Abstract
New strategies to fight bacteria and fungi are necessary in view of the problem of iatrogenic and nosocomial infections combined with the growing threat of increased antimicrobial resistance. Recently, our group has prepared and described two new readily available materials based on the combination of Rose Bengal (singlet oxygen photosensitizer) and commercially available cationic polystyrene (macroporous resin Amberlite® IRA 900 or gel-type resin IRA 400). These materials showed high efficacy in the antimicrobial photodynamic inactivation (aPDI) of Pseudomonas aeruginosa. Here, we present the photobactericidal effect of these polymers against an extended group of pathogens like Escherichia coli, Enterococcus faecalis, Staphylococcus aureus, and the opportunistic yeast Candida albicans using green light. The most interesting finding is that the studied materials are able to reduce the population of both Gram-positive and Gram-negative bacteria with good activity, although, for C. albicans, in a moderate manner. In view of the results achieved and especially considering the inexpensiveness of these two types of photoactive polymers, we believe that they could be used as the starting point for the development of coatings for self-disinfecting surfaces.
Keywords: ESKAPE, antimicrobials, polystyrene, broad-spectrum, photodynamic inactivation, singlet oxygen
Introduction
Nosocomial infections are growing in importance day by day and constitute a serious problem for public health, causing important human and economical loses. In the future, it is expected that bacterial and fungal infections will be a major cause of death worldwide (1). These infections are mainly originated by a growing number of bacteria and fungi with strong resistance to chemotherapeutical drugs, and special attention is paid to the development of strategies that deal with the well-defined group of ESKAPE pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species) (2).
Other important sources of nosocomial infections are opportunistic fungal pathogens, especially in immunocompromised patients (3). In particular, several Candida spp. are widely recognized as majorly responsible for the morbidity and mortality caused by opportunistic microbes in healthcare settings (4). Similarly, the emergence of Candida spp. resistant to antifungal drugs is also widely recognized and therefore has become a global health problem (5). Despite the intensive work carried out in order to develop alternatives to the current drug treatments (6, 7), the most realistic approach to fighting antimicrobial-resistant microorganisms continues to be the prevention of contagion.
Nosocomial infections arise mainly from the growth of microorganisms in surfaces in close contact to patients, for instance orthopedic implants, catheters, and gastroesophageal tubes. Therefore, the development of antimicrobial coatings engineered for use in medical devices is of great practical interest. Several strategies have been developed in the past to make surfaces with antimicrobial properties, and the literature is abundant in reviews about this topic (8, 9). Thus, it is possible to design surfaces with antifouling properties that inhibit the adherence of microorganisms by controlling, for example, the surface hydrophobicity (10). Also, there is relevant research on the development of coatings with intrinsic antimicrobial features by the incorporation of biocide compounds (11–13).
An emerging strategy to fight hospital-acquired infections is the so-called antimicrobial photodynamic inactivation (aPDI) (14–17). This approach has been developed in parallel with the photodynamic therapy (PDT) of cancer (18, 19), although in recent times it has attracted a renewed interest (14, 15, 20–26). It is based on the killing of microorganisms by reactive oxygen species (ROS), for instance singlet oxygen and radicals, which in turn are generated due to the absorption of light by a photosensitizer in the presence of oxygen. Since the mechanistic aspects of the processes involved are very well-described elsewhere, the reader is referred to any of the excellent reviews published in the literature about photosensitization (27–33).
Based on this strategy, we reported recently (34) on simple and inexpensive photosensitizing materials based on the ionic attachment of the anionic singlet oxygen photosensitizer Rose Bengal (RB) on commercial cationic polystyrene (Amberlite® IRA 900 and IRA 400). The materials previously described by our group were able to eradicate completely the population of P. aeruginosa under irradiation [reduction of 8 log10 colony forming units (CFU) per milliliter]. In the present work, we extend the evaluation of these materials as aPDI agents against other relevant pathogens as well as the yeast C. albicans. The results presented here indicate that these photoactive polymers could be good starting points for the development of coatings for medical devices that prevent hospital-acquired infections. It has to be noted that the use of ionic exchange for the preparation of photoactive materials can be traced back to the pioneering work of Williams et al. on polymers for photocatalytic applications (35).
The present investigation can be enclosed within the interdisciplinary emerging field of materials for aPDI, which use typically biopolymers or synthetic organic macromolecules as supports (20, 22, 36–39).
Materials and Methods
Synthesis and Characterization of the Polymeric Photosensitizers
The photosensitizing polymers RB@Pmp and RB@Pgel were prepared from RB sodium salt (Sigma-Aldrich) and the ion exchange resins Amberlite® IRA-900 (Pmp) and Amberlite® IRA-400 (Pgel), respectively (chloride forms, both from Sigma-Aldrich). The synthesis and characterization are reported elsewhere (34).
Microorganisms and Growth Conditions
The Gram-positive bacterial strains E. faecalis ATCC 29212 and S. aureus ATCC 29213, Gram-negative E. coli ATCC 25922, as well as the yeast strains of C. albicans ATCC 10231 were acquired from the American Type Culture Collection (ATCC, Rockville, MD, USA).
Microorganisms seeded on Columbia Blood Agar (Oxoid® Madrid, Spain) were cultured aerobically overnight at 35°C.
Antimicrobial Photodynamic Inactivation Experiments
The inoculum was prepared by adding colonies in distilled water (Gibco®, Thermo Fisher, Spain) and adjusted to 0.50 ± 0.03 on the McFarland scale for bacteria and to 5.00 ± 0.03 on the McFarland scale for C. albicans (microbial suspensions containing >108 bacteria/ml and >106 yeasts/ml, respectively).
Ten experimental groups for each strain were prepared with the inocula. They were prepared using 10 different RODAC plates and dropping a volume of 5 ml of the microbial suspensions into each one and then 200 mg of the photoactive polymer RB@Pmp (group I), or the same amount of control Pmp resin (without RB; group II), or 200 mg of the photoactive polymer RB@Pgel (group III), or the same amount of control Pgel resin (without RB; group IV), or no resin was added (group V). These five groups were subjected to irradiation, and in parallel, another five groups were kept in darkness as controls (groups VI to X).
Supplementary Figures 1A,C show the setup used.
The samples were shaken (mode: orbital 15 rpm; Grant Bio™ PS-M3D 3D Multi-Function Rotator) during the irradiation (groups I to V) or during the time corresponding to the irradiation period (groups VI to X).
The source light used was a light-emitting diode lamp (Showtec LED Par 64 Short 18 × RGB 3-in-1 LED, Highlite International B.V., Spain) emitting at 515 ± 10 nm (green range matching the excitation spectrum of RB in the polymers; Supplementary Figure 1D). Supplementary Figure 1B shows the LED emission spectrum. The irradiation was performed using a total light dose up to 200 J/cm2, keeping a 17-cm distance between the LEDs and the RODAC plates (light irradiance, 5.8 mW/cm2).
Final loading of RB in the polymers was 1.5 mg RB/g resin, that is, a concentration of 60 μg/ml or 5.9 × 10−5 M (200 mg of RB@Pmp or RB@Pgel in 5 ml of microbial suspension).
No incubation time after the addition of the polymers to the microbial suspension was used, that is, when the polymers are added is when t = 0 is established and the irradiation or darkness time begins to be counted.
Aliquots from the RODAC plates were taken every time equivalent to a 20-J/cm2 light dose (57.6 min of illumination or darkness) up to a maximum of 200 J/cm2 (9.6 h of illumination); the appropriate dilutions were made and they were seeded in blood agar plates and incubated overnight at 35°C. The antimicrobial effect was determined by counting the number of CFU per milliliter on the plate using the Flash & Go automatic colony counter (IUL, S.A, Spain). The aliquots had a volume of 10 μl (0.2% of the initial sample volume). The dilutions or the direct seeding in the plates for counting were carried out according to previous experiments in order to count the range {>0, <200} CFU/agar plate. Higher volumes of aliquots were taken in cases where, according to the preliminary experiments, the CFU number in the plates from the aliquot of 10 μl planted undiluted was 0 CFU/agar plate (i.e., bacterial or fungal growth is expected to be <100 CFU/ml; this equates to bacterial samples where the logarithmic reduction reaches or exceeds 6 log10 or ≥4 log10 for C. albicans).
In these cases, the volume removed was 100 μl (2% of the initial sample volume) and the maximum volume taken was 1 ml (20% of the initial sample volume) in the points where there were <10 CFU/ml (the logarithmic reduction reaches or exceeds 7 log10 in the bacterial samples or ≥5 log10 for the yeast samples).
All experiments were performed three times: five groups for irradiation + five groups for darkness (=10) for each type of polymer (×2); it was performed for each microorganism (×6) in three replicates of the experiment (×3). Graphs of the results and statistical analysis were done using GraphPad Prism 8. The results are expressed as mean and standard deviation. Differences between groups were compared by analysis of variance.
Results and Discussion
The polymeric supports used in this study, Amberlite® IRA-900 and IRA-400, are commercially available ion exchange resins used in diverse fields, from catalysis to chromatography. They consist of cross-linked polystyrene with appended trimethylammonium groups (with chloride anions). The difference between both resins is the degree of cross-linking: Amberlite® IRA-900 (Pmp) presents a high degree of cross-linking, and hence permanent porosity, giving rise to a macroporous structure. On the other hand, Amberlite® IRA-400 (Pgel) presents a lower degree of cross-linking and lacks permanent porosity, thus presenting a gel-type structure in the presence of the appropriate compatible solvent. Preparation of the photo-antimicrobial conjugates involving these resins and RB was easily done by the exchange of chloride ions present in the original Amberlite® polymers (Pmp and Pgel), by RB anions, yielding the final polymers RB@Pmp and RB@Pgel, respectively. More details about the synthesis and characterization of the materials can be found in our previous work (34).
The photodynamic activity of the materials using green light (515 nm) was tested against two strains of Gram-positive bacteria such as E. faecalis and S. aureus and two strains of Gram-negative bacteria, specifically E. coli and P. aeruginosa. We have recently reported on the photodynamic activity of both Amberlite® polymers (Pmp and Pgel) against P. aeruginosa (34), and therefore the results for this Gram-negative bacteria are included in the present work for comparison purposes. In addition, the photoactivity against C. albicans is also presented in this study in order to have a fungal representative. Overall, we present a broad-spectrum photo-antimicrobial analysis of these polymers based on cationic polystyrene and RB.
Activity Against Gram-Positive Bacteria
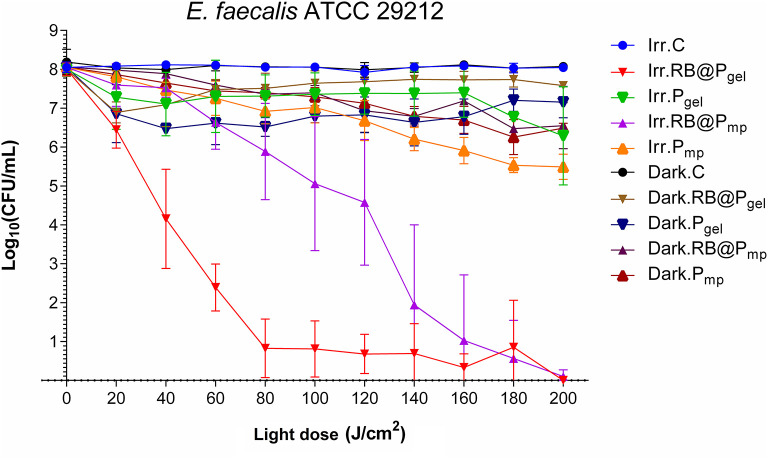
Both RB@Pmp and RB@Pgel materials present a high efficiency against Gram-positive E. faecalis at a total light dose of 200 J/cm2, with a total eradication of the bacterium population (8 log10 CFU/ml). At lower exposures to light (100 J/cm2), differences between both polymers can be noticed, showing the gel matrix to have a better performance than the macroporous one (Figure 1 and Supplementary Figures 2, 3). Additionally, the corresponding controls in the dark as well as the polymeric matrices Pmp and Pgel without a photosensitizer show some activity, with log10 reductions of CFU per milliliter in the range of 0.5–2 units. This partial activity can be, in principle, attributed to the presence of ammonium groups in the polymeric matrices, which are known to have antimicrobial effects by triggering bacterial envelope destruction (40).
Figure 1.
Survival curves corresponding to the photodynamic inactivation of Enterococcus faecalis. Every point is the average of three independent experiments. Error bars correspond to the standard deviations. Legend titles: Irr, irradiated samples; Dark, controls in the darkness; C, control, only microbial suspension; RB@Pgel, Amberlite® IRA-400 (Pgel) loaded with Rose Bengal (RB); Pgel, Pgel resin without RB; RB@Pmp, Amberlite® IRA-900 (Pmp) loaded with RB; Pmp, Pmp resin without RB.
Several studies have described the photodynamic killing of planktonic suspensions of E. faecalis by different photosensitizing materials (Table 1 shows some representative examples). Although the different experimental setups used make a direct comparison of bibliographic data difficult, we would like to illustrate the effectiveness of our systems against different bacterial pathogens in the context of other materials studied for the same goal. It is worth noting the activity of chitosan nanoparticles functionalized with RB (CS-RB) (43) causing a notable reduction of E. faecalis viability. Moreover, the dark toxicity of the reported nanoparticles was significant, indicating that the cationic matrix is also playing an important role in such bactericidal effect.
Table 1.
Representative examples reported in the literature of Enterococcus faecalis inactivation caused by photosensitizing materials.
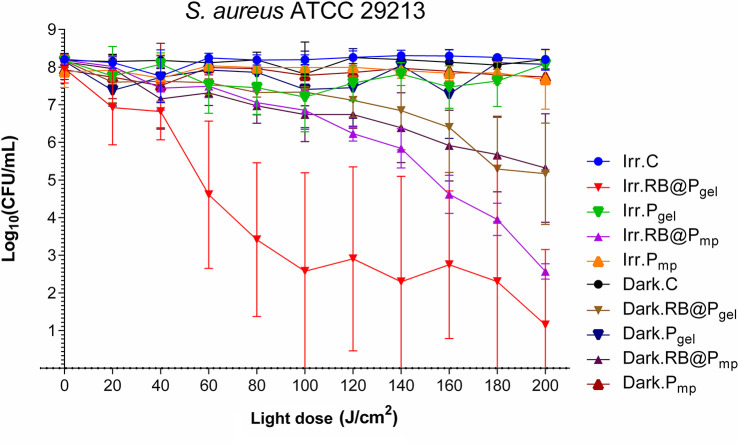
For S. aureus, the bacterial viability reduction after irradiation is dependent on the polymer used. The activity for RB@Pgel is better than for RB@Pmp at lower light doses, but similar at 200 J/cm2 (5.5–7 log10) (Figure 2 and Supplementary Figures 2, 3). The results of the RB-containing polymers in the dark also demonstrate a significant activity, as denoted by a reduction of 3 log10 in the count of S. aureus population (at the end of the kinetics). It can be hypothesized that part of the dark toxicity of RB@Pgel and RB@Pmp could be originated by the fact that RB was recently found to be a potent inhibitor for SecA ATPase activity, which is essential in protein translocation in bacteria (44). Thus, if some photosensitizer is transferred from the polymers to the bacteria during the course of the experiments, this could originate some reduction of the CFU per milliliter. However, more experiments are needed in order to confirm this activity. This process seems very unlikely since, according to preliminary assays, no leaching out of RB takes place, as determined spectrophotometrically, after keeping both RB@Pmp and RB@Pgel submerged in water for several weeks.
Figure 2.
Survival curves corresponding to the photodynamic inactivation of Staphylococcus aureus. Every point is the average of three independent experiments. Error bars correspond to the standard deviations. Legend titles: Irr, irradiated samples; Dark, controls in the darkness; C, control, only microbial suspension; RB@Pgel, Amberlite® IRA-400 (Pgel) loaded with Rose Bengal (RB); Pgel, Pgel resin without RB; RB@Pmp, Amberlite® IRA-900 (Pmp) loaded with RB; Pmp, Pmp resin without RB.
The photoinactivation of this pathogen by different photoactive materials has been extensively reported in the literature. Some recent representative examples of planktonic studies are shown in Table 2. Typical reductions of the bacterial population range from 4 to 6 log10 CFU/ml. We have previously reported the notable activity of the hexanuclear molybdenum cluster [Mo6I8Ac6]2− when loaded in the same polymeric matrices used in the present work for both Gram-positive and Gram-negative bacteria. These polymers exhibited a slightly better performance than RB@Pmp and RB@Pgel, with a 7–8 log10 reduction in the populations of S. aureus (49). Some questions are still open regarding the use of molybdenum hybrid polymers for the coating of medical devices, in front of the RB-loaded polymers presented here, like the unknown toxicity of the molybdenum clusters as well as the higher cost of preparation.
Table 2.
Recent examples reported in the literature of Staphylococcus aureus inactivation caused by photosensitizing materials.
| Photosensitizer | Support | Initial load (log10 CFU/ml) | Load reduction (Δlog10 CFU/ml) | References |
|---|---|---|---|---|
| Porphyrin | Dipyrromethane polymeric films | 7–7.8 | 4–5 | (45) |
| Electropolymerizable Zn(II) porphyrin containing carbazoyl groups | Polymeric films from polymerization of the porphyrin | 6 | 6 | (46) |
| Methylene blue | Methacrylate polymer doped with montmorillonite | 8–8.7 | 4.8 | (47) |
| Rose Bengal | Sol–gel hybrid coatings based on alkyl silanes | 4.4 | 4.4 | (48) |
| Pmp (IRA900) | 8 | 8 | (49) | |
| Pgel (IRA400) | 8 | 7 | (49) | |
| Rose Bengal | Pmp (IRA900) | 8 | 5.5 | This work |
| Rose Bengal | Pgel (IRA400) | 8 | 7 | This work |
Activity Against Gram-Negative Bacteria
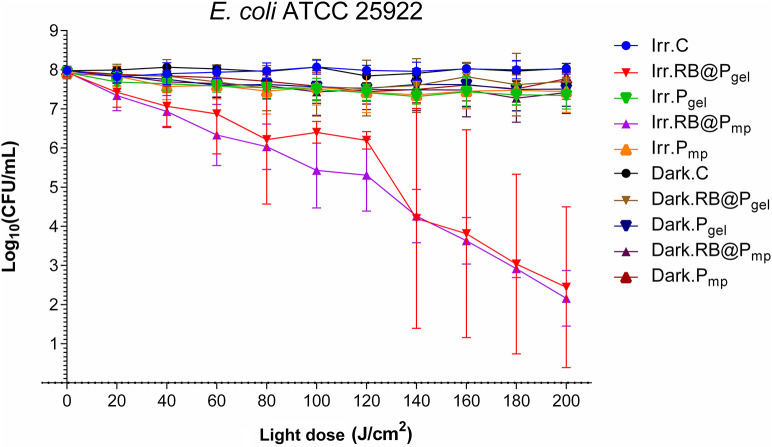
It is known that Gram-negative bacteria are more resistant to photodynamic inactivation than Gram-positive bacteria due to their highly organized outer wall (22). It has been reported that an effective inactivation of Gram-negative bacteria requires the presence of cationic photosensitizers, and in consequence, it has been found that RB is relatively inefficient against these bacteria in its free form, but highly effective in combination with adjuvants like cationic peptides (50) or core–shell silver–silica nanoparticles (51). It must be noted that the positive effect of cationic residues (not belonging strictly to the photosensitizer) was described earlier for chlorin e6 conjugated to poly-l-lysine (52). Thus, we decided to investigate the RB inhibitory effect when it is supported on the cationic Amberlite resins. The results obtained using RB@Pmp and RB@Pgel demonstrate that RB becomes an efficient photosensitizer against the Gram-negative bacteria E. coli at a total light dose of 200 J/cm2, with a reduction of CFU per milliliter of ~5.5 log10 units (Figure 3 and Supplementary Figure 3). In this case, no important differences were detected between the gel and macroporous polymers, as can be seen from the data at 100 J/cm2 (Supplementary Figure 2).
Figure 3.
Survival curves corresponding to the photodynamic inactivation of Escherichia coli. Every point is the average of three independent experiments. Error bars correspond to the standard deviations. Legend titles: Irr, irradiated samples; Dark, controls in the darkness; C, control, only microbial suspension; RB@Pgel, Amberlite® IRA-400 (Pgel) loaded with Rose Bengal (RB); Pgel, Pgel resin without RB; RB@Pmp, Amberlite® IRA-900 (Pmp) loaded with RB; Pmp, Pmp resin without RB.
The photoinactivation of E. coli as a model of Gram-negative bacterium has been thoroughly studied in the past. Some recent examples using photosensitizing materials are shown in Table 3. Interestingly, Bilici et al. (53) reported a remarkable activity of indocyanine green loaded in superparamagnetic iron oxide nanoparticles. However, they combined photodynamic therapy with photothermal therapy to trigger antibacterial phototoxicity, which cannot be comparable with our system or any of the other studies presented in Table 3.
Table 3.
Recent examples reported in literature of Escherichia coli inactivation caused by photosensitizing materials.
| Photosensitizer | Support | Initial load (log10 CFU/ml) | Load reduction (Δlog10 CFU/ml) | References |
|---|---|---|---|---|
| Indocyanine green | Superparamagnetic iron oxide NPs | 12 | 12 | (53) |
| Porphyrin | Metal organic framework/cotton fabrics | 8 | 6 | (54) |
| Porphyrin | Silica-coated magnetite NPs | 6 | 3.1 | (55) |
| Cationic Pd(II) porphyrin | Polyacrylamide hydrogel | 6 | 2.93 | (56) |
| Rose Bengal | Sol–gel hybrid coatings based on alkyl silanes | 4.4 | 4.4 | (48) |
| Rose Bengal | Pmp (IRA900) | 8 | 5.5 | This work |
| Rose Bengal | Pgel (IRA400) | 8 | 5.5 | This work |
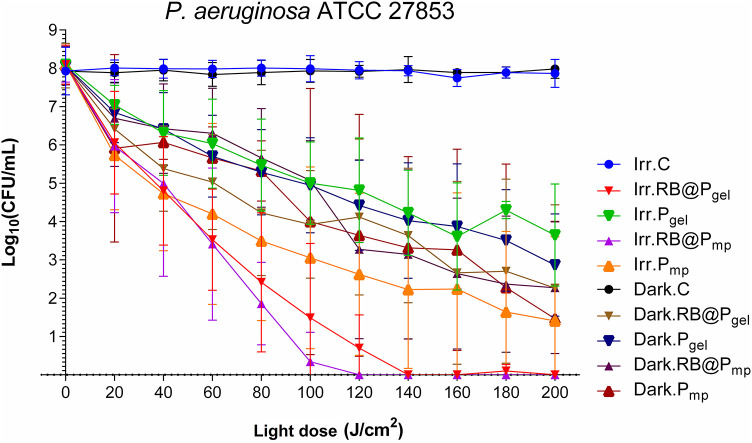
The activity against P. aeruginosa of RB@Pmp and RB@Pgel was recently reported by us (34) and is included in this study for comparison to the rest of the pathogens. A complete eradication of this species (8 log10 CFU/ml) was observed with both polymers when light was applied (Figure 4 and Supplementary Figures 2, 3). Also, an important dark toxicity of the polymers (~6 log10 CFU/ml reduction) was observed, indicating that the polymeric matrix is also playing an important role, probably due to the presence of the ammonium groups that can interact efficiently with the external wall of the bacterium cell (57). This activity is comparable to that reported for methylene blue encapsulated in porous silica nanoparticles (58) and for chitosan used as a carrier of Toluidine blue O (59) that also induced a reduction of 8 log10 CFU/ml, and for the aforementioned system involving indocyanine green loaded in superparamagnetic iron oxide nanoparticles, which induced a reduction of 12 log10 CFU/ml (53). Nevertheless, in these cases, the activity of the materials in the dark is negligible or very low. The corresponding comparative table for this bacterium can be found in the cited publication (34).
Figure 4.
Survival curves corresponding to the photodynamic inactivation of Pseudomonas aeruginosa. Every point is the average of three independent experiments. Error bars correspond to the standard deviations. Legend titles: Irr, irradiated samples; Dark, controls in the darkness; C, control, only microbial suspension; RB@Pgel, Amberlite® IRA-400 (Pgel) loaded with Rose Bengal (RB); Pgel, Pgel resin without RB; RB@Pmp, Amberlite® IRA-900 (Pmp) loaded with RB; Pmp, Pmp resin without RB. Adapted from (34). Copyright 2020 with permission from Elsevier.
Activity Against Candida albicans
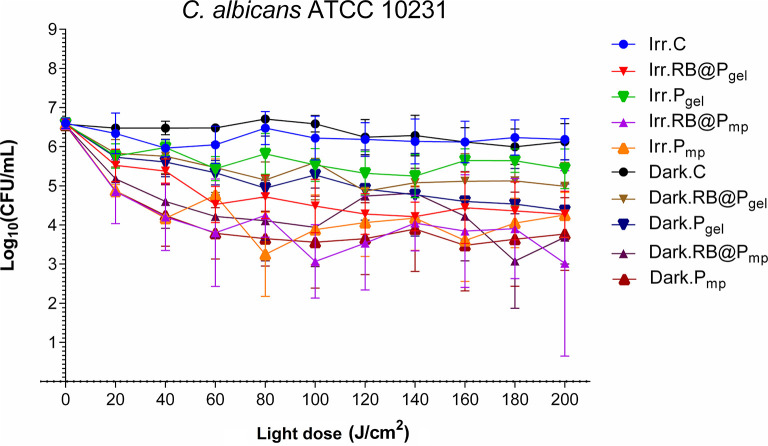
The antifungal activity of polymers RB@Pmp and RB@Pgel was evaluated and the CFU per milliliter values after aPDI treatment presented in Figure 5 and Supplementary Figures 4, 5. Reductions of 1.5–3.0 log10 CFU/ml are observed against C. albicans for all the polymers, in both irradiated and dark conditions. It seems that some toxicity is related to the polymeric matrices Pmp and Pgel; hence, RB direct photodynamic action seems to be not very important for C. albicans. As expected, light alone did not show any inhibition. The dark activity of the polymers (around 2.5 log10 CFU/ml) is probably connected to the presence of the positively charged groups on the surface of the polymer since several materials containing ammonium compounds have been reported to exhibit antifungal properties (60, 61). On the other hand, the scarce photoactivity of RB against C. albicans has been reported previously (62), which might probably rely on features such as the thickness of the yeast cell wall. However, it is not discarded for future studies that an increase in the concentration of the photosensitizer would lead to enhanced photoactivities. Finally, it can be said that a slightly better performance of the macroporous resin is observed in Figure 5 as compared to the gel-type one, probably due to the higher specific surface of the Pmp material.
Figure 5.
Survival curves corresponding to the photodynamic inactivation of Candida albicans. Every point is the average of three independent experiments. Error bars correspond to the standard deviations. Legend titles: Irr, irradiated samples; Dark, controls in the darkness; C, control, only microbial suspension; RB@Pgel, Amberlite® IRA-400 (Pgel) loaded with Rose Bengal (RB); Pgel, Pgel resin without RB; RB@Pmp, Amberlite® IRA-900 (Pmp) loaded with RB; Pmp, Pmp resin without RB.
Reports on the photoinactivation of C. albicans and other opportunistic Candida non-albicans species using photoactive solid materials are scarce. Table 4 summarizes some representative examples. The best results are obtained with a cationic phthalocyanine electrostatically attached to poly(propylene)-based films, which caused a 4 log10 decrease of the C. albicans population (65). Good results were observed as well when anionic porphyrin was used as a photosensitizer, but mainly when it was conjugated with platinum nanoparticles, showing a 3.95 log10 CFU/ml decrease (64).
Table 4.
Representative examples reported in literature of Candida albicans inactivation caused by photosensitizing materials.
| Photosensitizer | Support | Initial load (log10 CFU/ml) | Load reduction (Δlog10 CFU/ml) | References |
|---|---|---|---|---|
| Porphyrin | Polysilsesquioxane | 6 | 2.5 | (63) |
| Anionic porphyrin | Pt nanoparticles | 8 | 3.95 | (64) |
| Porphyrin | Silica-coated magnetite NPs | 6 | 2.5 | (55) |
| Cationic phthalocyanine | Poly(propylene) | 6 | 4 | (65) |
| Toluidine blue/Rose Bengal | Cellulose acetate | 5.3 | 0.9 | (66) |
| Rose Bengal | Pmp (IRA900) | 6 | 3 | This work |
| Rose Bengal | Pgel (IRA400) | 6 | 1.5 | This work |
An important question that can arise, for all the microorganisms studied, is the potential formation of biofilms during the time that the experiment is running. Although this is always possible, (a) the continuous agitation of the samples minimizes this possibility and (b) typical conditions for biofilm formation like extended incubations (24–72 h) are avoided. Nevertheless, this fact should always be taken into account in studies involving surfaces.
Throughout this study, we are assuming that the killing of the microorganisms involves, very likely, singlet oxygen (type II mechanism), provided that RB is a well-known generator of this ROS upon visible light excitation in solution (67, 68). However, since some type I photoactivity has also been described for this photosensitizer (via superoxide anion) (69), this pathway cannot be ruled out completely in the complex environment created by the polymer matrix. Nevertheless, the existence of natural defensive agents like superoxide dismutase (SOD) makes the involvement of this ROS in the mechanism of cell death very unlikely. A more in-depth study would be needed to afford some clarification on this question, but this is out of the scope of this work.
Conclusion
The aPDI capacity of RB@Pmp and RB@Pgel was addressed against both Gram-positive (S. aureus and E. faecalis) and Gram-negative (E. coli and P. aeruginosa) bacteria as well as the pathogenic yeast C. albicans. At a high total light dose (200 J/cm2), both groups of bacteria reduced their populations (5–8 log10 CFU/ml) in the presence of the photoactive polymers and light in a statistically significant manner (p < 0.01 to p < 0.0001, depending on the specific case; see Supplementary Material). Only for C. albicans was the observed photodynamic action scarce, although the effect of the polymeric matrix in the dark is the cause of around 2.5 log10 of CFU/ml reduction (statistically significant, with p < 0.05) and could be of interest for further studies.
Finally, we would like to stress that, only as a proof-of-concept, despite anionic photosensitizers, like RB, being largely considered ineffective for the inactivation of Gram-negative bacteria, we have shown that, when combined with commercial supports like cationic exchange resins, the resultant systems can be efficient materials against bacterial pathogens. The polymers described here lack the complexity of the other systems described in the literature, but it is precisely the accessibility of the starting materials that makes this combination an appealing option for new practical developments.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
AR and FG designed and supervised the study. CAdV synthesized the materials under study. VP-L performed the biological experiments and conducted the statistical analysis of the data. RG, RdL, VP-L, JM, AR, and FG wrote parts of the manuscript. RG edited the manuscript. All authors read and approved the final version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. FG thanks Universitat Jaume I (grant UJI-B2018-30) for financial support. This study was also supported by the Aragón Government: Infectious Diseases of Difficult Diagnosis and Treatment research group (GIIS-023). RG thanks Universitat Jaume I for a postdoctoral fellowship (POSDOC-B/2018/09). RL was funded through a Beatriz Galindo Fellowship of the Ministerio de Ciencia e Innovación, Spanish Government.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.641646/full#supplementary-material
References
- 1.Bush K, Courvalin P, Dantas G, Davies J, Eisenstein B, Huovinen P, et al. Tackling antibiotic resistance. Nat Rev Microbiol. (2011) 9:894–6. 10.1038/nrmicro2693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rice LB. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: no ESKAPE. J Infect Dis. (2008) 197:1079–81. 10.1086/533452 [DOI] [PubMed] [Google Scholar]
- 3.Bassetti M, Ansaldi F, Nicolini L, Malfatto E, Molinari MP, Mussap M, et al. Incidence of candidaemia and relationship with fluconazole use in an intensive care unit. J Antimicrob Chemother. (2009) 64:625–9. 10.1093/jac/dkp251 [DOI] [PubMed] [Google Scholar]
- 4.Pappas PG, Lionakis MS, Arendrup MC, Ostrosky-Zeichner L, Kullberg BJ. Invasive candidiasis. Nat Rev Dis Prim. (2018) 4:1–20. 10.1038/nrdp.2018.26 [DOI] [PubMed] [Google Scholar]
- 5.Pristov KE, Ghannoum MA. Resistance of Candida to azoles and echinocandins worldwide. Clin Microbiol Infect. (2019) 25:792–8. 10.1016/j.cmi.2019.03.028 [DOI] [PubMed] [Google Scholar]
- 6.Otis G, Bhattacharya S, Malka O, Kolusheva S, Bolel P, Porgador A, et al. Selective labeling and growth inhibition of pseudomonas aeruginosa by aminoguanidine carbon dots. ACS Infect Dis. (2019) 5:292–302. 10.1021/acsinfecdis.8b00270 [DOI] [PubMed] [Google Scholar]
- 7.Cândido ES, Cardoso MH, Chan LY, Torres MDT, Oshiro KGN, Porto WF, et al. Short cationic peptide derived from archaea with dual antibacterial properties and anti-infective potential. ACS Infect Dis. (2019) 5:1081–6. 10.1021/acsinfecdis.9b00073 [DOI] [PubMed] [Google Scholar]
- 8.Hasan J, Crawford RJ, Ivanova EP. Antibacterial surfaces: the quest for a new generation of biomaterials. Trends Biotechnol. (2013) 31:295–304. 10.1016/j.tibtech.2013.01.017 [DOI] [PubMed] [Google Scholar]
- 9.Ghilini F, Pissinis DE, Miñán A, Schilardi PL, Diaz C. How functionalized surfaces can inhibit bacterial adhesion and viability. ACS Biomater Sci Eng. (2019) 5:4920–36. 10.1021/acsbiomaterials.9b00849 [DOI] [PubMed] [Google Scholar]
- 10.Balaure PC, Grumezescu AM. Recent advances in surface nanoengineering for biofilm prevention and control. Part II: active, combined active and passive, and smart bacteria-responsive antibiofilm nanocoatings. Nanomaterials. (2020) 10:1–53. 10.3390/nano10081527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kazemzadeh-Narbat M, Cheng H, Chabok R, Alvarez MM, de la Fuente-Nunez C, Phillips KS, et al. Strategies for antimicrobial peptide coatings on medical devices: a review and regulatory science perspective. Crit Rev Biotechnol. (2021) 41:94–120. 10.1080/07388551.2020.1828810 [DOI] [PubMed] [Google Scholar]
- 12.Zhu Z, Wang Z, Li S, Yuan X. Antimicrobial strategies for urinary catheters. J Biomed Mater Res - Part A. (2019) 107:445–467. 10.1002/jbm.a.36561 [DOI] [PubMed] [Google Scholar]
- 13.Cloutier M, Mantovani D, Rosei F. Antibacterial Coatings: Challenges, Perspectives, and Opportunities. Trends Biotechnol. (2015) 33:637–652. 10.1016/j.tibtech.2015.09.002 [DOI] [PubMed] [Google Scholar]
- 14.Nakonieczna J, Wozniak A, Pieranski M, Rapacka-Zdonczyk A, Ogonowska P, Grinholc M. Photoinactivation of ESKAPE pathogens: overview of novel therapeutic strategy. Future Med Chem. (2019) 11:443–61. 10.4155/fmc-2018-0329 [DOI] [PubMed] [Google Scholar]
- 15.Wainwright M, Maisch T, Nonell S, Plaetzer K, Almeida A, Tegos GP, Hamblin MR. Photoantimicrobials—are we afraid of the light? Lancet Infect Dis. (2017) 17:e49–55. 10.1016/S1473-3099(16)30268-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yin R, Agrawal T, Khan U, Gupta GK, Rai V, Huang YY, Hamblin MR. Antimicrobial photodynamic inactivation in nanomedicine: small light strides against bad bugs. Nanomedicine. (2015) 10:2379–404. 10.2217/nnm.15.67 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Pérez-Laguna V, García-Luque I, Ballesta S, Pérez-Artiaga L, Lampaya-Pérez V, Rezusta A, Gilaberte Y. Photodynamic therapy using methylene blue, combined or not with gentamicin, against Staphylococcus aureus and Pseudomonas aeruginosa. Photodiagnosis Photodyn Ther. (2020) 31:101810. 10.1016/j.pdpdt.2020.101810 [DOI] [PubMed] [Google Scholar]
- 18.Moan J, Peng Q. An outline of the hundred-year history of PDT. Anticancer Res. (2003) 23:3591–3600. [PubMed] [Google Scholar]
- 19.Shi H, Sadler PJ. How promising is phototherapy for cancer? Br J Cancer. (2020) 123:871–3. 10.1038/s41416-020-0926-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spagnul C, Turner LC, Boyle RW. Immobilized photosensitizers for antimicrobial applications. J Photochem Photobiol B Biol. (2015) 150:11–30. 10.1016/j.jphotobiol.2015.04.021 [DOI] [PubMed] [Google Scholar]
- 21.González-Delgado JA, Kennedy PJ, Ferreira M, Tomé JPC, Sarmento B. Use of photosensitizers in semisolid formulations for microbial photodynamic inactivation. J Med Chem. (2016) 59:4428–42. 10.1021/acs.jmedchem.5b01129 [DOI] [PubMed] [Google Scholar]
- 22.Mesquita MQ, Dias CJ, Neves MGPMS, Almeida A, Faustino MAF. Revisiting current photoactive materials for antimicrobial photodynamic therapy. Molecules. (2018) 23:2424. 10.3390/molecules23102424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jia Q, Song Q, Li P, Huang W. Rejuvenated photodynamic therapy for bacterial infections. Adv Healthc Mater. (2019) 1900608:1–19. 10.1002/adhm.201900608 [DOI] [PubMed] [Google Scholar]
- 24.Page K, Wilson M, Parkin IP. Antimicrobial surfaces and their potential in reducing the role of the inanimate environment in the incidence of hospital-acquired infections. J Mater Chem. (2009) 19:3818–3831. 10.1039/b818698g [DOI] [Google Scholar]
- 25.Wiehe A, O'brien JM, Senge MO. Trends and targets in antiviral phototherapy. Photochem Photobiol Sci. (2019) 18:2565–2612. 10.1039/c9pp00211a [DOI] [PubMed] [Google Scholar]
- 26.Pérez-Laguna V, Gilaberte Y, Millán-Lou MI, Agut M, Nonell S, Rezusta A, et al. A combination of photodynamic therapy and antimicrobial compounds to treat skin and mucosal infections: a systematic review. Photochem Photobiol Sci. (2019) 18:1020–9. 10.1039/c8pp00534f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.MacDonald IJ, Dougherty TJ. Basic principles of photodynamic therapy. J Porphyr Phthalocyanines. (2001) 5:105–29. 10.1002/jpp.328 [DOI] [Google Scholar]
- 28.Juzeniene A, Peng Q, Moan J. Milestones in the development of photodynamic therapy and fluorescence diagnosis. Photochem Photobiol Sci. (2007) 6:1234–45. 10.1039/b705461k [DOI] [PubMed] [Google Scholar]
- 29.Zhao J, Wu W, Sun J, Guo S. Triplet photosensitizers: from molecular design to applications. Chem Soc Rev. (2013) 42:5323–51. 10.1039/c3cs35531d [DOI] [PubMed] [Google Scholar]
- 30.Abrahamse H, Hamblin MR. New photosensitizers for photodynamic therapy. Biochem J. (2016) 473:347–64. 10.1042/BJ20150942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wainwright M. Synthetic, small-molecule photoantimicrobials—a realistic approach. Photochem Photobiol Sci. (2018) 17:1767–79. 10.1039/c8pp00145f [DOI] [PubMed] [Google Scholar]
- 32.Cieplik F, Deng D, Crielaard W, Buchalla W, Hellwig E, Al-Ahmad A, et al. Antimicrobial photodynamic therapy–what we know and what we don't. Crit Rev Microbiol. (2018) 44:571–89. 10.1080/1040841X.2018.1467876 [DOI] [PubMed] [Google Scholar]
- 33.Agazzi ML, Ballatore MB, Durantini AM, Durantini EN, Tomé AC. BODIPYs in antitumoral and antimicrobial photodynamic therapy: An integrating review. J Photochem Photobiol C Photochem Rev. (2019) 40:21–48. 10.1016/j.jphotochemrev.2019.04.001 [DOI] [Google Scholar]
- 34.Arnau del Valle C, Pérez-Laguna V, Resta IM, Gavara R, Felip-León C, Miravet JF, et al. A cost-effective combination of Rose Bengal and off-the-shelf cationic polystyrene for the photodynamic inactivation of Pseudomonas aeruginosa. Mater Sci Eng C. (2020) 117:111302. 10.1016/j.msec.2020.111302 [DOI] [PubMed] [Google Scholar]
- 35.Williams JR, Orton G, Unger LR. Preparation of singlet oxiygen by heterogeneous photosensitisation. Tetrahedron Lett. (1973) 14:4603–6. [Google Scholar]
- 36.Plíštil L, Henke P, Kubát P, Mosinger J. Anion exchange nanofiber materials activated by daylight with a dual antibacterial effect. Photochem Photobiol Sci. (2014) 13:1321–9. 10.1039/c4pp00157e [DOI] [PubMed] [Google Scholar]
- 37.Baigorria E, Milanesio ME, Durantini EN. Synthesis, spectroscopic properties and photodynamic activity of Zn(II) phthalocyanine-polymer conjugates as antimicrobial agents. Eur Polym J. (2020) 134:109816. 10.1016/j.eurpolymj.2020.109816 [DOI] [Google Scholar]
- 38.Maldonado-Carmona N, Ouk TS, Calvete MJF, Pereira MM, Villandier N, Leroy-Lhez S. Conjugating biomaterials with photosensitizers: advances and perspectives for photodynamic antimicrobial chemotherapy. Photochem Photobiol Sci. (2020) 19:445–61. 10.1039/c9pp00398c [DOI] [PubMed] [Google Scholar]
- 39.Wylie MP, Irwin NJ, Howard D, Heydon K, McCoy CP. Hot-melt extrusion of photodynamic antimicrobial polymers for prevention of microbial contamination. J Photochem Photobiol B Biol. (2021) 214:112098. 10.1016/j.jphotobiol.2020.112098 [DOI] [PubMed] [Google Scholar]
- 40.Kenawy ER, Worley SD, Broughton R. The chemistry and applications of antimicrobial polymers: a state-of-the-art review. Biomacromolecules. (2007) 8:1359–84. 10.1021/bm061150q [DOI] [PubMed] [Google Scholar]
- 41.Akbari T, Pourhajibagher M, Hosseini F, Chiniforush N, Gholibegloo E, Khoobi M, et al. The effect of indocyanine green loaded on a novel nano-graphene oxide for high performance of photodynamic therapy against Enterococcus faecalis. Photodiagnosis Photodyn Ther. (2017) 20:148–53. 10.1016/j.pdpdt.2017.08.017 [DOI] [PubMed] [Google Scholar]
- 42.Carvalho CMB, Alves E, Costa L, Tomé JPC, Faustino MAF, Neves MGPMS, et al. Functional cationic nanomagnet—porphyrin hybrids for the photoinactivation of microorganisms. ACS Nano. (2010) 4:7133–40. 10.1021/nn1026092 [DOI] [PubMed] [Google Scholar]
- 43.Shrestha A, Hamblin MR, Kishen A. Photoactivated rose bengal functionalized chitosan nanoparticles produce antibacterial/biofilm activity and stabilize dentin-collagen. Nanomedicine Nanotechnology, Biol Med. (2014) 10:491–501. 10.1016/j.nano.2013.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang YJ, Wang H, Gao FB, Li M, Yang H, Wang B, et al. Fluorescein analogues inhibit SecA ATPase: the first sub-micromolar inhibitor of bacterial protein translocation. ChemMedChem. (2012) 7:571–7. 10.1002/cmdc.201100594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Comuzzi C, Fiorot A, Baggio A, Maifreni M, Strazzolini P, Marino M, et al. Imprinting pentaphyrin on conductive electropolymerized dipyrromethane films: a new strategy towards the synthesis of photokilling materials. Chempluschem. (2020) 85:776–82. 10.1002/cplu.202000137 [DOI] [PubMed] [Google Scholar]
- 46.Heredia DA, Martínez SR, Durantini AM, Pérez ME, Mangione MI, Durantini JE, et al. Antimicrobial photodynamic polymeric films bearing biscarbazol triphenylamine end-capped dendrimeric Zn(II) porphyrin. ACS Appl Mater Interfaces. (2019) 11:27574–87. 10.1021/acsami.9b09119 [DOI] [PubMed] [Google Scholar]
- 47.Wang Q, Chen W, Zhang Q, Ghiladi RA, Wei Q. Preparation of photodynamic P(MMA-co-MAA) composite nanofibers doped with MMT: a facile method for increasing antimicrobial efficiency. Appl Surf Sci. (2018) 457:247–55. 10.1016/j.apsusc.2018.06.041 [DOI] [Google Scholar]
- 48.Akarsu E, Uslu R. Light-activated hybrid organic/inorganic antimicrobial coatings. J Sol-Gel Sci Technol. (2018) 87:183–94. 10.1007/s10971-018-4714-y [DOI] [Google Scholar]
- 49.Felip-León C, Arnau Del Valle C, Pérez-Laguna V, Isabel Millán-Lou M, Miravet JF, Mikhailov M, et al. Superior performance of macroporous over gel type polystyrene as a support for the development of photo-bactericidal materials. J Mater Chem B. (2017) 5:6058–64. 10.1039/c7tb01478c [DOI] [PubMed] [Google Scholar]
- 50.Nakonieczna J, Wolnikowska K, Ogonowska P, Neubauer D, Bernat A, Kamysz W. Rose bengal-mediated photoinactivation of multidrug resistant pseudomonas aeruginosa is enhanced in the presence of antimicrobial peptides. Front Microbiol. (2018) 9:1–15. 10.3389/fmicb.2018.01949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Planas O, Macia N, Agut M, Nonell S, Heyne B. Distance-dependent plasmon-enhanced singlet oxygen production and emission for bacterial inactivation. J Am Chem Soc. (2016) 138:2762–8. 10.1021/jacs.5b12704 [DOI] [PubMed] [Google Scholar]
- 52.Soukos NS, Ximenez-Fyvie LA, Hamblin MR, Socransky SS, Hasan T. Targeted antimicrobial photochemotherapy. Antimicrob Agents Chemother. (1998) 42:2595–601. 10.1128/aac.42.10.2595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bilici K, Atac N, Muti A, Baylam I, Dogan O, Sennaroglu A, et al. Broad spectrum antibacterial photodynamic and photothermal therapy achieved with indocyanine green loaded SPIONs under near infrared irradiation. Biomater Sci. (2020) 8:4616–25. 10.1039/d0bm00821d [DOI] [PubMed] [Google Scholar]
- 54.Nie X, Wu S, Mensah A, Wang Q, Huang F, Li D, et al. Insight into light-driven antibacterial cotton fabrics decorated by in situ growth strategy. J Colloid Interface Sci. (2020) 579:233–42. 10.1016/j.jcis.2020.06.038 [DOI] [PubMed] [Google Scholar]
- 55.Scanone AC, Gsponer NS, Alvarez MG, Durantini EN. Photodynamic properties and photoinactivation of microorganisms mediated by 5,10,15,20-tetrakis(4-carboxyphenyl)porphyrin covalently linked to silica-coated magnetite nanoparticles. J Photochem Photobiol A Chem. (2017) 346:452–61. 10.1016/j.jphotochem.2017.06.039 [DOI] [Google Scholar]
- 56.Spagnul C, Turner LC, Giuntini F, Greenman J, Boyle RW. Synthesis and bactericidal properties of porphyrins immobilized in a polyacrylamide support: influence of metal complexation on photoactivity. J Mater Chem B. (2017) 5:1834–45. 10.1039/c6tb03198f [DOI] [PubMed] [Google Scholar]
- 57.Muñoz-Bonilla A, Fernández-García M. The roadmap of antimicrobial polymeric materials in macromolecular nanotechnology. Eur Polym J. (2015) 65:46–62. 10.1016/j.eurpolymj.2015.01.030 [DOI] [Google Scholar]
- 58.Planas O, Bresolí-Obach R, Nos J, Gallavardin T, Ruiz-González R, Agut M, et al. Synthesis, photophysical characterization, and photoinduced antibacterial activity of methylene blue-loaded amino- and mannose-targeted mesoporous silica nanoparticles. Molecules. (2015) 20:6284–98. 10.3390/molecules20046284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tsai T, Chien HF, Wang TH, Huang CT, Ker YB, Chen CT. Chitosan augments photodynamic inactivation of gram-positive and gram-negative bacteria. Antimicrob Agents Chemother. (2011) 55:1883–90. 10.1128/AAC.00550-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ng CKL, Obando D, Widmer F, Wright LC, Sorrell TC, Jolliffe KA. Correlation of antifungal activity with fungal phospholipase inhibition using a series of bisquaternary ammonium salts. J Med Chem. (2006) 49:811–16. 10.1021/jm0508843 [DOI] [PubMed] [Google Scholar]
- 61.Kenawy ER, Mahmoud YA-G. Biologically active polymers: 6a. Synthesis and antimicrobial activity of some crosslinked copolymers with quaternary ammonium and phosphonium groups. Macromol Biosci. (2003) 3:107–16. 10.1016/j.reactfunctpolym.2005.09.002 [DOI] [Google Scholar]
- 62.Wen X, Zhang X, Szewczyk G, El-Hussein A, Huang Y-Y, Sarna T, et al. Potassium iodide potentiates antimicrobial photodynamic inactivation mediated by rose bengal in in vitro and in vivo studies. Antimicrob Agents Chemother. (2017) 61:e00467–17. 10.1128/AAC.00467-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alvarez MG, Gómez ML, Mora SJ, Milanesio ME, Durantini EN. Photodynamic inactivation of Candida albicans using bridged polysilsesquioxane films doped with porphyrin. Bioorganic Med Chem. (2012) 20:4032–9. 10.1016/j.bmc.2012.05.012 [DOI] [PubMed] [Google Scholar]
- 64.Managa M, Amuhaya EK, Nyokong T. Photodynamic antimicrobial chemotherapy activity of (5,10,15,20-tetrakis(4-(4-carboxyphenycarbonoimidoyl)phenyl)porphyrinato) chloro gallium(III). Spectrochim Acta—Part A Mol Biomol Spectrosc. (2015) 151:867–74. 10.1016/j.saa.2015.06.088 [DOI] [PubMed] [Google Scholar]
- 65.Tempesti T, Alvarez MG, Gómez C, Strumia M, Durantini EN. Poly(propylene)-based films modified with a tetracationic phthalocyanine with applications in photodynamic inactivation of candida albicans. Polym—Plast Technol Eng. (2018) 57:166–74. 10.1080/03602559.2017.1315643 [DOI] [Google Scholar]
- 66.Decraene V, Pratten J, Wilson M. Cellulose acetate containing toluidine blue and rose bengal is an effective antimicrobial coating when exposed to white light. Appl Environ Microbiol. (2006) 72:4436–9. 10.1128/AEM.02945-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kochevar IE, Lambert CR, Lynch MC, Tedesco AC. Comparison of photosensitized plasma membrane damage caused by singlet oxygen and free radicals. Biochim Biophys Acta—Biomembr. (1996) 1280:223–30. 10.1016/0005-2736(95)00297-9 [DOI] [PubMed] [Google Scholar]
- 68.Lambert CR, Kochevar IE. Does rose bengal triplet generate superoxide anion? J Am Chem Soc. (1996) 118:3297–8. 10.1021/ja9600800 [DOI] [Google Scholar]
- 69.Lee PCC, Rodgers MAJ. Laser flash photokinetic studies of rose bengal sensitized photodynamic interactions of nucleotides and DNA. Photochem Photobiol. (1987) 45:79–86. 10.1111/j.1751-1097.1987.tb08407.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.