Abstract
Background
The emergence of increasing reports worldwide of a severe inflammatory process and shock in pediatric patients resembling Kawasaki disease (KD)—and, more specifically, Kawasaki disease shock syndrome (KDSS)—prompted us to explore KDSS in a preamble of a systematic comparison between the 2 conditions.
Methods
We completed a systematic review of KDSS and performed a meta-analysis comparison between reported KDSS cases and KD controls.
Results
A total of 10 case-control series were included in the meta-analysis. Patients with KDSS were older (38.4 ± 30.6 vs 21.9 ± 19.5 months; P < 0.001) compared with standard KD with equal sex distribution and completeness of clinical diagnostic criteria. KDSS present higher C-reactive protein (59.4 ± 29.2 mg/dL vs 20.8 ± 14.8 mg/dL; P < 0.001), lower albumin (2.7 ± 0.5 g/dL vs 3.3 ± 0.5 g/dL; P < 0.01), and lower platelets (255 ± 149 109/L vs 394 ± 132 109/L; P < 0.001) but only borderline higher white blood cells (P = 0.06). Differences in alanine transaminase, aspartate aminotransferase, and erythrocyte sedimentation rate were nonsignificant. The odds of intravenous immunoglobulin resistance (44.4% vs 9.6%; (P < 0.001) and the hospital length of stay (10.9 ± 5.8 vs 5.0 ± 3.0 days; P < 0.001) were higher in KDSS, as were the odds of coronary-artery abnormalities (33.9% vs 8.6%; P < 0.001).
Conclusions
This first meta-analysis on KDSS vs KD represents a basis for future works on KDSS and opens the opportunity for future multicentre studies in the search of causal relationships between presenting elements and the eventual complications of KDSS. The similarities between SARS-CoV-2 multisystem inflammatory syndrome in children and KDSS open new horizons to the understanding of the etiology and pathophysiology related to KDSS.
RÉSUMÉ
Contexte
À l’échelle mondiale, de plus en plus d'enfants sont touchés par un processus inflammatoire grave avec état de choc mimant la maladie de Kawasaki – nommément le syndrome de choc de la maladie de Kawasaki (Kawasaki disease shock syndrome ou KDSS). Cette situation nous a incités à mieux caractériser le KDSS en préambule à une comparaison systématique des deux pathologies.
Méthodologie
Nous avons passé systématiquement en revue les cas de KDSS et effectué une méta-analyse comparant les cas déclarés de KDSS à des témoins atteints de la maladie de Kawasaki.
Résultats
La méta-analyse a porté sur 10 séries cas/témoins en tout. Les patients atteints de KDSS étaient plus âgés (38,4 ± 30,6 mois vs 21,9 ± 19,5 mois; p < 0,001) que les patients présentant la forme classique de la maladie de Kawasaki, il y avait répartition égale par sexe et sur le taux des critères de diagnostic clinique réunis. Le KDSS se caractérise par une concentration plus élevée de protéine C-réactive (59,4 ± 29,2 mg/dl vs 20,8 ± 14,8 mg/dl; p < 0,001), une concentration plus faible d'albumine (2,7 ± 0,5 g/dl vs 3,3 ± 0,5 g/dl; p < 0,01) et un nombre moins élevé de plaquettes (255 ± 149 109/L vs 394 ± 132 109/L; p < 0,001), mais les taux de globules blancs sont à peine plus élevés (p = 0,06). Les différences relatives à l'alanine transaminase, à l'aspartate aminotransférase et à la vitesse de sédimentation érythrocytaire n’étaient pas significatives. Le risque de résistance aux immunoglobulines intraveineuses (44,4 % vs 9,6 %; (p < 0,001) et la durée d'hospitalisation (10,9 ± 5,8 vs 5,0 ± 3,0 jours; p < 0,001) étaient plus grands chez les patients atteints de KDSS, tout comme le risque d'anomalies coronariennes (33,9 % vs 8,6 %; p < 0,001).
Conclusions
Cette première méta-analyse comparative portant sur le KDSS et la maladie de Kawasaki jette les bases de travaux ultérieurs sur le KDSS et prépare la voie pour des études multicentriques axées sur la recherche des relations causales entre les signes cliniques et les complications possibles du KDSS. Les similitudes entre le syndrome inflammatoire multisystémique pédiatrique lié au SRAS-CoV-2 et le KDSS ouvrent de nouvelles perspectives en matière de compréhension des aspects étiologiques et physiopathologiques du KDSS.
Kawasaki disease (KD) is the leading cause of acquired childhood heart diseases in developed countries, mostly affecting children below the age of 5. It is classified among the acute immune vasculitis and may result in coronary-artery aneurysms, as they account for the most severe complication; they occur in 25% of untreated cases of KD.1 A single high dose of intravenous immunoglobulin (IVIG), combined with aspirin, is the most efficient primary treatment for KD, as it reduces the risk of coronary-artery abnormalities and resolves inflammation.1 The generalized aspect of KD sometimes implies a multiorgan involvement, which might include cerebral involvement (eg, aseptic meningitis, cerebral stroke),2 renal involvement (eg, proteinuria, hematuria, sterile pyuria, renal failure),3 gastrointestinal involvement (eg, diarrhea, vomiting, abdominal pain), hepatic involvement (raised liver enzymes), and other organs.4 This multiorgan dysfunction is also observed in a rare form of KD called Kawasaki disease shock syndrome (KDSS). Cases of KDSS were reported over the last 15 years but remain rare, representing 1% to 7% of KD.5 KDSS is described as KD involving a patient with complete or incomplete criteria who requires resuscitation, inotropic or support with vasoactive agents, or extracorporeal membrane oxygenation (ECMO), with the shock being unrelated to an acute coronary event.
From another perspective, the etiology of KD remains unknown, but the widely accepted hypothesis is that KD is a result of immunologic response to nonspecific infection.6 , 7 Previous studies have shown associations between viral respiratory infections and KD.8, 9, 10 Thus, preceding exposure to an environmental trigger, such as a viral antigen in a genetically susceptible child, persists as the prevailing hypothesis to explain the hyperimmune response characteristic of KD. In 2005, a circumstantial association between the New Haven coronavirus outbreak and KD was advanced but was not supported by further assessment.11 Subsequent reports unsuccessfully attempted to link KD to other types of coronaviruses.8 , 12, 13, 14
In late 2019 to early 2020, the severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) pandemic caused lower respiratory tract infection and an acute respiratory syndrome in adults (COVID-19).15 Initial pediatric case series focused on characterizing SARS-CoV-2 rare respiratory illness in children, as less than 4% of pediatric cases require admission to intensive care units for inotropic or circulatory mechanical support.16 In April 2020, a group of KD experts published a warning letter regarding the possibility of a missed or delayed KD diagnosis amid the SARS-CoV-2 pandemic crisis, either by underdiagnosis or by parental sheltering of their children for fear of contracting the virus while consulting. The concern was that worse outcomes would ensue in severe cardiac consequences, such as coronary artery abnormalities.17 There were subsequent reports on children developing other types of critical illness, secondary to SARS-CoV-2 exposure, caused by a hyperimmune reaction with features of KD.18, 19, 20 This clinical syndrome is now termed by the US Centers for Disease Prevention and Control (CDC) as the multisystem inflammatory syndrome in children (MIS-C) and requires evidence of SARS-CoV-2 exposure to fulfill the case definition.21 , 22
As MIS-C reports emerged, similarities with KDSS surfaced from the clinical and the biological perspectives. Therefore, the increased prevalence of KDSS-like cases during the SARS-CoV-2 pandemic is an opportunity to investigate KDSS and its possible complications further. We herein review and summarize case series and reports pertaining to KDSS before the SARS-CoV-2 pandemic as a basis for a better understanding of KDSS and future comparison between the 2 entities. The objective of this paper was to describe the features of KDSS by performing a systematic review and a meta-analysis comparing KDSS with their KD controls. As the immunogenic triggers of the 2 conditions (KDSS/KD and MIS-C/SARS-CoV-2) bear some similarities, this article displays qualitative comparison between the 2 entities.
Methods
For the KDSS systematic review, and to perform the meta-analysis, we searched PubMed for case reports and case series reporting Kawasaki disease with shock. The search terms used were “Kawasaki disease,” “Kawasaki disease shock syndrome,” “Kawasaki disease + shock,” and “Kawasaki disease +hypotension.” Abstracts were reviewed to identify relevant articles. Studies were excluded if they were not in English or if they did not present sufficient information about their patients with shock (eg, shock intervention and treatment). The following data were collected from each article: patient demographics (including age, sex, ethnicity), clinical criteria for KD (complete or incomplete), elements of shock (low blood pressure, low perfusion, myocardial dysfunction), laboratory findings, treatment received for KD and shock, intensive care unit (ICU) length of stay and outcome, cardiac complications (coronary-artery aneurysms or dilatation, residual cardiac dysfunction).
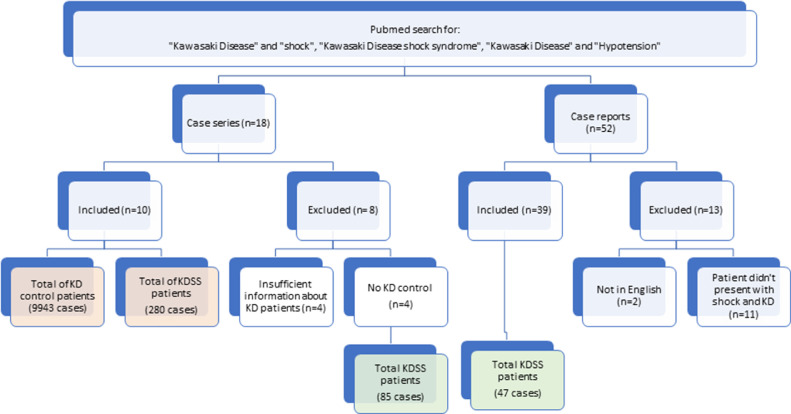
A total of 70 publications relating to KDSS were identified, including 52 case reports and 18 case series. After review, 39 case reports presenting 47 patients were included in the aggregated data collection, whereas 14 case reports were excluded (2 were not in English [1 was in Chinese and the other in German], and 11 included patients without shock). There were 18 case series, 4 of which were excluded for insufficient information. Of the remaining 14 case series, 4 case series did not have KD controls and were excluded from the meta-analysis but used for the aggregated data collection and presented a total of 85 patients with KDSS.23, 24, 25, 26 The following 10 case series comprised the elements for meta-analysis5 , 27, 28, 29, 30, 31, 32, 33, 34, 35 (Fig. 1 ). The KDSS group was composed of 280 patients, and the control group was composed of 9943 patients (9350 controls from Lin et al.35) KDSS diagnostic criteria used across the 10 studies were either patients with KD admitted to the ICU for hypotension or shock or patients with KD presenting with systolic hypotension for age following Kanegaye's definition.36
Figure 1.
Breakdown of systematic review and meta-analysis. Orange frames: total cases included in the meta-analysis; green frames: 132 total cases included in the aggregated data. KD, Kawasaki disease; KDSS, Kawasaki disease shock syndrome.
We only included case-control studies for our meta-analysis. Therefore, the KDSS/control comparison includes only data from the 10 reports that included a control group from the same population.5 , 27, 28, 29, 30, 31, 32, 33, 34, 35 Data from the 4 case series without KD controls were excluded from meta-analysis.24, 25, 26 , 36 For the meta-regression, a sensitivity analysis was done without the series by Lin et al.; given the high control N, the standard error of the difference is very low. The results are comparable, and thus the sensitivity analysis is not reported. Meta-regression was performed only if at least 5 of 10 studies reported a specific parameter. Meta-regressions were performed using the Metafor package for R. For each parameter, we report separately the weighted average for the 10 reports that are included in the KDSS/control comparisons and the weighted average for the 14 series and the combined case reports. A weighted average was performed for the aggregated data, which included the case reports and the excluded case series from the meta-analysis. When necessary, mean and standard deviations were estimated from median and interquartile range (IQR) or median and minimum/maximum, using the method proposed in Wan et al.37 Mean, standard deviation (SD), median, and frequencies were extracted and presented as number (percentage), median (range), or mean ± SD. The combined effect P value was calculated. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed for appropriate meta-analysis methodology.38 This study was exempt from ethics review.
Results
Patient characteristics
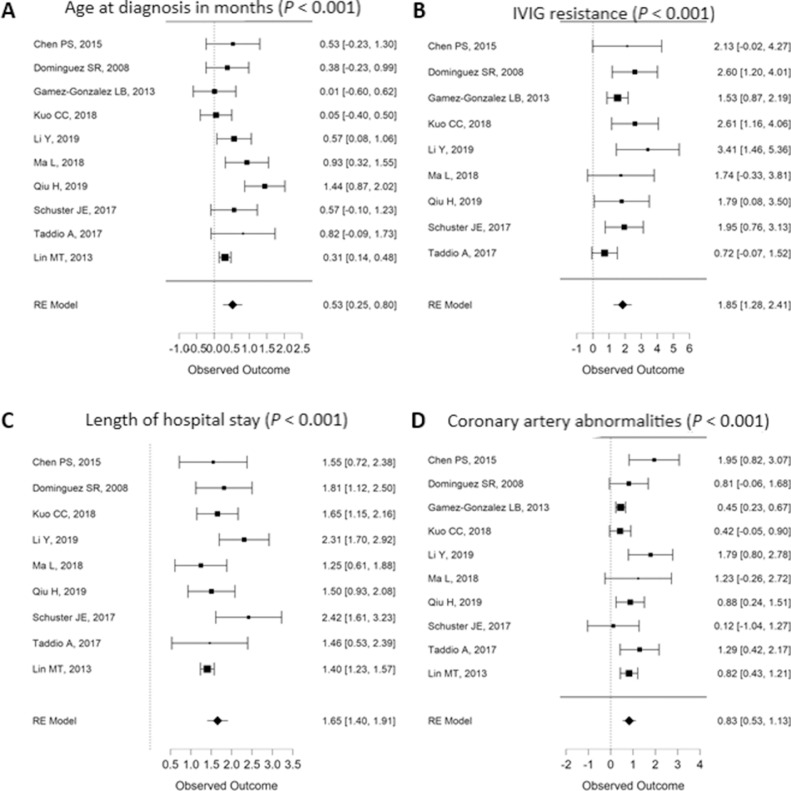
Patients with KDSS are, on average, older than their KD control counterparts, with an average age of 38.4 ± 30.6 months compared with 21.9 ± 19.5 months (Fig. 2 A). There seems to be no association between sex and KDSS, as female patients represent 35.7% of patients with KDSS and 38.2% of KD (P = 0.95). Overall, 9 publications originate from East Asia, whereas 4 case reports do not report ethnicity. Only 2 studies report data from a diverse group of patient ancestry-wise, and therefore it is not possible to conduct an unbiased analysis. There was no association between the type of KD based on the classical diagnostic clinical criteria (complete vs incomplete) and KDSS (P = 0.37) (Figs. 2 and 3 ).
Figure 2.
Meta-regression results reporting clinical aspects of Kawasaki disease shock syndrome/Kawasaki disease(observed outcome) by incorporated study and by combined effect representing significant factors (P values for combined effect). IVIG, intravenous immunoglobulin; RE, random-effect.
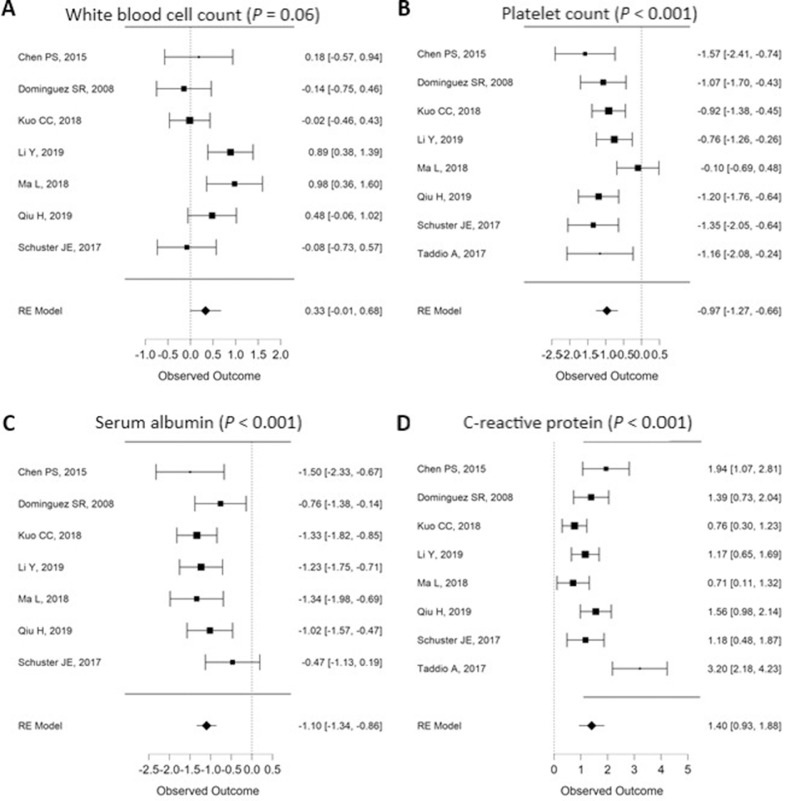
Figure 3.
Meta-regression results reporting laboratory data of Kawasaki disease shock syndrome/Kawasaki disease (observed outcome) of Kawasaki disease shock syndrome by incorporated study and by combined effect representing significant factors (P values for combined effect). RE, random-effect.
Clinical manifestations and laboratory results
The admitting diagnosis of KDSS cases is not KD in general, possibly leading to longer hospital stay, with the lowest study27 having 22% of their patients presenting with KD as initial diagnosis and the highest study31 presenting with 65%. KDSS is associated with slightly higher white blood cells (WBCs) and significantly lower platelet counts than controls (Fig. 3, A and B), lower serum albumin levels (Fig. 3C), and higher C-reactive protein (CRP) (Fig. 3D). KDSS was not associated with higher erythrocyte sedimentation rate (ESR) (P = 0.78), alanine transaminase (ALT) (P = 0.22), or aspartate aminotransferase (AST) (P = 0.15) (Table 1 ). Other cardiac biomarkers, such as troponin, were only reported in 2 studies, and γ-glutamyl transferase was only reported in a single study. Elevated D-dimers, although rarely reported owing to unavailable data, were particularly present in KDSS, accompanying the cytokine storm. Only recent reports presented patients with the macrophage-activation syndrome (MAS).31
Table 1.
Characteristics of cumulated cases from meta-analysis including treatment received and main outcomes
| KDSS | KD controls | P value | Studies included* | |
|---|---|---|---|---|
| Age (months) | 38.4 ± 30.6 | 21.9 ± 19.5 | < 0.001 | a,b,c,d,e,f,g,h,i,j |
| Male | 64.3% | 61.4% | 0.95 | a,b,c,d,e,f,g,h,i,j |
| Incomplete KD | 34.7 % | 23.5% | 0.37 | a,b,c,d,e,g,h,i |
| Treatment | NA | |||
| IVIG | 98.9% (n = 280) | 99.9% (n = 9593) | ||
| Aspirin | 98.8% (n = 252) | 100.0% (n = 9514) | ||
| Steroids | 36.7% (n = 98) | 11.0% (n = 219) | ||
| Shock treatment | NA | NA | ||
| Mechanical ventilation | 37.8% (n = 74) | |||
| Fluid resuscitation | 85.0% (n = 127) | |||
| Inotropes | 64.3% (n = 126) | |||
| Vasopressors | 54.3% (n = 127) | |||
| ECMO | 4.3% (n = 47) | |||
| Combination | 69.1% (n = 28) | |||
| Laboratory findings | ||||
| Albumin (g/dL) | 2.71 ± 0.54 | 3.33 ± 0.5 | < 0.001 | a,b,d,e,g,h,i |
| WBC (109/L) | 17.6 ± 8.5 | 14.7 ± 5.8 | 0.06 | a,b,d,e,g,h,i |
| Platelets (109/L) | 255 ± 149 | 394 ± 132 | < 0.001 | a,b,d,e,g,h,i,j |
| ESR (mm/h) | 67.7 ± 27.0 | 66.3 ± 30.2 | 0.78 | a,b,e,g,i,j |
| CRP (mg/dL) | 59.4 ± 29.2 | 20.8 ± 14.8 | < 0.001 | a,b,d,e,g,h,i,j |
| ALT (U/L) | 131 ± 88 | 59.4 ± 29.2 | 0.22 | b,e,g,h,i |
| AST(U/L) | 93 ± 76 | 62 ± 50 | 0.15 | b,e,g,h,i |
| IVIG resistance | 44.4% | 9.6% | < 0.001 | a,b,c,d,e,g,h,i,j |
| Length of hospital stay (days) | 10.9 ± 5.8 | 5.0 ± 3.0 | < 0.001 | a,b,d,e,f,g,h,i,j |
| Coronary artery abnormalities | 33.9% | 8.6% | < 0.001 | a,b,c,d,e,f,g,h,i,j |
| Cardiac findings | NA | |||
| Systolic dysfunction | 12.4% (n = 193) | 1.9% (n = 207) | ||
| Pericarditis | 22.2% (n = 45) | 5.7% (n = 386) | ||
| AV valve regurgitation | 32.8% (n = 64) | 9.2% (n = 207) | ||
| Death | 1.3% (n = 78) | 0.0% (n = 396) | NA |
Data are mean ± standard deviation (SD) and percentage (n = denominator).
ALT, alanine transaminase; AST, aspartate aminotransferase; AV, atrioventricular; CRP, C-reactive protein; ECMO, extracorporeal membrane oxygenation; ESR, erythrocyte sedimentation rate; IVIG, intravenous immunoglobulin; KD, Kawasaki disease; WBC, white blood cell.
Multiorgan components
Multiorgan dysfunction and myocardial systolic dysfunction were reported in a small number of studies and were therefore insufficient for comparative analysis. In general, patients with KDSS were reported to develop higher proportions of depressed left- ventricular systolic function (12.4%), pericardial effusions (22.2%), or atrioventricular valve regurgitation (32.8%) compared with KD (Table 1). Because of an overall low reporting on cardiac involvement in the references, we did not perform statistical comparison. From the pathophysiology perspective, 5 patients presented with a cardiogenic shock, whereas 3 presented with a distributive shock, suggesting that KDSS might cause various mechanisms of shock.25 The series from the Children's Hospital of Mexico City established that 90% of KDSS cases presented with gastrointestinal (GI) abnormalities (abdominal pain, vomiting, diarrhea, GI bleeding).29
Treatment and outcome
KDSS was associated with increased odds of occurrence of coronary-artery abnormality (CAA) compared with controls (prevalence of 33.9% vs 8.6%) (Table 1, Fig. 2D). The definition of CAA was not uniformly defined among the source series, however, which limits the qualitative discrimination between aneurysms and dilatation as well as the quantitative determination of size of the CAA. For instance, Gamez-Gonzalez et al.39 reported a very high prevalence of CAA in the control group (58%), which makes the CAA rate in that group questionable.
IVIG therapy was reported in all but 1 study for KDSS. In many studies, treatment with IVIG was among the inclusion criteria, and, as such, the rate of IVIG is universally high. No meta-regression analyses were performed for the use of IVIG, aspirin, and corticosteroid because of insufficient variance among studies. Overall, patients with KDSS received IVIG in 98.9% (n = 280), aspirin in 98.8% (n = 252), and corticosteroids in 36.7% (n = 98), whereas KD controls received IVIG in 99.9% (n = 9593), aspirin in 100% (n = 9514), and corticosteroids in 11.0% (n = 219). KDSS was associated with increased odds of IVIG resistance (44.4%) compared with controls (9.6%) (Fig. 2B). The length of hospital stay was significantly higher in KDSS 10.9 ± 5.8 days compared with 5.0 ± 3.0 days (Table 1, Fig. 2C). In terms of shock, when specific management was detailed, 97.1% received fluid resuscitation, 50% mechanical ventilation, 63.2% inotropic support, 63.8% vasopressors, and 78.6% a combination of treatment.
Aggregated data
The 38 case reports and the 4 case series were combined (Fig. 1) to present aggregated data on 132 patients with KDSS (Table 2 ). In general, these patients presented a very similar profile to the patients with KDSS from our meta-analysis. There was identical male predominance (58%, 77 of 132); high IVIG resistance (44%); similar shock treatment, with a predominance of fluid resuscitation and inotropic support with 35% receiving ventilation support. Some differences were seen between the group of the meta-analysis and the aggregated data. More patients with KDSS presented with incomplete KD (50%) in the aggregated data compared with meta-analysis estimate (34.7%). Use of aspirin for the treatment of KDSS was much less reported in the aggregated data group compared with the meta-analysis. From the cardiac-involvement perspective, systolic dysfunction was present in higher proportion in the aggregated data (34%) compared with the meta-analysis (12.4%), and CAA as well (reporting “abnormalities” in 15%, specifying dilatations and aneurysms in 31% and 22% respectively).
Table 2.
Characteristics of aggregated data of 132 patients with KDSS from 39 case reports and 4 case series
| Age (months) | 41.9 ± 31.3 |
| Male / total | 77/132 (58%) |
| Ethnicity | |
| White | 3/132 (2%) |
| Asian | 90/132 (68%) |
| African American | 2/132 (2%) |
| Hispanic | 1/132 (1%) |
| Other | 3/132 (2%) |
| Not specified | 33/132 (25%) |
| Incomplete KD | 29/58 (50%) |
| Type of shock | |
| KDSS, not specified otherwise | 117/132 (89%) |
| Myocardial | 10/132 (8%) |
| TSS | 4/132 (3%) |
| Treatment | |
| IVIG | 113/115 (98%) |
| Aspirin | 41/115 (36%) |
| Corticosteroids | 18/115 (16%) |
| Other immunosuppressive therapy | 12/115 (10%) |
| IVIG resistance | 51/115 (44%) |
| Admitted ICU | 128/132 (97%) |
| Treatment for shock | |
| Ventilation support | 20/65 (31%) |
| Fluid resuscitation | 41/65 (63%) |
| Inotropic support | 38/65 (58%) |
| Vasoactive agents | 25/65 (38%) |
| ECMO | 2/65 (3%) |
| Combination of treatment | 45/65 (69%) |
| Multiorgan dysfunction | 53/69 (77%) |
| Uveitis | 3/69 (5%) |
| Irritability | 5/69 (9%) |
| Gastrointestinal symptoms | 22/69 (38%) |
| Diarrhea | 13/69 (22%) |
| Vomiting | 23/69 (40%) |
| Abdominal pain | 14/69 (24%) |
| Hepatomegaly | 10/69 (17%) |
| Ascites | 2/69 (3%) |
| Renal dysfunction/failure | 15/69 (26%) |
| Respiratory difficulties | 23/69 (40%) |
| Cardiac involvement | |
| Coronary abnormality | 20/132 (15%) |
| Coronary dilatation | 41/132 (31%) |
| Coronary aneurysm | 29/132 (22%) |
| Giant coronary aneurysm | 0/132 (0%) |
| Systolic dysfunction | 20/58 (34%) |
| Mitral/tricuspid regurgitation | 9/132 (13%) |
| Laboratory findings | |
| Albumin (g/dL) | 2.28 ± 0.51 |
| WBC (109/L) | 15.2 ± 6.6 |
| Platelets (109/L) | 309 ± 148 |
| ESR (mm/h) | 71.3 ± 38.2 |
| CRP (mg/dL) | 87.6 ± 76.3 |
| ALT (U/L) | 120 ± 81 |
| AST(U/L) | 130.8 ± 96.8 |
| Death | 4/132 (3%) |
Mean ± SD, n (%), or median (range).
ALT, alanine transaminase; AST, aspartate aminotransferase; CRP, C-reactive protein; ECMO, extracorporeal membrane oxygenation; ESR, erythrocyte sedimentation rate; ICU, intensive care unit; IVIG, intravenous immunoglobulin; KD, Kawasaki disease; KDSS, KD shock syndrome; SD, standard deviation; TSS, toxic shock syndrome; WBC, white blood cell.
Discussion
KD, in its acute phase, can lead to KDSS, a relatively rare entity summarized in 15 studies5 , 23, 24, 25, 26, 27, 28 , 30, 31, 32, 33, 34, 35, 36 and 39 case reports published since 1993 on the subject. The cumulative information from the 10 papers included in the meta-analysis established the following features that enable distinguishing KDSS from the usual KD case presentation: (1) Usually, the admitting diagnosis of KDSS cases is not KD, leading possibly to longer hospital stay; (2) patients with KDSS are older than the usual patient with KD and have a higher prevalence and severity of GI symptoms; (3) patients with KDSS present with worse biological and inflammatory markers, (4) exhibit a higher resistance rate to IVIG, (5) have a higher rate of CAA, and (6) have greater number of reported cases of ventricular systolic dysfunction and atrioventricular valve regurgitation.
There are no explanations or attempts to explaining causal reasons for KDSS to occur beyond the observational clinical and laboratory features. Until now, only few papers report MAS and multiorgan failure in association with KDSS, suggestive of cytokine storm. Given the featured similarities with MIS-C, the current SARS-CoV-2 pandemic is a valuable opportunity to better understand enigmatic features of an old disease from the exhaustive dynamic research on a current new disease and vice versa.
Our meta-analysis on KDSS and KD highlights a few important points that can be taken into account by physicians to better distinguish those diseases in a clinical setting. Therefore, we present a few clinical investigations that should be performed routinely when evaluating patients with KD, KDSS, or MIS-C. First of all, when evaluating a patient presenting to the clinic or the hospital with shock, it is important to include KD as differential diagnosis, as it is often missed, leading to longer hospitalization and severe complications. The same is to be done with patients admitted with MIS-C. It is also important to assess for previous infections or viral triggers that could have potentially started the inflammation. To obtain a better profile of patients with KD and KDSS across various locations, it is important to order the appropriate laboratory tests when presented with a patient with shock or a patient with features of KD. Albumin, WBC, platelets, and ESR are essential to assess inflammation, CRP, B-type natriuretic peptide (BNP)/N-terminal (NT)-proBNP and troponins for cardiac function, ALT and AST for liver function, interleukins and D-dimers to assess the cytokine storm. A study by Park et al. found that patients with KDSS presented with conjunctival injection or cervical lymphadenopathy and higher ESR and CRP compared with patients with toxic shock syndrome (TSS), which could help better distinguish KDSS.40 Patients with KDSS also tend to have higher ESR and CRP in comparison with patients with TSS. Adherence to a standard set of investigations could help form a structured registry of patients with KDSS and MIS-C, which could help better understand those syndromes and promote better patient outcomes.
The initial circumstantial evidence suggesting a causal link between MIS-C and COVID-19 is now supported by serology data and immunologic mechanisms. Patients initially presented with MIS-C within 2 to 6 weeks following the outbreak of the pandemic. The main characteristics of MIS-C present many similarities with KDSS, which have been qualitatively compared in Table 3 . Although MIS-C is reportedly associated with lymphopenia, reports on KDSS do not provide lymphocyte counts.
Table 3.
Comparison between KDSS and MIS-C according to the MMWR CDC report41
| KDSS* | MIS-C† | |
|---|---|---|
| Clinical |
|
|
| Biological |
|
|
BNP/NT-proBNP, B-type natriuretic peptide-N-terminal pro-BNP; CDC, US Centers for Disease Prevention and Control; CRP, C-reactive protein; GI, gastrointestinal; IQR, interquartile range; IVIG, intravenous immunoglobulin; KD, Kawasaki disease; KDSS, KD shock syndrome; MIS-C, multisystem inflammatory syndrome in children; MMWR, Morbidity Mortality Weekly Report; PICU, pediatric intensive care unit; SARS-CoV-2, severe acute respiratory syndrome-coronavirus-2 TNF, tumour necrosis factor; WBC, white blood cell.
Age, male to female ratio, and complete vs incomplete diagnostic criteria for KD are based on the current meta-analysis.
As reported by the CDC.41
“Of 2143 Chinese children diagnosed with laboratory-verified or clinically diagnosed COVID-19, 5.2% had severe disease, and 0.6% had critical disease.”50
“From 2.6 to 6.95% in Western countries (vs) 1.45% and 1.9% in Taiwan.”5
In the spring of 2020, the CDC collected 570 suspected MIS-C cases in the United States.41 The ensuing report described 3 distinct classes based on Latent Class Analysis. Class 1, (203 patients [35.6%], median age 9 years) had severe multiorgan involvement; one-half of them with 6 or more organ-system involvement, with a predominance for the GI tract in 97.5% and the cardiovascular system in 100%. This group had the highest rate of complications and high markers of inflammation and cardiac involvement (rise in troponins, elevated BNP, shock, congestive heart failure, cardiac dysfunction, myocarditis, coronary abnormalities, pericardial effusion, and mitral regurgitation). Class 2, (169 patients [29.6%], median age: 10 years), in which the respiratory system involvement was predominant, including the acute respiratory distress syndrome, had the highest death toll (5.3%). Class 3, (198 patients [34.7%], median age 6 years) was the youngest age group, had the lowest comorbidities, organ system involvement (including cardiac damage), and markers of inflammation. This latter class presented the highest prevalence of skin rash and mucocutaneous lesions, was “less severe and [presented] clinical manifestations that overlapped with Kawasaki disease, [where] distinguishing [those] patients from those with true Kawasaki disease could be difficult.” From the biomarkers’ detection perspective, Class 1 cases had the highest positive serology (98%) but not necessarily positive SARS-CoV-2 real-time reverse transcriptase-polymerase chain reaction (RT-PCR) tests. Class 2 cases had the highest RT-PCR positivity without seropositivity instead (84%), along with the acute respiratory clinical pictures. Class 3 cases figured between Class 1 and Class 2 in SARS-CoV-2 seropositivity alone in 63.1% and seropositivity with positive RT-PCR results in 33.8%. From the cardiac perspective, the 3 classes had no statistically significant difference in the prevalence of CAA (21.1%, 15.8%, and 18.2%, for the respective classes); instead, the prevalence was decremental between Classes 1 to 2 to 3 for shock, congestive heart failure, cardiac dysfunction, myocarditis, hypotension, pericardial effusion, and mitral regurgitation.
A comprehensive comparison between KDSS and MIS-C was reported on the UK series vs a series of patients with KDSS diagnosed between 2002 and 2019 in San Diego, California.42 Accordingly, MIS-C and KDSS are thought be 2 different entities. Although we agree, in general, with the latter, one should be conscious of the fact that the 2 series are drawn from 2 different populations, with different social-ethnic makeup, in 2 remote geographic areas, which were exposed to totally different infectious and environmental triggers.43 The comparison between the results from our meta-analysis and MIS-C literature identified striking similarities, with higher inflammatory response compared with KD, elevated myocardial markers, and both reporting MAS and elements of cytokine storm response. As lymphopenia is not typically searched for in KD, there are no available data in the KDSS literature to verify whether this occurs, in what frequency, and at what intensity. At this state of knowledge surrounding the various case definitions of MIS-C, the lack of precision discerning KD and KD-like presentation associated to SARS-CoV2, and the potential mixing between the 2 entities in this early MIS-C reporting, we elected to refrain from quantitative comparison between KDSS and MIS-C. It would be more appropriate to perform in-depth quantitative comparison once the case definition of MIS-C is upgraded to higher standards of certainty. The clinical pictures of MIS-C and KDSS are also very similar, with a few differences, such as older age, sex distribution as well as ethnic backgrounds and race. The latter observation, including the relative rarity in the Asian populations, raises questions concerning the etiology and genetic component of both conditions. Clinical outcome related to the impact on the coronary arteries (dilatations or aneurysms) and the myocardial function (typically responsive to IVIG), poor perfusion and shock, as well as the low mortality rate are other common grounds of similarities between MIS-C and KDSS.
The association between KD and respiratory viruses have always existed, including corona viruses in particular. For instance, pathology specimen from deceased patients with KD were extensively studied by Rowley et al., advancing that RNA respiratory viruses could be the infectious trigger of KD.9 , 44 In 2004, van Der Hoek et al. discovered HCoV-NL63, a novel human coronavirus, which caused many respiratory tract illnesses.45 The following year, Esper et al. suggested an association between the New Haven coronavirus (HCoV-NH) and a small series of KD.11 The association between HCoV-NH and KD was refuted in the following years in Japan, the United States, and Taiwan, 46, 47, 48 as the association between HCoV-NL63 and KD was likewise refuted.13 , 14 , 49 Since then, in 2014 Chang et al. proposed that patients with KD had higher chances of being infected by coronavirus than temporal local controls,8 and their hypothesis was supported by Shirato et al., who found a possible association between another strain of coronavirus (HCoV-229E or the Sendai-H serotype) and KD.12 In this line of thought, KDSS should be maintained in the list of differential diagnoses of MIS-C or shock with or without presenting features of KD, notwithstanding the circumstantial association between the clinical presentation and the exposure and or infection with SARS-CoV-2.
Risk of bias
KDSS data are from retrospective series of consecutive cases in single institutions, which exclude selection bias. Controls varied between random selection,5 , 31 , 34 , 35 , 39 seasonal, sex and age matching,27 , 30 or date of admission.28 , 32 , 33
Limitations
The main limitation of the meta-analysis is related to the rarity of KDSS and therefore the number of eligible studies. As such, some information is not readily available for proper statistics. The case definition of coronary lesions and precision among dilatation, aneurysm, and giant aneurysm are not uniformly considered in the reports. The numbers for coronary artery “abnormalities” may be overestimated, as a result of the mix of types of lesions in some reports. Finally, we could not perform a cause-to-effect analysis in the meta-analysis because basic characteristics and baseline biological and laboratory data were not available in association with outcomes in a patient-by-patient–tabulated format in most series. We intend, however, to study cause-to-effect relationship further in a multicentre retrospective study.
Conclusions
This first meta-analysis represents a basis for future works on KDSS and opens the opportunity for future multicentre studies in the search of causal relationships between presenting elements and the eventual complications of KDSS. KDSS is currently acknowledged to be a subentity of KD. However, the similarities among the new pediatric manifestations of SARS-CoV-2, MIS-C, and KDSS opens new horizons to the understanding of the etiology and pathophysiology related to KDSS.
Funding Sources
No funding was received for this study.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
See page 1627 for disclosure information.
References
- 1.McCrindle BW, Rowley AH, Newburger JW, et al. diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. 2017;135:e927–ee99. doi: 10.1161/CIR.0000000000000484. [DOI] [PubMed] [Google Scholar]
- 2.Yeom JS, Cho JY, Woo HO. Understanding the importance of cerebrovascular involvement in Kawasaki disease. Korean J Pediatr. 2019;62:334–339. doi: 10.3345/kjp.2019.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonany PJ, Bilkis MD, Gallo G, et al. Acute renal failure in typical Kawasaki disease. Pediatr Nephrol. 2002;17:329–331. doi: 10.1007/s00467-002-0844-z. [DOI] [PubMed] [Google Scholar]
- 4.Zulian F, Falcini F, Zancan L, et al. Acute surgical abdomen as presenting manifestation of Kawasaki disease. J Pediatr. 2003;142:731–735. doi: 10.1067/mpd.2003.232. [DOI] [PubMed] [Google Scholar]
- 5.Li Y, Zheng Q, Zou L, et al. Kawasaki disease shock syndrome: clinical characteristics and possible use of IL-6, IL-10 and IFN-γ as biomarkers for early recognition. Pediatr Rheumatol Online J. 2019;17:1. doi: 10.1186/s12969-018-0303-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagata S. Causes of Kawasaki disease; from past to present. Front Pediatr. 2019;7:18. doi: 10.3389/fped.2019.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuo HC, Pan CT, Huang YH, et al. Global investigation of immune repertoire suggests Kawasaki disease has infectious cause. Circ J. 2019;83:2070–2078. doi: 10.1253/circj.CJ-19-0206. [DOI] [PubMed] [Google Scholar]
- 8.Chang LY, Lu CY, Shao PL, et al. Viral infections associated with Kawasaki disease. J Formos Med Assoc. 2014;113:148–154. doi: 10.1016/j.jfma.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rowley AH, Shulman ST. The epidemiology and pathogenesis of Kawasaki disease. Front Pediatr. 2018;6:374. doi: 10.3389/fped.2018.00374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.L'Huillier AG, Brito F, Wagner N, et al. Identification of viral signatures using high-throughput sequencing on blood of patients with Kawasaki disease. Front Pediatr. 2019;7:524. doi: 10.3389/fped.2019.00524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Esper F, Shapiro ED, Weibel C, et al. Association between a novel human coronavirus and Kawasaki disease. J Infect Dis. 2005;191:499–502. doi: 10.1086/428291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shirato K, Imada Y, Kawase M, et al. Possible involvement of infection with human coronavirus 229E, but not NL63, in Kawasaki disease. J Med Virol. 2014;86:2146–2153. doi: 10.1002/jmv.23950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dominguez SR, Anderson MS, Glodé MP, Robinson CC, Holmes KV. Blinded case-control study of the relationship between human coronavirus NL63 and Kawasaki syndrome. J Infect Dis. 2006;194:1697–1701. doi: 10.1086/509509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lehmann C, Klar R, Lindner J, et al. Kawasaki disease lacks association with human coronavirus NL63 and human bocavirus. Pediatr Infect Dis J. 2009;28:553–554. doi: 10.1097/inf.0b013e31819f41b6. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. Multisystem inflammatory syndrome in children and adolescents with COVID-19: scientific brief. Available at:https://www.who.int/news-room/commentaries/detail/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19. Accessed September 23, 2021.
- 16.Götzinger F, Santiago-García B, Noguera-Julián A, et al. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health. 2020;4:653–661. doi: 10.1016/S2352-4642(20)30177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harahsheh AS, Dahdah N, Newburger JW, et al. Missed or delayed diagnosis of Kawasaki disease during the 2019 novel coronavirus disease (COVID-19) pandemic. J Pediatr. 2020;222:261–262. doi: 10.1016/j.jpeds.2020.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395:1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395:1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Belhadjer Z, Méot M, Bajolle F, et al. Acute heart failure in multisystem inflammatory syndrome in children in the context of global SARS-CoV-2 pandemic. Circulation. 2020;142:429–436. doi: 10.1161/CIRCULATIONAHA.120.048360. [DOI] [PubMed] [Google Scholar]
- 21.Loke YH, Berul CI, Harahsheh AS. Multisystem inflammatory syndrome in children: Is there a linkage to Kawasaki disease? Trends Cardiovasc Med. 2020;30:389–396. doi: 10.1016/j.tcm.2020.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention Health Alert Network. Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with Coronavirus Disease 2019 (COVID-19). 2020. Available at: https://emergency.cdc.gov/han/2020/han00432.asp. Accessed September 23, 2021.
- 23.Lin YJ, Cheng MC, Lo MH, Chien SJ. Early differentiation of Kawasaki disease shock syndrome and toxic shock syndrome in a pediatric intensive care unit. Pediatr Infect Dis J. 2015;34:1163–1167. doi: 10.1097/INF.0000000000000852. [DOI] [PubMed] [Google Scholar]
- 24.Liang YC, Chang CH, Lin MT, Kao FY, Huang SK, Wu MH. Shock and unresponsiveness to repeated courses of intravenous immunoglobulin in Kawasaki disease: a nationwide database study. Pediatr Res. 2020;87:961–966. doi: 10.1038/s41390-019-0668-1. [DOI] [PubMed] [Google Scholar]
- 25.Gatterre P, Oualha M, Dupic L, et al. Kawasaki disease: an unexpected etiology of shock and multiple organ dysfunction syndrome. Intensive Care Med. 2012;38:872–878. doi: 10.1007/s00134-012-2473-8. [DOI] [PubMed] [Google Scholar]
- 26.Zhang MM, Shi L, Li XH, Lin Y, Liu Y. Clinical analysis of Kawasaki disease shock syndrome. Chin Med J (Engl) 2017;130:2891–2892. doi: 10.4103/0366-6999.219151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen PS, Chi H, Huang FY, et al. Clinical manifestations of Kawasaki disease shock syndrome: a case-control study. J Microbiol Immunol Infect. 2015;48:43–50. doi: 10.1016/j.jmii.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 28.Dominguez SR, Friedman K, Seewald R, et al. Kawasaki disease in a pediatric intensive care unit: a case-control study. Pediatrics. 2008;122:e786–e790. doi: 10.1542/peds.2008-1275. [DOI] [PubMed] [Google Scholar]
- 29.Gámez-González LB, Murata C, Muñoz-Ramírez M, Yamazaki-Nakashimada M. Clinical manifestations associated with Kawasaki disease shock syndrome in Mexican children. Eur J Pediatr. 2013;172:337–342. doi: 10.1007/s00431-012-1879-1. [DOI] [PubMed] [Google Scholar]
- 30.Kuo CC, Lee YS, Lin MR, et al. Characteristics of children with Kawasaki disease requiring intensive care: 10 years' experience at a tertiary pediatric hospital. J Microbiol Immunol Infect. 2018;51:184–190. doi: 10.1016/j.jmii.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 31.Ma L, Zhang YY, Yu HG. Clinical manifestations of Kawasaki disease shock syndrome. Clin Pediatr (Phila) 2018;57:428–435. doi: 10.1177/0009922817729483. [DOI] [PubMed] [Google Scholar]
- 32.Qiu H, Li C, He Y, Weng F, et al. Association between left ventricular ejection fraction and Kawasaki disease shock syndrome. Cardiol Young. 2019;29:178–184. doi: 10.1017/S1047951118002056. [DOI] [PubMed] [Google Scholar]
- 33.Schuster JE, Palac HL, Innocentini N, et al. Hyponatremia is a feature of Kawasaki disease shock syndrome: a case-control study. J Pediatric Infect Dis Soc. 2017;6:386–388. doi: 10.1093/jpids/piw081. [DOI] [PubMed] [Google Scholar]
- 34.Taddio A, Rossi ED, Monasta L, et al. Describing Kawasaki shock syndrome: results from a retrospective study and literature review. Clin Rheumatol. 2017;36:223–228. doi: 10.1007/s10067-016-3316-8. [DOI] [PubMed] [Google Scholar]
- 35.Lin MT, Fu CM, Huang SK, Huang SC, Wu MH. Population-based study of Kawasaki disease shock syndrome in Taiwan. Pediatr Infect Dis J. 2013;32:1384–1386. doi: 10.1097/INF.0b013e31829efae6. [DOI] [PubMed] [Google Scholar]
- 36.Kanegaye JT, Wilder MS, Molkara D, et al. Recognition of a Kawasaki disease shock syndrome. Pediatrics. 2009;123:e783–e789. doi: 10.1542/peds.2008-1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gamez-Gonzalez LB, Moribe-Quintero I, Cisneros-Castolo M, et al. Kawasaki disease shock syndrome: unique and severe subtype of Kawasaki disease. Pediatr Int. 2018;60:781–790. doi: 10.1111/ped.13614. [DOI] [PubMed] [Google Scholar]
- 40.Park WY, Lee SY, Kim GB, et al. Clinical aspects for differential diagnosis of Kawasaki disease shock syndrome: a case control study. BMC Pediatr. 2021;21:25. doi: 10.1186/s12887-020-02488-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Godfred-Cato S, Bryant B, Leung J, et al. COVID-19-associated multisystem inflammatory syndrome in children: United States, March-July 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1074–1080. doi: 10.15585/mmwr.mm6932e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Whittaker E, Bamford A, Kenny J, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324:259–269. doi: 10.1001/jama.2020.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Portman MA, Cimaz R. Should coronavirus disease 2019-associated inflammatory syndromes in children affect social reintegration? JAMA Pediatr. 2020;174:827–828. doi: 10.1001/jamapediatrics.2020.2810. [DOI] [PubMed] [Google Scholar]
- 44.Rowley AH, Baker SC, Shulman ST, et al. RNA-containing cytoplasmic inclusion bodies in ciliated bronchial epithelium months to years after acute Kawasaki disease. PLoS One. 2008;3:e1582. doi: 10.1371/journal.pone.0001582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van der Hoek L, Pyrc K, Jebbink MF, et al. Identification of a new human coronavirus. Nat Med. 2004;10:368–373. doi: 10.1038/nm1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ebihara T, Endo R, Ma X, Ishiguro N, Kikuta H. Lack of association between New Haven coronavirus and Kawasaki disease. J Infect Dis. 2005;192:351–352. doi: 10.1086/430797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Belay ED, Erdman DD, Anderson LJ, et al. Kawasaki disease and human coronavirus. J Infect Dis. 2005;192:352–353. doi: 10.1086/431609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chang LY, Chiang BL, Kao CL, et al. Lack of association between infection with a novel human coronavirus (HCoV), HCoV-NH, and Kawasaki disease in Taiwan. J Infect Dis. 2006;193:283–286. doi: 10.1086/498875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baker SC, Shimizu C, Shike H, et al. Human coronavirus-NL63 infection is not associated with acute Kawasaki disease. Adv Exp Med Biol. 2006;581:523–526. doi: 10.1007/978-0-387-33012-9_94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dong Y, Mo X, Hu Y, et al. Epidemiology of COVID-19 among children in China. Pediatrics. 2020:145. doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]