Abstract
When chill-susceptible insects are exposed to low temperatures they enter a temporary state of paralysis referred to as a chill coma. The most well-studied physiological mechanism of chill coma onset and recovery involves regulation of ion homeostasis. Previous studies show that changes in metabolism may also underlie the ability to recovery quickly, but the roles of genes that regulate metabolic homeostasis in chill coma recovery time (CCRT) are not well understood. Here, we investigate the roles of Sestrin and Spargel (Drosophila homolog of PGC-1α), which are involved in metabolic homeostasis and substrate oxidation, on CCRT in Drosophila melanogaster. We find that sestrin and spargel mutants have impaired CCRT. sestrin is required in the muscle and nervous system tissue for normal CCRT and spargel is required in muscle and adipose. On the basis that exercise induces sestrin and spargel, we also test the interaction of cold and exercise. We find that pre-treatment with one of these stressors does not consistently confer acute protection against the other. We conclude that Sestrin and Spargel are important in the chill coma response, independent of their role in exercise.
Keywords: Chill coma, Recovery, Metabolism, Exercise
1. Introduction
The ability to tolerate cold stress is a determining factor in survival and distribution in ectotherms (Andersen et al., 2015b; Bale, 1996). During acute, nonlethal cold exposure, chill-susceptible insects enter a temporary state of paralysis referred to as a chill coma (Mellanby and Gardiner, 1939). Many physiological changes contribute to the onset of a chill coma but the primary mechanism involves perturbation of water and ion homeostasis (Koštál et al., 2004; MacMillan and Sinclair, 2011). Acute cold stress increases water and sodium secretion into the gut lumen from the Malpighian tubules, which reduces haemolymph volume. As a result, the haemolymph becomes saturated with potassium, which depolarizes cell membranes such as muscle tissue (Andersen et al., 2015a; Koštál et al., 2004; MacMillan et al., 2015a). Consequently, when muscle resting membrane potential is depolarized to a certain threshold during cold exposure, (below −37 to −45 mV) action potential generation ceases, which prevents muscle contraction and marks the onset of muscle paralysis (Hosler et al., 2000).
Chill coma recovery time (CCRT) is a common method to study cold tolerance in chill-susceptible insects and is dependent on numerous factors (Andersen et al., 2015b). For instance, cold exposure in Drosophila has been shown to increase mRNA of neuropeptides, such as CAPA, which influence recovery time by restoring fluid and ion homeostasis at the Malpighian tubules (Lubawy et al., 2020). Additionally, the activity of active transporters such as Na/K-ATPases and Ca-ATPases have been implicated in chill coma onset as their activity decreases with cold exposure, thus altering membrane potential (MacMillan et al., 2015a, 2015b; MacMillan and Sinclair, 2011). Recovery from a chill coma involves restoration of ion homeostasis and is a metabolically expensive process (MacMillan et al., 2012). The speed by which an individual fly resumes active transport and other energetically demanding processes during chill coma recovery may depend on ATP availability/production. However, recent work has shown no difference in ATP availability during acute cold exposure between cold-resistant and cold-susceptible flies, suggesting that ATP reduction may not be a determining factor in recovery (MacMillan and Sinclair, 2011; Williams et al., 2018).
Despite similar ATP availability, cold-resistant flies have greater metabolic turnover than cold-susceptible flies during chill coma recovery, as evidenced by faster substrate oxidation before cold exposure and during recovery (Williams et al., 2016). It has been proposed that higher tricarboxylic acid (TCA) cycle flux and substrate oxidation may be important in the synthesis of metabolic intermediates that have cryoprotective functions or are involved in cellular repair of damage incurred from cold stress (e.g. membrane repair) (Williams et al., 2016, 2018). Several metabolomics studies have found higher concentrations of metabolic intermediates in cold-resistant flies after acute cold exposure, indicating altered metabolism (Enriquez et al., 2018; MacMillan et al., 2016; Williams et al., 2014). Therefore, proteins that are involved in metabolism and nutrient flux, such as Sestrin or Spargel, might also be predicted to affect CCRT.
Sestrin is a small, conserved protein that is induced by stressors such as DNA damage, oxidative stress, and metabolic stress (Budanov et al., 2004; Lee et al., 2012; Velasco-Miguel et al., 1999). When induced, Sestrin can promote autophagy through activation of AMPK and reduce reactive oxygen species (ROS) accumulation (Budanov et al., 2010; Ho et al., 2016). Sestrin is also implicated in metabolic homeostasis which is regulated through its downstream target AMPK (Ho et al., 2016). sestrin mutant mice are insulin resistant and sestrin-null flies have elevated levels of trehalose in haemolymph (Lee et al., 2012). Additionally, in the context of endurance exercise, a potent metabolic stressor, sestrin is required for acute exercise performance and adaptation to chronic exercise in both mice and flies (Kim et al., 2020). These effects are dependent on spargel (Drosophila homolog of peroxisome proliferator-activated receptor-gamma co-activator 1a; PGC-1α), a downstream target of AMPK that regulates fatty acid oxidation and mitochondrial biogenesis (Scarpulla, 2011). Given that cold stress affects metabolism and increases ROS production, it is reasonable to hypothesize that Sestrin may be an important protector against cold stress. In fact, a recent study showed sestrin was upregulated 2.5 fold in wild type flies after six days of 6 °C cold exposure (MacMillan et al., 2016), consistent with a role in cold stress. However, the role of Sestrin in acute chill coma recovery has not been directly tested.
In the current study, we assessed the role of Sestrin and its downstream target Spargel in CCRT in Drosophila melanogaster. We hypothesized that knockdown of sestrin and spargel in muscle, adipose, and nervous tissue would increase CCRT whereas overexpression would decrease recovery time. Because Sestrin is induced by both exercise and cold, we also assessed the interaction between these two treatments. To test this we acutely exercised flies and assessed cold tolerance immediately after. We also tested the converse interaction by subjecting flies to 48 h of cold exposure and assessing the effects of cold on acute exercise performance. We hypothesized that acute exercise would enhance CCRT and 48 h of cold exposure could enhance acute exercise performance, and that this interaction would be dependent on Sestrin. We further asked if three weeks of chronic exercise would confer additional protection against cold stress.
2. Methods
2.1. Fly Stocks and maintenance
All flies were maintained at 25 °C and 50% humidity on a 12-h light/dark cycle and fed a standard 10% sugar 10% yeast diet unless otherwise stated. All experiments were conducted on male flies because female D. melanogaster have a blunted response to exercise, compared to males (Sujkowski et al., 2017). All UAS lines and srl1 were obtained from Drosophila Stock Center (Bloomington, IN), with the following exceptions. sestrin transgenic stocks were gifted from Jun Hee Lee. GS-Mhc-Gal4 were gifted from Rolf Bodmer. GS-S106-Gal4 were gifted from Marc Tatar. GS-Elav-Gal4 were gifted from Scott Pletcher. All driver constructs have been previously described (Giannakou et al., 2004; Osterwalder et al., 2001). Sestrin8A11 and srl1 mutants have been previously characterized (Lee et al., 2010; Tiefenböck et al., 2010) as a null mutant and strong hypomorph, respectively.
2.2. Drug treatment
Tissue-specific experiments were performed using mifepristone (RU486)-inducible Gal4 drivers. Larvae were reared to adulthood on standard food. After eclosion, adult male flies were collected into food vials containing either 100 μM mifepristone (RU+) or 70% ethanol vehicle (RU−). Flies were fed RU486 continuously for at least 3 days to allow accumulation of induced expression prior to experimentation.
2.3. Chill coma
Five vials of flies were transferred from their food vials to empty vials 10 min prior to cold exposure. Chill coma experiments used flies that were 5 days old, except in chronic exercise pretreatment experiments, when they were 28 days old. Each vial contained 7–10 flies for optimal visual scoring. Vials were then submerged in a cooler full of ice at 0 °C for 2 h. After 2 h, vials were removed and immediately placed on a countertop at 23–25 °C on a white sheet of paper. Time to recovery was visually scored using a timer, which was started when all vials were placed on the white sheet of paper. Each vial was scored in the same order and recovery for each individual fly was determined when it was standing upright on all six legs. The time for each fly to recover was recorded and data was analyzed using a log-rank test for significance and graphed in GraphPad Prism. Significance level was set at p < 0.05.
2.4. Exercise
Flies were exercised as previously described (Damschroder et al., 2018). Exercise was induced by repeatedly stimulating negative geotaxis using a motorized machine to repeatedly lift and drop vials of flies. For acute exercise experiments, flies were placed on the machine and subjected to exercise for 2 h prior to cold assessment. For chronic exercise, flies were exercised daily for five consecutive days per week followed by two days rest for three weeks. Exercise was 120 min daily for the first week, 150 min daily for the second week, and 180 min daily for the third week. In all exercise experiments, unexercised flies were also placed on the machine but the plugs in the vials were pushed down into the vial to restrict movement.
2.5. Assessment of endurance
A minimum of six vials containing 10–20 flies each per group were placed on the exercise machine and subjected to run until fatigue. Fatigue was visually scored by the experimenter and was determined when 80% of the flies in the vial could no longer climb 1 cm off the food for three consecutive drops. A vial was removed when it was determined fatigued and the time was recorded. Each vial of 10–20 flies was scored as a single unit. Data were analyzed using a log-rank test for significance and the significance level was set at p < 0.05.
2.6. RT-qPCR
Total RNA was extracted from whole flies using Trizol (Invitrogen). One-step RT-qPCR was done using Power SYBR Green PCR master mix (Applied Biosystems) and performed using an ABI 7300 Real Time PCR System (Applied Biosystems). Three independent biological replicates with three technical repetitions were performed for each experimental group. Each 20 μl reaction contained 4 μl of RNA (25 ng/μl), 1 μl each of forward and reverse primers (1 μM), 10 μl of Power SYBR Green PCR master mix, 0.1 μl of reverse transcriptase, .025 μl of inhibitor, and 3.875 μl of dH2O.The qPCR program ran for 40 cycles of 95 °C for 15 s followed by 60 °C for 1 min mRNA data were normalized to Act5C. Spargel primers have been previously published (Kim et al., 2020). Primer sequences:
Sestrin F: AGAGCATCAACACGTTTCGC
Sestrin R: GACGTGTCCACATTCTTGATGC
Spargel F: GGATTCACGAATGCTAAATGTGTTCC
Spargel R: GATGGGTAGGATGCCGCTCAG
Act5C F: CGCAGAGCAAGCGTGGTA
Act5C R: GTGCCACACGCAGCTCAT
3. Results
3.1. Sestrin and spargel are required for normal chill coma recovery response
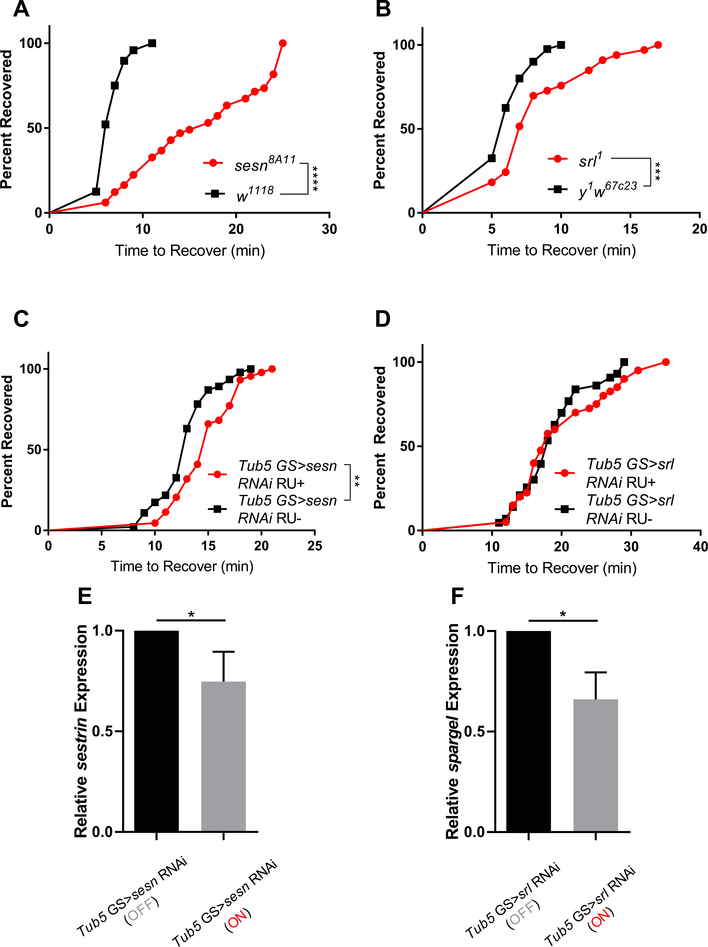
To assess the role of Sestrin on CCRT, sestrin mutants (sestrin8A11) and background controls (w1118) were subjected to 2 h of cold exposure at 0 °C then individually monitored for time to recovery. sestrin mutant recovery time was significantly longer than background controls (Fig. 1A), indicating that sestrin is required for normal recovery from acute cold exposure. To verify these effects were due to lack of sestrin, we performed the same experiment in adult flies expressing sestrin RNAi ubiquitously under control of a GS-Tub5-Gal4 driver (Fig. 1C), and again found significant extension of CCRT, in the presence of a modest knockdown of sestrin transcript (Fig. 1E).
Fig. 1.
sestrin and spargel are required for normal chill coma recovery. sestrin (sesn8A11) and spargel (srl1) mutants were subjected to 2 h of cold exposure at 0 °C and monitored for recovery. (A) sestrin mutants took significantly longer to recover from chill coma (n ≥ 47 each group; p < 0.0001; log-rank test). (B) spargel mutants took significantly longer to recover from chill coma (n ≥ 33 each group; p < 0.0003; log-rank test). (C) Ubiquitous knockdown of sestrin under control of GS-Tub5-Gal4 driver significantly increased chill coma recovery time (n ≥ 44 each group; p = 0.001; log-rank test) (D) Ubiquitous knockdown of spargel did not significantly extend recovery time. (E,F) Relative sestrin and spargel transcript normalized to vehicle treated controls. Flies were 5–7 days old (n = 3 biological replicates each group; p = 0.04 and p = 0.01 for Tub5 GS > sesn RNAi groups and Tub5 GS > srl RNAi groups, respectively; unpaired t-test). ‘On’ indicates RU486 treated groups and ‘Off’ indicates vehicle treated groups.
Spargel (Drosophila homolog of PGC-1α) is a conserved downstream target of Sestrin that promotes fatty acid oxidation and mitochondrial respiration (Cheng et al., 2018; Rera et al., 2011). Since Sestrin acts through Spargel in the context of exercise (Kim et al., 2020), and spargel overexpression can mimic the effects of Sestrin, we next asked if spargel was required for chill coma recovery. We subjected spargel mutants (srl1) to 2 h of cold exposure and measured the time for individual flies to recover. We found that srl1 mutants had reduced cold tolerance compared to background control flies (Fig. 1B). We expressed spargel RNAi under control of GS-Tub5-Gal4 driver and found that CCRT was not affected (Fig. 1D) by a partial reduction of spargel mRNA (Fig. 1F).
3.2. Tissue-specific effects of sestrin in chill coma recovery
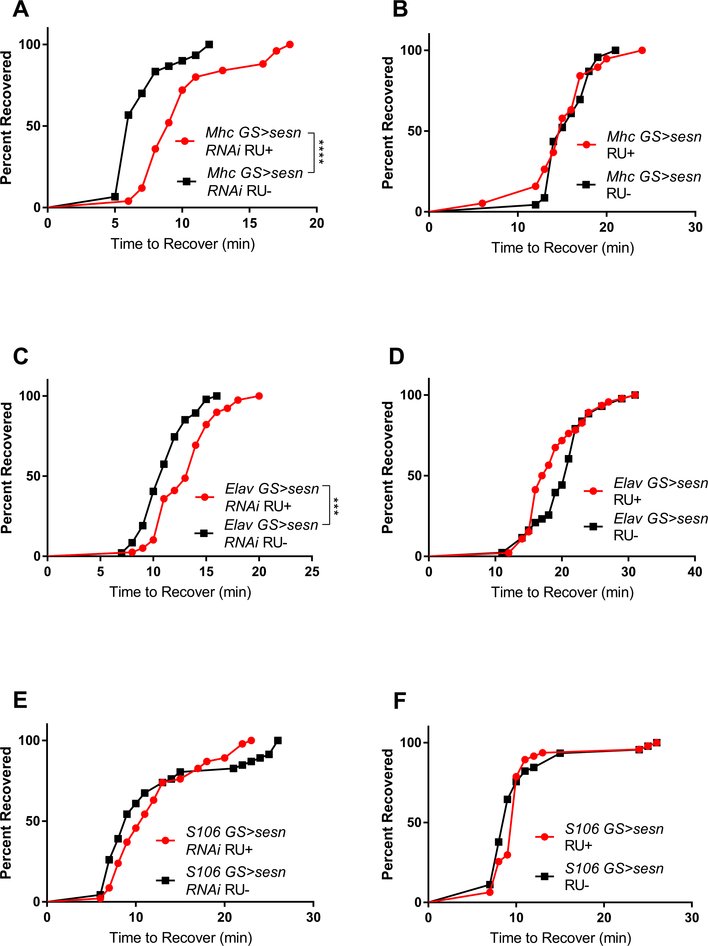
Since sestrin mutants had impaired recovery from chill coma, we next sought to determine which tissues required sestrin expression in this context. We first looked at the effects of sestrin knockdown in muscle because depolarization of muscle resting membrane potential is a primary cause of muscle paralysis in chill coma. We expressed sestrin RNAi under control of a muscle-specific, inducible GS-Mhc-Gal4 driver in adult flies and assessed chill coma recovery after 2 h of cold exposure at 0 °C. Muscle-specific knockdown of sestrin impaired chill coma recovery (Fig. 2A) but overexpression of sestrin in muscle did not confer any benefits (Fig. 2B), suggesting that a threshold level of Sestrin is required in muscle for efficient chill coma recovery.
Fig. 2.
Tissue-specific effects of sestrin on chill coma recovery. (A) Muscle-specific knockdown of sestrin significantly increased time to recovery (n ≥ 25 each group; p < 0.0001; log-rank test). (B) Overexpression of sestrin in muscle did not affect recovery time. (C) sestrin knockdown in nervous system significantly increased time to recovery (n ≥ 39 each group; p = 0.0002; log-rank test). (D) Overexpression of sestrin in the nervous system did not affect time to recovery. (E–F) Manipulating sestrin expression in fat body tissue had no effect on time to recovery.
Recovery of membrane potential in the nervous system is also important in recovery from a cold shock (Andersen and Overgaard, 2019). Therefore, we next looked at the effects of nervous tissue-specific knockdown and overexpression of sestrin using a pan-neuronal GS-Elav-Gal4 driver in adult flies. Knockdown of sestrin throughout the nervous system impaired CCRT while overexpression of sestrin had no benefit (Fig. 2C–D). These findings show that Sestrin is also required in the nervous system for proper chill coma recovery.
Lastly, we examined the effects of Sestrin in the adipose tissue on chill coma recovery. sestrin mutant Drosophila larvae have elevated triglyceride accumulation in fat bodies (Lee et al., 2010). Additionally, knockdown of genes that affect whole body triglyceride levels (Heinrichsen et al., 2014) or fat accumulation in the adipose tissue (Moraru et al., 2017) have been shown to affect cold tolerance. Therefore, we hypothesized that knockdown of sestrin in adipose tissue would increase CCRT while overexpression would reduce recovery time. We knocked down or overexpressed sestrin using a GS-S106-Gal4 driver in adult flies and, contrary to our hypothesis, we found that neither knockdown nor overexpression of sestrin in the adipose significantly affected chill coma recovery (Fig. 2E–F).
3.3. Tissue-specific effects of spargel in chill coma recovery
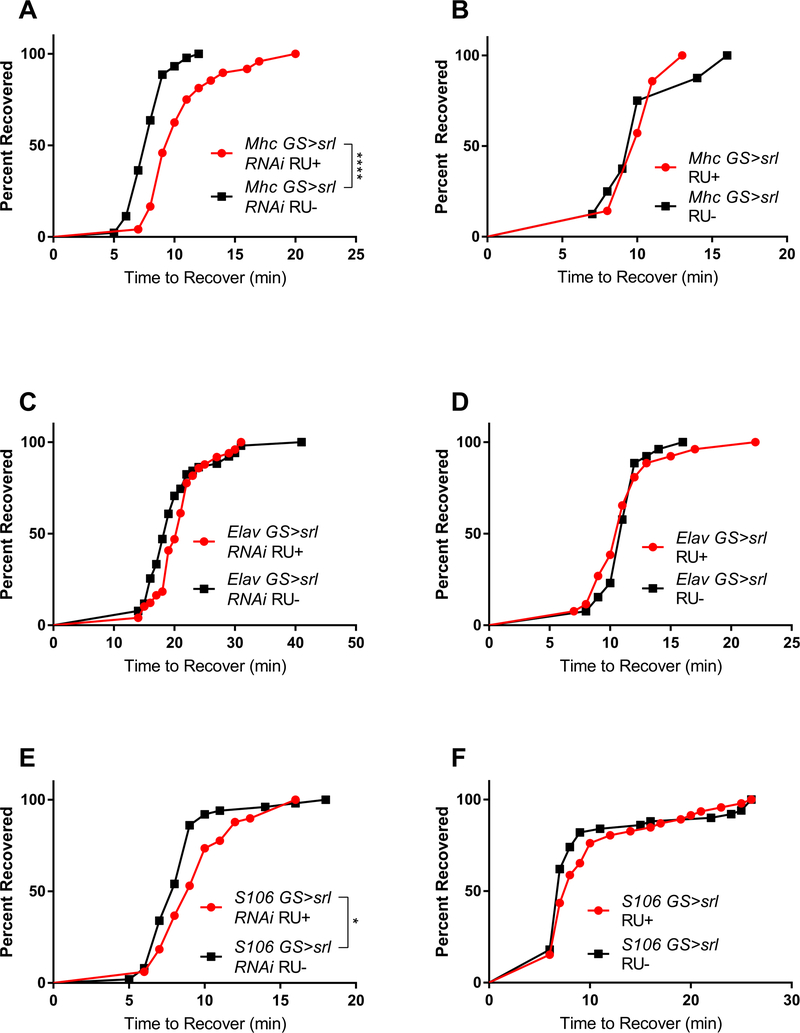
Because we have observed muscle-specific effects of spargel RNAi in different contexts (Kim et al., 2020) and srl1 mutants had impaired recovery from chill coma, we tested the tissue-specific requirements of spargel, despite no effect of ubiquitous knockdown of spargel on CCRT (Fig. 1D). Knockdown of spargel in muscle significantly increased CCRT (Fig. 3A). Overexpression in muscle did not affect CCRT (Fig. 3B). Neither knockdown nor overexpression of spargel in the nervous system affect CCRT (Fig. 3C–D). Adipose-specific knockdown of spargel significantly increased CCRT, but overexpression had no effect (Fig. 3E–F).
Fig. 3.
Tissue-specific effects of spargel on chill coma recovery. (A) Muscle-specific knockdown of spargel significantly increased chill coma recovery time (n ≥ 44 each group; p < 0.0001; log-rank test). (B) Overexpression of spargel in muscle did not affect recovery time. Neither (C) knockdown nor (D) overexpression of spargel in nervous system affected chill coma recovery. (E) Knockdown of spargel in the fat body significantly increased time to recovery (n ≥ 49 each group; p = 0.01; log-rank test). (F) Overexpression of spargel in the fat body had no effect on chill coma recovery.
3.4. The interaction of cold and exercise treatment
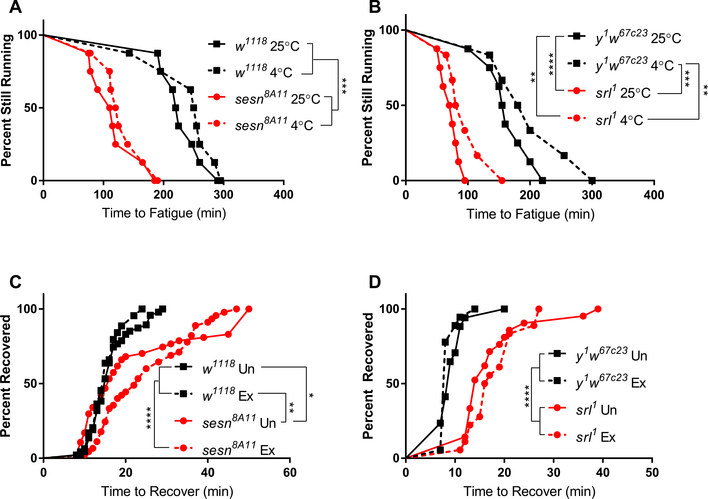
Both cold and exercise have been shown to induce sestrin in Drosophila (Kim et al., 2020; MacMillan et al., 2016). Given the importance of Sestrin and Spargel in both chill coma recovery and the exercise response, we asked whether cold treatment could enhance acute exercise performance and if acute exercise could enhance CCRT. To test the former, sestrin and spargel mutants and their respective background controls were reared at 25 °C for three days after eclosion and then equally divided to be housed at either 4 °C or 25 °C for 48 h. All flies at 4 °C were returned to 25 °C after 48 h and were allowed to recover for 14 h. The recovery period was employed to reduce immediate negative effects of cold on mobility (Garcia and Teets, 2019). After 14 h, endurance was assessed between groups to test the effects of cold pre-treatment on exercise performance. We found that 48 h of pre-treatment at 4 °C did not affect endurance in sestrin or spargel mutants, or in their background controls (Fig. 4A–B). Endurance was reduced in both mutants regardless of temperature, consistent with previous findings (Kim et al., 2020; Tinkerhess et al., 2012).
Fig. 4.
The interaction of cold and exercise treatments. sestrin and spargel mutants and background controls were housed at 4 °C for 48 h and exercised until fatigued following 14 h of recovery at 25 °C. (A) Cold treatment did not affect endurance in either sestrin mutants or controls. Mutants had significantly lower endurance than controls whether pre-treated or not (n = 8 vials of 10–20 flies each group; p = 0.0002; log-rank test). (B) Cold treatment did not significantly affect endurance in spargel mutants, but mutants had significantly lower endurance whether pre-treated or not (n ≥ 6 vials of 20 flies each group; p = 0.006; log-rank test). sestrin and spargel mutants were exercised for 2 h and immediately transferred to an ice bath for 2 h and CCRT was assessed. Acute exercise did not affect CCRT in either (C) sestrin or (D) spargel mutants. In both cases, mutants had longer CCRT than controls, whether pre-treated or not.
To test the role of acute exercise on CCRT, sestrin and spargel mutants and their background controls were exercised for 2 h and immediately transferred to empty vials and submerged into ice for 2 h. Time to recovery between exercised and unexercised flies was measured after 2 h. Acute exercise did not affect CCRT in sestrin or spargel mutants, nor did it affect recovery time in control lines (Fig. 4C–D), indicating that pre-treatment with acute exercise does not significantly affect CCRT. Taken together, these results fail to detect a significant interaction between cold and exercise treatments.
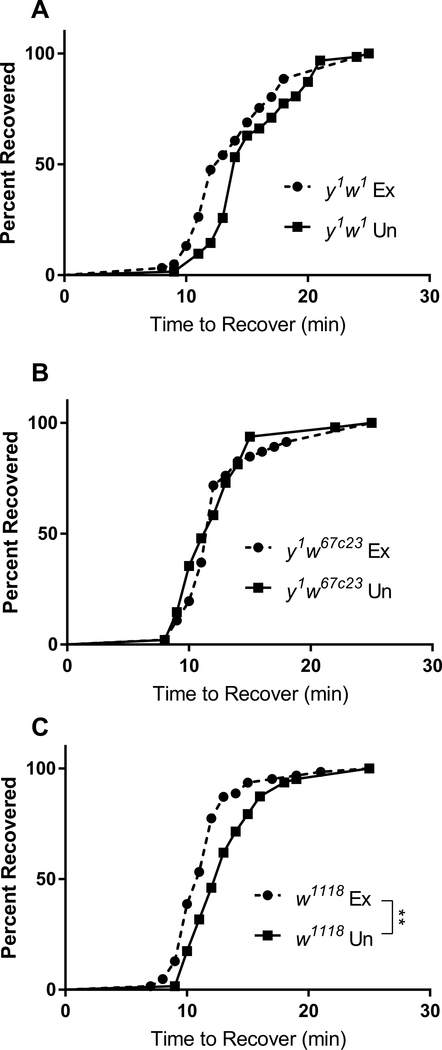
Although acute exercise did not affect CCRT, it is possible that long-term, chronic exercise training may affect CCRT. To test this, we exercised flies from three different genetic backgrounds (y1w1, y1w67c23, and w1118) for three weeks following a ramped protocol. CCRT was measured three days after the final exercise session. Chronic exercise training did not affect CCRT in y1w1 or y1w67c23 genotypes (Fig. 5A–B), but did enhance time to recovery in the w1118 line (Fig. 5C). Based on these findings, we conclude that chronic exercise pre-treatment has a small, genotype-dependent effect on CCRT.
Fig. 5.
Effects of chronic exercise training on CCRT. CCRT was measured after three weeks of exercise training in three lines of control flies. (A,B) Chronic exercise training did not affect CCRT in y1w1 or y1w67c23 flies. (C) Chronic exercise significantly improved CCRT in w1118 flies (n = 62 each group; p = 0.0003; log-rank test).
4. Discussion
In the current study, we find that both Sestrin and Spargel are required for normal chill coma recovery after acute exposure to cold. Tissue-specific requirements varied between the two genes, with sestrin required in muscle and nervous system, and spargel required in muscle and adipose tissue. Although both of these genes are also required for adaptation to chronic exercise, we found no consistent interaction between exercise and cold tolerance across genotypes, although pre-treatment with chronic exercise did improve CCRT in one genotype.
Sestrin is a stress-inducible protein that is upregulated in response to genotoxic, oxidative, and metabolic stress (Budanov et al., 2004; Lee et al., 2012; Velasco-Miguel et al., 1999). Here, we found that Sestrin is also required in the chill coma recovery response. Although more work will be required to identify the specific mechanisms of Sestrin’s effects in this context, the pivotal role of Sestrin in coordinating a variety of metabolic responses to stress is likely mediated by its interactions with other key regulatory molecules, including TORC1 and FoxO.
Both acute and chronic exposure to low temperatures can influence energy metabolism (Williams et al., 2014). Cold-resistant flies have greater metabolic plasticity and have elevated levels of metabolic intermediates during acute cold stress despite no changes in ATP concentration (MacMillan et al., 2016; Williams et al., 2016, 2018). It has been proposed that this is due to increased metabolic turnover to support the energetic demands of cryoprotectant biosynthesis or cellular repair (Williams et al., 2018). If this is the case, Sestrin may be an important regulator of energy metabolism by signaling through AMPK and Spargel to stimulate substrate uptake, TCA flux, and substrate oxidation during cold stress (Supruniuk et al., 2017).
Cold exposure has also been associated with changes in membrane fluidity, which can cause proton leak leading to increased ROS and oxidative stress (MacMillan and Sinclair, 2011; Williams et al., 2014). Oxidative stress can damage and impair protein function, enzyme activity, and lead to apoptosis (Celi and Gabai, 2015; Chainy et al., 2016; Maiuri et al., 2007). Sestrin has intrinsic peroxidase activity (Ho et al., 2016) which may act to reduce ROS accumulation during cold stress. Additionally, Sestrin can indirectly reduce ROS by promoting autophagy through AMPK activation and upregulation of other antioxidants through regulation of Nrf2, a transcription factor that promotes transcription of antioxidant genes (Maiuri et al., 2009; Rhee and Bae, 2015). This may help reduce protein or lipid damage that occurs during cold stress.
Clearing of damaged materials in the cell via autophagy has also been proposed as an important response during cold stress (Gerken et al., 2015). In support of this, mutations in genes that regulate autophagy have been shown to adversely affect short-term cold adaptation (Gerken et al., 2015). Given Sestrin’s ability to promote autophagy through inhibition of TORC1 activity, it may help coordinate the removal of damaged material in the cell through autophagy to prevent apoptosis during cold stress. This is supported by findings from desiccation stress studies that found sestrin transcript to be increased 11-fold during dehydration (Teets et al., 2012). This response was hypothesized to be a mechanism to prevent apoptosis due to ROS accumulation during dehydration (Teets and Denlinger, 2013a). Given that desiccation and cold stress share similar physiological responses and have cross-protective effects (Sinclair et al., 2013; Yi et al., 2017), Sestrin may act as a key protein that regulates environmental stress responses through the induction of autophagy to prevent apoptosis and promote cell survival.
Acute cold exposure is also associated with disrupted ion homeostasis in the brain in Drosophila and Locusta migratoria (Armstrong et al., 2012; Rogers et al., 2004). The onset of chill coma has been shown to be initiated first in the central nervous system and then affect the muscular system (Andersen and Overgaard, 2019). We initially hypothesized that both sestrin and spargel would be required in the nervous system for normal chill coma recovery. However, we found that nervous system-specific knockdown of sestrin, but not spargel, increased time to recovery from a chill coma. This is in contrast to what we found in muscle where both genes are required. Recovery of resting membrane potentials in the central nervous system precedes recovery of the musculature during a chill coma, suggesting different requirements for recovery (Andersen and Overgaard, 2019). Our findings support this, as Spargel had no effect in neurons but was required in muscle and adipose tissue. One potential explanation for these findings is that the muscle may rely more on changes to mitochondrial content and/or fatty acid metabolism that are regulated by the Sestrin-Spargel axis. In neurons, Sestrin might instead exert its effects in through alternative mechanisms such as ROS detoxification, autophagy, alternative metabolic targets, or other unknown mechanisms. Follow-up studies will be needed to distinguish which of the many functions and genetic interactions of Sestrin are key in which tissues during CCRT.
Many metabolic benefits of chronic exercise are highly conserved. For instance, sestrin is upregulated in muscle upon exercise training and both sestrin and spargel are required for exercise adaptations in vertebrates and invertebrates (Baar, 2007; Kim et al., 2020; Little et al., 2010; Tinkerhess et al., 2012). Similarly, longer-term cold exposure has been shown to upregulate sestrin, but not spargel, in Drosophila (MacMillan et al., 2016). Since both stressors upregulate Sestrin, we initially hypothesized that acute exposure to one stressor may have a protective effect on the other. Contrary to our hypothesis, we found that acute exercise did not affect CCRT and cold exposure did not affect endurance. This is in line with the tissue-specific overexpression experiments in this study. Overexpression of sestrin or spargel did not affect CCRT, consistent with a threshold effect where higher levels of these genes will not provide any additional benefit.
The basis of long-term adaptation to endurance exercise training is to protect the animal from future bouts of metabolic perturbations during exercise. Similarly, brief cold exposure enhances CCRT of future cold shocks in invertebrates (Lee et al., 1987; Teets and Denlinger, 2013b). Previous studies have investigated the interactions between seemingly different stimuli and have found cross-protective effects in the long-term. For instance, long-term hypergravity and cold stimuli provide resistance to heat stress in Drosophila (Le Bourg and Polesello, 2019) indicating possible overlap in the stress response of these stimuli. We tested the interaction of long-term exercise training on CCRT and, similar to our results from the acute studies, found no significant effect on CCRT in two of three genotypes tested.
In summary, we show that Sestrin and Spargel are required for chill coma recovery in Drosophila. Sestrin appears to be required in muscle and nervous system tissue while Spargel is required in muscle and adipose for normal chill coma recovery. Future research should focus on elucidating the specific mechanisms of these proteins in various tissues during the chill coma response.
Acknowledgments
Funding
This work was supported by an NIH/NIA award to RW (1RO1AG059683-02) and an AHA award to DD (19PRE34380493).
Footnotes
Declaration of competing interest
None to declare.
References
- Andersen JL, MacMillan HA, Overgaard J, 2015a. Muscle membrane potential and insect chill coma. J. Exp. Biol 218, 2492–2495. [DOI] [PubMed] [Google Scholar]
- Andersen JL, Manenti T, Sørensen JG, MacMillan HA, Loeschcke V, Overgaard J, 2015b. How to assess Drosophila cold tolerance: chill coma temperature and lower lethal temperature are the best predictors of cold distribution limits. Funct. Ecol 29, 55–65. [Google Scholar]
- Andersen MK, Overgaard J, 2019. The central nervous system and muscular system play different roles for chill coma onset and recovery in insects. Comp. Biochem. Physiol. Mol. Integr. Physiol 233, 10–16. [DOI] [PubMed] [Google Scholar]
- Armstrong GAB, Rodríguez EC, Meldrum Robertson R, 2012. Cold hardening modulates K+ homeostasis in the brain of Drosophila melanogaster during chill coma. J. Insect Physiol. 58, 1511–1516. [DOI] [PubMed] [Google Scholar]
- Baar K, 2007. Involvement of PPARγ co-activator-1, nuclear respiratory factors 1 and 2, and PPARα in the adaptive response to endurance exercise. Proc. Nutr. Soc 63, 269–273. [DOI] [PubMed] [Google Scholar]
- Bale JS, 1996. Insect cold hardiness: a matter of life and death. EJE (Eur. J. Epidemiol.) 93, 369–382. [Google Scholar]
- Budanov AV, Lee JH, Karin M, 2010. Stressin’ Sestrins take an aging fight. EMBO Mol. Med. 2, 388–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budanov AV, Sablina AA, Feinstein E, Koonin EV, Chumakov PM, 2004. Regeneration of peroxiredoxins by p53-regulated sestrins, homologs of bacterial AhpD. Science 304, 596–600. [DOI] [PubMed] [Google Scholar]
- Celi P, Gabai G, 2015. Oxidant/antioxidant balance in animal nutrition and health: the role of protein oxidation. Frontiers in Veterinary Science 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chainy GBN, Paital B, Dandapat J, 2016. An overview of seasonal changes in oxidative stress and antioxidant defence parameters in some invertebrate and vertebrate species. Sci. Tech. Rep 2016, 6126570–6126570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C-F, Ku H-C, Lin H, 2018. PGC-1α as a pivotal factor in lipid and metabolic regulation. Int. J. Mol. Sci 19, 3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damschroder D, Cobb T, Sujkowski A, Wessells R, 2018. Drosophila endurance training and assessment of its effects on systemic adaptations. Bio Protoc 8, e3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enriquez T, Renault D, Charrier M, Colinet H, 2018. Cold acclimation favors metabolic stability in Drosophila suzukii. Front. Physiol 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia MJ, Teets NM, 2019. Cold stress results in sustained locomotor and behavioral deficits in Drosophila melanogaster. J. Exp. Zool. Part A, Ecological and integrative physiology 331, 192–200. [DOI] [PubMed] [Google Scholar]
- Gerken AR, Eller OC, Hahn DA, Morgan TJ, 2015. Constraints, independence, and evolution of thermal plasticity: probing genetic architecture of long- and short-term thermal acclimation. Proc. Natl. Acad. Sci. U. S. A 112, 4399–4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannakou ME, Goss M, Jünger MA, Hafen E, Leevers SJ, Partridge L, 2004. Long-lived Drosophila with overexpressed dFOXO in adult fat body. Science 305, 361–361. [DOI] [PubMed] [Google Scholar]
- Heinrichsen ET, Zhang H, Robinson JE, Ngo J, Diop S, Bodmer R, Joiner WJ, Metallo CM, Haddad GG, 2014. Metabolic and transcriptional response to a high-fat diet in Drosophila melanogaster. Molecular Metabolism 3, 42–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho A, Cho C-S, Namkoong S, Cho U-S, Lee JH, 2016. Biochemical basis of sestrin physiological activities. Trends Biochem. Sci 41, 621–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosler JS, Burns JE, Esch HE, 2000. Flight muscle resting potential and species-specific differences in chill-coma. J. Insect Physiol 46, 621–627. [DOI] [PubMed] [Google Scholar]
- Kim M, Sujkowski A, Namkoong S, Gu B, Cobb T, Kim B, Kowalsky AH, Cho C-S, Semple I, Ro S-H, Davis C, Brooks SV, Karin M, Wessells RJ, Lee JH, 2020. Sestrins are evolutionarily conserved mediators of exercise benefits. Nat. Commun 11, 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koštál V, Vambera J, Bastl J, 2004. On the nature of pre-freeze mortality in insects: water balance, ion homeostasis and energy charge in the adults of Pyrrhocoris apterus. J. Exp. Biol 207, 1509. [DOI] [PubMed] [Google Scholar]
- Le Bourg E, Polesello C, 2019. Hypergravity increases resistance to heat in dFOXO Drosophila melanogaster mutants and can lower FOXO translocation in wild-type males. Biogerontology 20, 883–891. [DOI] [PubMed] [Google Scholar]
- Lee JH, Budanov AV, Park EJ, Birse R, Kim TE, Perkins GA, Ocorr K, Ellisman MH, Bodmer R, Bier E, Karin M, 2010. Sestrin as a feedback inhibitor of TOR that prevents age-related pathologies. Science 327, 1223–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Budanov AV, Talukdar S, Park EJ, Park HL, Park H-W, Bandyopadhyay G, Li N, Aghajan M, Jang I, Wolfe AM, Perkins GA, Ellisman MH, Bier E, Scadeng M, Foretz M, Viollet B, Olefsky J, Karin M, 2012. Maintenance of metabolic homeostasis by Sestrin2 and Sestrin3. Cell Metabol. 16, 311–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RE Jr., Chen CP, Denlinger DL, 1987. A rapid cold-hardening process in insects. Science 238, 1415–1417. [DOI] [PubMed] [Google Scholar]
- Little JP, Safdar A, Cermak N, Tarnopolsky MA, Gibala MJ, 2010. Acute endurance exercise increases the nuclear abundance of PGC-1α in trained human skeletal muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol 298, R912–R917. [DOI] [PubMed] [Google Scholar]
- Lubawy J, Urbański A, Colinet H, Pflüger H-J, Marciniak P, 2020. Role of the insect neuroendocrine system in the response to cold stress. Front. Physiol 11, 376–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMillan HA, Andersen JL, Davies SA, Overgaard J, 2015a. The capacity to maintain ion and water homeostasis underlies interspecific variation in Drosophila cold tolerance. Sci. Rep 5, 18607–18607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMillan HA, Ferguson LV, Nicolai A, Donini A, Staples JF, Sinclair BJ, 2015b. Parallel ionoregulatory adjustments underlie phenotypic plasticity and evolution of Drosophila cold tolerance. J. Exp. Biol. 218, 423–432. [DOI] [PubMed] [Google Scholar]
- MacMillan HA, Knee JM, Dennis AB, Udaka H, Marshall KE, Merritt TJS, Sinclair BJ, 2016. Cold acclimation wholly reorganizes the Drosophila melanogaster transcriptome and metabolome. Sci. Rep 6, 28999–28999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMillan HA, Sinclair BJ, 2011. Mechanisms underlying insect chill-coma. J. Insect Physiol. 57, 12–20. [DOI] [PubMed] [Google Scholar]
- MacMillan HA, Williams CM, Staples JF, Sinclair BJ, 2012. Reestablishment of ion homeostasis during chill-coma recovery in the cricket Gryllus pennsylvanicus. Proc. Natl. Acad. Sci. U. S. A 109, 20750–20755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiuri MC, Malik SA, Morselli E, Kepp O, Criollo A, Mouchel PL, Carnuccio R, Kroemer G, 2009. Stimulation of autophagy by the p53 target gene Sestrin2. Cell Cycle 8, 1571–1576. [DOI] [PubMed] [Google Scholar]
- Maiuri MC, Zalckvar E, Kimchi A, Kroemer G, 2007. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 8, 741–752. [DOI] [PubMed] [Google Scholar]
- Mellanby K, Gardiner JS, 1939. Low temperature and insect activity. Proc. Roy. Soc. Lond. B Biol. Sci 127, 473–487. [Google Scholar]
- Moraru A, Cakan-Akdogan G, Strassburger K, Males M, Mueller S, Jabs M, Muelleder M, Frejno M, Braeckman BP, Ralser M, Teleman AA, 2017. THADA regulates the organismal balance between energy storage and heat production. Dev. Cell 41, 72–81 e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterwalder T, Yoon KS, White BH, Keshishian H, 2001. A conditional tissue-specific transgene expression system using inducible GAL4. Proc. Natl. Acad. Sci. U. S. A 98, 12596–12601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rera M, Bahadorani S, Cho J, Koehler CL, Ulgherait M, Hur JH, Ansari WS, Lo T Jr., Jones DL, Walker DW, 2011. Modulation of longevity and tissue homeostasis by the Drosophila PGC-1 homolog. Cell Metabol. 14, 623–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee SG, Bae SH, 2015. The antioxidant function of sestrins is mediated by promotion of autophagic degradation of Keap1 and Nrf2 activation and by inhibition of mTORC1. Free Radic. Biol. Med 88, 205–211. [DOI] [PubMed] [Google Scholar]
- Rogers AS, Escher SA, Pasetto C, Rosato E, Costa R, Kyriacou CP, 2004. A mutation in Drosophila simulans that lengthens the circadian period of locomotor activity. Genetica 120, 223–232. [DOI] [PubMed] [Google Scholar]
- Scarpulla RC, 2011. Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochim. Biophys. Acta 1813, 1269–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair BJ, Ferguson LV, Salehipour-shirazi G, MacMillan HA, 2013. Cross-tolerance and cross-talk in the cold: relating low temperatures to desiccation and immune stress in insects. Integr. Comp. Biol. 53, 545–556. [DOI] [PubMed] [Google Scholar]
- Sujkowski A, Ramesh D, Brockmann A, Wessells R, 2017. Octopamine drives endurance exercise adaptations in Drosophila. Cell Rep. 21, 1809–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supruniuk E, Mikłosz A, Chabowski A, 2017. The implication of PGC-1α on fatty acid transport across plasma and mitochondrial membranes in the insulin sensitive tissues. Front. Physiol 8, 923–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teets NM, Denlinger DL, 2013a. Autophagy in Antarctica: combating dehydration stress in the world’s southernmost insect. Autophagy 9, 629–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teets NM, Denlinger DL, 2013b. Physiological mechanisms of seasonal and rapid cold-hardening in insects. Physiol. Entomol 38, 105–116. [Google Scholar]
- Teets NM, Peyton JT, Colinet H, Renault D, Kelley JL, Kawarasaki Y, Lee RE Jr., Denlinger DL, 2012. Gene expression changes governing extreme dehydration tolerance in an Antarctic insect. Proc. Natl. Acad. Sci. U. S. A 109, 20744–20749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiefenböck SK, Baltzer C, Egli NA, Frei C, 2010. The Drosophila PGC-1 homologue Spargel coordinates mitochondrial activity to insulin signalling. EMBO J. 29, 171–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinkerhess MJ, Healy L, Morgan M, Sujkowski A, Matthys E, Zheng L, Wessells RJ, 2012. The Drosophila PGC-1α homolog spargel modulates the physiological effects of endurance exercise. PloS One 7 e31633–e31633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasco-Miguel S, Buckbinder L, Jean P, Gelbert L, Talbott R, Laidlaw J, Seizinger B, Kley N, 1999. PA26, a novel target of the p53 tumor suppressor and member of the GADD family of DNA damage and growth arrest inducible genes. Oncogene 18, 127–137. [DOI] [PubMed] [Google Scholar]
- Williams CM, McCue MD, Sunny NE, Szejner-Sigal A, Morgan TJ, Allison DB, Hahn DA, 2016. Cold adaptation increases rates of nutrient flow and metabolic plasticity during cold exposure in Drosophila melanogaster. Proc. Biol. Sci 283, 20161317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CM, Rocca JR, Edison AS, Allison DB, Morgan TJ, Hahn DA, 2018. Cold adaptation does not alter ATP homeostasis during cold exposure in Drosophila melanogaster. Integr. Zool 13, 471–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CM, Watanabe M, Guarracino MR, Ferraro MB, Edison AS, Morgan TJ, Boroujerdi AFB, Hahn DA, 2014. Cold adaptation shapes the robustness of metabolic networks in Drosophila melanogaster. Evolution 68, 3505–3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi S-X, Gantz JD, Lee RE, 2017. Desiccation enhances rapid cold-hardening in the flesh fly Sarcophaga bullata: evidence for cross tolerance between rapid physiological responses. J. Comp. Physiol. B 187, 79–86. [DOI] [PubMed] [Google Scholar]