Abstract
BACKGROUND:
Worldwide methamphetamine (METH) use has increased significantly over the last 10 years, and in the US, METH dependence has sky-rocketed among individuals with opioid use disorder. Of significant concern, METH use is gaining popularity among groups with susceptibility to developing severe substance use disorders, such as women and adolescents. Nevertheless, there is no established pharmacotherapy for METH addiction. Emerging evidence has identified the orexin/hypocretin system as an important modulator of reward-driven behavior and a potential target for the treatment of drug addiction and relapse. However, to date, there have been no investigations into the therapeutic efficacy of orexin/hypocretin receptor antagonists for METH-motivated behavior in adolescents or adults. In the present study, we examined the effects of selective antagonists of the orexin-1 (SB-334867, 20 mg/kg) and orexin-2 (TCS-OX2-29, 20 mg/kg) receptors on the reinstatement of METH seeking in both adolescent and adult male and female rats.
METHODS:
Rats were trained to self-administer METH (0.05 mg/kg/inf, iv) during two 2-h sessions/day for 5 days. Following 20 sessions of extinction over 10 days, a within-subjects design was used to test for METH seeking precipitated by METH (1 mg/kg, ip) or METH cues after systemic pretreatment with SB-334867 or TCS-OX2-29. RESULTS: SB-334867 reduced cue-induced reinstatement in males and females, regardless of age. Additionally, METH-induced METH seeking was attenuated by SB-334867 in adolescents and by TCS-OX2-29 in adults.
CONCLUSION:
Selective orexin/hypocretin receptor antagonists have significant therapeutic potential for diminishing METH-seeking behavior, although their treatment efficacy may be influenced by age.
Keywords: adolescent, hypocretin, methamphetamine, orexin, reinstatement, sex differences
1. Introduction
Psychostimulants are among the most widely used classes of illicit drugs in the world, accounting for nearly 70 million past-year consumers (World Drug Report, 2019). Among them, methamphetamine (METH) is gaining popularity, especially with groups at significant risk of developing severe substance use disorders, such as women (Anker and Carroll, 2010a; Becker and Koob, 2016; Ellis et al., 2018; Hudson and Stamp, 2011; World Drug Report, 2019) and adolescents (Chomchai and Chomchai, 2015; Luikinga et al., 2018; Spear, 2016; World Drug Report, 2019). Both women and adolescents have shorter time from initial use to the onset of dependence for most drugs of abuse (Clark et al. 1998, Chen and Kandel 2002), and once dependence develops, they are more likely to engage in harmful and potentially lethal drug binges compared to their respective counterparts (i.e., men, adults) (Baumeister and Tossmann, 2005; Brady and Randall, 1999; Estroff et al., 1989; Mann et al., 2005; McCambridge and Strang, 2005; Randall et al., 1999).
Within the US, increases in METH potency and affordability over the last decade have coincided with surges in METH treatment admissions (US Department of Justice, 2017) and overdose fatalities (CDC, 2018; Seth et al., 2018). Non-medical use of psychostimulants is highest among those aged 18–25 more than any other age group (World Drug Report, 2019), and about 20% of those admitted to a treatment program for METH abuse or dependence are adolescents (Gonzales et al., 2008; Rawson et al., 2007). Additionally, of significant concern is the dramatic increase in METH use among individuals with opioid use disorder (Ellis et al., 2018; Strickland et al., 2019; The Lancet, 2018). From 2011 to 2017, past month METH use increased by 80% among all opioid users and by 98% among women (Ellis et al., 2018), and comorbid METH-opioid use accounted for an 11-fold increase in overdose rates (CDC, 2018). While much attention and resources are focused on finding solutions for the US opioid crisis, there remains no effective pharmacotherapy for METH addiction (Siefried et al., 2020), and this puts groups with heightened susceptibility for the development and persistence of drug addiction, such as women and adolescents, at a particular disadvantage.
The orexin (hypocretin) system is emerging as a promising target for the treatment of drug addiction and relapse. The neuropeptides orexin A (hypocretin 1) and orexin B (hypocretin 2) are released by hypothalamic afferents targeting neurons widely throughout the brain via two G-protein-coupled receptors, orexin type 1 (OX1) and orexin type 2 (OX2) (hypocretin type 1 and hypocretin type 2, respectively) (de Lecea et al., 1998; Sakurai et al., 1998). Aside from a prominent role for orexins in arousal (Alexandre et al., 2013; Kilduff and Peyron, 2000; Sakurai, 2007) and feeding (Berthoud and Münzberg, 2011; Burdakov et al., 2013; Cason et al., 2010; Sheng et al., 2014), a growing body of evidence identifies orexins as important modulators of reward processing and drug-motivated behaviors (Baimel et al., 2015; James et al., 2016; Simmons and Gentile, 2020). In the context of drug addiction, orexin neurons are not specifically involved in mediating the primary reinforcing effects of most drugs of abuse (España et al., 2010; Hutcheson et al., 2011; Smith et al., 2009) (but see LeSage et al., 2010; Moorman and Aston-Jones, 2009; Smith and Aston-Jones, 2012). Rather, they are theorized to control motivated responding for drugs (Borgland et al., 2009; Boutrel et al., 2010; Mahler et al., 2014) and are engaged by drug-associated stimuli, such as discrete cues and contexts paired with drug administration (Cason et al., 2010; James et al., 2011; Mahler et al., 2013; Smith et al., 2010).
Preclinical animal models have demonstrated that orexin receptor antagonists are highly effective in attenuating relapse-related behavior (James et al., 2016) for all drugs of abuse tested, including cocaine (Bentzley and Aston-Jones, 2015; Martin-Fardon and Weiss, 2014a; Smith et al., 2009, 2010; Zhou et al., 2012), alcohol (Brown et al., 2013; Martin-Fardon and Weiss, 2014b; Moorman et al., 2017), nicotine (Plaza-Zabala et al., 2010; Uslaner et al., 2014), remifentanil (Porter-Stransky et al., 2017), and heroin (Smith and Aston-Jones, 2012). In 2018, the National Institute on Drug Abuse listed antagonists of the orexin neuropeptide system among the pharmacotherapeutic strategies with the “highest probability” of Federal Drug Administration approval for the treatment of opioid use disorder in the near future (Rasmussen et al., 2019). Many of the early preclinical investigations focused on OX1 receptor antagonists, based on initial studies showing little therapeutic benefit from OX2 receptor blockade for cocaine seeking (Zhou et al., 2012) and generalizing from an apparent dichotomy in orexin function, where OX1 receptors primarily participate in reward processing while OX2 receptors primarily participate in arousal (Akanmu and Honda, 2005; Marcus et al., 2001; Willie et al., 2003). However, several studies have now demonstrated reduction of drug-seeking behavior by OX2 receptor antagonists for drugs such as nicotine (Uslaner et al., 2014) and alcohol (Brown et al., 2013). These data suggest potential therapeutic benefit may be derived by blockade of both receptors, and several groups have called for repurposing the dual orexin receptor antagonist suvorexant (approved as a therapeutic for insomnia) for the treatment of opioid addiction (James et al., 2020; Valentino and Volkow, 2020; Volkow, 2020). Supporting this notion, a recent preliminary examination of suvorexant in patients with cocaine use disorder reported improvement in relapse-related measures, including cocaine craving (Suchting et al., 2020).
Despite encouraging outcomes for combating motivated behavior for various drugs of abuse, no investigations into the treatment efficacy of orexin receptor antagonists for METH addiction have been undertaken. Nevertheless, several studies suggest a role for orexin peptide signaling in METH’s mechanism of action. For example, acute or chronic administration of METH induces fos expression in orexin neurons (Cornish et al., 2012; Estabrooke et al., 2001), and serum levels of orexin A are elevated in individuals with methamphetamine use disorder (Chen et al., 2016). Therefore, the aim of the present investigation was to examine the therapeutic potential of selective orexin receptor antagonists for METH-seeking behavior in a rodent model of relapse in male and female adolescents and adults. Rats were trained to self-administer i.v. METH and subsequently underwent a multiple-component reinstatement procedure in which they were treated systemically with selective OX1 or OX2 receptor antagonists and exposed to either a METH priming injection or METH-related cues. Selective antagonists for both OX1 and OX2 receptors were examined separately to evaluate their individual treatment effects and contributions to cue- vs. METH-induced drug seeking.
2. Methods and materials
2.1. Subjects
A total of 43 Wistar rats served as subjects in this study. Early adolescence to late adolescence/emerging adulthood in rats has been estimated to range from postnatal day (PND) 25–65 (Spear, 2015). Twelve adolescent female and 8 adolescent male rats were bred in our laboratory at the University of Minnesota from parents obtained from Harlan Sprague-Dawley, Inc. (Madison, WI, USA) and began testing on PND 23. Thirteen adult female and 10 adult male rats were obtained from Harlan Sprague-Dawley, Inc. around PND 80–100, and they began testing around PND 90–120. Although adolescent and adults animals were reared in separate facilities, adult rats were shipped from the vendor during adulthood to mitigate the impact of early developmental stress. Due to the difficulty of moving subjects through the lengthy self-administration procedure, however, adolescent rats that completed the procedure by PND 75 were included in the final analysis (Zlebnik et al., 2012). When adolescent rats that finished around PND 75 were compared to those that finished around PND 60, no differences were found in their behavioral data. Mean group ages (PND) at the conclusion of behavioral testing were as follows: adolescent females = 65, adolescent males = 65, adult females = 163, and adult males = 144.
After arrival at the laboratory, adult rats were pair-housed in plastic cages and allowed 3–10 days to acclimate before testing. Adults and adolescents had free access to laboratory chow (Teklad 2018, Harlan Laboratories, Madison, WI, USA) and water. Upon commencement of behavioral testing, all rats were housed in individual operant conditioning chambers where they remained for the duration of the study. While in the chambers, rats continued to have free access to water. Adults were food-restricted (females, 16 g; males, 20 g) to maintain them at approximately 85% of their free-feeding body weight. To allow for normal development, adolescents were initially fed ad libitum after session until they began consuming the daily allotment of their adult counterparts; thereafter, they were restricted to the adult allotment. All adolescents were restricted to the adult allotment by the end of self-administration training. All rodent holding rooms were maintained at 24°C and at 40–50% humidity under a light/dark cycle (12/12-h) with room lights on at 6:00 am. The experimental protocol was approved by the University of Minnesota Institutional Animal Care and Use Committee. The study was conducted in compliance with the Principles of Laboratory Animal Care (National Academies Press, 2011), and all laboratory facilities were accredited by the American Association for the Accreditation of Laboratory Animal Care.
2.2. Apparatus
Rats were housed and tested in custom-built operant conditioning chambers as previously described (Holtz and Carroll, 2013; Zlebnik et al., 2012). Data collection and programming were conducted using PC computers with a Med-PC interface (MedAssociates, Inc., St. Albans, VT, USA).
2.3. Drugs
D-Methamphetamine HCl (METH) (National Institute of Drug Abuse, Research Triangle Institute, Research Triangle Park, NC, USA) was dissolved in 0.9% NaCl at a concentration of 0.2 mg/ml, and heparin (5 USP/ml) was added to the solution to prevent catheter occlusion from thrombin accumulation. Consistent with our previous work examining iv drug self-administration in adolescent and adult rats (Anker et al., 2012, 2011; Anker and Carroll, 2010b; Holtz and Carroll, 2015, 2013; Zlebnik et al., 2012), the flow rate of each METH infusion was 0.025 ml/sec, and the duration of pump activation (1 sec/100 g of body weight) was adjusted daily to provide a 0.05 mg/kg METH dose throughout self-administration testing. Mean pump durations (sec) per group were as follows: adol males = 1.55, adol females = 1.43, adult males = 3.66, adult females = 2.88. Priming injections of METH during reinstatement were delivered ip at a dose of 1 mg/kg (Holtz et al., 2012).
The OX1 receptor (SB-334867, 20 mg/kg) and the OX2 receptor (TCS-OX2-29, 20 mg/kg) antagonists were synthesized by Dr. Yanan Zhang at Research Triangle Institute and were dissolved in 10% dimethyl sulfoxide (DMSO) (Sigma-Aldrich, St. Louis, MO, USA). A 20 mg/kg (ip) dose was chosen because prior work by others demonstrated that it was sufficient to reduce cue-primed reinstatement of cocaine seeking (Smith et al., 2009). Due to time constraints of testing adolescents, only one dose of each antagonist was examined, and all experimental conclusions are limited to the doses examined.
2.4. Catheterization Surgery
Rats were implanted with chronic indwelling jugular catheters in the right jugular vein by methods previously described (Zlebnik et al., 2010). Antibiotics (enrofloxacin, 10 mg/kg, sc) and analgesics (buprenorphine, 0.05 mg/kg, sc) were administered daily during the 3-day recovery period. Catheters were flushed daily with a solution (0.3 ml, iv) of heparinized saline (20 USP/ml) to prevent catheter blockage and infection. Catheter patency was assessed weekly with a solution of ketamine (60 mg/kg), midazolam (3 mg/kg), and saline (volume ratio: 1:1:2). If loss of the righting reflex did not manifest following iv administration of this solution, a second catheter was implanted in the left jugular vein, and the experiment was resumed following a 3-day recovery period and re-establishment of baseline behavior. Due to the limited window of adolescent testing, no adolescent rat underwent a second catheter surgery; one female rat and two male rats were re-implanted following such methods.
2.5. Maintenance, extinction, and reinstatement
Rats were trained to self-administer METH following established methods (Zlebnik et al., 2012), and all rats acquired METH self-administration. In brief, three experimenter-delivered priming infusions of 0.4 mg/kg cocaine were administered periodically (9:00 am, 11:00 am, and 1:00 pm) during initial training sessions, followed by placement of a small amount of ground food on the active/drug-paired lever. During initial training, METH self-administration was limited to 40 infusions/session under a fixed-ratio 1 schedule of reinforcement for 6 h/day (Table 1). House light illumination signaled the start of the session; responses on the active lever delivered METH infusions and turned on the stimulus lights directly about the lever, and responses on the inactive lever turned on the stimulus lights directly about the lever to control for lever pressing for stimulus light presentations (Barry and Symmes, 1963; Goodrick, 1970; Roberts et al., 1958) but had no other consequences. Responses during infusions were recorded but did not result in additional infusions. When rats self-administered 40 infusions for 2 consecutive days in the absence of experimenter-delivered infusions, the infusion limit was removed, and sessions were changed to two 2-h sessions/day (9–11 am, 1–3 pm) for 5 days. Due to the brief duration of adolescence, 2 sessions/day were used to augment the number of METH self-administration sessions in as few days as possible following established methods (Anker and Carroll, 2010b). Following 5 days of maintenance of self-administration under these conditions, METH access and METH-paired cues were discontinued to allow rats to extinguish responding for 10 days. Subsequently, animals were exposed to a within-subjects reinstatement period where METH-seeking behavior was precipitated by METH or METH-related cues. All rats were tested under both priming conditions (e.g., METH injection, cues), and the order of presentation was counter-balanced across animals. Within each priming condition, the treatment sequence was nonsystematic. Thirty min prior to the am session, animals were pretreated with SB-334867, TCS-OX2-29, or vehicle (ip); at the start of session, animals were primed with METH (ip) or given access to METH cues (e.g., noncontingent illumination of house light and response-contingent illumination of stimulus lights and activation of the sound of the infusion pump). The pm sessions served as extinction sessions, and rats were pretreated and primed with vehicle (ip) and did not have exposure to METH cues.
Table 1.
Experimental design.
| Phase | Training | Maintenance | Extinction | Reinstatement | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cue-Induced | METH-Induced | ||||||||||
| VEH | VEH | SB-334867 | TCS-OX2-29 | VEH | VEH | SB-334867 | TCS-OX2-29 | ||||
| No Cues | Cues | Cues | Cues | VEH | METH | METH | METH | ||||
| Days | ~5 | 5 | 10 | 4 | 4 | ||||||
| Session | 6 h | 2, 2 h | |||||||||
2.6. Data analysis
The primary dependent measures were responses, infusions, and responses during infusions during maintenance and responses during extinction and reinstatement. For maintenance and extinction, data were grouped into 2-session blocks to reduce time-of-day variability (e.g., am vs. pm session) and the number of post-hoc contrasts. Measures were analyzed with 3-factor mixed analyses of variance (ANOVA) with age and sex as the between-subjects factors and blocks of sessions or treatment session as the repeated measure. Separate 3-factor ANOVA were performed for each priming condition (e.g., cues, METH injection). Following significant interactions, post hoc tests were performed with Fisher’s least significant difference (LSD) protected t-tests or Tukey’s multiple comparisons test, and results were considered significant if p < 0.05. Based on previous studies (Holtz and Carroll, 2015), an a priori decision was made to use Grubb’s extreme studentized deviate test (GraphPad, San Diego, CA, USA) was used to remove up to 1 outlying point per group during the reinstatement conditions, and other statistical analyses were performed using GB Stat (Dynamic Microsystems, Inc., Silver Spring, MD, USA) and Prism (version 9.0 for Mac, Graphpad). Early trials suggested that the abbreviated experimental procedure did not yield reinstatement responding as robust as previous work (Holtz et al. 2012); therefore, per analytical methods of Ramirez-Niño et al. (Ramirez-Niño et al., 2013), rats were removed from all analyses if they failed to respond at > 175% of extinction responding (mean of extinction sessions 11–20) during reinstatement sessions with vehicle treatment (e.g., veh/cues, veh/METH). Similar numbers were removed from each group: adol males = 1, adol females = 3, adult males = 3, adult females = 3.
3. Results
3.1. Maintenance
Fig. 1 displays responses for METH infusions during the 5-day maintenance period. Results from the 3-factor ANOVA revealed a significant age X sex X day interaction (F4,214 = 6.23, p = 0.0001) but no other significant main effects or interactions. Both age and sex differences in maintenance responding were indicated by the post hoc analyses. Adolescent males escalated their responding (Fig. 1A, day 1 vs. day 5, p < 0.05) and made significantly more responses than adolescent females (day 4 and day 5, p < 0.01) and adult males (Fig. 1D, day 5, p < 0.01) by the end of the maintenance period. On day 2, adult males made a greater number of responses than on days 1 and 3–5 (Fig. 1B, p < 0.05) and also made significantly more responses than adult females (p < 0.01). There were no differences among adolescent and adult females (Fig. 1C).
Figure 1.
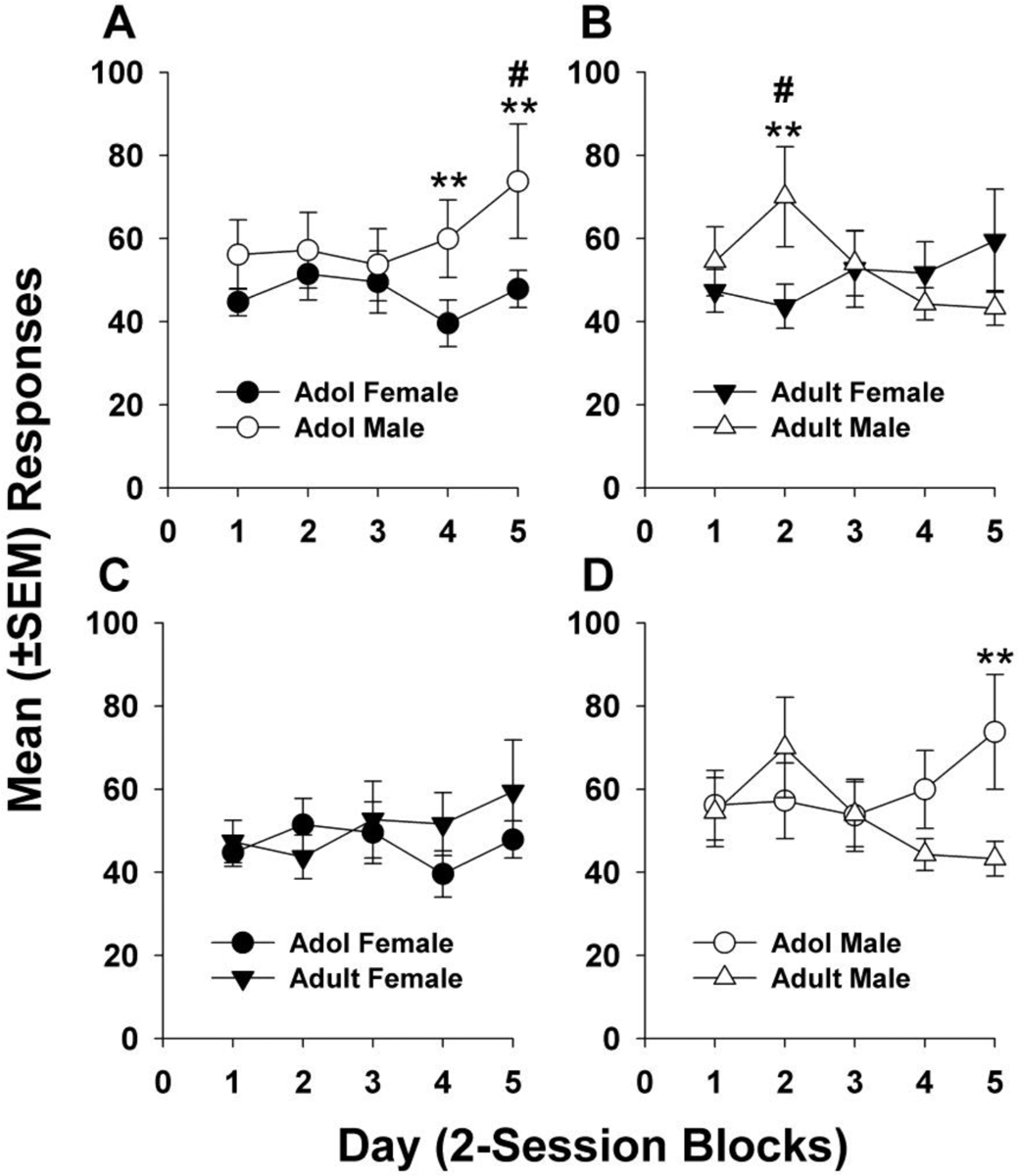
(A-D) Mean (± SEM) maintenance responses for METH (0.05 mg/kg, iv) averaged into 2-session blocks. Data from (A) and (B) replotted in (C) and (D) to highlight age comparisons. (A) Adolescent male rats responded significantly more than adolescent female rats during days 4 and 5 (** p < 0.01) and escalated their responding for METH over the 5-day maintenance period (day 1 vs. day 5, # p < 0.05). (B) On day 2 of maintenance, adult male rats responded significantly more than on days 1 and 3–5 (# p < 0.05) and also significantly more than female rats on day 2 (** p < 0.01). (C) No differences were found in maintenance responses among adolescent and adult female rats. (D) Adolescent male rats emitted a greater number of responses during day 5 of maintenance compared to adult male rats (** p < 0.01). Group numbers: adol male = 8, adol female = 12, adult male = 10, adult female = 12.
Infusions during the maintenance period are displayed in Fig. 2. Post hoc analyses following a significant main effect of day (F4,214 = 3.82, p = 0.0055) and a significant age X sex X day interaction (F4,214 = 5.88, p = 0.0002) also confirmed age and sex differences in infusions over the maintenance period. Like for responses, adolescent males escalated their infusions (Fig. 2A, day 1 vs. day 5, p < 0.01) and earned significantly more infusions than adolescent females and adult males (Fig. 2D, day 5, p < 0.01) by the end of the 5-day period. While adult males had a greater number of infusions than adult females on day 2 (Fig. 2B, p < 0.05), adult females escalated their infusions (day 1 vs. day 5, p < 0.01) and also earned significantly more infusions than males by day 5 (p < 0.01). However, despite a notable increase in infusions over the maintenance period for adult females, there were no differences among adolescent and adult females on any day (Fig. 2C). Inactive lever presses throughout the maintenance period were low and did not differ among the groups. Mean (± SEM) inactive presses on the last day of the maintenance period were as follows: adol male = 16.17 (± 9.13), adol female = 17.56 (± 6.63), adult male = 18.35 (± 8.35), adult female = 13.19 (± 4.09).
Figure 2.
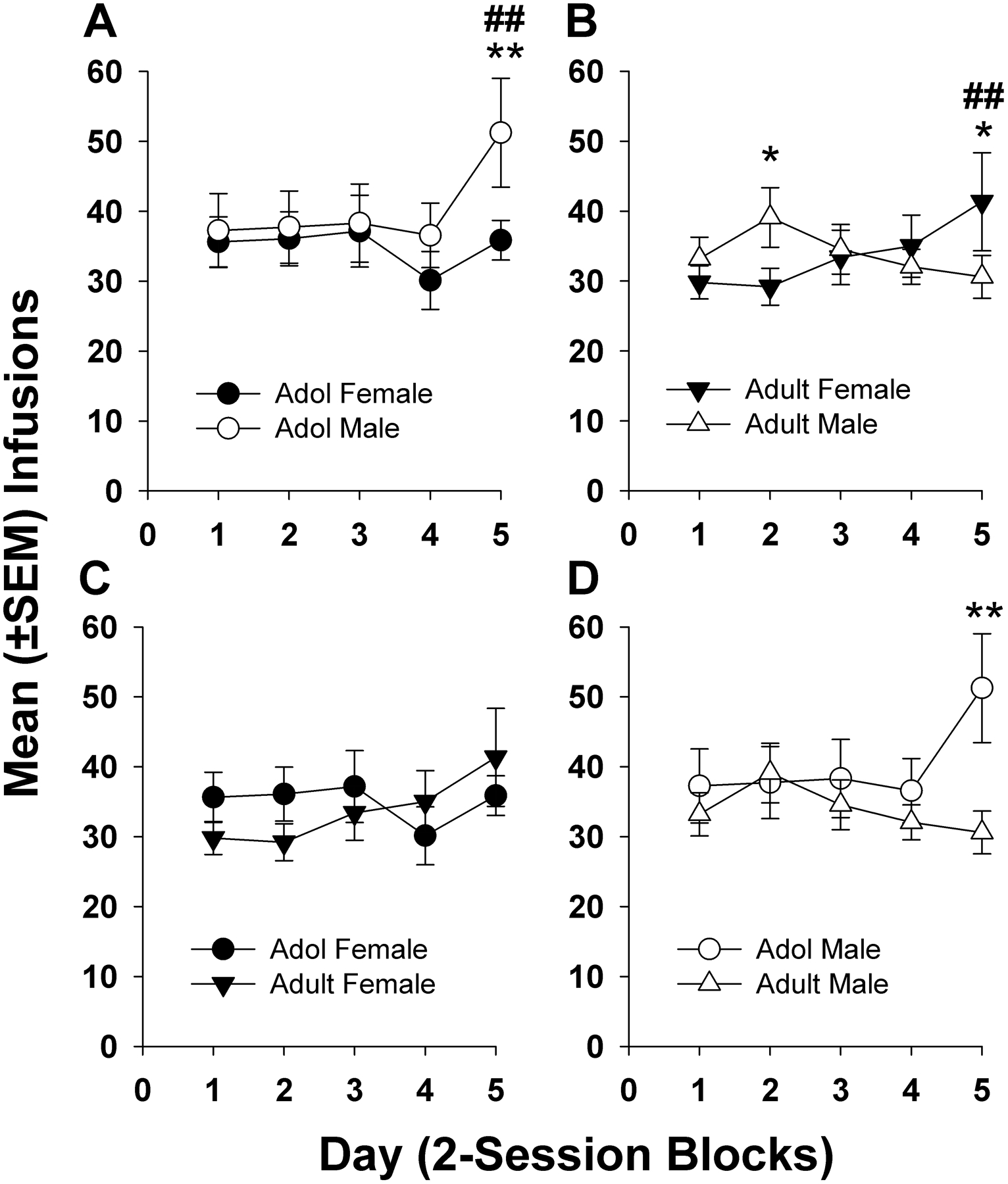
(A-D) Mean (± SEM) maintenance infusions for METH (0.05 mg/kg, iv) averaged into 2-session blocks. Data from (A) and (B) replotted in (C) and (D) to highlight age comparisons. (A) Adolescent male rats infused significantly more METH than adolescent female rats on day 5 (** p < 0.01) and escalated their intake over the 5-day maintenance period (day 1 vs. day 5, ## p < 0.01). (B) On day 2 of maintenance, adult male rats self-administered significantly more METH than female rats (* p < 0.05). Adult female rats significantly escalated their intake of METH over the 5-day maintenance period (day 1 vs. day 5, ## p < 0.01) and self-administered more METH than male rats on day 5 (* p < 0.05). (C) No differences were found in METH infusions among adolescent and adult female rats. (D) Adolescent male rats had greater intake of METH on day 5 of maintenance compared to adult male rats (** p < 0.01). Group numbers: adol male = 8, adol female = 12, adult male = 10, adult female = 12.
Responses during infusions (data not shown) did not differ between the groups or over the 5-day maintenance period. Following a significant age X sex X day interaction (F4.152 = 2.952, p < 0.05) but no significant main effects of age, sex, or day, there were no significant post-hoc comparisons. The mean (± SEM) responses during infusions for each group over the maintenance period were as follows: adol male = 19.73 (± 5.31), adol female = 11.70 (± 2.73), adult male = 19.74 (± 4.52), adult female = 17.20 (± 3.80).
3.2. Extinction
Analysis of extinction responses (Fig. 3) showed a significant main effect of day (F9,419 = 8.56, p < 0.0001) and also significant age X day (F9,419 = 2.57, p = 0.007), sex X day (F9,419 = 3.35, p = 0.0006), and age X sex X day (F9,419 = 2.59, p = 0.0067) interactions. Most notably, adult males demonstrated resistance to extinction with more responding on day 1 compared to adult females (Fig. 3B, p < 0.01) and adolescent males (Fig. 3D, p < 0.01). Adolescent females made more responses than adolescent males on day 2 (Fig. 3A, p < 0.05), but this trend was reversed on days 4 and 6, as adolescent males made more responses than adolescent females (p < 0.05). While adolescent males also made more extinction responses than adult males on days 4 and 6 (Fig. 3D, p < 0.05), there were no differences between adolescent and adult females throughout the extinction period (Fig. 3C). Inactive lever presses were low and did not differ among the groups. Mean (± SEM) inactive presses on the last day of extinction were as follows: adol male = 11.19 (± 0.39), adol female = 3.67 (± 1.24), adult male = 3.22 (± 1.12), adult female = 2.33 (± 1.21).
Figure 3.
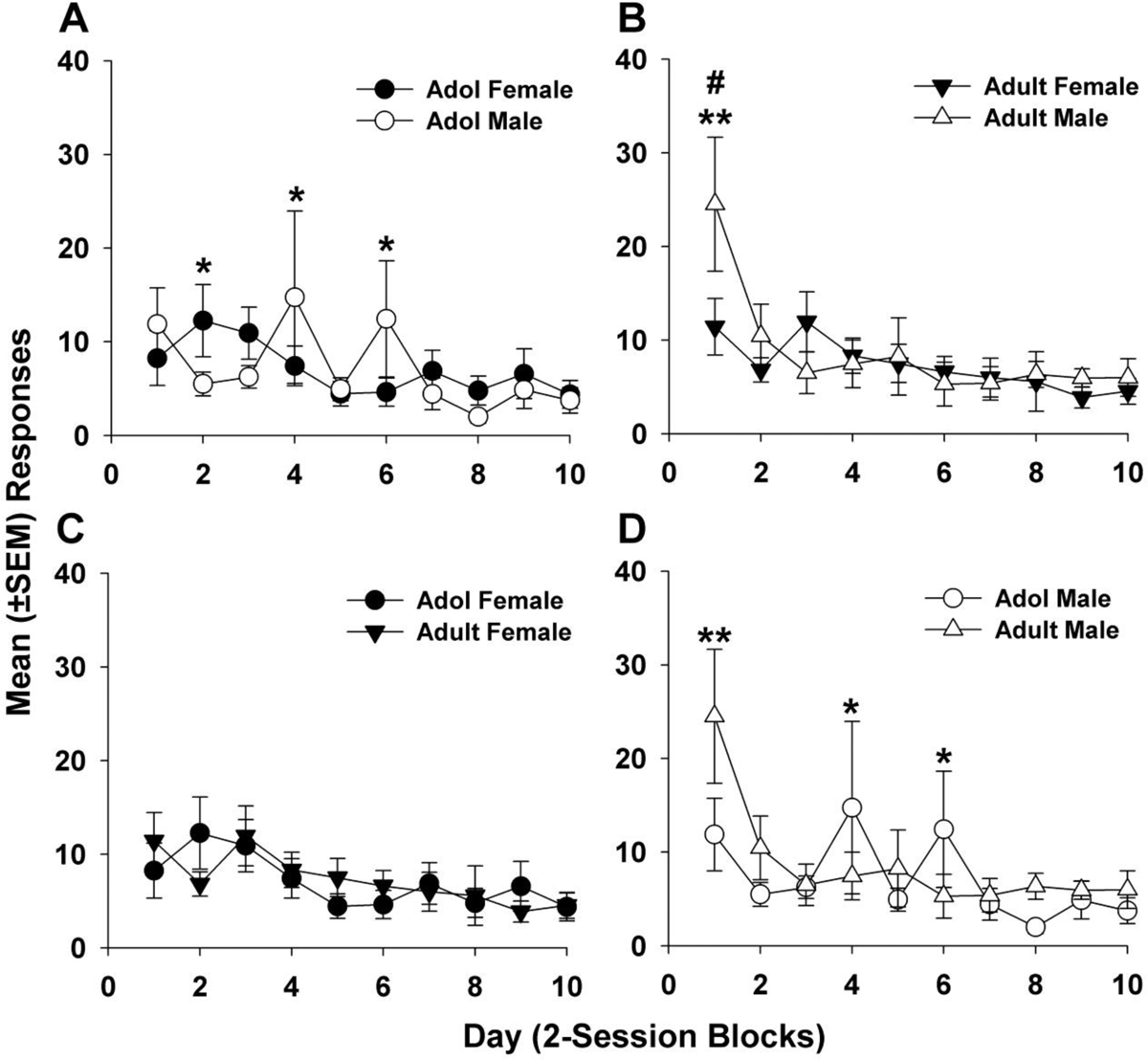
(A-D) Mean (± SEM) extinction responses averaged into 2-session blocks. Data from (A) and (B) replotted in (C) and (D) to highlight age comparisons. (A) Adolescent female rats responded significantly more than adolescent male rats on day 2 (* p < 0.05), while adolescent male rats responded significantly more than adolescent female rats on days 4 and 6. (B) Adult males were more resistant to extinction on day 1 than adult females (** p < 0.01) and responded more on day 1 than on days 2–10 (# p < 0.01). (C) No differences were found in extinction among adolescent and adult female rats. (D) Adult male rats responded more than adolescent male rats on day 1 of extinction (** p < 0.01), while adolescent male rats responded more than adult male rats on days 4 and 6 (* p < 0.05). Group numbers: adol male = 8, adol female = 12, adult male = 10, adult female = 12.
3.3. Cue-induced reinstatement
METH cue-induced reinstatement responding is displayed in Fig. 4A, and analyses illustrated significant attenuation of METH seeking by orexin receptor antagonism, regardless of age or sex. A 3-factor ANOVA resulted in a significant main effect of treatment session (F3,127 = 16.48, p < 0.0001) and a significant age X sex interaction (F1,127 = 5.40, p = 0.028). In order to further probe potential treatment effects across age and sex, additional 3-factor ANOVA were conducted to examine each orexin receptor antagonist separately (e.g., VEH/no cues, VEH/cues, and SB-334867/cues or VEH/no cues, VEH/cues, and TCS-OX2-29/cues).
Figure 4.
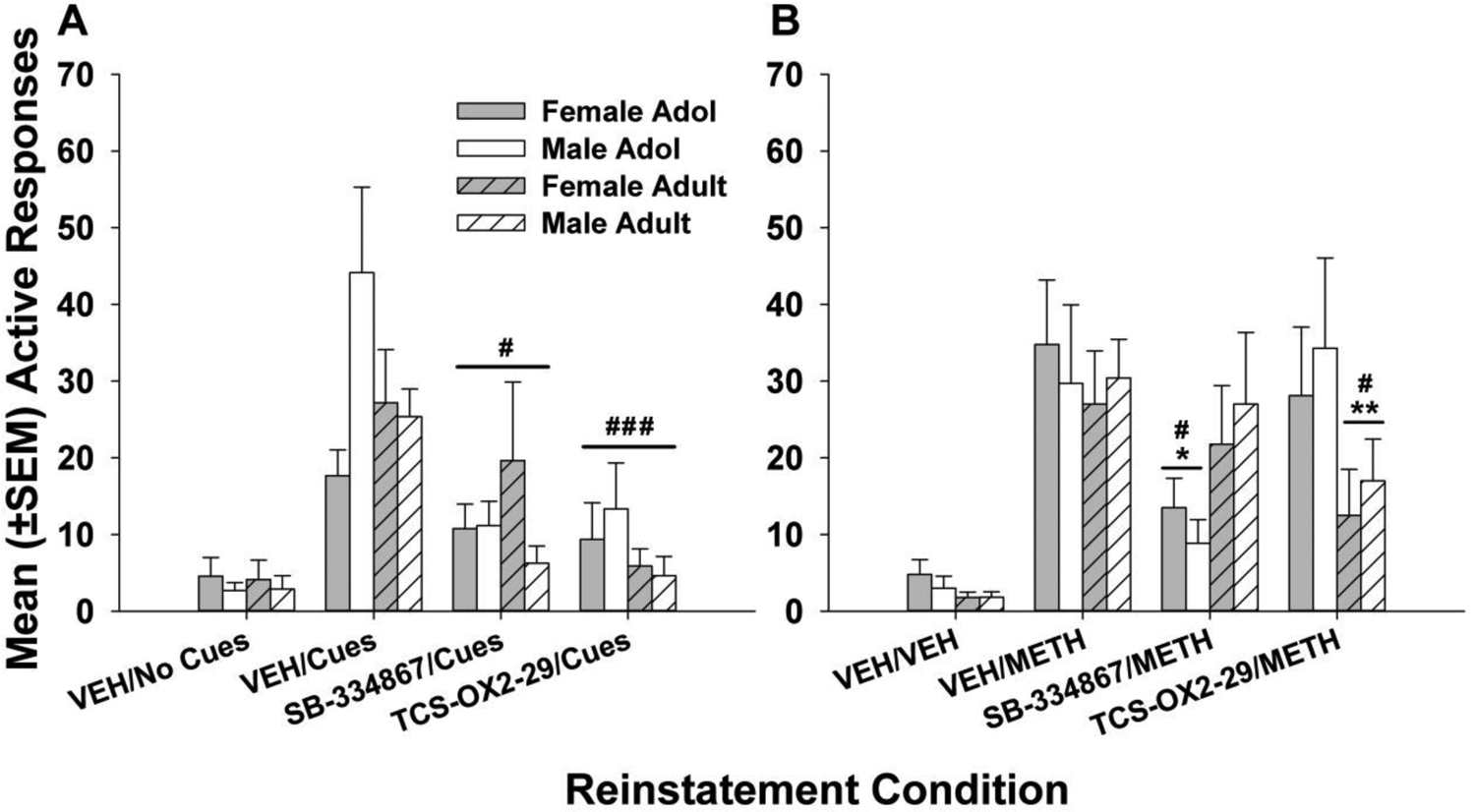
Mean (± SEM) reinstatement active responses induced by (A) METH-related cues or (B) METH injections (1 mg/kg, ip). (A) There were no significant age or sex differences in the attenuation of cue-induced reinstatement by orexin receptor antagonism. Both the selective OX1 receptor antagonist SB-334867 (vs. VEH/cue, # p < 0.05) and the selective OX2 receptor antagonist TCS-OX2-29 (vs. VEH/cue, ### p < 0.001) reduced reinstatement responding in adolescent and adult males and females. (B) Regardless of sex, SB-334867 suppressed METH-induced reinstatement selectively in adolescents (vs. VEH/cue, # p < 0.05; vs. adults, * p < 0.05), and TCS-OX2-29 suppressed reinstatement selectively in adults (vs. VEH/cue, # p < 0.05; vs. adolescents, ** p < 0.01). Group numbers: adol male = 7, adol female = 9–10, adult male = 7–8, adult female = 9.
Analyses of the selective OX1 receptor antagonist SB-334867 revealed a significant main effect of treatment session (F1.431,40.07 = 16.50, p < 0.0001) but no significant interactions or other main effects. Data were collapsed across age and sex, and following a single factor repeated-measures ANOVA (F1.423,44.13 = 14.99, p < 0.0001), post hoc comparisons revealed significant reduction of cue-induced METH seeking-behavior by SB-334867 in all groups (Fig. 4A, p < 0.05 vs. vehicle treatment). Similar analyses of the selective OX2 receptor antagonist TCS-OX2-29 showed a significant main effect of treatment session (F1.315,26.83 = 22.01, p < 0.0001) but no significant interactions or other main effects. A single-factor repeated measures ANOVA was conducted after collapsing across age and sex (F1.211,40.65 = 20.19, p < 0.0001), revealing notable reductions in cue-induced METH reinstatement in all groups following treatment with TCS-OX2-29 (Fig. 4A, p < 0.001 vs. vehicle treatment). Finally, although there was a significant main effect of treatment session (F3,127 = 11.30, p < 0.0001), inactive lever pressing during cue-induced reinstatement (Fig. 5A) did not differ significantly among the groups or individual treatment conditions.
Figure 5.
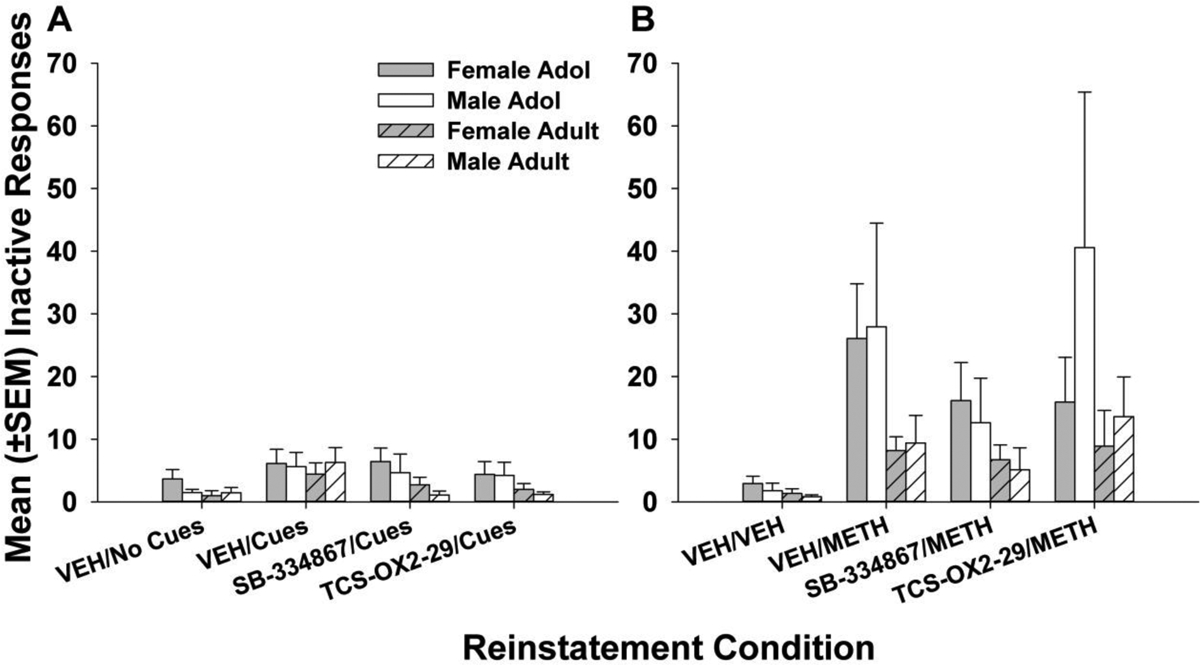
Mean (± SEM) reinstatement inactive responses primed by (A) METH cues or (B) METH injections (1 mg/kg, ip). There were no significant differences among the groups, treatments (e.g., VEH, SB-334867, TCS-OX2-29), or individual reinstatement conditions. Group numbers: adol male = 7, adol female = 9–10, adult male = 7–8, adult female = 9.
3.4. METH-primed reinstatement
Treatment effects of SB-334867 and TCS-OX2-29 on reinstatement responding precipitated by METH priming injections (Fig. 4B) were significantly influenced by age. Results of the 3-factor ANOVA revealed a significant main effect of treatment condition (F3,127 = 21.96, p < 0.0001) and a significant age X treatment condition interaction (F3,127 = 5.93, p = 0.001). Data were collapsed across sex, and the resulting 2-factor ANOVA showed a significant main effect of treatment condition (F3,127 = 18.10, p <0.0001) and a significant age X treatment condition interaction (F3,127 = 4.73, p = 0.0041). SB-334867 reduced METH-seeking behavior in adolescents compared to adults (p < 0.05) and control treatment conditions (p < 0.01). Inversely, TCS-OX2-29 reduced METH-seeking behavior in adults compared to adolescents (p < 0.01) and control treatment conditions (p < 0.05).
Inactive lever presses (Fig. 5B) did not differ among the groups or individual priming conditions. However, compared to inactive lever presses during cue-induced reinstatement sessions, inactive lever presses during METH-induced reinstatement sessions were elevated, and this may reflect stimulus generalization where drug seeking was directed toward both levers in the absence of METH-related cues, general increases in locomotion, or a combination of both.
4. Discussion
The present study expanded on earlier work investigating the role of orexin neurotransmission in relapse to drug seeking by examining both OX1 and OX2 receptor antagonists on METH-motivated behavior in adolescent and adult male and female rats. Our results revealed age- but not sex-dependent reduction of METH-seeking behavior following systemic blockade of orexin receptors. Specifically, for METH seeking precipitated by METH-related cues (Fig. 4A), we found that the selective OX1 receptor antagonist SB-334867 and the selective OX2 receptor antagonist TCS-OX2-29 attenuated reinstatement in both adolescent and adult males and females. However, for reinstatement of METH seeking precipitated by a METH priming injection (Fig. 4B), SB-334867 reduced reinstatement responding only in adolescents, whereas TCS-OX2-29 reduced reinstatement responding only in adults. While previous results demonstrated involvement of the OX1 but not OX2 receptor in cue-induced cocaine seeking (Smith et al., 2009) and sex-specific treatment effects of OX1 receptor antagonism (Zhou et al., 2012), findings from the current investigation illustrate that the complementary attenuating effects of OX1 and OX2 by age but not sex. Overall, these results demonstrate differential treatment effects in an animal model of adolescent addiction vulnerability and suggest a possible role for the orexin system in mediating reward processes across development.
Prior work in adult rats suggests that the OX1 receptor may play a greater role in conditioned cue- vs. drug-induced drug seeking (Zhou et al., 2012), and the present study supports these findings by showing that OX1 receptor antagonism attenuated cue-induced but not METH-induced reinstatement of drug seeking in adult male and female rats. However, we found that OX2 antagonism robustly attenuated METH-induced reinstatement in these groups, contrasting with previous research showing a lack of an effect of OX2 antagonism on cocaine- and alcohol-induced drug seeking in adults (Brown et al., 2013; Smith et al., 2009) (but see Uslaner et al., 2014). Conversely, in adolescent rats, OX1 receptor antagonism reduced METH-induced reinstatement responding, while OX2 antagonism had no effect. These opposing results are intriguing, as they suggest that OX1 and OX2 receptors may play distinct roles in mediating cue- and drug-induced reinstatement of drug seeking over various stages of development in the rat. A number of studies suggest dynamic changes in orexin peptide (Desarnaud et al., 2004; Kessler et al., 2011; Sawai et al., 2010; Van Den Pol et al., 2001) and receptor (Terao et al., 2002; Van Den Pol et al., 2001) expression across the lifespan. In particular, early in postnatal development until early adulthood (~ 8 weeks), hypothalamic orexin A and orexin B peptide expression increases (Sawai et al., 2010), while OX1 and OX2 receptors are expressed at their highest levels before decreasing across distributed brain regions (e.g., hypothalamus, hippocampus, cortex) (Van Den Pol et al., 2001). Critical ongoing maturation of the orexin peptide system over adolescent development may not only help explain differential treatment efficacy of orexin receptor antagonists but may point toward a possible mechanism of age-related differences in drug addiction vulnerability.
Another difference between adolescents and adults in the present study may relate to potential locomotor-depressant effects of the OX2 antagonist. In adults, its administration reduced both cue- and METH-induced METH seeking (Fig. 4), and – given the prominent role of the OX2 receptor in arousal – this could be interpreted as a non-specific decrease in behavior vs. a decrease in drug-seeking behavior specifically. However, this was not the case in adolescents, in which the OX2 antagonist decreased only cue-induced, but not METH-induced, METH-seeking behavior. Regulation of locomotor activity may be differentially regulated by OX2 receptors in adolescents and adults, but levels of inactive lever-pressing argue against this view. There were no notable differences in activity directed toward the inactive lever following TCS-OX2-29 treatment compared to vehicle treatment (Fig. 5), and inactive lever-pressing, especially under METH-priming conditions, did not reach floor levels in either adolescent or adult rats. Therefore, similar to other investigations of drug-motivated behavior (Brown et al., 2013; Schmeichel et al., 2015; Uslaner et al., 2014), we conclude that blockade of the OX2 receptor attenuates METH seeking precipitated by cues and/or METH priming injection in both adolescents and adults.
Previously, Zhou et al. (2012) observed that OX1 receptor antagonism attenuated cue-induced reinstatement of cocaine-seeking behavior in male but not female rats, but these findings did not extend to METH-seeking behavior in the present study. The current investigation established that pretreatment with the OX1 receptor antagonist reduced responding in the presence of METH cues in both adolescent and adult male and female rats. The reasons for differences between cue-induced cocaine and METH seeking are unclear. A recent study examining incubation of METH seeking after 30 days of forced abstinence found that female rats expressed higher levels of nucleus accumbens OX1 and OX2 receptor mRNA than male rats, and METH seeking in females vs. males was negatively associated with mRNA levels (Daiwile et al., 2019). This implicates the orexin system in sex differences that are found in drug seeking, which may reflect general sex differences in the orexin system. For example, expression of OX1 and OX2 receptors (Silveyra et al., 2010), as well as the orexin A and B peptides (Porkka-Heiskanen et al., 2004), vary over the estrous cycle in female rats. Both the present study and previous work (Zhou et al., 2012) examined orexin antagonism of drug seeking in freely-cycling female rats, but future studies should explore an interaction of estrous cycle or gonadal hormones with orexin signaling in the context of drug-seeking behavior.
Although the primary focus of this study was the effect of orexin receptor antagonism on reinstatement of METH seeking, this study also allowed for examination of age and sex differences in METH self-administration, extinction, and reinstatement. During the maintenance period, both adolescent males (Fig. 1A, Fig. 2A) and adult females (Fig. 2B) escalated their intake of METH over the 5-day period, highlighting their vulnerability to METH self-administration compared to their adult male counterparts. Although previous studies did not identify the same differences in short-access METH self-administration compared to adult males (Anker et al., 2012; Hankosky et al., 2018; Reichel et al., 2012; Westbrook et al., 2020 but see Anker and Carroll, 2010b), these discrepancies may be related to the two 2-h daily METH self-administration sessions in the present study. Compared to typical short-access (2 h/day) self-administration sessions, the 4 h of total daily METH self-administration in the present study may be more similar to long-access (generally > 6 h/day) conditions that promote the development of behavioral neuroadaptations resulting in escalation of intake (Edwards and Koob, 2013).
The abbreviated protocol used in the current study may also explain other inconsistencies with earlier findings. For example, Fig. 3 displays low rates of extinction responding for all groups throughout the 10-day period, with the only exception being robust responding for adult males on Day 1 and adolescent males on Days 4 and 6. However, a similar study examining cocaine reinstatement in adolescent and adult male rats using an identical protocol demonstrated greater levels of extinction, including overall higher rates in adolescent males, as well as differential cocaine reinstatement among adolescents and adults (Anker and Carroll, 2010b). Overall, the lack of robust age and sex differences in drug seeking presented in the current investigation are nevertheless supported by other methamphetamine studies in adolescents and adults (Westbrook and Gulley, 2020) as well as males and females (Holtz et al., 2012; Reichel et al., 2012; Westbrook and Gulley, 2020).
The present study demonstrated age-dependent effects of OX1 and OX2 antagonists on reinstatement of METH-seeking behavior in rats. These results add to a growing body of literature that establishes promising therapeutic potential for orexin receptor blockade in the treatment of drug relapse. Our findings suggest ways in which the orexin system can be harnessed to develop personalized treatment for drug addiction; preferential antagonism of either the OX1 or OX2 receptor may help to target not only specific drugs of abuse (e.g., METH, cocaine, alcohol, etc.) (Gotter et al., 2012; Perrey and Zhang, 2020) and relapse triggers (e.g., cues, drugs) (Brown et al., 2013; Smith et al., 2009; Zhou et al., 2012) but also individual vulnerability factors (e.g., adolescence) (Zhou et al., 2012). However, at least for METH relapse-related behavior, a more generalized benefit may be derived from a dual orexin receptor antagonist (James et al., 2020; Perrey and Zhang, 2020; Winrow et al., 2010), such as suvorexant. A recent study demonstrated suvorexant to be safe and well-tolerated in adolescent humans with sleep disorders (Kawabe et al., 2017), and future work should investigate its efficacy for METH relapse in adolescent and adult populations. Overall, it is clear that an enhanced understanding of developmental differences in the orexin system may guide more effective pharmacotherapeutic intervention for addiction to METH and other drugs of abuse.
Highlights.
Age affects treatment of methamphetamine seeking by orexin receptor antagonism
Blockade of the orexin-1 receptor reduced cued METH seeking in all rats
In adolescents, orexin-1 receptor antagonism decreased METH-primed METH seeking
In adults, orexin-2 receptor antagonism attenuated METH-primed METH seeking
Acknowledgements
This work was supported by the National Institute on Drug Abuse (NIDA) grants K99 DA047419 (NEZ), R01 DA019942 (MEC), R01 DA003240 (MEC), and K05 DA015267 (MEC). The authors would like to thank Vanessa Adamson, Yosef Amrami, Tom Baron, Cole Batty, Katie Bressler, Luke Bushman, Clare Chamberlain, Alex Claxton, Seth Johnson, Brandon Knight, Sarah Korthauer, Nathan Omdalen, Aneal Rege, Tyler Rehbein, Rachael Turner, Heather Veglahn, and Ashley Xiong for technical assistance and Krista Walkowiak, DVM, for veterinary care.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
No conflict declared
References
- Akanmu MA, Honda K, 2005. Selective stimulation of orexin receptor type 2 promotes wakefulness in freely behaving rats. Brain Res. 1048, 138–145. 10.1016/j.brainres.2005.04.064 [DOI] [PubMed] [Google Scholar]
- Alexandre C, Andermann ML, Scammell TE, 2013. Control of arousal by the orexin neurons. Curr. Opin. Neurobiol 23, 752–759. 10.1016/j.conb.2013.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker JJ, Baron TR, Zlebnik NE, Carroll ME, 2012. Escalation of methamphetamine self-administration in adolescent and adult rats. Drug and Alcohol Dependence 124, 149–153. 10.1016/j.drugalcdep.2012.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker JJ, Carroll ME, 2010a. Females Are More Vulnerable to Drug Abuse than Males: Evidence from Preclinical Studies and the Role of Ovarian Hormones, in: Neill JC, Kulkarni J (Eds.), Biological Basis of Sex Differences in Psychopharmacology, Current Topics in Behavioral Neurosciences. Springer Berlin Heidelberg, Berlin, Heidelberg, pp. 73–96. 10.1007/7854_2010_93 [DOI] [PubMed] [Google Scholar]
- Anker JJ, Carroll ME, 2010b. Reinstatement of cocaine seeking induced by drugs, cues, and stress in adolescent and adult rats. Psychopharmacology (Berl.) 208, 211–222. 10.1007/s00213-009-1721-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker JJ, Zlebnik NE, Navin SF, Carroll ME, 2011. Responding during signaled availability and nonavailability of iv cocaine and food in rats: age and sex differences. Psychopharmacology 215, 785–799. 10.1007/s00213-011-2181-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baimel C, Bartlett SE, Chiou L-C, Lawrence AJ, Muschamp JW, Patkar O, Tung L-W, Borgland SL, 2015. Orexin/hypocretin role in reward: implications for opioid and other addictions: Orexin/hypocretin role in addiction. Br J Pharmacol 172, 334–348. 10.1111/bph.12639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry HI, Symmes D, 1963. Reinforcing effects of illumination change in different phases of the rat’s diurnal cycle. Journal of Comparative and Physiological Psychology 56, 117–119. 10.1037/h0045033 [DOI] [Google Scholar]
- Baumeister SE, Tossmann P, 2005. Association between early onset of cigarette, alcohol and cannabis use and later drug use patterns: an analysis of a survey in European metropolises. Eur Addict Res 11, 92–98. 10.1159/000083038 [DOI] [PubMed] [Google Scholar]
- Becker JB, Koob GF, 2016. Sex Differences in Animal Models: Focus on Addiction. Pharmacol Rev 68, 242–263. 10.1124/pr.115.011163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzley BS, Aston-Jones G, 2015. Orexin-1 receptor signaling increases motivation for cocaine-associated cues. Eur. J. Neurosci 41, 1149–1156. 10.1111/ejn.12866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud H-R, Münzberg H, 2011. The lateral hypothalamus as integrator of metabolic and environmental needs: from electrical self-stimulation to opto-genetics. Physiol. Behav 104, 29–39. 10.1016/j.physbeh.2011.04.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Chang S-J, Bowers MS, Thompson JL, Vittoz N, Floresco SB, Chou J, Chen BT, Bonci A, 2009. Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers. J. Neurosci 29, 11215–11225. 10.1523/JNEUROSCI.6096-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutrel B, Cannella N, de Lecea L, 2010. The role of hypocretin in driving arousal and goal-oriented behaviors. Brain Res. 1314, 103–111. 10.1016/j.brainres.2009.11.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady KT, Randall CL, 1999. Gender differences in substance use disorders. Psychiatr. Clin. North Am 22, 241–252. 10.1016/s0193-953x(05)70074-5 [DOI] [PubMed] [Google Scholar]
- Brown RM, Khoo SY-S, Lawrence AJ, 2013. Central orexin (hypocretin) 2 receptor antagonism reduces ethanol self-administration, but not cue-conditioned ethanol-seeking, in ethanol-preferring rats. Int. J. Neuropsychopharmacol 16, 2067–2079. 10.1017/S1461145713000333 [DOI] [PubMed] [Google Scholar]
- Burdakov D, Karnani MM, Gonzalez A, 2013. Lateral hypothalamus as a sensor-regulator in respiratory and metabolic control. Physiol. Behav 121, 117–124. 10.1016/j.physbeh.2013.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cason AM, Smith RJ, Tahsili-Fahadan P, Moorman DE, Sartor GC, Aston-Jones G, 2010. Role of orexin/hypocretin in reward-seeking and addiction: implications for obesity. Physiol. Behav 100, 419–428. 10.1016/j.physbeh.2010.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC, 2018. Multiple Cause of Death 1999–2017 on CDC WONDER Online Database.
- Chen W-Y, Kao C-F, Chen P-Y, Lin S-K, Huang M-C, 2016. Orexin-A level elevation in recently abstinent male methamphetamine abusers. Psychiatry Research 239, 9–11. 10.1016/j.psychres.2016.02.059 [DOI] [PubMed] [Google Scholar]
- Chomchai C, Chomchai S, 2015. Global patterns of methamphetamine use: Current Opinion in Psychiatry 28, 269–274. 10.1097/YCO.0000000000000168 [DOI] [PubMed] [Google Scholar]
- Cornish JL, Hunt GE, Robins L, McGregor IS, 2012. Regional c-Fos and FosB/ΔFosB expression associated with chronic methamphetamine self-administration and methamphetamine-seeking behavior in rats. Neuroscience 206, 100–114. 10.1016/j.neuroscience.2012.01.004 [DOI] [PubMed] [Google Scholar]
- Daiwile AP, Jayanthi S, Ladenheim B, McCoy MT, Brannock C, Schroeder J, Cadet JL, 2019. Sex Differences in Escalated Methamphetamine Self-Administration and Altered Gene Expression Associated With Incubation of Methamphetamine Seeking. International Journal of Neuropsychopharmacology 22, 710–723. 10.1093/ijnp/pyz050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG, 1998. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc. Natl. Acad. Sci. U.S.A 95, 322–327. 10.1073/pnas.95.1.322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desarnaud F, Murillo-Rodriguez E, Lin L, Xu M, Gerashchenko D, Shiromani SN, Nishino S, Mignot E, Shiromani PJ, 2004. The diurnal rhythm of hypocretin in young and old F344 rats. Sleep 27, 851–856. 10.1093/sleep/27.5.851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S, Koob GF, 2013. Escalation of drug self-administration as a hallmark of persistent addiction liability. Behav Pharmacol 24, 356–362. 10.1097/FBP.0b013e3283644d15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis MS, Kasper ZA, Cicero TJ, 2018. Twin epidemics: The surging rise of methamphetamine use in chronic opioid users. Drug and Alcohol Dependence 193, 14–20. 10.1016/j.drugalcdep.2018.08.029 [DOI] [PubMed] [Google Scholar]
- España RA, Oleson EB, Locke JL, Brookshire BR, Roberts DCS, Jones SR, 2010. The hypocretin-orexin system regulates cocaine self-administration via actions on the mesolimbic dopamine system. European Journal of Neuroscience 31, 336–348. 10.1111/j.1460-9568.2009.07065.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estabrooke IV, McCarthy MT, Ko E, Chou TC, Chemelli RM, Yanagisawa M, Saper CB, Scammell TE, 2001. Fos Expression in Orexin Neurons Varies with Behavioral State. J. Neurosci 21, 1656–1662. 10.1523/JNEUROSCI.21-05-01656.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estroff TW, Schwartz RH, Hoffmann NG, 1989. Adolescent cocaine abuse. Addictive potential, behavioral and psychiatric effects. Clin Pediatr (Phila) 28, 550–555. 10.1177/000992288902801201 [DOI] [PubMed] [Google Scholar]
- Gonzales R, Ang A, McCann MJ, Rawson RA, 2008. An emerging problem: methamphetamine abuse among treatment seeking youth. Subst Abus 29, 71–80. 10.1080/08897070802093312 [DOI] [PubMed] [Google Scholar]
- Goodrick C, 1970. Light- and dark-contingent bar pressing in the rat as a function of age and motivation. Journal of Comparative and Physiological Psychology 73, 100–104. 10.1037/h0030017 [DOI] [Google Scholar]
- Gotter AL, Roecker AJ, Hargreaves R, Coleman PJ, Winrow CJ, Renger JJ, 2012. Orexin receptors as therapeutic drug targets. Prog. Brain Res 198, 163–188. 10.1016/B978-0-444-59489-1.00010-0 [DOI] [PubMed] [Google Scholar]
- Hankosky ER, Westbrook SR, Haake RM, Marinelli M, Gulley JM, 2018. Reduced sensitivity to reinforcement in adolescent compared to adult Sprague-Dawley rats of both sexes. Psychopharmacology (Berl) 235, 861–871. 10.1007/s00213-017-4804-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtz NA, Carroll ME, 2015. Cocaine self-administration punished by intravenous histamine in adolescent and adult rats. Behav Pharmacol 26, 393–397. 10.1097/FBP.0000000000000136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtz NA, Carroll ME, 2013. Escalation of i.v. cocaine intake in peri-adolescent vs. adult rats selectively bred for high (HiS) vs. low (LoS) saccharin intake. Psychopharmacology (Berl.) 227, 243–250. 10.1007/s00213-012-2958-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtz NA, Lozama A, Prisinzano TE, Carroll ME, 2012. Reinstatement of methamphetamine seeking in male and female rats treated with modafinil and allopregnanolone. Drug Alcohol Depend 120, 233–237. 10.1016/j.drugalcdep.2011.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson A, Stamp JA, 2011. Ovarian hormones and propensity to drug relapse: A review. Neuroscience & Biobehavioral Reviews 35, 427–436. 10.1016/j.neubiorev.2010.05.001 [DOI] [PubMed] [Google Scholar]
- Hutcheson DM, Quarta D, Halbout B, Rigal A, Valerio E, Heidbreder C, 2011. Orexin-1 receptor antagonist SB-334867 reduces the acquisition and expression of cocaine-conditioned reinforcement and the expression of amphetamine-conditioned reward. Behav Pharmacol 22, 173–181. 10.1097/FBP.0b013e328343d761 [DOI] [PubMed] [Google Scholar]
- James MH, Charnley JL, Levi EM, Jones E, Yeoh JW, Smith DW, Dayas CV, 2011. Orexin-1 receptor signalling within the ventral tegmental area, but not the paraventricular thalamus, is critical to regulating cue-induced reinstatement of cocaine-seeking. Int. J. Neuropsychopharmacol 14, 684–690. 10.1017/S1461145711000423 [DOI] [PubMed] [Google Scholar]
- James MH, Fragale JE, Aurora RN, Cooperman NA, Langleben DD, Aston-Jones G, 2020. Repurposing the dual orexin receptor antagonist suvorexant for the treatment of opioid use disorder: why sleep on this any longer? Neuropsychopharmacol. 45, 717–719. 10.1038/s41386-020-0619-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MH, Mahler SV, Moorman DE, Aston-Jones G, 2016. A Decade of Orexin/Hypocretin and Addiction: Where Are We Now?, in: Lawrence AJ, de Lecea L (Eds.), Behavioral Neuroscience of Orexin/Hypocretin, Current Topics in Behavioral Neurosciences. Springer International Publishing, Cham, pp. 247–281. 10.1007/7854_2016_57 [DOI] [Google Scholar]
- Kawabe K, Horiuchi F, Ochi M, Nishimoto K, Ueno S, Oka Y, 2017. Suvorexant for the Treatment of Insomnia in Adolescents. Journal of Child and Adolescent Psychopharmacology 27, 792–795. 10.1089/cap.2016.0206 [DOI] [PubMed] [Google Scholar]
- Kessler BA, Stanley EM, Frederick-Duus D, Fadel J, 2011. Age-related loss of orexin/hypocretin neurons. Neuroscience 178, 82–88. 10.1016/j.neuroscience.2011.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilduff TS, Peyron C, 2000. The hypocretin/orexin ligand-receptor system: implications for sleep and sleep disorders. Trends Neurosci. 23, 359–365. 10.1016/s0166-2236(00)01594-0 [DOI] [PubMed] [Google Scholar]
- LeSage MG, Perry JL, Kotz CM, Shelley D, Corrigall WA, 2010. Nicotine self-administration in the rat: effects of hypocretin antagonists and changes in hypocretin mRNA. Psychopharmacology (Berl.) 209, 203–212. 10.1007/s00213-010-1792-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luikinga SJ, Kim JH, Perry CJ, 2018. Developmental perspectives on methamphetamine abuse: Exploring adolescent vulnerabilities on brain and behavior. Progress in Neuro-Psychopharmacology and Biological Psychiatry 87, 78–84. 10.1016/j.pnpbp.2017.11.010 [DOI] [PubMed] [Google Scholar]
- Mahler SV, Moorman DE, Smith RJ, James MH, Aston-Jones G, 2014. Motivational activation: a unifying hypothesis of orexin/hypocretin function. Nat. Neurosci 17, 1298–1303. 10.1038/nn.3810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Smith RJ, Aston-Jones G, 2013. Interactions between VTA orexin and glutamate in cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl.) 226, 687–698. 10.1007/s00213-012-2681-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann K, Ackermann K, Croissant B, Mundle G, Nakovics H, Diehl A, 2005. Neuroimaging of gender differences in alcohol dependence: are women more vulnerable? Alcohol. Clin. Exp. Res 29, 896–901. 10.1097/01.alc.0000164376.69978.6b [DOI] [PubMed] [Google Scholar]
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK, 2001. Differential expression of orexin receptors 1 and 2 in the rat brain. J. Comp. Neurol 435, 6–25. 10.1002/cne.1190 [DOI] [PubMed] [Google Scholar]
- Martin-Fardon R, Weiss F, 2014a. Blockade of hypocretin receptor-1 preferentially prevents cocaine seeking: comparison with natural reward seeking. Neuroreport 25, 485–488. 10.1097/WNR.0000000000000120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Fardon R, Weiss F, 2014b. N-(2-methyl-6-benzoxazolyl)-N’−1,5-naphthyridin-4-yl urea (SB334867), a hypocretin receptor-1 antagonist, preferentially prevents ethanol seeking: comparison with natural reward seeking. Addict Biol 19, 233–236. 10.1111/j.1369-1600.2012.00480.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCambridge J, Strang J, 2005. Age of first use and ongoing patterns of legal and illegal drug use in a sample of young Londoners. Subst Use Misuse 40, 313–319. 10.1081/ja-200049333 [DOI] [PubMed] [Google Scholar]
- Moorman DE, Aston-Jones G, 2009. Orexin-1 receptor antagonism decreases ethanol consumption and preference selectively in high-ethanol--preferring Sprague--Dawley rats. Alcohol 43, 379–386. 10.1016/j.alcohol.2009.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman DE, James MH, Kilroy EA, Aston-Jones G, 2017. Orexin/hypocretin-1 receptor antagonism reduces ethanol self-administration and reinstatement selectively in highly-motivated rats. Brain Res. 1654, 34–42. 10.1016/j.brainres.2016.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Academies Press, 2011. Guide for the Care and Use of Laboratory Animals, 8th ed, The National Academies Collection: Reports funded by National Institutes of Health. National Academies Press (US), Washington (DC). [Google Scholar]
- Perrey DA, Zhang Y, 2020. Therapeutics development for addiction: Orexin-1 receptor antagonists. Brain Research 1731, 145922. 10.1016/j.brainres.2018.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaza-Zabala A, Martín-García E, de Lecea L, Maldonado R, Berrendero F, 2010. Hypocretins regulate the anxiogenic-like effects of nicotine and induce reinstatement of nicotine-seeking behavior. J. Neurosci 30, 2300–2310. 10.1523/JNEUROSCI.5724-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porkka-Heiskanen T, Kalinchuk A, Alanko L, Huhtaniemi I, Stenberg D, 2004. Orexin A and B levels in the hypothalamus of female rats: the effects of the estrous cycle and age. Eur. J. Endocrinol 150, 737–742. 10.1530/eje.0.1500737 [DOI] [PubMed] [Google Scholar]
- Porter-Stransky KA, Bentzley BS, Aston-Jones G, 2017. Individual differences in orexin-I receptor modulation of motivation for the opioid remifentanil. Addict Biol 22, 303–317. 10.1111/adb.12323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Niño AM, D’Souza MS, Markou A, 2013. N-acetylcysteine decreased nicotine self-administration and cue-induced reinstatement of nicotine seeking in rats: comparison with the effects of N-acetylcysteine on food responding and food seeking. Psychopharmacology (Berl) 225, 473–482. 10.1007/s00213-012-2837-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall CL, Roberts JS, Del Boca FK, Carroll KM, Connors GJ, Mattson ME, 1999. Telescoping of landmark events associated with drinking: a gender comparison. J. Stud. Alcohol 60, 252–260. 10.15288/jsa.1999.60.252 [DOI] [PubMed] [Google Scholar]
- Rasmussen K, White DA, Acri JB, 2019. NIDA’s medication development priorities in response to the Opioid Crisis: ten most wanted. Neuropsychopharmacology 44, 657–659. 10.1038/s41386-018-0292-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawson RA, Gonzales R, Mccann M, Ling W, 2007. Use of methamphetamine by young people: is there reason for concern? Addiction 102, 1021–1022. 10.1111/j.1360-0443.2007.01899.x [DOI] [PubMed] [Google Scholar]
- Reichel CM, Chan CH, Ghee SM, See RE, 2012. Sex differences in escalation of methamphetamine self-administration: cognitive and motivational consequences in rats. Psychopharmacology (Berl) 223, 371–380. 10.1007/s00213-012-2727-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts CL, Marx MH, Collier G, 1958. Light onset and light offset as reinforcers for the albino rat. J Comp Physiol Psychol 51, 575–579. 10.1037/h0042974 [DOI] [PubMed] [Google Scholar]
- Sakurai T, 2007. The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat. Rev. Neurosci 8, 171–181. 10.1038/nrn2092 [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M, 1998. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92, 573–585. 10.1016/s0092-8674(00)80949-6 [DOI] [PubMed] [Google Scholar]
- Sawai N, Ueta Y, Nakazato M, Ozawa H, 2010. Developmental and aging change of orexin-A and -B immunoreactive neurons in the male rat hypothalamus. Neurosci. Lett 468, 51–55. 10.1016/j.neulet.2009.10.061 [DOI] [PubMed] [Google Scholar]
- Schmeichel BE, Barbier E, Misra KK, Contet C, Schlosburg JE, Grigoriadis D, Williams JP, Karlsson C, Pitcairn C, Heilig M, Koob GF, Vendruscolo LF, 2015. Hypocretin Receptor 2 Antagonism Dose-Dependently Reduces Escalated Heroin Self-Administration in Rats. Neuropsychopharmacol 40, 1123–1129. 10.1038/npp.2014.293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth P, Scholl L, Rudd RA, Bacon S, 2018. Overdose Deaths Involving Opioids, Cocaine, and Psychostimulants - United States, 2015–2016. MMWR Morb. Mortal. Wkly. Rep 67, 349–358. 10.15585/mmwr.mm6712a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng Z, Santiago AM, Thomas MP, Routh VH, 2014. Metabolic regulation of lateral hypothalamic glucose-inhibited orexin neurons may influence midbrain reward neurocircuitry. Mol. Cell. Neurosci 62, 30–41. 10.1016/j.mcn.2014.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siefried KJ, Acheson LS, Lintzeris N, Ezard N, 2020. Pharmacological Treatment of Methamphetamine/Amphetamine Dependence: A Systematic Review. CNS Drugs 34, 337–365. 10.1007/s40263-020-00711-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveyra P, Cataldi NI, Lux-Lantos VA, Libertun C, 2010. Role of orexins in the hypothalamic-pituitary-ovarian relationships. Acta Physiol (Oxf) 198, 355–360. 10.1111/j.1748-1716.2009.02049.x [DOI] [PubMed] [Google Scholar]
- Simmons SJ, Gentile TA, 2020. Cocaine abuse and midbrain circuits: Functional anatomy of hypocretin/orexin transmission and therapeutic prospect. Brain Research 1731, 146164. 10.1016/j.brainres.2019.02.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, Aston-Jones G, 2012. Orexin / hypocretin 1 receptor antagonist reduces heroin self-administration and cue-induced heroin seeking. Eur. J. Neurosci 35, 798–804. 10.1111/j.1460-9568.2012.08013.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, See RE, Aston-Jones G, 2009. Orexin/hypocretin signaling at the orexin 1 receptor regulates cue-elicited cocaine-seeking. European Journal of Neuroscience 30, 493–503. 10.1111/j.1460-9568.2009.06844.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, Tahsili-Fahadan P, Aston-Jones G, 2010. Orexin/hypocretin is necessary for context-driven cocaine-seeking. Neuropharmacology 58, 179–184. 10.1016/j.neuropharm.2009.06.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP, 2016. Consequences of adolescent use of alcohol and other drugs: Studies using rodent models. Neuroscience & Biobehavioral Reviews 70, 228–243. 10.1016/j.neubiorev.2016.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP, 2015. Adolescent alcohol exposure: Are there separable vulnerable periods within adolescence? Physiol Behav 148, 122–130. 10.1016/j.physbeh.2015.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland JC, Havens JR, Stoops WW, 2019. A nationally representative analysis of “twin epidemics”: Rising rates of methamphetamine use among persons who use opioids. Drug and Alcohol Dependence 204, 107592. 10.1016/j.drugalcdep.2019.107592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchting R, Yoon JH, Miguel GGS, Green CE, Weaver MF, Vincent JN, Fries GR, Schmitz JM, Lane SD, 2020. Preliminary examination of the orexin system on relapse-related factors in cocaine use disorder. Brain Research 1731, 146359. 10.1016/j.brainres.2019.146359 [DOI] [PubMed] [Google Scholar]
- Terao A, Apte-Deshpande A, Morairty S, Freund YR, Kilduff TS, 2002. Age-related decline in hypocretin (orexin) receptor 2 messenger RNA levels in the mouse brain. Neurosci. Lett 332, 190–194. 10.1016/s0304-3940(02)00953-9 [DOI] [PubMed] [Google Scholar]
- Lancet The, 2018. Opioids and methamphetamine: a tale of two crises. The Lancet 391, 713. 10.1016/S0140-6736(18)30319-2 [DOI] [PubMed] [Google Scholar]
- US Department of Justice, 2017. 2017 National Drug Threat Assessment [WWW Document]. URL https://www.dea.gov/documents/2017/10/01/2017-national-drug-threat-assessment (accessed 4.27.20).
- Uslaner JM, Winrow CJ, Gotter AL, Roecker AJ, Coleman PJ, Hutson PH, Le AD, Renger JJ, 2014. Selective orexin 2 receptor antagonism blocks cue-induced reinstatement, but not nicotine self-administration or nicotine-induced reinstatement. Behav. Brain Res 269, 61–65. 10.1016/j.bbr.2014.04.012 [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Volkow ND, 2020. Drugs, sleep, and the addicted brain. Neuropsychopharmacol. 45, 3–5. 10.1038/s41386-019-0465-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Pol AN, Patrylo PR, Ghosh PK, Gao XB, 2001. Lateral hypothalamus: early developmental expression and response to hypocretin (orexin). J. Comp. Neurol 433, 349–363. 10.1002/cne.1144 [DOI] [PubMed] [Google Scholar]
- Volkow ND, 2020. Personalizing the Treatment of Substance Use Disorders. AJP 177, 113–116. 10.1176/appi.ajp.2019.19121284 [DOI] [PubMed] [Google Scholar]
- Westbrook SR, Dwyer MR, Cortes LR, Gulley JM, 2020. Extended access self-administration of methamphetamine is associated with age- and sex-dependent differences in drug taking behavior and recognition memory in rats. Behav Brain Res 390, 112659. 10.1016/j.bbr.2020.112659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westbrook SR, Gulley JM, 2020. Effects of the GluN2B antagonist, Ro 25–6981, on extinction consolidation following adolescent- or adult-onset methamphetamine self-administration in male and female rats. Behav Pharmacol 31, 748–758. 10.1097/FBP.0000000000000586 [DOI] [PubMed] [Google Scholar]
- Willie JT, Chemelli RM, Sinton CM, Tokita S, Williams SC, Kisanuki YY, Marcus JN, Lee C, Elmquist JK, Kohlmeier KA, Leonard CS, Richardson JA, Hammer RE, Yanagisawa M, 2003. Distinct narcolepsy syndromes in Orexin receptor-2 and Orexin null mice: molecular genetic dissection of Non-REM and REM sleep regulatory processes. Neuron 38, 715–730. 10.1016/s0896-6273(03)00330-1 [DOI] [PubMed] [Google Scholar]
- Winrow CJ, Tanis KQ, Reiss DR, Rigby AM, Uslaner JM, Uebele VN, Doran SM, Fox SV, Garson SL, Gotter AL, Levine DM, Roecker AJ, Coleman PJ, Koblan KS, Renger JJ, 2010. Orexin receptor antagonism prevents transcriptional and behavioral plasticity resulting from stimulant exposure. Neuropharmacology 58, 185–194. 10.1016/j.neuropharm.2009.07.008 [DOI] [PubMed] [Google Scholar]
- World Drug Report, 2019. World Drug Report 2019.
- Zhou L, Ghee SM, Chan C, Lin L, Cameron MD, Kenny PJ, See RE, 2012. Orexin-1 receptor mediation of cocaine seeking in male and female rats. J. Pharmacol. Exp. Ther 340, 801–809. 10.1124/jpet.111.187567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlebnik NE, Anker JJ, Carroll ME, 2012. Exercise to reduce the escalation of cocaine self-administration in adolescent and adult rats. Psychopharmacology 224, 387–400. 10.1007/s00213-012-2760-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlebnik NE, Anker JJ, Gliddon LA, Carroll ME, 2010. Reduction of extinction and reinstatement of cocaine seeking by wheel running in female rats. Psychopharmacology 209, 113–125. 10.1007/s00213-010-1776-0 [DOI] [PMC free article] [PubMed] [Google Scholar]


