | 93.6 | 0.5155 | |
| pN ≥ 1 | 247 | 0.8 | 3.6 | 4.5 | 91.1 | ||
| pM0 | 174 | 1.1 | 2.3 | 2.3 | 94.3 | 0.1374 | |
| pM1 | 80 | 0.0 | 3.8 | 7.5 | 88.8 | ||
| HER2 negative | 563 | 1.1 | 2.5 | 4.4 | 92.0 | 0.2221 | |
| HER2 positive | 77 | 0.0 | 1.3 | 1.3 | 97.4 | ||
| ER negative | 133 | 1.5 | 3.8 | 6.8 | 88.0 | 0.1269 | |
| ER positve | 475 | 0.8 | 1.7 | 3.2 | 94.3 | ||
| PR negative | 250 | 1.2 | 2.0 | 5.2 | 91.6 | 0.7046 | |
| PR positive | 388 | 1.0 | 2.3 | 3.4 | 93.3 | ||
| not Triple negative | 499 | 0.8 | 1.6 | 3.2 | 94.4 | 0.0336 | |
| Triple negative | 93 | 2.2 | 4.3 | 8.6 | 84.9 | ||
| Lobular carcinoma of the breast | all cancers | 93 | 65.6 | 9.7 | 9.7 | 15.1 | |
| pT1 | 40 | 72.5 | 10.0 | 5.0 | 12.5 | 0.7552 | |
| pT2 | 35 | 62.9 | 8.6 | 11.4 | 17.1 | ||
| pT3–4 | 16 | 56.3 | 6.3 | 18.8 | 18.8 | ||
| Grade 1 | 4 | 50.0 | 25.0 | 0.0 | 25.0 | 0.3431 | |
| Grade 2 | 76 | 67.1 | 10.5 | 10.5 | 11.8 | ||
| Grade 3 | 13 | 61.5 | 0.0 | 7.7 | 30.8 | ||
| pN0 | 54 | 64.8 | 9.3 | 11.1 | 14.8 | 0.5989 | |
| pN ≥ 1 | 11 | 70.0 | 3.3 | 6.7 | 20.0 | ||
| pM0 | 21 | 71.4 | 0.0 | 9.5 | 19.0 | 0.9970 | |
| pM1 | 11 | 72.7 | 0.0 | 9.1 | 18.2 | ||
| HER2 negative | 65 | 69.2 | 7.7 | 12.3 | 10.8 | 0.8664 | |
| HER2 positive | 1 | 100.0 | 0.0 | 0.0 | 0.0 | ||
| ER negative | 8 | 62.5 | 0.0 | 37.5 | 0.0 | 0.0909 | |
| ER positve | 47 | 70.2 | 8.5 | 8.5 | 12.8 | ||
| PR negative | 30 | 73.3 | 0.0 | 16.7 | 10.0 | 0.0672 | |
| PR positive | 34 | 67.6 | 11.8 | 5.9 | 14.7 | ||
| not Triple negative | 47 | 70.2 | 8.5 | 8.5 | 12.8 | 0.0454 | |
| Triple negative | 5 | 40.0 | 0.0 | 60.0 | 0.0 | ||
| Colorectal cancers | all cancers | 1570 | 0.5 | 5.5 | 9.7 | 84.3 | |
| pT1 | 63 | 0.0 | 6.3 | 4.8 | 88.9 | 0.0047 | |
| pT2 | 313 | 0.0 | 4.8 | 5.4 | 89.8 | ||
| pT3 | 859 | 0.7 | 5.2 | 9.8 | 84.3 | ||
| pT4 | 321 | 0.6 | 6.9 | 14.3 | 78.2 | ||
| pN0 | 808 | 0.4 | 4.7 | 5.9 | 89.0 | < 0.0001 | |
| pN+ | 732 | 0.7 | 6.4 | 13.8 | 79.1 | ||
| V0 | 1135 | 0.3 | 5.2 | 7.8 | 86.8 | < 0.0001 | |
| V+ | 396 | 1.0 | 6.8 | 15.2 | 77.0 | ||
| L0 | 595 | 0.3 | 5.2 | 5.5 | 88.9 | < 0.0001 | |
| L1 | 922 | 0.7 | 6.0 | 12.5 | 80.9 | ||
| left | 1142 | 0.4 | 6.0 | 9.5 | 84.1 | 0.4828 | |
| right | 422 | 0.7 | 4.3 | 10.2 | 84.8 | ||
| microsatellite instable | 80 | 1.3 | 10.0 | 7.5 | 81.3 | 0.1616 | |
| microsatellite stable | 1096 | 0.3 | 4.6 | 8.1 | 87.0 | ||
| RAS mutation | 328 | 0.9 | 7.3 | 8.5 | 83.2 | 0.1609 | |
| RAS wild type | 420 | 0.0 | 7.1 | 9.5 | 83.3 | ||
| BRAF V600E mutation | 18 | 0.0 | 16.7 | 11.1 | 72.2 | 0.3486 | |
| BRAF wild type | 122 | 0.8 | 4.9 | 8.2 | 86.1 | ||
| Clear cell renal cell carcinoma | all cancers | 569 | 51.1 | 25.1 | 14.2 | 9.5 | |
| ISUP 1 | 179 | 62.0 | 20.7 | 11.7 | 5.6 | < 0.0001 | |
| ISUP 2 | 185 | 45.9 | 30.8 | 14.6 | 8.6 | ||
| ISUP 3 | 163 | 42.3 | 27.6 | 16.6 | 13.5 | ||
| ISUP 4 | 34 | 70.6 | 11.8 | 11.8 | 5.9 | ||
| Fuhrmann 1 | 25 | 72.0 | 20.0 | 8.0 | 0.0 | 0.1532 | |
| Fuhrmann 2 | 334 | 51.5 | 26.3 | 13.8 | 8.4 | ||
| Fuhrmann 3 | 169 | 45.6 | 25.4 | 16.6 | 12.4 | ||
| Fuhrmann 4 | 40 | 60.0 | 17.5 | 12.5 | 10.0 | ||
| Thoenes 1 | 194 | 61.3 | 22.7 | 10.8 | 5.2 | 0.0036 | |
| Thoenes 2 | 318 | 44.0 | 28.0 | 16.7 | 11.3 | ||
| Thoenes 3 | 56 | 57.1 | 17.9 | 12.5 | 12.5 | ||
| UICC 1 | 238 | 50.8 | 26.1 | 15.5 | 7.6 | 0.1201 | |
| UICC 2 | 27 | 51.9 | 25.9 | 7.4 | 14.8 | ||
| UICC 3 | 76 | 31.6 | 36.8 | 15.8 | 15.8 | ||
| UICC 4 | 62 | 53.2 | 24.2 | 12.9 | 9.7 | ||
| pT1 | 319 | 52.4 | 23.8 | 16.0 | 7.8 | 0.0276 | |
| pT2 | 60 | 66.7 | 16.7 | 6.7 | 10.0 | ||
| pT3–4 | 185 | 44.3 | 30.3 | 13.5 | 11.9 | ||
| pN0 | 100 | 50.0 | 25.0 | 12.0 | 13.0 | 0.4847 | |
| pN ≥ 1 | 14 | 28.6 | 35.7 | 14.3 | 21.4 | ||
| pM0 | 86 | 44.2 | 32.6 | 14.0 | 9.3 | 0.5788 | |
| pM ≥ 1 | 64 | 54.7 | 23.4 | 12.5 | 9.4 | ||
| Papillary renal cell carcinoma | all cancers | 131 | 59.0 | 25.9 | 5.8 | 9.3 | |
| ISUP 1 | 26 | 69.2 | 23.1 | 3.8 | 3.8 | 0.0207 | |
| ISUP 2 | 65 | 52.3 | 29.2 | 6.2 | 12.3 | ||
| ISUP 3 | 46 | 63.0 | 23.9 | 4.3 | 8.7 | ||
| ISUP 4 | 1 | 0.0 | 0.0 | 100.0 | 0.0 | ||
| Fuhrmann 1 | 1 | 100.0 | 0.0 | 0.0 | 0.0 | 0.7761 | |
| Fuhrmann 2 | 92 | 58.7 | 27.2 | 4.3 | 9.8 | ||
| Fuhrmann 3 | 42 | 57.1 | 26.2 | 7.1 | 9.5 | ||
| Fuhrmann 4 | 3 | 66.7 | 0.0 | 33.3 | 0.0 | ||
| Thoenes 1 | 33 | 63.6 | 30.3 | 6.1 | 0.0 | 0.018 | |
| Thoenes 2 | 96 | 56.3 | 27.1 | 4.2 | 12.5 | ||
| Thoenes 3 | 9 | 66.7 | 0.0 | 22.2 | 11.1 | ||
| UICC 1 | 77 | 54.5 | 31.2 | 5.2 | 9.1 | 0.0067 | |
| UICC 2 | 10 | 100.0 | 0.0 | 0.0 | 0.0 | ||
| UICC 3 | 3 | 33.3 | 33.3 | 33.3 | 0.0 | ||
| UICC 4 | 9 | 55.6 | 0.0 | 11.1 | 33.3 | ||
| pT1 | 98 | 56.1 | 31.6 | 4.1 | 8.2 | 0.0424 | |
| pT2 | 26 | 76.9 | 11.5 | 3.8 | 7.7 | ||
| pT3–4 | 10 | 40.0 | 10.0 | 20.0 | 30.0 | ||
| pN0 | 14 | 50.0 | 42.9 | 0.0 | 7.1 | 0.0368 | |
| pN ≥ 1 | 7 | 57.1 | 0.0 | 14.3 | 28.6 | ||
| pM0 | 23 | 60.9 | 26.1 | 4.3 | 8.7 | 0.2792 | |
| pM ≥ 1 | 5 | 60.0 | 0.0 | 20.0 | 20.0 | ||
Fig. 4.
E-Cadherin immunostaining and patient prognosis. a in invasive breast carcinoma of no special type, b in lobular carcinoma of the breast, c papillary renal cell carcinoma, overall survival, (OS), d clear cell renal cell carcinoma (OS), e papillary renal cell carcinoma, recurrence-free survival (RFS), f clear cell renal cell carcinoma (RFS), g papillary renal cell carcinoma, cancer-specific survival (CSS), h clear cell renal cell carcinoma (CSS)
Discussion
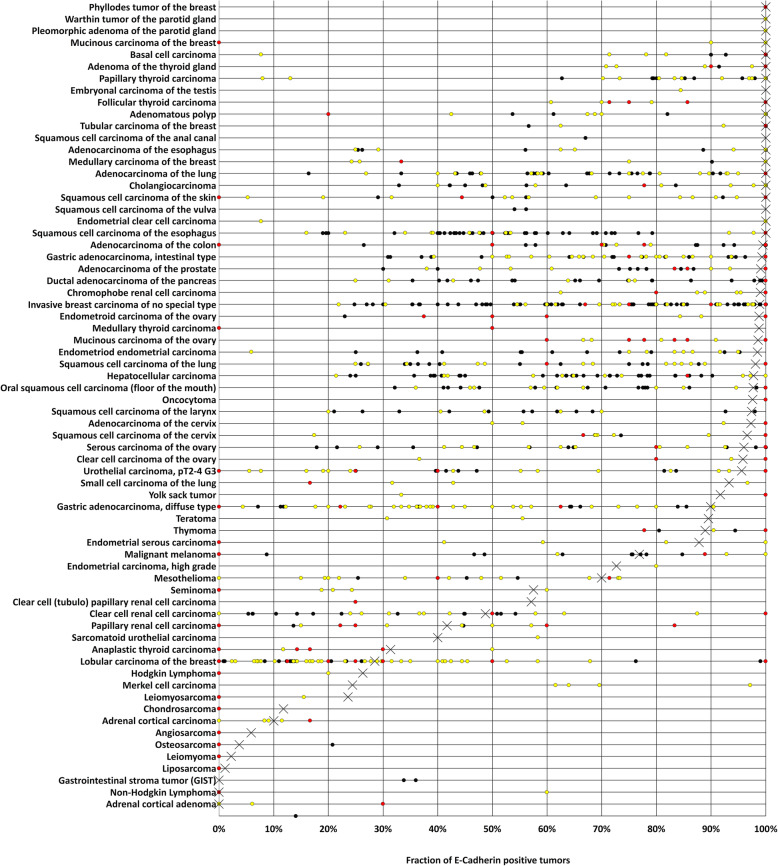
More than 1000 studies have described E-Cadherin immunohistochemical expression in cancer. This abundance of data obtained by using varying staining protocols and criteria for interpretation have made it difficult to easily understand the relative importance of E-Cadherin expression in various cancer types. This standardized analysis of 10,851 cancers by IHC provides a comprehensive overview of E-Cadherin immunostaining in 112 different tumor types. The most significant result of our study is a rank order of cancers according to their frequency of E-Cadherin expression, which is shown in Fig. 5 together with earlier data from the literature. The finding that most tumor types show either very high or very low E-Cadherin expression frequencies reflects the fact that frequent and intense E-Cadherin immunostaining is commonly seen in cancers derived from E-Cadherin positive normal cell types while neo-expression of E-Cadherin is rare and usually low in neoplasia derived from E-Cadherin negative normal cells.
Fig. 5.
Graphical representation of E-Cadherin data from this study (x) in comparison with the previous literature. Red dots are used for studies involving 2–10 cases, yellow dots are used for studies involving 11–50 cases, and black dots are used for studies involving > 50 cases
The group of cancers derived from E-cadherin positive normal cells and showing a particularly frequent loss of E-Cadherin expression included highly dedifferentiated cancers such as Merkel cell carcinoma, anaplastic thyroid cancer, dedifferentiated endometrium carcinoma, and sarcomatoid and small cell carcinomas of the urinary bladder. Together with other tumor types known to frequently show reduced E-Cadherin expression, such as invasive lobular breast cancer [34, 35], diffuse gastric carcinoma [28, 36], pseudopapillary neoplasm of the pancreas [37, 38] and plasmocytoid urothelial carcinoma [39, 40] these tumors morphologically have the loss of tumor cell cohesion in common. Given the pivotal role of E-Cadherin for cell-cell adhesion and maintenance of epithelial polarity [41, 42], it is tempting to speculate that noticeable effects of E-Cadherin downregulation on tumor morphology can appear. Molecular mechanisms for impaired E-Cadherin function include inactivating gene mutations, chromosomal deletions, and promotor hypermethylation which can occur in various combinations and can vary in frequency between cancer types [43–49]. Alternatively, the E-Cadherin function can be impaired by defects in other members of the E-Cadherin-Catenin complex – especially of alpha-catenin. Loss of alpha-catenin disrupts the structure of the E-Cadherin-Catenin complex, preventing the formation of cell-cell junctions between the actin cytoskeleton of adjacent cells (adherens junctions), thereby decreasing cell-cell adhesion [50–52].
The particularly high frequency of E-Cadherin loss in highly lethal cancers with dedifferentiated morphology already argues for a negative impact of E-Cadherin loss on the prognosis of cancer patients. Several aspects of our data support the concept that a reduced E-Cadherin expression may also be linked to unfavorable cancer features in tumors with less conspicuous morphology. In our study, reduced E-Cadherin expression was linked to high grade, triple negative receptor status and poor prognosis in invasive breast carcinoma of no special type, triple negative receptor status in lobular breast cancer, advanced pT stage and lymph node metastasis in colorectal cancer as well as prostate cancer progression. Significant associations of reduced E-Cadherin expression with poor outcome in invasive breast cancer [10–12, 53–57], triple receptor negativity in breast cancer [58], poor outcome and unfavorable tumor phenotype in colorectal cancer [29, 30, 59, 60], and adverse features in prostate cancer [13, 14] have been reported by various other investigators. However, several other studies have not found associations between reduced E-Cadherin expression and unfavorable patient prognosis or tumor phenotype in breast [20, 34, 54, 61–63], colorectal [31, 64] and prostate cancer [65]. Furthermore, previous studies in bladder and pancreatic cancer, tumors for which we did not find links to unfavorable tumor features, have provided inconsistent results, either suggesting [66–68] or rejecting [19, 69, 70] a prognostic role of reduced E-Cadherin expression. Overall, these data seem to suggest that reduced E-Cadherin expression is linked to unfavorable tumor outcome to some extent but cannot be considered a key indicator for aggressive disease course. This notion is also supported by the somewhat better prognosis of lobular breast cancer, a tumor with a particularly high rate of E-Cadherin loss, as compared to invasive breast cancer of no special type, a tumor which is mostly E-Cadherin positive [35, 71]. Also, the fact that two benign tumors - oncocytoma and non-invasive papillary urothelial pTaG2 low grade carcinoma - showed occasional E-Cadherin loss suggests that reduced or absent E-Cadherin immunostaining is not invariably linked to tumor malignancy. Given the high E-Cadherin expression in normal urothelium and the high positivity rate in invasive urothelial cancer, the comparatively high number of non-invasive papillary urothelial pTaG2 low grade carcinoma with loss of E-Cadherin immunostaining was highly unexpected. However, since large non-invasive papillary urothelial pTa tumors are often transported in containers with more tumor than formalin, it cannot be excluded, that these findings are caused by fixation artifacts [72, 73].
Upregulation of E-Cadherin as compared to normal cells was observed in 24 different tumor types in this study. The fact that the highest frequency of E-Cadherin positivity was seen in germ cell tumors may reflect the pluripotency of their precursor cells, which often results in a variable degree of epithelial differentiation in these tumors. The next large tumor categories with frequent E-Cadherin upregulation are papillary and clear cell RCCs derived from E-Cadherin negative proximal tubuli, melanocytic tumors, as well as several sarcoma types derived from E-Cadherin negative mesenchymal cells. Our data suggest that E-Cadherin upregulation is associated with increased cancer aggressiveness in these tumors. High E-Cadherin levels were more commonly seen in malignant than in benign soft tissue tumors, more frequent in leiomyosarcoma than in leiomyoma, associated with high grade in clear cell RCC and linked to poor prognosis in papillary RCC in this study. These observations are in line with one earlier study reporting unfavorable tumor properties in RCC with high E-Cadherin expression [17]. However, other authors could not confirm these results [74, 75]. What cellular function of E-Cadherin may be driving cancer progression in the case of protein upregulation is unclear. Two studies demonstrated, that E-Cadherin upregulation may lead to anoikis suppression, rapid formation of multicellular spheroids and allows therefore anchorage-independent cell growth in Ewing tumor cells [76] and oral squamous cell carcinoma cells [77]. It is also possible, that E-cadherin upregulation simply reflects aberrant differentiation or dedifferentiation of cancer cells and does not itself play a specific biological role.
Conclusion
E-Cadherin is consistently expressed in the vast majority of epithelial cancers. Both loss of E-Cadherin expression in cancers derived from E-Cadherin positive normal cells and upregulation in malignancies derived from E-cadherin negative normal tend to be linked to unfavorable tumor phenotype and disease outcome. Diagnosis of lobular breast cancer and distinction of chromophobe from clear cell carcinoma remain the best diagnostic applications of E-Cadherin IHC.
Supplementary Information
Additional file 1 : Supplementary Figure 1. Difference of E-Cadherin expression between benigne (including hemangiomas, ganglioneuromas, glomus tumors, granular cell tumors, myopericytomas, neurofibromas, paragangliomas and schwannomas) and maligne (including all types of sarcomas, dermatofibrosarcoma protuberans, mesotheliomas, and primitive neuroectodermal tumors) soft tissue tumors.
Acknowledgements
We are grateful to Melanie Witt, Inge Brandt, Maren Eisenberg, and Sünje Seekamp for excellent technical assistance and to Wilfrid Fehrle for proof-reading of the manuscript.
Abbreviations
- IHC
Immunohistochemistry
- p120-ctn
P120-catenin
- pN
Pathological lymph node status
- pT
Pathological tumor stage
- RCC
Renal cell carcinoma
- TMA
Tissue microarray
Authors’ contributions
EB, CB, RS, and GS designed the study. EB, FL, NG, FB, AML, AM, AH, DH, SW, CF, KM, FJ, PL, TSC, RU, WW, SS, SM, DD, RK, TK, and AHM performed the immunohistochemical analyses and/or contributed to the pathological validation of the tumors, the tissue microarray construction, and data collection. MK, CHM, and RS carried out the data analyses. GS, RS, EB, CB, and MK wrote the first draft of the manuscript. All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to the published, and agree to be accountable for all aspects of the work.
Funding
Not applicable. Open Access funding enabled and organized by Projekt DEAL.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The use of archived remnants of diagnostic tissues for manufacturing of TMAs and their analysis for research purposes as well as patient data analysis has been approved by local laws (HmbKHG, §12) and by the local ethics committee (Ethics Commission Hamburg, WF-049/09). All work has been carried out in compliance with the Helsinki Declaration.
Consent for publication
Not applicable.
Competing interests
The Institute of Pathology of the UKE receives royalties on the sale of E-Cadherin clone MSVA-035 from MS Validated Antibodies GmbH (owned by a family member of GS).
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.van Roy F, Berx G. The cell-cell adhesion molecule E-cadherin. Cell Mol Life Sci. 2008;65(23):3756–3788. doi: 10.1007/s00018-008-8281-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daugherty RL, Gottardi CJ. Phospho-regulation of Beta-catenin adhesion and signaling functions. Physiology (Bethesda) 2007;22:303–309. doi: 10.1152/physiol.00020.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoshida-Noro C, Suzuki N, Takeichi M. Molecular nature of the calcium-dependent cell-cell adhesion system in mouse teratocarcinoma and embryonic cells studied with a monoclonal antibody. Dev Biol. 1984;101(1):19–27. doi: 10.1016/0012-1606(84)90112-X. [DOI] [PubMed] [Google Scholar]
- 4.Hatta K, Okada TS, Takeichi M. A monoclonal antibody disrupting calcium-dependent cell-cell adhesion of brain tissues: possible role of its target antigen in animal pattern formation. Proc Natl Acad Sci U S A. 1985;82(9):2789–2793. doi: 10.1073/pnas.82.9.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Niessen CM, Leckband D, Yap AS. Tissue organization by cadherin adhesion molecules: dynamic molecular and cellular mechanisms of morphogenetic regulation. Physiol Rev. 2011;91(2):691–731. doi: 10.1152/physrev.00004.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larue L, Ohsugi M, Hirchenhain J, Kemler R. E-cadherin null mutant embryos fail to form a trophectoderm epithelium. Proc Natl Acad Sci U S A. 1994;91(17):8263–8267. doi: 10.1073/pnas.91.17.8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mendonsa AM, Na TY, Gumbiner BM. E-cadherin in contact inhibition and cancer. Oncogene. 2018;37(35):4769–4780. doi: 10.1038/s41388-018-0304-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu MN, Hu SH, Zhang XW, Xiong SM, Deng H. Overview on new progress of hereditary diffuse gastric cancer with CDH1 variants. Tumori J. 2020;106(5):34–55. [DOI] [PubMed]
- 9.Luveta J, Parks RM, Heery DM, Cheung KL, Johnston SJ. Invasive lobular breast cancer as a distinct disease: implications for therapeutic strategy. Oncol Ther. 2020;8(1):1–11. doi: 10.1007/s40487-019-00105-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brzozowska A, Sodolski T, Duma D, Mazurkiewicz T, Mazurkiewicz M. Evaluation of prognostic parameters of E-cadherin status in breast cancer treatment. Ann Agric Environ Med. 2012;19(3):541–546. [PubMed] [Google Scholar]
- 11.Tang D, Xu S, Zhang Q, Zhao W. The expression and clinical significance of the androgen receptor and E-cadherin in triple-negative breast cancer. Med Oncol. 2012;29(2):526–533. doi: 10.1007/s12032-011-9948-2. [DOI] [PubMed] [Google Scholar]
- 12.Zhang YG, Du J, Tian XX, Zhong YF, Fang WG. Expression of E-cadherin, beta-catenin, cathepsin D, gelatinases and their inhibitors in invasive ductal breast carcinomas. Chin Med J. 2007;120(18):1597–1605. doi: 10.1097/00029330-200709020-00010. [DOI] [PubMed] [Google Scholar]
- 13.Abdelrahman AE, Arafa SA, Ahmed RA. Prognostic value of twist-1, E-cadherin and EZH2 in prostate cancer: an immunohistochemical study. Turk Patoloji Derg. 2017;1(1):198–210. doi: 10.5146/tjpath.2016.01392. [DOI] [PubMed] [Google Scholar]
- 14.Cheng L, Nagabhushan M, Pretlow TP, Amini SB, Pretlow TG. Expression of E-cadherin in primary and metastatic prostate cancer. Am J Pathol. 1996;148(5):1375–1380. [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao XJ, Li H, Chen H, Liu YX, Zhang LH, Liu SX, Feng QL. Expression of e-cadherin and beta-catenin in human esophageal squamous cell carcinoma: relationships with prognosis. World J Gastroenterol. 2003;9(2):225–232. doi: 10.3748/wjg.v9.i2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Araki K, Shimura T, Suzuki H, Tsutsumi S, Wada W, Yajima T, Kobayahi T, Kubo N, Kuwano H. E/N-cadherin switch mediates cancer progression via TGF-beta-induced epithelial-to-mesenchymal transition in extrahepatic cholangiocarcinoma. Br J Cancer. 2011;105(12):1885–1893. doi: 10.1038/bjc.2011.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Langner C, Ratschek M, Rehak P, Schips L, Zigeuner R. Expression of MUC1 (EMA) and E-cadherin in renal cell carcinoma: a systematic immunohistochemical analysis of 188 cases. Mod Pathol. 2004;17(2):180–188. doi: 10.1038/modpathol.3800032. [DOI] [PubMed] [Google Scholar]
- 18.Uzquiano MC, Prieto VG, Nash JW, Ivan DS, Gong Y, Lazar AJ, et al. Metastatic basal cell carcinoma exhibits reduced actin expression. Mod Pathol. 2008;21(5):540–543. doi: 10.1038/modpathol.3801051. [DOI] [PubMed] [Google Scholar]
- 19.Reis ST, Leite KR, Mosconi Neto A, Pontes Junior J, Viana NI, Antunes AA, et al. Immune expression of E-cadherin and alpha, beta and gamma-catenin adhesion molecules and prognosis for upper urinary tract urothelial carcinomas. Int Braz J Urol. 2012;38(4):466–473. doi: 10.1590/S1677-55382012000400005. [DOI] [PubMed] [Google Scholar]
- 20.Asgeirsson KS, Jonasson JG, Tryggvadottir L, Olafsdottir K, Sigurgeirsdottir JR, Ingvarsson S, et al. Altered expression of E-cadherin in breast cancer. Patterns, mechanisms and clinical significance. Eur J Cancer. 2000;36(9):1098–1106. doi: 10.1016/S0959-8049(00)00062-9. [DOI] [PubMed] [Google Scholar]
- 21.Garcia S, Dales JP, Jacquemier J, Charafe-Jauffret E, Birnbaum D, Andrac-Meyer L, et al. c-Met overexpression in inflammatory breast carcinomas: automated quantification on tissue microarrays. Br J Cancer. 2007;96(2):329–335. doi: 10.1038/sj.bjc.6603569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horne HN, Oh H, Sherman ME, Palakal M, Hewitt SM, Schmidt MK, Milne RL, Hardisson D, Benitez J, Blomqvist C, Bolla MK, Brenner H, Chang-Claude J, Cora R, Couch FJ, Cuk K, Devilee P, Easton DF, Eccles DM, Eilber U, Hartikainen JM, Heikkilä P, Holleczek B, Hooning MJ, Jones M, Keeman R, Mannermaa A, Martens JWM, Muranen TA, Nevanlinna H, Olson JE, Orr N, Perez JIA, Pharoah PDP, Ruddy KJ, Saum KU, Schoemaker MJ, Seynaeve C, Sironen R, Smit VTHBM, Swerdlow AJ, Tengström M, Thomas AS, Timmermans AM, Tollenaar RAEM, Troester MA, van Asperen CJ, van Deurzen CHM, van Leeuwen FF, van’t Veer LJ, García-Closas M, Figueroa JD. E-cadherin breast tumor expression, risk factors and survival: pooled analysis of 5,933 cases from 12 studies in the breast cancer association consortium. Sci Rep. 2018;8(1):6574. doi: 10.1038/s41598-018-23733-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lim SC, Zhang S, Ishii G, Endoh Y, Kodama K, Miyamoto S, Hayashi R, Ebihara S, Cho JS, Ochiai A. Predictive markers for late cervical metastasis in stage I and II invasive squamous cell carcinoma of the oral tongue. Clin Cancer Res. 2004;10(1 Pt 1):166–172. doi: 10.1158/1078-0432.CCR-0533-3. [DOI] [PubMed] [Google Scholar]
- 24.Driemel O, Muller-Richter UD, Hakim SG, Bauer R, Berndt A, Kleinheinz J, et al. Oral acantholytic squamous cell carcinoma shares clinical and histological features with angiosarcoma. Head Face Med. 2008;4(1):17. doi: 10.1186/1746-160X-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu PF, Kang BH, Wu YM, Sun JH, Yen LM, Fu TY, Lin YC, Liou HH, Lin YS, Sie HC, Hsieh IC, Tseng YK, Shu CW, Hsieh YD, Ger LP. Vimentin is a potential prognostic factor for tongue squamous cell carcinoma among five epithelial-mesenchymal transition-related proteins. PLoS One. 2017;12(6):e0178581. doi: 10.1371/journal.pone.0178581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou Y, Li G, Wu J, Zhang Z, Wu Z, Fan P, Hao T, Zhang X, Li M, Zhang F, Li Q, Lu B, Qiao L. Clinicopathological significance of E-cadherin, VEGF, and MMPs in gastric cancer. Tumour Biol. 2010;31(6):549–558. doi: 10.1007/s13277-010-0068-y. [DOI] [PubMed] [Google Scholar]
- 27.Ascano JJ, Frierson H, Jr, Moskaluk CA, Harper JC, Roviello F, Jackson CE, et al. Inactivation of the E-cadherin gene in sporadic diffuse-type gastric cancer. Mod Pathol. 2001;14(10):942–949. doi: 10.1038/modpathol.3880416. [DOI] [PubMed] [Google Scholar]
- 28.Begnami MD, Fregnani JH, Brentani H, Torres C, Costa WL, Jr, Montagnini A, et al. Identification of protein expression signatures in gastric carcinomas using clustering analysis. J Gastroenterol Hepatol. 2012;27(2):378–384. doi: 10.1111/j.1440-1746.2011.06849.x. [DOI] [PubMed] [Google Scholar]
- 29.Senol S, Ceyran AB, Kosemetin D, Gobanoglu B, Aydin D, Duran EA, et al. Immunohistochemical profile of tumor pathways and prognostic significance in colon adenocarcinomas. J Environ Pathol Toxicol Oncol. 2017;36(1):29–41. doi: 10.1615/JEnvironPatholToxicolOncol.2017016530. [DOI] [PubMed] [Google Scholar]
- 30.Sayar I, Akbas EM, Isik A, Gokce A, Peker K, Demirtas L, Gürbüzel M. Relationship among mismatch repair deficiency, CDX2 loss, p53 and E-cadherin in colon carcinoma and suitability of using a double panel of mismatch repair proteins by immunohistochemistry. Pol J Pathol. 2015;66(3):246–253. doi: 10.5114/pjp.2015.54958. [DOI] [PubMed] [Google Scholar]
- 31.Bondi J, Bukholm G, Nesland JM, Bakka A, Bukholm IR. An increase in the number of adhesion proteins with altered expression is associated with an increased risk of cancer death for colon carcinoma patients. Int J Color Dis. 2006;21(3):231–237. doi: 10.1007/s00384-005-0762-1. [DOI] [PubMed] [Google Scholar]
- 32.Mikami S, Katsube K, Oya M, Ishida M, Kosaka T, Mizuno R, et al. Expression of snail and slug in renal cell carcinoma: E-cadherin repressor snail is associated with cancer invasion and prognosis. Lab Investig. 2011;91(10):1443–1458. doi: 10.1038/labinvest.2011.111. [DOI] [PubMed] [Google Scholar]
- 33.Pan CC, Chen PC, Ho DM. The diagnostic utility of MOC31, BerEP4, RCC marker and CD10 in the classification of renal cell carcinoma and renal oncocytoma: an immunohistochemical analysis of 328 cases. Histopathology. 2004;45(5):452–459. doi: 10.1111/j.1365-2559.2004.01962.x. [DOI] [PubMed] [Google Scholar]
- 34.Lobo J, Petronilho S, Newell AH, Coach J, Harlow G, Cruz A, Lopes P, Antunes L, Bai I, Walker E, Henrique R. E-cadherin clone 36 nuclear staining dictates adverse disease outcome in lobular breast cancer patients. Mod Pathol. 2019;32(11):1574–1586. doi: 10.1038/s41379-019-0294-9. [DOI] [PubMed] [Google Scholar]
- 35.Christgen M, Steinemann D, Kuhnle E, Langer F, Gluz O, Harbeck N, et al. Lobular breast cancer: clinical, molecular and morphological characteristics. Pathol Res Pract. 2016;212(7):583–597. doi: 10.1016/j.prp.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 36.Ansari S, Gantuya B, Tuan VP, Yamaoka Y. Diffuse gastric cancer: a summary of analogous contributing factors for its molecular pathogenicity. Int J Mol Sci. 2018;19(8):2424. [DOI] [PMC free article] [PubMed]
- 37.Kim MJ, Jang SJ, Yu E. Loss of E-cadherin and cytoplasmic-nuclear expression of beta-catenin are the most useful immunoprofiles in the diagnosis of solid-pseudopapillary neoplasm of the pancreas. Hum Pathol. 2008;39(2):251–258. doi: 10.1016/j.humpath.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 38.Liu BA, Li ZM, Su ZS, She XL. Pathological differential diagnosis of solid-pseudopapillary neoplasm and endocrine tumors of the pancreas. World J Gastroenterol. 2010;16(8):1025–1030. doi: 10.3748/wjg.v16.i8.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fox MD, Xiao L, Zhang M, Kamat AM, Siefker-Radtke A, Zhang L, Dinney CP, Czerniak B, Guo CC. Plasmacytoid urothelial carcinoma of the urinary bladder: a clinicopathologic and immunohistochemical analysis of 49 cases. Am J Clin Pathol. 2017;147(5):500–506. doi: 10.1093/ajcp/aqx029. [DOI] [PubMed] [Google Scholar]
- 40.Sangoi AR, Chan E, Stohr BA, Kunju LP. Invasive plasmacytoid urothelial carcinoma: a comparative study of E-cadherin and P120 catenin. Hum Pathol. 2020;102:54–59. doi: 10.1016/j.humpath.2020.07.006. [DOI] [PubMed] [Google Scholar]
- 41.Desai RA, Gao L, Raghavan S, Liu WF, Chen CS. Cell polarity triggered by cell-cell adhesion via E-cadherin. J Cell Sci. 2009;122(Pt 7):905–911. doi: 10.1242/jcs.028183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coradini D, Casarsa C, Oriana S. Epithelial cell polarity and tumorigenesis: new perspectives for cancer detection and treatment. Acta Pharmacol Sin. 2011;32(5):552–564. doi: 10.1038/aps.2011.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hansford S, Kaurah P, Li-Chang H, Woo M, Senz J, Pinheiro H, Schrader KA, Schaeffer DF, Shumansky K, Zogopoulos G, Santos TA, Claro I, Carvalho J, Nielsen C, Padilla S, Lum A, Talhouk A, Baker-Lange K, Richardson S, Lewis I, Lindor NM, Pennell E, MacMillan A, Fernandez B, Keller G, Lynch H, Shah SP, Guilford P, Gallinger S, Corso G, Roviello F, Caldas C, Oliveira C, Pharoah PDP, Huntsman DG. Hereditary diffuse gastric cancer syndrome: CDH1 mutations and beyond. JAMA Oncol. 2015;1(1):23–32. doi: 10.1001/jamaoncol.2014.168. [DOI] [PubMed] [Google Scholar]
- 44.Corso G, Figueiredo J, La Vecchia C, Veronesi P, Pravettoni G, Macis D, et al. Hereditary lobular breast cancer with an emphasis on E-cadherin genetic defect. J Med Genet. 2018;55(7):431–441. doi: 10.1136/jmedgenet-2018-105337. [DOI] [PubMed] [Google Scholar]
- 45.Abida W, Armenia J, Gopalan A, Brennan R, Walsh M, Barron D, et al. Prospective genomic profiling of prostate cancer across disease states reveals germline and somatic alterations that May affect clinical decision making. JCO Precis Oncol. 2017;2017(1):1–16. [DOI] [PMC free article] [PubMed]
- 46.Zehir A, Benayed R, Shah RH, Syed A, Middha S, Kim HR, Srinivasan P, Gao J, Chakravarty D, Devlin SM, Hellmann MD, Barron DA, Schram AM, Hameed M, Dogan S, Ross DS, Hechtman JF, DeLair DF, Yao JJ, Mandelker DL, Cheng DT, Chandramohan R, Mohanty AS, Ptashkin RN, Jayakumaran G, Prasad M, Syed MH, Rema AB, Liu ZY, Nafa K, Borsu L, Sadowska J, Casanova J, Bacares R, Kiecka IJ, Razumova A, Son JB, Stewart L, Baldi T, Mullaney KA, al-Ahmadie H, Vakiani E, Abeshouse AA, Penson AV, Jonsson P, Camacho N, Chang MT, Won HH, Gross BE, Kundra R, Heins ZJ, Chen HW, Phillips S, Zhang H, Wang J, Ochoa A, Wills J, Eubank M, Thomas SB, Gardos SM, Reales DN, Galle J, Durany R, Cambria R, Abida W, Cercek A, Feldman DR, Gounder MM, Hakimi AA, Harding JJ, Iyer G, Janjigian YY, Jordan EJ, Kelly CM, Lowery MA, Morris LGT, Omuro AM, Raj N, Razavi P, Shoushtari AN, Shukla N, Soumerai TE, Varghese AM, Yaeger R, Coleman J, Bochner B, Riely GJ, Saltz LB, Scher HI, Sabbatini PJ, Robson ME, Klimstra DS, Taylor BS, Baselga J, Schultz N, Hyman DM, Arcila ME, Solit DB, Ladanyi M, Berger MF. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med. 2017;23(6):703–713. doi: 10.1038/nm.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pozdeyev N, Gay LM, Sokol ES, Hartmaier R, Deaver KE, Davis S, French JD, Borre PV, LaBarbera DV, Tan AC, Schweppe RE, Fishbein L, Ross JS, Haugen BR, Bowles DW. Genetic analysis of 779 advanced differentiated and anaplastic thyroid cancers. Clin Cancer Res. 2018;24(13):3059–3068. doi: 10.1158/1078-0432.CCR-18-0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dulak AM, Stojanov P, Peng S, Lawrence MS, Fox C, Stewart C, Bandla S, Imamura Y, Schumacher SE, Shefler E, McKenna A, Carter SL, Cibulskis K, Sivachenko A, Saksena G, Voet D, Ramos AH, Auclair D, Thompson K, Sougnez C, Onofrio RC, Guiducci C, Beroukhim R, Zhou Z, Lin L, Lin J, Reddy R, Chang A, Landrenau R, Pennathur A, Ogino S, Luketich JD, Golub TR, Gabriel SB, Lander ES, Beer DG, Godfrey TE, Getz G, Bass AJ. Exome and whole-genome sequencing of esophageal adenocarcinoma identifies recurrent driver events and mutational complexity. Nat Genet. 2013;45(5):478–486. doi: 10.1038/ng.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang K, Russell JS, McDermott JD, Elvin JA, Khaira D, Johnson A, et al. Profiling of 149 salivary duct carcinomas, carcinoma ex pleomorphic adenomas, and adenocarcinomas, not otherwise specified reveals actionable genomic alterations. Clin Cancer Res. 2016;22(24):6061–6068. doi: 10.1158/1078-0432.CCR-15-2568. [DOI] [PubMed] [Google Scholar]
- 50.Gloushankova NA, Rubtsova SN, Zhitnyak IY. Cadherin-mediated cell-cell interactions in normal and cancer cells. Tissue Barriers. 2017;5(3):e1356900. doi: 10.1080/21688370.2017.1356900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakopoulou L, Gakiopoulou-Givalou H, Karayiannakis AJ, Giannopoulou I, Keramopoulos A, Davaris P, Pignatelli M. Abnormal alpha-catenin expression in invasive breast cancer correlates with poor patient survival. Histopathology. 2002;40(6):536–546. doi: 10.1046/j.1365-2559.2002.01392.x. [DOI] [PubMed] [Google Scholar]
- 52.de Groot JS, Ratze MA, van Amersfoort M, Eisemann T, Vlug EJ, Niklaas MT, et al. alphaE-catenin is a candidate tumor suppressor for the development of E-cadherin-expressing lobular-type breast cancer. J Pathol. 2018;245(4):456–467. doi: 10.1002/path.5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kashiwagi S, Yashiro M, Takashima T, Nomura S, Noda S, Kawajiri H, Ishikawa T, Wakasa K, Hirakawa K. Significance of E-cadherin expression in triple-negative breast cancer. Br J Cancer. 2010;103(2):249–255. doi: 10.1038/sj.bjc.6605735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Engstrom MJ, Opdahl S, Vatten LJ, Haugen OA, Bofin AM. Invasive lobular breast cancer: the prognostic impact of histopathological grade, E-cadherin and molecular subtypes. Histopathology. 2015;66(3):409–419. doi: 10.1111/his.12572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kashiwagi S, Yashiro M, Takashima T, Aomatsu N, Ikeda K, Ogawa Y, Ishikawa T, Hirakawa K. Advantages of adjuvant chemotherapy for patients with triple-negative breast cancer at stage II: usefulness of prognostic markers E-cadherin and Ki67. Breast Cancer Res. 2011;13(6):R122. doi: 10.1186/bcr3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bankfalvi A, Terpe HJ, Breukelmann D, Bier B, Rempe D, Pschadka G, et al. Immunophenotypic and prognostic analysis of E-cadherin and beta-catenin expression during breast carcinogenesis and tumour progression: a comparative study with CD44. Histopathology. 1999;34(1):25–34. doi: 10.1046/j.1365-2559.1999.00540.x. [DOI] [PubMed] [Google Scholar]
- 57.Siitonen SM, Kononen JT, Helin HJ, Rantala IS, Holli KA, Isola JJ. Reduced E-cadherin expression is associated with invasiveness and unfavorable prognosis in breast cancer. Am J Clin Pathol. 1996;105(4):394–402. doi: 10.1093/ajcp/105.4.394. [DOI] [PubMed] [Google Scholar]
- 58.Ionescu Popescu C, Giusca SE, Liliac L, Avadanei R, Ceausu R, Cimpean AM, et al. E-cadherin expression in molecular types of breast carcinoma. Romanian J Morphol Embryol. 2013;54(2):267–273. [PubMed] [Google Scholar]
- 59.Cai X, Qi WX, Wang L, Zhang Z. Correlation of multiple proteins with clinic-pathological features and its prognostic significance in colorectal cancer with signet-ring cell component. Eur Rev Med Pharmacol Sci. 2016;20(16):3358–3367. [PubMed] [Google Scholar]
- 60.Yu XW, Rong W, Xu FL, Xu GY, Sun YR, Feng MY. Expression and clinical significance of mucin and E-cadherin in colorectal tumors. Ai Zheng. 2007;26(11):1204–1210. [PubMed] [Google Scholar]
- 61.Makdissi FB, Machado LV, Oliveira AG, Benvenuti TT, Katayama ML, Brentani MM, et al. Expression of E-cadherin, snail and hakai in epithelial cells isolated from the primary tumor and from peritumoral tissue of invasive ductal breast carcinomas. Braz J Med Biol Res. 2009;42(12):1128–1137. doi: 10.1590/S0100-879X2009001200002. [DOI] [PubMed] [Google Scholar]
- 62.Yamashita N, Tokunaga E, Iimori M, Inoue Y, Tanaka K, Kitao H, Saeki H, Oki E, Maehara Y. Epithelial paradox: clinical significance of coexpression of E-cadherin and vimentin with regard to invasion and metastasis of breast cancer. Clin Breast Cancer. 2018;18(5):e1003–e10e9. doi: 10.1016/j.clbc.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 63.Pang H, Lu H, Song H, Meng Q, Zhao Y, Liu N, Lan F, Liu Y, Yan S, Dong X, Cai L. Prognostic values of osteopontin-c, E-cadherin and beta-catenin in breast cancer. Cancer Epidemiol. 2013;37(6):985–992. doi: 10.1016/j.canep.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 64.Van Marck V, Stove C, Jacobs K, Van den Eynden G, Bracke M. P-cadherin in adhesion and invasion: opposite roles in colon and bladder carcinoma. Int J Cancer. 2011;128(5):1031–1044. doi: 10.1002/ijc.25427. [DOI] [PubMed] [Google Scholar]
- 65.Rubin MA, Mucci NR, Figurski J, Fecko A, Pienta KJ, Day ML. E-cadherin expression in prostate cancer: a broad survey using high-density tissue microarray technology. Hum Pathol. 2001;32(7):690–697. doi: 10.1053/hupa.2001.25902. [DOI] [PubMed] [Google Scholar]
- 66.Favaretto RL, Bahadori A, Mathieu R, Haitel A, Grubmuller B, Margulis V, et al. Prognostic role of decreased E-cadherin expression in patients with upper tract urothelial carcinoma: a multi-institutional study. World J Urol. 2017;35(1):113–120. doi: 10.1007/s00345-016-1835-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hussein S, Mosaad H, Rashed HE, Ahmed S, Ragab A, Ismail EI. Molecular factors regulating E-cadherin expression in urothelial bladder cancer and their correlations with the clinicopathological features. Mol Biol Rep. 2017;44(4):365–377. doi: 10.1007/s11033-017-4118-z. [DOI] [PubMed] [Google Scholar]
- 68.Shimamura T, Sakamoto M, Ino Y, Sato Y, Shimada K, Kosuge T, Sekihara H, Hirohashi S. Dysadherin overexpression in pancreatic ductal adenocarcinoma reflects tumor aggressiveness: relationship to e-cadherin expression. J Clin Oncol. 2003;21(4):659–667. doi: 10.1200/JCO.2003.06.179. [DOI] [PubMed] [Google Scholar]
- 69.Matsumoto H, Munemori M, Shimizu K, Fujii N, Kobayashi K, Inoue R, Yamamoto Y, Nagao K, Matsuyama H. Risk stratification using Bmi-1 and snail expression is a useful prognostic tool for patients with upper tract urothelial carcinoma. Int J Urol. 2016;23(12):1030–1037. doi: 10.1111/iju.13229. [DOI] [PubMed] [Google Scholar]
- 70.Handra-Luca A, Hammel P, Sauvanet A, Lesty C, Ruszniewski P, Couvelard A. EGFR expression in pancreatic adenocarcinoma. Relationship to tumour morphology and cell adhesion proteins. J Clin Pathol. 2014;67(4):295–300. doi: 10.1136/jclinpath-2013-201662. [DOI] [PubMed] [Google Scholar]
- 71.Ashaie MA, Chowdhury EH. Cadherins: the superfamily critically involved in breast cancer. Curr Pharm Des. 2016;22(5):616–638. doi: 10.2174/138161282205160127095338. [DOI] [PubMed] [Google Scholar]
- 72.Thavarajah R, Mudimbaimannar VK, Elizabeth J, Rao UK, Ranganathan K. Chemical and physical basics of routine formaldehyde fixation. J Oral Maxillofac Pathol. 2012;16(3):400–405. doi: 10.4103/0973-029X.102496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Werner M, Chott A, Fabiano A, Battifora H. Effect of formalin tissue fixation and processing on immunohistochemistry. Am J Surg Pathol. 2000;24(7):1016–1019. doi: 10.1097/00000478-200007000-00014. [DOI] [PubMed] [Google Scholar]
- 74.Harb OA, Elfeky MA, El Shafaay BS, Taha HF, Osman G, Harera IS, et al. SPOP, ZEB-1 and E-cadherin expression in clear cell renal cell carcinoma (cc-RCC): Clinicopathological and prognostic significance. Pathophysiology. 2018;25(4):335–345. doi: 10.1016/j.pathophys.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 75.Zhang X, Yang M, Shi H, Hu J, Wang Y, Sun Z, Xu S. Reduced E-cadherin facilitates renal cell carcinoma progression by WNT/beta-catenin signaling activation. Oncotarget. 2017;8(12):19566–19576. doi: 10.18632/oncotarget.15361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kang HG, Jenabi JM, Zhang J, Keshelava N, Shimada H, May WA, Ng T, Reynolds CP, Triche TJ, Sorensen PHB. E-cadherin cell-cell adhesion in Ewing tumor cells mediates suppression of anoikis through activation of the ErbB4 tyrosine kinase. Cancer Res. 2007;67(7):3094–3105. doi: 10.1158/0008-5472.CAN-06-3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kantak SS, Kramer RH. E-cadherin regulates anchorage-independent growth and survival in oral squamous cell carcinoma cells. J Biol Chem. 1998;273(27):16953–16961. doi: 10.1074/jbc.273.27.16953. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1 : Supplementary Figure 1. Difference of E-Cadherin expression between benigne (including hemangiomas, ganglioneuromas, glomus tumors, granular cell tumors, myopericytomas, neurofibromas, paragangliomas and schwannomas) and maligne (including all types of sarcomas, dermatofibrosarcoma protuberans, mesotheliomas, and primitive neuroectodermal tumors) soft tissue tumors.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from corresponding author on reasonable request.