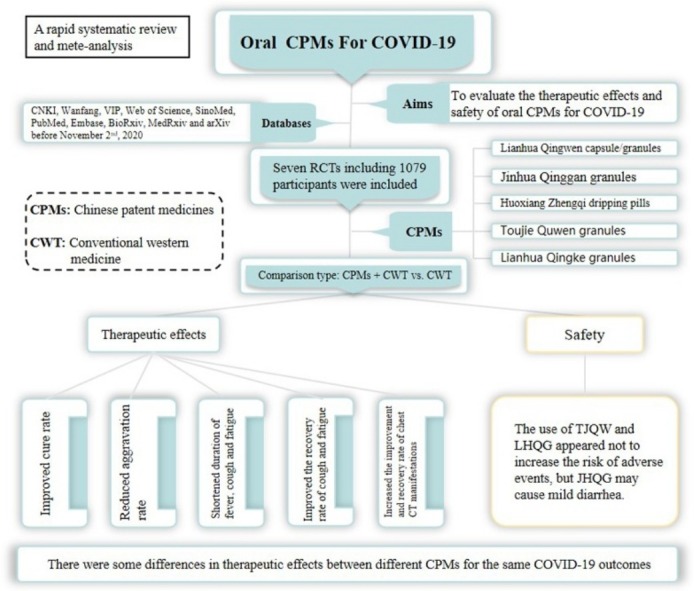
Graphical abstract
Abbreviations: CPM, Chinese patent medicine; COVID-19, Coronavirus Disease 2019; RCTs, randomized controlled trials; WHO, World Health Organization; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; PHEIC, public health emergency of international concern; TCM, traditional Chinese medicine; CNKI, China National Knowledge Infrastructure; VIP, the China Science Technology Journal Database; RR, Risk ratio; MD, mean difference; CI, confidence intervals; GRADE, Grading of Recommendations Assessment, Development and Evaluation criteria; FAS, Full-analysis set; HR, Hazards ratio; LHQW, Lianhua Qingwen capsule/granules; JHQG, Jinhua Qinggan granules; HXZQ, Huoxiang Zhengqi dripping pills; TJQW, Toujie Quwen granules; LHQK, Lianhua Qingke granules; SFJD, Shufeng Jiedu capsule; XBJ, Xue Bi Jing injection
Keywords: Coronavirus Disease 2019, COVID-19, Chinese patent medicine, Chinese herbal medicine, Systematic review, Meta-analysis
Abstract
Introduction
Chinese patent medicine (CPM) is an indispensable part of traditional Chinese medicine. Coronavirus Disease 2019 (COVID-19) manifests is an acute respiratory infectious disease. This systematic review aimed to evaluate the therapeutic effects and safety of oral CPM for COVID-19.
Methods
We included randomized controlled trials (RCTs) that tested oral CPM for the treatment of COVID-19 identified from publications in CNKI, Wanfang, VIP, Web of Science, SinoMed, PubMed, Embase, BioRxiv, MedRxiv and arXiv before November 2nd, 2020. The risk of bias for each trial was assessed using the Cochrane Risk of Bias Tool 2.0. RevMan 5.4 software was used for data analyses. The certainty of the evidence was assessed using the online GRADEpro tool.
Results
Seven RCTs including 1079 participants were identified. The overall bias was assessed as “-high risk of bias” for all included trials. Oral CPM investigated were: Lianhua Qingwen capsule/granules (LHQW), Jinhua Qinggan granules (JHQG), Huoxiang Zhengqi dripping pills (HXZQ), Toujie Quwen granules (TJQW) and Lianhua Qingke granules (LHQK). Compared with conventional western therapy alone for people with COVID-19: regarding the main outcomes, the results showed that oral CPM combined with conventional western therapy improved cure rate (RR = 1.20, 95 % CI 1.04–1.38, involving LHQW and TJQW), reduced aggravation rate (RR = 0.50, 95 % CI 0.29 – 0.85, involving LHQW, JHQG, LHQK and TJQW); with regard to additional outcomes, the results showed that add-on oral CPM shortened the duration of fever, cough and fatigue, improved the recovery rate of cough and fatigue, and increased the improvement and recovery rate of chest CT manifestations. There were some differences in therapeutic effects among various CPMs for the same COVID-19 outcome. The use of TJQW and LHQG appeared not to increase the risk of adverse events, but JHQG may cause mild diarrhea.
Conclusion
Low-certainty or very low-certainty evidence demonstrated that oral CPM may have add-on potential therapeutic effects for patients with non-serious COVID-19. These findings need to be further confirmed by well-designed clinical trials with adequate sample sizes.
1. Introduction
In December 2019, an outbreak of serious pneumonia cases of unknown cause emerged in Wuhan, Hubei, China, with clinical presentations greatly resembling viral pneumonia.1 , 2 By January 9th, 2020, World Health Organization (WHO) reported that Chinese authorities had determined that the outbreak was caused by a novel coronavirus of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).3 Later, on February 11th 2020, WHO announced that the disease should be named Coronavirus Disease 2019 (COVID-19).4 The SARS-CoV-2, causes acute respiratory tract infections, is highly contagious in nature causing high mortality.5 The main symptoms of COVID-19 are fever, dry cough and fatigue, and may be accompanied with nasal congestion, runny nose, sore throat, diarrhea, or loss of taste and smell (anosmia).6 , 7 Almost all of the patients with COVID-19 have pneumonia, and their chest CT scans show abnormalities.8, 9, 10, 11, 12 Complications include acute respiratory distress syndrome, acute heart injury, and secondary infections.8 COVID-19 is the third recent outbreak, following SARS and MERS, that a coronavirus has caused a widespread epidemic and created a public health emergency of international concern (PHEIC).8 On 11th March 2020, the director-general of WHO, Dr Tedros Adhanom Ghebreyesus, declared that COVID-19 was now characterized as a pandemic,13 that is, COVID-19 had spread worldwide. Given the rapid spread of SARS-CoV-2 through human-to-human transmission, the COVID-19 cases currently continue to rise worldwide. So far, although the spread in China has gradually been controlled, one year later, the infection rate outside China had risen rapidly, especially in the United States, India and Brazil. As of 8th January 2021, more than 86 million confirmed COVID-19 cases have been reported to WHO worldwide, including more than 1.88 million deaths.14 To prevent and control the spread of COVID-19, the development of vaccines is indispensable and luckily, their development been successful within a short time period, e.g., Sinopharm’s two inactivated vaccines, which were separately developed in collaboration with Wuhan Institute of Biological Products and Beijing Institute of Biological Products, BioNTech/FosunPharm/Pfizer’s BNT162 vaccine and Oxford-AstraZeneca vaccine.15 , 16 Also, there are now treatments that can reduce the severity and help early hospital discharge, e.g., a trial established that a moderate dose of dexamethasone (6 mg daily for 10 days) reduced mortality in hospitalised patients with COVID-19 and respiratory failure who required therapy with supplemental oxygen or mechanical ventilation.17 The current epidemic situation remains very serious as new variants emerge and poses a great challenge and threat to the existing public health resources.
COVID-19 is an infectious disease and is categorized as "pestilence" (Yibing, 疫病) in TCM terms.18 Traditional Chinese Medicine (TCM) has a long history and has played an indispensable role in the prevention and treatment of several epidemics.19 It is thought that one of the main reasons that China was able to gradually control the epidemic was the early use and full participation of TCM in the prevention and treatment of COVID-19 and public knowledge on how to use TCM. During the outbreak, the National Health Commission of the People’s Republic of China released multiple editions of guidelines for the diagnosis and treatment (GDT) of COVID-19 (hereinafter referred to as GDT of COVID-19). In the third edition, prescribed herbal decoctions of CHM are recommended for the treatment of COVID-19.20 In the fourth edition, oral Chinese patent medicines (CPM such as Jinhua Qinggan granules (JHQG, 金花清感颗粒), Lianhua Qingwen capsule/granules (LHQW, 连花清瘟胶囊/颗粒)), have also been recommended for the treatment of COVID-19.21 Oral CPM as an indispensable part of TCM mainly refers to a kind of medication with a certain specification, which can be directly used for disease prevention and treatment. CPM has the advantages of being locally available and accessible suitable for emergency needs, convenient for storage, easy to carry and not requiring decoction.22 , 23 They are highly valued and critically acclaimed in China for their use in containing and responding to the epidemic, demonstrating success in treating both suspected and confirmed cases.24
A previous systematic review25 conducted by Liu AH et al. that included one randomized controlled trial (RCT) and six retrospective non-randomized controlled trials indicated that CPM combined with usual care (or western medicine) was superior to usual care (or western medicine) in reducing aggravation or hospitalization rate, improving total effective rate, increasing the improvement rate of chest CT manifestations and improving the main symptoms (fever, cough, fatigue and expectoration). However, the review did not carry out subgroup analysis of the different oral CPMs in use (e.g., LHQW, JHQG). Additionally, one RCT conducted by Hu et al.26 obtained a conflicting result with the above review, that is, LHQW plus usual care had no difference in the aggravation rate compared with usual care. Given this, and considering that many high level evidence RCTs regarding oral CPM in COVID-19 have been published since the retrieval date (April 17, 2020) of the above previous review,25 it was timely to conduct a rapid systematic review and meta-analysis that included only RCTs to examine the therapeutic effects and safety of oral CPM for COVID-19.
2. Materials and methods
2.1. Inclusion and exclusion criteria of studies
2.1.1. Inclusion criteria
The PICOST framework was used to develop the inclusion criteria. The criteria were as follows,
(P) Participants: Populations diagnosed with COVID-19, regardless of the severity of the disease (It could be mild, common, severe or critical, as prescribed in the GDT of COVID-19), participants' age, gender and ethnicity. (I) Interventions: Oral CPM alone or combined with comparator(s) (C) Comparators: Conventional western therapy, such as the treatment recommended by GDT of COVID-19 released by the National Health Commission of the People’s Republic of China, Clinical management of COVID-19 by WHO, et al.; or placebo of CPM combined with conventional western therapy. (O) Outcomes: Main outcomes including cure rate, mortality rate, aggravation rate (the change in the disease severity category, or patients were admitted to the ICU, etc.). Additional outcomes included the recovery rate or the duration of main symptoms (fever, cough and fatigue), the negative conversion rate of nucleic acid test for SARS-CoV-2, the improvement or recovery of chest CT manifestations, the length of hospitalization and adverse events. (S) Study design: Only RCTs were included in this review. (T) Time periods: All lengths of treatment time and duration of follow-up were eligible. For outcomes reported at multiple time points, we used the longest reported follow-up time point.
2.1.2. Exclusion criteria
The full text of publications could not be obtained, duplicate publications and clinical trial protocols were excluded.
2.2. Retrieval platforms and search strategies of studies
In order to complete this review as soon as possible without compromising the quality of our research, this rapid review was carried out on the basis of our previous research,27 an evidence review of clinical studies regarding CHM for COVID-19. In the previous research,27 we systematically searched for clinical trials of CHM which included oral CPM, Chinese herbal medicine injection and prescribed herbal decoctions used for COVID-19. We screened studies included in the previous research27 to identify RCTs that only use oral CPM for COVID-19. Since the previous search date was up to April 30th, 2020, the official start date of this rapid review was November 2nd, 2020, we updated and searched for publications during the period between April 30th, 2020 and November 2nd, 2020. Databases searched included: China National Knowledge Infrastructure (CNKI), Wanfang Database, the China Science Technology Journal Database (VIP), Web of Science, SinoMed, PubMed, Embase, BioRxiv, MedRxiv and arXiv. The subject/Mesh terms used for the update searches were: Xinxing Guanzhuang Bingdu Bing (新型冠状病毒病), Xinguan Feiyan (新冠肺炎), 2019 Guanzhuang Bingdu Bing (2019冠状病毒病), coronavirus disease-19, COVID-19, 2019 novel coronavirus, 2019-nCOV, NCP, Zhongyi (中医), Zhongyao (中药), Zhongchengyao (中成药), Zhongxiyi Jiehe (中西医结合), Chinese medicine, patent medicine, integrated Chinese and western medicine, and adjusted for use in the different databases. For example, Pubmed was searched with the following terms: (((corona virus disease-19 OR COVID-19 OR 2019 novel coronavirus OR 2019-nCOV OR NCP[MeSH Major Topic])) AND (patent medicine OR Chinese medicine[MeSH Major Topic])) AND ("2020/04/30"[Date - Publication] : "2020/11/02"[Date - Publication]). In order not to omit RCTs that potentially met the inclusion criteria, we also checked the reference lists of relevant included publications.
2.3. Study selection and data extraction
Firstly, we included the RCTs on oral CPM from our previous research which met the inclusion criteria. Then updated trials were selected according to the inclusion/exclusion criteria by reading titles, abstracts and (or) full texts of the newly identified publications.
Two authors extracted data independently from the included publications using a pre-designed data extraction sheet, including publication titles, authors’ information, characteristics of participants (e.g., sample size, age and disease severity), details of interventions and comparators, outcomes, information relevant to RCTs’ design, etc. Any disagreements were resolved by discussions with a third author (JPL).
2.4. Risk of bias for each trial
The risk of bias for each trial was assessed using the Cochrane Risk of Bias Tool 2.028 by two authors independently. Inconsistencies were discussed with a third author (JPL). The tool consists of the following five domains: randomization process, deviations from the intended interventions, missing outcome data, measurement of the outcome and selection of the reported result.
Each domain was evaluated as one of the following response options: (a) low risk of bias, (b) high risk of bias, or (c) some concerns.
2.5. Data synthesis
The data were synthesized descriptively, including summary statistics and detailed tables of trial characteristics. In terms of the outcomes, we conducted meta-analyses to pool the data using Review Manager 5.4 (Revman 5.4, Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration) software,29 if data allowed. Risk ratio (RR) and mean difference (MD) with their 95 % confidence intervals (CI) were used for dichotomous outcomes and continuous outcomes, separately. The random-effect model was used for meta-analysis considering potential sources of clinical heterogeneity. If P < 0.10, it suggested that there is heterogeneity among the included RCTs, and we referred to the value of I 2 to judge the size of heterogeneity30: When I 2 < 50 %, we checked the accuracy of the data first. If the data were accurate and appropriate, subgroup analysis would be carried out to explore the source of heterogeneity. Otherwise, the results would be carefully interpreted.
We planned to conduct subgroup analyses, if appropriate, in light of the following aspects: (a) the severity of COVID-19, to detect whether it had an impact on the effects; (b) the different CPMs, to explore the effect difference of CPM in the treatment of COVID-19.
Although planned, we did not construct funnel plots to evaluate publication bias because these are inaccurate when fewer than 10 trials are included in the analysis.31 In addition, the evidence certainty for outcomes of effectiveness was evaluated by using the GRADE (Grading of Recommendations Assessment, Development and Evaluation criteria) approach.32
3. Results
3.1. Number and characteristics of included RCTs
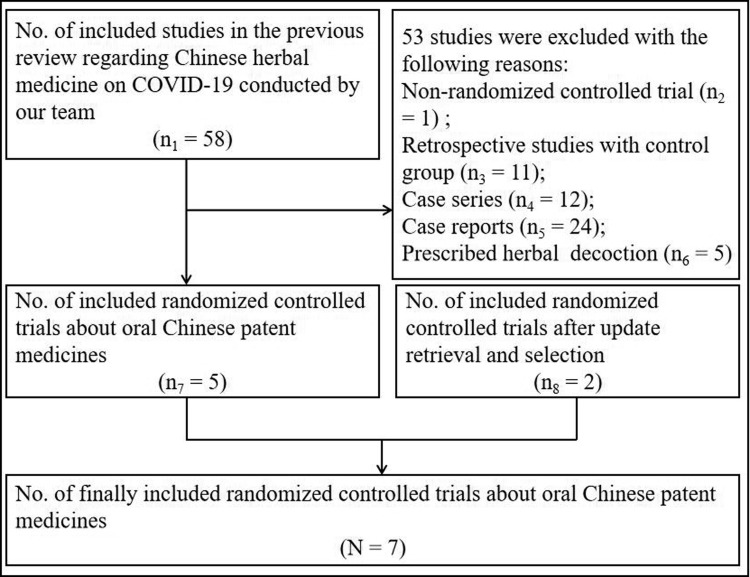
Searches identified 6 two-26 , 33 , 35, 36, 37, 38 and 1 three-armed34 RCTs (1079 participants) which met the inclusion criteria and could be included in this review. Fig. 1 shows the flow diagram of studies selection for this review. Supplement material (Supplement-Figs. 1 and 2) shows the flow diagram of studies selection for the previous review regarding Chinese herbal medicine on COVID-19.
Fig. 1.
Flow diagram of studies selection.
All of these were conducted in China and published in Chinese33 , 35, 36, 37, 38 or English.26 , 34All but one trial34 reported information regarding the severity of COVID-19 and that all patients were not serious. The ratio of male to female was about 1.2: 1 in light of all the included trials. The comparator in each included trial was conventional western therapy developed based on the GDT of COVID-19, and the intervention was to apply CPM on the basis of the comparator (hereinafter referred to as add-on CPM). Oral CPM in the included trials contained LHQW, JHQG, Huoxiang Zhengqi dripping pills (HXZQ, 藿香正气滴丸), Toujie Quwen granules (TJQW, 透解祛瘟颗粒), Lianhua Qingke granules (LHQK, 连花清咳颗粒). For LHQW, two dosage forms were involved, one form was capsule,26 the other form was granules.34 , 37 All but one three-armed trial34 used only a single CPM. In the three-arm trial,34 the CPM used in intervention group-1 was LHQW, and the CPM used in intervention group-2 was LHQW combined with HXZQ (hereinafter referred to as “LHQW+ HXZQ”). In the evaluation of effectiveness, in order to facilitate statistical analysis and take into account that it will not have a favorable positive impact on the treatment group, we divided the three-armed trial34 into 2 two-armed trials, namely Xiao MZ-1 and Xiao MZ-2. The duration of treatment in all trials was varied from 5 to 15 days.
The characteristics of the included trials are summarized in Table 1 .
Table 1.
The characteristics of included studies of Chinese patent medicine for COVID-19.
| Study ID | Sample size (M/F) | Age (year) | Severity* of COVID-19 | Name and administration of CPM | Conventional western therapy(Yes/No) | Course of CPM treatment | Outcomes | Author’s conclusion towards the role of CPM for COVID-19 (positive/negative) | |
|---|---|---|---|---|---|---|---|---|---|
| Hu K26 | T:79/63 | T:50.4 ± 15.2 | Non-serious | Lianhua Qingwen capsule. (连花清瘟胶囊, 4 capsules, thrice daily, take orally) | Yes. It generally consisted of the supportive treatment such as oxygen therapy, antiviral medications and symptomatic therapies. | 14 days | ①②⑦⑧⑨⑩⑪⑬ | Positive | |
| C:71/71 | C:51.8 ± 14.8 | ||||||||
| Duan C33 | T:39/43 | T:51.99 ± 13.88 | Non-serious | Jinhua Qinggan granules. (金花清感颗粒, 10 g, thrice daily, take orally) | Yes. Antiviral, anti-infection and other symptomatic therapies. | 5 days | ②④⑤⑥⑬ | Positive | |
| C:23/18 | C:50.29 ± 13.17 | ||||||||
| Xiao MZa34 | 1 | T1:35/23 | T1:52.86 ± 13.95 | Not reported | Lianhua Qingwen granules. (连花清瘟颗粒, 6 g, thrice daily, take orally) | Yes. Antiviral treatment with oral oseltamivir (75 mg per tablet) one tablet, once daily; Arbidol (100 mg per tablet) taken orally, two tablets, thrice daily; Ribavirin (100 mg per tablet) taken orally, one and a half tablets, thrice daily; Antimicrobial therapy: strengthen bacteriological monitoring and use antibiotics when there is evidence of a secondary bacterial infection using oral penicillins, cephalosporins, ofloxacin, and macrolide etc. | 14 days | ② | Positive |
| C:35/28 | C:53.90 ± 13.92 | ||||||||
| 2 | T2:33/28 | T2:56.07 ± 12.10 | Not reported | Huoxiang Zhengqi dripping pills (藿香正气滴丸, 2.6 g, twice daily, take orally) + Lianhua Qingwen granules (连花清瘟颗粒, 6 g, thrice daily, take orally) | 14 days | ② | Positive | ||
| C:35/28 | C:53.90 ± 13.92 | ||||||||
| Fu XXa35 | T:17/15 | T:43.26 ± 7.15 | Non-serious | Toujie Quwen granules (透解祛瘟颗粒, twice daily, take orally) | Yes. Abidor tablet, 0.2 g, thrice daily; Moxifloxacin tablet, 0.4 g, once daily; Ambroxol tablets, 30 mg, thrice daily. | 10 days | ②⑪⑬ | Positive | |
| C:19/14 | C:43.68 ± 6.45 | ||||||||
| Sun HM36 | T:17/15 | T:45.4 ± 14.10 | Non-serious | Lianhua Qingke granules (连花清咳颗粒, twice daily, take orally) | Yes. Lopinavir (200 mg/pill) or Ritonab (50 mg/pill), 2 pills, twice daily; IFN-α 5 million U each time, added to 2 mL sterile water for injection, twice daily; symptomatic and supportive treatment. | 14 days | ②④⑤⑥⑧⑪ | Positive | |
| C:11/14 | C:42.0 ± 11.70 | ||||||||
| Yu P37 | T:82/65 | T:48.27 ± 9.56 | Non-serious | Lianhua Qingwen granules (连花清瘟颗粒, 6 g, thrice daily, take orally) | Yes. Abidor tablet, 0.2 g, thrice daily; Moxifloxacin tablet, 0.4 g, once daily; Ambroxol tablets, 30 mg, thrice daily. | 7 days | ②③⑪⑬ | Positive | |
| C:89/59 | C:47.25 ± 8.67 | ||||||||
| Fu XXb38 | T:19/18 | T:45.26 ± 7.25 | Non-serious | Toujie Quwen granules (透解祛瘟颗粒, twice daily, take orally) | Yes. Abidor tablet, 0.2 g, thrice daily, 10 days; Ambroxol tablets, 30 mg, thrice daily, 15 days. | 15 days | ①②⑬ | Positive | |
| C:19/17 | C:44.68 ± 7.45 | ||||||||
Note: CPM, Chinese patent medicine; M, male; F, female; T, treatment group involving CPM; C, controlled group not involving CPM; Yes, intervention involved in the trial was CPM combined with conventional western therapy; No, intervention involved in the trial was CPM alone, not combined with conventional western therapy; Positive, add-on CPM has benefits for COVID-19; negative, add-on CPM has no benefits for COVID-19, or can even make the disease worse.
The severity (*) was classified according to the guidelines for the diagnosis and treatment of COVID-19 released by the National Health Commission of the People’s Republic of China. We divide them into two categories of non-serious (including mild and common) and serious (including severe and critical). Although one two-armed trial33 involving LHQW conducted by Yu P et al. reported aggravation rate, we did not enroll the data on this outcome in our statistical analysis due to the inconsistency between the data presented in the table and the text of Yu P et al.’ publication.
Outcomes: ① Cure rate; ② Aggravation rate; ③ Mortality rate; ④ The recovery of fever; ⑤ The recovery rate of cough; ⑥ The recovery of fatigue; ⑦ The duration of fever; ⑧ The duration of cough; ⑨ The duration of fatigue; ⑩ The negative conversion rate of nucleic acid test; ⑪ The improvement or recovery rate of chest CT manifestations; ⑫ The length of hospitalization; ⑬ Adverse events.
No included trial report the outcome of the length of hospitalization.
In the three-arm trial, the CPM used T1 was Lianhua Qingwen granules, and in T2 was Lianhua Qingwen granules and Huoxiang Zhengqi dripping pills.
3.2. Risk of bias assessment of included trials
The risk of bias assessment of included trials is given in Fig. 2 .
Fig. 2.
Risk of bias assessment of included trials.
The overall bias was assessed as “high risk of bias” in all the included trials.26 , 33, 34, 35, 36, 37, 38
-
a)
Domain 1: Randomization process
Among the seven included trials, one trial35 only mentioned “random” without further clarification and six trials26 , 33, 34, 35, 36, 37 declared the specific methods of random sequence generation. The baseline data were comparable for all the included trials. However, none of the included trials reported any information about allocation concealment. If allocation concealment was not implemented, there is reason to suspect that the enrolling investigator or the participant had knowledge of the forthcoming allocation. Therefore, all trials were judged as “some concerns” in the randomization process.
-
b)
Domain 2: Deviations from intended interventions
Judged in the light of the interventions and comparators, all trials26 , 33, 34, 35, 36, 37, 38 failed to conduct blinding for participants and clinicians delivering the interventions. Among all the trials, one26 reported that three patients in each group had a major protocol deviation and were therefore excluded from the per protocol set, six33, 34, 35, 36, 37, 38 did not report whether deviations arose because of the trial context. Apart from that, three trials26 , 33 , 34 used an appropriate analysis to estimate the effect of assignment to intervention, four trials35, 36, 37, 38 probably used an appropriate analysis. Taking the above into consideration, all trials were judged as “some concerns” in this domain of deviations from intended interventions.
-
c)
Domain 3: Missing outcome data
All trials26 , 33, 34, 35, 36, 37, 38 were considered as “low risk of bias” in missing outcome data domain due to the complete, or nearly complete outcome data available.
-
d)
Domain 4: Measurement of the outcome
All trials26 , 33, 34, 35, 36, 37, 38 failed to conduct blinding for clinicians delivering the interventions. Moreover, none of the trials reported whether the outcome assessors were independent of the clinicians. If the outcome assessors and the clinicians were the same, the outcomes that need to be evaluated by the clinicians (such as the aggravation rate) were likely influenced by clinicians’ awareness of the interventions received by participants. Therefore, all trials were assessed as “some concerns” in this domain.
-
e)
Domain 5: Selection of the reported result
Two trials26 , 34 reported the information about their protocol, and we found that there was no selective reporting of outcomes in its publication, but they were all lack of pre-specified analysis method plan for outcomes in their registered protocol. For the other five trials33 , 35, 36, 37, 38 we could not obtain their protocol. Therefore, all trials were assessed as “some concerns” in this domain.
3.3. Effectiveness and safety of CPM in the treatment of COVID-19
3.3.1. Primary outcomes
3.3.1.1. Cure rate
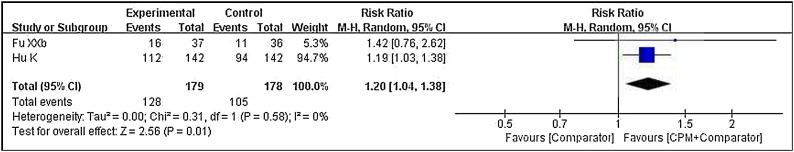
With regard to cure rate, a pooled result (Fig. 3 ) from two trials26 , 38 demonstrated that there was a statistical difference between the two groups (RR = 1.20, 95 % CI 1.04–1.38, P = 0.01, involving LHQW and TJQW). That is, add-on CPM was superior to conventional western therapy alone in increasing the cure rate of patients with COVID-19.
Fig. 3.
Forest plot of cure rate.
3.3.1.2. Aggravation rate
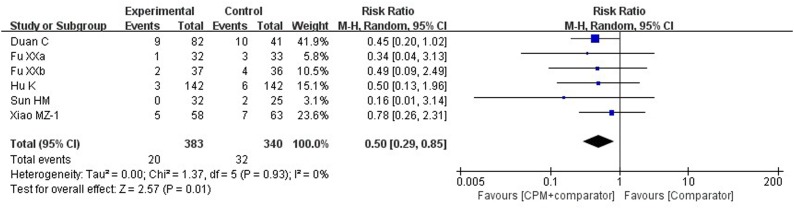
One three-34 and 6 two-armed26 , 33 , 35, 36, 37, 38 trials reported this outcome. Although one trial37 involving LHQW reported this outcome, we did not include the data on this outcome in our statistical analysis due to the inconsistency between the data presented in the table and the text of the trial’s publication. One trial34 of Xiao MZ-2 that with two CPMs (LHQW + HXZQ), its result showed that there was no difference (RR = 0.15, 95 % CI 0.02–1.16, P = 0.07) between the add-on CPM group and the control group in reducing the aggravation rate. A pooled result (Fig. 4 ) from the other six trials (single CPM was used in each trial)26 , 33, 34, 35, 36 , 38 showed that there was a statistical difference between the two groups (RR = 0.50, 95 % CI 0.29–0.85, P = 0.01, involving LHQW, JHQG, LHQK and TJQW). That means the aggravation rate in the add-on CPM group was less likely than in the conventional western therapy group.
Fig. 4.
Forest plot of aggravation rate.
3.3.1.3. Mortality rate
Only Yu P et al.37 reported this outcome. The result showed that there was no statistical difference between the add-on CPM (LHQW) group and the comparator group (RR = 0.50, 95 % CI 0.05–5.49, P = 0.57) in reducing the mortality rate.
3.3.2. Secondary outcomes
3.3.2.1. The recovery rate and the duration of main symptoms (fever, cough and fatigue)
-
a
The recovery rate of main symptoms
A total of 2 two-armed trials33 , 36 reported the recovery of main symptoms. Pooled results demonstrated that the add-on CPM were probably superior to the conventional western therapy alone in improving the recovery rate from cough (RR = 1.45, 95 % CI 1.12–1.89, P = 0.005, involving JHQG and LHQK) and fatigue (RR = 1.33, 95 % CI 1.03–1.71, P = 0.03, involving JHQG and LHQK), but not for improving the recovery rate from fever (RR = 1.24, 95 % CI 0.77–1.99, P = 0.038, large statistical heterogeneity (I 2 = 71 %), involving JHQG and LHQK). We explored the source of large statistical heterogeneity through subgroup analyses based on a covariate of different CPMs and found that the heterogeneity may be related to the covariate (Supplement material, Supplement-Fig. 5).
-
b
The duration of main symptoms
Hu K et al.26 reported that the add-on CPM (LHQW) group demonstrated a significantly shorter time to the recovery of fever (Full-analysis set (FAS): 2 days vs. 3 days, Hazards ratio (HR): 1.39, 95 % CI 1.00–1.94, P = 0.017), fatigue (FAS: 3 days vs. 6 days, HR: 1.78, 95 % CI 1.26–2.54, P < 0.001) and cough (FAS: 7 days vs. 10 days, HR: 1.71, 95 % CI 1.30–2.23, P < 0.001). This means that add-on LHQW was superior to conventional western therapy alone in shortening the duration of fever, cough and fatigue.
Sun HM et al.36 reported that add-on CPM (LHQK) was superior to conventional western therapy alone in shortening the duration of cough. The median duration of cough was 4 days in the add-on CPM group and 7 days in the control group (P < 0.05).
3.3.2.2. The negative conversion rate of nucleic acid test for SARS-CoV-19
Only Hu K et al.26 reported this outcome and the result showed that there was no statistical difference between the add-on CPM (LHQW) group and the comparator group in increasing the negative conversion rate (RR = 1.08, 95 % CI 0.49–1.24, P = 0.28).
3.3.2.3. The improvement or recovery of chest CT manifestations
Three two-armed trials35, 36, 37 reported the improvement rate of chest CT manifestations (improvement rate = the number of patients with improvement of chest CT manifestations / the total number of patients in the experimental or the control group × 100 %). A pooled result from the three trials demonstrated that add-on CPM was superior to conventional western therapy alone in increasing the improvement rate of chest CT manifestations (RR = 1.20, 95 % CI 1.05–1.37, P = 0.007, involving LHQW, LHQK and TJQW).
In terms of the recovery from chest CT manifestations, 3 two-armed trials26 , 35 , 37 reported the recovery rate (= the number of patients with the recovery of chest CT manifestations / the total number of patients in the experimental or the control group × 100 %). A pooled result from the three trials indicated that add-on CPM had a potential advantage over conventional western therapy alone (RR = 1.32, 95 % CI 1.16–1.51, P < 0.001, involving LHQW and TJQW).
3.3.2.4. The length of hospitalization
No trial reported this outcome.
3.3.2.5. Adverse events
Five trials26 , 33 , 35 , 37 , 38 reported this outcome. Of these, three trials35 , 37 , 38 reported that no adverse events occurred in both the add-on CPM (involving TJQW and LHQW) group and the control group.
One trial conducted by Hu K et al.26 reported that 45.8 % (65/142) cases with adverse events (including abnormal liver function, renal dysfunction, headache, nausea, vomiting, diarrhea and loss of appetite) occurred in the add-on LHQW group and 54.2 % (77/142) cases with adverse events (including abnormal liver function, renal dysfunction, headache, nausea, vomiting, diarrhea and loss of appetite) occurred in the comparator group. This trial also reported that no serious adverse events occurred in either of the groups.
The remaining trial33 reported that 32.93 % (27/82) cases with adverse events of diarrhea occurred in the add-on JHQG group and no adverse events occurred (0/41) in the comparator group. Among the 27 cases, 19 patients’ diarrhea recovered after 1–2 days without any special treatment, eight patients stopped taking medicine and then their diarrhea improved.
3.3.3. Subgroup analysis
One pre-planned subgroup analysis that aimed to detect whether the severity of COVID-19 has an impact on the effects were not performed due to: all included trials either reported the severity of COVID-19 as non-serious (six RCTs26 , 33 , 35, 36, 37, 38) or did not report the severity of COVID-19 (one RCT34).
Therefore, we only performed another predefined subgroup analysis according to the currently available information to explore the therapeutic effects of different CPMs for COVID-19. The results of subgroup analyses are presented in summary Table 2 . The forest plots of all subgroup analyses can be found in the Supplement material (Supplement-Figs. 3–9).
Table 2.
The results of subgroup analyses based on different Chinese patent medicines.
| Outcomes | Comparisons |
|||
|---|---|---|---|---|
| LHQW + CWT (VS CWT) | JHQG + CWT (VS CWT) | LHQK + CWT (VS CWT) | TJQW + CWT (VS CWT) | |
| Cure rate | RR = 1.19, 95 % CI 1.03–1.38, P = 0.02, 1RCT | RR = 1.42, 95 % CI 0.76–2.62, P = 0.27, 1 RCT | ||
| Aggravation rate | RR = 0.65, 95 % CI 0.28–1.53, P = 0.33, 2 RCTs | RR = 0.45, 95 % CI 0.20–1.02, P = 0.06, 1 RCT | RR = 0.16, 95 % CI 0.01–3.14, P = 0.23, 1 RCT | RR = 0.43, 95 % CI 0.12–1.60, P = 0.21, 2 RCTs |
| The recovery rate of fever | RR = 1.51, 95 % CI 1.07–2.14, P = 0.02, 1 RCT | RR = 1.00, 95 % CI 0.68–1.46, P = 1.00, 1 RCT | ||
| The recovery rate of cough | RR = 1.54, 95 % CI 0.97–2.45, P = 0.07, 1 RCT | RR = 1.42, 95 % CI 1.03–1.94, P = 0.03, 1 RCT | ||
| The recovery rate of fatigue | RR = 1.44, 95 % CI 0.98–2.11, P = 0.06, 1 RCT | RR = 1.25, 95 % CI 0.90–1.75, P = 0.19, 1 RCT | ||
| Recovery rate of chest CT manifestations | RR = 1.32, 95 % CI 1.15–1.51, P < 0.0001, 2 RCTs | RR = 1.43, 95 % CI 0.63–3.25, P = 0.40, 1 RCT | ||
| Improvement rate of chest CT manifestations | RR = 1.10, 95 % CI 0.94–1.30, P = 0.24, 1 RCT | RR = 1.35, 95 % CI 1.05–1.73, P = 0.02, 1 RCT | RR = 1.30, 95 % CI 0.97–1.74, P = 0.08, 1 RCT | |
LHQW: Lianhua Qingwen; HXZQ: Huoxiang Zhengqi; JHQG: Jinhua Qinggan; LHQK: Lianhua Qingke; TJQW: ToujieQuwen; CI: Confidence interval; RR: Risk ratio; RCT: Randomized controlled trial; VS: versus; CWT: conventional western therapy.
The forest plots of all subgroup analyses can be found in Supplement material (Supplement-Fig.3 to Supplement-Fig.9).
3.4. Certainty assessment of evidence by using GRADE
The certainty of the available evidence for outcomes was evaluated as low-certainty or very low-certainty by using the GRADE system recommendation approach. The details of the certainty of available evidence can be found in Table 3 . The certainty of the evidence was downgraded mainly for the following reasons: (a) Risk of bias (high risk of detection bias and/or attrition bias); (b) Inconsistency (significant statistical heterogeneity and/or small overlap of 95 % CI of different trial results); and (c) imprecision (small sample size or only one trial was included).
Table 3.
GRADE evaluation form of evidence certainty.
| Patient or population: Patients diagnosed with COVID-19 | |||||
|---|---|---|---|---|---|
| Setting: Hospital | |||||
| Intervention: CPM + conventional western therapy | |||||
| Comparison: Conventional western therapy | |||||
| Outcomes | Anticipated absolute effects* (95% CI) |
Relative effect (95 % CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with Comparator | Risk with CPM + Comparator | ||||
| Cure rate | 590 per 1,000 | 708 per 1,000 (613–814) | RR 1.20 (1.04–1.38) | 357 (2 two-armed RCTs) | ⨁⨁◯◯a.b |
| LOW | |||||
| Aggravation rate | 94 per 1,000 | 47 per 1,000 (27–80) | RR 0.50 (0.29–0.85) | 723 (6 RCTs) | ⨁⨁◯◯a.b |
| LOW | |||||
| Mortality rate | 14 per 1,000 | 7 per 1,000 (1–74) | RR 0.50 (0.05–5.49) | 295 (1 RCT) | ⨁◯◯◯a.c |
| VERY LOW | |||||
| The recovery rate of fever | 595 per 1,000 | 737 per 1,000 (458 to 1,000) | RR 1.24 (0.77–1.99) | 107 (2 RCTs) | ⨁◯◯◯a.b |
| VERY LOW | |||||
| The recovery rate of cough | 528 per 1,000 | 766 per 1,000 (592–998) | RR 1.45 (1.12–1.89) | 147 (2 RCTs) | ⨁◯◯◯a.b |
| VERY LOW | |||||
| The recovery rate of fatigue | 611 per 1,000 | 813 per 1,000 (629 to 1,000) | RR 1.33 (1.03–1.71) | 108 (2 RCTs) | ⨁⨁◯◯a.b |
| LOW | |||||
| Negative conversion rate of nucleic acid test for SARS-CoV-19 | 711 per 1,000 | 768 per 1,000 (669–882) | RR 1.08 (0.94–1.24) | 284 (1 RCT) | ⨁⨁◯◯a.c |
| LOW | |||||
| The recovery rate of chest CT manifestations | 406 per 1,000 | 536 per 1,000 (471–613) | RR 1.32 (1.16–1.51) | 639 (3 t RCTs) | ⨁⨁◯◯a.b |
| LOW | |||||
| The improvement rate of chest CT manifestations | 645 per 1000 | 774 per 1000 (678–884) | RR 1.20 (1.05–1.37) | 412 (3 RCTs) | ⨁⨁◯◯a.b |
| LOW | |||||
Factors of downgrade:
a. Risk of bias (high risk of detection bias and/or attrition bias).
b. Inconsistency (significant statistical heterogeneity and/or small overlap of 95 % CI of different trial results).
c. imprecision (small sample size or only one trial were included).
GRADE Working Group grades of evidence: High certainty (We are very confident that the true effect lies close to that of the estimate of the effect) Moderate certainty (We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different) Low certainty (Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect) Very low certainty (We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect).
The risk in the intervention group (and its 95 % confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95 % CI). CI: Confidence interval; RR: Risk ratio; CPM: Chinese patent medicine; RCT: Randomized controlled trial.
4. Discussion
The use of CHM to prevent infections can be traced back to ancient Chinese practices cited in Huangdi's Internal Classic (Huang Di Nei Jing, 黄帝内经) where preventive effects were recorded. CPM are a kind of CHM preparation that can be conveniently taken according to certain specifications. The purpose of our review was to confirm whether CPM was effective and safe when used in the treatment of COVID-19.
4.1. Summary of the main findings
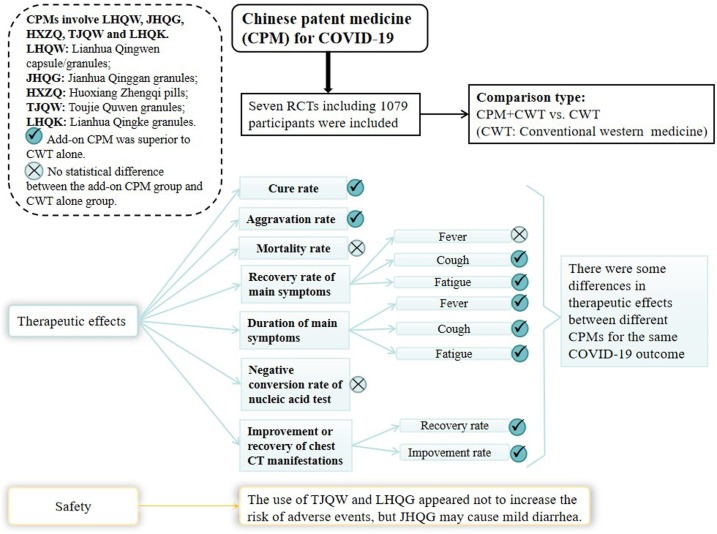
Our review suggests that there maybe a tendency that CPM plus conventional western therapy was better than conventional western alone in the treatment of non-serious COVID-19. The potential of an add-on CPM in the treatment of COVID-19 were as follows: improving cure rate; reducing aggravation rate; shortening the duration of fever, cough and fatigue; improving the recovery rate of cough and fatigue, and increasing the improvement and recovery rate of chest CT manifestations. However, the evidence also indicated that there were probably no differences between the add-on CPM group and the control group in reducing mortality rate, improving the recovery rate of fever, and reducing the negative conversion rate of nucleic acid test for SARS-CoV-19. It should be noted that, there are some differences in therapeutic effects between different oral CPMs for the same outcome of COVID-19. We would like to mention that all the findings of different CPMs were based on a single RCT, except for outcomes on aggravation rate and recovery rate of chest CT manifestations in LHQW were based on two RCTs respectively. All in all, the use of CPM provided add-on benefits to the treatment of COVID-19.
With regard to the safety of CPM, the evidence demonstrated that the use of TJQW and LHQW probably does not increase the risk of adverse events, but JHQG may cause mild diarrhea in patients.
The summary of the main findings is presented in Fig. 5 .
Fig. 5.
Summary of the main findings.
4.2. Implications for the clinical practice
In this rapid review, except for one trial which did not report the severity of COVID-19, all the participants in the other trials were diagnosed as having non-serious COVID-19. Based on the findings of our review, CPM has some potential and probably is safe for patients with non-serious COVID-19, from the RCTs regarding the therapeutic effects and safety of CPM on patients with severe COVID-19. In addition, we found that there were some differences in the therapeutic effects and safety of different CPMs for COVID-19. Therefore, we would recommend doctors choose the appropriate CPM in clinic practice. For example, for patients with obvious lung inflammation on chest CT manifestations, LHQW or LHQK plus conventional western therapy may be preferred over TJQW, because add-on LHQW or LHQK has the potential in increasing the recovery or improvement rate of chest CT manifestations compared with conventional western therapy alone, but add-on TJQW does not seem to have such a potential. For the dosage and treatment course of CPM use, clinicians can refer not only to the RCTs included in our review but also to the instructions of each CPM.
However, due to the small number of included trials and the high risk of bias of each trial, these rapid review findings need to be further verified by included more high-quality clinical studies in the future. Therefore, our findings can only be used as a reference for clinicians but not be directly used as guidelines in clinical practice.
4.3. Comparison with the previous review(s)
A previous systematic review25 enrolled 1 RCT and 6 retrospective non-randomized controlled trials indicated that CPM combined with usual care (or western medicine) was superior to usual care (or western medicine) in reducing the aggravation (hospitalization) rate (LHQW, JHQG), improving the total effective rate (LHQW, SFJD, XBJ), increasing the improvement rate of chest CT manifestations (LHQW, SFJD, XBJ), improving the recovery rate of main symptoms (fever, cough, fatigue and expectoration; LHQW, JHQG) and shortening the duration of fever (LHQW, SFJD). No other outcomes were reported. [ Note: SFJD = Shufeng Jiedu capsule (疏风解毒胶囊); XBJ = Xue Bi Jing injection (血必净注射液).]
However, our review included 1 three- and 6 two-armed RCTs obtained a different result that there were probably no differences between the add-on CPM group and the conventional western therapy group in improving the recovery rate of fever (JHQG, LHQK). With regard to the aggravation rate, our review is the same as the previous review,25 find that the add-on CPM (LHQW, LHQW + HXZQ, JHQG, LHQK, TJQW) was superior to conventional western therapy in reducing it. But, for different CPMs, the results of subgroup analysis in our review showed that there seems to be no statistical difference between the add-on CPM group and the control group. It should be noted that we cannot be sure that this finding is certain because the number of trials included for each CPM and the sample size of participants in each trial is small. Besides, our review also found that the add-on CPM has greater potential than conventional western therapy alone in improving the cure rate (LHQW, TJQW), increasing the recovery rate from chest CT manifestations (LHQW) and shortening the duration of cough (LHQW, LHQK) and fatigue (LHQW). The safety of CPM should be the key point for consideration. The previous review,25 however, did not report this information. Our review found that the use of TJQW and LHQG probably does not increase the risk of adverse events, but JHQG may cause mild diarrhea in patients.
Due to multiple CPMs being included in the previous25 and this review, it is necessary to perform subgroup analyses in light of different CPMs in order to find the differences between them on the same outcome of COVID-19. By this, we can obtain evidence to follow when choosing CPM to treat COVID-19. Nevertheless, the previous review25 did not carry out the relevant subgroup analyses, but the relevant subgroup analyses were conducted in this review.
4.4. Strengths and limitations
As far as we know, this is the first rapid systematic review related to RCTs on the therapeutic effects and safety of CPM for COVID-19, and we have performed subgroup analyses according to different CPMs. GRADE assessment of the certainty of the evidence was also carried out in our review. There are also limitations. First, we did not construct funnel plots to evaluate publication bias as few trials could be included. Second, although we performed subgroup analyses in light of different CPMs, the conclusions of our review still need to be further verified by more high-quality studies in the future as the number of included trials was small. Additionally, the sample sizes were small in most of the included trials, and there was a high risk of bias in all of the included trials.
4.5. Implications for further research
More high-quality clinical trials (such as the use of an appropriate random allocation method to avoid selection bias, ensure blinding of participants, clinicians, outcome assessors and data analysts to remove performance bias and detection bias, etc.39) with a large sample size of participants need to be carried out. Secondly, the safety of CPM needs to be considered with future studies reporting the occurrence of adverse events in detail. In addition to the safety of CPM, some key outcomes such as cure rate, aggravation rate, mortality rate, and the recovery rate or duration of fever, cough and fatigue, should also be reported. The use of TCM in the treatment of diseases usually requires a long course of treatment; however, the longest course of treatment included in our review was 14 days. Therefore, it is recommended to carry out trials with longer treatment courses and to follow up with the participants regularly for a period of time after the end of treatment.
5. Conclusion
Low-certainty or very low-certainty evidence demonstrated that oral CPM may have add-on potential therapeutic effects for patients with non-serious COVID-19. There are some differences in therapeutic effects between different oral CPMs for the same outcome of COVID-19. The use of TJQW and LHQG probably does not increase the risk of adverse events, but JHQG may cause mild diarrhea in patients. The conclusion of this review needs to be further confirmed by well-designed clinical trials with adequate sample sizes.
Author contributions
JPL and SBL conceived and designed the review. SBL, MF and HDL were responsible for the searching, screening and selection of studies. MF, HDL and SBL participated in the data extraction. SBL and CHL were responsible for the risk of bias assessment. SBL performed the statistical analysis. SBL drafted the manuscript. CHL and MF assisted in the writing of the manuscript. HDL and CS completed the PRISMA checklist. JPL, NR, LJY, XYH and MH were involved in critically revising the manuscript. All authors have read, revised and approved the manuscript, including the authorship list.
Registration and protocol
The review was not registered.
Funding
Prof. Jian-Ping Liu is supported by the National Natural Science Foundation project (No. 81830115) in China. Prof. Nicola Robinson (visiting Professor of Beijing University of Chinese Medicine) is funded by the Project of International Development and Capability Improvement of Evidence-based Chinese Medicine (G20200001187). Dr Xiao-Yang Hu is supported by the National Institute for Health Research (SPCR-143).
Declaration of Competing Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgements
We greatly thank Ying-Ying Zhang, Bao-Yong Lai, Ning Dai, Yu-Qi Li, Zi-Yu Tian, Xiao-Wen Zhang, Yue Jiang, Min Xiong and Ya-Peng Zhang from Centre for Evidence-Based Chinese Medicine, Beijing University of Chinese Medicine for their assistance on study selection and data extraction. We also greatly thank the reviewers for their feedback and comments on this paper. Prof. Nicola Robinson (visiting professor of Beijing University of Chinese Medicine) is funded by International development and capacity enhancement of evidence-based Chinese medicine Project, Ministry of Science and Technology of the People's Republic of China (G20200001187).
Note: Acknowledgments are developed with the explicit statement that this does not constitute an endorsement of the report.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.ctim.2021.102744.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.World Health Organization (WHO) 2020. Novel coronavirus – China.http://www.who.int/csr/don/12-january-2020-novel-coronavirus-china/en/ Jan 12. [Google Scholar]
- 2.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(February (10223)):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization (WHO) 2020. WHO Statement regarding cluster of pneumonia cases in Wuhan, China.https://www.who.int/china/news/detail/09-01-2020-who-statement-regarding-cluster-of-pneumonia-cases-in-wuhan-china Jan 9. [Google Scholar]
- 4.World Health Organization (WHO) 2020. WHO Director-General’s remarks at the media briefing on 2019-nCo.https://www.who.int/dg/speeches/detail/who-director-general-s-remarks-at-the-media-briefing-on-2019-ncov-on-11-february-2020 Feb 11. [Google Scholar]
- 5.Khan M., Adil S.F., Alkhathlan H.Z., et al. COVID-19: a global challenge with old history, epidemiology and progress so far. Molecules. 2020;26(December (1)):E39. doi: 10.3390/molecules26010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Health Commission of the People’s Republic of China . 7th edition. 2020. Diagnosis and treatment protocol for novel coronavirus pneumonia.http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml Mar 4. [Google Scholar]
- 7.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(February (10223)):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang X.Y., Huang H.J., Zhuang D.L., et al. Biological, clinical and epidemiological features of COVID-19, SARS and MERS and AutoDock simulation of ACE2. Infect Dis Poverty. 2020;9(July (1)):99. doi: 10.1186/s40249-020-00691-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paules C.I., Marston H.D., Fauci A.S. Coronavirus infections-more than just the common cold. JAMA. 2020;323(February (8)):707–708. doi: 10.1001/jama.2020.0757. [DOI] [PubMed] [Google Scholar]
- 10.Zhu N., Zhang D., Wang W., et al. China Novel Coronavirus Investigating and Research Team. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382(February (8)):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holshue M.L., DeBolt C., Lindquist S., et al. Washington State 2019-nCoV Case Investigation Team. First Case of 2019 Novel Coronavirus in the United States. N Engl J Med. 2020;382(March (10)):929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lei J., Li J., Li X., et al. CT imaging of the 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology. 2020;295(April (1)):18. doi: 10.1148/radiol.2020200236. Epub 2020 Jan 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization (WHO) 2020. WHO Director-General’s opening remarks at the media briefing on COVID-19. March 11. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 [Accessed June 12, 2020] [Google Scholar]
- 14.World Health Organization (WHO) 2021. WHO coronavirus disease (COVID-19) dashboard.https://covid19.who.int/ Jan 8. [Google Scholar]
- 15.Sharma O., Sultan A.A., Ding H., et al. A review of the progress and challenges of developing a vaccine for COVID-19. Front Immunol. 2020;11(October):585354. doi: 10.3389/fimmu.2020.585354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knoll M.D., Wonodi C. Oxford-AstraZeneca COVID-19 vaccine efficacy. Lancet. 2021;397(January (10269)):72–74. doi: 10.1016/S0140-6736(20)32623-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matthay M.A., Thompson B.T. Dexamethasone in hospitalised patients with COVID-19: addressing uncertainties. Lancet Respir Med. 2020;8(December (12)):1170–1172. doi: 10.1016/S2213-2600(20)30503-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi Y., Gao H., Zhao L., et al. Preliminary discussion for COVID-19 and wind cold dampness plague. Zhong Hua Zhong Yi Yao Xue Kan. 2020;38(03):4–6. doi: 10.13193/j.issn.1673-7717.2020.03.002. [DOI] [Google Scholar]
- 19.Ren J.L., Zhang A.H., Wang X.J. Traditional Chinese medicine for COVID-19 treatment. Pharmacol Res. 2020;155 doi: 10.1016/j.phrs.2020.104743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Health Commission of the People’s Republic of China . 3rd edition. 2020. Notice on diagnosis and treatment protocol for novel coronavirus pneumonia.http://www.nhc.gov.cn/xcs/yqfkdt/202001/f492c9153ea9437bb587ce2ffcbee1fa.shtml Jan 23. [Google Scholar]
- 21.National Health Commission of the People’s Republic of China . 4th edition. 2020. Notice on diagnosis and treatment protocol for novel coronavirus pneumonia.http://www.nhc.gov.cn/xcs/yqfkdt/202001/4294563ed35b43209b31739bd0785e67.shtml Jan 27. [Google Scholar]
- 22.National Medical Products Administration . 2017. What in Chinese patent medicine.https://www.nmpa.gov.cn/xxgk/kpzhsh/kpzhshyp/20171024101101251.html Oct 24. [Google Scholar]
- 23.National Medical Products Administration . 2017. What are the commonly used dosage forms of Chinese patent medicine?https://www.nmpa.gov.cn/xxgk/kpzhsh/kpzhshyp/20171024101101627.html Oct 24. [Google Scholar]
- 24.Zhang D., Zhang B., Lv Jt, et al. The clinical benefits of Chinese patent medicine against COVID-19 based on current evidence. Pharmacol Res. 2020;157(July):104882. doi: 10.1016/j.phrs.2020.104882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Ah, Dong Jc. Chinese patent medicine used with or without western medicine for COVID-19: a systematic review and meta-analysis. Chinnese patent medicine: 1-6 [online]. http://kns.cnki.net/kcms/detail/31.1368.R.20200615.1633.002.html.
- 26.Hu K., Guan W.J., Bi Y., et al. Efficacy and safety of Lianhuaqingwen capsule (连花清瘟胶囊), a repurposed Chinese herb, in patients with coronavirus disease 2019: a multicenter, prospective, randomized controlled trial. Phytomedicine. 2020;(May) doi: 10.1016/j.phymed.2020.153242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang S.B., Zhang Y.Y., Shen C., et al. Chinese herbal medicine used with or without conventional therapy for COVID-19: an evidence review of clinical studies. Front Pharmacol. 2021;26(11):583450. doi: 10.3389/fphar.2020.583450. Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.The Cochrane Collaboration; 2020. Review manager (RevMan) [computer program]. Version5.4. [Google Scholar]
- 30.Higgins J.P.T., Thomas J., Chandler J., et al. Cochrane; 2020. Cochrane handbook for systematic reviews of interventions version 6.1 (updated September 2020)www.training.cochrane.org/handbook Available from. [Google Scholar]
- 31.Egger M., Davey Smith G., Schneider M., et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(September (7109)):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guyatt G.H., Oxman A.D., Kunz R., et al. What is "quality of evidence" and why is it important to clinicians? BMJ. 2008;336(May (7651)):995–998. doi: 10.1136/bmj.39490.551019.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duan C., Xia G.W., Zheng C.J., et al. Clinical observation on Jinhua Qinggan granules (金花清感颗粒) combined with conventional Western medicine therapy in treating mild cases of Coronavirus Disease 2019. J Chin Trad Med. 2020;61(17):1473–1477. [Google Scholar]
- 34.Xiao M., Tian J., Zhou Y., et al. Efficacy of Huoxiang Zhengqi dropping pills (藿香正气滴丸) and Lianhua Qingwen granules (连花清瘟颗粒) in treatment of COVID-19: a randomized controlled trial. Pharmacol Res. 2020;161(November) doi: 10.1016/j.phrs.2020.105126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fu X.X., Lin L.P., Tan X.H. Clinical study on treatment of cases of COVID-19 with toujie quwen granules (透解祛瘟颗粒) Chin J Exp Tradit Med Form. 2020;26(12):44–48. doi: 10.13422/j.cnki.syfjx.20201314. [DOI] [Google Scholar]
- 36.Sun H.M., Xu F., Zhang L., et al. Study on clinical efficacy of Lianhua Qingke Granules (连花清瘟颗粒) in treatment of mild and ordinary COVID-19. Chin J Exp Tradit Med Form. 2020;26(14):29–34. doi: 10.13422/j.cnki.syfjx.20201438. [DOI] [Google Scholar]
- 37.Yu P., Li Y.Z., Wan S.B., et al. Effects of Lianhua Qingwen granules (连花清瘟颗粒) plus arbidol on treatment of mild corona virus disease-19. Chin Pharm J. 2020;55(12):1042–1045. doi: 10.11669/cpj.2020.12.014. [DOI] [Google Scholar]
- 38.Fu X.X., Lin L.P., Tan X.H. Clinical study on 37 case of COVID-19 treated with integrated traditional Chinese and Western Medicine. Trad Chin Drug Res Pharmacol. 2020;31(05):600–604. doi: 10.13422/j.cnki.syfjx.20201314. [DOI] [Google Scholar]
- 39.Flower A., Witt C., Liu J.P., et al. GP-TCM Unabridged guidelines for randomised controlled trials investigating Chinese herbal medicine (CHM) Eur J Integr Med. 2014;6(2):186–210. doi: 10.1016/j.eujim.2013.07.011. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.