Abstract
Dihydroorotate dehydrogenase (DHODH) is rate-limiting enzyme in biosynthesis of pyrimidone which catalyzes the oxidation of dihydro-orotate to orotate. Orotate is utilized in the biosynthesis of uridine-monophosphate. DHODH inhibitors have shown promise as antiviral agent against Cytomegalovirus, Ebola, Influenza, Epstein Barr and Picornavirus. Anti-SARS-CoV-2 action of DHODH inhibitors are also coming up. In this review, we have reviewed the safety and efficacy of approved DHODH inhibitors (leflunomide and teriflunomide) against COVID-19. In target-centered in silico studies, leflunomide showed favorable binding to active site of MPro and spike: ACE2 interface. In artificial-intelligence/machine-learning based studies, leflunomide was among the top 50 ligands targeting spike: ACE2 interaction. Leflunomide is also found to interact with differentially regulated pathways [identified by KEGG (Kyoto Encyclopedia of Genes and Genomes) and reactome pathway analysis of host transcriptome data] in cogena based drug-repurposing studies. Based on GSEA (gene set enrichment analysis), leflunomide was found to target pathways enriched in COVID-19. In vitro, both leflunomide (EC50 41.49 ± 8.8 μmol/L) and teriflunomide (EC50 26 μmol/L) showed SARS-CoV-2 inhibition. In clinical studies, leflunomide showed significant benefit in terms of decreasing the duration of viral shredding, duration of hospital stay and severity of infection. However, no advantage was seen while combining leflunomide and IFN alpha-2a among patients with prolonged post symptomatic viral shredding. Common adverse effects of leflunomide were hyperlipidemia, leucopenia, neutropenia and liver-function alteration. Leflunomide/teriflunomide may serve as an agent of importance to achieve faster virological clearance in COVID-19, however, findings needs to be validated in bigger sized placebo controlled studies.
Keywords: DHODH inhibitor, Leflunomide, Teriflunomide, SARS-CoV-2, COVID-19, Drug repurposing
1. Introduction
COVID-19 pandemic has become a major cause of mortality and morbidity at this current point of time across the world (Prajapat et al., 2020a; Vm et al., 2020). The coronavirus invades the host via binding of S1-domain of the spike protein of SARS-CoV-2 with the host receptor angiotensin Converting enzyme-2 (ACE-2) leading to conformational changes in spike protein (S1 and S2 domains) (Prajapat et al., 2020b, p. 2), which leads to exposure of “fusion peptide” of S2 domain and subsequent fusion of the virus and host cell membranes, thereby leading to release of viral RNA genome into the host (Prajapat et al., 2020a; Shekhar et al., 2020). Host-cell machinery is then utilized for the synthesis of polyproteins which further releases structural (S, M, E proteins) and nonstructural proteins (NSP 1–16) after processing by proteases. NSPs participates further in the replicase transcriptase complex formation and viral RNA replication. All these processes in coherence leads to generation of new viral progenies, which are released into extracellular environment by exocytosis (Prajapat et al., 2020a). The associated cytokine storm, sequestration of inflammatory cells in lung tissue, host cell apoptosis and resultant loss of type I and II pneumocytes all takes part in the pathogenesis of COVID-19 and results in clinical manifestations like respiratory distress, sepsis, coagulopathy, organ injury and other complications (Parasher, 2020).
Various virus and host directed therapeutic measures are being tried e.g. favipiravir (Prajapat et al., 2020b, p. 2; Vm et al., 2020), tocilizumab (Gupta et al., 2021, p. 19), ivermectin (Harpinder Kaur et al., 2021) etc. The questionable efficacy of hydroxychloroquine (Sarma et al., n.d.) and Lopinavir/Ritonavir is under scanner now (Bhattacharyya et al., 2020). Among all, steroid therapy is showing some promise especially among severe and critical patients (Sarma et al., 2020a). Role of povidone iodine is also coming up as a prophylactic agent (Khan et al., 2020; Sarma et al., 2020b, 2020c, p. 2). Other agents under evaluation are remdesivir (Rezagholizadeh et al., 2021) folic acid (Bhattacharyya et al., 2021, p. 19; Hardeep Kaur et al., 2021) etc. Various vaccine candidates are also under clinical trials for the same. However, we are far behind from finding a potent and clinically effective anti-COVID-19 medication with low toxicity profile and this is the need of the day.
Apart from various therapies being explored for their possible-role in therapeutics against COVID-19, the role of dihydroorotate dehydrogenase (DHODH) inhibitors are coming up gradually. DHODH is an important rate limiting enzyme in the “pyrimidine biosynthesis” pathway and thus play a vital role in viral replication. Here, in this review we narrate the mechanism of antiviral action of DHODH inhibitors and the evidence of safety and efficacy of two FDA approved DHOH inhibitors: leflunomide and its active metabolite teriflunomide for their possible role in the treatment and management of COVID-19.
1.1. Pyrimidine biosynthesis and the role of dihydroorotate dehydrogenase (DHODH)
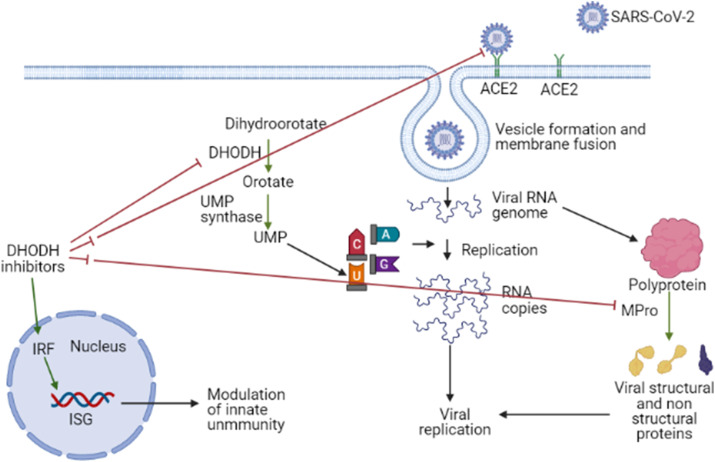
The viral replication inside host cells is reliant upon the “pyrimidine synthesis” bio-mechanics of the host. Pyrimidine building blocks are the structural basis of many physiologically important biomolecules e.g. RNA, DNA, glycoproteins and phospholipids and these important biomolecules are critically important for cell survival and proliferation (Xu and Jiang, 2020). In the pyrimidine biosynthesis process, dihydroorotate dehydrogenase (DHODH) is a rate-limiting enzyme (Mei-jiao et al., 2019) and catalyzes the oxidation of dihydro-orotate, which results in formation of orotate (Xu and Jiang, 2020), which is utilized in the bio-synthesis of uridine-monophosphate (UMP) by the enzyme uridine-monophosphate synthase subsequently (Mei-jiao et al., 2019). The same is represented in <b>Fig. 1 .
Fig. 1.
Virus directed and host directed actions of DHODH inhibitors as antiviral agents. DHODH plays an important role in generation of UMP, which is used by the virus for its replication. Thus DHODH inhibitors may hamper the RNA replication process of the virus. By acting on the interferon system, DHODH inhibitors also plays crucial role in activation of innate immune system. Leflunomide/teriflunomide shows binding to the spike: ACE2 interface (in silico evidence), binds to active site of M-Pro of SARS-CoV-2 (in silico evidence). ┤indicates inhibition and ┤┤indicates possible inhibition (only in silico evidence). Green arrow → indicates activation of a pathway. ISG: Interferon stimulated genes, IRF: Interferon regulatory factors. MPro: Main protease, DHODH: Dihydroorotate dehydrogenase, UMP: Uridine monophosphate.
1.2. </b>DHODH inhibitors and innate immunity
Interferons play a major role in the innate immunity system. Interferon inducible genes are mediators of antiviral effect of IFNs. Interferon regulatory factor-1 (IRF-1) and IRF-2 are major regulators of interferon genes (Harada et al., 1994). IRF-1 acts as a transcription repressor or activator on a number of genes by binding to specific response element in their promoters. IRFs are also essential for adaptive immunity through their role in elicitation of innate pattern recognition receptors (Yanai et al., 2012) and thus takes important part in immune-regulation and induction of expression of interferon genes (Brien et al., 2011).
DHODH inhibitors induce interferon simulated genes (ISG) and thus strengthens the innate immune system and can act as host directed therapy against viral infections (Lucas-Hourani et al., 2013). A DHODH inhibitor FA-613, which is active against influenza A & B, SARS and MERS (Cheung et al., 2017; Coelho and Oliveira, 2020), induces expression of IFN-β1 and ISG-15. Antiviral efficacy of FA-613 was lost in interferon-deficient vero-cells (Cheung et al., 2017; Coelho and Oliveira, 2020). Other DHODH inhibitors like DD264 (Brequinar) and SW835 also stimulated the production of IRF1 mediated expression of antiviral genes in human cells (Lucas-Hourani et al., 2013; Luthra et al., 2018). GSK-983, which also target activity of DHODH, activates immune response through IRF-1 and ATM mediated immune system stimulation (Coelho and Oliveira, 2020). The possible mechanism of stimulation of innate immunity by DHODH inhibitors is showed in Fig. 1.
1.3. Antiviral effect of DHODH inhibitors
In animal model (RAG−/− mice), two DHODH inhibitors (FK778 & Cmp1) inhibited the replication of CMV (Xiong et al., 2020). Other viruses/viral diseases against which efficacy of DHODH inhibitors are reported are Newcastle disease, Ebola, EBV and Picornavirus (Maghzi et al., 2020). On the basis of structure based virtual screening (against the ubiquinone-binding site of DHODH) and in vitro studies, Xiong R et al., 2020 identified two potent DHODH inhibitors (S416 and S312) which were found to be active against influenza-A virus (Xiong et al., 2020). Another DHODH inhibitor FA-613 was found to be active against influenza A & B, SARS and MERS (Cheung et al., 2017; Coelho and Oliveira, 2020).
1.4. Antiviral effects of approved DHODH inhibitors (leflunomide and teriflunomide)
DHODH inhibitors approved by FDA are leflunomide and teriflunomide. Leflunomide [N-(4- trifluoromethylphenyl)-5-methylisoxazole-4-carboxamide] belongs to the category of isoxazole compounds. After oral administration, it is rapidly metabolized to the active-metabolite teriflunomide (A77 1726) and hepatic cytosolic & microsomal fractions are implicated in its metabolism. In kinetic studies, the metabolite teriflunomide is primarily evaluated for PK-PD correlations (Rozman, 2002). These agents are approved as immunomodulators for the treatment of rheumatoid arthritis (Xu and Jiang, 2020) and multiple sclerosis (Xu and Jiang, 2020). These agents are also reported to have antiviral effect against different viruses e.g. cytomegalovirus (Gokarn et al., 2019; Silva et al., 2018), BK viremia (Chen et al., 2013; Nesselhauf et al., 2016), HIV-1 (Read et al., 2010), Junín virus (Sepúlveda et al., 2018) and Epstein-Barr virus (Zivadinov et al., 2019).
2. LEFLUNOMIDE/TERIFLUNOMIDE (approved DHODH inhibitors) in COVID-19
DHODH inhibitors are reported to have anti-SARS-CoV-2 effect (Xiong et al., 2020) and clinical case reports and studies are increasingly coming up on the same (Maghzi et al., 2020, p. 1). In this context, we have reviewed the safety and efficacy of FDA approved DHODH inhibitors (leflunomide and its metabolite teriflunomide) against “SARS-CoV-2” and in the evidence generation process; we reviewed data from in silico, in vitro, preclinical in-vivo and clinical studies.
2.1. Computational drug re-propositioning studies
2.1.1. Target-centered in silico based screening studies
Leflunomide was found to bind to two important targets of SARS-CoV-2, which are M-pro (Farag et al., 2020; Sencanski et al., 2020) and spike protein: ACE2 interface (Smith and Smith, 2020). In case of SARS-CoV-2 main protease (MPro), leflunomide was found to bind with both the central site of M-pro (S score of −7.1231 kcal/mol) and the allosteric pocket (binding energy −5.7 kcal/mol). In case of binding of leflunomide to spike protein: ACE2 interface, Smith et al. found leflunomide to be among the top posers among the 8000 screened candidates for binding to host recognition region of S-protein. However, none of the studies provided details of amino acid level interactions. [Data showed in Table 1 ].
Table 1.
Details of studies based upon target centered in-silico approach, AI/ML based screening approach, host transcriptional response and network biology based approach and in-vitro studies.
| S. No. | Type of study/Study design | Details of study/Study methodology | Result | References |
|---|---|---|---|---|
| 1 | Target-centered in-silico based screening |
Target: MPro Site targeted for in-silico design: Allosteric site of MPro |
Leflunomide binding energy −5.7 kcal/mol | Sencanski et al. (2020) |
| 2 | Target-centered in-silico based screening | Target: MPro (PDB: 6LU7) Site targeted for in-silico design: Inhibitory peptide (N3) binds to the substrate binding pocket of COVID-19 MPro. Central site of protease domain |
Leflunomide S score −7.1231 kcal/mol, Leflunomide was one of the top 100 drugs with good binding profile. However, they did not evaluate leflunomide in-vitro. |
Farag et al. (2020) |
| 3 | Target-centered in-silico based screening | Target: Spike protein: ACE2 interaction | Leflunomide was among the top 50 ligands. | Smith and Smith (2020) |
| 4 | AI/ML based screening |
Target: S Protein and S: ACE2 interaction surface Methodology: Autodock Vina scores of ligands against S-protein (n = 8120) and of S-Protein: human ACE2 (n = 5478) were used for training Random Forest (RF) machine learning model. The RF models were validated by conducting docking calculations. |
Leflunomide was one of the top 50 candidates | Batra et al. (2020) |
| 5 | Drug repurposing studies based upon host transcriptional response and network biology | The bronchoalveolar lavage fluid (BALF) of 8 COVID-19 patients and 20 healthy controls were analysed for transcriptomic changes. Differential expression analysis was performed with Bioconducter edgeR and limma packages and visualized with Principal Component Analysis (PCA). Pathway and drug repurposing analysis was performed on the cogena package. | Leflunomide was one of the top 20 enriched drugs. | Jia et al. (2020b) |
| 6 | Drug repurposing studies based upon host transcriptional response and network biology | RNA seq data from normal human bronchial epithelial cells, cells without ACE2 expression, Lung cancer expressing ACE2, and SARS-CoV-2 infected cells were obtained from Geo database. Significant fold changes (Fischer exact test, P = 0.1) was used to identify significant Gene Ontologies (GOs). Activated GOs were divided in subgroups by affinity propagation clustering (APclustering). The genes upregulated in each subgroup were analysed in the connectivity map (CMAP) database to identify potential drug candidates. | Leflunomide is among the top 100 identified possible candidates. | (Li et al., 2020, p. 19) |
| 7 | Drug repurposing studies based upon host transcriptional response and network biology | 380 unique Covid-19 drug targets were identified from 1) ClinicalTrials.gov, 2) Human proteins interacting with Sars-Cov-2, and 3) genes associated with SARS-CoV. The tissue specific gene expression data were obtained from databases e.g. GTEx. OpenTargets, ChEMBL, DrugBank and DGI platforms were used to map the drug targets with potential drugs. 726 target disease associations were prioritized by scoring on basis of 1) omics 2) Infection score, 3) Druggable score and 4) Target score. |
DHODH as a target gene to abate COVID-19. Leflunomide and teriflunomide are known and approved DHODH inhibitors. | Zheng et al. (2020) |
| 8 | In vitro study |
Drugs evaluated: Leflunomide and teriflunomide. Cell line model: Vero E6 cell line SARS-CoV-2 (MOI = 0.05) |
Leflunomide: EC50: 41.49 ± 8.84 μmol/L, CC50: 879 ± 62.58 S.I.: 21.19 Teriflunomide: EC50: 26.06 ± 6.4 CC50: 850 ± 67 S.I.: 32.64 EC50 (μmol/L) of other drugs evaluated: Favipiravir: 67 μmol/L, Remdesivir: 0.77 μmol/L, Chloroquine: 0.017 μmol/L, |
Xiong et al. (2020) |
|
Cell line model: Vero E6 cell line SARS-CoV-2 (MOI = 0.03) |
Teriflunomide: EC50: 6.00 ± 0.77 CC50: 850 ± 67 SI: 141.75 |
2.1.2. Artificial intelligence (AI) and machine learning (ML) based screening studies
AI/ML based approach is increasingly being used to identify novel drugs or to repurpose existing drugs against COVID-19. Batra R et al., 2020 (Batra et al., 2020) used a sequential machine learning based combined methodology to screen the ligand databases against spike protein of SARS-CoV-2 or the S:ACE2 interaction surface, which was followed by validation using Autodock. For training of the ML-model and validation, they used dataset from Smith and Smith (2020) (Smith and Smith, 2020).Testing the trained model against “FDA approved CureFFI” dataset, common active ingredient from “DrugCentral” and “BindingDB dataset” of small molecules revealed a total of 187 ligands with good binding scores. Out of the 187 ligands, 87 were from FDA approved dataset. After performing ensemble docking study of the selected 187 ligands, 175 ligands out of 187 showed favorable profile. Leflunomide was one of the top 50 candidates. Data showed in Table 1.
2.1.3. Host transcriptional response and network biology based drug re-purposing
Biological networks provide an excellent platform for understanding and characterizing complex multilevel biological interactions i.e. interactomes and is increasingly being used in the new drug discovery and drug repurposing process (Zhang et al., 2014). Jha Z et al., 2020 (Jia et al., 2020), used transcriptomic data of broncho-alveolar lavage fluid from healthy controls and COVID-19 cases and a differential expression analysis was performed. “KEGG pathway analysis”, “reactome pathway analysis” and “computational repositioning” was then performed for the co-expressed genes. For the pathway analysis they used “KEGG” and “reactome gene sets” and the drug-induced transcriptome was used for cogena-based drug-repositioning. Leflunomide was identified as one of the top 20 enriched drugs (leflunomide ranked 15th).
In another transcriptional response of host-cell to SARS-CoV2 based drug re-purposing, Li et al. (2020) used RNA sequence data of normal human bronchial cells, lung cancer cell line without ACE2 expression (A549), lung cancer cells with ACE2 expression (CALU-3) and SARS-CoV-2 infected cells and signaling network analysis was done. Identification of potential gene candidates was identified through signaling network analysis. On the basis of gene ontology (GO) analysis, genes that were upregulated within each super GO were used as signatures to identify potential drugs that can inhibit activation of these super GOs. Drug-target interaction analysis was done with the FDA approved drug bank database. These gene signatures were fed into the connectivity map database to identify potential drugs and drugs were categorized based upon gene set enrichment analysis (GSEA) scores. They have highlighted top 100 FDA approved drugs based on GSEA score to identify potential drugs against COVID-19. Leflunomide was one of them. Among the identified drugs they identified 16 drugs, the clinical trial of which was also running simultaneously for the treatment of COVID-19. Leflunomide was one of these 16 drugs.
In another multi-omics based study, disease-atlas of drug-targets for COVID-19 were constructed and using this 726 target disease associations were prioritized (on the basis of mendelian randomization and co-localization evidence). The whole study resulted in identification of three potential genes as targets of COVID-19 therapy, which included DHODH and leflunomide and teriflunomide are known inhibitors of DHODH (Zheng et al., 2020). Data from all these studies is showed in Table 1.
2.2. In vitro efficacy of leflunomide/teriflunomide against SARS-COV-2
Efficacy of leflunomide and teriflunomide was evaluated in vitro settings (Xiong et al., 2020) and both Leflunomide and teriflunomide showed inhibitory action against “SARS-CoV-2” in “vero E6 cells” (MOI 0.05) with EC50 of 41.49 ± 8.84 μmol/L. However, teriflunomide was found to be more potent with EC50 of 26 μmol/L (at MOI 0.05) and 6 μmol/L (at MOI 0.03). Notably, EC50 of leflunomide was found to be higher than fevipiravir, but lower than remdesivir and chloroquine. At MOI 0.05, leflunomide had higher selectivity index compared to teriflunomide, whereas at MOI 0.03, teriflunomide showed a higher selectivity index. Data showed in Table 1.
2.3. Efficacy of leflunomide/teriflunomide against SARS-COV-2 in pre-clinical IN-VIVO studies
After exhaustive search of different databases, we couldn't find a single study which evaluated the “safety and efficacy” of leflunomide/teriflunomide in animal models of “COVID-19”.
2.4. Clinical safety and efficacy: leflunomide/teriflunomide versus control/standard of care (SOC)
We could find two comparative clinical studies, where leflunomide was compared against control/standard of care (Ke et al., 2020; Wang et al., 2020), and one dedicated case series on teriflunomide in COVID-19, which evaluated the “safety and efficacy” of leflunomide/teriflunomide in “COVID-19” (Mantero et al., 2020a) [Details shown in Table 2 ]. Among these studies, the study by Wang Q et al., 2020 is a pre-print (Wang et al., 2020).
Table 2.
Details of clinical trials, observational studies and dedicated case series reporting safety and efficacy of leflunomide and teriflunomide in COVID-19. (N.A. = not applicable).
| S. No | Study design | Population | Intervention | Control | Co-interventions | Outcome | Comment | References |
|---|---|---|---|---|---|---|---|---|
| 1 | Open label Randomized controlled trial | Lab confirmed (RT-PCR) moderate COVID-19 patients (Moderate as per NHC, 2020; China), | Leflunomide 50 mgm 12 hourly three times daily than 20 mg every day for total 10 days. N = 5 |
Blank control N = 5 |
Arbidol, Lianhua qingwen capsule (traditional medicine), magnesium isoglycyrrhizinate & cefoperazone. | Time from leflunomide initiation to discharge, length of hospitalization, duration of viral shredding and safety. | 2 patients in each group showed COVID-19 PCR negativity, however, there were obvious lung inflammatory opacities, so these four patients were excluded while calculating “viral shredding time analysis”. | (Ke et al., 2020) |
| 2 | Open label controlled study/Allocated as per patients' choice | PCR+, Radiologically confirmed COVID-19 cases positive for the virus for >28 days despite standard of care. | Leflunomide 30 mg/day in patients <64 years and 20 mg/day in patients ≥65 years N = 15 |
Standard of care N = 12 |
Standard of care was given to both the groups, which included antibiotics, antivirals, glucocorticoids and Lianhua capsules | Rate and time of SARS-CoCV-2 clearance, 14 and 30 day hospital discharge rate, flare incidence, Adverse event | The trial was initiated and registered as RCT (ChiCTR2000030058), but later the allocation to different groups were made on the basis of patients' choice. | Wang et al. (2020b) |
| 3 | Case series | COVI-19 positive multiple sclerosis patients on teriflunomide | 6 Patients already on Teriflunomide for multiple sclerosis with mean duration 2.1 ± 1.6 years. | N.A. | N.A. | All the 6 cases had mild self-limiting disease. | None had other co-morbidities, PCR diagnosis in only three cases, rest three diagnosis was done based on typical symptomatology following a contact. | (Mantero et al., 2020b, p. 19, p. 19) |
| 4 | Case series | Multiple sclerosis patients on teriflunomide developing COVID-19 | 5 patients already on teriflunomide for multiple sclerosis. | N.A. | N.A. | In all 5 patients, disease was mild disease. | Symptoms ranged from mild fever to mild dyspnea. In 4 patients, there was no requirement of hospitalization, while the 5th patient developed the disease during hospitalization. |
Mantero et al. (2020b) |
2.4.1. Duration of viral shredding
Duration of viral shredding represents an important endpoint to evaluate the clinical antiviral activity of an agent under investigation. In the study by Ke et al. (2020) (Ke et al., 2020), leflunomide treatment showed significantly “shorter duration of viral shredding time” (median 5 days in leflunomide group vs. 11 days in control, mean ± S.D. 5.67 ± 2 days in leflunomide and 10.33 ± 1.155 days in control group, (mean derived from original data provided in the article n = 3 in each arm, p < 0.05).
In the open label controlled study by Wang et al., 2020 (Wang et al., 2020), median time of viral shredding in the leflunomide group (n = 15) among clearers till 14th day post enrollment (n = 12) was 6 days (IQR 1–12). Among 3 patients who did not clear the virus in leflunomide group till day 14 post enrolment, leflunomide was further continued and virus clearance was noted with 1, 2 and 5 days of additional leflunomide. However, in the standard of care group (n = 12), only two patients showed clearing of the virus on day 14 post enrollment. After 14 days of standard of care, the control patients (n = 9) were crossed over to leflunomide, and median duration of viral clearance in the cross over group was 9 days (IQR 1–13) following initiation of leflunomide.
2.4.2. SARS-CoV-2 clearance
Number of patients who became cleared of the virus at specific time points represents an important clinical endpoint to evaluate the antiviral activity of an agent under evaluation (Jomah et al., 2020).Two comparative studies reported “clearance of SARS-CoV-2” following treatment with leflunomide. Wang et al., 2020 reported higher proportion of patients (80%) showed better clearance of the virus compared to standard of care (16.7%) on day 14 post initiation of therapy (P < 0.05) (Wang et al., 2020). Another study by Ke et al. (2020), all the 3 patients (100%) showed virological clearance in the leflunomide arm, whereas, in the control arm none (0%) showed virological clearance on day 8 post initiation of therapy (Ke et al., 2020).
2.4.3. Effect of leflunomide on level of C reactive protein (CRP) level
C reactive protein is an important biomarker of inflammatory status in different diseases e.g. rheumatoid arthritis, sepsis etc. (Dhingra et al., 2007). CRP also serves an important prognostic biomarker for COVID-19 with higher level correlating well with disease severity (Ali, 2020, p. 19). In the study by Ke et al. (2020) (Ke et al., 2020), compared to baseline (median 37.4, IQR: 7.8–120.6) a significant decrease in CRP level was seen in the leflunomide treatment group post treatment (median 5, IQR 5-5).
2.4.4. Duration of hospital stay
Duration of hospital stay and hospital discharge rate at specific day can serve as important surrogates of the health status of patients under different disease conditions (Baek et al., 2018). In a single study (Wang et al., 2020), “median duration of hospital stay” was 11 days (IQR 7–19) in the leflunomide group and it was median 24 days in the SOC group (P < 0.05) showing lower duration of hospital stay in leflunomide group.
2.4.5. 14 day and 30 day hospital discharge-rate
In a single study (Wang et al., 2020),14-day hospital discharge rate was 73.3% in leflunomide group compared to only 8.3% in the SOC arm (P < 0.05). Similarly, 30 day discharge rate was also higher in the leflunomide group compared to the SOC group (100% in leflunomide group, 66.7% in the SOC arm, P < 0.05).
2.4.6. Effect of leflunomide/Teriflunomide on severity of infection
Although we don't have data from dedicated controlled studies, two case series on teriflunomide addressed this issue. In a case series of five “COVID-19” infected cases among teriflunomide treated patients with multiple sclerosis (Maghzi et al., 2020), All the patients had mild disease (100%, 5/5) and 4 patients recovered with medications and no hospital admission was required, while the 5th patient developed the disease while in hospital for other disease condition, however after restarting teriflunomide, his fever didn't exceed 37.5° (Maghzi et al., 2020). In another case series of 6 multiple sclerosis patients on teriflunomide therapy (Mantero et al., 2020a) with average duration of teriflunomide use of 2.1 ± 1.6 years (no additional co-morbidity), all had a self-limiting mild disease. None of the patients developed leucopenia, neutropenia or lymphopenia. None of the patient required hospital admission.
2.4.7. Adverse effects
In s single study, 73% in the leflunomide group showed adverse events, however, more adverse events were seen in the control group (83.3%) (Wang et al., 2020).
2.4.7.1. Treatment emergent adverse effect (TEAE)
In a single study, 40% patients in the leflunomide group showed treatment emergent adverse event, whereas only 25% in the SOC group showed TEAE (Wang et al., 2020).
2.4.7.2. Alteration in lipid profile
Hyperlipidemia was seen in 20% of the population in the leflunomide group (3/15) and 16.7% (2/12) in the SOC group.
2.4.7.3. Alteration in hematological profile
Alterations in hematological profile reported in the leflunomide group (single study) were leucopenia (20%) and Neutropenia (13.3%) (Wang et al., 2020). Other less common TEAEs were lymphopenia, thrombocytopenia each of which occurred in the leflunomide Group (6.7% for both). However, no such adverse effects were noted in the control group (Wang et al., 2020).
2.4.7.4. Alteration in liver function
One study reported higher level of liver enzymes (AST and ALT) in the leflunomide group (p < 0.05 for each) when compared to the control/SOC group (Ke et al., 2020). In the study by Wang et al., 2020 (Wang et al., 2020) elevation of ALT was seen in 6.7% of case (1/15) in the leflunomide and 0% cases in the SOC group. These changes were commonly reversible after stopping of leflunomide and standard clearance protocols were sufficient for dealing with such an increase (Ke et al., 2020).
2.5. Clinical safety and efficacy: leflunomide as add on to other therapy
One single RCT evaluated leflunomide as add on to nebulized interferon alpha-2a (Mantero et al., 2020b) among patients with prolonged post symptomatic viral shredding. However, no difference was seen between the interferon alpha-2a + leflunomide arm compared to interferon alpha-2a alone arm with regards to “duration of viral shredding (confirmed by RT-PCR)” and “duration of hospital stay”. It is to be noted that viral shredding was not confirmed by culture (Wang et al., n.d.). Interestingly, two patients in the leflunomide arm couldn't complete owing to occurrence of adverse events.
2.6. Ongoing clinical studies
Three studies are registered in “COVID-19”, out of which 2 studies addressed leflunomide and the third study was a case control study addressing the safety and efficacy of traditional anti-rheumatoid drugs against “COVID-19”. [Details of ongoing studies showed in Table 3 ].
Table 3.
Details of ongoing studies evaluating safety and efficacy of leflunomide and teriflunomide in COVID-19 (Data collected from Clinicaltrials.gov).
| NCT no | Population | Intervention | Control | Outcome | Reference |
|---|---|---|---|---|---|
| NCT04361214/Phase 1/Single group trial | Mild COVID patients | Leflunomide | Single group study | Tolerability, Rate of hospitalization, Time to defervescence, Resolution of other COVID-19 symptoms | (University of Chicago, 2020) |
| NCT04532372/Phase 2/RCT | Severe COVID-19 in Patients With concurrent malignancy | Leflunomide | Placebo | Toxicity, time to clinical response, survival, O2 saturation improvement, SARS-CoV-2 resolution, hospitalization, requirement of mechanical ventilation. | (City of Hope Medical Center, 2021) |
| NCT04434118/Case control study | Rheumatoid Arthritis Patients on anti-rheumatoid drugs | Traditional anti-rheumatoid drugs | N.A. | Risk of SARS_CoV-2 infection among patients on different anti-rheumatoid drugs. Incidence of hospitalization. |
(Abdallah, 2021) |
3. Summary and conclusion
DHODH inhibitors are important class of antivirals which can act as both host-directed, and virus directed therapy. The primary antiviral mechanism of DHODH inhibitors involve both inhibition of pyrimidine synthesis and stimulation of innate immunity (through stimulation of interferon stimulated genes e.g., ISGs). In case of SARS-CoV-2, however, other mechanisms like binding to the active site of M-Pro and Spike: ACE2 interface can also play role. The possible virus directed actions of DHODH inhibitors are showed in Fig. 1. However standard precautions are to be exercised while using these agents e.g. in patients having interstitial lung disease and among patients with previous methotrexate exposure (Chikura et al., 2009; Sawada et al., 2009).
To summarize the evidence of efficacy, in vitro studies, both leflunomide and teriflunomide showed inhibitory action against “SARS-CoV-2” in vero E6 cells [multiplicity of infection (MOI) 0.05] with EC50 of 41.49 ± 8.84 μmol/L. However, teriflunomide was found to be more potent with EC50 of 26 μmol/L (at MOI 0.05) and 6 μmol/L (at MOI 0.03). Notably, EC50 of leflunomide was found to be higher than Favipiravir, but lower than remdesivir and chloroquine.
In clinical studies, compared to control/standard of care, leflunomide showed significant benefit in terms of decreasing the duration of viral shredding (2 studies), decreasing CRP level (1 study, pre post comparison), duration of hospital stay (1 study), 14-day and 30-day hospital discharge rate (1 study) and severity of infection (2 case series on teriflunomide). However, among the four clinical studies included, 1 was a small sample size RCT (n = 5 in each arm), one was a non-randomized controlled trial, and rest two were case series (in both teriflunomide was used, n ≥ 5 for both the cases). However, no add on advantage was seen with leflunomide treatment when it was added to (IFN alpha-2a) and the combination did not decrease the duration of viral shredding among patients with prolonged post symptomatic viral shredding compared to IFN alpha-2a alone. However, this fact can be attributed to the involvement of interferon pathway in the efficacy of the DHODH inhibitors, such that addition of additional IFN alpha-2a was not associated with additional benefit.
Among adverse effects, leflunomide use was associated with alteration in lipid profile (hyperlipidemia), alteration in hematological profile (leucopenia, neutropenia and rare ones like lymphopenia and thrombocytopenia), alteration in liver function test (in most cases reversible after stopping of the drug) were commonly encountered adverse effects in “COVID-19” population.
To conclude, leflunomide seems promising against “COVID-19” across all range of studies ranging from in silico, in vitro and clinical studies. Although seems promising, we need more targeted evaluation of leflunomide as an “anti-COVID-19” agent in larger sized blinded placebo/standard controlled RCTs before coming to a final conclusion.
Funding
Nil.
Ethical clearance
Not Applicable.
CRediT authorship contribution statement
Hardeep Kaur: Conceptualization, Data curation, Formal analysis, and/or interpretation, Writing – original draft, Visualization, Writing – review & editing. Phulen Sarma: Conceptualization, Data curation, Formal analysis, and/or interpretation, Writing – original draft, Visualization, Writing – review & editing. Anusuya Bhattacharyya: Conceptualization, Data curation, Formal analysis, and/or interpretation, Writing – original draft, Visualization, Writing – review & editing. Saurabh Sharma: Conceptualization, Data curation, Formal analysis, and/or interpretation, Writing – original draft, Visualization, Writing – review & editing. Neeraj Chhimpa: Conceptualization, Data curation, Formal analysis, and/or interpretation, Writing – original draft, Visualization, Writing – review & editing. Manisha Prajapat: Conceptualization, Data curation, Formal analysis, and/or interpretation, Writing – original draft, Visualization, Writing – review & editing. Ajay Prakash: Conceptualization, Data curation, Formal analysis, and/or interpretation, Writing – original draft, Visualization, Writing – review & editing. Subodh Kumar: Conceptualization, Data curation, Formal analysis, and/or interpretation, Writing – original draft, Visualization, Writing – review & editing. Ashutosh Singh: Conceptualization, Data curation, Formal analysis, and/or interpretation, Writing – original draft, Visualization, Writing – review & editing. Rahul Singh: Conceptualization, Data curation, Formal analysis, and/or interpretation, Writing – original draft, Visualization, Writing – review & editing. Pramod Avti: Conceptualization, Data curation, Formal analysis, and/or interpretation, Writing – original draft, Visualization, Writing – review & editing. Prasad Thota: Conceptualization, Data curation, Formal analysis, and/or interpretation, Writing – original draft, Visualization, Writing – review & editing. Bikash Medhi: Conceptualization, Data curation, Formal analysis, and/or interpretation, Writing – original draft, Visualization, Writing – review & editing, All authors have reviewed the manuscript and approved the submission.
Declaration of competing interest
None declared.
Acknowledgement
The authors acknowledge Mr. Nripendra Bhatta for his kind help in logistic issues.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ejphar.2021.174233.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Abdallah M.S. 2021. Anti-rheumatic Drug Use and Risk of COVID-19 Infection in Rheumatoid Arthritis Patients: A Retrospective, Case-control Study (Clinical trial registration No. NCT04434118). clinicaltrials.gov. [Google Scholar]
- Ali N. Elevated level of C‐reactive protein may be an early marker to predict risk for severity of COVID‐19. J. Med. Virol. 2020 doi: 10.1002/jmv.26097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek H., Cho M., Kim S., Hwang H., Song M., Yoo S. Analysis of length of hospital stay using electronic health records: a statistical and data mining approach. PloS One. 2018;13 doi: 10.1371/journal.pone.0195901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batra R., Chan H., Kamath G., Ramprasad R., Cherukara M.J., Sankaranarayanan S.K.R.S. Screening of therapeutic agents for COVID-19 using machine learning and ensemble docking studies. J. Phys. Chem. Lett. 2020;11:7058–7065. doi: 10.1021/acs.jpclett.0c02278. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya A., Kumar S., Sarma P., Kaur H., Prajapat M., Shekhar N., Bansal S., Avti P., Hazarika M., Sharma S., Mahendru D., Prakash A., Medhi B. Safety and efficacy of lopinavir/ritonavir combination in COVID-19: a systematic review, meta-analysis, and meta-regression analysis. Indian J. Pharmacol. 2020;52:313. doi: 10.4103/ijp.IJP_627_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya A., Sarma P., Kaur H., Medhi B. Hydroxychloroquine in nonhospitalized adults with early COVID-19. Ann. Intern. Med. 2021;174:434. doi: 10.7326/L21-0001. [DOI] [PubMed] [Google Scholar]
- Brien J.D., Daffis S., Lazear H.M., Cho H., Suthar M.S., Gale M., Diamond M.S. Interferon regulatory factor-1 (IRF-1) shapes both innate and CD8+ T cell immune responses against west nile virus infection. PLoS Pathog. 2011;7 doi: 10.1371/journal.ppat.1002230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X.-C., Liu T., Li J.-J., He C., Meng W.-T., Huang R. Efficacy and safety of leflunomide for the treatment of BK virus-associated hemorrhagic cystitis in allogeneic hematopoietic stem cell transplantation recipients. Acta Haematol. 2013;130:52–56. doi: 10.1159/000345852. [DOI] [PubMed] [Google Scholar]
- Cheung N.N., Lai K.K., Dai J., Kok K.H., Chen H., Chan K.-H., Yuen K.-Y., Kao R.Y.T. Broad-spectrum inhibition of common respiratory RNA viruses by a pyrimidine synthesis inhibitor with involvement of the host antiviral response. J. Gen. Virol. 2017;98:946–954. doi: 10.1099/jgv.0.000758. [DOI] [PubMed] [Google Scholar]
- Chikura B., Lane S., Dawson J.K. Clinical expression of leflunomide-induced pneumonitis. Rheumatology. 2009;48:1065–1068. doi: 10.1093/rheumatology/kep050. [DOI] [PubMed] [Google Scholar]
- Coelho A.R., Oliveira P.J. Dihydroorotate dehydrogenase inhibitors in SARS-CoV-2 infection. Eur. J. Clin. Invest. 2020;50 doi: 10.1111/eci.13366. e13366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- City of Hope Medical Center . 2021. A Phase 1/2 Trial of Leflunomide for the Treatment of Severe COVID-19 in Patients With a Concurrent Malignancy (Clinical trial registration No. NCT04532372). clinicaltrials.gov. [Google Scholar]
- Dhingra R., Gona P., Nam B.-H., D'Agostino R.B., Wilson P.W.F., Benjamin E.J., O'Donnell C.J. C - reactive protein, inflammatory conditions and cardiovascular disease risk. Am. J. Med. 2007;120:1054–1062. doi: 10.1016/j.amjmed.2007.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farag A., Wang P., Ahmed M., Sadek H. 2020. Identification of FDA Approved Drugs Targeting COVID-19 Virus by Structure-Based Drug Repositioning. [DOI] [Google Scholar]
- Gokarn A., Toshniwal A., Pathak A., Arora S., Bonda A., Punatar S., Nayak L., Dwivedi P., Bhat V., Biswas S., Kelkar R., Kannan S., Khattry N. Use of leflunomide for treatment of cytomegalovirus infection in recipients of allogeneic stem cell transplant. Biol. Blood Marrow Transplant. 2019;25:1832–1836. doi: 10.1016/j.bbmt.2019.04.028. [DOI] [PubMed] [Google Scholar]
- Gupta S., Wang W., Hayek S.S., Chan L., Mathews K.S., Melamed M.L., Brenner S.K., Leonberg-Yoo A., Schenck E.J., Radbel J., Reiser J., Bansal A., Srivastava A., Zhou Y., Finkel D., Green A., Mallappallil M., Faugno A.J., Zhang J., Velez J.C.Q., Shaefi S., Parikh C.R., Charytan D.M., Athavale A.M., Friedman A.N., Redfern R.E., Short S.A.P., Correa S., Pokharel K.K., Admon A.J., Donnelly J.P., Gershengorn H.B., Douin D.J., Semler M.W., Hernán M.A., Leaf D.E. STOP-COVID Investigators. Association Between Early Treatment With Tocilizumab and Mortality Among Critically Ill Patients With COVID-19. JAMA Intern Med. 2021 Jan 1;181(1):41–51. doi: 10.1001/jamainternmed.2020.6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada H., Takahashi E., Itoh S., Harada K., Hori T.A., Taniguchi T. Structure and regulation of the human interferon regulatory factor 1 (IRF-1) and IRF-2 genes: implications for a gene network in the interferon system. Mol. Cell Biol. 1994;14:1500–1509. doi: 10.1128/mcb.14.2.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Z., Song X., Shi J., Wang W., He K. Transcriptome-based drug repositioning for coronavirus disease 2019 (COVID-19) Pathog Dis. 2020;78 doi: 10.1093/femspd/ftaa036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jomah S., Asdaq S.M.B., Al-Yamani M.J. Clinical efficacy of antivirals against novel coronavirus (COVID-19): a review. Journal of Infection and Public Health. 2020;13:1187–1195. doi: 10.1016/j.jiph.2020.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur Hardeep, Sarma P., Bhattacharyya A., Prajapat M., Kumar S., Prakash A., Medhi B. Folic acid as placebo in controlled clinical trials of hydroxychloroquine prophylaxis in COVID-19: is it scientifically justifiable? Med. Hypotheses. 2021;149:110539. doi: 10.1016/j.mehy.2021.110539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur Harpinder, Shekhar N., Sharma S., Sarma P., Prakash A., Medhi B. Ivermectin as a potential drug for treatment of COVID-19: an in-sync review with clinical and computational attributes. Pharmacol. Rep. 2021 doi: 10.1007/s43440-020-00195-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke H., Wang M., Zhao Y., Zhang Y., Wang T., Zheng Z., Li X., Zeng S., Zhao D., Li H., Xu K., Lan K. A small-scale medication of leflunomide as a treatment of COVID-19 in an open-label blank-controlled clinical trial. Virol. Sin. 2020;35(6):725–733. doi: 10.1007/s12250-020-00258-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M.M., Parab S.R., Paranjape M. Repurposing 0.5% povidone iodine solution in otorhinolaryngology practice in Covid 19 pandemic. Am. J. Otolaryngol. 2020;41:102618. doi: 10.1016/j.amjoto.2020.102618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas-Hourani M., Dauzonne D., Jorda P., Cousin G., Lupan A., Helynck O., Caignard G., Janvier G., André-Leroux G., Khiar S., Escriou N., Desprès P., Jacob Y., Munier-Lehmann H., Tangy F., Vidalain P.-O. Inhibition of pyrimidine biosynthesis pathway suppresses viral growth through innate immunity. PLoS Pathog. 2013;9 doi: 10.1371/journal.ppat.1003678. e1003678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthra P., Naidoo J., Pietzsch C.A., De S., Khadka S., Anantpadma M., Williams C.G., Edwards M.R., Davey R.A., Bukreyev A., Ready J.M., Basler C.F. Inhibiting pyrimidine biosynthesis impairs Ebola virus replication through depletion of nucleoside pools and activation of innate immune responses. Antivir. Res. 2018;158:288–302. doi: 10.1016/j.antiviral.2018.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maghzi A.H., Houtchens M.K., Preziosa P., Ionete C., Beretich B.D., Stankiewicz J.M., Tauhid S., Cabot A., Berriosmorales I., Schwartz T.H.W., Sloane J.A., Freedman M.S., Filippi M., Weiner H.L., Bakshi R. COVID-19 in teriflunomide-treated patients with multiple sclerosis. J. Neurol. 2020:1–7. doi: 10.1007/s00415-020-09944-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantero V., Baroncini D., Balgera R., Guaschino C., Basilico P., Annovazzi P., Zaffaroni M., Salmaggi A., Cordano C. Mild COVID‐19 infection in a group of teriflunomide‐treated patients with multiple sclerosis. J. Neurol. 2020:1–2. doi: 10.1007/s00415-020-10196-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantero V., Baroncini D., Balgera R., Guaschino C., Basilico P., Annovazzi P., Zaffaroni M., Salmaggi A., Cordano C. Mild COVID‐19 infection in a group of teriflunomide‐treated patients with multiple sclerosis. J. Neurol. 2020:1–2. doi: 10.1007/s00415-020-10196-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei-jiao G., Shi-fang L., Yan-yan C., Jun-jun S., Yue-feng S., Ting-ting R., Yong-guang Z., Hui-yun C. Antiviral effects of selected IMPDH and DHODH inhibitors against foot and mouth disease virus. Biomed. Pharmacother. 2019;118:109305. doi: 10.1016/j.biopha.2019.109305. [DOI] [PubMed] [Google Scholar]
- Nesselhauf N., Strutt J., Bastani B. Evaluation of leflunomide for the treatment of BK viremia and biopsy proven BK nephropathy; a single center experience. J Nephropathol. 2016;5:34–37. doi: 10.15171/jnp.2016.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parasher A. COVID-19: current understanding of its pathophysiology, clinical presentation and treatment. Postgrad. Med. 2020 doi: 10.1136/postgradmedj-2020-138577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prajapat M., Sarma P., Shekhar N., Prakash A., Avti P., Bhattacharyya A., Kaur H., Kumar S., Bansal S., Sharma A.R., Medhi B. Update on the target structures of SARS-CoV-2: a systematic review. Indian J. Pharmacol. 2020;52:142. doi: 10.4103/ijp.IJP_338_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prajapat M., Shekhar N., Sarma P., Avti P., Singh S., Kaur H., Bhattacharyya A., Kumar S., Sharma S., Prakash A., Medhi B. Virtual screening and molecular dynamics study of approved drugs as inhibitors of spike protein S1 domain and ACE2 interaction in SARS-CoV-2. J. Mol. Graph. Model. 2020;101:107716. doi: 10.1016/j.jmgm.2020.107716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read S.W., DeGrezia M., Ciccone E.J., DerSimonian R., Higgins J., Adelsberger J.W., Starling J.M., Rehm C., Sereti I. The effect of leflunomide on cycling and activation of T-cells in HIV-1-infected participants. PloS One. 2010;5 doi: 10.1371/journal.pone.0011937. e11937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezagholizadeh A., Khiali S., Sarbakhsh P., Entezari-Maleki T. Remdesivir for treatment of COVID-19; an updated systematic review and meta-analysis. Eur. J. Pharmacol. 2021;897:173926. doi: 10.1016/j.ejphar.2021.173926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozman B. Clinical pharmacokinetics of leflunomide. Clin. Pharmacokinet. 2002;41:421–430. doi: 10.2165/00003088-200241060-00003. [DOI] [PubMed] [Google Scholar]
- Sarma P., Bhattacharyya A., Kaur H., Prajapat M., Prakash A., Kumar S., Bansal S., Kirubakaran R., Reddy D.H., Muktesh G., Kaushal K., Sharma S., Shekhar N., Avti P., Thota P., Medhi B. Efficacy and safety of steroid therapy in COVID-19: a rapid systematic review and Meta-analysis. Indian J. Pharmacol. 2020;52:535. doi: 10.4103/ijp.ijp_1146_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, F., Michelson, A.P., Foraker, R., Zhan, M., Payne, P.R.O., 2020. Repurposing drugs for COVID-19 based on transcriptional response of host cells to SARS-CoV-2. arXiv:2006.01226 [q-bio]. [DOI] [PMC free article] [PubMed]
- Sarma, P., Kaur, H., Kumar, H., Mahendru, D., Avti, P., Bhattacharyya, A., Prajapat, M., Shekhar, N., Kumar, S., Singh, R., Singh, A., Dhibar, D.P., Prakash, A., Medhi, B., n.d. Virological and clinical cure in COVID‐19 patients treated with hydroxychloroquine: a systematic review and meta‐analysis. J. Med. Virol.. 10.1002/jmv.25898. [DOI] [PMC free article] [PubMed]
- Sarma P., Kaur H., Medhi B., Bhattacharyya A. Possible prophylactic or preventive role of topical povidone iodine during accidental ocular exposure to 2019-nCoV. Graefes Arch. Clin. Exp. Ophthalmol. 2020:1–3. doi: 10.1007/s00417-020-04752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma P., Kaur H., Medhi B., Bhattacharyya A. Letter to the editor: possible role of topical povidone iodine in case of accidental ocular exposure to SARS-CoV-2. Graefes Arch. Clin. Exp. Ophthalmol. 2020:1–4. doi: 10.1007/s00417-020-04864-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada T., Inokuma S., Sato T., Otsuka T., Saeki Y., Takeuchi T., Matsuda T., Takemura T., Sagawa A. vol. 48. Rheumatology (Oxford); 2009. Study committee for leflunomide-induced lung injury, Japan college of rheumatology; pp. 1069–1072. (Leflunomide-induced Interstitial Lung Disease: Prevalence and Risk Factors in Japanese Patients with Rheumatoid Arthritis). [DOI] [PubMed] [Google Scholar]
- Sencanski M., Perovic V., Pajovic S.B., Adzic M., Paessler S., Glisic S. Drug repurposing for candidate SARS-CoV-2 main protease inhibitors by a novel in silico method. Molecules. 2020;25 doi: 10.3390/molecules25173830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepúlveda C.S., García C.C., Damonte E.B. Antiviral activity of A771726, the active metabolite of leflunomide, against Junín virus. J. Med. Virol. 2018;90:819–827. doi: 10.1002/jmv.25024. [DOI] [PubMed] [Google Scholar]
- Shekhar N., Sarma P., Prajapat M., Avti P., Kaur H., Raja A., Singh H., Bhattacharya A., Sharma S., Kumar S., Prakash A., Medhi B. vol. 5. 2020. (Silico Structure-Based Repositioning of Approved Drugs for Spike Glycoprotein S2 Domain Fusion Peptide of SARS-CoV-2: Rationale from Molecular Dynamics and Binding Free Energy Calculations. mSystems). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva J.T., Pérez-González V., Lopez-Medrano F., Alonso-Moralejo R., Fernández-Ruiz M., San-Juan R., Brañas P., Folgueira M.D., Aguado J.M., de Pablo-Gafas A. Experience with leflunomide as treatment and as secondary prophylaxis for cytomegalovirus infection in lung transplant recipients: a case series and review of the literature. Clin. Transplant. 2018;32 doi: 10.1111/ctr.13176. [DOI] [PubMed] [Google Scholar]
- Smith M., Smith J.C. Repurposing therapeutics for COVID-19: supercomputer-based docking to the SARS-CoV-2 viral spike protein and viral spike protein-human ACE2 interface. 2020. [DOI]
- University of Chicago . 2020. Leflunomide for the Treatment of Ambulatory Patients With Mild COVID-19 (Clinical trial registration No. NCT04361214). clinicaltrials.gov. [Google Scholar]
- Vm M., D M., A S., S K., H K., P S., A P., B M. COVID-19 pandemic: a review based on current evidence [WWW Document] Indian J. Pharmacol. 2020 doi: 10.4103/ijp.IJP_310_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, M., Zhao, Y., Hu, W., Zhao, D., Zhang, Y., Wang, T., Zheng, Z., Li, X., Zeng, S., Liu, Z., Lu, L., Wan, Z., Hu, K., n.d. Treatment of COVID-19 patients with prolonged post-symptomatic viral shedding with leflunomide -- a single-center, randomized, controlled clinical trial. Clin. Infect. Dis.: An Official Publication of the Infectious Diseases Society of America. 10.1093/cid/ciaa1417. [DOI] [PMC free article] [PubMed]
- Wang Q., Guo H., Li Yu, Jian X., Hou X., Zhong N., Fei J., Su D., Bian Z., Zhang Y., Hu Y., Sun Y., Yu X., Li Yuan, Jiang B., Li Yan, Qin F., Wu Y., Gao Y., Hu Z. Efficacy and safety of leflunomide for refractory COVID-19: an open-label controlled study. medRxiv 2020.05.29.20114223. 2020 doi: 10.1101/2020.05.29.20114223. [DOI] [Google Scholar]
- Xiong R., Zhang L., Li S., Sun Y., Ding M., Wang Y., Zhao Y., Wu Y., Shang W., Jiang X., Shan J., Shen Z., Tong Y., Xu L., Chen Y., Liu Y., Zou G., Lavillete D., Zhao Z., Wang R., Zhu L., Xiao G., Lan K., Li H., Xu K. 2020. Novel and Potent Inhibitors Targeting DHODH Are Broad-Spectrum Antivirals against RNA Viruses Including Newly-Emerged Coronavirus SARS-CoV-2. Protein Cell 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Jiang H. Potential treatment of COVID-19 by inhibitors of human dihydroorotate dehydrogenase. Protein Cell. 2020:1–4. doi: 10.1007/s13238-020-00769-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanai H., Negishi H., Taniguchi T. The IRF family of transcription factors. OncoImmunology. 2012;1:1376–1386. doi: 10.4161/onci.22475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Tian Y., Zhang Z. Network biology in medicine and beyond. Circ Cardiovasc Genet. 2014;7:536–547. doi: 10.1161/CIRCGENETICS.113.000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J., Zhang Y., Liu Y., Baird D., Liu X., Wang L., Zhang H., Davey Smith G., Gaunt T. Multi-omics study revealing tissue-dependent putative mechanisms of SARS-CoV-2 drug targets on viral infections and complex diseases. 2020. [DOI]
- Zivadinov R., Ramanathan M., Hagemeier J., Bergsland N., Ramasamy D.P., Durfee J., Kolb C., Weinstock-Guttman B. Teriflunomide's effect on humoral response to Epstein-Barr virus and development of cortical gray matter pathology in multiple sclerosis. Mult Scler Relat Disord. 2019;36:101388. doi: 10.1016/j.msard.2019.101388. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.