Abstract
Objectives
To assess the prevalence of and factors associated with post-coronavirus disease 2019 (COVID-19) syndrome 6 months after the onset.
Methods
A bidirectional prospective study. Interviews investigated symptoms potentially associated with COVID-19 6 months after the disease onset of all consecutive adult inpatients and outpatients with COVID-19 attending Udine Hospital (Italy) from March to May 2020. IgG antibodies against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) were also evaluated 6 months after the onset of symptoms, at the time of the interview.
Results
A total of 599 individuals were included (320 female, 53.4%; mean age 53 years, SD 15.8) and interviewed 187 days (22 SD) after onset. The prevalence of post-COVID-19 syndrome was 40.2% (241/599). The presence of IgG antibodies was significantly associated with the occurrence of post-COVID-19 syndrome (OR 2.56, 95% CI 1.48–4.38, p 0.001) and median SARS-CoV-2 IgG titres were significantly higher in patients with post-COVID-19 syndrome than in patients without symptoms (42.1, IQR 17.1–78.4 vs. 29.1, IQR 12.1–54.2 kAU/L, p 0.004). Female gender (OR 1.55, 95% CI 1.05–2.27), a proportional increase in the number of symptoms at the onset of COVID-19 (OR 1.81, 95% CI 1.59–2.05) and ICU admission OR 3.10, 95% CI 1.18–8.11) were all independent risk factors for post-COVID-19 syndrome. The same predictors also emerged in a subgroup of 231 patients with the serological follow-up available at the time of the interview alongside the proportional increase in anti-SARS-CoV-2 IgG (OR 1.01, 95% CI 1.00–1.02, p 0.04).
Discussion
Prospective follow-up could be offered to specific subgroups of COVID-10 patients, to identify typical symptoms and persistently high anti-SARS-CoV-2 IgG titres as a means of early detection of post-COVID-19 long-term sequelae.
Keywords: Chronic COVID, COVID survivors, COVID-19, Patients with post-COVID-19 syndrome, Long COVID, Post-COVID syndrome, SARS-CoV-2 antibodies, SARS-CoV-2 serology
Introduction
Post-COVID-19 syndrome describing the experience of persistent symptoms after recovering from the initial acute COVID-19 has recently attracted increased attention [1,2] given that it might be expected according to previous coronavirus epidemics [3,4]. The pathophysiology of post-viral syndromes has been reported to involve a complex interplay between the viral loads and the humoral and cellular immune responses that could elicit both direct non-immunological and indirect immune effects [3,5]. However, the available knowledge of post-COVID-19 syndrome is still poor and controversial due to the lack of a consensus definition [6], the heterogeneity of the investigated population and diagnostic follow-ups [1,2,7,8]. A better understanding of post-COVID-19 syndrome and the dynamics and the nature of immune response to SARS-CoV-2 infection might assist in identifying long-haulers and appropriate pathways of care. Therefore, the aim of this study was to comprehensively characterise predictors of post-COVID-19 syndrome, including the serological responses, among survivors.
Material and methods
Setting, patients and data collection
We performed a bidirectional cohort study design in a tertiary care teaching hospital (Udine, Italy, 1000 beds) designated as a regional centre for COVID-19 patients and serving approximately 350 000 citizens. The reference Ethics Committee (CEUR-2020-OS-219 and CEUR-2020-OS-205) approved the study and all procedures were in accordance with the ethical standards of the Health Care Trust.
A cohort of all consecutive adult in- and out-patients (≥18 years) attending the Infectious Disease Department with a diagnosis of COVID-19 from 1 March to 30 May 2020 were eligible. Patients who were willing to participate were included and a database concerning their demographic, clinical and laboratory data was populated. Six months after disease onset (September and November 2020), patients were telephone interviewed by trained nurses with a pilot-tested questionnaire investigating specific persistent or emerging symptoms potentially associated with COVID-19. Participants were free to answer in their own words according to the patient-reported outcomes (PROs) framework [9]; the interview took from 10 to 30 minutes. Their narratives were categorized by four independent physicians: post-COVID-19 syndrome was defined as symptoms that developed during or after COVID-19, continued for ≥12 weeks, and were not explained by an alternative diagnosis. Furthermore, SARS-CoV-2 antibody concentrations were measured for a subgroup of patients who agreed to participate in a parallel study on monthly serological follow-up; available serological data at the time of the interview (±15 days) were also recorded in the database.
Acute COVID-19 definitions
A patient with a positive nucleic acid amplification test (NAAT) for SARS-CoV-2 in respiratory tract specimens was considered a confirmed COVID-19 case whereas a suspected COVID-19 case was considered as having COVID-19 with a negative SARS-CoV-2 NAAT reporting laboratory or imaging findings and/or positive serology [11]. Based on the COVID-19 disease severity scale, patients were classified into five groups, from asymptomatic to critical disease [11]. Symptomatic and asymptomatic patients were categorized into three groups: (a) intensive care unit (ICU), (b) hospital-ward, and (c) outpatients, when they had been never hospitalized.
Laboratory methods
Identifying cases with the COVID-19 virus was based on the detection of unique sequences of virus RNA by NAATs such as real-time PCR (RT-PCR) on respiratory samples. Cycle threshold (Ct) values of first positive NAAT were collected. Serial measurements of SARS-CoV-2 anti-nucleocapsid and anti-spike IgM/IgG were obtained using iFlash-SARS-CoV-2 (Yhlo), a paramagnetic particle based chemiluminescence immunoassay (CLIA). According to the manufacturer's information, the IgM and IgG cut-off is 10.0 kAU/L. Ct, IgG and IgM were considered in order to identify associations, if any, between the anti-SARS-CoV-2 and post-COVID syndrome. Further test procedures are summarized in Table S2.
Statistical analysis
Patients were divided into two groups (with or without post-COVID-19 syndrome) at the time of interview. Absolute values, percentages, mean and median (standard deviation (SD) or interquartile range (IQR)) were calculated. Categorical variables were compared using the chi-squared test or Fisher's exact test, while continuous variables were compared using a Student t-test or Mann–Whitney U test for two groups, one-way ANOVA or the Kruskal–Wallis test for more than two groups, according to the Shapiro–Wilk test establishing whether data were normally or non-normally distributed. A multivariable logistic regression was performed to explore variables associated with post-COVID-19 syndrome, estimating the odds ratios (OR; 95% CI). All clinically or microbiologically relevant variables or those which were significant at p ≤ 0.10 in univariable analysis were included, taking into account potential collinearities (e.g. the severity of acute COVID-19 and the ICU admission). Given that only 231 patients performed the serological follow-up at the time of interview, univariable and multivariable logistic regression was performed on this sub-group; moreover, this sub-group was compared with the remaining population (Tables S3–S5). Seronegative patients and those with seroreversion of IgM and IgG were excluded from the serological follow-up at the time of interview, but were included in the overall count only to evaluate the presence of IgG antibodies against SARS-CoV-2 6 months after the onset. Analyses were performed by STATA 16.
Results
Patients' baseline characteristics
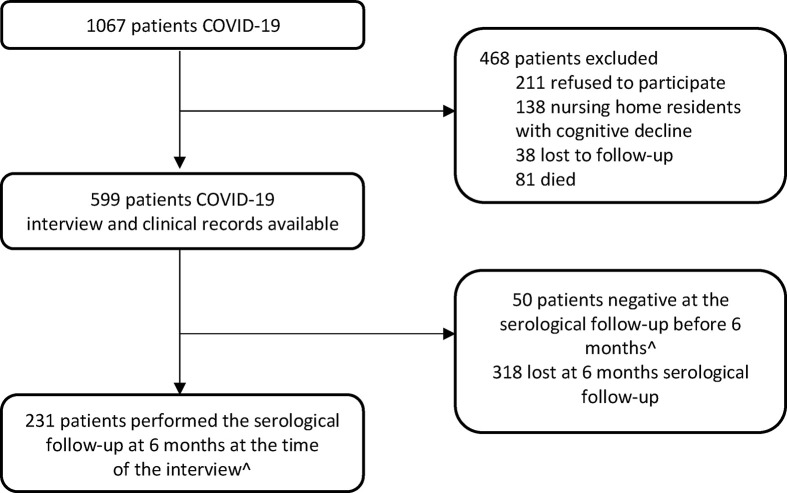
Overall, during the study period, 1067 COVID-19 patients were diagnosed in our hospital (Fig. 1 ); a total of 231 out of 599 (38.6%) completed the serological follow-up at the time of the interview.
Fig. 1.
Flow chart of in- and out-patients with COVID-19 attending the Infection Disease Department between March and May 2020. ∧ CORMOR 3-4 protocol.
Baseline demographic and clinical characteristics are summarized in Table 1 . In brief, the mean age was 53 years (SD 15.8; range 18‒94), 320/599 (53.4%) were female and the majority (524/573, 91.4%) were native Italian. A total of 314/599 (52.4%) reported at least one baseline co-morbidity, most often hypertension (135/599, 23%) and obesity (body mass index >30 = 98/599, 16.4%).
Table 1.
Post-COVID-19 syndrome according to patients' baseline characteristics (n = 599)
| Post-COVID-19 syndromea | |||
|---|---|---|---|
| Yes N = 241 |
No N = 358 |
p | |
| Gender, n (%) | 0.004 | ||
| Female | 146 (60.6) | 174 (48.6) | |
| Male | 95 (39.4) | 184 (51.4) | |
| Age group, n (%) | 0.22 | ||
| 18‒40 | 50 (20.8) | 91 (25.4) | |
| 41‒60 | 109 (45.2) | 138 (38.6) | |
| >60 | 82 (34.0) | 129 (36.0) | |
| Ethnicity, n/N (%) | 0.74 | ||
| Native Italian | 215/235 (91.5) | 309/338 (91.4) | |
| European | 19/235 (8.1) | 25/338 (7.4) | |
| Non-European | 1/235 (0.4) | 4/338 (1.2) | |
| Smoking habit, n/N (%) | 0.33 | ||
| Non-smoker | 161 (66.8) | 228/356 (64.0) | |
| Smoker | 27 (11.2) | 55/356 (15.5) | |
| Ex-smoker | 53 (22.0) | 73/356 (20.5) | |
| Alcohol habit, n/N (%) | 0.35 | ||
| Non-drinker | 129 (53.5) | 170/355 (47.9) | |
| Drinker | 111 (46.1) | 183/355 (51.5) | |
| Alcohol use disordere | 1 (0.4) | 2/355 (0.6) | |
| Work, n/N (%) | 0.32 | ||
| Work in contact with public and HCWs | 101/230 (43.9) | 132/326 (40.5) | |
| Work not in contact with the public | 68/230 (29.6) | 84/326 (25.8) | |
| Retired | 37/230 (16.1) | 70/326 (21.5) | |
| Other | 24/230 (10.4) | 40/236 (12.3) | |
| Co-morbidities, number, n (%) | 0.31 | ||
| 0 | 108 (44.8) | 177 (49.4) | |
| 1 | 74 (30.7) | 101 (28.2) | |
| 2 | 33 (13.7) | 46 (12.8) | |
| 3 | 15 (6.2) | 22 (6.2) | |
| ≥4 | 11 (4.6) | 12 (3.4) | |
| Co-morbidities, n (%) | |||
| Hypertension | 52 (22.0) | 83 (23.2) | 0.64 |
| Obesity | 36 (14.9) | 62 (17.3) | 0.44 |
| Diabetes | 15 (6.2) | 18 (5.03) | 0.23 |
| Chronic respiratory diseasec | 11 (4.6) | 10 (2.8) | 0.26 |
| Cardiovascular diseaseb | 1 (0.4) | 6 (1.7) | 0.25 |
| Liver disease | 8 (3.3) | 2 (0.5) | 0.22 |
| Psychiatric disordersd | 2 (0.8) | 4 (1.1) | 0.73 |
| Renal impairment | 0 (0.0) | 0 (0.0) | — |
| Under chronic medication, n/N (%) | 0.37 | ||
| Yes | 118/239 (49.4) | 187/352 (53.1) | |
| No | 121/239 (50.6) | 165/352 (46.9) | |
HCWs, health care workers; n, number, N, number as a denominator.
Patient's narratives collected with the telephonic interview were categorized by four independent physicians, who then agreed upon the findings. Post-COVID-19 syndrome was defined as symptoms that developed during or after COVID-19, continued for ≥12 weeks, not explained by an alternative diagnosis [10] (conjunctivitis, visual changes) in at least one of the following clinical presentations: dyspnoea, cough, fatigue (asthenia, myalgia), chest pain, anosmia/dysgeusia, headache, rheumatological involvement (back pain, arthralgia), neurological disorders (hypoesthesia, dizziness, hypoacusia), psychiatric/mood disorders (anxiety, sleep disorders, depression), inability to concentrate/brain fog, gastrointestinal disorders (diarrhoea, vomiting, nausea, epigastric pain, constipation and abdominal pain), skin lesions, hair loss, upper respiratory infection involvement (nose cold, sneezing, odynophagia) and ocular involvement.
Cardiovascular disease: heart failure, ischaemic heart disease, tachyarrhythmias, valvular heart disease, venous thromboembolism.
Pulmonary disease: asthma, chronic obstructive pulmonary disease.
Depression, anxiety.
Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) definition [36].
Acute COVID-19
Most patients were symptomatic (541/596, 90.8%) and presented mild (409/541, 75.6%) and moderate (93/541, 17.2%) disease. A total of 157/599 (26.2%) had been hospitalized (23/599; 3.8% in ICU) (Table 2 ), reporting a median in-hospital stay of 7 days (IQR 3‒11).
Table 2.
Post-COVID-19 syndrome according to the clinical presentation and the onset and the microbiological evolution (n = 599)
| Post-COVID-19 syndromea |
p | ||
|---|---|---|---|
| Yes N = 241 |
No N = 358 |
||
| Acute COVID-19 severity§, n/N (%) | <0.001 | ||
| Asymptomatic | 3/239 (1.2) | 52/357 (14.6) | |
| Mild | 166/239 (69.5) | 243/357 (68.1) | |
| Moderate | 49/239 (20.5) | 44/357 (12.3) | |
| Severe | 10/239 (4.2) | 14/357 (3.9) | |
| Critical | 11/239 (4.6) | 4/357 (1.1) | |
| Symptoms at onset, number, n (%) | <0.001 | ||
| 0 | 3 (1.2) | 52 (14.5) | |
| 1 | 22 (9.1) | 104 (29.0) | |
| 2 | 35 (14.5) | 78 (21.8) | |
| 3 | 49 (20.3) | 51 (14.2) | |
| 4 | 53 (22.0) | 36 (10.1) | |
| ≥5 | 79 (32.8) | 37 (10.3) | |
| Management, n (%) | <0.001 | ||
| Outpatients | 157 (65.1) | 285 (79.6) | |
| Inpatients | |||
| Wardˆ | 70 (29.1) | 64 (17.9) | |
| ICU | 14 (5.8) | 9 (2.5) | |
|
Length of in-hospital stay, days, median (IQR) |
7 (3–11) |
7 (3–12) |
0.48 |
| Viral shedding, days, median (IQR) | 20 (14–26) | 19 (14–25) | 0.21 |
| Ct-values, median (IQR) | 28.6 (24.7–33.5) | 29.5 (23.8–33.2) | 0.81 |
ct, cycle threshold; IQR, interquartile range; ICU, intensive care unit; n, number, N, number as a denominator.
§ asymptomatic; mild (without pneumonia); moderate (with pneumonia); severe (with severe pneumonia); critical including Acute Respiratory Distress Syndrome (ARDS), sepsis and/or septic shock [11].
Patient's narratives collected with the telephonic interview were categorized by four independent physicians, who then agreed upon the findings. Post-COVID-19 syndrome was defined as symptoms that developed during or after COVID-19, continued for ≥12 weeks, not explained by an alternative diagnosis [10] (conjunctivitis, visual changes) in at least one of the following clinical presentations: dyspnoea, cough, fatigue (asthenia, myalgia), chest pain, anosmia/dysgeusia, headache, rheumatological involvement (back pain, arthralgia), neurological disorders (hypoesthesia, dizziness, hypoacusia), psychiatric/mood disorders (anxiety, sleep disorders, depression), inability to concentrate/brain fog, gastrointestinal disorders (diarrhoea, vomiting, nausea, epigastric pain, constipation and abdominal pain), skin lesions, hair loss, upper respiratory infection involvement (nose cold, sneezing, odynophagia) and ocular involvement.
Infectious Disease or Pneumology Department.
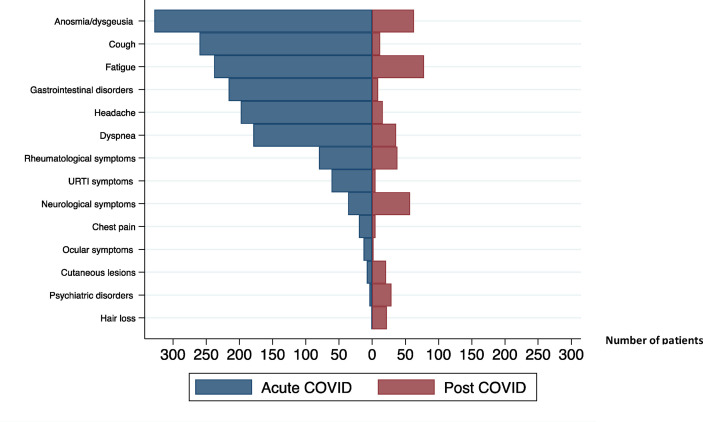
Among those that were symptomatic, the median number of symptoms was three (IQR 1–4) and the most common clinical manifestations included fever (399/541, 73.7%), anosmia/dysgeusia (326/541, 60.3%), cough (259/541, 47.9%), fatigue (237/541, 43.8%) (Fig. 2 ). During the follow-up, we detected SARS-CoV-2 viral shedding for a median of 19 (IQR 14‒25) days after the symptom(s) onset (Table 2).
Fig. 2.
Acute- and post-COVID related sympthoms. Abbreviations: COVID, coronavirus disease; URTI, upper respiratory tract infection.
Post-COVID-19 syndrome
Patient evaluation was performed on day 191 (median, IQR 172–204) after acute COVID-19 onset. At this time, post-COVID-19 syndrome prevalence was 40.2% (241/599), (95% CI 0.36–0.44). Among them, 22.9% (137/599) reported at least one symptom, 10.8% (65/599) two symptoms and 6.5% (39/599) three or more symptoms. The most frequently reported persistent symptom was fatigue (78/599, 13.1%) (Table 3 ; Fig. 2). The persistence of fatigue, dyspnoea and neurological disorders was significantly associated with disease severity at onset (p < 0.05), whereas anosmia/dysgeusia occurred significantly more often among mild and acute COVID-19 patients (p < 0.001) (Table 3).
Table 3.
Symptoms of post-COVID-19 syndrome according to grade of severity of acute COVID-19 (n = 599)
| Total N = 596 |
Severity of disease at the onset§ |
p | |||||
|---|---|---|---|---|---|---|---|
| Asymptomatic N = 55 |
Mild N = 409 |
Moderate N = 93 |
Severe N = 24 |
Critical N = 15 |
|||
| Post-COVID symptoms, number, n (%) | <0.001 | ||||||
| 0 | 357 (59.9) | 52 (94.5) | 243 (59.4) | 44 (47.3) | 14 (58.3) | 4 (26.7) | |
| 1 | 136 (22.8) | 3 (5.4) | 96 (23.5) | 28 (30.1) | 5 (20.8) | 4 (26.7) | |
| 2 | 64 (10.7) | 0 (0.0) | 46 (11.2) | 12 (12.9) | 3 (12.5) | 3 (20.0) | |
| 3 | 28 (4.7) | 0 (0.0) | 18 (4.4) | 7 (7.5) | 1 (4.2) | 2 (13.3) | |
| 4 | 7 (1.2) | 0 (0.0) | 4 (1.0) | 2 (2.1) | 0 (0.0) | 1 (6.7) | |
| 5 | 4 (0.7) | 0 (0.0) | 2 (0.5) | 0 (0.0) | 1 (4.2) | 1 (6.7) | |
| Fatigue, n (%) | 78 (13.1) | 1 (1.8) | 45 (11.0) | 21 (22.6) | 5 (20.8) | 6 (40.0) | <0.001 |
| Anosmia/dysgeusia, n (%) | 62 (10.4) | 0 (0.0) | 57 (13.9) | 4 (4.3) | 1 (4.2) | 0 (0.0) | <0.001 |
| Neurological disorders, n (%) | 57 (9.6) | 0 (0.0) | 32 (7.8) | 17 (18.3) | 3 (12.5) | 5 (33.3) | <0.001 |
| Rheumatological disorders n/N (%) | 38/463 (8.2) | 0 (0.0) | 25/311 (8.0) | 6/67 (9.0) | 2/17 (11.8) | 4/10 (0.8) | 0.002 |
| Dyspnoea, n (%) | 36 (6.0) | 0 (0.0) | 22 (5.4) | 10 (10.7) | 1 (4.2) | 3 (20.0) | 0.011 |
| Psychiatric disorders, n (%) | 29 (4.9) | 0 (0.0) | 20 (4.9) | 6 (6.4) | 1 (4.2) | 2 (13.3) | 0.14 |
| Hair loss, n (%) | 22 (3.7) | 1 (1.8) | 16 (3.9) | 2 (2.1) | 2 (8.3) | 1 (6.7) | 0.39 |
| Cutaneous lesions, n (%) | 20 (3.4) | 1 (1.8) | 15 (3.7) | 3 (3.2) | 0 (0.0) | 1 (6.7) | 0.77 |
| URTI symptoms, n (%) | 20 (3.4) | 0 (0.0) | 3 (0.7) | 1 (1.1) | 0 (0.0) | 1 (6.7) | 0.23 |
| Headache, n (%) | 16 (2.7) | 0 (0.0) | 15 (3.4) | 1 (1.1) | 0 (0.0) | 0 (0.0) | 0.51 |
| Cough, n (%) | 12 (2.0) | 0 (0.0) | 7 (1.7) | 3 (3.2) | 1 (4.2) | 1 (6.7) | 0.18 |
| Gastrointestinal disorders, n (%) | 9 (1.5) | 0 (0.0) | 4 (1.0) | 3 (3.2) | 2 (8.3) | 0 (0.0) | 0.05 |
| Chest pain, n (%) | 5 (0.8) | 0 (0.0) | 3 (0.7) | 1 (1.1) | 1 (4.2) | 0 (0.0) | 0.36 |
| Ocular symptoms, n (%) | 2 (0.3) | 0 (0.0) | 2 (0.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1.00 |
COVID-19, coronavirus disease-19; n, number, N, number as a denominator; URTI, upper respiratory tract infection.
& The question was missed in some interviews.
Asymptomatic; mild (without pneumonia); moderate (with pneumonia); severe (with severe pneumonia); critical including Acute Respiratory Distress Syndrome (ARDS), sepsis and/or septic shock [11].
Fifty patients were seronegative or seroconverted at serological follow-up before 6 months and 231 patients underwent a serological test at the time of the interview (±15 days); among 193/281 (68.7%), a persistence of anti-SARS-CoV-2 IgG emerged. The median SARS-CoV-2 IgG antibody titre was 33 kAU/L (IQR 15‒65.8 kAU/L). At the time of the interview three out of 55 initially asymptomatic patients reported symptoms (including fatigue, skin lesions and hair loss) and two of these same three maintained a stable serological response (Table 3).
Post-COVID-19 syndrome associated factors
Risk factors for post-COVID-19 syndrome were evaluated for the total population (n = 599) and the sub-group with the serological follow-up available at the time of the interview (n = 231) (Table 4 and Tables S3–S5).
Table 4.
Multivariable analysis of risk factors associated with post-COVID-19 syndrome (n = 599)
| Risk factors | OR | 95% CI | p |
|---|---|---|---|
| Gender | |||
| Female | 0.025 | 1.05–2.27 | |
| Age group | |||
| 41‒60 vs. 18‒40 | 1.00 | 0.61–1.62 | 0.99 |
| >60 vs. 18‒40 | 1.03 | 0.62–1.74 | 0.90 |
| >60 vs. 41‒60 | 1.04 | 0.67 – 1.60 | 0.87 |
| Symptoms of acute COVID-19 at the onset, number | 1.81 | 1.59–2.05 | <0.001 |
| Management | |||
| Ward vs. outpatients | 1.87 | 1.19–2.94 | 0.007 |
| ICU vs. outpatients | 3.10 | 1.18–8.11 | 0.021 |
| ICU vs. ward | 1.65 | 0.61–4.46 | 0.32 |
CI, confidence of interval; Ct, cycle threshold; ICU, intensive care unit; OR, odds ratio.
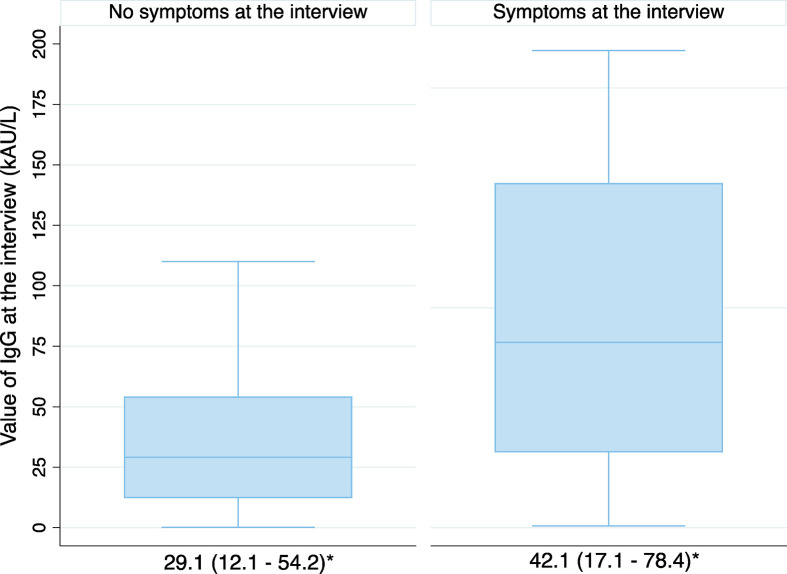
Presence of IgG antibodies against SARS-CoV-2 6 months after acute COVID-19 onset was significantly associated with the risk of post-COVID syndrome (n = 281, 88 seronegative patients vs. 193 seropositive patients, OR 2.56, 95% CI 1.48–4.38, p 0.001). Focusing on the subgroup of 231 patients, with regard to the titre magnitude among seropositive patients, median SARS-CoV-2 IgG antibody titres were significantly higher in long-haulers than in patients without sequelae (42.1, IQR 17.1–78.4 vs. 29.1, IQR 12.1–54.2 kAU/L) (Fig. 3 ).
Fig. 3.
Serological response againts SARS-CoV-2 in patients with or without post-COVID-19 syndrome at 6 months. ∗median (IQR).
In the multivariable logistic regression analysis, female gender (total population: OR 1.55, 95% CI 1.05–2.27, p 0.025; sub-group OR 2.82, 95% CI 1.46–5.43, p 0.002), a proportional increase in the number of symptoms (quantitative variable) at the onset of COVID-19 (total population: OR 1.81, 95% CI 1.59–2.05, p < 0.001; subgroup: OR 1.51, 95% CI 1.23–1.85, p < 0.001), ICU admission (total population: OR 3.10, 95% CI 1.18–8.11, p 0.021; subgroup: OR 8.29, 95% CI 1.43–48.00, p 0.018) and a proportional increase in anti-SARS-CoV-2 IgG at the time of the interview in the subgroup (OR 1.01, 95% CI 1.00–1.02, p 0.040) were all independent risk factors for the development of post-COVID-19 syndrome (Table 4 and Table S5).
Discussion
Possible long-term effects associated with coronavirus outbreaks documented to date have concerned severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) [5]. However, with regard to the recent COVID-19 outbreak, the available studies provided evidence on hospitalized patients [2,8,12,13], mainly with short follow-ups (<3 months) [1,12], leaving the long-term implications among community-dwelling individuals underinvestigated. Considering symptoms as perceived by patients and their associations with some serological parameters, this study has integrated the PROs framework [9] into routine clinical care, which might enhance symptom managements [6].
Findings suggest that the persistent high titres of the serological response against SARS-CoV-2 may play a significant role as an independent risk factor for post-COVID-19 symptoms alongside female gender, the number of symptoms at onset and ICU admission. Specifically, the overall incidence of post-COVID-19 syndrome was 40.3% with a high burden of symptoms (17.3% manifested ≥2 sequelae); although it is challenging to compare findings emerged in studies with different follow-ups (from 15 days to 7 months) [1,5,7,8,13,15] and populations, the overall incidence of post-COVID-19 syndrome was documented to range from 35% to 94% [1,2,7,[14], [15], [16]].
Findings suggest also that SARS-CoV-2 has several manifestations at 6 months regardless of the onset manifestation, from asymptomatic through to critical life-threatening disease. As previously reported [2,7], the presence of post-COVID-19 symptoms was significantly associated with the number of symptoms at onset and with the grade of disease requiring an ICU admission; however, as documented [14,15], our findings indicate that COVID-19 can also cause prolonged illness among individuals with milder disease and those requiring ambulatory care. In addition, we found significant differences regarding persistent symptoms with a higher incidence of anosmia/dysgeusia in patients with mild illness, and fatigue, dyspnoea, neurological disorders and rheumatological symptoms in patients with severe disease. Fatigue and dyspnoea have been reported among SARS and MERS patients, and are currently being described in COVID-19 survivors, mainly after hospitalization [3,7,17,18]. SARS-CoV-2 has high neuroinvasive potential compared with known coronavirus, causing variable prolonged sequelae, including headache, chemosensory dysfunction and neurocognitive issues [14,19]. It is interesting that unlike in SARS and MERS, anosmia and dysgeusia are frequently reported at acute onset and persisted in about 10% of the patients in our cohort. Rheumatological symptoms have also been reported in COVID-19 patients both at onset and over time [7,20], with the most common symptom in our study being arthralgia. The multi-organ chronic COVID-19 disease suggests that a multidisciplinary approach is required when implementing the care pathway, as recommended in NICE guidelines [10].
The underlying mechanism of acute and long-term consequences of COVID-19 is likely to be multifactorial. COVID-19 infection has been found to have equal prevalence in men and women, but male gender has shown to be a risk factor for higher severity and mortality [21]. In our cohort, females reported a higher prevalence of post-COVID-19 symptoms than males, consistent with previous long-haulers studies [1,2]. Physiological and social factors have been reported as possible explanation [1,8,22]; however, further data are needed. In contrast with available studies [8,15,23], we did not find any association with age and pre-existing co-morbidities, suggesting that the long-term effects of COVID-19 can be debilitating, even among young and healthy individuals.
The reasons why some recovered patients report chronic-COVID-19 are unknown [14,24]. Changes in viral load and the ability to interfere with the human immune system may play a role. A positive outcome implies the eradication of infection by immune response while providing immune protection from reinfection. On the other hand, a poor immune response with the persistence of viral triggers may promote a chronic phase of the disease [25]. However, knowledge regarding the dynamics and nature of the immune response to SARS-CoV-2 infection and its association with long COVID-19 is still limited. Huang and colleagues, including COVID-19 patients 6 months after hospital discharge, found a decline in seropositivity and in the median titres of the neutralizing antibodies when compared with the acute phase. Unfortunately, due to the limited serological data, the authors could not associate these findings with post-COVID syndrome [2]. In our study, a significant association between the presence and the persistence of acquired immune function and the association of increasing titres of SARS-CoV-2 antibodies and post-COVID syndrome emerged. Interestingly, multisystem inflammatory syndrome (MIS) has been reported to be a unique aspect of post-acute SARS-CoV-2 infection. In a large study describing this entity, approximately three-quarters of subjects presenting with MIS reported a serological response [26]. In our study, viral replication was rarely recovered 3 weeks after symptom onset [27] and the duration of viral shedding was not associated with a higher risk of post-COVID-19 symptoms. Despite this, it is well known that SARS-CoV-2 may persist in other difficult-to-eradicate tissues [6,28]. Our data and literature [5,6,25] suggest that SARS-CoV-2 could cause damage through the extent of cytopathic virus replication, creating an inflammatory environment, especially in immunologically privileged sites where it can be difficult for the immune system to eradicate. Hence, post-acute COVID may be driven mainly by allo-immune and auto-immune responses. Cytokine storm and the dysregulated immune response with the exaggerated inflammatory reaction associated with symptomatic COVID-19 [29], microangiopathy and endothelial injury and post-viral syndrome, as described for other viral infections, could promote a chronic disease [30]. A better understanding of the potential mechanism of post-COVID-19 is needed to establish effective treatments.
To the best of our knowledge, no data regarding asymptomatic patients over time are available. Three out of 55 asymptomatic patients complained symptoms at follow-up, and taking into consideration the limited size, two out of three maintained serological response at the follow-up.
This study has several limitations. It was performed in a single centre, introducing a potential selection bias; it has relied on patient self-reported symptoms [17], without any objective assessment introducing an information bias [[32], [33], [34]]. Only one serological test was performed, which may have an assay-dependent rate of sensitivity, specificity and antibody decline. However, we chose this antibody assay (iFlash-SARS-CoV-2) on the basis of a preliminary validation study and the literature [35]. The neutralizing antibodies test was not performed: its complexity prevents routine testing on a large scale while its utility in long-term immunity against SARS-CoV-2 remains undetermined.
In conclusion, a prospective follow-up of a large cohort of recovered COVID-19 patients with different grade severities and cared for in different settings suggested that 40.2% of them experienced post-COVID-19 syndrome up to 6 months after the onset. Several long-term pandemic clinical and health care service management issues should be considered. Moreover, the association of post-COVID-19 symptoms with the serological response triggers several insights into underlying pathogenesis mechanisms and possible therapeutic strategies.
Transparency declaration
Maddalena Peghin reports receiving grants and personal fees from Pfizer, MSD and Dia Sorin outside the submitted work. Carlo Tascini has received grants in the last two years from Correvio, Biotest, Biomerieux, Gilead, Angelini, MSD, Pfizer, Thermofisher, Zambon, Shionogi, Avir Pharma and Hikma outside the submitted work.
Funding
This research was funded by PRIN 2017 n.20178S4EK9 ‒ “Innovative statistical methods in biomedical research on biomarkers: from their identification to their use in clinical practice”.
Authors contributions
Conceptualization: M. Peghin, A. Palese, M. Isola, C. Tascini; Methodology: M. Peghin, A. Palese, M. Isola, C. Tascini; Software: M. De Martino, M. Isola; Validation: M. De Martino, M. Isola; Formal Analysis: M. De Martino M. Isola; Investigation: M. Peghin, A. Palese, M. Isola, M. De Martino, C. Tascini; Resources: M. Fabris, F. Curcio; Data curation: M. Venturini, V. Gerussi, E. Graziano, G. Bontempo, F. Marrella; Writing – Original Draft: M. Peghin, A. Palese; Writing – Review & Editing: M. Peghin, A. Palese, M. Isola, M. De Martino, M. Fabris, F. Curcio, A. Tommasini; C. Tascini; Visualization: M. Peghin, M. Isola, M. De Martino; Supervision: M. Peghin, A. Palese, M. Isola, C. Tascini; Project Administration: M. Peghin, A. Palese, M. Isola, F. Curcio, C. Tascini; Funding acquisition: M. Isola.
Acknowledgements
The authors are grateful to all patients for their collaboration. The authors would like to thank all clinical and nursing staff who cared for the patients at Udine Infectious Disease Clinic during hospitalization and ambulatory management. We also thank the umarells for their precious and strenuous support in the fight against COVID-19.
Editor: J. Bielicki
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2021.05.033.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Goertz Y.M.J., Van Herck M., Delbressine J.M., Vaes A.W., Meys R., Machado F.V.C., et al. Persistent symptoms 3 months after a SARS-CoV-2 infection: the post-COVID-19 syndrome? ERJ Open Res. 2020;6:542–2020. doi: 10.1183/23120541.00542-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C., Huang L., Wang Y., Li X., Ren L., Gu X., et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmed H., Patel K., Greenwood D.C., Halpin S., Lewthwaite P., Salawu A., et al. Long-term clinical outcomes in survivors of severe acute respiratory syndrome and Middle East respiratory syndrome coronavirus outbreaks after hospitalisation or ICU admission: a systematic review and meta-analysis. J Rehabil Med. 2020;52 doi: 10.2340/16501977-2694. [DOI] [PubMed] [Google Scholar]
- 4.Honigsbaum M., Krishnan L. Taking pandemic sequelae seriously: from the Russian influenza to COVID-19 long-haulers. Lancet. 2020;396:1389–1391. doi: 10.1016/S0140-6736(20)32134-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peghin M., Los-Arcos I., Hirsch H.H., Codina G., Monforte V., Bravo C., et al. Community-acquired respiratory viruses are a risk factor for chronic lung allograft dysfunction. Clin Infect Dis. 2019;69:1192–1197. doi: 10.1093/cid/ciy1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amenta E.M., Spallone A., Rodriguez-Barradas M.C., El Sahly H.M., Atmar R.L., Kulkarni P.A. Postacute COVID-19: an overview and approach to classification. Open Forum Infect Dis. 2020;7:ofaa509. doi: 10.1093/ofid/ofaa509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carfi A., Bernabei R., Landi F., Gemelli Against C-P-ACSG Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324:603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobs L.G., Gourna Paleoudis E., Lesky-Di Bari D., Nyirenda T., Friedman T., Gupta A., et al. Persistence of symptoms and quality of life at 35 days after hospitalization for COVID-19 infection. PLoS One. 2020;15 doi: 10.1371/journal.pone.0243882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Powers J.H., III, Howard K., Saretsky T., Clifford S., Hoffmann S., Llorens L., et al. Patient-reported outcome assessments as endpoints in studies in infectious diseases. Clin Infect Dis. 2016;63(Suppl 2):S52–S56. doi: 10.1093/cid/ciw317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.NICE guideline [NG188] COVID-19 rapid guideline: managing the long-term effects of COVID-19 2020. https://www.nice.org.uk/guidance/ng188. Published: 18 December. 2020 [Google Scholar]
- 11.World Health Organization . 2021. Clinical management of COVID-19: interim guidance.https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-1 (No. WHO/2019-nCoV/clinical/2021.1) 2021. Available at: [Google Scholar]
- 12.World Health Organization . 2020. Laboratory testing for coronavirus disease (COVID-19) in suspected human cases interim guidance.https://www.who.int/publications-detail/laboratory-testing-for-2019-novel-coronavirus-in-suspected-human-cases-20200117 (No. WHO/2019-nCoV/laboratory/2020.2) 2020. Available from: [Google Scholar]
- 13.Center for Health Security Serology-based tests for COVID-19 2020. https://www.centerforhealthsecurity.org/resources/COVID-19/serology/Serology-based-tests-for-COVID-19.html Available at:
- 14.Del Rio C., Collins L.F., Malani P. Long-term health consequences of COVID-19. JAMA. 2020;324(17):1723–1724 doi: 10.1001/jama.2020.19719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carvalho-Schneider C., Laurent E., Lemaignen A., Beaufils E., Bourbao-Tournois C., Laribi S., et al. Follow-up of adults with noncritical COVID-19 two months after symptom onset. Clin Microbiol Infect. 2021;27(2):258–263 doi: 10.1016/j.cmi.2020.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis H.E., Assaf G.S., McCorkell L., Wei H., Low R.J., Re’em Y., et al. Characterizing Long COVID in an international cohort: 7 months of symptoms and their impact. medRxiv 2020.12.24.20248802. 2020 doi: 10.1016/j.eclinm.2021.101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mandal S., Barnett J., Brill S.E., Brown J.S., Denneny E.K., Hare S.S., et al. Long-COVID’: a cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax. 2020;thoraxjnl-2020-215818 doi: 10.1136/thoraxjnl-2020-215818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Halpin S.J., McIvor C., Whyatt G., Adams A., Harvey O., McLean L., et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: a cross-sectional evaluation. J Med Virol. 2021;93:1013–1022. doi: 10.1002/jmv.26368. [DOI] [PubMed] [Google Scholar]
- 19.Wijeratne T., Crewther S. Post-COVID 19 Neurological Syndrome (PCNS); a novel syndrome with challenges for the global neurology community. J Neurol Sci. 2020;419:117179. doi: 10.1016/j.jns.2020.117179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ciaffi J., Meliconi R., Ruscitti P., Berardicurti O., Giacomelli R., Ursini F. Rheumatic manifestations of COVID-19: a systematic review and meta-analysis. BMC Rheumatol. 2020;4:65. doi: 10.1186/s41927-020-00165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gebhard C., Regitz-Zagrosek V., Neuhauser H.K., Morgan R., Klein S.L. Impact of sex and gender on COVID-19 outcomes in Europe. Biol Sex Differ. 2020;11:29. doi: 10.1186/s13293-020-00304-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bardel A., Wallander M.A., Wallman T., Rosengren A., Johansson S., Eriksson H., et al. Age and sex related self-reported symptoms in a general population across 30 years: patterns of reporting and secular trend. PLoS One. 2019;14 doi: 10.1371/journal.pone.0211532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tenforde M.W., Kim S.S., Lindsell C.J., Billig Rose E., Shapiro N.I., Files D.C., et al. Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems Network – United States, March-June 2020. MMWR Morb Mortal Wkly Rep. 2020;69:993–998. doi: 10.15585/mmwr.mm6930e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davido B., Seang S., Tubiana R., de Truchis P. Post-COVID-19 chronic symptoms: a postinfectious entity? Clin Microbiol Infect. 2020;26:1448–1449. doi: 10.1016/j.cmi.2020.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rouse B.T., Sehrawat S. Immunity and immunopathology to viruses: what decides the outcome? Nat Rev Immunol. 2010;10:514–526. doi: 10.1038/nri2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Godfred-Cato S., Bryant B., Leung J., Oster M.E., Conklin L., Abrams J., et al. COVID-19-associated multisystem inflammatory syndrome in children – United States, March-July 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1074–1080. doi: 10.15585/mmwr.mm6932e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiao A.T., Tong Y.X., Zhang S. Profile of RT-PCR for SARS-CoV-2: a preliminary study from 56 COVID-19 patients. Clin Infect Dis. 2020;71:2249–2251. doi: 10.1093/cid/ciaa460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y., Geng X., Tan Y., Li Q., Xu C., Xu J., et al. New understanding of the damage of SARS-CoV-2 infection outside the respiratory system. Biomed Pharmacother. 2020;127:110195. doi: 10.1016/j.biopha.2020.110195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Broderick G., Fuite J., Kreitz A., Vernon S.D., Klimas N., Fletcher M.A. A formal analysis of cytokine networks in chronic fatigue syndrome. Brain Behav Immun. 2010;24:1209–1217. doi: 10.1016/j.bbi.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Needham D.M., Davidson J., Cohen H., Hopkins R.O., Weinert C., Wunsch H., et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders' conference. Crit Care Med. 2012;40:502–509. doi: 10.1097/CCM.0b013e318232da75. [DOI] [PubMed] [Google Scholar]
- 33.Rossi R., Socci V., Talevi D., Mensi S., Niolu C., Pacitti F., et al. COVID-19 pandemic and lockdown measures impact on mental health among the general population in Italy. Front Psychiatr. 2020;11:790. doi: 10.3389/fpsyt.2020.00790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aiyegbusi O.L., Calvert M.J. Patient-reported outcomes: central to the management of COVID-19. Lancet. 2020;396:531. doi: 10.1016/S0140-6736(20)31724-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Plebani M., Padoan A., Negrini D., Carpinteri B., Sciacovelli L. Diagnostic performances and thresholds: the key to harmonization in serological SARS-CoV-2 assays? Clin Chim Acta. 2020;509:1–7. doi: 10.1016/j.cca.2020.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.American Psychiatric Association . vol. 10. APA; Washington, DC: 2013. (DSM-5 diagnostic classification). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.