Abstract
Objectives:
Lung cancer has the highest cancer-related mortality in the U.S. and among Veterans. Screening of high-risk individuals with low-dose CT (LDCT) can improve survival through detection of early stage lung cancer. Organizational factors that aid or impede implementation of this evidence-based practice in diverse populations are not well described. We evaluated organizational readiness for change and change valence (belief that change is beneficial and valuable) for implementation of LDCT screening.
Methods:
We performed a cross-sectional survey of providers, staff, and administrators in radiology and primary care at a single Veterans Affairs Medical Center. Survey measures included Shea’s validated Organizational Readiness for Implementing Change (ORIC) scale and Shea’s ten items to assess change valence. ORIC and change valence were scored on a scale from 1–7 (higher scores representing higher readiness for change or valence). Multi-variable linear regressions were conducted to determine predictors of ORIC and change valence.
Results:
Of 523 employees contacted, 282 completed survey items (53.9% overall response rate). Higher ORIC scores were associated with radiology vs. primary care (mean 5.48, standard deviation [SD] 1.42 vs 5.07, SD 1.22, Beta=0.37, p=0.039). Self-identified leaders in lung cancer screening had both higher ORIC (5.56, SD 1.39 vs 5.11, SD 1.26, Beta=0.43, p=0.050) and change valence scores (5.89, SD 1.21 vs 5.36, SD 1.19, Beta=0.51, p=0.012).
Discussion:
Radiology health professionals have higher levels of readiness for change for implementation of LDCT screening than those in primary care. Understanding health professionals’ behavioral determinants for change can inform future lung cancer screening implementation strategies.
Keywords: lung cancer, lung cancer screening, organizational readiness, implementation science, Veteran
Summary Sentence:
Findings of this pilot cross-sectional study suggest that health professionals working in radiology have higher levels of readiness for change than those in primary care for implementing lung cancer screening.
Introduction:
Lung cancer has the highest cancer-related mortality in the U.S. and among Veterans.[1,2] Lung cancer screening with low-dose CT (LDCT) can improve survival in high-risk individuals through detection of early stage lung cancer.[3,4] LDCT screening is currently being implemented across the U.S. healthcare system including the Veterans Health Administration (VHA).[5,6]
LDCT is considered an evidence-based practice by the U.S. Preventive Services Task Force and considered a Grade B recommendation.[7] Yet, it is only utilized in 2%−14% of US patients and Veterans.[8–12] The organizational factors that aid or impede the utilization of LDCT in diverse populations are not well described. The U.S. Department of Veterans Affairs (VA) Quality Enhancement Research Initiative (QUERI) advocates for rigorous evaluation of organizational attributes that may impact the implementation of a new evidenced-based practice (such as lung cancer screening).[13,14] Broadly speaking, organizational effectiveness and implementation success are a function of organizational structure and organizational processes.[15]
The Consolidated Framework for Implementation Research (CFIR) defines several operational constructs likely to influence the implementation of evidence-based practices.[16] Constructs include an organization’s inner and outer settings, individual and team characteristics, processes of implementation and measures of implementation.[16] Implementation success is more likely when an organization and its members are ready for change and believe the change has positive value.[17] For this reason, our pilot study focused on a single Veterans Affairs Medical Center’s (VAMC) inner setting. We specifically chose to evaluate organizational readiness for change and change valence. Organizational readiness for change is defined as the “extent to which organizational members are psychologically and behaviorally prepared to implement organizational change” and reflects the knowledge, attitudes, and beliefs of providers, staff, and leadership.[18] Change valence is defined as the organization’s members belief that pursuing change is beneficial and valuable to the organization.[17] Weiner has described change valence as the beliefs that are the precursor to the “commitment to change.”
In this pilot study, we evaluated organizational readiness for change and change valence among clinical providers, staff, and administrators affiliated with radiology and primary care at a single VAMC. Primary care and radiology are interdependent in the delivery of lung cancer screening services. Primary care is paramount in identification of eligible patients for lung cancer screening, follow-up of screening results and coordination of downstream testing and treatment. Radiology performs and interprets LDCT screening examinations. We hypothesized that radiology would have higher readiness for change and change valence than primary care for a number of reasons. Primarily, the screening test is a radiological examination that is familiar to this service line. Radiologists have been involved in imaging screening (i.e. screening mammography) for many years. Furthermore, LDCT represents an annual yearly service provided to patients. In contrast, primary care provides a wide range of general health services, of which cancer screening is only a small component. Additionally, primary care providers may have differing levels of experience with the follow up of abnormal LDCT exams and care coordination for patients with abnormalities. Based on prior literature supporting the importance of leadership during implementation of new evidence-based practices, we further hypothesized that those who self-identify as holding leadership roles would have higher readiness for change and change valence than those who did not identify as holding leadership roles.[19–21]
Methods:
Study Design/Setting/ Population:
In this cross-sectional study, we surveyed clinical providers, staff, and administrators in radiology and primary care at a single VAMC that offers hospital-based, community-based, and specialty care. The survey was administered in August 2019. VAMC primary care and radiology team members at the main hospital and community-based outpatient clinics (CBOC) were included. Participants were identified through administrative staff and email listservs. The VAMC Institutional Review Board, Research and Development Committee, and local union approved this study and a waiver of written consent was granted. Consent was implied by participant completion of the survey after reading the study information. Respondents were offered the opportunity to win 1 of 20 Amazon $50 gift cards upon completion of the questionnaire.
This VAMC centrally-organized lung cancer screening program is overseen by an inter-professional steering committee. The lung cancer screening program coordinator (a nurse practitioner whose efforts are dedicated to the program) officially began to organize screening efforts in February 2019. From June to August 2019, the program began offering lung cancer screening to Veterans referred by a small group of 5–10 primary care providers. The program screened less than 20 Veterans during the 3 month planning period. In August, the program activated a referral order to the screening program which increased the number of referrals to approximately 30 per week. The program performed provider outreach through a Grand Rounds event and in-person visits to primary care clinics. Additionally, all providers were able to order the screening exam for eligible Veterans outside of this program.
Study Procedures:
VHA clinical providers and research team members pilot tested the survey for content and clarity and minor word revisions were made to make the survey specific to the VAMC. In August 2019, potential participants were emailed the internet-based questionnaire through VA-REDCap.[22,23] Non-responders and participants who had partially completed the survey received weekly reminders for up to 12 weeks or until survey completion.
Survey Content:
The survey (Appendix 1) was developed to explore the inner setting constructs of the CFIR and adapted from Shea’s validated Organizational Readiness for Implementing Change (ORIC) scale along with additional items developed by Shea to assess change valence.[16,24] The ORIC scale contains two subscales, change commitment and change efficacy. Change commitment is the organizational desire to support a particular course of action. Change efficacy is the belief in the ability to engage in those action necessary to implement a change. We defined our primary outcomes as total ORIC score and change valence score.
Independent Variables:
The primary independent variable was defined as the employee belonging to either the primary care or radiology service line. Primary care was inclusive of physicians, advanced practice providers, nurses, staff, administrators, and service line leadership. Radiology was inclusive of physicians, technologists, staff, and service line leadership. In a separate question we asked respondents to report whether they self-identified as holding a leadership role in the implementation of lung cancer screening in this VAMC. Those self-identifying as holding a leadership role were defined as leaders and those that did not identify as holding a leadership role were defined as non-leaders. A secondary analysis defined the independent variable as those who self-identified as leaders vs. non-leaders. Participants also self-reported age, gender, and information related to their clinical position. This included current position, duration in this position, and clinical setting (main hospital vs. CBOC).
Co-Primary Outcomes:
Organizational Readiness for Change:
The ORIC scale consists of nine items on two subscales (four items on change commitment and five items on change efficacy). The language of the nine ORIC items was minimally adapted to reflect organizational readiness for change specific to implementation of lung cancer screening and rated on a 7-point Likert-type scale ranging from strongly disagree (1) to strongly agree (7). A mean ORIC score for each participant was calculated by summing the scores for each of the nine items in the organizational readiness for change scale and dividing by nine (possible range 1–7). A mean ORIC change commitment subscale score for each participant was calculated by summing the scores of the four change commitment items and dividing by four (possible range 1–7). A mean ORIC change efficacy subscale score for each participant was calculated by summing the scores of the five change efficacy items and dividing by five (possible range 1–7). Higher ORIC scores indicated higher organizational readiness for change, change commitment, and change efficacy.
Change Valence:
We adapted the language of Shea et al.’s ten change valence items to reflect valence or how the organization values lung cancer screening implementation.[24] These items were also rated on the same 7-point Likert-type scale. The mean change valence score for each participant was calculated by summing the scores for each of the 10 change valence items and dividing by ten (possible range 1–7). Higher scores indicated higher change valence.
Scale Reliability:
We calculated Cronbach’s alpha to assess scale reliability and inter-item correlation for ORIC, ORIC subscales (change commitment and change efficacy), and the ten change valence items.[25,26] Each scale demonstrated very good scale reliability indicated by high alpha scores: 0.965 (ORIC), 0.982 (ORIC change commitment), 0.963 (ORIC change efficacy), and 0.969 (change valence). We performed Spearman’s tests to assess correlation amongst scales. Highest scale correlation was seen between the parent ORIC scale and ORIC change commitment and the parent ORIC scale and ORIC change efficacy subscales: ORIC-ORIC change commitment (0.895), ORIC-ORIC change efficacy (0.943), ORIC change commitment-ORIC change efficacy (0.706), ORIC-change valence (0.824), ORIC change commitment-change valence (0.815) and ORIC change efficacy-change valence (0.742).
Sensitivity Analysis:
A sensitivity analysis explored ORIC and change valence scores by work position as the independent variable. We categorized work position into three groups. Staff included clinic scheduler, clerical employee, RN, LPN, nursing assistant, nursing-other, diagnostic imaging technician, other-direct patient care. Providers included advanced practice providers, psychologist, social worker, physician assistant, physician-attending, and physician-in training. The final category, administrator, included clinical informatics, executive leaders, division chiefs, section chiefs, and administrative-other.
Statistical Analysis:
The final analytic sample included participants who completed one or both outcome scales (ORIC or change valence). Descriptive statistics were used to characterize demographic characteristics for participants who provided complete responses. For ORIC and change valence scores, we report both mean with standard deviation (SD) and median with interquartile range [IQR].[27–29] Multi-variable linear regressions were conducted to determine the predictors of ORIC and change valence. Respondent gender, age (continuous), and clinical position (staff, provider, or administrator) were also entered as covariates in the adjusted regression models. Respondents who reported “Other” for gender (n=1) or who were missing a response for gender (n=4) were excluded from adjusted multivariable analyses. Additionally, we determined if ORIC or change valence scores differed among the clinical positions (staff, provider, or administrator).
All analyses were performed using Stata (release 14, 2015) (StataCorp, College Station, TX). A two-sided p-value <0.05 was considered statistically significant.
Role of the Funding Source:
Funding was providing by the Veterans Health Administration Office of Rural Health. The lottery incentives to the providers were funded through the Vanderbilt-Ingram Cancer Center.
Study design, data collection, and data analysis/interpretation was independent of the Office of Rural Health and the Vanderbilt-Ingram Cancer Center. The Office of Rural Health had the opportunity to review the manuscript prior to submission. Study results and the decision to publish are solely the responsibility of the authors and do not necessarily represent official views of the funder.
Results:
Analytic Sample
We contacted 523 individuals (395 primary care; 128 radiology) via email and asked them to voluntarily participate in the survey. Of these, 282 individuals provided data (53.9% overall response rate). Within primary care the response rate was 52.2% (206/395) and within radiology the response rate was 59.4% (76/128). Twelve surveys were incomplete and excluded from the analysis (11 primary care; 1 radiology). One respondent indicated their primary job position was not within radiology or primary care and was also excluded. The final analytic sample included 195 individuals from primary care (49.4% final response rate) and 74 individuals from radiology (57.8% final response rate). Refer to Figure 1 for the full flow chart of survey distribution and responses.
Figure 1.
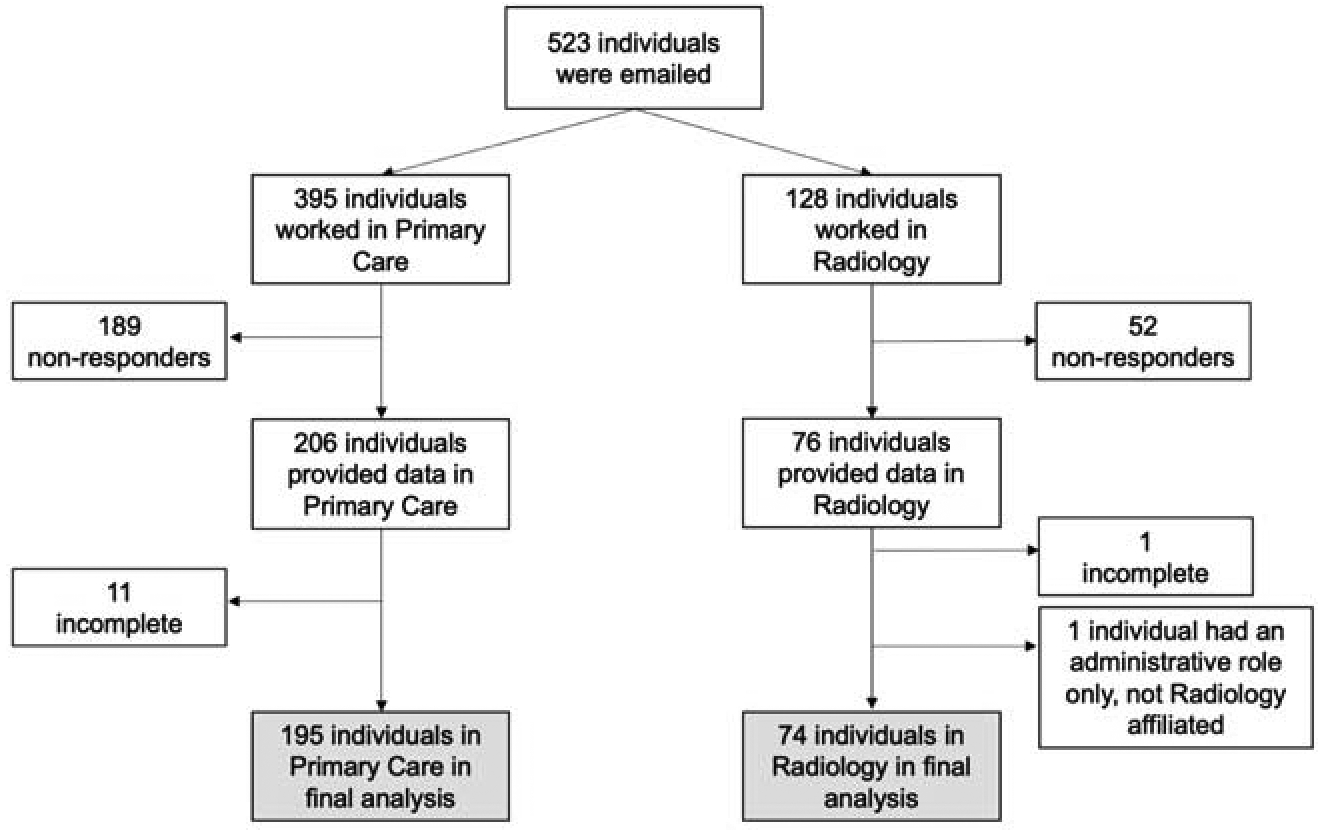
Flow chart of survey respondents.
Respondent Characteristics
Descriptive statistics summarizing respondents’ demographics, self-identified leadership role in lung cancer screening, position, and clinical setting are presented in Table 1 stratified by service line. There were minor differences between the two groups. Primary care respondents were more often female and associated with the community-based clinical setting while radiology respondents were more often male and associated with a hospital-based clinical setting, reflective of differences in these services lines. Available characteristics for the 241 non-responders (clinical position, clinical setting, and affiliation with radiology or primary care) are also included in Table 1. Non-responder age, gender, self-identified leadership role, duration in position, and clinical area could not be obtained as this data was only available from survey responses.
Table 1.
Characteristics of Respondents
| Characteristic | Non-Responders | All Responders | Primary Care | Radiology |
|---|---|---|---|---|
| *N=241 (%) | N= 269 (%) | N=195 (%) | N=74 (%) | |
| Age, years Mean(Standard deviation [SD]) | - | 47.1(11.3) | 47.1(11.2) | 47.0(11.5) |
| Gender | - | |||
| Female | 192(71.4) | 147(75.4) | 45(60.8) | |
| Male | 72(26.7) | 43(22.0) | 29(39.2) | |
| Unknown | 5(1.9) | 5(2.6) | 0(0) | |
| Self-identified leadership role in lung cancer screening, yes | - | 44(16.4) | 33(16.9) | 11(14.9) |
| Clinical Position | ||||
| Staff | 147(61.0) | 171(63.6) | 116(59.5) | 55(74.3) |
| Provider | 62(25.7) | 65(24.2) | 51(26.2) | 14(18.9) |
| Administrator | 8(3.3) | 31(11.5) | 26(13.3) | 5(6.8) |
| Unknown | 24(10.0) | 2(<1) | 2(1.0) | 0(0) |
| Duration in current position, years | - | 7.0(7.3) | 6.2(7.0) | 9.3(7.7) |
| Mean(SD) | ||||
| Clinic setting | ||||
| Community-Based Outpatient Center | 136(56.4) | 153(56.9) | 147(75.4) | 6(8.1) |
| Hospital-Based | 90(37.3) | 116(43.1) | 48(24.6) | 68(91.9) |
| Unknown | 15(6.2) | 0(0) | 0(0) | 0(0) |
| Clinical Area (Primary Care only) | - | |||
| Family Medicine | 113(57.9) | |||
| General Internal Medicine | 43(22.1) | |||
| Geriatrics | 5(2.6) | |||
| Women’s Health | 4(2.1) | |||
| Health Behavior Coordinator | 4(2.1) | |||
| Hospitalist | 1(<1) | |||
| “I do not provide direct clinical care” | 24(12.3) | |||
| Unknown | 1(<1) |
Of all non-responders 189 were affiliated with primary care (78.4%) and 52 were affiliated with radiology (21.6%).
Co- Primary Outcomes: ORIC and Change Valence
The outcomes for linear and multivariable regressions including means, Beta coefficients with 95% Confidence Intervals (CI), and p-values are summarized in Table 2. All participants in the analytic sample answered all nine ORIC questions. The overall mean ORIC score was 5.18 (SD 1.29; median 5.11 [4.33–6.22]); higher scores suggest higher readiness for change), with differences between radiology (mean 5.48 (1.42); median 6.0 [4.44–6.67]) and primary care (mean 5.07 (1.22); median 5.00 [4.33–6.00]) (p=0.020). This difference remained statistically significant in a model adjusting for age, gender, and clinical position (p=0.039). See Figure 2A for the distribution of organizational readiness scores by service line. The mean ORIC score in self-reported leaders (mean 5.56 (1.39); median 5.83 [4.94–6.78]) was significantly higher than in non-leaders (mean 5.11 (1.26); median 5.00 [4.22–6.00]); p=0.036) in unadjusted analyses. In a model adjusting for age, gender, and clinical position results remained statistically significant (p=0.050). See Figure 2B for the distribution of organizational readiness scores by leadership role status.
Table 2.
Outcomes
| Primary Outcomes, by service line | Service Line | Mean Score (SD) | Linear Regression | Multivariable Regression | ||
|---|---|---|---|---|---|---|
| Beta [95% CI] | p-value | Beta [95%CI] | p-value | |||
| ORIC | Primary Care | 5.07 (1.22) | REF | - | REF | - |
| Radiology | 5.48 (1.42) | 0.41 [0.07, 0.75] | 0.020 | 0.37 [0.02, 0.73] | 0.039 | |
| ORIC - change commitment | Primary Care | 5.04 (1.35) | REF | - | REF | - |
| Radiology | 5.64 (1.42) | 0.59 [0.23, 0.96] | 0.002 | 0.59 [0.21, 0.98] | 0.003 | |
| ORIC - change efficacy | Primary Care | 5.09 (1.33) | REF | - | REF | - |
| Radiology | 5.35 (1.53) | 0.26 [−0.11, 0.64] | 0.165 | 0.20 [−0.18, 0.58] | 0.311 | |
| Change Valence | Primary Care | 5.37 (1.14) | REF | - | REF | - |
| Radiology | 5.65 (1.34) | 0.28 [−0.04, 0.61] | 0.089 | 0.26 [−0.08, 0.60] | 0.127 | |
| Primary Outcomes, by self-identified leadership role | Leader status | Linear Regression | Multivariable Regression | |||
| Beta [95% CI] | p-value | Beta | p-value | |||
| ORIC | Non-Leader | 5.11 (1.26) | REF | - | REF | - |
| Leader | 5.56 (1.39) | 0.45 [0.03, 0.87] | 0.036 | 0.43 [0.00, 0.85] | 0.050 | |
| ORIC - change commitment | Non-Leader | 5.11 (1.36) | REF | - | REF | - |
| Leader | 5.71 (1.52) | 0.61 [0.15, 1.06] | 0.009 | 0.58 [0.11, 1.04] | 0.014 | |
| ORIC - change efficacy | Non-Leader | 5.11 (1.37) | REF | - | REF | - |
| Leader | 5.43 (1.45) | 0.32 [−0.13, 0.77] | 0.162 | 0.30 [−0.15, 0.76] | 0.191 | |
| Change Valence | Non-Leader | 5.36 (1.19) | REF | - | REF | - |
| Leader | 5.89 (1.21) | 0.53 [0.14, 0.92] | 0.008 | 0.51 [0.11, 0.91] | 0.012 | |
| Sensitivity Analysis | Clinical Role | One-way ANOVA for difference in groups p-value | ||||
| ORIC | Staff | 5.2 (1.25) | 0.859 | |||
| Provider | 5.15 (1.35) | |||||
| Administrator | 5.07 (1.40) | |||||
| ORIC - change commitment | Staff | 5.17 (1.36) | 0.793 | |||
| Provider | 5.30 (1.38) | |||||
| Administrator | 5.12 (1.62) | |||||
| ORIC - change efficacy | Staff | 5.22 (1.32) | 0.566 | |||
| Provider | 5.04 (1.51) | |||||
| Administrator | 5.03 (1.51) | |||||
| Change Valence | Staff | 5.43 (1.16) | 0.883 | |||
| Provider | 5.41 (1.19) | |||||
| Administrator | 5.54 (1.47) | |||||
ORIC = Organizational Readiness for Implementing Change
CI = Confidence Interval
Beta = the absolute difference in means on the original scale
Figure 2.
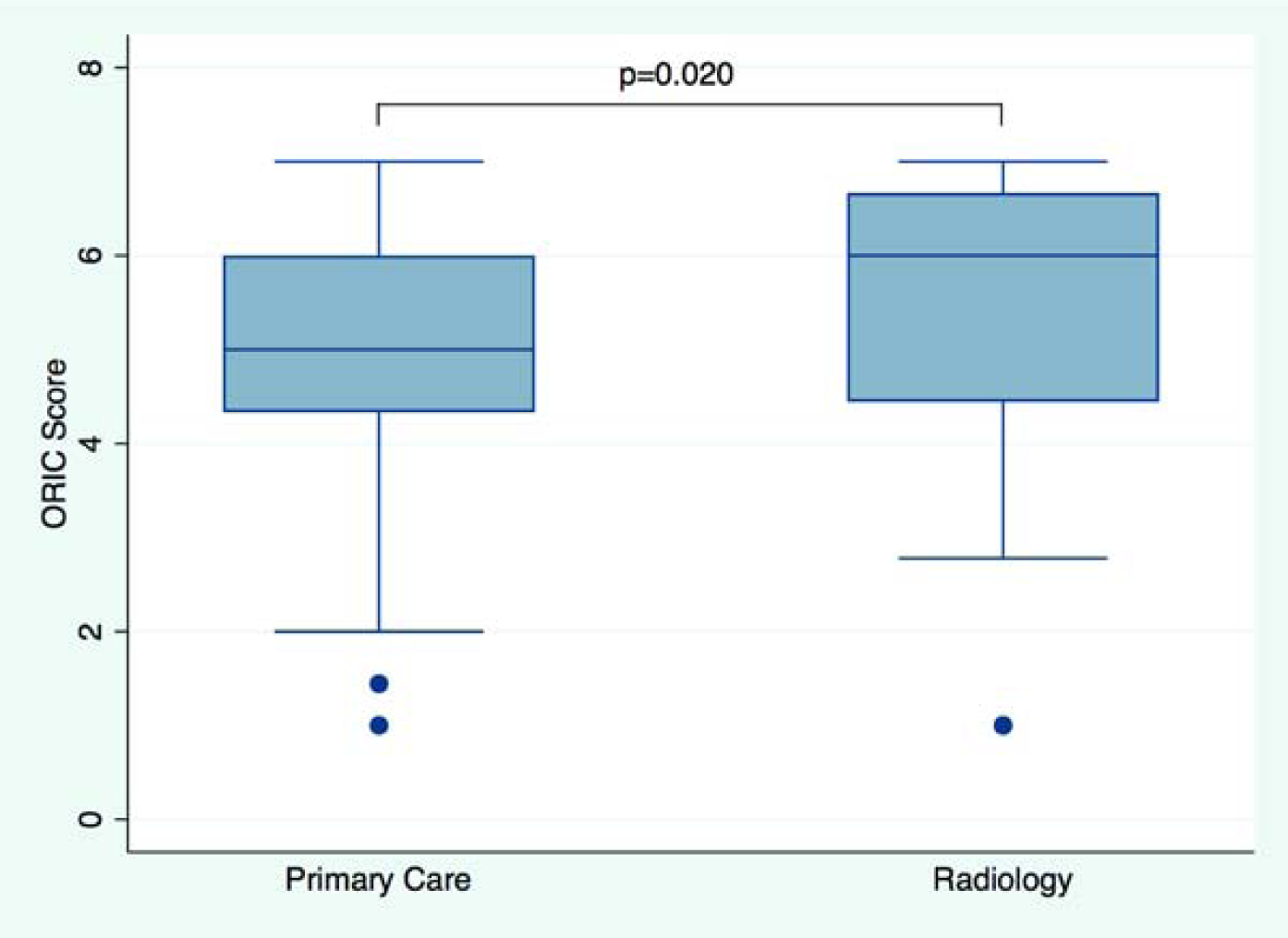
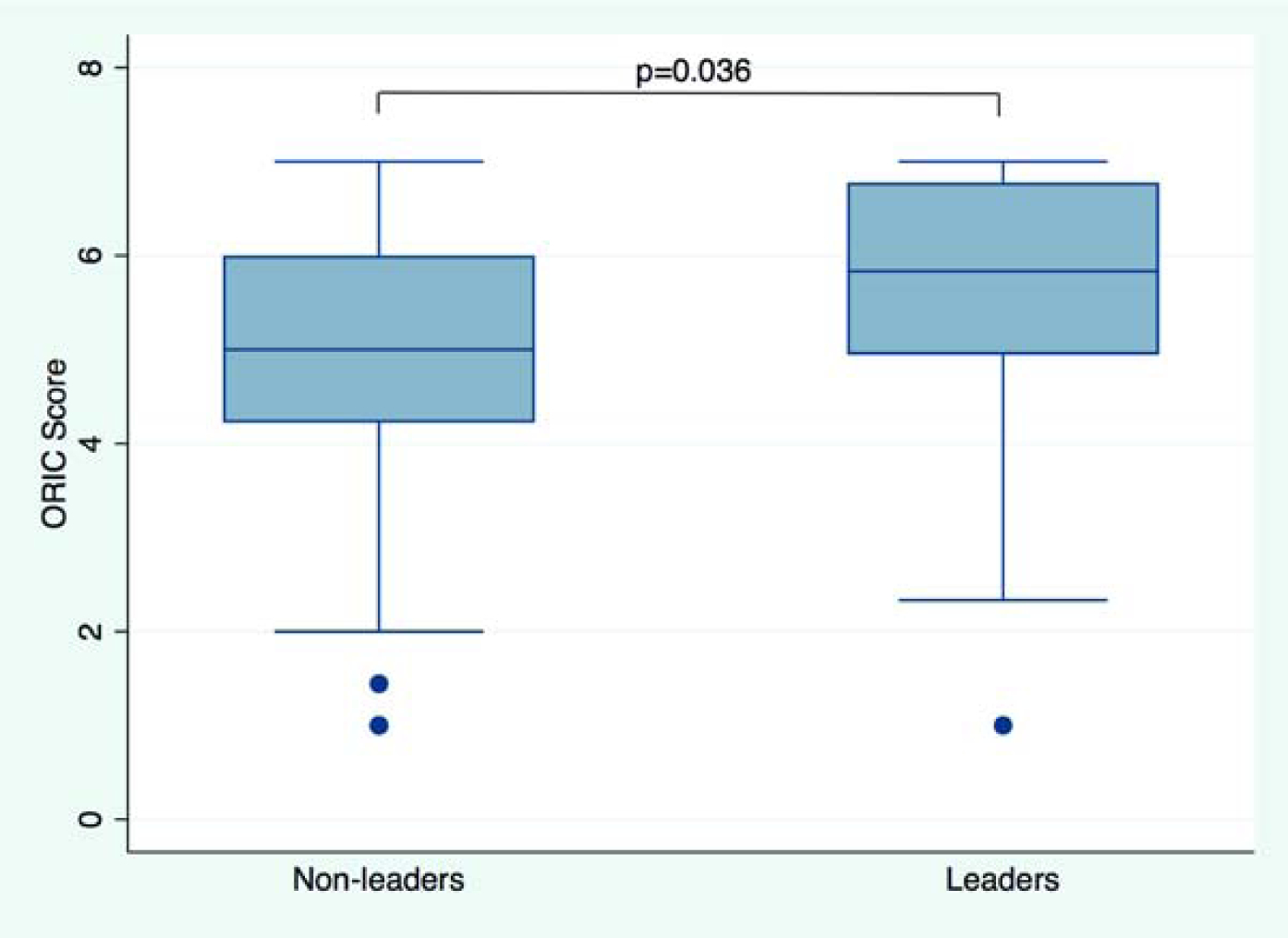
Organizational Readiness for Implementing Change (ORIC). Boxplot shows ORIC score by service line (Figure 2A) and in non-leaders vs. leaders (Figure 2B). Median scores and interquartile ranges (whiskers) are shown. P-value from unadjusted linear regression.
The overall mean ORIC change commitment score was 5.20 (SD 1.39; median 5.00 [4.00–6.25]) with differences between radiology (mean 5.64 (1.4); median 6.00 [4.50–7.00]) and primary care (mean 5.04 (1.35); median 5.00 [4.00–6.00]) in unadjusted (p=0.002) and adjusted (p=0.003) models. Change commitment also differed in leaders (mean 5.71 (1.52); median 6.13 [5.00–7.00]) and non-leaders (mean 5.11 (1.36); median 5.00 [4.00–6.00]) in unadjusted (p=0.009) and adjusted (p=0.014) models. The overall mean ORIC change efficacy score was 5.16 (SD 1.39; median 5.00 [4.00–6.20]) with no difference between radiology (mean 5.35 (1.53); median 5.80 [4.00–7.00]) and primary care (mean 5.09 (1.33); median 5.00 [4.00–6.00]) in unadjusted (p=0.165) or adjusted (p=0.311) models. No difference in change efficacy was noted amongst leaders (mean 5.43 (1.45); median 5.60 [4.90–6.90]) and non-leaders (mean 5.11 (1.37); median 5.00 [4.00–6.00]) in unadjusted (p=0.162) or adjusted (p=0.191) models.
All participants in the analytics sample answered all ten change valence questions. The overall mean change valence score was 5.45 (SD 1.20; median 5.40 [4.60–6.50]; higher scores suggest higher change valence). Change valence in radiology (mean 5.65 (1.34); median 6.00 [4.80–6.85] was similar to primary care (mean 5.37 (1.14); median 5.30 [4.60–6.20]) (p=0.089). In a multivariable model adjusting for age, gender, and clinical position results remained similar (p=0.127). See Figure 3A for the distribution of change valence scores by service line. Change valence in self-reported leaders (mean 5.89 (1.21); 6.10 [5.25–6.95]) was higher than in non-leaders (mean 5.36 (1.19); median 5.30 [4.50–6.30]) (p=0.008), and remained statistically significant in multivariable regression (p=0.012). See Figure 3B for the distribution of change valence scores by leadership role status.
Figure 3.
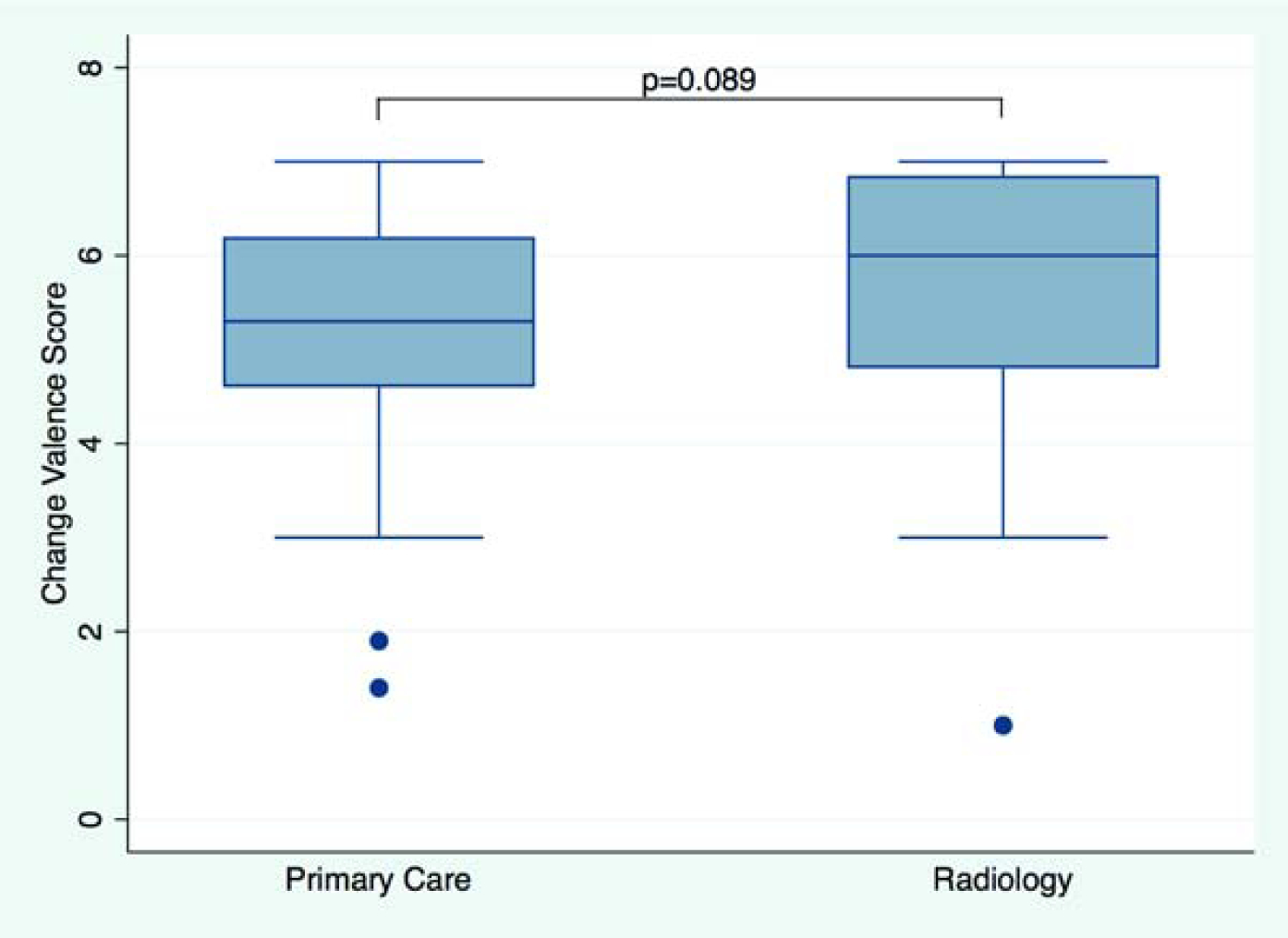
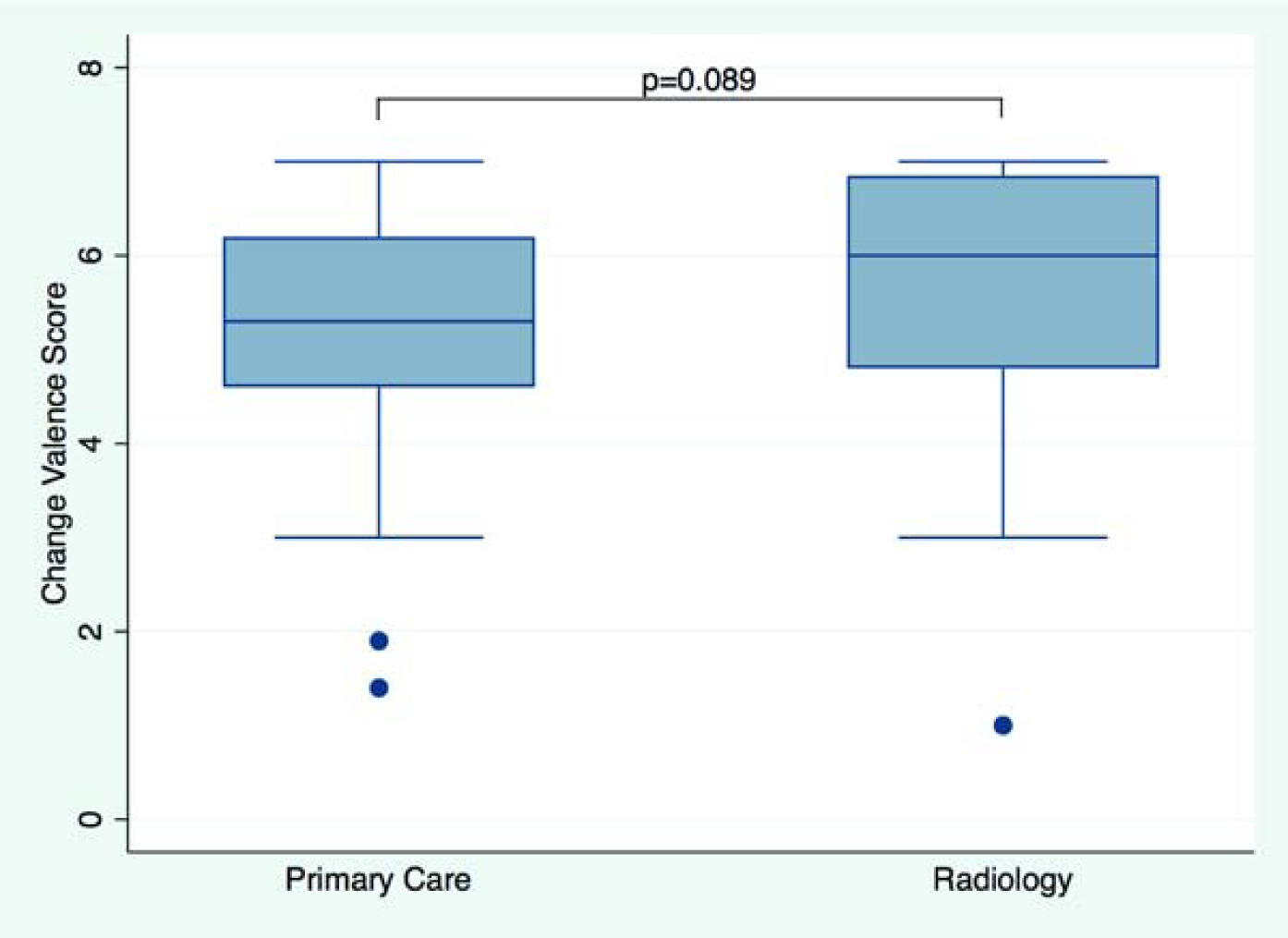
Change Valence. Boxplot shows change valence score by service line (Figure 3A) and in non-leaders vs. leaders (Figure 3B). Median scores and interquartile ranges (whiskers) are shown. P-value from unadjusted linear regression.
The sensitivity analysis (Table 2) found no difference in ORIC score among staff (n=171), providers (n=65) and administrators (n=31) (p=0.859). Sensitivity analysis also found no difference in change valence score among staff (n=170), providers (n=62), and administrators (n=31) (p=0.883).
Discussion:
Our study assessed organizational readiness for change among health professionals working in radiology or primary care in the early implementation of an organized lung cancer screening program. Higher levels of organizational readiness for change, primarily due to change commitment, were associated with health professionals working in radiology and having a leadership role in lung cancer screening. Higher levels of change valence were associated with those who self-identified as holding a leadership role in lung cancer screening. To our knowledge, this is the first assessment of radiology and primary care readiness and change valence for implementation of lung cancer screening.
This study provides novel results to inform implementation strategies for deployment of lung cancer screening activities within VHA. Deployment of lung cancer screening is complex. Additionally, VHA serves a diverse population of Veterans, including those in rural and urban locations where needs and readiness may vary according to available resources. Understanding the influencers of behavioral change in healthcare professionals from a variety of backgrounds and leadership roles can help to allocate appropriate resources for education and engagement in lung cancer screening. The three key drivers of behavioral change include capability, opportunity, and motivation.[30] Recognizing these drivers of change and differences in organizational readiness can inform the potential to change behavior and the sustainability of new interventions to meet the needs of VHA’s geographically diverse population.[18]
As with many innovations, the implementation of a new evidenced-based practice such as lung cancer screening is a complex endeavor that can benefit from a strategic and systematic approach.[6,31] The evaluation of this approach through the study of the internal setting (the networks, communications, and culture) of an organization can improve the processes and outcomes of the implementation.[16] Understanding organizational readiness for change prior to implementation of complex evidence-based practices is of critical importance, as individuals in organizations with higher readiness for change are more likely to initiate change, be collaborative and cooperative, and exert greater effort to implement new evidence-based practices.[32] Dym and Hutson described three states of readiness.[33] The first is a foray which “support and augment forces for alignment that are already in motion.” The second type is a responsive state of readiness, such as curiosity, receptiveness, urgency, and determination. This change is best served through information, advice, and guidance. Finally, the third type is a state of instability and crisis which makes the need for change urgent. In this study we postulate that radiology and primary care fall into two of these different states. Radiology (most likely a foray) was more aware of lung cancer screening, had previously informally engaged in screening patients, and had purchased an additional scanner to meet the anticipated need of increases in screening volumes. Whereas, primary care (responsive readiness) had not formally been trained in the process or procedures involved and had little guidance on what the processes were for lung cancer screening. Other challenges, specific to primary care, include identifying eligible patients, conducting shared-decision making, and the resources necessary to manage abnormal screening results.[5,6,31]
Recognizing variation in readiness for change amongst different medical specialties who each have different roles and responsibilities in a complex evidenced-based practice such as lung cancer screening can help to inform strategies for implementation. When there are limitations on resources, it can be helpful to understand organizational readiness so that strategically focused education and support can be targeted to those at highest need; where there is less readiness for change. We have disseminated our findings to leadership within our primary care service line to elicit input into how to improve primary care readiness for lung cancer screening. Strategies currently being deployed to improve primary care readiness included a series of on-site meetings with community-based outpatient clinic staff and delivering focused provider education.
Our second finding was that there is variation in how leaders vs. non-leaders value change. Leaders reflect an organization’s culture, patterns of thought, and behaviors. Many leaders plan and implement change efforts without knowledge or thought to the readiness of their staff. Often the assumption is that persuasion and reason will lead to the necessary change. This idea is reflected in our study results which demonstrate that commitment is quite high among leaders with a lower change efficacy score; which represents the confidence and belief in the actions for change. Recognizing this difference and supporting the front line is important for developing implementation strategies targeted to members of the healthcare team. Leadership should balance enthusiasm with knowledge of barriers. A previous study performed in VHA found that the majority of pulmonologists have favorable perceptions of the evidence supporting lung cancer screening, however, several barriers to implementation of lung cancer screening exist.[34] In this study by Tukey and colleagues, the recommended infrastructure for comprehensive lung cancer screening programs (i.e. CT scanner, PET scanner, medical oncologist, radiation oncologist, thoracic surgeon) were present in 36 of 106 VAMC facilities (34.0%). Overall, only 26.5% of Veterans Health Administration facilities were ideally prepared for lung cancer screening implementation with adequate onsite resources.
This pilot study has several strengths including understanding organizational readiness in two different service lines that should ideally work together to implement lung cancer screening. Further, the study used an established implementation framework (CFIR) and validated measurement scale for readiness for change.[16,24] Our overall response rate of 53.6% is a reasonable response rate for internet-based surveys of health professionals, which has varied from 9–94%.[35] This study was performed at a single VAMC, therefore the generalizability is limited, but may translate to other VAMCs. Additional potential limitations include use of a survey which had not previously been validated in this setting, a definition of leadership which may not capture one’s informal leadership influence in practice, social desirability bias and selection bias. We do not believe that our results, which demonstrated that radiology was more ready to implement lung cancer screening than primary care, were due to a response or selection bias. Rather we believe that it is more likely due to more organizational barriers perceived in primary care as detailed above. Similarly, we do not believe that our findings that leaders were more ready to implement lung cancer screening than non-leaders were due to response or selection bias; however, a response bias may exist.
Currently, there is a lack of information in the published literature on what represents an important and meaningful clinical difference in magnitude in ORIC and change valence. Our future work will focus on evaluating organizational readiness as a predictor of lung cancer screening practices.
Lung cancer screening implementation is a complex process requiring coordination of care between multiple specialties. Understanding readiness for change, value of change, and the other behavioral determinants of health professionals from different specialties and with different patient care roles can help to inform future lung cancer screening implementation strategies. The higher levels of readiness for change found amongst radiology health professionals and self-identified leaders can be leveraged to develop strategies to engage other specialties with lower levels of readiness and lower value of change. The findings of this study will directly inform our strategies and resource allocation to educate, inform, and engage health professionals outside of radiology in lung cancer screening.
Supplementary Material
Take Home Points:
Lung cancer screening implementation is a complex process requiring coordination of care between multiple specialties.
Findings of this pilot cross-sectional study suggest that health professionals working in radiology have higher levels of readiness for change than those in primary care for implementing lung cancer screening.
These findings are an important first step in the understanding of implementation of lung cancer screening throughout the VHA and beyond.
Understanding health professionals’ readiness for change, value of change, and other behavioral determinants can help to inform future lung cancer screening implementation strategies.
Future studies to evaluate organizational processes and design robust implementation strategies can help to achieve high-quality evidence-based lung cancer screening.
Acknowledgments:
This study was supported in part by the VA Office of Rural Health (LBS, JAL, CLR), Office of Academic Affiliations, Department of Veterans Affairs, VA National Quality Scholars Program (JAL, CLR,) with resources and use of facilities at VA Tennessee Valley Healthcare System, Nashville TN (LBS, JAL, VMY, CCL, CLR, PPM), the VA Boston Healthcare System (RSW), the VA Portland Health Care System (CGS), and the Phoenix Veterans Healthcare System (CIH). The study was also supported in part by the Vanderbilt-Ingram Cancer Center Support Grant CA68485 (LBS, JAL, PPM), Vanderbilt Scholars in T4 Translational Research (VSTTaR) K12 Program, funded by the National Heart, Lung, and Blood Institute K12HL137943 (JAL and CLR) and Agency for Healthcare Research and Quality K01 HS25486-01 (DS) and Agency for Healthcare Research and Quality/Patient Centered Outcomes Research Institute K12 HS026395 (TV and CLR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data access/integrity: The author(s) declare(s) that they had full access to all of the data in this study and the author(s) take(s) complete responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of Interest:
LBS, JAL, and CCL serve on the Steering Committee for this VAMC lung screening program. JAL and CCL are clinical co-directors of the clinical lung cancer screening program. Neither receive financial compensation for these roles.
CGS is the co-director of his facility’s lung cancer screening program and does not receive financial compensation for that role.
DFY is a named inventor on a number of patents and patent applications relating to the evaluation of diseases of the chest including measurement of nodules. Some of these, which are owned by Cornell Research Foundation (CRF), are non-exclusively licensed to General Electric. As an inventor of these patents, DFY is entitled to a share of any compensation which CRF may receive from its commercialization of these patents. He is also an equity owner in Accumetra, a privately held technology company committed to improving the science and practice of image-based decision making. DFY also serves on the advisory board of GRAIL.
CIH is the President and serves on the board of the Early Diagnosis and Treatment Research Foundation. She receives no compensation from the Foundation. The Foundation is established to provide grants for projects, conferences, and public databases for research on early diagnosis and treatment of diseases. CIH is also a named inventor on a number of patents and patent applications relating to the evaluation of pulmonary nodules on CT scans of the chest which are owned by Cornell Research Foundation (CRF). Since 2009, CIH does not accept any financial benefit from these patents including royalties and any other proceeds related to the patents or patent applications owned by CRF.
REFERENCES:
- [1].American Cancer Society. Cancer Facts & Figures 2019. Atlanta: American Cancer Society; 2019. [Google Scholar]
- [2].Moghanaki D, Williams CD. Lung Cancer in the VA at a National Level. Fed Pract. 2019;Cancer Data Trends:S26–28. [Google Scholar]
- [3].de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N Engl J Med. 2020;382:503–513. [DOI] [PubMed] [Google Scholar]
- [4].The National Lung Screening Trial Research Team. Reduced Lung-Cancer Mortality with Low-Dose Computed Tomographic Screening. N Engl J Med. 2011;365:395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].McKee BJ, McKee AB, Flacke S, et al. Initial Experience With a Free, High-Volume, Low-Dose CT Lung Cancer Screening Program. J Am Coll Radiol. 2013;10:586–592. [DOI] [PubMed] [Google Scholar]
- [6].Kinsinger LS, Anderson C, Kim J, et al. Implementation of Lung Cancer Screening in the Veterans Health Administration. JAMA Intern Med. 2017;177:399–406. [DOI] [PubMed] [Google Scholar]
- [7].US Preventive Services Taskforce. Lung Cancer Screening. Available from: https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/lung-cancer-screening. Accessed November 11, 2020.
- [8].Jemal A, Fedewa SA. Lung Cancer Screening With Low-Dose Computed Tomography in the United States-2010 to 2015. JAMA Oncol. 2017;3:1278–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Pham D, Bhandari S, Oechsli M, et al. Lung cancer screening rates: Data from the lung cancer screening registry. J Clin Oncol. 2018;36:6504–6504. [Google Scholar]
- [10].Lewis JA, Denton J, Matheny ME, et al. National lung cancer screening utilization trends in the Veterans Health Administration. J Clin Oncol. 2019;37:6547–6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Liu B, Dharmarajan K, Henschke CI, et al. State-Level Variations in the Utilization of Lung Cancer Screening Among Medicare Fee-for-Service Beneficiaries. Chest. 2020;157:1012–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zahnd WE, Eberth JM. Lung Cancer Screening Utilization: A Behavioral Risk Factor Surveillance System Analysis. Am J Prev Med. 2019;57:250–255. [DOI] [PubMed] [Google Scholar]
- [13].Yano EM. The role of organizational research in implementing evidence-based practice: QUERI Series. Implement Sci. 2008;3:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].McQueen L, Mittman BS, Demakis JG. Overview of the Veterans Health Administration (VHA) Quality Enhancement Research Initiative (QUERI): Table 1. J Am Med Inform Assoc. 2004;11:339–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Moore L, Lavoie A, Bourgeois G, et al. Donabedian’s structure-process-outcome quality of care model: Validation in an integrated trauma system. J Trauma Acute Care Surg. 2015;78:1168–1175. [DOI] [PubMed] [Google Scholar]
- [16].Damschroder LJ, Aron DC, Keith RE, et al. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci IS. 2009;4:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Weiner BJ. A theory of organizational readiness for change. Implement Sci. 2009;4:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Weiner BJ, Amick H, Lee S-YD. Conceptualization and measurement of organizational readiness for change: a review of the literature in health services research and other fields. Med Care Res Rev MCRR. 2008;65:379–436. [DOI] [PubMed] [Google Scholar]
- [19].Edmondson AC. Speaking Up in the Operating Room: How Team Leaders Promote Learning in Interdisciplinary Action Teams. J Manag Stud. 2003;40:1419–1452. [Google Scholar]
- [20].Nembhard IM, Edmondson AC. Making it safe: the effects of leader inclusiveness and professional status on psychological safety and improvement efforts in health care teams. J Organ Behav. 2006;27:941–966. [Google Scholar]
- [21].Rokstad AMM, Vatne S, Engedal K, et al. The role of leadership in the implementation of person-centred care using Dementia Care Mapping: a study in three nursing homes. J Nurs Manag. 2015;23:15–26. [DOI] [PubMed] [Google Scholar]
- [22].Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].U.S. Department of Veterans Affairs. VA Research Electronic Data Capture (REDCap). Available from: https://www.virec.research.va.gov/Resources/REDCap.asp. Accessed November 11, 2020.
- [24].Shea CM, Jacobs SR, Esserman DA, et al. Organizational readiness for implementing change: a psychometric assessment of a new measure. Implement Sci. 2014;9:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Cronbach LJ. Coefficient alpha and the internal structure of tests. Psychometrika. 1951;16:297–334. [Google Scholar]
- [26].Tavakol M, Dennick R. Making sense of Cronbach’s alpha. Int J Med Educ. 2011;2:53–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sanders KA, Wolcott MD, McLaughlin JE, et al. Organizational readiness for change: Preceptor perceptions regarding early immersion of student pharmacists in health-system practice. Res Soc Adm Pharm. 2017;13:1028–1035. [DOI] [PubMed] [Google Scholar]
- [28].Cunha-Cruz J, Milgrom P, Huebner CE, et al. Care delivery and compensation system changes: a case study of organizational readiness within a large dental care practice organization in the United States. BMC Oral Health. 2017;17:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Becker WC, Mattocks KM, Frank JW, et al. Mixed methods formative evaluation of a collaborative care program to decrease risky opioid prescribing and increase non-pharmacologic approaches to pain management. Addict Behav. 2018;86:138–145. [DOI] [PubMed] [Google Scholar]
- [30].Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci IS. 2011;6:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].McKee BJ, McKee AB, Kitts AB, et al. Low-dose Computed Tomography Screening for Lung Cancer in a Clinical Setting: Essential Elements of a Screening Program. J Thorac Imaging. 2015;30:115–129. [DOI] [PubMed] [Google Scholar]
- [32].Weiner BJ, Lewis MA, Linnan LA. Using organization theory to understand the determinants of effective implementation of worksite health promotion programs. Health Educ Res. 2009;24:292–305. [DOI] [PubMed] [Google Scholar]
- [33].Dym B, Hutson H. Leveraging Organizational Readiness for Change. Syst. Thinkier Available from: https://thesystemsthinker.com/leveraging-organizational-readiness-for-change/. Accessed November 12, 2020.
- [34].Tukey MH, Clark JA, Bolton R, et al. Readiness for Implementation of Lung Cancer Screening. A National Survey of Veterans Affairs Pulmonologists. Ann Am Thorac Soc. 2016;13:1794–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Braithwaite D, Emery J, De Lusignan S, et al. Using the Internet to conduct surveys of health professionals: a valid alternative? Fam Pract. 2003;20:545–551. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


