Abstract
Background:
Electronic nicotine delivery systems (ENDS) differ from combustible cigarettes given that nicotine-salt or nicotine-freebase may be used depending on the product. We have investigated how nicotine-salt and freebase formulations alter e-Vape® self-administration (EVSA) behavior and plasma cotinine levels in male and female mice.
Methods:
Adult C57/BL6J mice were used in EVSA and assigned vaping e-liquids (50:50 PGVG, 6 mg/mL nicotine-freebase, or 6 mg/mL nicotine-salt). Mice were escalated on a fixed ratio 1 (FR1) schedule in daily 2 hr sessions and then transitioned to a FR3 to examine reinforcement-related behaviors.
Results:
Here we observed that mice assigned nicotine-salt exhibited increased EVSA on a FR3 schedule compared to nicotine-freebase. Additionally, mice assigned nicotine-salt exhibited higher plasma cotinine concentrations following delivery-controlled passive-inhalation sessions.
Conclusions:
These data provide evidence nicotine-salt formulations may contribute to greater reinforcement-related behavior and highlight the need for further investigations regarding nicotine formulation in ENDS.
Keywords: Animal models, nicotine formulations, self-administration, reinforcement
1. INTRODUCTION
Since the introduction of ENDS in 2003 [1], their use has grown among life-long cigarette smokers as a safer alternative to smoking, as well as teens naïve to smoking. Ongoing research has demonstrated that ENDS have fewer toxic chemicals than combustible cigarettes [2], but there are numerous novel chemicals and constituents in ENDS that need to be evaluated. Thus far, some ENDS-specific chemical constituents have been identified to include heavy metals, carcinogenic chemicals, chemical flavorants, and chemical odorants that have been shown to cause various levels of toxicity in cell lines [3–7]. Despite this, it should be noted that many of these studies are ongoing and there is a great need to evaluate these chemicals at levels relevant to human vaping. Vaping, similar to smoking, presents a distinct pharmacokinetic profile due to the rapid delivery of inhaled chemicals to the brain [8]. ENDS products are different from combustible cigarettes as some products utilize nicotine-salts instead of nicotine-freebase. The increasingly popular Juul devices are the most prominent ENDS product to use nicotine-salts and their success could be due to the cigarette-like pulmonary delivery of nicotine due to the use of salt formulations [9]. There are few investigations into the impact that nicotine formulations have on reinforcement-related behaviors [9, 10]. To fill this gap, we have utilized a e-Vape® self-administration (EVSA) paradigm to model human-related vaping behaviors. Many products that utilize nicotine-salts contain higher concentrations of nicotine (JUUL, ~60 mg/mL). To avoid nicotine concentration as an additional variable, we selected a nicotine dose of 6 mg/mL as this is both amenable to mouse EVSA assays [11] but is also a dose that is popular among humans that use tank-based ENDS [12]. Accordingly, we utilized e-liquids made in-lab to contain 6 mg/mL nicotine using either freebase or salt formulations. We then evaluated male and female adult mice for EVSA on a fixed-ratio 3 (FR3) schedule following a previously described protocol [11]. Here we observed that in both males and females, mice that were assigned nicotine-salt exhibited significantly more aerosol deliveries than mice assigned nicotine-freebase. Additionally, plasma cotinine analysis revealed that mice assigned nicotine-salt exhibited a significantly higher plasma value for cotinine following passive E-Vape® sessions. These data highlight that nicotine formulations may facilitate different levels of reinforcement related behaviors and one reason may be due to distinct pharmacokinetics.
2. MATERIAL AND METHODS
2.1. Mice —
All experiments were conducted in accordance with the guidelines for care and use of animals provided by the National Institutes of Health. Protocols were approved by the Institutional Animal Care and Use Committee at Marshall University. Adult (3 months old) male and female C57BL/6J mice were obtained in-house from breeding colonies (wildtype littermates of a6-GFP mice). Mice were group-housed on a standard 12/12 hr light/dark cycle and allowed food and water ad libitum. For self-administration assays, mice were singly housed and all experiments were conducted during the light cycle.
2.2. Drugs —
(−)-nicotine (N2472–100ML, lot#: 2AH0278) was obtained from Spectrum. Nicotine-salt (ditartrate dihydrate) was obtained from Acros Organics (AC415660500). (−)-menthol was obtained from Alfa Aesar (A10474). Both nicotine formulations were mixed with propylene glycol and vegetable glycerin (PGVG) at a 50:50 ratio at a final concentration of 6 mg/mL. This dose was selected based on our previous investigation [11]. For both formulations, nicotine content was based upon molecular weight of freebase to ensure equal amounts of nicotine were present.
2.3. Self-administration —
EVSA was conducted in four air-tight chambers with interior dimensions of 21 cm L × 19 cm W × 12.5 cm H La Jolla Alcohol Research, Inc. (LJARI), La Jolla, CA, USA) [11]. Two nosepoke holes with cue lights were mounted above the floor on the back-side walls of the chamber. Airflow was vacuum controlled by an electric pump that allowed air flow at 1 L/min. SMOK® baby beast TFV8 X-baby Q2 atomizer tank (0.40 ohms dual coil; Shenzhen IVPS Technology Co., Ltd., Shenzhen, China) were activated by a custom e-cigarette mod box (LJARI, La Jolla, CA, USA). Vapor delivery settings were controlled by an e-Vape® custom controller at 400 oF and 65 W (LJARI, La Jolla, CA, USA).
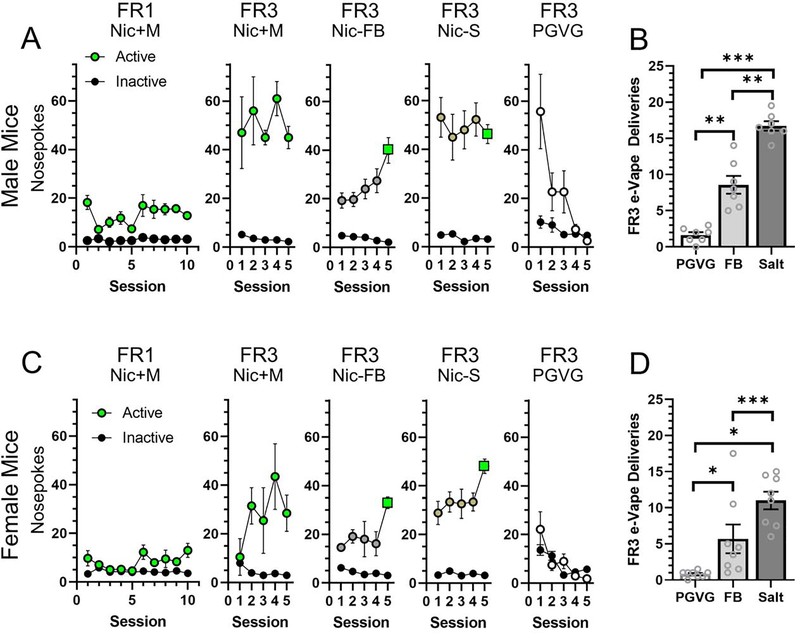
Adult (3 months old), male (n = 14) and female (n = 14) mice began EVSA on a fixed-ratio (FR1) schedule on a Monday for 10 daily, 2 hr sessions, with a weekend break. Mice were singly housed in operant chambers [11]. Nose-pokes in the active hole of the operant chamber resulted in a 3 s delivery of vaporized e-liquids through the vapor entrance port. Inactive nose-pokes were recorded with no consequences. Following a nose-poke and 3 s vapor delivery, a 30 s timeout period was initiated and signaled by a cue light in the nose-poke hole. Mice were trained on nicotine (6 mg/mL freebase) with 15 mg/mL menthol as this provides the most robust and consistent acquisition of self-administration behaviors [11]. Using menthol-nicotine e-liquids, 50–60% of mice acquire self-administration behavior based on the criteria of active:inactive responding ≥2 (average responses on last 2 days). This is an improvement over training with nicotine-alone (6 mg/mL freebase) as this results in a ~28% success rate (unpublished data). For this cohort, 7 males and 8 females escalated on the FR1 schedule (the remainder were excluded from further behavioral assays). Following the 10-day FR1 protocol, mice continued on the same e-liquid but moved to a FR3 schedule for five days. After this training period, all vaping-related exposure for mice involved menthol-free e-liquids with the exception of the day they were re-baselined (Figure 1). Mice were randomly assigned to 6 mg/mL nicotine-freebase or 6 mg/mL nicotine-salt using a within-subjects design. Mice were maintained on these e-liquids for 4 days to establish stable FR3 responding with their respective assignments. On day 5, mice were re-baselined with their training e-liquid (nicotine + menthol). After both nicotine formulations, mice were assigned PGVG. Thus, all mice were used in all conditions. The mean of the final twosessionswereused to compare reinforcement-related behavior between e-liquid assignments.
Figure 1.
Daily FR1 and FR3 EVSA nosepokes for male (A) and female (C) mice. Symbol colors indicate e-liquid assignment (green, nicotine + menthol; grey, nicotine-freebase (FB); bronze, nicotine-salt; white, PGVG (vehicle control); and black, inactive nosepokes). Green squares indicate sessions where mice were re-baselined to nicotine + menthol. Mean FR3 EVSA deliveries for male (B) and female (D) mice that were assigned PGVG (vehicle), nicotine- FB, or nicotine-salt. *, p<0.05; **, p<0.01; and ***, p<0.001. All data are Mean ± SEM.
2.4. Plasma Cotinine Assays —
Examining the plasma cotinine values in mice assigned to self-administration assays would vary greatly depending on the number of earned deliveries per mouse, and would complicate any observations into the impact that formulation exerts on plasma cotinine concentration. To overcome this issue, a separate group of mice (n = 13 males and 13 females) naïve to e-Vape® assays were assigned to PGVG, 6 mg/mL nicotine-freebase, and 6 mg/mL nicotine-salt treatment groups and exposed to 15 non-contingent e-Vape® deliveries (3 s vapor delivery every 8 min for 2 h). We used a plasma cotinine enzyme-linked immunosorbent assay (ELISA) kit (Origene, EA100902) to examine concentrations after vapor inhalation sessions. Mice were then immediately removed from chambers and were anesthetized with CO2 and plasma was drawn via cardiac puncture and placed on ice. Immediately after, plasma was separated via centrifugation (1.5 rpm for 15 minutes at 4°C). We followed the established Origene cotinine ELISA kit protocol to assay plasma cotinine levels. Measurements were taken using a Flexstation III (Molecular Devices).
2.5. Statistical Analysis —
All results are presented as mean ± SEM and all statistical analyses were performed using GraphPad Prism 9. Data were analyzed using a Two-Way Repeated Measures ANOVA and a post hoc Tukey was used for multiple comparisons.
3. Results
Using previously established protocols [11], we trained male and female mice to acquire EVSA behaviors on a FR1 schedule (Figure 1). Mice were randomly assigned to either 6 mg/mL nicotine-freebase or 6 mg/mL nicotine-salt using a within-subjects design. After assignment to both formulations, mice were assigned to PGVG. Using a two-way ANOVA we detected a significant main-effect on sex (F(1,39) = 10.9, p<0.01), formulation (F(2,39) = 58.7, p<0.001), but not on interaction (F(2,39) = 2.2, p>0.05)). In male mice, both nicotine formulations produced FR3 deliveries that were significantly different from PGVG (Figure 1A, p<0.01 and 0.001 for nicotine-freebase and nicotine-salt, respectively). In female mice, both formulations exhibited a significant difference compared to PGVG (Figure 1B, p<0.05 and 0.001 for freebase and salt, respectively). In both male and female mice, mice assigned nicotine-salt earned significantly more FR3 deliveries compared to nicotine-freebase (p<0.01 and 0.05, respectively). Comparing the mean earned FR3 deliveries between males and females, we noted a significant difference for mice assigned nicotine-salt (p<0.05).
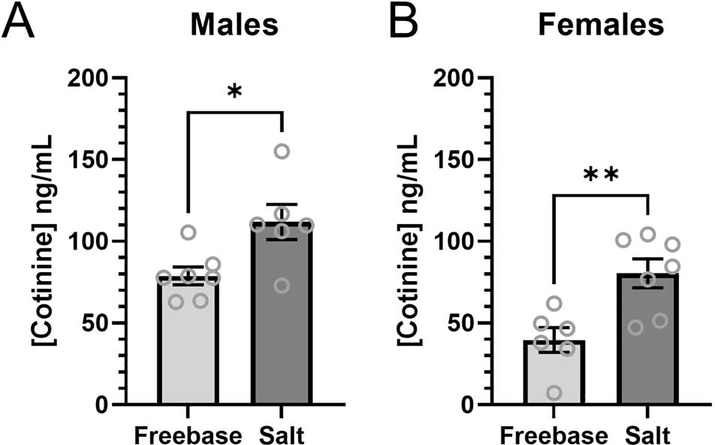
In both male and female mice we observed a significantly higher concentration of plasma cotinine in mice assigned to nicotine-salt when compared to nicotine-freebase (Figure 2, p<0.05 and p<0.01, respectively). Using a two-way ANOVA weobserved a significant main-effect on sex (F(1,22) = 18.3, p<0.001), formulation (F(1,22) = 20.0, p<0.001), but not on interaction (F(1,22) = 0.22, p>0.05)). Additionally, we noted a significant difference between the plasma cotinine values in male versus female mice assigned nicotine-freebase or nicotine-salt (p<0.01 and p<0.05, respectively).
Figure 2.
Plasma cotinine levels of male (A) and female (B) mice that were assigned PGVG, nicotine-FB,or nicotine-salt following a single passive e-Vape® session. Data are mean ± SEM. *, p<0.05; **, p<0.01.
4. Discussion
Our data suggest that nicotine-salt produces greater reinforcement-related behaviors in male and female mice when compared to nicotine-freebase. Additionally, non-contingent exposure to nicotine-salt produced higher plasma cotinine values compared to nicotine-freebase. Previous human studies reported that nicotine-salts in ENDS enables cigarette-like pulmonary delivery of nicotine that reduced the desire to smoke [9]. O’Connell et al., observed that nicotine lactate at all doses was delivered more rapidly into the systemic circulation when compared to nicotine-freebase. While it is difficult to separate the precise mechanisms of subjective measures like ‘desire to smoke’, the pharmacokinetics presented by O’Connell et al. show salt-based formulations facilitate a more-rapid delivery of nicotine with a higher peak and area-under-the-curve. Therefore, our results in mice are in agreement with this prior human study regarding plasma nicotine/cotinine levels. We believe this difference innicotine/cotinine pharmacokinetics may partially explain the observed differences in reinforcement-related behavior. However, we must also consider that nicotine is less rewarding than other addictive drugs (i.e., cocaine) and many equate continual nicotine use to be maintained by circumventing withdrawal-related symptoms [13]. Additionally, there may be other subjective influences between nicotine freebase and salt such as novelty or even pH chemistry [10] that may contribute to the behavior we observed. This highlights the need to continue this investigation into formulation-based effects and determine if withdrawal-related behaviors may differ as well.
While we did not directly measure nicotine levels, cotinine has been a widely used biomarker of nicotine exposure in both humans and animal models for decades [14–16]. One reason for this is the longer half-life of cotinine (~1 hr in mice) [17] compared to nicotine (~7 min in mice) [18]. An additional limitation of this study is the use of a single dose of nicotine (6 mg/ml). At this dose, plasma cotinine values ranged up to ~100 ng/mL (males, nicotine-salt). While this matches the 0–300 ng/mL seen in humans [19] this highlights the need to examine lower nicotine doses to identify the threshold for smoking/vaping-relevant cotinine values. Nicotine has produced inverted-U behavioral responses in both rats and mice in several paradigms [20]. Thus, higher doses need to be examined as well. Examining a full range of nicotine doses in the absence or presence of salt formulations may reveal a formulation-specific effect regarding the sensitivity to nicotine’s rewarding and reinforcing properties. We postulate 0.1 – 60 mg/mL (60 mg/mL nicotine-lactate is present in Juuls) nicotine will need to be examined to observe the threshold for reward/reinforcement and the presence of nicotine’s wellcharacterized aversive properties [21]. As a final note, we also must highlight our cotinine measurements were observed after a single day of nicotine exposure and cannot account for nicotine tolerance that may occur over long-term exposure.
A final limitation to this study is the brief period used for training (10 days) and reinforcement-related behavior (4 days for each e-liquid assignment). We have used this brief period in the process of developing a behavioral paradigm that can be used with adolescent mice. Adolescence in mice is an extremely short period of time (~2 weeks [22]). These assays were designed to be utilized to examine both adult and adolescent vaping-related behavior. We also acknowledge that our protocol uses menthol + nicotine. We specifically chose this combination because menthol enhances nicotine reward [23] and EVSA acquisition rates [11] by triggering the same nAChR-related mechanisms as nicotine [23]. We view this as similar to prior intravenous self-administration assays that utilize stronger reinforcers (food or cocaine) to acquire operant behavior prior to transitioning to contingent nicotine delivery [24]. We admit this does introduce some confounds, as menthol does interact with other Cys-loop ion channels; but this is at concentrations higher than what is vaping-relevant [25–27]. Additionally, menthol may facilitate attenuates nicotine airway irritation through TrpM8-mediated mechanisms [28].
Highlights:
Nicotine salt formulations produce more self-administration in both male and female mice compared to nicotine freebase
Nicotine salt formulations produce higher plasma cotinine concentrations when compared to nicotine freebase in both male and female mice.
ACKNOWLEDGEMENTS
This work was supported by NIDA (DA040047 to PrBJH). Research reported in this publication was supported by NIDA and FDA Center for Tobacco Products (DA046335 to BJH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the Food and Drug administration. This work was also supported by the PhRMA Foundation (Predoctoral fellowship to SYC).
Role of Funding Source:
Our funding body (NIH) did not have any role in the design of this study, collection, analysis, or interpretation of the data. Our funding body did not have any role in writing this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest:
No conflicts declared
References
- 1.Bhatnagar A, Payne TJ, and Robertson RM, Is There A Role for Electronic Cigarettes in Tobacco Cessation? J Am Heart Assoc, 2019. 8(12): p. e012742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Papaefstathiou E, Stylianou M, and Agapiou A, Main and side stream effects of electronic cigarettes. J Environ Manage, 2019. 238: p. 10–17. [DOI] [PubMed] [Google Scholar]
- 3.Hua M, Omaiye EE, Luo W, McWhirter KJ, Pankow JF, and Talbot P, Identification of Cytotoxic Flavor Chemicals in Top-Selling Electronic Cigarette Refill Fluids. Sci Rep, 2019. 9(1): p. 2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koopsamy Naidoo SV, Bester MJ, Arbi S, Venter C, Dhanraj P, and Oberholzer HM, Oral exposure to cadmium and mercury alone and in combination causes damage to the lung tissue of Sprague-Dawley rats. Environ Toxicol Pharmacol, 2019. 69: p. 86–94. [DOI] [PubMed] [Google Scholar]
- 5.Osei AD, Mirbolouk M, Orimoloye OA, Dzaye O, Uddin SMI, Dardari ZA, DeFilippis AP, Bhatnagar A, and Blaha MJ, The association between e-cigarette use and asthma among never combustible cigarette smokers: behavioral risk factor surveillance system (BRFSS) 2016 & 2017. BMC Pulm Med, 2019. 19(1): p. 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Talih S, Salman R, El-Hage R, Karam E, Karaoghlanian N, El-Hellani A, Saliba N, and Shihadeh A, Characteristics and toxicant emissions of JUUL electronic cigarettes. Tob Control, 2019. 28(6): p. 678–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zahedi A,Phandthong R, Chaili A, Leung S, Omaiye E, and Talbot P, Mitochondrial Stress Response in Neural Stem Cells Exposed to Electronic Cigarettes. iScience, 2019. 16: p. 250–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benowitz NL, Pharmacology of nicotine: addiction, smoking-induced disease, and therapeutics. Annu Rev Pharmacol Toxicol, 2009. 49: p. 57–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Connell G, Pritchard JD, Prue C, Thompson J, Verron T, Graff D, and Walele T, A randomised, open-label, cross-over clinical study to evaluate the pharmacokinetic profiles of cigarettes and e-cigarettes with nicotine salt formulations in US adult smokers. Intern Emerg Med, 2019. 14(6): p. 853–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shao XM and Friedman TC, Pod-mod vs. conventional e-cigarettes: nicotine chemistry, pH, and health effects. J Appl Physiol (1985), 2020. 128(4): p. 1056–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooper SY, Akers AT, and Henderson BJ, Flavors enhance nicotine vapor self-administration in male mice. Nicotine Tob Res, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Omaiye EE, McWhirter KJ, Luo W, Tierney PA, Pankow JF, and Talbot P, High concentrations of flavor chemicals are present in electronic cigarette refill fluids. Sci Rep, 2019. 9(1): p. 2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McLaughlin I, Dani JA, and De Biasi M, Nicotine withdrawal. Curr Top Behav Neurosci, 2015. 24: p. 99–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Montanari C, Kelley LK, Kerr TM, Cole M, and Gilpin NW, Nicotine e-cigarette vapor inhalation effects on nicotine & cotinine plasma levels and somatic withdrawal signs in adult male Wistar rats. Psychopharm, 2020. 237(3): p. 613–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Omelchenko N, Roy P, Balcita-Pedicino JJ, Poloyac S, and Sesack SR, Impact of prenatal nicotine on the structure of midbrain dopamine regions in the rat. Brain Struct Funct, 2016. 221(4): p. 1939–53. [DOI] [PubMed] [Google Scholar]
- 16.Petersen DR, Norris KJ, and Thompson JA, A comparative study of the disposition of nicotine and its metabolites in three inbred strains of mice. Drug Metab Dispos, 1984. 12(6): p. 725–31. [PubMed] [Google Scholar]
- 17.Siu EC and Tyndale RF, Characterization and comparison of nicotine and cotinine metabolism in vitro and in vivo in DBA/2 and C57BL/6 mice. Mol Pharmacol, 2007. 71 (3): p. 826–34. [DOI] [PubMed] [Google Scholar]
- 18.Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, Craig CR, Collins AC, Damaj M.l., Donny EC, Gardiner PS, Grady SR, Heberlein U, Leonard SS, Levin ED, Lukas RJ, Markou A, Marks MJ, McCallum SE, Parameswaran N, Perkins KA, Picciotto MR, Quik M, Rose JE, Rothenfluh A, Schafer WR, Stolerman IP, Tyndale RF, Wehner JM, and Zirger JM, Guidelines on nicotine dose selection for in vivo research. Psychopharm, 2007. 190(3): p. 269–319.. [DOI] [PubMed] [Google Scholar]
- 19.Marsot A and Simon N, Nicotine and Cotinine Levels With Electronic Cigarette: A Review. Int J Toxicol, 2016. 35(2): p. 179–85. [DOI] [PubMed] [Google Scholar]
- 20.Torres OV, Tejeda HA, Natividad LA, and O’Dell LE, Enhanced vulnerability to the rewarding effects of nicotine during the adolescent period of development. Pharmacol Biochem Behav, 2008. 90(4): p. 658–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Griede TE, Besson M, Maal-Bared G, Pons S, Maskos U, and van der Kooy D, beta2* nAChRs on VTA dopamine and GABA neurons separately mediate nicotine aversion and reward. Proc Natl Acad Sci USA, 2019. 116(51): p. 25968–25973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laviola G, Macri S, Morley-Fletcher S, and Adriani W, Risk-taking behavior in adolescent mice: psychobiological determinants and early epigenetic influence. Neurosci Biobehav Rev, 2003. 27(1–2): p. 19–31. [DOI] [PubMed] [Google Scholar]
- 23.Henderson BJ, Wall TR, Henley BM, Kim CH, McKinney S, and Lester HA Menthol enhances nicotine reward-related behavior by potentiating nicotine-induced changes in nAChR function, nAChR upregulation, and DA neuron excitability. Neuropsychopharm, 2017. 10.1038/npp.2017.72. [DOI] [PMC free article] [PubMed]
- 24.Goenaga J, Powell GL, Leyrer-Jackson JM, Pina J, Phan S, Prakapenka AV, Koebele SV, Namba MD, McClure EA, Bimonte-Nelson HA, and Gipson CD, N-acetyicysteine yields sex-specific efficacy for cue-induced reinstatement of nicotine seeking. Addict Biol, 2020. 25(1): p. e12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ashoor A, Nordman JC, Veltri D, Yang KH, Shuba Y, Al Kury L, Sadek B, Howarth FC, Shehu A, Kabbani N, and Oz M, Menthol inhibits 5-HT3 receptor-mediated currents. J Pharmacol Exp Ther, 2013. 347(2): p. 398–409. [DOI] [PubMed] [Google Scholar]
- 26.Corvalan NA, Zygadlo JA, and Garcia DA, Stereo-selective activity of menthol on GABA(A) receptor. Chirality, 2009. 21(5): p. 525–30. [DOI] [PubMed] [Google Scholar]
- 27.Hall AC, Turcotte CM, Betts BA, Yeung WY, Agyeman AS, and Burk LA, Modulation of human GABAA and glycine receptor currents by menthol and related monoterpenoids. Eur J Pharmacol, 2004. 506(1): p. 9–16. [DOI] [PubMed] [Google Scholar]
- 28.Fan L, Balakrishna S, Jabba SV, Bonner PE, Taylor SR, Picciotto MR, and Jordt S-E, Menthol decreases oral nicotine aversion in C57BL/6 mice through a TRPM8-dependent mechanism. Tob Control, 2016. 0: p. 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]