Abstract
Background
Air pollution and poor ambient air quality are significantly related to multiple health risks. One associated disease is chronic obstructive pulmonary disease (COPD), a preventable disease with several contributing factors and one of the leading causes of morbidity/mortality locally and globally. A potentially high-risk population are traffic enforcers who are constantly exposed to air pollution. In the Philippines, the MMDA has the widest coverage in traffic management. The study determined the risk of COPD among Metro Manila Development Authority (MMDA) traffic enforcers in relation to ambient air quality level, as well as identified other factors that increase the risk of developing COPD.
Methods
Fifty-two MMDA traffic enforcers deployed in PM2.5 air quality sensor areas in Metro Manila from 2016 to 2018 were recruited through stratified sampling. The International Primary Airways Guidelines (IPAG) questionnaire was utilized to measure risk of COPD. Respiratory health and working history were obtained through questionnaires. Department of environment and natural resources provided PM2.5 ambient air quality data which aided in the construction of the Exposure-Month Index. Ordinal logistic regression was used to examine the association of PM2.5 together with the relevant factors and the risk of COPD.
Results
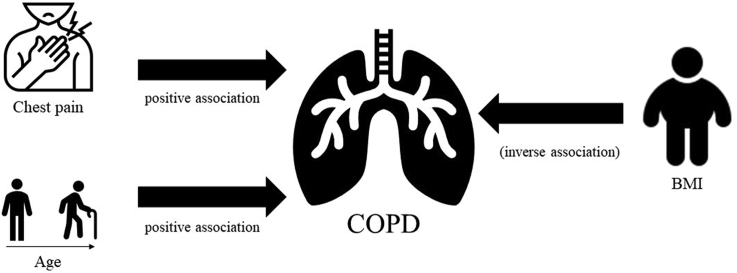
We found statistically significant associations between PM2.5 and COPD among high risk category [odds risk (OR): 1.24, 95% confidence interval (CI): 1.07–1.44]. Age (Moderate, OR: 1.16, 95% CI: 0.98–1.38 and High, OR: 10.06, 95% CI: 4.02–25.17) and chest pain (Moderate, OR: 68.65, 95% CI: 1.71–2.75 × 103) were potential risk factors, whereas body mass index (BMI) (OR: 0.05, 95% CI: 0.01–0.53) exhibited protective effect.
Conclusions
Exposure to PM2.5 was associated with an increased risk of COPD among high-risk category MMDA traffic enforcers. Age and chest pain were potential risk factors to risk of COPD, whereas BMI exhibited a potential protective effect. Results of this study can be used for clinical management of high-risk populations, such that of MMDA traffic enforcers.
Keywords: Chronic obstructive pulmonary disease, Traffic enforcers, Air pollution, Occupational risk, Philippines
Graphical abstract
Introduction
Globally, air pollution has negatively affected air quality and has become a threat to public health. In fact, 4.2 million deaths annually were estimated to be due to ambient air pollution.1 Morbidity and mortality from cardiovascular and respiratory causes, in particular, have been observed to be associated with particulate air pollution.2,3 Among the various criteria air pollutants, particulate matter (PM) is found to be responsible for the majority of associated health risks.2,4 An increasing emphasis was thus placed on PM with a size less than 2.5 microns in diameter (PM2.5) because of its ability to reach distal regions of the lung.5
One of the diseases associated with poor air quality is chronic obstructive pulmonary disease (COPD), which is a preventable yet commonly undiagnosed and life-threatening respiratory condition.6 It is characterized by persistent limitation of airflow and enhanced chronic inflammatory response in the airways to noxious particles or gases.7 Mainstay symptoms of COPD are chronic, progressive dyspnea, cough, sputum, or mucus production—these usually become apparent after 40–50 years of age.8
The primary cause of COPD remains to be exposure to tobacco smoke. However, recent scientific developments revealed the substantial role of PM2.5 on the exacerbation of COPD.8, 9, 10, 11, 12, 13 Other modifiable risk factors include obesity and occupational exposures to organic and inorganic dusts, chemicals, fumes, and irritants. Meanwhile, non-modifiable factors include increasing age, male gender, lung development abnormalities, bronchial hyperreactivity, long-term asthma, childhood respiratory infections, and family history of respiratory diseases.7,14,15
In the Philippines, the Department of Health recognizes COPD as the seventh leading cause of mortality.16 In 2007, an estimated 14% of Metro Manila's population alone reportedly had COPD.17 Several risk factors are present in the country—the most significant of which are air pollution (96%) in urban settings and biomass exposure (87%) in rural settings.18 A significant contributor to air pollution are motor vehicles—this is especially true in Metro Manila where there is a 2.5 million annual average daily traffic.19 In fact, the region's average PM2.5 level (30.44 μg/m3) from 2016 to 2018 lies beyond the recommended annual PM2.5 level (20 μg/m3) by the World Health Organization.20 With considerably high ambient PM2.5 levels, outdoor occupations are mostly hazardous in nature given the prolonged exposure.21
Traffic enforcers had the highest exposure, who were found to have an increased risk of developing respiratory and cardiac diseases.22, 23, 24 In Metro Manila, the Metro Manila Development Authority (MMDA) remains to be the agency with the widest coverage in traffic management.19 On the job, MMDA traffic enforcers work on 8-hour shifts on weekdays. Given the nature and scope of the work, the MMDA may then adequately represent traffic enforcers with a high risk of exposure to high levels air pollution in Metro Manila.
In response to a growing need for evidence linking air quality to health outcomes in the country, specifically among high risk groups, this study explored the association between ambient air quality and risk of COPD among MMDA traffic enforcers in Metro Manila.
Methods
We utilized a cross-sectional study design in elucidating the risk of developing COPD due to ambient air quality. The list of variables with their corresponding units, definitions, type, and sources of information are summarized in Table S1.
Ethical approval
Ethical clearance was obtained from the ASMPH Ethical Research Board with Ethical Approval number: SMPH 2019 Group 02. Permission to conduct the study in the selected MMDA satellites was also coordinated with the main headquarters in Makati City and each MMDA satellite head prior to each visit. Prior to assessment, all participants were briefed and given informed written consent forms (see Supplementary Document 3), detailing the purpose and methods of the study and their rights as study participants. Gathered data were kept confidential throughout the entire study, in compliance with the Data Privacy Act of 2012.
Independent variable
For the independent variable, an Exposure-Month Index (EMI) which is calculated as the average PM2.5 exposure of the participant in the month/s where s/he was deployed. Since traffic enforcers are assigned to different areas in varying time periods, EMI captures the cumulative exposure of the traffic enforcers, which is dependent on the length of time the subject stayed in the location. The EMI was assessed with the aid of a working history form (see Supplementary Document 1) and was computed as follows:
whereby, k represents the work location; PM2.5 represents the PM2.5 concentration in the work location; duration is the length of time the traffic officer was assigned in that location. The summation of the duration weighted PM2.5 is the exposure index () for traffic officer y.
PM 2.5 levels were obtained from air quality ground monitoring data from the department of environment and natural resources-air quality management section (DENR-AQMS) measured via BAM (Thermos, Met 1) and TEOM (Thermos) sensors from 2016 to 2018 (see Table S2 for PM 2.5 Yearly Average).
Dependent variable
For the dependent variable, risk of COPD was measured using the International Primary Airways Guidelines (IPAG) risk score, which was later transformed into a categorical variable with three levels: namely, low, moderate, and high risk. The IPAG Questionnaire, highlighted in Appendix 1 of Sichletidis, Spyratos, Papaioannou et al,25 is the most widely validated screening questionnaire aimed at determining risk of COPD (sensitivity = 64.5%; specificity = 65.2%).26 This tool includes questions regarding respiratory symptoms, smoking history, allergies, age, and anthropometrics. There are a total of 38 points, with a cut-off score of greater than 17 points as high risk of COPD.27 The questionnaire was translated to Tagalog for comprehensibility and incorporated in the Respiratory Health Questionnaire (see Supplementary Document 2).
Study sites
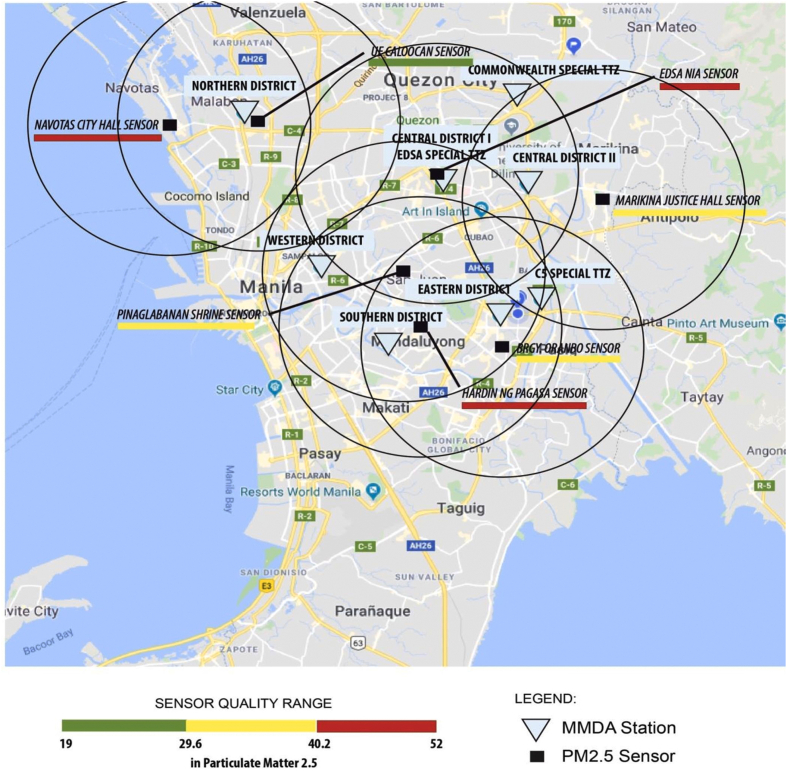
The study was conducted in nine selected MMDA satellite stations with an active PM2.5 air quality sensor installed and monitored by the DENR-AQMS. Fig. 1 illustrates the coverage area of PM2.5 sensors within a 5-kilometer radius and the location of the different MMDA satellites. Three-year PM2.5 levels in these sites were obtained from DENR-AQMS and processed with the assistance of environmental health experts at the ASMPH Center for Research and Innovation (ACRI), Environmental Health unit.
Fig. 1.
Map of Metro Manila showing air quality sensors' area coverage (in circles) and selected MMDA satellites.
Study population
MMDA traffic enforcers satisfying any of the following criteria were excluded from the study population: (1) were not currently assigned to the field, (2) were assigned to the field for more than 6 months in an area that does not have a PM 2.5 air quality sensor, (3) were newly assigned to the field from January 2019 onwards, and (4) previously diagnosed with COPD before January 2016. Study sample consisted of 52 MMDA traffic enforcers (prevalence = 0.883%, power = 80, design effect = 1, confidence interval = 95%) assigned to field jobs for at least 6 months.28
Data collection procedure
Pre-testing of questionnaires was done to three participants in the EDSA-MMDA satellite for the purpose of training the researchers and testing the data collection instruments. During actual data collection, a minimum of three researchers went per study site and requested for available participants who were currently not on duty. All data gathered were transcribed and encrypted to ensure confidentiality and, afterwards, will be permanently deleted once the study has concluded.
Statistical analyses
Data obtained was encoded in Excel version 2016 (Microsoft, USA). We utilized an ordinal logistic regression to assess association of the risk of COPD and PM2.5, after adjusting for several covariates. A bivariable analysis was utilized to examine the association of IPAG variable and the covariates. Covariates which exhibited statistical significance were then included to the full model in the multivariable regression. The full model was then subjected to a stepwise multivariable regression via backward elimination, whereby variables which did not show statistical significance in the full model were dropped one-by-one. The final model favored the inclusion of body mass index (BMI), age, symptoms of chest pain and PM2.5. The subsequent interpretations of the odds risk (OR) for moderate and high categories are relative to the low category, which is set as the base level of comparison in the ordinal logistic regression. All statistical analyses were done using R Statistical Programming (R Development Core Team, USA).29
Results
In Table 1, we observed strong statistically significant association of the IPAG categories with age (High, OR: 1.48, 95% CI: 1.05–2.07), pack years (Moderate, OR:1.14, 95% CI: 0.03–50.94 and High, OR:1.20, 95% CI: 1.05–1.38), chest pain (Moderate, OR: 19.50, 95% CI: 6.97–54.57). Whereas, BMI (High, OR: 0.76, 95% CI: 0.57–1.02), years of service (High, OR: 1.11, 95% CI: 0.98–1.25), past respiratory disease (High, OR: 6.17, 95% CI: 0.78–48.64) and PM2.5 (High, OR: 1.06, 95% CI: 0.99–1.13) exhibited a relatively moderate statistical significance. All statistically significant variables exhibited a positive association with IPAG categories, except for BMI, which indicated a possible negative association.
Table 1.
Bivariable, ordinal logistic regression results of International Primary Airways Guidelines categories with age.
| Variables | Moderate |
High |
||
|---|---|---|---|---|
| OR (95% CI) | P values | OR (95% CI) | P values | |
| BMIa | 0.90 (0.00–915) | 0.36 | 0.76 (0.57–1.02) | 0.07b |
| Agea | 1.09 (0.00–7.5 × 107) | 0.14 | 1.48 (1.05–2.07) | 0.02b |
| Current Smoker | 0.31 (0.09–1.03) | 0.31 | 1.04 (0.16–6.94) | 0.97 |
| Past Smoker | 0.51 (0.07–4.01) | 0.51 | 1.54 (0.12–19.5) | 0.74 |
| Years of Servicea | 1.09 (0.13–9.09) | 0.12 | 1.11 (0.98–1.25) | 0.10b |
| Pack Yearsa | 1.14 (0.03–50.94) | 0.03b | 1.20 (1.05–1.38) | 0.01b |
| Difficulty of Breathing | 1.55 (0.47–5.07) | 0.64 | 2.07 (14.18–0.46) | 0.46 |
| Sneezing | 0.87 (0.26–2.86) | 0.88 | 1.16 (0.17–7.72) | 0.88 |
| Colds | 1.55 (0.55–4.39) | 0.64 | 0.78 (0.08–7.76) | 0.83 |
| Atopy | – | – | 2.31 (0.21–26.05) | 0.50 |
| Cough | 0.83 (0.29–2.33) | 0.87 | 1.03 (0.10–10.53) | 0.98 |
| Chest Paina | 19.50 (6.97–54.57) | 0.01b | 4.88 (0.36–66.41) | 0.23 |
| Shortness of Breath | 0.39 (0.12–1.27) | 0.40 | 1.29 (0.19–8.61) | 0.80 |
| Current Respiratory Disease | 3.90 (1.39–10.91) | 0.30 | 4.87 (0.36–66.41) | 0.23 |
| Past Respiratory Diseasea | 4.62 (1.43–15.00) | 0.13 | 6.17 (0.78–48.64) | 0.08b |
| Symptoms in family | 2.56 (0.35–18.92) | 0.31 | 5.11 (0.52–49.80) | 0.16 |
| Family history of respiratory disease | 1.35 × 104 (4.68 × 103–3.92 × 104) | 0.82 | 0.32 (0.03–3.11) | 0.33 |
| PM2.5a | 1.03 (0.00–2.93 × 103) | 0.32 | 1.06 (0.99–1.13) | 0.10b |
BMI: Body Mass Index; OR: Odds Ratio; 95% CI: 95% Confidence Interval; PM2.5: particulate matter with a size of 2.5 microns or less in diameter.
Reference level of COPD risk is low.
Variables which were included in the full model of the subsequent multivariable, multinomial analysis.
P value ≤ 0.10 indicate statistical significance.
Further multivariable, ordinal logistic regression analysis favored a simpler model with the inclusion of BMI (High, OR: 0.05, 95% CI: 0.01–0.53), age (Moderate, OR: 1.16, 95% CI: 0.98–1.38 and High, OR: 10.06, 95% CI: 4.02–25.17), chest pain (Moderate, OR: 68.65, 95% CI: 1.71–2.75 × 103) and PM2.5 (High, OR: 1.24, 95% CI: 1.07–1.44) as shown in Table 2. While age and chest pain exhibited statistical significance in the high and moderate categories, respectively, results should be interpreted with caution due to the substantially wide confidence intervals. We observe a consistent statistically significant and negative association of BMI with IPAG category.
Table 2.
Multivariate, ordinal logistic regression results of final model.
| Variable | Moderate |
High |
||
|---|---|---|---|---|
| OR (95% CI) | P values | OR (95% CI) | P values | |
| BMI | 0.87 (0.65–1.18) | 0.37 | 0.05 (0.01–0.53) | 0.01a |
| Age | 1.16 (0.98–1.38) | 0.09a | 10.06 (4.02–25.17) | <0.001a |
| Chest Pain | 68.65 (1.71–2.75 × 103) | 0.03a | 0.12 (6.33 × 10−7 – 2.28 × 104) | 0.73 |
| PM2.5 | 1.01 (0.94–1.08) | 0.87 | 1.24 (1.07–1.44) | 0.01a |
BMI: Body Mass Index; OR: Odds Ratio; 95% CI: 95% Confidence Interval; PM2.5: particulate matter with a size of 2.5 microns or less in diameter.
P value ≤ 0.10 indicate statistical significance.
Discussion
In this study, we examined the effects of ambient air pollution on the risk of COPD and observed statistically significant association between PM2.5 exposure and the risk of COPD among MMDA traffic enforcers, after adjusting for relevant covariates. Several risk and protective factors, aside from PM2.5, were also observed.
PM2.5 and COPD
We observed statistically significant association between PM2.5 exposure and the risk of COPD. A unit increase in PM2.5 exposure was associated with a statistically significant OR in high-risk category (OR: 1.24, 95% CI: 1.07–1.44) relative to the low-risk category, in Table 2. Among high risk populations, such as traffic enforcers, studies have indicated a decline in the workers’ lung function due to traffic-generated PM2.5.30 In India, taxi motorcyclists were observed to have substantial risk in developing restrictive syndrome, a form of impairment in the pulmonary function.31 In Zagreb and Croatia, Zuskin et al32 noted the higher risks in developing respiratory symptoms for bus drivers and mechanics. Epidemiological studies have demonstrated an association between short-term ambient PM2.5 exposure and lung function decline. Short-term exposure to ambient air pollutants are said to result in oxidative stress and pulmonary and systemic inflammatory responses such as reduction in the ciliary activity in the airways and acute decrease of pulmonary function.9 Schikowski et al9 argued that if repeated acute exacerbations of COPD were considered a factor in the development of COPD, then the causal role of air pollution in aggravation of COPD is substantial. That being said, the effect of long-term exposure to PM2.5 is slowly but considerably being investigated and has been additionally associated with increased morbidity and mortality of COPD. A recent longitudinal cohort study by Guo et al33 found that PM2.5 was consistently associated with reduction in lung function with respect to four parameters—namely FEV1, FVC, MMEF, and FEV1/FVC ratio—as well as increased risk of COPD development. Prolonged and chronic exposure may potentially induce pulmonary inflammation, decrease lung function, and cause emphysematous changes.34 Likewise, this study supports the reported specificity and sensitivity of IPAG Questionnaire as the most widely validated screening questionnaire for COPD.
COPD risk factors: age and chest pain
Age was positively associated with the risk of COPD in the high-risk category (OR: 10.06, 95% CI: 4.02–25.17) compared to the low-risk category; Table 2. The prevalence and manifestations of COPD increases with age for both men and women throughout most of the lifespan, especially among those above 40–50 years old.35 Since COPD is a progressive lung disease, symptoms worsen over time and often do not appear until there is significant lung damage. Aside from age, we also observed that symptom of chest pain was associated with statistically significant, yet with highly uncertain effects estimates. HajGhanbari et al36 noted that the hyper-expanded and relatively rigid chest wall, which affects the thoracic articulations in a hyper extended position, may contribute to thoracic pain experienced by people with COPD. Whereas, Bordoni et al37 posits the role of costal muscles, which face an alteration of their physiologic length (shortening or lengthening) caused by the change of costal biomechanics, influenced in turn by lung movements. However, due to the relatively uncertain effects estimate of chest pain, further studies are warranted.
COPD protective factor: BMI
Aside from risk factors, we observed statistically significant protective factor of BMI on the risk of COPD. A unit increase in BMI was associated with a decrease in the risk for COPD among high-risk category (OR: 0.05, 95%CI: 0.01–0.53). Galesanu et al38 observed similar findings noting that overweight/obese patients had better lung function and a larger midthigh muscle cross-sectional area obtained using computed tomography (MTCSACT) than those with normal BMI as well as significantly higher peak work rate than patients with normal BMI. A likely mechanism proposed by Divo et al39 posits the role of adipose tissue plays a modulating role in disease expression in the two (normal and obese) groups as the disease begins and progresses. Sun et al,40 on the other hand, postulates a possible reverse-causation, where increased COPD disease activity or severity leads to weight loss and cachexia. We invite the readers to read the systematic review by Sun et al40 for a detailed discussion regarding the inverse association of BMI and COPD.
The study recognizes the following limitations. First, this study only included ambient air quality data from designated PM2.5 sensors of the DENR-AQMS. Indoor air pollution was not quantified. Other gaseous pollutants (e.g. nitric oxide) aside from PM2.5 were not taken into account. Second, the developed Exposure-Month Index incorporates only the three most recent years of the participants' working history. Additionally, with a cross-sectional study design, exposure and outcomes were assessed simultaneously, thereby only capturing a snapshot of the participants’ health. Third, although the IPAG Questionnaire is a widely used screening tool, it does not account other risk factors of COPD. Additional information regarding respiratory health as well as candidate risk factors is encouraged. Fourth, because of the number of associated risk factors, a larger sample size may be more appropriate for future studies. Fifth, this study does not aim to clinically diagnose COPD. The clinical diagnosis of COPD requires a more extensive history taking and physical examination by a certified and experienced medical practitioner. Other subsequent tests may also aid in the accurate diagnosis of COPD, such as a post-bronchodilator spirometry assessment, arterial blood gas, and chest radiograph, all of which our study did not include.
In conclusion, exposure to PM2.5 was associated with an increased risk of COPD among high-risk category MMDA traffic enforcers. While age and symptom of chest pain may potentially pose a risk in developing COPD, caution should be exercised due to substantial uncertainty in the effect estimates. On the other hand, BMI exhibited a potential protective effect on the risk of COPD. Results of this study can be used for clinical management of high-risk populations, such that of MMDA traffic enforcers.
Conflicts of interest
None.
Edited by Yi Cui
Footnotes
Peer review under responsibility of Chinese Medical Association.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cdtm.2021.01.002.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.WHO. Ambient (outdoor) air pollution and health. 2018. https://www.who.int/en/news-room/fact-sheets/detail/ambient-(outdoor)-air-quality-and-health
- 2.Kelly F.J., Fussell J.C. Air pollution and public health: emerging hazards and improved understanding of risk. Environ Geochem Health. 2015;37:631–649. doi: 10.1007/s10653-015-9720-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shah A.S.V., Lee K.K., McAllister D.A. Short term exposure to air pollution and stroke: systematic review and meta-analysis. BMJ. 2015;350:h1295. doi: 10.1136/bmj.h1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Achilleos S., Kioumourtzoglou M.A., Wu C.D., Schwartz J.D., Koutrakis P., Papatheodorou S.I. Acute effects of fine particulate matter constituents on mortality: a systematic review and meta-regression analysis. Environ Int. 2017;109:89–100. doi: 10.1016/j.envint.2017.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dagher Z., Garçon G., Gosset P. Pro-inflammatory effects of Dunkerque city air pollution particulate matter 2.5 in human epithelial lung cells (L132) in culture. J Appl Toxicol. 2005;25:166–175. doi: 10.1002/jat.1050. [DOI] [PubMed] [Google Scholar]
- 6.WHO. Chronic obstructive pulmonary disease (COPD) 2017. https://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd)
- 7.Vestbo J., Hurd S.S., Agustí A.G. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187:347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 8.Ko F.W.S., Hui D.S.C. Air pollution and chronic obstructive pulmonary disease. Respirology. 2012;17:395–401. doi: 10.1111/j.1440-1843.2011.02112.x. [DOI] [PubMed] [Google Scholar]
- 9.Schikowski T., Mills I.C., Anderson H.R. Ambient air pollution: a cause of COPD? Eur Respir J. 2014;43:250–263. doi: 10.1183/09031936.00100112. [DOI] [PubMed] [Google Scholar]
- 10.Huang H.-C., Lin F.C.-F., Wu M.-F. Association between chronic obstructive pulmonary disease and PM2.5 in Taiwanese nonsmokers. Int J Hyg Environ Health. 2019;222:884–888. doi: 10.1016/j.ijheh.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 11.Wen C.P., Gao W. PM2.5: an important cause for chronic obstructive pulmonary disease? Lancet Planet Health. 2018;2:e105–e106. doi: 10.1016/S2542-5196(18)30025-1. [DOI] [PubMed] [Google Scholar]
- 12.Jo Y.S., Lim M.N., Han Y.-J., Kim W.J. Epidemiological study of PM2.5 and risk of COPD-related hospital visits in association with particle constituents in Chuncheon, Korea. Int J Chronic Obstr Pulm Dis. 2018;13:299–307. doi: 10.2147/COPD.S149469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao N., Xu W., Ji J. Lung function and systemic inflammation associated with short-term air pollution exposure in chronic obstructive pulmonary disease patients in Beijing, China. Environ Health. 2020;19:12. doi: 10.1186/s12940-020-0568-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mannino D.M., Buist A.S. Global burden of COPD: risk factors, prevalence, and future trends. Lancet. 2007;370:765–773. doi: 10.1016/S0140-6736(07)61380-4. [DOI] [PubMed] [Google Scholar]
- 15.Poulain M., Doucet M., Major G.C. The effect of obesity on chronic respiratory diseases: pathophysiology and therapeutic strategies. CMAJ (Can Med Assoc J) 2006;174:1293–1299. doi: 10.1503/cmaj.051299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Idolor L.F., De Guia T.S., Francisco N.A. Burden of obstructive lung disease in a rural setting in the Philippines. Respirology. 2011;16:1111–1118. doi: 10.1111/j.1440-1843.2011.02027.x. [DOI] [PubMed] [Google Scholar]
- 17.PCCP . Clinical practice guidelines in the diagnosis and management of chronic obstructive pulmonary disease (COPD) in the Philippines 2009. In: Physicians P.C.o.C., editor. Philippine College of Chest Physicians. 2009. [Google Scholar]
- 18.Regional COPD Working Group COPD prevalence in 12 Asia–Pacific countries and regions: projections based on the COPD prevalence estimation model. Respirology. 2003;8:192–198. doi: 10.1046/j.1440-1843.2003.00460.x. [DOI] [PubMed] [Google Scholar]
- 19.Villas-Alvaren A.L. MMDA faces greatest challenge: managing 2.5M vehicles in MM. Manila Bulletin. 2016 [Google Scholar]
- 20.WHO WHO Air quality guidelines for particulate matter, ozone, nitrogen dioxide and sulfur dioxide: summary of risk assessment (Global Update 2005) 2005. http://apps.who.int/iris/bitstream/handle/10665/69477/WHO_SDE_PHE_OEH_06.02_eng.pdf;jsessionid=BAA40CB3B2D29632CCE8E9227F72246B?sequence=1
- 21.Mills N.L., Miller M.R., Lucking A.J. Combustion-derived nanoparticulate induces the adverse vascular effects of diesel exhaust inhalation. Eur Heart J. 2011;32:2660–2671. doi: 10.1093/eurheartj/ehr195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmed S., Akter Q., Eva H., Bhowmik M. Effect of air pollution on FVC, FEV1 and FEV1/FVC% of the traffic policemen in Dhaka city. J Bangladesh Soc Physiol. 2016;11:39–42. [Google Scholar]
- 23.Patil R.R. SRM University; 2016. Respiratory Health Status of Traffic Police Working in Vehicular Pollution Setting. [Google Scholar]
- 24.Rahman M.A., Badruzzaman A.B.M., Rahman M. Assessing the impact of exposure to polluted air on the pulmonary systems of service personnel using a peak flow meter. Aust J Basic Appl Sci. 2010;4:5533–5549. [Google Scholar]
- 25.Sichletidis L., Spyratos D., Papaioannou M. A combination of the IPAG questionnaire and PiKo-6® flow meter is a valuable screening tool for COPD in the primary care setting. Prim Care Respir J. 2011;20:184–189. doi: 10.4104/pcrj.2011.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haroon S., Jordan R., Takwoingi Y., Adab P. Diagnostic accuracy of screening tests for COPD: a systematic review and meta-analysis. BMJ Open. 2015;5 doi: 10.1136/bmjopen-2015-008133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kotaki K., Ikeda H., Fukuda T. Effectiveness of diagnostic screening tests in mass screening for COPD using a cooperative regional system in a region with heavy air pollution: a cross-sectional study. BMJ Open. 2017;7 doi: 10.1136/bmjopen-2016-012923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.IHME. Global burden of disease. 2019. https://vizhub.healthdata.org/gbd-compare/
- 29.R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2013. R: A Language and Environment for Statistical Computing. [Google Scholar]
- 30.Santos U.P., Garcia M.L.S.B., Braga A.L.F. Association between traffic air pollution and reduced forced vital capacity: a study using personal monitors for outdoor workers. PloS One. 2016;11 doi: 10.1371/journal.pone.0163225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mbelambela E.P., Hirota R., Eitoku M. Occupation exposed to road-traffic emissions and respiratory health among Congolese transit workers, particularly bus conductors, in Kinshasa: a cross-sectional study. Environ Health Prev Med. 2017;22:11. doi: 10.1186/s12199-017-0608-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zuskin E., Mustajbegovic J., Schachter E.N. Respiratory symptoms and lung function in bus drivers and mechanics. Am J Ind Med. 1994;26:771–783. doi: 10.1002/ajim.4700260606. [DOI] [PubMed] [Google Scholar]
- 33.Guo C., Zhang Z., Lau A.K.H. Effect of long-term exposure to fine particulate matter on lung function decline and risk of chronic obstructive pulmonary disease in Taiwan: a longitudinal, cohort study. Lancet Planet Health. 2018;2:e114–e125. doi: 10.1016/S2542-5196(18)30028-7. [DOI] [PubMed] [Google Scholar]
- 34.Zhao J., Li M., Wang Z. Role of PM2.5 in the development and progression of COPD and its mechanisms. Respir Res. 2019;20:120. doi: 10.1186/s12931-019-1081-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buist A.S., Vollmer W.M., McBurnie M.A. Worldwide burden of COPD in high- and low-income countries. Part I. The burden of obstructive lung disease (BOLD) initiative. Int J Tubercul Lung Dis. 2008;12:703–708. [PubMed] [Google Scholar]
- 36.HajGhanbari B., Holsti L., Road J.D., Darlene Reid W. Pain in people with chronic obstructive pulmonary disease (COPD) Respir Med. 2012;106:998–1005. doi: 10.1016/j.rmed.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 37.Bordoni B., Marelli F., Morabito B., Castagna R. Chest pain in patients with COPD: the fascia's subtle silence. Int J Chronic Obstr Pulm Dis. 2018;13:1157–1165. doi: 10.2147/COPD.S156729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Galesanu R.G., Bernard S., Marquis K. Obesity in chronic obstructive pulmonary disease: is fatter really better? Cancer Respir J. 2014;21:297–301. doi: 10.1155/2014/181074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Divo M.J., Cabrera C., Casanova C. Comorbidity distribution, clinical expression and survival in COPD patients with different Body mass index. Chronic Obstr Pulm Dis. 2014;1:229–238. doi: 10.15326/jcopdf.1.2.2014.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun Y., Milne S., Jaw J.E. BMI is associated with FEV1 decline in chronic obstructive pulmonary disease: a meta-analysis of clinical trials. Respir Res. 2019;20:236. doi: 10.1186/s12931-019-1209-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.