Abstract
Objective:
To develop a contemporary profile of infective endocarditis (IE) among a population in six counties of Olmsted, Dodge, Mower, Steele, Waseca, and Freeborn in southern Minnesota between 2014 and 2018.
Patients and Methods:
All possible and definite IE cases (≥18 years) among residents of six counties in southern Minnesota, including Olmsted County, diagnosed between January 1, 2014, and December 31, 2018,were included in this retrospective, population-based investigation using the Expanded Rochester Epidemiology Project (E-REP).
Results:
Overall, 137 IE patients developed incident IE in the six-county region, corresponding to an age-and sex-adjusted incidence rate of 11.9 per 100,000 person-years. Men had significantly higher IE incidence (17.9 vs. 6.8 per 100,000 person-years), and rates increased exponentially with age in both sexes. The median age of incident cases was 68.2 years and 67.9% were males. The percentage of patients with a history of injection drug use was low at 6.7%. Bicuspid aortic valve was the most common (9.6%) native valve predisposing condition. Staphylococcus aureus was identified as the predominant pathogen in the overall group (34.8%) with viridans group streptococci accounting for only 19.3% cases. Central nervous system and musculoskeletal complications were common. The 30-day readmission rate was 27.9% and the 6-month mortality rate was 31.8%.
Conclusions:
To our knowledge, this is the first time that the population-based E-REP has been used to determine an age- and sex-adjusted IE incidence. Older male patients predominated and S. aureus was the most common pathogen. Based on these findings, it is not surprising that IE complications were frequently seen.
Keywords: infective endocarditis, incidence, epidemiology, outcomes, Rochester Epidemiology Project, trends, population-based
INTRODUCTION
Infective endocarditis (IE) is life-threatening and although classified as a cardiac infection, complications are not limited to the heart and can involve any anatomical site. Multiple cardiac valve conditions predispose to IE. Prosthetic valves and previously infected valves are at highest risk of infection1. Injection drug use complicating the opioid epidemic has also been a risk factor associated with the development of IE. Management has traditionally included prolonged (≥4 weeks) intravenous antimicrobial therapy and many patients will require valve surgery for local complications (e.g. perivalvular abscess formation, severe valve deficiency causing heart failure) due to IE. Hospital readmission and mortality are frequently seen.
Our group has serially characterized IE in population-based analyses of all adult IE cases in Olmsted County between 1970 and 2013 using the Rochester Epidemiology Project (REP) 2–4. No significant change was observed in overall IE incidence or six-month mortality, with an incidence ranging from 5 to 9.4 per 100,000 person-years and a six-month mortality of 14% to 29%2–4. Staphylococcus aureus, however, surpassed viridans group streptococci as a leading causative pathogen, and age at diagnosis increased over time. There was also an increase in proportion of IE hospitalizations in patients who inject drugs (PWID) from 3% in 1970–2000, to 10% in 2007–2013. However, due to the small number of PWID-related cases seen in both periods (n=3 and =5, respectively), the conclusion that an actual increase occurred is problematic. With the increase in IE due to the opioid epidemic, continued evaluation for this complication is warranted considering the burden on healthcare, including need for surgical intervention5.
Common criticism regarding use of the REP in analyses of IE has been the limited size of the Olmsted County population available to examine an uncommon infection such as IE, with a small number of annual cases of IE as compared to that of nationwide populations. Another limitation has been that residents of Olmsted County are predominately Caucasian, more highly educated, and wealthier than the US population6. Thus, generalizability of our findings may be limited in areas with different population characteristics.
Therefore, a more contemporary population-based analysis of IE in the Upper Midwest is warranted. To increase the cohort size, the “expanded” REP (E-REP) was used in this investigation to approximately double the size (~300,000 inhabitants) of the study cohort by adding five additional counties in southern Minnesota where electronic health records for >90% of the population were available. We included all adult patients with incident IE diagnoses between January 1, 2014 and December 31, 2018.
METHODS
Using the E-REP resources, all potential adult (≥ 18 years) IE incident cases in six counties between Jan 1, 2014, and December 31, 2018, were reviewed. The Endocarditis Registry of the Division of Infectious Diseases in Mayo Clinic Rochester, Minnesota, and E-REP were used to verify first-time episode cases of possible and definite IE defined by modified Duke criteria7. Detailed data regarding demographics, clinical and microbiological characteristics, outcome, and mortality were collected. REDCap (Research Electronic Data Capture) was used to securely collect individual patient data for research purposes8. Patient follow-up extended until six months after completion of antimicrobial therapy for IE.
Patients with cardiovascular implantable electronic devices (CIED) and two Federal Medical Center residents were excluded from the study. The Mayo Clinic Institutional Review Board reviewed and approved our research protocol.
The REP is a collaboration between health care providers in southern Minnesota and western Wisconsin that links together the medical records of persons living in this region for medical research studies. The REP research infrastructure has been funded by NIH since 19669,10, and has supported over 3,000 peer-reviewed publications on a wide range of research topics. The REP links together the health records of all community members in Olmsted County who have agreed to share their medical records for research (>95% of the population). More recently, the REP has expanded to include 27 counties in southern Minnesota and western Wisconsin (“E-REP”). Six counties (Olmsted, Dodge, Mover, Waseca, Freeborn, and Steele) with greater than 90% resident participation in the E-REP were selected for analysis in the current study. The person per square mile has ranged between 24 to 86, and white alone population has ranged between 80.8% to 96.8% based on Minnesota Health statistics annual summary 201711. By adding five rural counties to Olmsted County, the population investigated was doubled, and also enhanced our ability to evaluate a predominately white rural population, which has characterized other areas in the United States severely impacted by the opioid epidemic.
Crude incidence rates of IE were calculated for women and men by dividing the number of IE cases by the corresponding age- and sex-specific person-years in the population. Because the demographic characteristics in this study setting may not be representative of other populations, adjusted incidence rates were computed using direct standardization against the overall age and sex distribution of the United States white population in 2010. Both the crude and standardized rates are presented per 100,000 person-years and are accompanied by 95% confidence intervals (CI) that assume a Poisson distribution. To test the influence of age and sex on crude incidence rates, a Poisson regression model was fit to the data arranged in single-year age- and sex-specific strata. The number of IE cases was modeled as the response and the corresponding counts of person-years (log-scale) was included as an offset to account for the different strata-specific number at risk. Age was modeled as a continuous variable with nonlinear transformation via 4-knot restricted cubic spline function and with allowance for interaction with sex. A partial effects plot was used to illustrate the modeled incidence rate as a function of age and sex by anti-logging the predicted log-rate values to obtain estimates on the rate scale.
Descriptive statistics were reported as median (interquartile range [IQR]) and percentage (N), as appropriate, for all incident IE cases and separately for those residing in Olmsted County vs. the 5 other southeastern MN counties. This was done to extend our investigations of IE in Olmsted County, which date as far back as 1970, and to compare the epidemiology of IE in Olmsted County, which is more heavily populated than the five other counties combined. Comparisons of baseline characteristics between these groups were performed using the Wilcoxon rank sum test for continuous variables and the Pearson χ2 test for categorical variables. All tests were two-tailed, with a significance level of 0.05. Statistical analyses were done using SAS (version 9.4; SAS Institute Inc., Cary, NC) and the ‘rms’ package (‘Glm’ function) in R (version 3.6.2; R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Between 2014 and 2018, a total of 137 patients aged ≥ 18 years developed incident IE in the six-county region, of whom 67 (48.9%) resided in Olmsted County. Overall, the age- and sex-adjusted annual incidence rate of IE (per 100,000 persons) was 11.9 (95% CI, 9.9 – 13.9), with similar rates in Olmsted County (11.8 [95% CI, 9.0 – 14.7]) compared with the five remaining counties (11.9 [95% CI, 9.1 – 14.7]). IE incidence increased markedly with age in both sexes, and the age-adjusted rate was higher in men (17.9 [95% CI, 14.3 – 21.6]) compared with women (6.8 ([95% CI, 4.7 – 8.8]) (Table 1).
Table 1.
Annual incidence of IE (per 100,000 persons) in 6-county area in SE Minnesota between 2014 and 2018.
| Age categories | Women | Men | Total |
|---|---|---|---|
| No. of pts (crude IR) | |||
| 18–39 years | 2 (0.9) | 9 (4.5) | 11 (2.6) |
| 40–59 years | 7 (3.7) | 24 (13.5) | 31 (8.4) |
| 60–74 years | 19 (16.8) | 29 (28.1) | 48 (22.2) |
| ≥75 years | 16 (24.3) | 31 (68.0) | 47 (42.1) |
| All ages | 44 (7.4) | 93 (17.7) | 139 (12.4) |
| Adjusted IR (95% CI) | |||
| Overall | 6.8 (4.7, 8.8) a | 17.9 (14.3, 21.6)a | 11.9 (9.9, 13..9)b |
Rate is directly standardized to the age distribution of the 2010 U.S. white population
Rate is directly standardized to the age and sex distributions of the 2010 U.S. white population
A multivariable Poisson regression model demonstrated that older age and male sex were strongly associated with IE (both P<0.001; Figure 1. Tests for nonlinearity (on modeled log-rate scale) and interaction were both decisively nonsignificant (P>0.6), implying that rates increased exponentially (on plotted rate scale) with age and that male incidence was consistently higher across all ages. At the median age, for example, the estimated IE incidence rate was more than two-fold higher (incidence ratio [IR]=2.36 [95% CI, 1.42 – 3.92]) in men compared with women. For age, the increase in incidence associated with a 22-year (IQR-based) age increase was more than three-fold in men (IR=3.20 [95% CI, 2.05 – 4.99]) and women (IR=3.75 [95% CI, 1.83 – 7.68]). Patient demographics, comorbid conditions, and IE risk factors among the 137 incident cases are summarized in Table 2. Of note, the median age of the overall group was 68.2 years and 67.9% were males. The percentage of patients with histories of IE and injection drug use was low. When comparing the patient characteristics by setting (Olmsted vs. other counties), there were slight differences in the percentage with mitral valve prolapse and rheumatic heart disease, although incident cases in the two regions were otherwise similar. There were no statistical differences in the prevalence of organ (respiratory, cardiac, renal, hepatic) failure among the two cohorts (data not shown).
Figure 1.
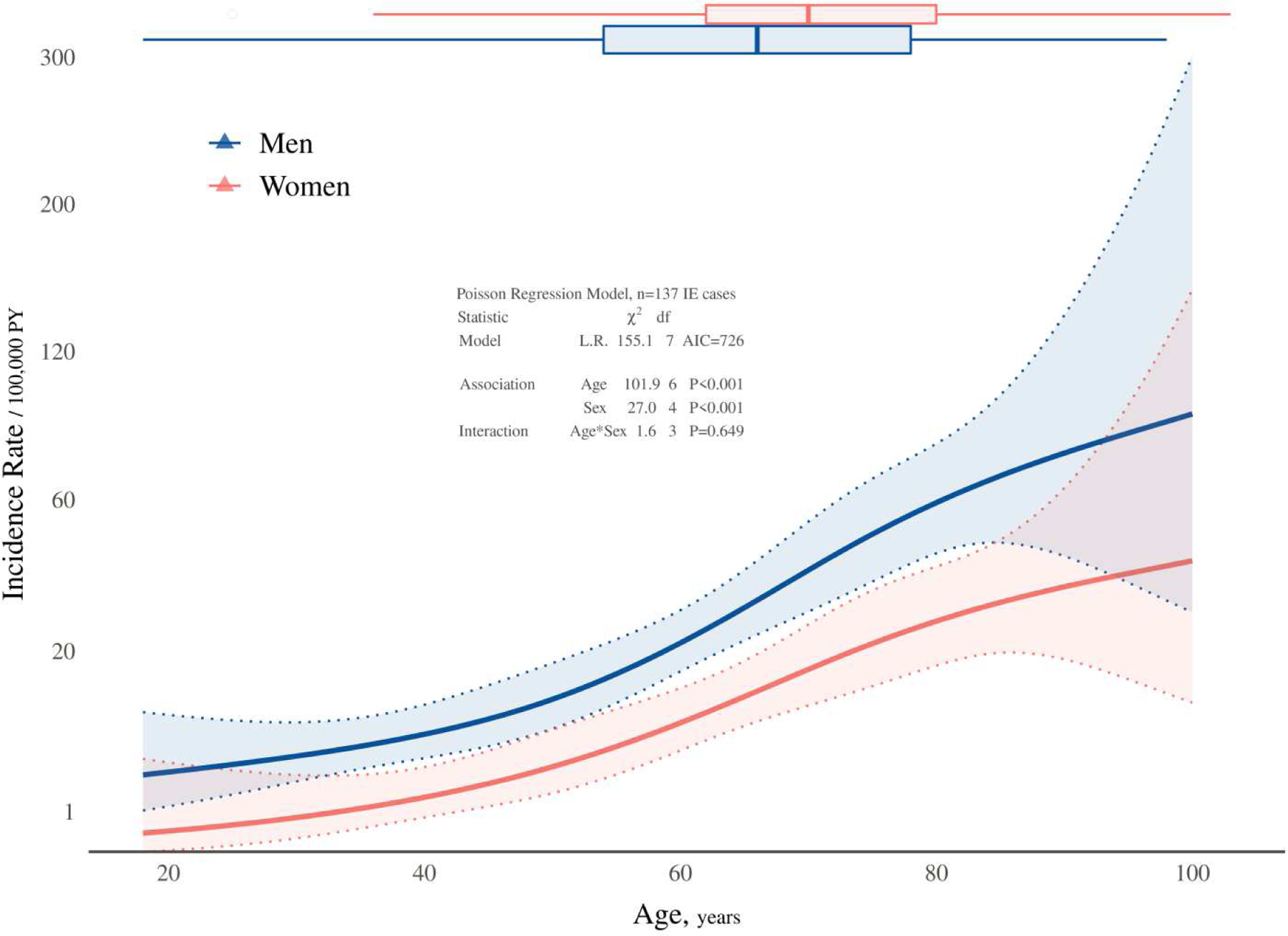
IE incidence according to age and sex. Solid lines represent model-predicted incidence rates of IE as a function of age and sex, and shaded bands represent pointwise 95% confidence limits. Symbols in the plot are observed incidence rates stratified by sex and age categories to provide a crude verification of the model fit. Box plots at the top depict the age distribution of incident cases by sex.
Table 2.
Patient Characteristics and Outcomes of Incident IE Cases According to Population.
| Variable | N | Total 6-County Population (n=137) | Olmsted County (n=67) | Other 5 Counties (n=70) | P-value |
|---|---|---|---|---|---|
| Demographics | |||||
| Age at index hospitalization^ | 137 | 68.2 (58.3, 78.6) | 68.2 (51.9, 78.6) | 68.8 (60.0, 79.2) | 0.36^ |
| Male gender | 137 | 67.9% (93) | 70.1% (47) | 65.7% (46) | 0.58 |
| Comorbid conditions | |||||
| Hemodialysis | 136 | 7.4% (10) | 7.6% (5) | 7.1% (5) | 0.92 |
| Immunocompromising condition | 136 | 13.2% (18) | 12.1% (8) | 14.3% (10) | 0.71 |
| Peripheral vascular disease | 135 | 20.7% (28) | 21.5% (14) | 20.0% (14) | 0.83 |
| Cerebrovascular disease | 135 | 17.0% (23) | 21.5% (14) | 12.9% (9) | 0.18 |
| Prior stroke | 135 | 10.4% (14) | 13.8% (9) | 7.1% (5) | 0.20 |
| Risk factors | |||||
| History of IV drug use | 135 | 6.7% (9) | 9.2% (6) | 4.3% (3) | 0.25 |
| Substance Abuse--Non IV | 136 | 10.3% (14) | 13.6% (9) | 7.1% (5) | 0.21 |
| Mitral Valve Prolapse | 135 | 6.7% (9) | 12.3% (8) | 1.4% (1) | 0.01 |
| Rheumatic Heart Disease | 135 | 3.7% (5) | 7.7% (5) | 0.0% (0) | 0.02 |
| Congenital Heart Disease | 135 | 3.0% (4) | 3.1% (2) | 2.9% (2) | 0.94 |
| Hypertrophic Cardiomyopathy | 135 | 1.5% (2) | 1.5% (1) | 1.4% (1) | >0.99` |
| Bicuspid aortic valve | 135 | 9.6% (13) | 9.2% (6) | 10.0% (7) | 0.88 |
| Indwelling Venous Catheter | 136 | 5.1% (7) | 3.0% (2) | 7.1% (5) | 0.28 |
| Skin & Soft Tissue Infection | 136 | 14.0% (19) | 13.6% (9) | 14.3% (10) | 0.91 |
| Microbiology | |||||
| Organism | 135 | 0.80` | |||
| • Staphylococcus aureus | 34.8% (47) | 37.9% (25) | 31.9% (22) | ||
| • Viridans group streptococci | 19.3% (26) | 19.7% (13) | 18.8% (13) | ||
| • Enterococcus species | 11.9% (16) | 12.1% (8) | 11.6% (8) | ||
| • Coagulase-negative Staph | 7.4% (10) | 4.5% (3) | 10.1% (7) | ||
| • Other streptococci | 8.1% (11) | 7.6% (5) | 8.7% (6) | ||
| • HACEK | 1.5% (2) | 3.0% (2) | 0.0% (0) | ||
| • Gram negatives | 1.5% (2) | 0.0% (0) | 2.9% (2) | ||
| • Culture-negative | 7.4% (10) | 6.1% (4) | 8.7% (6) | ||
| • Streptococcus bovis | 3.0% (4) | 3.0% (2) | 2.9% (2) | ||
| • Other organism | 5.2% (7) | 6.1% (4) | 4.3% (3) | ||
| Time to positivity^ | 98 | 14.0 (11.0, 18.0) | 13.0 (10.0, 16.0) | 14.0 (11.0, 23.0) | 0.15^ |
| Echocardiographic findings | |||||
| Native valve | 135 | 77.0% (104) | 76.9% (50) | 77.1% (54) | 0.98 |
| Prosthetic valve | 135 | 25.9% (35) | 23.1% (15) | 28.6% (20) | 0.47 |
| Both native and prosthetic valve | 137 | 2.9% (4) | 0.0% (0) | 5.7% (4) | 0.05 |
| Definite IE | 137 | 60.6% (83) | 59.7% (40) | 61.4% (43) | 0.84 |
| Cardiac abscess | 135 | 4.4% (6) | 4.6% (3) | 4.3% (3) | 0.93 |
| Mobile vegetation | 135 | 70.4% (95) | 70.8% (46) | 70.0% (49) | 0.92 |
| Vegetation on anterior MV leaflet | 135 | 15.6% (21) | 21.5% (14) | 10.0% (7) | 0.06 |
| Valve fistula | 135 | 0.7% (1) | 1.5% (1) | 0.0% (0) | 0.48` |
| Valve perforation | 135 | 11.9% (16) | 16.9% (11) | 7.1% (5) | 0.08 |
| Perivalvular extension | 135 | 3.7% (5) | 4.6% (3) | 2.9% (2) | 0.59 |
| Leaflet aneurysm | 135 | 0.7% (1) | 0.0% (0) | 1.4% (1) | >0.99` |
| Valve flail | 135 | 5.9% (8) | 7.7% (5) | 4.3% (3) | 0.40 |
| Valve prolapse | 135 | 9.6% (13) | 12.3% (8) | 7.1% (5) | 0.31 |
| Dehiscence | 135 | 0.0% (0) | 0.0% (0) | 0.0% (0) | |
| LAA thrombus | 135 | 0.7% (1) | 0.0% (0) | 1.4% (1) | >0.99` |
| Aortic valve | 137 | 43.1% (59) | 38.8% (26) | 47.1% (33) | 0.32 |
| Mitral valve | 137 | 32.8% (45) | 31.3% (21) | 34.3% (24) | 0.71 |
| Pulmonic valve | 137 | 1.5% (2) | 1.5% (1) | 1.4% (1) | >0.99` |
| Tricuspid valve | 137 | 4.4% (6) | 3.0% (2) | 5.7% (4) | 0.44 |
| Other | 137 | 5.1% (7) | 3.0% (2) | 7.1% (5) | 0.27 |
| None identified | 137 | 26.3% (36) | 28.4% (19) | 24.3% (17) | 0.59 |
| Regurgitation severity | 96 | 0.85 | |||
| • Severe | 22.9% (22) | 25.0% (11) | 21.2% (11) | ||
| • Moderate to severe | 6.3% (6) | 6.8% (3) | 5.8% (3) | ||
| • Moderate | 14.6% (14) | 11.4% (5) | 17.3% (9) | ||
| • Mild to moderate | 10.4% (10) | 6.8% (3) | 13.5% (7) | ||
| • Mild | 10.4% (10) | 13.6% (6) | 7.7% (4) | ||
| • Trivial | 25.0% (24) | 25.0% (11) | 25.0% (13) | ||
| • None | 10.4% (10) | 11.4% (5) | 9.6% (5) | ||
| Ascending aortic aneurysm | 135 | 10.4% (14) | 9.2% (6) | 11.4% (8) | 0.68 |
| Vegetation size | 77 | 0.48 | |||
| • Less than 5 mm | 14.3% (11) | 19.4% (7) | 9.8% (4) | ||
| • Between 5–10 mm | 39.0% (30) | 36.1% (13) | 41.5% (17) | ||
| • Greater than 10 mm | 46.8% (36) | 44.4% (16) | 48.8% (20) | ||
| Extracardiac complications | |||||
| Stroke | 134 | 10.4% (14) | 6.2% (4) | 14.5% (10) | 0.11 |
| CNS emboli | 134 | 31.3% (42) | 29.2% (19) | 33.3% (23) | 0.61 |
| CNS hemorrhage | 134 | 7.5% (10) | 6.2% (4) | 8.7% (6) | 0.58 |
| Lung emboli | 134 | 9.0% (12) | 10.8% (7) | 7.2% (5) | 0.48 |
| Lung abscess | 134 | 2.2% (3) | 3.1% (2) | 1.4% (1) | 0.52 |
| Spleen emboli | 134 | 13.4% (18) | 12.3% (8) | 14.5% (10) | 0.71 |
| Spleen abscess | 134 | 1.5% (2) | 1.5% (1) | 1.4% (1) | >0.99` |
| Liver emboli | 134 | 0.7% (1) | 0.0% (0) | 1.4% (1) | >0.99` |
| Renal emboli | 134 | 3.7% (5) | 3.1% (2) | 4.3% (3) | 0.70 |
| Vertebral osteomyelitis | 134 | 15.7% (21) | 18.5% (12) | 13.0% (9) | 0.39 |
| Epidural abscess | 134 | 3.7% (5) | 4.6% (3) | 2.9% (2) | 0.60 |
| Muscle abscess | 134 | 7.5% (10) | 9.2% (6) | 5.8% (4) | 0.45 |
| Septic joint | 134 | 13.4% (18) | 13.8% (9) | 13.0% (9) | 0.89 |
| Endophthalmitis | 134 | 2.2% (3) | 1.5% (1) | 2.9% (2) | 0.59 |
| Outcomes | |||||
| 6-month IE relapse or recurrence | 135 | 5.9% (8) | 6.2% (4) | 5.7% (4) | 0.91 |
| 30-day readmission from post-discharge | 136 | 27.9% (38) | 24.2% (16) | 31.4% (22) | 0.35 |
| 30-day death | 135 | 16.3% (22) | 15.4% (10) | 17.1% (12) | 0.78 |
| 6-month death | 132 | 31.8% (42) | 27.0% (17) | 36.2% (25) | 0.25 |
N is the number of non-missing observations
The number within the parentheses following the % is the number of patients with the listed variable.
Unless footnoted, variables are summarized as percentages and numbers, with P-values for testing population differences based on the Pearson χ2 test
Values are reported as median (25th, 75th percentiles) and compared between groups with Wilcoxon rank sum test
P-value from Fisher exact test
S. aureus was identified as the most common pathogen in the overall group (34.8%), followed by viridans group streptococci (19.3%) and enterococcal species (11.9%). Coagulase-negative staphylococci were infrequently identified.
The majority of IE cases involved native valves (Table 2). Based on echocardiographic findings and other modified Duke criteria, 60.6% of patients had “definite IE”. Cardiac abscess was seen in 4.4% of cases. Vegetations were identified in over two-thirds of cases.
Of the extracardiac complications observed, central nervous system (CNS) emboli were the most common, occurring in nearly one-third of patients. Splenic emboli were more commonly seen than renal or hepatic emboli in both groups; vertebral osteomyelitis was also common, being diagnosed in 15.7% of the overall group.
Outcomes assessed over 30-day and 6-month follow-up were generally comparable for patients from the two regions (Table 2). For the 135 patients in whom data were available, eight patients (5.9%) had either IE relapse (n=4) or recurrence (n=4), with numbers equally distributed between the 2 settings. Thirty-day readmission rates were common (Table 2) and the difference in 6-month mortality between the two cohorts was not significantly different.
DISCUSSION
This is the first population-based survey describing the profile of IE from southeastern Minnesota that included not only the previously studied population of Olmsted County2–4, but also that of five additional counties. Because there are differences in demographics of the populations in these six counties that include the percent of persons living below the poverty line, non-white race, college education, and rural versus urban designation9, the findings of the current investigation will likely be more applicable to other communities in the United States. Moreover, the larger cohort size by the inclusion of five counties in addition to Olmsted County and the contemporary nature of the cohort enhances the validity of our findings. We anticipate that additional counties in Minnesota and Wisconsin will be included in future evaluations of IE incidence following the expansion of available electronic health records in the E-REP.
The age- and sex-adjusted annual incidence rate of 11.9 per 100,000 patients identified in the current investigation is similar to that reported by Bor et al.12 and Pant et al.13. Both studies are from the United States and used the Nationwide Inpatient Sample (NIS) with considerable overlap regarding the study periods. Keller et al.14 used an national inpatient database in Germany and demonstrated an annual incidence of 14.4 cases per 100,000 citizens in the most recent year (2014) of the study period. In comparison, in our earlier work2–4, the adjusted annual incidence of IE in Olmsted County, MN was lower and ranged from 5.0 to 7.9 per 100,000 citizens, which has been in the range reported from California and New York State15 and the Netherlands16. Studies from France and England found adjusted incidence rates that were either similar to17 or lower than18 that described in the current study with rates in England increasing in recent years19, 20,21.
The prevalence of S. aureus as an IE pathogen deserves additional comment. S. aureus IE mirrors the findings of a prior investigation of Olmsted County cases that included patients seen between 2007 and 20133. Because the total number of IE cases seen annually in Olmsted County has been relatively small, firm conclusions about pathogen distribution were limited. Now with the inclusion of five additional counties in the E-REP evaluation, the number of cases approximately doubled that seen in Olmsted County alone. Factors that were responsible remain undefined; however, we suspect that an aging population with multiple comorbid conditions and increased healthcare exposures combined with expanded indications for an array of cardiovascular devices may explain, in part, the predominance of S. aureus as an IE pathogen. This predominance is noteworthy in a cohort with few PWID.
Readmission within 30 days post-discharge was noteworthy and occurred in 27.9% of the overall E-REP cohort. The prevalence of 30-day readmission has been the subject of recent attention that included use of the Nationwide Readmission Database (NRD).22, 23 The all-cause non-elective readmission rate in the E-REP group was similar to that seen in both (24.8% and 25.4%, respectively) NRD-related studies. Further details were not collected in the E-REP cohort regarding factors related to 30-day readmission or its cost. This outcome metric had not been evaluated in our earlier Olmsted County investigations.
Rates of six-month mortality have been evaluated in the Olmsted County IE populations for decades, and despite the relatively small number of deaths recorded, the rates of mortality have remained similar and have ranged ~25% to 33%2–4, despite major advances in cardiovascular surgery, post-operative care, and medical therapies. It is tempting to speculate that survival benefits related to these advances may have been blunted in mortality calculations due to the increasing prevalence of S. aureus as an IE pathogen in more recent years.
LIMITATIONS
There are potential limitations to our investigation. The work was conducted retrospectively and was dependent on accurate and available electronic health records with proper documentation and diagnostic coding for capture of IE cases. Since IE patients require hospitalization, with rare exception, the likelihood for missing cases should be reduced. There was no protocol employed at our institution in the diagnosis of IE and was left up to the managing physicians, which could result in underreporting of cases. Although the E-REP was used to increase both the cohort size and its diversity as compared to use of the REP that has focused on one county (Olmsted) in the past, the predominance of white people of middle socioeconomic income may not be applicable to all populations.
CONCLUSIONS
This investigation represents the first time the population-based E-REP has been used to determine the age-and sex-adjusted incidence of IE. The older male predominance was striking and S. aureus was most commonly identified among pathogens. Injection drug use was infrequently identified, but this IE risk factor deserves continued surveillance due to the ongoing opioid epidemic. The findings of this work will serve as a valuable benchmark for future comparisons of IE using the E-REP.
ACKNOWLEDGEMENTS
The authors are extremely grateful for the philanthropic support provided by a gift from Eva and Gene Lane (L.M.B.), and a Mayo Named Professorship, the Edward C. Rosenow III, M.D. Professorship in the Art of Medicine (W.R.W), both of which were paramount in our work to advance the science of cardiovascular infections, which has been an ongoing focus of investigation at Mayo Clinic for over 60 years.
Grant support:
This study was made possible using the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health (NIH) under Award Number RO1AG034676. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. In addition, NIH grant support (UL1 TR000135) was available for REDCap use.
Abbreviations and Acronyms:
- CNS
central nervous system
- CIED
cardiovascular implantable electronic device
- E-REP
Expanded-Rochester Epidemiology Project
- IE
infective endocarditis
- IE
infective endocarditis
- NIS
Nationwide Inpatient Sample
- NIH
National Institutes of Health
- NRD
National Readmission Database
- PWID
Persons [Patients] who inject drugs
Footnotes
Financial support/Conflict of interest: LMB—UpToDate, Inc., Boston Scientific; MRS Honoraria/Consulting fee: Medtronic Inc. and Aziyo Biologics, Inc. (All < US$10K), Research Grant: Medtronic. All other authors report no financial support. All authors report no COI.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Thornhill MH, Jones S, Prendergast B, Baddour LM, Chambers JB, Lockhart PB and Dayer MJ. Quantifying infective endocarditis risk in patients with predisposing cardiac conditions. European heart journal 2018;39:586–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Correa de Sa DD, Tleyjeh IM, Anavekar NS, Schultz JC, Thomas JM, Lahr BD, Bachuwar A, Pazdernik M, Steckelberg JM, Wilson WR and Baddour LM. Epidemiological trends of infective endocarditis: a population-based study in Olmsted County, Minnesota. Mayo Clin Proc 2010;85:422–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeSimone DC, Tleyjeh IM, Correa de Sa DD, Anavekar NS, Lahr BD, Sohail MR, Steckelberg JM, Wilson WR and Baddour LM. Temporal trends in infective endocarditis epidemiology from 2007 to 2013 in Olmsted County, MN. Am Heart J 2015;170:830–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tleyjeh IM, Steckelberg JM, Murad HS, Anavekar NS, Ghomrawi HM, Mirzoyev Z, Moustafa SE, Hoskin TL, Mandrekar JN, Wilson WR and Baddour LM. Temporal trends in infective endocarditis: a population-based study in Olmsted County, Minnesota. Jama 2005;293:3022–8. [DOI] [PubMed] [Google Scholar]
- 5.Schranz AJ, Fleischauer A, Chu VH, Wu LT and Rosen DL. Trends in Drug Use-Associated Infective Endocarditis and Heart Valve Surgery, 2007 to 2017: A Study of Statewide Discharge Data. Ann Intern Med 2019;170:31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sauver JL St, Grossardt BR, Leibson CL, Yawn BP, Melton LJ 3rd and Rocca WA. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc 2012;87:151–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG Jr., Ryan T, Bashore T and Corey GR. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000;30:633–8. [DOI] [PubMed] [Google Scholar]
- 8.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N and Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rocca WA, Grossardt BR, Brue SM, Bock-Goodner CM, Chamberlain AM, Wilson PM, Finney Rutten LJ and St Sauver JL. Data Resource Profile: Expansion of the Rochester Epidemiology Project medical records-linkage system (E-REP). Int J Epidemiol 2018;47:368–368j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR and Melton LJ 3rd. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clinic proceedings 2012;87:1202–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Minnesota Center for Health Statistics. Minnesota Health Statistics Annual Summary 2017.
- 12.Bor DH, Woolhandler S, Nardin R, Brusch J and Himmelstein DU. Infective endocarditis in the U.S., 1998–2009: a nationwide study. PLoS One 2013;8:e60033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pant S, Patel NJ, Deshmukh A, Golwala H, Patel N, Badheka A, Hirsch GA and Mehta JL. Trends in infective endocarditis incidence, microbiology, and valve replacement in the United States from 2000 to 2011. J Am Coll Cardiol 2015;65:2070–6. [DOI] [PubMed] [Google Scholar]
- 14.Keller K, von Bardeleben RS, Ostad MA, Hobohm L, Munzel T, Konstantinides S and Lankeit M. Temporal Trends in the Prevalence of Infective Endocarditis in Germany Between 2005 and 2014. Am J Cardiol 2017;119:317–322. [DOI] [PubMed] [Google Scholar]
- 15.Toyoda N, Chikwe J, Itagaki S, Gelijns AC, Adams DH and Egorova NN. Trends in Infective Endocarditis in California and New York State, 1998–2013. Jama 2017;317:1652–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van den Brink FS, Swaans MJ, Hoogendijk MG, Alipour A, Kelder JC, Jaarsma W, Eefting FD, Groenmeijer B, Kupper AJF and Ten Berg JM. Increased incidence of infective endocarditis after the 2009 European Society of Cardiology guideline update: a nationwide study in the Netherlands. Eur Heart J Qual Care Clin Outcomes 2017;3:141–147. [DOI] [PubMed] [Google Scholar]
- 17.Sunder S, Grammatico-Guillon L, Lemaignen A, Lacasse M, Gaborit C, Boutoille D, Tattevin P, Denes E, Guimard T, Dupont M, Fauchier L and Bernard L. Incidence, characteristics, and mortality of infective endocarditis in France in 2011. PLoS One 2019;14:e0223857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duval X, Delahaye F, Alla F, Tattevin P, Obadia JF, Le Moing V, Doco-Lecompte T, Celard M, Poyart C, Strady C, Chirouze C, Bes M, Cambau E, Iung B, Selton-Suty C and Hoen B. Temporal trends in infective endocarditis in the context of prophylaxis guideline modifications: three successive population-based surveys. J Am Coll Cardiol 2012;59:1968–76. [DOI] [PubMed] [Google Scholar]
- 19.Thornhill MH, Dayer MJ, Forde JM, Corey GR, Chu VH, Couper DJ and Lockhart PB. Impact of the NICE guideline recommending cessation of antibiotic prophylaxis for prevention of infective endocarditis: before and after study. Bmj 2011;342:d2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dayer MJ, Jones S, Prendergast B, Baddour LM, Lockhart PB and Thornhill MH. Incidence of infective endocarditis in England, 2000–13: a secular trend, interrupted time-series analysis. Lancet 2015;385:1219–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thornhill MH, Dayer MJ, Nicholl J, Prendergast BD, Lockhart PB and Baddour LM. An alarming rise in incidence of infective endocarditis in England since 2009: why? Lancet 2020;395:1325–1327. [DOI] [PubMed] [Google Scholar]
- 22.Morita Y, Haruna T, Haruna Y, Nakane E, Yamaji Y, Hayashi H, Hanyu M and Inoko M. Thirty-Day Readmission After Infective Endocarditis: Analysis From a Nationwide Readmission Database. J Am Heart Assoc 2019;8:e011598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pasupula DK, Bhat AG, Siddappa Malleshappa SK, Lotfi A, Slawsky M, Buffer S, Pack Q and Saba S. Trends and Predictors of 30-day Readmission Among Patients Hospitalized with Infective Endocarditis in the United States. Cureus 2019;11:e4962. [DOI] [PMC free article] [PubMed] [Google Scholar]


