Abstract
Background
Congenital portosystemic shunt (CPSS) is a rare malformation in which splanchnic venous flow bypasses the liver. CPSS is associated with other congenital anomalies and syndromes and can be associated with life-threatening complications. CPSS and their management remain underreported in the literature. Here, we review the clinical characteristics, management, and outcomes of a cohort of children and young adults with CPSS from two pediatric centers.
Methods
Cases of CPSS from Cincinnati Children’s Hospital Medical Center and C.S. Mott Children’s Hospital were reviewed to define CPSS anatomy, associated anomalies, complications, interventions, and outcomes. The imaging features and histopathology of liver lesions were characterized in detail.
Results
A total of 11 cases were identified. Median age was 10 years (range 0–26); 8 (73%) cases were female. Associated anomalies included six patients with heterotaxy (55%), five patients with congenital heart disease (45%), three patients with Turner syndrome (27%), and two patients with omphalocele, exstrophy, imperforate anus, spinal defects (OEIS) complex (18%). Eight (73%) cases had hyperammonemia ± encephalopathy. A 4-month-old presented with hepatopulmonary syndrome, and 12-year-old presented with pulmonary hypertension. Eight patients (73%) had liver lesions including five with premalignant adenomas and three with well-differentiated hepatocellular carcinoma (HCC). Four children underwent successful CPSS occlusion/ligation. Three children underwent liver transplant (2) or resection (1) for HCC without recurrence at extended follow-up.
Conclusions
CPSS is associated with multiple anomalies (heterotaxy, congenital heart disease) and syndromes (Turner syndrome). CPSS liver lesions should be very carefully evaluated due to risk of premalignant adenomas and HCC. Serious complications of CPSS can occur at a young age but can be managed endovascularly or with open surgery.
Keywords: Abernethy, Hepatocellular adenoma, Hepatocellular carcinoma, Portosystemic shunt, Liver
Introduction
Congenital portosystemic shunt (CPSS) is a malformation in which venous outflow from the intestines and spleen is diverted directly into the systemic circulation via an anomalous vascular connection (shunt), thereby bypassing the liver. The shunt may arise from any root of the portal vein (e.g., superior mesenteric vein, inferior mesenteric vein, or splenic vein), from the main portal vein, or from a branch of the portal vein. The systemic termination site is commonly the inferior vena cava but can be any systemic vein including a branch of the hepatic veins, the renal veins, or the iliac veins. The location of the shunt vessel may be intrahepatic or extrahepatic. The historical term for extrahepatic CPSS is “Abernethy malformation.” In patients with extrahepatic CPSS, the portal vein and intrahepatic portal venous branches may be absent (type 1) or patent (type 2) [1].
CPSS is regarded as a rare anomaly, but the prevalence of CPSS is unknown, as diagnosis requires clinical suspicion. The number of reported cases has increased over time, due to heightened awareness and improved imaging techniques [1]. Recognition of CPSS is essential since, in the appropriate circumstances, intervention in the form of obliterating the shunt or liver transplantation can improve clinical outcomes. Decisions regarding the timing and type of intervention are impacted by many factors including the anatomy of the shunt and portal vein, complications of CPSS (including masses in the liver) or the estimated risk of complications over time, and the presence of comorbidities that might influence the risk/benefit ratio of an intervention.
CPSS has been associated with complications during childhood including neonatal cholestasis, hyperammonemia and encephalopathy, hepatopulmonary syndrome, pulmonary hypertension, and benign and malignant liver masses [1, 2]. A recent review identified 82 published cases of liver masses associated with CPSS, most commonly focal nodular hyperplasia and nodular regenerative hyperplasia, but also hepatocellular adenoma, hepatoblastoma, and hepatocellular carcinoma (HCC) [2].
Knowledge of the various clinical presentations in children with CPSS and approaches to management are limited due to the rarity of CPSS. Here, we add to the existing literature by reviewing our experience caring for eleven children and young adults with CPSS, with details of clinical presentations, clinical associations, shunt anatomy, complications including liver masses, and outcomes following intervention.
Materials and Methods
This study was approved by the Institutional Review Board at Cincinnati Children’s Hospital Medical Center and C.S. Mott Children’s Hospital. We systematically queried the electronic medical record at CCHMC by available encounter codes likely to be associated with the diagnosis of CPSS, including all available codes containing the words portosystemic shunt, as well as those for known associations including inferior vena cava interruption, congenital bilateral superior vena cava, heterotaxy, polysplenia, and intestinal malrotation. For rigor, we included all pertinent encounter codes related to vascular disorders and anomalies.
After preliminary review of charts and cross-sectional imaging (CT or MR) to confirm a diagnosis of CPSS, we collected the following data: age at diagnosis of CPSS, gender, coexisting diagnoses, physical examination findings, laboratory and imaging results, complications, details of interventions, and clinical outcomes following interventions. Shunt anatomy was categorized according to the system proposed by Gauthier and colleagues [3]. The categories include: extrahepatic portosystemic (EHPS; origin is any root vessel draining into the portal vein, and termination is lower inferior vena cava or other systemic vein), portocaval (PC; origin is portal vein or branch of portal vein, and termination is inferior vena cava between the renal veins and hepatic veins), and portohepatic (PH; origin is portal vein or branch of portal vein, and termination is one of the hepatic veins).
Liver biopsies, resections, and explants in our cohort of CPSS patients with specimens were identified in the pathology archives. Liver lesions and background liver when available were reviewed by two or more pathologists (A.G., R.S., and K.B.). Specimens were evaluated using hematoxylin and eosin (H&E) stains, special stains including reticulin, and immunohistochemical stains related to evaluation of subtypes of hepatocellular adenoma (HCA), as previously described by Zucman-Rossi and colleagues [4–6]. Immunostains for glutamine synthetase and β-catenin (diffuse to heterogeneous strong glutamine synthetase positivity and ± nuclear β-catenin signal) were considered indicative of β-catenin-activated adenoma. Loss of fatty acid binding protein immunoexpression was considered consistent with hepatocyte nuclear factor 1-alpha (HNF1α)-inactivated adenoma. Diffuse overexpression of C-reactive protein and/or serum amyloid-A was considered consistent with inflammatory adenoma. In addition, we performed glypican-3 immunostaining which highlights an onco-fetal protein expressed in hepatoblastomas, some hepatocellular carcinomas, and dysplastic nodules, and is negative in all adenomas and macroregenerative nodules [7].
As per the International Consensus Group for Hepatocellular Neoplasia, early well-differentiated hepatocellular carcinoma (eHCC) was identified in cases of well-differentiated hepatocellular neoplasms lacking a well-defined capsule, containing some portal tracts, unpaired arteries, pseudoglandular structures, and either intratumoral lymphovascular invasion or portal tract invasion [8]. Well-differentiated HCC has similar findings as eHCC with the addition of being partially or completely encapsulated/pseudocapsule and showing morphologic variability of tumor cells.
Results
Our preliminary query generated a list of 345 individual patients. Of these, a total of 11 patients were confirmed by chart and imaging review to have CPSS, including eight females (73%) and three males (27%). Demographics, patency status of the portal vein, and shunt anatomy for each patient are summarized in Table 1. The median age at time of diagnosis of CPSS was 10 years, with a range from the neonatal period to 26 years of age.
Table 1.
Demographics, patency status of the portal vein, and shunt anatomy for each patient with CPSS
| Patient | Age at CPSS diagnosis/gender | Presenting concern | Imaging | PV statusa | Shunt anatomy | Shunt categor |
|---|---|---|---|---|---|---|
| 1 | Neonate/F | HLHS, heterotaxy | MR/IR | Absent | SV to left HV | EHPS |
| 2 | 4 mo/F | Hypoxia secondary to HPS | MR/OR | Absent | SMV to IVC | EHPS |
| 3 | 9 mo/M | Heterotaxy | MR/IR | Patent | SMV to left SVC | EHPS |
| 4 | 9/M | Liver lesion | MR/IR | Patent | SMV to left iliac vein | EHPS |
| 5 | 9/F | Leukopenia, thrombocytopenia, splenomegaly | CT/IR | Patent | PV to IVC | PC |
| 6 | 10/M | Liver lesions | IR/OR | Patent | PV to IVC | PC |
| 7 | 11/F | Liver lesions | MR/OR | Absent | SMV/SV to left renal vein | EHPS |
| 8 | 12/F | Liver lesions, occult PHTN | MR | Patent | Right PV to right HV | PH |
| 9 | 12/F | Liver lesions | MR/OR | Absent | SMV/SV to IVC | EHPS |
| 10 | 16/F | Chronic abdominal pain, liver lesions | CT/IR | Patent | SMV to IVC | EHPS |
| 11 | 26/F | Lymphaticovenous malformation, left leg | MR/IR | Absent | IMV to left internal iliac vein | EHPS |
CPSS congenital portosystemic shunt, HLHS hypoplastic left heart syndrome, HPS hepatopulmonary syndrome, PHTN pulmonary hypertension, MR magnetic resonance imaging, IR interventional angiography, CT computed tomography, OR direct visualization in the operating room, SV splenic vein, HV hepatic vein, PV portal vein, SMV superior mesenteric vein, IVC inferior vena cava, SVC superior vena cava, IMV inferior mesenteric vein, EHPS extrahepatic portosystemic, PC portocaval, PH portohepatic
Final assessment based on imaging
Associated anomalies and syndromes were present in nine patients (82%) and are summarized in Table 2. Congenital heart lesions were present in five of the patients, and one or more laterality defects (such as persistent left superior vena cava, malrotation, polysplenia, bilateral SVC, situs inversus) were present in six patients.
Table 2.
Anomalies and dominant clinical features in patients with CPSS
| Patient | Associated cardiac conditions | Associated other conditions | Liver lesion noted? | Other complications | Intervention | Outcome | Length of follow-up after CPSS diagnosis (mos) |
|---|---|---|---|---|---|---|---|
| 1 | HLHS Bilateral SVC |
Turner syndrome | No | ↑NH3 (108) | None | Died (complications of congenital heart disease) | 2 |
| 2 | ASD, VSD Bilateral SVC |
Situs inversus Intestinal malrotation Polysplenia | No | Hypoxemia (HPS) | OLT | Hypoxemia resolved by 3 months following OLT | 156 |
| 3 | Bilateral SVC Small IVC Left SVC to coronary sinus fistula |
None | No | ↑NH3 (112) | Lactulose Rifaximin | Surveillance | 79 |
| 4 | None | OEIS complex Ambiguous genitalia Intestinal malrotation |
Yes | ↑NH3 (113), HE | IR occlusion | NH3 normalized HE episodes resolved Regression of liver lesion |
36 |
| 5 | Aortic coarctation Bicuspid aortic valve PAPVR |
Turner syndrome | Yes | ↑NH3 (141) | IR occlusion | NH3 normalized Liver lesion too small to characterize |
39 |
| 6 | None | Neonatal cholestasis | Yes | Pruritusa | OR ligation; resection of eHCC | Pruritus resolved No evidence of recurrent liver lesion |
77 |
| 7 | ASD, VSD Aortic coarctation |
Turner syndrome Horseshoe kidney Neurogenic bladder Developmental delay |
Yes | Dev delay ↑NH3 (97) |
OLT | OLT NH3 normalized No evidence of recurrent liver lesion |
50 |
| 8 | None | None | Yes | PHTN | Treprostinil Sildenafil | OLT recommended | 49 |
| 9 | None | Turner syndrome Omphalocele Intestinal malrotation Developmental delay |
Yes | ↑NH3 (219) | OLT | OLT NH3 normalized No evidence of recurrent liver lesion |
48 |
| 10 | None | OEIS complex Intestinal malrotation |
Yes | ↑NH3 (195) | IR occlusion; resection and radiofrequency ablation of HCAs | Normalization of NH3 Regression of liver lesions |
26 |
| 11 | None | Capillary-venouslymphatic malformation, left leg | Yes | ↑NH3 (110) | Rifaximin | Listed for liver transplant | 63 |
SVC superior vena cava, ASD atrial septal defect, VSD ventricular septal defect, HPS hepatopulmonary syndrome, OLT orthotopic liver transplantation, IVC inferior vena cava, OEIS omphalocele–exstrophy–imperforate anus–spinal defects, HE hepatic encephalopathy, FNH focal nodular hyperplasia, IR interventional radiology, HLHS hypoplastic left heart syndrome, PAPVR partial anomalous pulmonary venous return, HCA hepatocellular adenoma, PHTN pulmonary hypertension, NH3 serum ammonia (peak level in parenthesis)
Secondary to biliary obstruction from a large mass in the left hemiliver
All patients underwent cross-sectional imaging with either CT or MRI, and ten patients (90%) were additionally assessed by catheter-based angiography or operative inspection. Five patients (45%) were found to have complete absence of the portal vein with an EHPS. Each had undergone either catheter-based angiography with wedge hepatic venography or operative inspection, which confirmed absence of the portal vein. Notably, for Patient 5, cross-sectional imaging was initially interpreted to show complete absence of the portal vein. Subsequent catheter-based venogram showed a hypoplastic but patent portal vein which was visible in retrospect on the CT scan (Fig. 1).
Fig. 1.
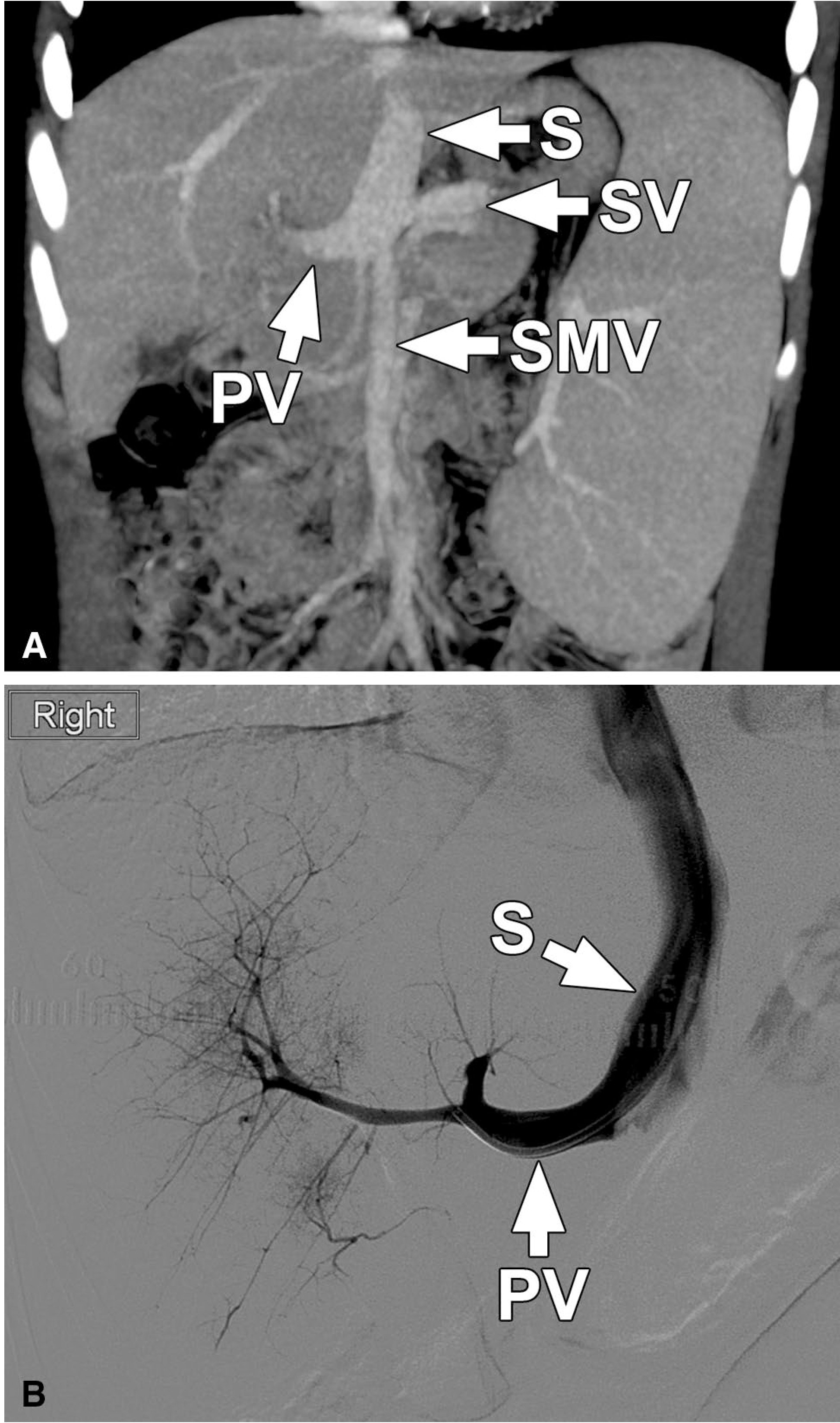
Patient 5. Coronal oblique maximum intensity projection (MIP) image (a) from a CT of the abdomen performed with intravenous contrast in the portal venous phase shows a large shunt (S) from the confluence of the superior mesenteric vein (SMV) and splenic vein (SV) coursing toward the left hepatic vein, where it terminates. An extrahepatic portal vein (PV) is present tapering toward the hepatic hilum. Mean portal vein pressure was 4 mm Hg. Other images (not shown) showed hypoplastic intrahepatic portal venous branches which were not recognized on the initial interpretation. Subsequently performed catheter venogram (b) with the catheter positioned across the abnormal shunt (S) and into the portal vein shows the tapering extrahepatic portal vein (PV) and hypoplastic but patent intrahepatic portal veins
Complications of CPSS
All patients had complications of CPSS. Hyperammonemia (maximum measured range, 97–219) was documented in eight patients (73%). Patients 7 and 9 had longstanding neurocognitive deficits ascribed to chronically elevated ammonia levels, and Patient 4 had discrete episodes of disorientation and irritability (encephalopathy) which were temporally linked to worsening elevations of serum ammonia. A total of four patients were given trials of rifaximin, with or without lactulose, without significant improvement in serum ammonia levels. Three patients, each with a patent portal vein, subsequently underwent endovascular occlusion of the CPSS, and two patients with absent portal veins underwent liver transplantation, with immediate normalization of serum ammonia levels in all and resolution of behavioral changes in Patient 4 following intervention to occlude the shunt. Patients 3 and 11 have had persistent hyperammonemia but have been maintained on rifaximin with or without lactulose at the discretion of their clinician, and remained without episodes of hepatic encephalopathy or neurocognitive disturbance.
Patient 2 presented with hepatopulmonary syndrome (HPS) with severe hypoxemia in the setting of diffuse, large pulmonary arteriovenous malformations. After liver transplantation, she was weaned to room air in 3 months. Patient 8 had severe pulmonary hypertension (cardiac catheterization showed mean pulmonary artery pressure of 52 mm Hg) and hypoxemia which has responded to intravenous treprostinil and sildenafil therapy. This patient also has innumerable liver lesions. Endovascular occlusion of the CPSS was considered but was deemed not to be technically possible due to the size and anatomy of the shunt. In view of this, plus the presence of at least one liver lesion with evidence of beta-catenin activation (high malignant potential), liver transplantation has been recommended.
Liver Tumors
Intrahepatic masses were identified in eight patients (73%), and six patients (55%) had multiple masses (Fig. 2). During our study review, Patient 5 was noted to have a 9-mm hypervascular liver lesion which was not reported at the time of her original CT scan; repeat MRI 2 years after endovascular occlusion of her CPSS demonstrated that the lesion had decreased to 6 mm in size. The remaining seven patients initially underwent biopsies of one or more lesions, followed by resection in Patients 6 and 10 (one lesion each), radiofrequency ablation in Patient 10 (one lesion), or liver transplantation (Patients 7 and 9) due to concern for hepatocellular carcinoma or adenomatosis. Paraffin-embedded blocks were available for six out of seven cases. A summary of the pertinent microscopic findings and abnormal immunostains, including histologic classification of lesions according to World Health Organization (WHO) system, is provided in Table 3.
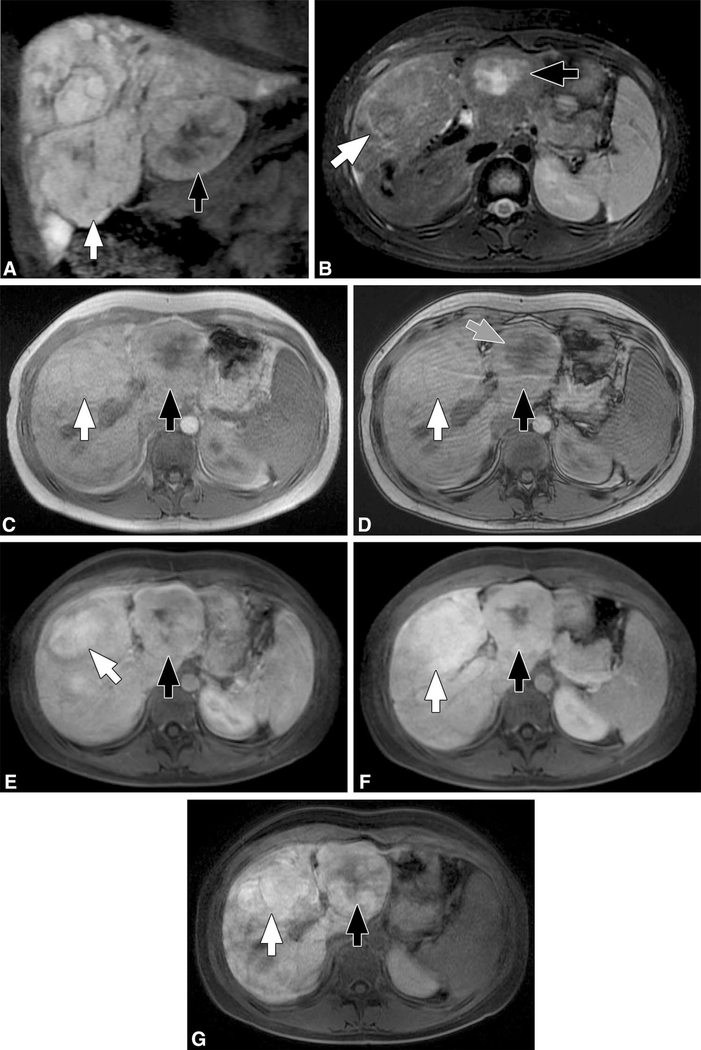
Fig. 2.
Patient 8. Coronal spoiled 3D gradient echo postcontrast image (a) obtained during the hepatocyte phase (20-min delay) following administration of gadoxetate disodium (Eovist®, Bayer, Whippany, NJ) shows innumerable hepatic lesions with variable retention of the contrast material. The two larger lesions (arrows) were biopsied. Both lesions were inflammatory adenomas with the lesion on the right (white arrow) showing β-catenin activation. Both lesions show some retention of contrast material during the hepatocyte phase, a feature of FNH. However, findings on other sequences including: suggestion of an atoll sign (rim of T2 hyperintense signal) surrounding the lesion on the right (white arrow) and with central irregular T2 signal in the lesion on the left (black arrow) on axial T2 weighted imaging (b) and focal loss of signal on opposed phase imaging in the lesion on the left (not shown) suggesting focal steatosis, suggests that these are, in fact, adenomas
Table 3.
Histopathologie description of biopsied liver lesions including microscopio findings and abnormal immunostains per the World Health Organization (WHO) System
| Patient | Lesion(s) | Features | Intervention |
|---|---|---|---|
| 4 | Inflammatory adenomaa | Hepatocellular lesion with foci of fibrous septae, ductular proliferation, absent portal veins, unpaired arteries, rare dystrophic artery profiles, foci of inflammation, pseudoacini change, foci of atypia, and sinusoidal dilatation | Catheter-based shunt occlusion |
| 6 | Early well-differentiated hepatocellular carcinoma with HNF1αb | No capsule, bland morphology, unpaired arteries, no steatosis, focal thickening of hepatocellular plates to 4–5 cells, areas with portal tract and vascular invasion, foci of pseudoacinar transformation, and necrosis (FABP—and patchy loss of reticulin) | Surgical resection of lesions and shunt ligation |
| 7 | 1. Multifocal well-differentiated (Edmondson grade 1–2) hepatocellular carcinoma, largest 4.5 cm, arising in β-catenin-mutated adenomas 2. HNF1α-inactivated adenoma (4.3 cm) 3. β-catenin-activated adenomas (innumerable) |
1. Capsule, nuclear atypia, portal tract invasion, patchy foci of reticulin loss (diffuse + GS and + nuclear β-catenin) 2. Bland morphology, glycogenated nuclei, patchy steatosis, unpaired arteries, no portal tracts (FABP −) 3. Pseudoacini, cytological atypia (diffuse + GS and nuclear β-catenin +) |
OLT |
| 8 | 1. Innumerable lesions, MR features of FNH 2. Biopsy: Inflammatory adenoma [right lobe] 3. Biopsy: Inflammatory adenoma with β-catenin activation [left lobe] |
2. Mild nuclear atypia, conspicuous nucleoli, foci of lobular inflammation, focal sinusoidal dilatation, ductular proliferation, CRP + 3. Mild nuclear atypia, conspicuous nucleoli, foci of lobular inflammation, focal sinusoidal dilatation, ductular proliferation, + GS, nuclear β-catenin +, and CRP + | OFT recommended |
| 9 | 1. Multifocal well-differentiated HCC (Edmondson grade 1–2) arising in β-catenin-activated [x3] and HNF1α-inactivated [x1] adenomas 2. HNF1α-inactivated adenoma [x1] 3. Multiple β-catenin-activated adenomas |
1. AFF had capsule, lymphovascular and portal tract invasion, patchy foci of reticulin loss (β-catenin-activated: diffuse + GS and nuclear β-catenin-Hand HNF1α-inactivated: FABP −) 2. Bland morphology, glycogenated nuclei, patchy steatosis, unpaired arteries, no portal tracts (FABP −) 3. Pseudoacini, cytological atypia (diffuse + GS and nuclear β-catenin +) |
OLT |
| 10 | 1. HNF1α-inactivated adenoma [x1, resection, left lobe, segment 3]b
2. Numerous microscopic nodules with β-catenin activation [resection, left lobe, segment 3 resection] 3. β-catenin-activated adenoma [x1, right lobe, biopsy only] |
1. Diffuse steatosis, foci of nuclear atypia (FABP −) 2. Not apparent on gross specimen examination. Bland morphology, no portal areas, disorganized hepatocyte plates (diffuse + GS and nuclear β-catenin −). No steatosis, unpaired arteries, bland morphology 3. Pseudoacini, cytological atypia (diffuse + GS and nuclear β-catenin +) |
Catheter-based shunt occlusion Surgical resection, left lobe, segment 3 Right lobe lesion radiofrequency ablation |
| 11 | 1. Inflammatory adenoma with β-catenin activation; suspicious for portal tract invasion [x1, right lower lobe periphery] 2. Inflammatory adenoma with β-catenin activation; suspicious for portal tract invasion [x1, right central lobe] |
Both lesions Well-differentiated liver tumor composed of mature hepatocytes arranged in 2–3-cell-thick mildly disorganized cell plates, mild cytological atypia, few scattered unpaired arteries, foci of inflammation, dilated sinusoids, and focal area suspicious for portal tract invasion (diffuse + GS, nuclear β-catenin −, CRP +, and patchy loss) | Fisted for OFT |
FNH focal nodular hyperplasia, FABP fatty acid binding protein, GS glutamine synthase, CRP C-reactive protein, HCC hepatocellular carcinoma, OLT orthotopic liver transplantation
Immunostains including C-reactive protein and others were not performed at the initial assessment, and tissue to perform further staining was not available
Differing from the initial biopsy, the tumor further showed acquired β-catenin activation by immunostains characterized by diffuse strong GS overexpression consistent with WNT/B-catenin pathway activation; β-catenin nuclear expression not seen
The most common liver lesion was hepatocellular adenoma (six of seven patients), with β-catenin-activated adenomas being the most common subtype (five of six patients) (Fig. 3a–e) [9]. A total of five inflammatory adenomas were identified in three patients (Fig. 3f). Three of the four inflammatory adenomas (case 4 could not be worked up) also demonstrated β-catenin activation (diffuse glutamine synthetase expression and ± nuclear β-catenin positivity) and both of the lesions in Patient 11 had foci suspicious for stromal portal invasion within the lesion (eHCC).
Fig. 3.
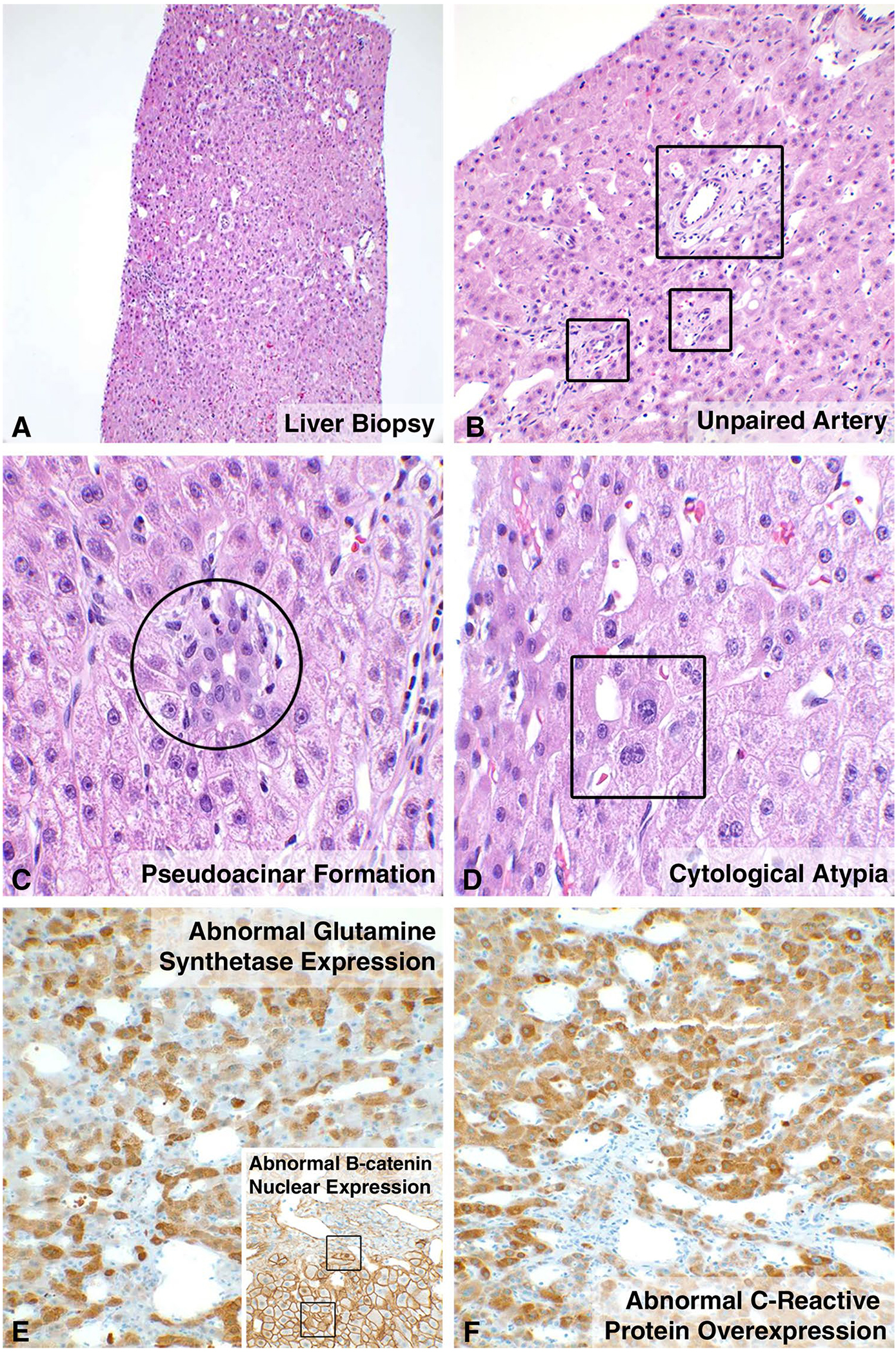
Histology of β-catenin-activated and inflammatory adenomas. Lesions from five patients showed β-catenin-activated adenomas. Liver needle core biopsy shows a lesion lacking portal tracts, with scattered mildly dilated sinusoids, suggestion of unpaired arteries, and foci of inflammation (a). High magnification confirms scattered unpaired arteries (b), pseudoacini (c), and mild cytological atypia (d). Immunostains for glutamine synthetase (e) and β-catenin (e insert) demonstrate an abnormal strong, checkboard-like pattern of expression and patchy nuclear positivity, respectively, within the tumor cells consistent with β-catenin-activated adenomas. Biopsy of right lobe lesion in Patient 8 showed the same histology findings as described above with the addition of diffuse, strong overexpression of C-reactive protein (f) with the lesional cells consistent with inflammatory adenoma with β-catenin activation
Three patients (Patients 6, 7, and 9) had lesions consistent with early to well-differentiated (Edmondson grade 1–2) hepatocellular carcinoma arising in either β-catenin-activated (Patients 7 and 9) or HNF1α-inactivated (Patients 6 and 9) adenomas (Fig. 4). The resected adenoma in Patient 6 showed expansion of the cell plates (greater than 2–3 cell plate thickness), patchy loss of reticulin, and strong, diffuse glutamine synthetase expression suggesting an acquired Wnt/β-catenin pathway activation. In addition, correlative next gene sequencing for Patient 7 demonstrated CTNNB1 (confirming β-catenin mutation) and BRIP1 alteration in the hepatocellular carcinoma, and for Patient 11 showed BCL6, MLL, and MYCN variants with unknown significance. In Patient 11, glutamine synthetase was diffusely positive within the adenoma in the biopsy consistent with β-catenin pathway activation in the absence of a CTNNB1 mutation [4, 6]. This may suggest another component of the WNT/β-catenin pathway is involved with tumorigenesis. All seven patients with liver lesions had normal serum alpha fetoprotein levels.
Fig. 4.
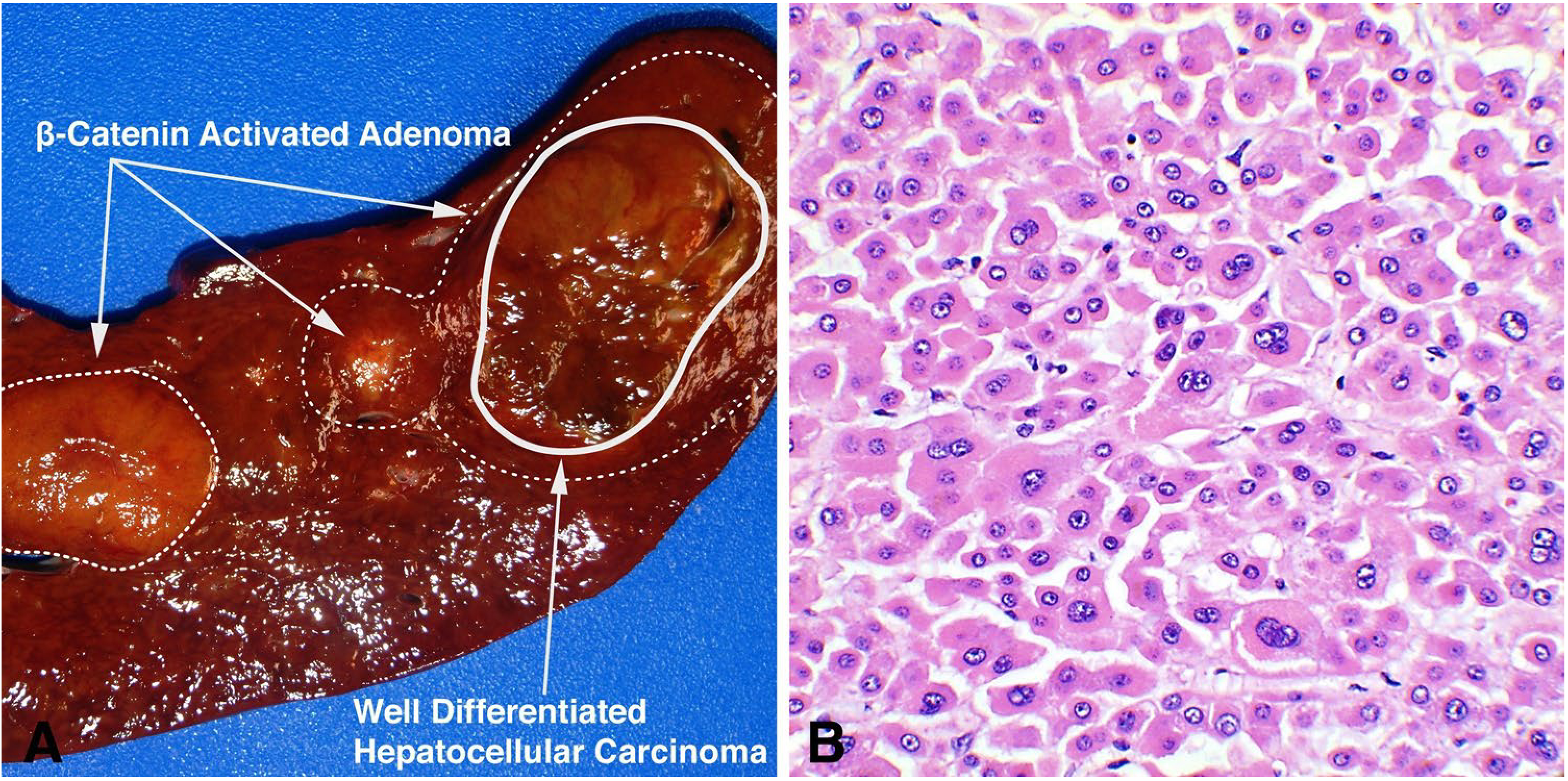
Hepatocellular carcinoma arising in a β-catenin-activated adenoma. Sections from a liver explant (Patient 7) demonstrates two β-catenin-activated adenomas with one of these lesions demonstrating a “nodule within a nodule” pattern characteristic of hepatocellular carcinoma arising within an adenoma (a). Histology confirms the central lesion as a well-differentiated hepatocellular carcinoma, Edmondson score of 1–2 (b)
Interventions to Occlude CPSS
Endovascular interventions targeting the shunt for occlusion were performed in three patients (36%). Patient 4 had a large circuitous shunt vessel (superior mesenteric vein to left iliac vein), which was occluded in two stages (Fig. 5). In stage 1, via femoral venous approach, two Amplatzer Vascular Plug II (AVPII; St. Jude Medical, St. Paul, MN) devices were deployed into the caudal aspect of the shunt resulting in decreased shunt flow. A second endovascular procedure was performed 6 weeks later, at which time complete shunt occlusion was achieved by endovascular deployment of Nester embolization coils (Cook Medical, Bloomington, IN) and Complex Helical-18 Fibered Platinum coils (Boston Scientific Corporation, Marlborough, MA). Follow-up MRI 5 months after complete shunt occlusion showed a decrease in size of a known inflammatory adenoma from 4.4 to 2.3 cm. Surveillance ultrasounds have shown no residual lesion.
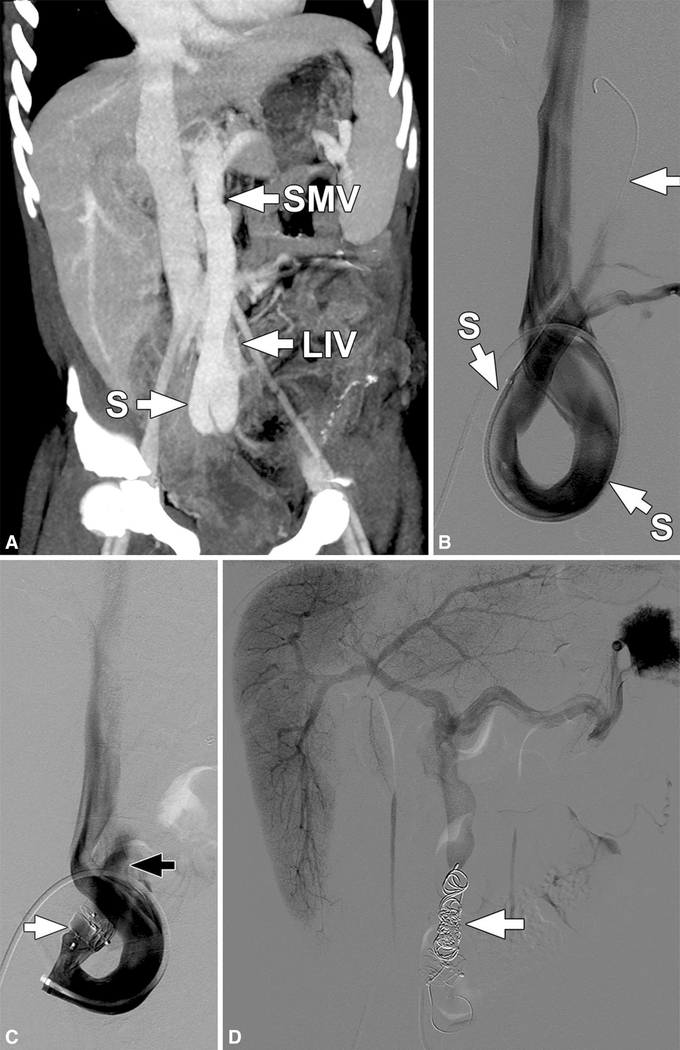
Fig. 5.
Patient 4. Coronal oblique maximum intensity projection (MIP) image (a) from a CT of the abdomen performed with intravenous contrast in the portal venous phase shows a large shunt (S) between the superior mesenteric vein (SMV) and the left common iliac vein (LIV). Subsequently performed catheter venogram (b) with the catheter coursing over the iliac vein confluence, through the left iliac vein and into the circuitous shunt (S). The wire (arrow) extends up through the shunt toward the SMV (not opacified). c Catheter venogram performed after deployment of two Amplatzer vascular plugs (white arrow) shows incomplete occlusion of the shunt with some contrast visible on the mesenteric venous side of the plugs (black arrow). d Splenoportogram performed after second-stage coil embolization (arrow) of the shunt shows no residual connection between the mesenteric venous system and left common iliac vein and demonstrates good opacification of the main portal vein and its intrahepatic branches
Patients 5 and 10 underwent endovascular shunt occlusion in single stages using AVPII, and both patients have had decreasing size of their liver lesions documented on surveillance MR imaging.
Patient 6 underwent surgical ligation of his large portocaval shunt at the time of resection of his large liver masses by a left lateral sectionectomy; he has had no recurrence of liver lesions at follow-up of nearly 6 years after resection.
Discussion
Congenital portosystemic shunts are rare. Associations with other syndromes and anomalies continue to be recognized. This is important since specific investigation for CPSS is often prompted by the recognition of known associations. Several forms of congenital heart disease, various vascular anomalies, and heterotaxy are clearly linked to CPSS in our series and in the literature [2, 10–18]. In addition, our series shows an association with Turner syndrome, and associations with other syndromes, such as Down syndrome and Noonan syndrome, have been reported [2, 18–23].
Genitourinary and abdominal wall anomalies may also be associated with CPSS, with our series being the first to show the association of CPSS with the omphalocele, exstrophy, imperforate anus, spinal defects (OEIS) complex. Several reported cases of CPSS have musculoskeletal abnormalities including polydactyly, thumb/radial hypoplasia, rib anomalies, vertebral and sacral anomalies, and spina bifida occulta [17, 19, 24–27].
When CPSS is suspected, cross-sectional imaging of the abdomen (CT or MRI) is indicated as the initial investigation to assess for the presence of a shunt, evaluate the anatomy of the shunt, evaluate the patency of the portal vein, evaluate for liver masses, and assess for associated anomalies. In patients with EHPS shunt, the portal vein may be absent or patent which is a critical distinction. Surgical ligation or endovascular occlusion of CPSS is a potential intervention for patients with a patent portal vein, even if the extra- and intrahepatic portal venous systems are extremely hypoplastic [3]. When ligation or endovascular occlusion is considered, the shunt may need to be occluded in stages, in order to avoid a dangerous increase in portal pressure. Protocols for staged closure of CPSS have been described by other centers [19, 28]. Liver transplantation is an important therapeutic option, especially in patients with innumerable liver masses with increased risk of malignant transformation, and in patients with an absent portal vein.
Patient 5 highlights the importance of careful characterization of the portal vein. This patient was initially thought to have an absent portal vein by computed tomography, but was found to have a patent but hypoplastic extrahepatic portal vein by transjugular retrograde cannulation of the shunt vessel with venography. This finding made endovascular occlusion of the CPSS an option, which was accomplished in one stage without an excessive increase in portal pressure. There are other reports of CPSS in which a hypoplastic native portal vein is identified only by angiography and some authors believe a hypoplastic portal vein is present in all cases [29]. Cases like these suggest that catheter-based venography should be strongly considered prior to intervention in patients with CPSS and an apparently absent portal vein, to definitively characterize the patency of the portal vein and thus determine options for intervention.
Hepatic encephalopathy, hepatopulmonary syndrome, and pulmonary hypertension are well-documented complications of CPSS and warrant intervention to obliterate the shunt (shunt occlusion if there is a patent portal vein or liver transplant if the portal vein is absent). Our data show that such complications can present at very early ages. We identified severe hepatopulmonary syndrome in a 4-month-old and severe pulmonary hypertension in a 12-year-old. Several reports of hepatopulmonary syndrome and pulmonary hypertension in children with CPSS within the first decade of life exist in the literature. Both hepatopulmonary syndrome and pulmonary hypertension have been reported to improve or resolve following surgical ligation or endovascular occlusion of the shunt or by liver transplantation [14–16, 19, 24, 29–32]. Indeed, Patient 2 in our series showed definitive reversal and resolution of hepatopulmonary syndrome following liver transplantation.
There is a known association between CPSS and intrahepatic masses [1]. The cause of lesion development in patients with CPSS is unknown. Hypothetically, the abnormal hepatic vasculature (i.e., abnormal systemic shunting, intermittent blood flow, regurgitation) may result in alternating hypoxia and/or hyperoxia, and disturbed micronutrient balance [33]. This in turn can result in altered glucose uptake, ATP, and pH imbalance, and it may provide decreased opportunity for nutrient and waste product exchange resulting in tissue acidosis. Previously reported liver lesions include focal nodular hyperplasia, regenerative nodules/nodular regenerative hyperplasia, hepatic adenomas, hemangiomas, and hepatocellular carcinoma [21, 34]. A recent report describes two young children with CPSS and hepatoblastoma. In one case, the primary mass was initially misdiagnosed as nodular regenerative hyperplasia by computed tomography (CT) scan prior to biopsy [35]. This mischaracterization by imaging exemplifies the fact that liver lesions may, to some degree, mimic one another on imaging, particularly with regard to retaining contrast during the hepatocyte phase of imaging when using a hepatocyte-specific contrast agent [36]. In our series, Patient 8 exemplifies this observation. This patient had innumerable liver lesions by imaging that had features that overlap with FNH; however, biopsied lesions have been shown to be inflammatory adenomas (Table 3) with and without β-catenin activation, the latter of which confers risk of malignant transformation [4, 6]. Imaging mimicry, the fact that in many reports the described imaging features are not typical for the reported lesion (e.g., T1 hyperintensity in focal nodular hyperplasia), and the fact that many lesions reported in the literature were incompletely microscopically characterized (no immunohistochemistry) raises questions about the correct classification of previously reported lesions in patients with CPSS. We recommend yearly screening liver imaging in patients with CPSS. When lesions are identified, strong consideration should be given to biopsy of liver lesions, in order to definitively characterize them and include/exclude premalignant adenomas and HCC.
To our knowledge, the histologic phenotypic properties of adenomas in children with CPSS have not been previously reported. In our series, there were five patients with β-catenin-activated adenomas in three of whom histological changes fulfilled criteria for a classification of early- to well-differentiated hepatocellular carcinoma according to WHO criteria. Our findings suggest that adenomas discovered in children with CPSS should be carefully evaluated for evidence of β-catenin activation. Once identified, β-catenin-activated adenomas should be ablated or resected, or liver transplant considered if such lesions cannot be safely ablated or resected, due to the risk of malignant transformation.
The association between CPSS and malignant lesions (hepatoblastoma and HCC) both in prior reports and in our series deserves expanded discussion. There are four reported cases of hepatoblastoma in patients with CPSS, all under the age of 10 [12, 37–39]. Hepatocellular carcinoma has been reported in 13 patients with CPSS [19, 21, 25, 34, 40–47]. All but three of the previously reported cases occurred in adult patients between 19 and 68 years of age. Three children with CPSS have been reported with HCC, a 10-year-old, an 8-year-old, and a 12-month-old; our series adds three additional pediatric patients ranging in age from 10 to 12 years. Nearly all reported tumors were moderately to well-differentiated where histology was reported with one reported to be focally poorly differentiated, and in no case was metastatic disease present at the time of diagnosis or during follow-up (maximum 4 years) [43]. Importantly, in one of the adult cases, evidence of hepatitis B infection was present in the background liver [44]. Of note, a 19-year-old patient with HCC was found at autopsy to have multifocal liver disease, similar to Patients 7 and 9 in our series, but no metastases.
Congenital portosystemic shunt is a rare malformation that is associated with multiple clinical associations. Intervention may not be warranted in the absence of complications. Serious complications of CPSS may manifest in early childhood years, and vigilance for signs and symptoms of encephalopathy, hepatopulmonary syndrome, and pulmonary hypertension should be maintained, as the occurrence of these complications warrants intervention to obliterate the shunt. Cross-sectional imaging is critical to identify and characterize the shunt, define patency of the portal vein, and to screen for liver lesions which may be benign or malignant. The most diminutive portal veins may require interventional venography to identify as this has significant implications for management. Vigilance should be maintained regarding identified liver lesions, and immunohistochemical staining should be routine in the CPSS population.
Funding
No funding was received for this work.
Footnotes
Extended author information available on the last page of the article
Compliance with Ethical Standards
Conflict of interest The authors do not have any relevant disclosures of potential conflicts of interest.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bernard O, Franchi-Abella S, Branchereau S, et al. Congenital portosystemic shunts in children: recognition, evaluation, and management. Semin Liver Dis. 2012;32:273–287. [DOI] [PubMed] [Google Scholar]
- 2.Sokollik C, Bandsma RH, Gana JC, et al. Congenital portosystemic shunt: characterization of a multisystem disease. J Pediatr Gastroenterol Nutr. 2013;56:675–681. [DOI] [PubMed] [Google Scholar]
- 3.Blanc T, Guerin F, Franchi-Abella S, et al. Congenital portosystemic shunts in children: a new anatomical classification correlated with surgical strategy. Ann Surg. 2014;260:188–198. [DOI] [PubMed] [Google Scholar]
- 4.Bioulac-Sage P, Rebouissou S, Thomas C, et al. Hepatocellular adenoma subtype classification using molecular markers and immunohistochemistry. Hepatology. 2007;46:740–748. [DOI] [PubMed] [Google Scholar]
- 5.Nault JC, Bioulac-Sage P, Zucman-Rossi J. Hepatocellular benign tumors-from molecular classification to personalized clinical care. Gastroenterology. 2013;144:888–902. [DOI] [PubMed] [Google Scholar]
- 6.Zucman-Rossi J, Jeannot E, Nhieu JT, et al. Genotype-phenotype correlation in hepatocellular adenoma: new classification and relationship with HCC. Hepatology. 2006;43:515–524. [DOI] [PubMed] [Google Scholar]
- 7.Wang HL, Anatelli F, Zhai QJ, et al. Glypican-3 as a useful diagnostic marker that distinguishes hepatocellular carcinoma from benign hepatocellular mass lesions. Arch Pathol Lab Med. 2008;132:1723–1728. [DOI] [PubMed] [Google Scholar]
- 8.International Consensus Group for Hepatocellular Neoplasia, The International Consensus Group for Hepatocellular N, Yuvi K. Pathologic diagnosis of early hepatocellular carcinoma: a report of the international consensus group for hepatocellular neoplasia. Hepatology. 2009;49:658–664. [DOI] [PubMed] [Google Scholar]
- 9.Sempoux C, Paradis V, Komuta M, et al. Hepatocellular nodules expressing markers of hepatocellular adenomas in Budd-Chiari syndrome and other rare hepatic vascular disorders. J Hepatol. 2015;63:1173–1180. [DOI] [PubMed] [Google Scholar]
- 10.Chiu SN, Chien YH, Wu MH, et al. Transcatheter closure of portal-systemic shunt combining congenital double extrahepatic inferior vena cava with vascular plug. J Pediatr. 2008;153:723. [DOI] [PubMed] [Google Scholar]
- 11.Howard ER, Davenport M. Congenital extrahepatic portocaval shunts—the Abernethy malformation. J Pediatr Surg. 1997;32:494–497. [DOI] [PubMed] [Google Scholar]
- 12.Murray CP, Yoo SJ, Babyn PS. Congenital extrahepatic portosystemic shunts. Pediatr Radiol. 2003;33:614–620. [DOI] [PubMed] [Google Scholar]
- 13.Nagata H, Yamamura K, Ikeda K, et al. Balloon-occluded retrograde transvenous obliteration for congenital portosystemic venous shunt: report of two cases. Pediatr Int. 2012;54:419–421. [DOI] [PubMed] [Google Scholar]
- 14.Newman B, Feinstein JA, Cohen RA, et al. Congenital extrahepatic portosystemic shunt associated with heterotaxy and polysplenia. Pediatr Radiol. 2010;40:1222–1230. [DOI] [PubMed] [Google Scholar]
- 15.Passalacqua M, Lie KT, Yarmohammadi H. Congenital extrahepatic portosystemic shunt (Abernethy malformation) treated endovascularly with vascular plug shunt closure. Pediatr Surg Int. 2012;28:79–83. [DOI] [PubMed] [Google Scholar]
- 16.Raghuram KA, Bijulal S, Krishnamoorthy KM, et al. Regression of pulmonary vascular disease after therapy of Abernethy malformation in visceral heterotaxy. Pediatr Cardiol. 2013;34:1882–1885. [DOI] [PubMed] [Google Scholar]
- 17.Ratnasamy C, Kurbegov A, Swaminathan S. Cardiac anomalies in the setting of the Abernethy malformation of the portal vein. Cardiol Young. 2007;17:212–214. [DOI] [PubMed] [Google Scholar]
- 18.Stringer MD. The clinical anatomy of congenital portosystemic venous shunts. Clin Anat. 2008;21:147–157. [DOI] [PubMed] [Google Scholar]
- 19.Franchi-Abella S, Branchereau S, Lambert V, et al. Complications of congenital portosystemic shunts in children: therapeutic options and outcomes. J Pediatr Gastroenterol Nutr. 2010;51:322–330. [DOI] [PubMed] [Google Scholar]
- 20.Konstas AA, Digumarthy SR, Avery LL, et al. Congenital portosystemic shunts: imaging findings and clinical presentations in 11 patients. Eur J Radiol. 2011;80:175–181. [DOI] [PubMed] [Google Scholar]
- 21.Morotti RA, Killackey M, Shneider BL, et al. Hepatocellular carcinoma and congenital absence of the portal vein in a child receiving growth hormone therapy for turner syndrome. Semin Liver Dis. 2007;27:427–431. [DOI] [PubMed] [Google Scholar]
- 22.Noe JA, Pittman HC, Burton EM. Congenital absence of the portal vein in a child with Turner syndrome. Pediatr Radiol. 2006;36:566–568. [DOI] [PubMed] [Google Scholar]
- 23.Pipitone S, Garofalo C, Corsello G, et al. Abnormalities of the umbilico-portal venous system in Down syndrome: a report of two new patients. Am J Med Genet A. 2003;120A:528–532. [DOI] [PubMed] [Google Scholar]
- 24.Hori T, Yonekawa Y, Okamoto S, et al. Pediatric orthotopic living-donor liver transplantation cures pulmonary hypertension caused by Abernethy malformation type Ib. Pediatr Transplant. 2011;15:e47–e52. [DOI] [PubMed] [Google Scholar]
- 25.Lisovsky M, Konstas AA, Misdraji J. Congenital extrahepatic portosystemic shunts (Abernethy malformation): a histopathologic evaluation. Am J Surg Pathol. 2011;35:1381–1390. [DOI] [PubMed] [Google Scholar]
- 26.Ogul H, Bayraktutan U, Yalcin A, et al. Congenital absence of the portal vein in a patient with multiple vascular anomalies. Surg Radiol Anat. 2013;35:529–534. [DOI] [PubMed] [Google Scholar]
- 27.Singhal M, Lal A, Thapa BR, et al. Congenital atresia of portal vein with portocaval shunt associated with cardiac defects, skeletal deformities, and skin lesions in a boy. J Pediatr Surg. 2008;43:e25–e28. [DOI] [PubMed] [Google Scholar]
- 28.Lautz TB, Tantemsapya N, Rowell E, et al. Management and classification of type II congenital portosystemic shunts. J Pediatr Surg. 2011;46:308–314. [DOI] [PubMed] [Google Scholar]
- 29.Kuo MD, Miller FJ, Lavine JE, et al. Exploiting phenotypic plasticity for the treatment of hepatopulmonary shunting in Abernethy malformation. J Vasc Interv Radiol. 2010;21:917–922. [DOI] [PubMed] [Google Scholar]
- 30.Iida T, Ogura Y, Doi H, et al. Successful treatment of pulmonary hypertension secondary to congenital extrahepatic portocaval shunts (Abernethy type 2) by living donor liver transplantation after surgical shunt ligation. Transpl Int. 2010;23:105–109. [DOI] [PubMed] [Google Scholar]
- 31.Morikawa N, Honna T, Kuroda T, et al. Resolution of hepatopulmonary syndrome after ligation of a portosystemic shunt in a pediatric patient with an Abernethy malformation. J Pediatr Surg. 2008;43:e35–e38. [DOI] [PubMed] [Google Scholar]
- 32.Tercier S, Delarue A, Rouault F, et al. Congenital portocaval fistula associated with hepatopulmonary syndrome: ligation vs liver transplantation. J Pediatr Surg. 2006;41:e1–e3. [DOI] [PubMed] [Google Scholar]
- 33.Vaupel P, Kallinowski F, Runkel S, et al. Blood flow and oxygen consumption rates of human gynecological tumors xenografted into rnu/rnu-rats. Strahlenther Onkol. 1989;165:502. [PubMed] [Google Scholar]
- 34.Sharma R, Suddle A, Quaglia A, et al. Congenital extrahepatic portosystemic shunt complicated by the development of hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2015;14:552–557. [DOI] [PubMed] [Google Scholar]
- 35.Lautz TB, Shah SA, Superina RA. Hepatoblastoma in children with congenital portosystemic shunts. J Pediatr Gastroenterol Nutr. 2016;62:542–545. [DOI] [PubMed] [Google Scholar]
- 36.Trout AT, Towbin AJ, Smith EA, et al. Hepatocyte-specific contrast media: not so simple. Pediatr Radiol. 2018;48:1245–1255. [DOI] [PubMed] [Google Scholar]
- 37.Barton JW 3rd, Keller MS. Liver transplantation for hepatoblastoma in a child with congenital absence of the portal vein. Pediatr Radiol. 1989;20:113–114. [DOI] [PubMed] [Google Scholar]
- 38.Kawano S, Hasegawa S, Urushihara N, et al. Hepatoblastoma with congenital absence of the portal vein—a case report. Eur J Pediatr Surg. 2007;17:292–294. [DOI] [PubMed] [Google Scholar]
- 39.Marois D, van Heerden JA, Carpenter HA, et al. Congenital absence of the portal vein. Mayo Clin Proc. 1979;54:55–59. [PubMed] [Google Scholar]
- 40.Asran MK, Loyer EM, Kaur H, et al. Case 177: congenital absence of the portal vein with hepatic adenomatosis. Radiology. 2012;262:364–367. [DOI] [PubMed] [Google Scholar]
- 41.Banz V, Olliff S, Taniere P, et al. Liver tumours in patients with Abernethy malformation. ANZ J Surg. 2011;81:640–641. [DOI] [PubMed] [Google Scholar]
- 42.Benedict M, Rodriguez-Davalos M, Emre S, et al. Congenital extrahepatic portosystemic shunt (Abernethy malformation type Ib) with associated hepatocellular carcinoma: case report and literature review. Pediatr Dev Pathol. 2017;20:354–362. [DOI] [PubMed] [Google Scholar]
- 43.Joyce AD, Howard ER. Rare congenital anomaly of the portal vein. Br J Surg. 1988;75:1038–1039. [DOI] [PubMed] [Google Scholar]
- 44.Lundstedt C, Lindell G, Tranberg KG, et al. Congenital absence of the intrahepatic portion of the portal vein in an adult male resected for hepatocellular carcinoma. Eur Radiol. 2001;11:2228–2231. [DOI] [PubMed] [Google Scholar]
- 45.Pichon N, Maisonnette F, Pichon-Lefievre F, et al. Hepatocarcinoma with congenital agenesis of the portal vein. Jpn J Clin Oncol. 2003;33:314–316. [DOI] [PubMed] [Google Scholar]
- 46.Scheuermann U, Foltys D, Otto G. Focal nodular hyperplasia precedes hepatocellular carcinoma in an adult with congenital absence of the portal vein. Transpl Int. 2012;25:e67–e68. [DOI] [PubMed] [Google Scholar]
- 47.Yoshidome H, Edwards MJ. An embryological perspective on congenital portacaval shunt: a rare anomaly in a patient with hepatocellular carcinoma. Am J Gastroenterol. 1999;94:2537–2539. [DOI] [PubMed] [Google Scholar]