Abstract
Background
There are no clear criteria for antifungal de-escalation after initial empirical treatments. We hypothesized that early de-escalation (ED) (within 5 days) to fluconazole is safe in fluconazole-susceptible candidemia with controlled source of infection.
Methods
This is a multicenter post hoc study that included consecutive patients from 3 prospective candidemia cohorts (2007–2016). The impact of ED and factors associated with mortality were assessed.
Results
Of 1023 candidemia episodes, 235 met inclusion criteria. Of these, 54 (23%) were classified as the ED group and 181 (77%) were classified as the non-ED group. ED was more common in catheter-related candidemia (51.9% vs 31.5%; P = .006) and episodes caused by Candida parapsilosis, yet it was less frequent in patients in the intensive care unit (24.1% vs 39.2%; P = .043), infections caused by Nakaseomyces glabrata (0% vs 9.9%; P = .016), and candidemia from an unknown source (24.1% vs 47%; P = .003). In the ED and non-ED groups, 30-day mortality was 11.1% and 29.8% (P = .006), respectively. Chronic obstructive pulmonary disease (odds ratio [OR], 3.97; 95% confidence interval [CI], 1.48–10.61), Pitt score > 2 (OR, 4.39; 95% CI, 1.94–9.20), unknown source of candidemia (OR, 2.59; 95% CI, 1.14–5.86), candidemia caused by Candida albicans (OR, 3.92; 95% CI, 1.48–10.61), and prior surgery (OR, 0.29; 95% CI, 0.08–0.97) were independent predictors of mortality. Similar results were found when a propensity score for receiving ED was incorporated into the model. ED had no significant impact on mortality (OR, 0.50; 95% CI, 0.16–1.53).
Conclusions
Early de-escalation is a safe strategy in patients with candidemia caused by fluconazole-susceptible strains with controlled source of bloodstream infection and hemodynamic stability. These results are important to apply antifungal stewardship strategies.
Keywords: antifungal, candidemia, de-escalation, invasive candidiasis, outcome
Early de-escalation (within 5 days of candidemia onset) was proven to be safe in episodes caused by fluconazole-susceptible strains with a controlled source of candidemia and hemodynamic stability. These results might help strengthen antifungal stewardship strategies.
Candidemia is a frequent nosocomial complication, which occurs especially in immunocompromised hosts and patients hospitalized in intensive care units (ICUs), with a prevalence of 6.9 per 1000 admitted patients according to a recent survey [1, 2]. High infection-related morbidity and mortality (attributable mortality ranging from 10% to 49%) [3], as well as suboptimal diagnostic tools, have driven the overuse of antifungal drugs as both therapy and prophylaxis for candidemia.
Current guidelines recommend echinocandins as initial empirical treatment in moderate-to-severe illness due to the improved prognosis in some studies [4], which could be related to its fungicidal effect and broad antifungal spectrum [5, 6]. Echinocandins are preferred to fluconazole, because of possible azole resistance in Candida species, which is minimal in Candida albicans and Candida parapsilosis (the most prevalent species in Southern Europe), yet more significant in Nakaseomyces glabrata (formerly known as Candida glabrata) and Candida guilliermondii (up to 45% of tested strains), and almost universal in Pichia kudriavzevii (formerly known as Candida krusei) [7].
However, there are no universal de-escalation criteria for fluconazole-susceptible strains. The European Society for Clinical Microbiology and Infectious Diseases (ESCMID) recommends a de-escalation strategy at day 3 in stable patients [6], whereas the Infectious Diseases Society of America (IDSA) guidelines advise de-escalation after 10 days of intravenous treatment [5]. The level of these recommendations is weak due to the lack of studies regarding antifungal de-escalation safety. De-escalation provides the possibility of implementing oral step-down therapy, diminishing costs, toxicity, and drug-drug interaction, and reducing the emergence of resistant strains.
Our hypothesis is that patients with candidemia caused by fluconazole-susceptible strains that reach hemodynamic stability can undergo early de-escalation (ED) to fluconazole. Therefore, we studied the mortality of patients who underwent ED (within 5 days of a positive blood culture) and compared it to that of patients who did not de-escalate.
MATERIALS AND METHODS
Patients, Setting, Data Collection, and Study Design
This a retrospective post hoc study that included subjects from 3 prospective, collaborative cohorts of patients with candidemia. First, we included patients from a prospective candidemia surveillance cohort from Hospital Clinic (a 700-bed tertiary university hospital in Barcelona). Second, we included patients from a prospective Spanish cohort used in a prior study to create a simple prediction score to estimate the risk of candidemia caused by fluconazole-nonsusceptible strains [8]. Third, we included patients prospectively collected from a prior study that aimed to assess the epidemiology of breakthrough candidemia in the era of broad-spectrum antifungal therapies [9].
All patients were adults diagnosed with candidemia between 2007 and 2016. No interventions to optimize the outcomes of these patients had been undertaken. All patients were followed up until day 30 after candidemia, death, or discharge. For the present study, the following patients were excluded: those with fluconazole-nonsusceptible strains; those receiving empirical monotherapy with fluconazole or without antifungal treatment; those with an uncontrolled source of candidemia; those with persistent fungemia; and those who died within the first 5 days of candidemia.
The following data were obtained for all patients: age, sex, pre-existing comorbidities, prognosis of the underlying disease, prior antimicrobial therapy, prior surgery (within the last month), Charlson comorbidity index, immunosuppressive drugs, source of candidemia, leukocyte count, length of hospitalization before candidemia diagnosis, ICU admission, need for mechanical ventilation, empirical and definitive antifungal treatment, presence of shock, and mortality.
Patients were divided into 2 groups: those who underwent ED (within 5 days of the fungemia) of any antifungal to fluconazole, and those who did not. For the purpose of this study, these groups will be referred to as the ED group and non-ED group. There was no official hospital policy concerning de-escalation.
Definitions
Candidemia was defined as 1 or more blood cultures positive for Candida species and the presence of clinically apparent signs and symptoms of sepsis. Empirical antifungal therapy was defined as that initiated before blood cultures yielded any result. The source of infection was determined by an infectious disease specialist who evaluated the patient’s medical history, performed a physical examination, and assessed results obtained from microbiological tests and complementary imaging. An intravenous catheter was considered to be the source of candidemia when, in the absence of any other clinically apparent focus, any of the following criteria was present: local inflammatory signs or suppuration at the insertion site; a positive catheter tip culture with the same Candida spp as that isolated in the blood cultures; and an earlier growth in catheter-drawn blood culture by at least 2 hours when compared with venipuncture-drawn cultures. An abdominal source was defined when candidemia was simultaneous with peritonitis or a Candida spp isolate in abdominal drainage. A urinary tract source was considered when candidemia occurred in a patient with urinary tract symptoms and concomitant candiduria due to the same Candida spp. When no focal infection could be demonstrated, the source was categorized as unknown. Comorbidity was defined as a disease or treatment that could predispose patients to infection, alter defense mechanisms, or cause functional impairment, such as the following items: diabetes, chronic liver disease, chronic renal failure, active neoplastic disease, severe chronic obstructive pulmonary disease, severe cardiac disease, severe dementia, and administration of immunosuppressive drugs, including corticosteroids. Septic shock was defined as a systolic pressure under 90 mmHg that was unresponsive to fluid treatment or required vasoactive drug therapy [10].
Microbiological Methods
The microbiological diagnosis followed in the whole cohort was similar. In brief, 2 sets of 2 blood samples were collected from patients with a suspected bloodstream infection. The blood samples were processed using either a BACTEC 9240 system (Becton-Dickinson Microbiology Systems, Franklin Lakes, NJ) or BacTAlert (BioMérieux SA, Marcy L’Etoile, France) with a 5-day incubation period. If yeast cells were observed after microscopic examination of a Gram stain, blood bottles were subcultured into Sabouraud agar plates (BD BBL Stacker Plates, Heidelberg, Germany) and chromogenic media (ChromAgar BioMerieux SA, Paris, France). Yeast isolates were identified by conventional methods (biochemical methods in the first years, and matrix-assisted laser desorption ionization time-of-flight or pan-fungal polymerase chain reaction after 2010). The antifungal susceptibility of the isolates was determined in accordance with the Clinical and Laboratory Standards Institute M27-S3 document [11]. In vitro antifungal activity was studied by applying a commercial microdilution method (YeastOne Sensititre, TREK Diagnostic Systems, Independence, OH) or an Etest (bioMérieux SA, Marcy L’Etoile, France). The fluconazole minimum inhibitory concentration (MIC) was defined as the lowest drug concentration to inhibit 50% of growth compared with the growth control after 24-hour incubation at 35°C for all Candida species [12]. However, N glabrata was an exception, which was determined after 48 hours to prevent misclassification bias among the isolates [13]. Of note, Candida species isolates with an MIC ≥4 mg/L to fluconazole were considered nonsusceptible to fluconazole, except for N. glabrata, which was considered susceptible-dose dependent to fluconazole when the MIC value was ≤32 mg/L. In addition, P. kudriavzevii was considered nonsusceptible to fluconazole, regardless of the MIC value. Quality controls were performed in each center using C. parapsilosis American Type Culture Collection (ATCC) 22019 and P. kudriavzevii ATCC 6258.
Statistical Analysis
Quantitative variables are reported as median with interquartile range (IQR); categorical variables are reported as absolute numbers and percentages. To detect significant differences between groups, we used the χ 2 test or Fisher’s exact test for categorical variables, and the Student’s t test or Mann-Whitney U test for continuous variables, when deemed appropriate. Factors associated with mortality were evaluated by univariate and multivariable analyses, with the multivariable analysis including all significant variables (P < .05) from the univariate analysis. Given the lack of randomization of initial therapies, a propensity score for antifungal de-escalation was estimated using a backward stepwise logistic regression model that included variables related with de-escalation with P ≤ .125 in the univariate analysis. The following variables were included based on their presence (yes/no) during the candidemia episode: Candida species (C. albicans, C. parapsilosis, N. glabrata); central catheter at diagnosis; catheter-related and unknown source of fungemia; ICU patient; breakthrough candidemia; and human immunodeficiency virus-positive serology. The propensity score for ED was then used as a covariate in a multivariable analysis to adjust for potential confounding factors. The goodness of fit of the final multivariable model was assessed again with the Hosmer-Lemeshow test and the area under the receiver operating characteristic curve. Finally, a multivariable regression model (step-forward procedure) was used to identify independent risk factors for mortality in episodes with candidemia. All data were analyzed using SPSS software version 20.0 (IBM SPSS, Chicago, IL). Statistical significance was established at α = 0.05, and all reported P values are 2-tailed.
Patient Consent Statement
This observational study was conducted in accordance with the Declaration of Helsinki. The design of the work has been approved by local ethical committees of each participating hospital. To protect personal privacy, identifiable information in the electronic database was encrypted for each patient. Informed consent was waived due to the retrospective nature of the study, the fact that no intervention was involved, and no patient-identifiable information was included.
RESULTS
Demographics, Epidemiology, and Microbiology
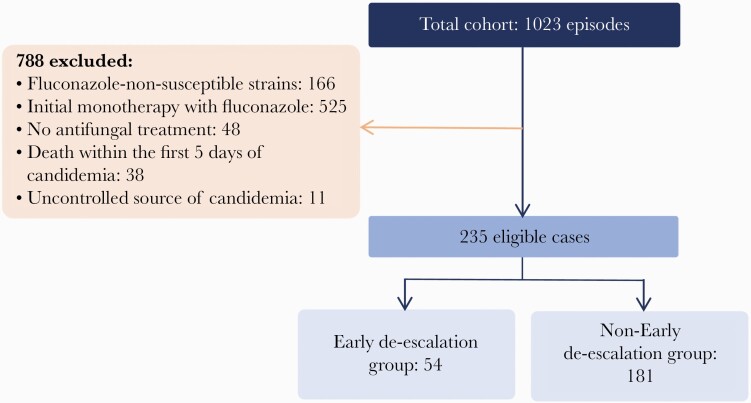
In the overall cohort, 1023 episodes of candidemia were documented over the study period. Of those, 235 patients met the inclusion criteria: 54 (23%) were classified into the ED group and 181 (77%) into the non-ED group. Figure 1 shows the study flowchart. Median time to de-escalation in the ED-group was 3.5 days (IQR, 3–5). Table 1 summarizes the demographic and clinical characteristics of the patients, as well as microbiological results, source of candidemia, and outcomes by study groups. No significant differences were documented in baseline demographics between groups. ED was less common in patients who developed candidemia in the ICU (24.1% vs 39.2%; P = .043).
Figure 1.
Study flowchart.
Table 1.
Main Characteristics and Outcomes of Patients Divided by Study Groups
| Characteristics | ED Group n = 54 (%) | Non-ED Group n = 181 (%) | Total N = 235 (%) | P Value |
|---|---|---|---|---|
| Demographics | ||||
| Male sex, n (%) | 36 (66.7) | 101 (55.8) | 137 (58.3) | .155 |
| Age (years), median (IQR) | 65.5 (55–75) | 62.2 (48–71) | 62.9 (49–72) | .617 |
| Age ≥65 years | 28 (51.9) | 83 (45.9) | 111 (47.2) | .439 |
| Comorbidities, n (%) | ||||
| Diabetes mellitus | 14 (25.9) | 37 (20.4) | 51 (21.7) | .391 |
| COPD | 7 (13) | 32 (17.7) | 39 (16.6) | .414 |
| Solid organ malignancy | 20 (37) | 58 (32.0) | 78 (33.2) | .494 |
| Hematological malignancy | 5 (9.3) | 26 (14.4) | 31 (13.2) | .331 |
| Hematopoietic stem cell transplantation | 3 (5.6) | 11 (6.1) | 14 (6.0) | 1.000 |
| Solid organ transplantation | 5 (9.3) | 21 (11.6) | 26 (11.1) | .630 |
| Chronic liver disease | 6 (11.1) | 29 (16) | 35 (14.9) | .374 |
| Chronic renal failure | 10 (18.5) | 42 (23.2) | 52 (22.1) | .467 |
| HIV infection | 0 (0.0) | 10 (5.5) | 10 (4.3) | .122 |
| Age-adjusted Charlson comorbidity index, median (IQR) | 2 (1–5) | 3 (2–4) | 3 (2–4) | .949 |
| Pitt score >2 | 14 (31.8) | 64 (42.1) | 78 (39.8) | .220 |
| Risk factors for candidemia | ||||
| Neutropenia (<500/mm3) | 5 (9.3) | 21 (11.6) | 26 (11.1) | .630 |
| Parenteral nutrition | 15 (27.8) | 75 (46) | 90 (38.3) | .157 |
| Immunosuppressant therapy | 15 (27.8) | 42 (23.1) | 57 (24.3) | .336 |
| Surgical patients | 14 (25.9) | 41 (22.6) | 55 (23.4) | .632 |
| Central venous catheter | 49 (90.7) | 173 (95.6) | 222 (94.5) | .073 |
| Previous antibiotic therapy (last month) | 41 (75.9) | 151 (83.4) | 192 (81.7) | .265 |
| ICU hospitalization | 13 (24.1) | 71 (39.2) | 84 (35.7) | .043 |
| Source of Candidemia | ||||
| Unknown | 13 (24.1) | 85 (47) | 98 (41.7) | .003 |
| Catheter-related | 28 (51.9) | 57 (31.5) | 85 (36.2) | .006 |
| Urological | 5 (9.3) | 9 (5) | 14 (6.0) | .243 |
| Abdominal | 5 (9.3) | 18 (9.9) | 23 (9.8) | .882 |
| Other sources | 3 (5.6) | 12 (6.6) | 15 (6.4) | 1.000 |
| Candida Species | ||||
| Candida albicans | 20 (37) | 91 (50.3) | 111 (47.2) | .087 |
| Nakaseomyces glabrata a | 0 (0.0) | 18 (9.9) | 18 (7.7) | .016 |
| Candida parapsilosis | 23 (42.6) | 40 (22.1) | 63 (26.8) | .003 |
| Candida tropicalis | 12 (22.2) | 27 (14.9) | 39 (16.6) | .205 |
| Other species | 2 (3.7) | 5 (2.8) | 7 (3.0) | .662 |
| Severity Indicators | ||||
| Shock | 14 (25.9) | 58 (32.2) | 72 (30.8) | .379 |
| Outcomes | ||||
| Length of hospital stay (days), median (IQR) | 19.5 (14–53) | 20 (10–37) | 20 (10–39) | .947 |
| Related mortality (7 days) | 3 (5.6) | 11 (6.1) | 14 (6.0) | 1.000 |
| Overall mortality (30 days) | 6 (11.1) | 54 (29.8) | 60 (25.5) | .006 |
Bold values indicate variables that were statistically significant.
Abbreviations: COPD, chronic obstructive pulmonary disease; ED, early de-escalation; HIV, human immunodeficiency virus; ICU, intensive care unit; IQR, interquartile range.
aFormerly known as Candida glabrata.
Most cases of candidemia were caused by C albicans (111 cases, 47%). Patients in the ED group more frequently had infection due to C. parapsilosis (42.6% vs 22.1%; P = .003) and catheter-related candidemia (51.9% vs 31.5%; P = .006), yet they less frequently presented with an infection caused by N. glabrata (0% vs 9.9%; P = .016) and candidemia from an unknown source (24.1% vs 47%; P = .003).
In addition, 30-day mortality was 25.5% (60 patients of 235): 11.1% in the ED group and 29.8% in the non-ED group (P = .006). In the ED-group, exact days until de-escalation had no impact on mortality (P = .665). The evolution of the rates of ED and mortality throughout the years is included in Supplementary Table 1 and Supplementary Table 2, respectively.
Risk Factors for Mortality
Table 2 summarizes univariate and multivariable analysis of risk factors related with 30-day mortality. Chronic obstructive pulmonary disease (OR, 3.97; 95% CI, 1.48–10.61), Pitt score > 2 (OR, 4.39; 95% CI, 1.94–9.20), unknown source of candidemia (OR, 2.59; 95% CI, 1.14–5.86), and candidemia caused by C albicans (OR, 3.92; 95% CI, 1.48–10.61) were independent predictors of mortality, whereas prior surgery (OR, 0.29; 95% CI, 0.08–0.97) was protective. The Hosmer-Lemeshow test value was 0.164. The discriminatory power of the model, as evaluated by the area under the receiver operating characteristic curve, was 0.824 (95% CI, 0.755–0.893), showing a strong ability to predict overall mortality. Similar results were found when the propensity score for receiving ED was incorporated into the model. Early de-escalation had no significant impact on the multivariable model (Supplementary Table 3 shows multivariable analysis after incorporating the propensity score to the model). Table 3 shows univariate and multivariable analyses of factors associated with early de-escalation. The goodness of fit of the propensity score was assessed with the Hosmer-Lemeshow test (P = .69). The discriminatory power of the de-escalation model, as evaluated by the area under the receiver operating curve, was 0.74 (95% CI, 0.66–0.82), showing a moderate ability to predict ED strategy.
Table 2.
Risk Factors for Overall Mortality: Univariate and Multivariable Analyses
| Risk Factor | Univariate OR (95% CI) | P Value | Multivariable OR (95% CI) | P Value |
|---|---|---|---|---|
| Male sex | 1.09 (0.60–1.98) | .766 | - | - |
| Age ≥65 years | 1.27 (0.71–2.28) | .425 | - | - |
| Diabetes mellitus | 1.46 (0.74–2.88) | .280 | - | - |
| COPD | 2.75 (1.34–5.64) | .005 | 3.97 (1.48–10.61) | .006 |
| Solid organ malignancy | 1.23 (0.67–2.27) | .508 | - | - |
| Hematological malignancy | 0.52 (0.19–1.42) | .198 | - | - |
| Hematopoietic stem cell transplantation | 1.68 (0.54–5.22) | .368 | - | - |
| Solid organ transplantation | 1.08 (0.43–2.72) | .863 | - | - |
| Chronic liver disease | 2.58 (1.22–5.45) | .011 | 2.47 (0.89–6.84) | .082 |
| Chronic renal failure | 2.50 (1.30–4.83) | .005 | 2.08 (0.83–5.19) | .118 |
| Charlson Comorbidity Index >3 | 2.24 (1.23–4.08) | .008 | 1.19 (0.48–2.94) | .714 |
| Pitt score >2 | 4.68 (2.34–9.34) | <.001 | 4.39 (1.94–9.20) | <.001 |
| Neutropenia (<500/mm3) | 1.64 (0.69–3.91) | .260 | - | - |
| Parenteral nutrition | 1.07 (0.58–1.98) | .837 | - | - |
| Immunosuppressant therapy | 2.16 (1.18–3.96) | .012 | 1.10 (0.46–2.61) | .830 |
| Surgical patients | 0.21 (0.08–0.57) | .001 | 0.29 (0.08–0.97) | .045 |
| Central venous catheter | 1.56 (0.33–7.41) | .734 | - | - |
| Previous antibiotic therapy (last month) | 1.67 (0.54–5.17) | .368 | - | - |
| ICU hospitalization | 2.54 (1.35–4.75) | .003 | 1.28 (0.50–3.31) | .607 |
| Unknown source of candidemia | 1.89 (1.04–3.41) | .034 | 2.59 (1.14–5.86) | .023 |
| Catheter-related candidemia | 0.56 (0.29–1.07) | .076 | - | - |
| Urinary source of candidemia | 0.79 (0.21–2.91) | 1.000 | - | - |
| Abdominal source of candidemia | 0.59 (0.19–1.80) | .454 | - | - |
| Candidemia by Candida albicans | 2.20 (1.20–4.00) | .009 | 3.92 (1.74–8.86) | .001 |
| Candidemia by Nakaesomyces glabrata | 0.34 (0.08–1.54) | .171 | - | - |
| Candidemia by Candida parapsilosis | 0.39 (0.18–0.86) | .017 | 1.39 (0.40–4.85) | .608 |
| Candidemia by Candida tropicalis | 1.01 (0.46–2.21) | .986 | - | - |
| Catheter removal | 0.92 (0.41–2.05) | .840 | - | - |
| Early de-escalation (within the first 5 days) | 0.29 (0.12–0.73) | .006 | 0.50 (0.16–1.53) | .223 |
| Shock | 2.44 (1.32–4.51) | .004 | 0.53 (0.18–1.55) | .248 |
Bold values indicate variables that were statistically significant.
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; ICU, intensive care unit; OR, odds ratio.
Table 3.
Univariate and Multivariable Analyses of Factors Associated With Early De-Escalation
| Risk Factor | Univariate OR (95% CI) | P Value | Multivariate OR (95% CI) | P Value |
|---|---|---|---|---|
| Male sex | 1.58 (0.84–2.37) | .155 | - | - |
| Age ≥65 years | 1.27 (0.69–2.34) | .439 | - | - |
| Diabetes mellitus | 1.36 (0.67–2.76) | .391 | - | - |
| COPD | 0.69 (0.29–1.67) | .414 | - | - |
| Solid organ malignancy | 1.25 (0.66–2.35) | .494 | - | - |
| Hematological malignancy | 0.61 (0.22–1.67) | .331 | - | - |
| Hematopoietic stem cell transplantation | 0.91 (0.24–3.38) | 1.000 | - | - |
| Solid organ transplantation | 0.78 (0.28–2.17) | .630 | - | - |
| Chronic liver disease | 0.66 (0.26–1.67) | .374 | - | - |
| HIV infection | 0.76 (0.71–0.82) | .122 | 0.01 (0.01-NE) | .999 |
| Chronic renal failure | 0.75 (0.35–1.62) | .467 | - | - |
| Charlson comorbidity index >3 | 0.97 (0.51–1.83) | .922 | - | - |
| Pitt score >2 | 0.64 (0.31–1.31) | .220 | - | - |
| Neutropenia (<500/mm3) | 0.78 (0.28–2.17) | .630 | - | - |
| Parenteral nutrition | 0.61 (0.30–1.22) | .157 | - | - |
| Immunosuppressant therapy | 1.44 (0.68–3.06) | .336 | - | - |
| Surgical patients | 1.19 (0.58–2.43) | .632 | - | - |
| Central venous catheter | 0.34 (0.10–1.16) | .073 | 0.49 (0.13–1.84) | .293 |
| Previous antibiotic therapy (last month) | 0.58 (0.22–1.52) | .265 | - | - |
| Breakthrough candidemia | 0.43 (0.18–1.03) | .053 | 0.38 (0.15–0.97) | .043 |
| ICU hospitalization | 0.49 (0.24–0.99) | .043 | 0.51 (0.24–1.08) | .079 |
| Unknown source of candidemia | 0.36 (0.18–0.71) | .003 | 0.26 (0.12–0.58) | .001 |
| Catheter-related candidemia | 2.34 (1.26–4.35) | .006 | 1.86 (0.72–4.82) | .198 |
| Urinary source of candidemia | 1.95 (0.62–6.09) | .243 | - | - |
| Abdominal source of candidemia | 0.92 (0.33–2.62) | .882 | - | - |
| Candidemia by Candida albicans | 0.58 (0.31–1.09) | .087 | 1.07 (0.44–2.62) | .885 |
| Candidemia by Nakaesomyces glabrata | 0.75 (0.70–0.81) | .016 | 0.01 (0.01-NE) | .998 |
| Candidemia by Candida parapsilosis | 2.61 (1.37–4.98) | .003 | 3.00 (1.43–6.30) | .004 |
| Candidemia by Candida tropicalis | 1.63 (0.76–3.49) | .205 | - | - |
| Catheter removal | 0.85 (0.37–1.95) | .695 | - | - |
| Shock | 0.74 (0.37–1.46) | .379 | - | - |
Bold values indicate variables that were statistically significant.
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; HIV, human immunodeficiency virus; ICU, intensive care unit; NE, not evaluable; OR, odds ratio.
DISCUSSION
The results in this study suggest that antifungal de-escalation could be a safe strategy in patients with candidemia caused by fluconazole-susceptible strains when source of infection is controlled and the patient is hemodynamically stable. Such de-escalation strategy could be implemented in patients admitted to both ICU and regular wards.
Some studies, with substantial methodological differences in comparison with ours, have tried to evaluate the impact of de-escalation in patients with candidemia or other forms of invasive candidiasis. Vazquez et al [14] evaluated an early step-down strategy from anidulafungin to an oral azole in 102 of 250 (40%) patients with candidemia or invasive candidiasis, showing response rates similar to nonearly switch. The population of early step-down strategy was less severely ill and de-escalation was performed within the first 7 days. Van der Geest et al [15] described 32 patients with invasive candidiasis belonging to a cohort of 56 patients admitted to the ICU who underwent step-down-to-fluconazole therapy (at a median of 5 days). Regarding the episodes, no differences were observed in characteristics or risk factors, and response rates and 28-day and 90-day mortality were similar in both patient groups. Furthermore, Bailly et al [16] reported a multicenter, observational study of 647 nonneutropenic patients with suspected or documented invasive candidiasis admitted to French ICUs in 2012–2013. ED was implemented in 142 (22%) patients; 70 presented with documented invasive candidiasis, 32 of whom had candidemia. No differences in 28-day mortality were documented among patients with or without early de-escalation. Only 2 prior investigations have included solely patients with candidemia. Bal et al [17] reported 19 patients with candidemia who underwent de-escalation from echinocandin or voriconazole to fluconazole within a median of 5 (3–9) days. One patient relapsed with a N. glabrata infection. Finally, in the study by Garnacho-Montero et al [4], 44 ICU patients underwent de-escalation from echinocandin to fluconazole, presenting lower 30-day and 90-day mortality. However, most of these studies had either too low of a number of patients to draw any conclusion or included different infection profiles, making extrapolation to patients with candidemia difficult. Nevertheless, this is the largest-to-date study focusing specifically on patients with candidemia.
The importance of de-escalation is the possibility of avoiding adverse events, lower treatment costs [18], and reduce selection pressure. It stands to reason that de-escalation to oral or intravenous fluconazole—as soon as when antifungal susceptibility testing states the nonresistance of an isolated strain—should not worsen the outcomes in terms of morbidity or mortality. In centers that were unable to perform on-site susceptibility testing, this form of de-escalation could also be considered when the isolated species is typically susceptible. More clinical data would be necessary to further support this strategy. In addition, independent of antifungal susceptibility, echinocandins have been shown to be superior to azoles in candidemia due to their increased fungicidal activity [19, 20]. However, this fungicidal effect is perhaps most important at infection onset when fungal load reaches its peak and decreases thereafter.
Nonetheless, our study has a clear and good methodology with some limitations. First, it was a nonrandomized, observational, retrospective, post hoc study. The absence of confounding factors cannot be assumed despite propensity score adjustment. Second, these results are only applicable in a selected group of patients. Finally, these results should be confirmed in a randomized clinical trial.
CONCLUSIONS
In summary, in adult patients with candidemia caused by fluconazole-susceptible strains who are clinically stable and have an appropriate source control, early de-escalation (within 5 day from the candidemia) seems to be a safe strategy that allows for oral step-down treatment, avoids side effects, reduces costs, and limits the emergence of resistance to drugs.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank Anthony Armenta for providing medical editing assistance for this manuscript.
Disclaimer. The funding bodies did not have a role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. This research forms part of an activity that has received funding from EIT Health. EIT Health is supported by the European Institute of Innovation and Technology (EIT), a body of the European Union that receives support from the European Union´s Horizon 2020 Research and Innovation Program. This study has been co-funded by the European Regional Development Fund. (EDRF, “A way to make Europe”). EM-G [PI18/01061], PP-A [“Juan Rodés” contract JR20/00012 and PI21/00498], MF-R [“Miguel Servet” contract CP18/00073]and CG-V [FIS PI15/00744] have received research grants from the Ministerio de Sanidad y Consumo, Instituto de Salud Carlos III. No funding bodies had any role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Potential conflicts of interest. C. G.-V. has received honoraria for talks on behalf of Gilead Science, MSD, Novartis, Pfizer, Janssen, Lilly as well as a grant from Gilead Science and MSD. A. S. has received honoraria for talks on behalf of Merck Sharp and Dohme, Pfizer, Novartis, Angellini, as well as grant support from Pfizer. P. P-A. has received honoraria for talks on behalf of Merck Sharp and Dohme, Pfizer, Gilead and Alexion. J. A. M. has received honoraria for talks on behalf of Merck Sharp and Dohme, Pfizer, Novartis and Angellini. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Kett DH, Azoulay E, Echeverria PM, Vincent JL. Candida bloodstream infections in intensive care units: analysis of the extended prevalence of infection in intensive care unit study. Crit Care Med 2011; 39:665–70. [DOI] [PubMed] [Google Scholar]
- 2. Horn DL, Neofytos D, Anaissie EJ, et al. Epidemiology and outcomes of candidemia in 2019 patients: data from the prospective antifungal therapy alliance registry. Clin Infect Dis 2009; 48:1695–703. [DOI] [PubMed] [Google Scholar]
- 3. Gudlaugsson O, Gillespie S, Lee K, et al. Attributable mortality of nosocomial candidemia, revisited. Clin Infect Dis 2003; 37:1172–7. [DOI] [PubMed] [Google Scholar]
- 4. Garnacho-Montero J, Díaz-Martín A, Cantón-Bulnes L, et al. Initial antifungal strategy reduces mortality in critically ill patients with candidemia: a propensity score-adjusted analysis of a multicenter study. Crit Care Med 2018; 46:384–93. [DOI] [PubMed] [Google Scholar]
- 5. Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 2016; 62:e1–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cornely OA, Bassetti M, Calandra T, et al. ; ESCMID Fungal Infection Study Group. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: non-neutropenic adult patients. Clin Microbiol Infect 2012; 18(Suppl 7):19–37. [DOI] [PubMed] [Google Scholar]
- 7. Pfaller MA, Moet GJ, Messer SA, et al. Candida bloodstream infections: comparison of species distributions and antifungal resistance patterns in community-onset and nosocomial isolates in the SENTRY Antimicrobial Surveillance Program, 2008–2009. Antimicrob Agents Chemother 2011; 55:561–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cuervo G, Puig-Asensio M, Garcia-Vidal C, et al. A simple prediction score for estimating the risk of candidaemia caused by fluconazole non-susceptible strains. Clin Microbiol Infect 2015; 21:684.e1–9. [DOI] [PubMed] [Google Scholar]
- 9. Cuervo G, Garcia-Vidal C, Nucci M, et al. Breakthrough candidaemia in the era of broad-spectrum antifungal therapies. Clin Microbiol Infect 2016; 22:181–8. [DOI] [PubMed] [Google Scholar]
- 10. Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med 2003; 22:530–8. [DOI] [PubMed] [Google Scholar]
- 11. CLSI. M27-A3. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standard. 3rd ed.Wayne, PA; Clinical and Laboratory Standards Institute; 2008. [Google Scholar]
- 12. Guinea J, Zaragoza Ó, Escribano P, et al. Molecular identification and antifungal susceptibility of yeast isolates causing fungemia collected in a population-based study in Spain in 2010 and 2011. Antimicrob Agents Chemother 2014; 58:1529–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ostrosky-Zeichner L, Rex JH, Pfaller MA, et al. Rationale for reading fluconazole MICs at 24 hours rather than 48 hours when testing Candida spp. by the CLSI M27-A2 standard method. Antimicrob Agents Chemother 2008; 52:4175–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vazquez J, Reboli AC, Pappas PG, et al. Evaluation of an early step-down strategy from intravenous anidulafungin to oral azole therapy for the treatment of candidemia and other forms of invasive candidiasis: results from an open-label trial. BMC Infect Dis 2014; 14:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van der Geest PJ, Rijnders BJ, Vonk AG, Groeneveld AB. Echinocandin to fluconazole step-down therapy in critically ill patients with invasive, susceptible Candida albicans infections. Mycoses 2016; 59:179–85. [DOI] [PubMed] [Google Scholar]
- 16. Bailly S, Leroy O, Montravers P, et al. Antifungal de-escalation was not associated with adverse outcome in critically ill patients treated for invasive candidiasis: post hoc analyses of the AmarCAND2 study data. Intensive Care Med 2015; 41:1931–40. [DOI] [PubMed] [Google Scholar]
- 17. Bal AM, Shankland GS, Scott G, et al. Antifungal step-down therapy based on hospital intravenous to oral switch policy and susceptibility testing in adult patients with candidaemia: a single centre experience. Int J Clin Pract 2014; 68:20–7. [DOI] [PubMed] [Google Scholar]
- 18. Masterton RG, Casamayor M, Musingarimi P, et al. De-escalation from micafungin is a cost-effective alternative to traditional escalation from fluconazole in the treatment of patients with systemic Candida infections. J Med Econ 2013; 16:1344–56. [DOI] [PubMed] [Google Scholar]
- 19. Reboli AC, Shorr AF, Rotstein C, et al. Anidulafungin compared with fluconazole for treatment of candidemia and other forms of invasive candidiasis caused by Candida albicans: a multivariate analysis of factors associated with improved outcome. BMC Infect Dis 2011; 11:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kullberg BJ, Viscoli C, Pappas PG, et al. Isavuconazole versus caspofungin in the treatment of candidemia and other invasive Candida infections: the ACTIVE trial. Clin Infect Dis 2019; 68:1981–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.