Abstract
The present pilot study had the objective to determine the effects of transcutaneous and transmucosal laser irradiation on arterial blood pressure (ABP), glucose (Glu) triglycerides (Tg), total cholesterol (Ch), high-density level cholesterol (HDL) and low-density cholesterol (LDL) immediately after treatment (T0) and after 30 (T30) and 60 (T60) days. Patients (n = 36) were selected and randomly distributed into 6 groups (n = 6/group; [G1] negative control, [G2] radial artery transcutaneous laser irradiation [G3] radial artery transcutaneous irradiation, [G4] transmucosal sublingual irradiation, [G5] transmucosal intra-nasal irradiation and [G6] extended radial artery transcutaneous irradiation). Blood exams were performed at T0, T30 and T60. Systolic and diastolic pressure results have indicated that patients' pressures ranged from 90 mmHg (P22, T30, G4) to 189 mmHg (P16, T0, G3) and 54 mmHg (P21, T60, G4) to 175 mmHg (P16, T30, G3). Levels of Glu at T30 and T60 varied from 5.53% (G1) to -5.78% (G6) and 1.21 (G2) to -8.69 (G6), respectively. Data was statistically assessed for normality and homogeneity of variances using the F-statistic and Bartlett's tests. Significant differences were determined using One-Way ANOVA and Fischer post hoc tests. Results indicated that treatments investigated can be safely used as an adjunct method to regulate blood pressure, glucose, triglycerides and cholesterol.
Keywords: Low intensity level laser, Transmucosal, Transcutaneous, Infrared, Blood pressure, Glucose, Triglycerides, Cholesterol
Low intensity level laser, Transmucosal, Transcutaneous, Infrared, Blood pressure, Glucose, Triglycerides, Cholesterol.
1. Introduction
The classical signs of inflammation are redness, heat, swelling, pain and loss of function [1]. Tissue and organ inflammation may be caused by a broad variety of etiological factors including injury, effects of chemicals or radiation, diseases, pathogenic microorganisms or chronic conditions (rheumatoid arthritis, plaque psoriasis and Chron's disease). Mainstream drugs (such as antibiotics, anti-inflammatories and opioids) have been used to treat inflammatory processes originated from a variety of sources. However, despite their widespread use and clinical acceptance [2], their long-term utilization is commonly associated with adverse side effects that include changes in salivary flow and saliva composition [3, 4], gastric ulceration, increased bleeding, nephrotoxicity, retention of sodium, development of drug-resistant bacterial strains [5] and drug abuse (heroin in particular) [6, 7].
Mortality rates associated with multidrug-resistant bacterial infections are currently very high. In 2009, twenty-five thousand patients in the European Union and more than 63,000 patients in the U.S. have lost their lives [2] due to hospital-acquired bacterial infections [8]. A study [9] investigating the correlation between xerostomia and medications in a long-term cross-sectional study, demonstrated that opioid (Vicodin® and Percocet®) abusers typically display diminished salivary flow, poor oral hygiene and increased sugar consumption [10] The combination of the factors cited results in the development of gross dental decay and other systemic conditions (e.g., insulin resistance and diabetes) [11] Therefore, the development of devices and techniques capable of effectively treating inflammation without the utilization of drugs is urgently needed.
One possible approach to overcome this problem is the utilization of intravenous laser irradiation of blood (ILIB). This technique was firstly reported by Meshalkin and Sergiewski [12]. Since then, several reports have described the positive effects of ILIB in cardiology-related areas (wound healing and angiology) [13]. According to these studies, ILIB has an outstanding ability to reduce the infarction area by increasing local microcirculation (neoangiogenesis). In fact, Stroev et al. [13] while investigating the treatment of angiopathies by endovascular low-intensity laser irradiation, have indicated that ILIB decreased thrombocyte aggregation levels, improved erythrocyte deformability, increased oxygen supply [14, 15], and upregulated the proliferation of lymphocytes and subpopulations of cells (B and T, in particular) [16, 17, 18], which in turn, further demonstrates ILIB's capability to improve blood's rheological properties [19, 20]. According to Gasparyan and Makela [19], laser irradiation of blood can be performed by direct or indirect approaches, wherein the former requires the immediate contact of light with blood (invasive technique), and the latter is performed in a minimally invasive fashion through the skin [19, 21, 22].
In the invasive technique, blood and its components (e.g., plasma, red cells, white cells and platelets) are directly exposed [23] to laser irradiation. Wirz-Ridolfi [24] while comparing intravenous laser blood irradiation with transcutaneous and transmucous techniques (sublingual), indicated that photons are typically absorbed at the mitochondrial cell wall and are capable to improve, in a wavelength-dependent manner [25], the citrate cycle within the respiratory chain of eukaryotic cells. The upregulation of these biochemical reactions result in immediate energy production increases, and patients displaying higher stamina. Despite these promising aspects, legal (license-related) and anatomical limitations (obese patients), local-risks (hemorrhage and infection) and patients’ personal preferences have limited the widespread utilization of invasive techniques. Minimally invasive techniques have completely overcome problems cited and are not limited anatomical characteristics or license-related regulations, and allow for a pleasant and anxiety-free treatment experience for patients. One possible drawback of minimally invasive techniques, is related to non-linear interactions (e.g., scattering, absorption and transmission) that take place once the laser beam interacts with different types of cells and tissues.
In these situations, the laser beam loses some of its characteristic properties (e.g., intensity, coherency and collimation) [26] before light can reach veins, arteries and capillaries [19]. Tiina Karu [27] while reviewing the cellular mechanisms of action of low-power laser therapy, indicated that coherency and collimation are not important for the attainment of positive clinical effects. Osipov et al. [28] indicated [29, 30], that cytochrome c oxidase is the main target of laser radiation techniques, because such molecule is the major responsible for photo-modulated biological responses. Other studies have shown that wavelengths within the red spectra are capable to alter the redox potential of exposed cells, thereby promoting subsequent chemical and enzymatic reactions, dissociation of internal cellular components and the release of nitric oxide (NO) [27]. Based on the context presented, the objective of the present pilot study in women was to determine the positive effects of low-intensity transcutaneous or transmucosal laser irradiation on the arterial blood pressure, and on blood-related biomarkers such as glucose, triglycerides and cholesterol (total, high-density and low-density) after 30 and 60 days of laser irradiation.
2. Materials and methods
2.1. Internal Review Board (IRB)
The present pilot study was reviewed and approved by the Internal Review Board of the University of São Paulo College of Dentistry at Ribeirão Preto (protocol # 45390715.2.0000.5419). The present cohort pilot study has been registered at the Brazilian Registry of Clinical Trials (ReBEC; registration # RBR-8vny3v, 05/21/2020) which is a publicly accessible primary register and an integral member of the World Health Organization international Clinical Trial Registry Platform. The present study followed the ethical principles for medical research involving human subjects established by the Declaration of Helsinki of 1975, and as revised by the World Medical Association in 2013 [31].
2.2. Inclusion and exclusion criteria
A total of 50 patients from the city of Ribeirão Preto (São Paulo State, Brazil) were subjected to clinical examination to determine their participation eligibility. The inclusion criteria were: females, ages between 35 and 55 with no history of systemic (hypertension and diabetes) or heart diseases. The exclusion criteria included the presence of uncontrolled systemic diseases, breast feeding, pregnant women and patients with oral tumors and/or temporomandibular joint disorder. The rationale for the inclusion criteria selected is based on the fact that women within 35 and 55 years typically display stronger variations on blood biomarkers investigated.
2.3. Experimental groups and conditions
Triaged volunteers (n = 36) were informed regarding the risks and benefits of the proposed laser irradiation therapy. Questions and doubts related to the laser irradiation protocols and treatment outcomes were fully addressed before patients signed an informed consent for their participation. Selected patients were then randomly distributed into 6 experimental groups (n = 6/group) as described in Table 1 and, as follows: (G1) negative control, (G2) radial artery transcutaneous laser irradiation (660 nm, 1 diode, 100 mW), (G3) radial artery transcutaneous irradiation (660 nm, 2 diodes, 50 mW/each), (G4) transmucosal sublingual irradiation (660 nm, 1 diode, 100 mW), (G5) transmucosal intra-nasal irradiation (660 nm, 1 diode, 100 mW) and (G6) extended radial artery transcutaneous irradiation (660 nm, 1 diode, 100 mW). It is important to note that the sample size used in the present pilot study served the purpose to determine the statistical power of data reported and to substantiate the execution of a subsequent large-scale clinical trial study considering longer periods of time.
Table 1.
Experimental conditions in terms of treatment time, delivery mode, wavelength and dosimetry.
| Groups | Patients | Treatment | Delivery Mode | λ (nm) | Dosimetry |
|---|---|---|---|---|---|
| G1 (control) | n = 6 | - | - | - | - |
| G2 | n = 6 | Twice a week, for 8 weeks (16 irradiations). | Transcutaneous | 660 | 1 diode laser with 100 mW, during 30 minutes in contact mode and CW |
| G3 | n = 6 | Twice a week, for 8 weeks (16 irradiations). | Transcutaneous | 660 | 2 diode laser with 50mW, during 30 minutes in contact mode and CW |
| G4 | n = 6 | Twice a week, for 8 weeks (16 irradiations). | Transmucosal (sublingual) | 660 | 1 diode laser with 100 mW, during 30 minutes in contact mode and CW |
| G5 | n = 6 | Twice a week, for 8 weeks (16 irradiations). | Transmucosal (intra-nasal) | 660 | 1 diode laser with 100 mW, during 30 minutes in contact mode and CW |
| G6 | n = 6 | 10 daily irradiations, 20 days interval followed by 10 daily irradiations. | Transcutaneous | 660 | 1 diode laser with 100mW, during 30 minutes in contact mode and CW |
2.4. Irradiation devices and dosimetry
A commercially available pen-shaped dual-wavelength low intensity level laser (Laser Duo, MMO, Brazil; 660 nm and 808 nm, 100 mW/each, spot size = 0.03 cm2) and a prototype bracelet-shaped single-wavelength low intensity level laser (660 nm, 100 mW, spot size = 4.0 cm2; LAT, São Carlos Physics Institute) were used in the present study. Patients from the control group (G1) did not receive any type of laser therapy. Patients in experimental groups G2-G5 were subjected to laser treatment (30 min, 180 J; 2x/week, total of 4 weeks). Patients in experimental group G6 were subjected to an extended treatment with laser irradiation that was composed of two irradiation cycles (10 consecutive days/cycle [30 min, 180 J daily]) spaced 20 days apart. Patients from all groups were examined with a thermographic system (Fluke Ti20, Fluke Co, U.S.A.) before and after irradiation with laser to qualitatively observe photo-induced temperature variations at treated areas. It is important to note, that even though a dual-wavelength system was used in the present study, the equipment was set to emit only 660 nm to allow for intragroup comparison of results.
2.5. Assessment of arterial blood pressure and blood biomarkers
Arterial blood pressure (ABP) was determined using the auscultatory method along with a digital sphygmomanometer (Elite HEM 7130BR, Omron, São Paulo, Brazil) [32]. Measurements of ABP were conducted immediately before or after laser irradiation (at 30 and 60 days; either transcutaneous of transmucosal). Patients were subjected to three blood exams performed at a professional laboratory (Laboratório Dr. Coutinho, Sabin Medicina Diagnóstica, Ribeirao Preto, Sao Paulo, Brazil) immediately before (T0), after 30 days (T30) and after 60 days (T60) of laser irradiation to determine photo-induced biomodulation of glucose, triglycerides and cholesterol (total, high-density and low-density).
2.6. Statistical analysis
Data obtained was assessed for normality and homogeneity of variances using the F-statistic and Bartlett's tests, respectively. Since data was normally distributed and variance among groups was not equal, statistical significant differences were determined using One-Way ANOVA and Fischer post hoc tests. Statistical analyses were performed using OriginLab 8 (Origin Corporation, U.S.A.).
3. Results
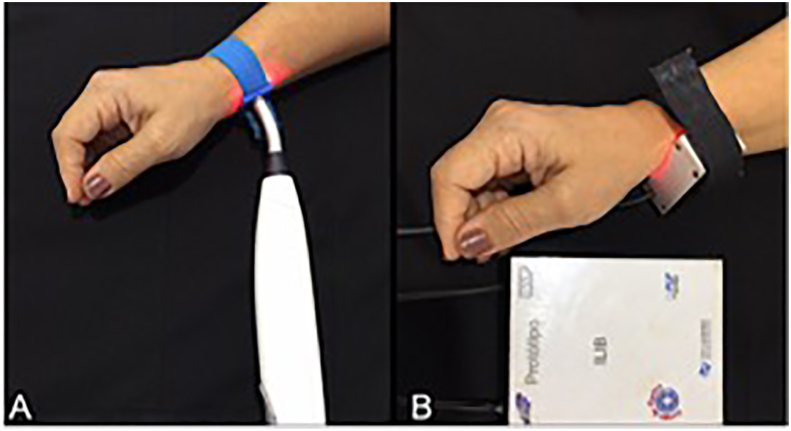
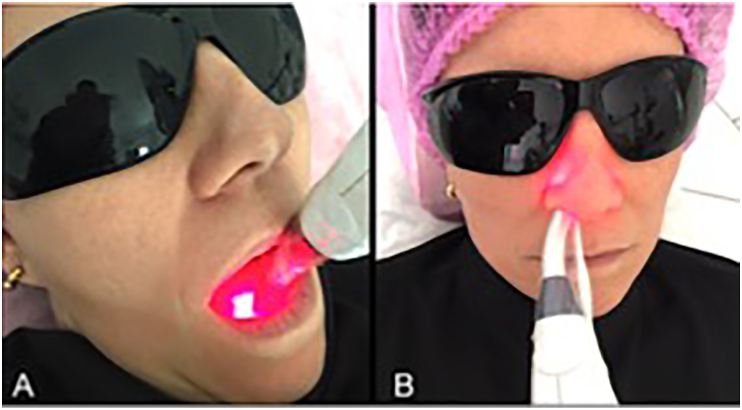
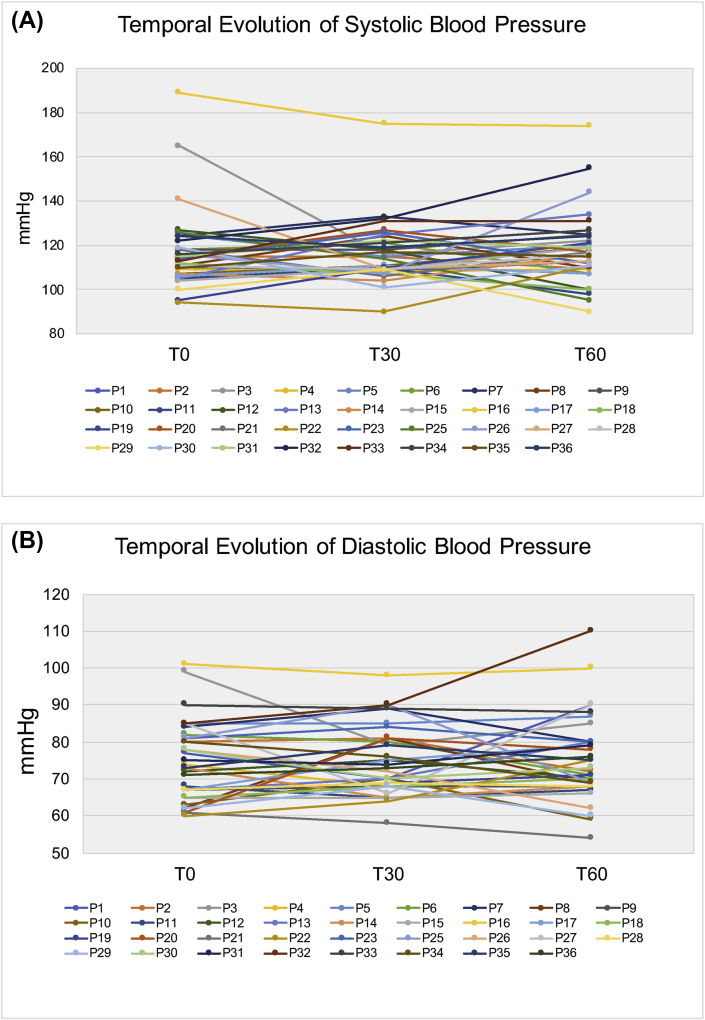
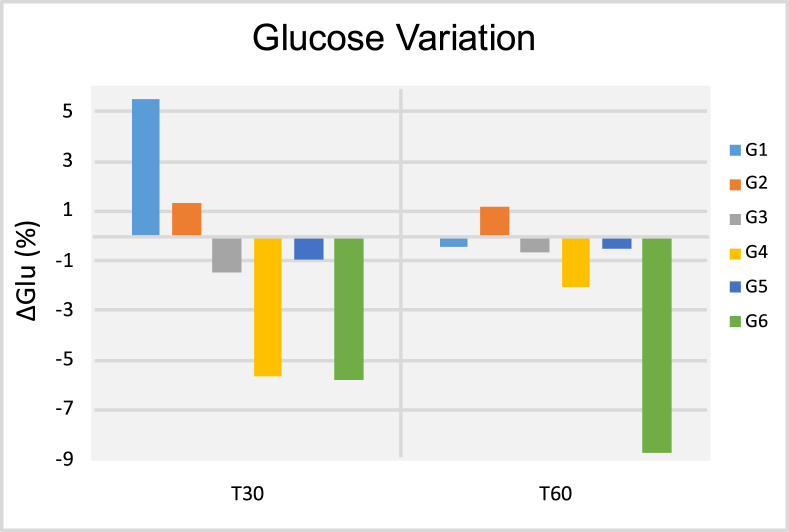
Figure 1 (A and B) illustrates transcutaneous irradiation using either a commercially available (1A) or prototype (1B) laser device. Figure 2 (A and B) illustrates sublingual transmucosal (2A) or intranasal (2B) irradiation. Figure 3 illustrates the temporal evolution (T0, T30 and T60) of systolic (3A) and diastolic (3B) blood pressures for patients (P, #1–36) pertaining to experimental groups G1-G6. Results obtained have indicated that patients' systolic and diastolic blood pressures were distributed within a range of values that varied from 90 mmHg (P22, T30, G4) to 189 mmHg (P16, T0, G3) and 54 mmHg (P21, T60, G4) to 175 mmHg (P16, T30, G3), respectively. It is also possible to observe that P16 (G3) displayed the highest systolic (T0, T30 and T60) and diastolic (T0 and T30) pressures amongst all patients investigated. Even though P32 was subjected to extended radial artery transcutaneous irradiation, P32's systolic (132 mmHg and 155 mmHg) and diastolic (90 mmHg and 110 mmHg) pressures at T30 and T60, respectively, were significantly higher when compared to P32's systolic and diastolic pressures at T0 (122 mmHg and 85 mmHg), which was a surprising and unexpected result. Figures 4, 5, 6, 7, and 8 demonstrate the average percentage variation (i.e., Δx = Xfinal-Xinitial) in glucose (ΔGlu), triglycerides (ΔTg), total cholesterol (ΔCh), high-density level cholesterol (ΔHDL) and low-density cholesterol (ΔLDL) observed at T30 and T60, respectively. It is possible to observe in Figure 4 that average glucose levels at T30 and T60 varied from 5.53% (G1) to -5.78% (G6), and 1.21 (G2) to -8.69 (G6), respectively. The lowest percent averages of glucose in circulating blood were observed in groups G4 and G6 at both T30 and T60 time-points. These findings suggest that transcutaneous ILIB is capable to promote down-regulation of glucose levels in blood at both time-points investigated.
Figure 1.
Radial artery transcutaneous irradiation using (A) pen-shaped dual-wavelength low intensity level laser (Laser Duo, MMO, Brazil; 660 nm and 808 nm, 100 mW/each, spot size = 0.03 cm2) and a prototype bracelet-shaped single-wavelength low intensity level laser (660 nm, 100 mW, spot size = 4.0 cm2; LAT, São Carlos Physics Institute).
Figure 2.
(A) Transmucosal sublingual irradiation and (B) transmucosal intra-nasal irradiation using pen-shaped dual-wavelength low intensity level laser (Laser Duo, MMO, Brazil; 660 nm and 808 nm, 100 mW/each, spot size = 0.03 cm2).
Figure 3.
Temporal evolution (T0, T30 and T60) of (A) systolic and (B) diastolic blood pressures for patients 1–36.
Figure 4.
Results of the glucose variation (ΔGlu [%]) after thirty (T30) and sixty days (T60) of treatment.
Figure 5.
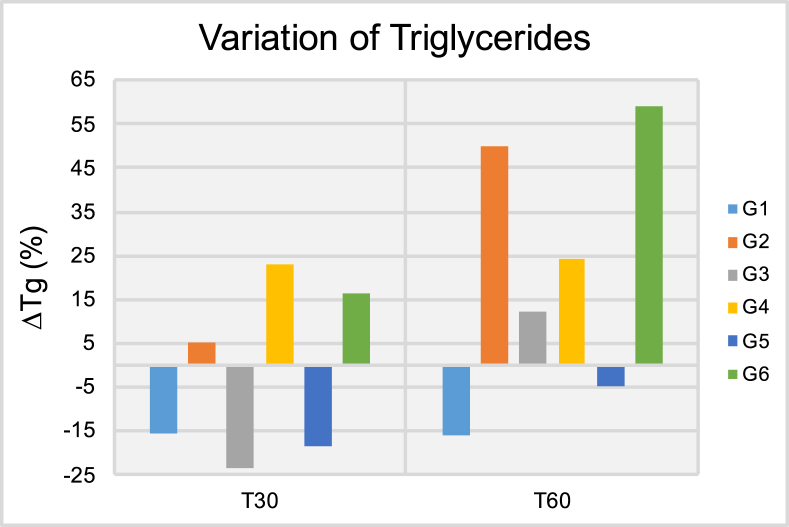
Results of the triglycerides variation (ΔTg [%]) after thirty (T30) and sixty days (T60) of treatment.
Figure 6.
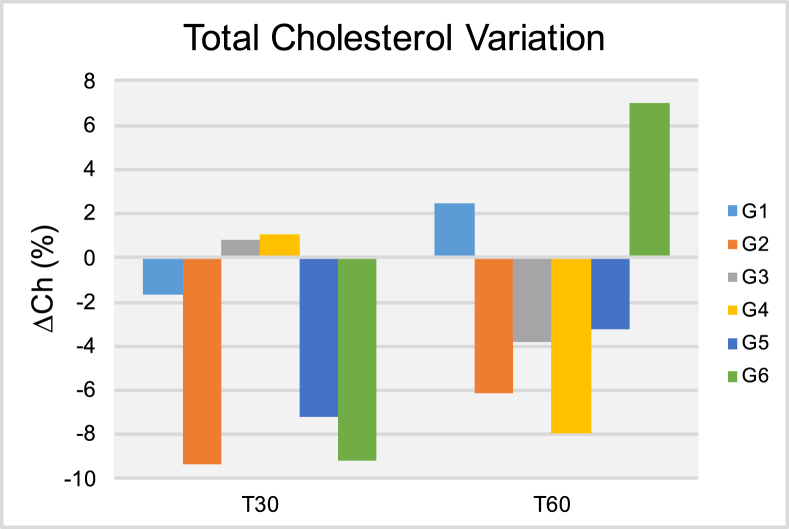
Results of the total cholesterol variation (ΔCh [%]) after thirty (T30) and sixty days (T60) of treatment.
Figure 7.
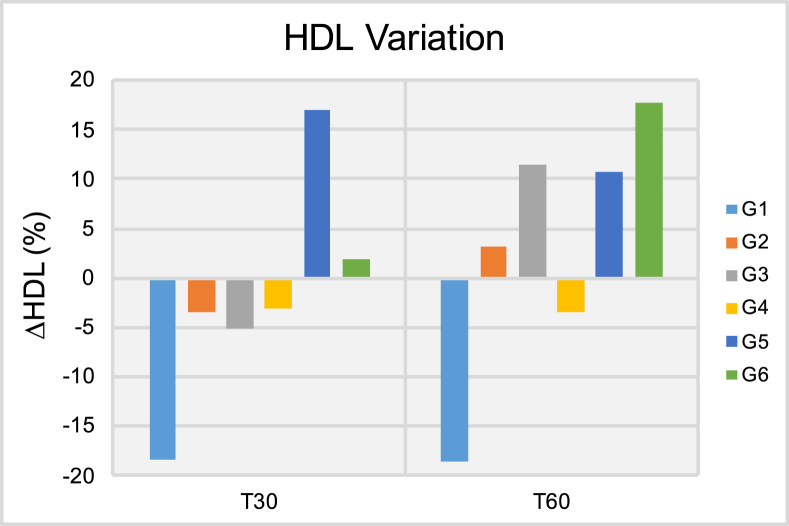
Results of the high-density cholesterol variation (ΔHDL [%]) after thirty (T30) and sixty days (T60) of treatment.
Figure 8.
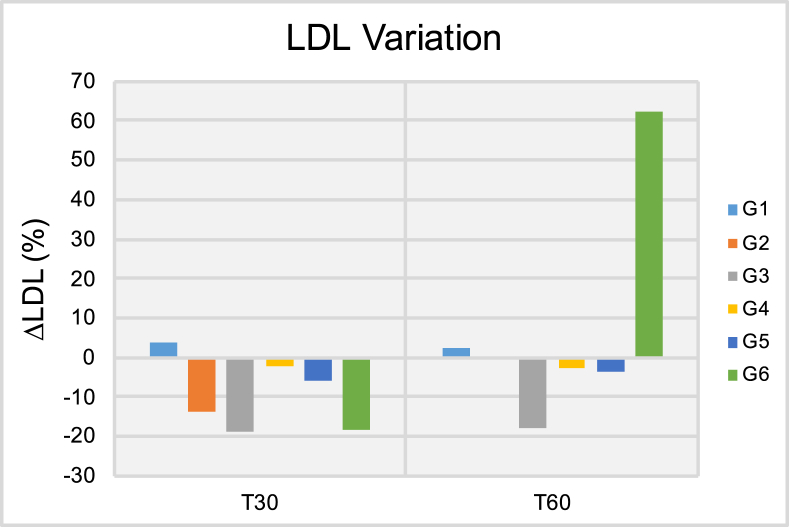
Results of the low-density cholesterol variation (ΔLDL [%]) after thirty (T30) and sixty days (T60) of treatment.
Figure 5 shows the ΔTg results in circulating blood at both T30 and T60 time-points. It is possible to observe at T30 that patients pertaining to groups G1, G3 and G5 have experienced significant variations in average triglycerides levels that ranged from -15.34% (G1) to -23.49% (G3). Despite these promising results, at T60, patients in groups G2-G4 and G6 have experienced positive and significant variations in triglycerides levels in circulating blood that ranged from 12.23% (G3) to 59.18% (G6). Taken together, these findings indicate that independently of delivery mode (either transcutaneous or transmucosal; single or extended application) ILIB was not able to display positive effects for triglycerides levels at T60. Figure 6 demonstrates the ΔCh results at both T30 and T60, where it is possible to observe that patients pertaining to groups G2, G5 and G6 have experienced significant reductions in total cholesterol levels T30. At T60, patients in G2 and G5 continued to experience lower cholesterol levels when compared to those of T0, however, the reduction experienced was of a smaller magnitude. Patients in G6 experienced significant variation in ΔCh results that ranged from -9.16% (T30) to 6.98% (T60). Figure 7 illustrate the ΔHDL results where it is possible to observe that patients in G1 experienced the most significant reductions in HDL levels among all groups investigated independently of time-point considered (either T30 or T60). At T60, patient in groups G2, G3, G5 and G6 displayed significant increases in HDL levels that varied from 3.15% (G2) to 17.78% (G6). Figure 8 indicates that patients from G2-G5 have experienced moderate variations in LDL levels at both T30 and T60 that ranged from -1.96% (G4, T30) to -18.60% (G3, T30). Patients in group G6 displayed strong variations in LDL levels that ranged from -18.26% (T30) to 62.15% (T60).
It is important to note that all data reported in the present pilot study have been normalized to patients’ initial conditions to allow better observation of outcomes promoted by the laser irradiation protocols investigated.
4. Discussion
The present study had the objective to investigate the effects (at 30 and 60 days) of ILIB (transcutaneous or transmucosal) on arterial blood pressure (systolic and diastolic) and on blood-related biomarkers such as glucose, triglycerides and cholesterol (total, high-density and low-density). The rationale for the selection of such experimental design was based on previous scientific evidence [13, 14, 15, 16, 17, 18, 19] that reported ILIB's capability to improve blood's rheological properties, improve wound healing and immune system, while modulating numerous chemical and enzymatic reactions in circulating blood [27]. The ABP (systolic and diastolic) results reported suggested that ILIB has the potential to biomodulate some hemodynamic variables of concern for hypertensive patients. These findings have been corroborated by Madi [33] who has demonstrated that intra-nasal transmucosal ILIB irradiation (808 nm, 100 mW, 120 s, 12 J, spot size = 0.2 cm2) of hypertense pregnant women (18–49 years old) was able to significantly (p < 0.005) decrease ABP by reducing the vascular and systemic resistance. Chavantes et al. [34] suggested that decreases in ABP following laser irradiation precipitates from an endothelial action that improves blood flow and reduces peripherical vascular resistance.
Makela [35] suggested that diabetes is not a single disease, but the result of a combination of pathological metabolic states caused by inadequate transport and breakdown of glucose. According to the author ILIB is capable of reducing glucose and cholesterol, and stabilizes hormonal and immune system status [35]. The results presented in Figures 4, 5, 6, 7, and 8 illustrate the average percentage variation in glucose (ΔGlu), triglycerides (ΔTg), total cholesterol (ΔCh), high-density level cholesterol (ΔHDL) and low-density cholesterol (ΔLDL) observed at T30 and T60, respectively. The ΔGlu results presented in Figure 4 have clearly indicated that ILIB is capable of biomodulating the quantity of glucose in circulating blood where glycemic indexes were lower at 30 days. The only exception to this trend was observed for patients in G6 (extended radial artery transcutaneous irradiation), where glycemic indexes at 60 days were lower as compared to glucose levels observed at 30 days. These results suggest that extended laser irradiation could be used as an adjunct therapy to treat patients with diabetes. Khoo et al. [36] investigated the effect of ILIB on diabetes mellitus using a metabolomics approach. Results reported [36] have indicated that ILIB was effective in significantly (p < 0.0018) decreasing blood sugar levels by photo-biomodulating the pentose phosphate pathway, starch and sucrose metabolism, thereby corroborating the findings of the present study. The ΔTg results are presented in Figure 5, where it is possible to observe that ILIB was more efficient in down-regulating levels of triglycerides in circulating blood after 30 days, and effects at T60 could not be observed. These findings are in agreement with a previous report that demonstrated that 84% of patients treated displayed reductions in total cholesterol serum levels (-1.00 mg/dL to -32.00 mg/dL; p < 0.01). The unexpected increase in triglyceride serum levels reported at T60 can be explained by intrinsic and patient-dependent variations, drastic changes in dietary habits, and have been previously reported by other research groups [37].
The results shown in Figures 6, 7, and 8 illustrate the percentage variation of total cholesterol, HDL and LDL in circulating blood, where it is possible to observe that ILIB significantly reduced the serum levels of total cholesterol at both T30 and T60. The ΔCh reported ranged from 6.98% (G6, T60) to -9.33% (G2, T30). In addition to that, it is possible to observe that ILIB was able to lower the serum levels of LDL (at T30 and T60) while increasing the serum levels of HDL, and therefore, are in agreement with previous reports [37]. The present manuscript is the first instance in dentistry to demonstrate the positive effects of ILIB after 30- and 60 days in ABP and blood-related biomarkers. Results reported have clearly indicated that treatments investigated were effective in biomodulating the parameters of choice after 30 and 60 days. In addition, treated patients have indicated significant improvements in physical performance, mental sharpness, cognitive levels, weight loss and quality of lives.
Even though healthy patients are constantly being subjected to several stressor agents that precipitate from normal aging processes, the improvement observed after transcutaneous ILIB tend to be less pronounced even after extended periods of laser irradiation, therefore, based on results reported an on our clinical experience, we hypothesize that patients in a disease-associated condition will display improvements in parameters investigated that will be more significant than those observed on healthy patients. We propose that ILIB protocols investigated be used as coadjutant methods to control blood pressure, glucose and cholesterol (total, LDL and HDL). Additional studies are made necessary to confirm the results of the present study and to determine longitudinal effects of ILIB for longer periods of times, such as months to years.
5. Conclusions and summary
The present study was successful in determining the effects of systemic transcutaneous and or transmucosal laser therapy on the arterial blood pressure (systolic and diastolic), glucose, triglycerides and cholesterol (total, LDL and HDL) at 30 and 60 days. Photo-biomodulation was successfully achieved independently of power intensity, wavelength or delivery mode (transcutaneous or transmucosal). The results of the present study indicate that ILIB can be safely used as an adjunct method to regulate blood pressure (systolic and diastolic), glucose, triglycerides and cholesterol (total, LDL and HDL). The results of the present study have clearly demonstrated that, independently of irradiation route (either transcutaneous or transmucosal), laser therapy with wavelengths within the visible spectrum (606 nm) are capable to facilitate patients to achieve homeostasis. In addition to that, patients’ reports regarding their qualities of lives during the execution of the present study have strongly suggested that the laser therapy protocols herein proposed are safe and could be used without any restriction as a coadjutant method to control concerning systemic conditions. Despite these promising results, the results of the present study also indicate the need for additional studies (I) focused on the elucidation of the mechanisms of action by which photophysical effects are modulating biochemical reactions in humans, and (II) to determine the longitudinal (6 months–1 year) effects of ILIB.
Declarations
Author contribution statement
Rosane de Fátima Zanirato Lizarelli: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Simone Cecilio Hallak Regalo: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Clovis Grecco and Fernando Esteban Florez: Analyzed and interpreted the data; Wrote the paper.
Vanderlei Salvador Bagnato: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by the São Carlos Physics Institute of the University of São Paulo.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
The clinical trial described in this paper was registered at The Brazilian Clinical Trials Registry under the registration number U1111-1252-4644.
Acknowledgements
Commercially available low intensity laser systems have been graciously provided by MMO Ltda., whereas prototype laser sources described in the present study have been designed and manufactured by the technological support laboratory of the São Carlos Physics Institute.
References
- 1.(IQWiG), I. f. Q. a. E. i. H. C. 2010. What Is an Inflammation?https://www.ncbi.nlm.nih.gov/books/NBK279298/ [Google Scholar]
- 2.Aminov R.I. A brief history of the antibiotic era: lessons learned and challenges for the future. Front. Microbiol. 2010;1 doi: 10.3389/fmicb.2010.00134. 134-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scully C. Drug effects on salivary glands: dry mouth. Oral Dis. 2003;9:165–176. doi: 10.1034/j.1601-0825.2003.03967.x. [DOI] [PubMed] [Google Scholar]
- 4.Visvanathan V., Nix P. Managing the patient presenting with xerostomia: a review. Int. J. Clin. Pract. 2010;64:404–407. doi: 10.1111/j.1742-1241.2009.02132.x. [DOI] [PubMed] [Google Scholar]
- 5.Tayem Y.I. Non-steroidal anti-inflammatory drugs and antibiotics prescription trends at a central west bank hospital. Sultan Qaboos Univ. Med. J. 2013;13:567–573. doi: 10.12816/0003317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prevention., C. f. D. C. a. 2015. Transcript for CDC Telebriefing: New Vital Signs Report: Today’s Heroin Epidemic.http://www.cdc.gov/media/releases/2015/t0707-heroin-epidemic.html.2015 [Google Scholar]
- 7.Abuse, N. I. o. D. 2014. Abuse of Prescription Pain Medications Risks Heroin Use.https://www.drugabuse.gov/related-topics/trendsstatistics/infographics/abuse-prescription-pain-medications-risksheroin-use.2014 [Google Scholar]
- 8.Aminov R.I., Mackie R.I. Evolution and ecology of antibiotic resistance genes. FEMS Microbiol. Lett. 2007;271:147–161. doi: 10.1111/j.1574-6968.2007.00757.x. [DOI] [PubMed] [Google Scholar]
- 9.Desoutter A. Xerostomia and medication: a cross-sectional study in long-term geriatric wards. J. Nutr. Health Aging. 2012;16:575–579. doi: 10.1007/s12603-012-0007-2. [DOI] [PubMed] [Google Scholar]
- 10.Mysels D.J., Sullivan M.A. The relationship between opioid and sugar intake: review of evidence and clinical applications. J. Opioid Manag. 2010;6:445–452. doi: 10.5055/jom.2010.0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fraser A.D., Zhang B., Khan H., Ma H., Hersh E.V. Prescription opioid abuse and its potential role in gross dental decay. Curr. Drug Saf. 2017;12:22–26. doi: 10.2174/1574886311666160803111401. [DOI] [PubMed] [Google Scholar]
- 12.Meshalkin E. Nauka Novosibirsk; 1981. Application of Direct Laser Irradiation in Experimental and Clinical Heart Surgery. [Google Scholar]
- 13.Stroev E.A., Larionov V.A., Grigor'eva L.P., Makarova V.G., Dubinina [The treatment of diabetic angiopathies by endovascular low-intensity laser irradiation] Probl. Endokrinol. 1990;36:23–25. [PubMed] [Google Scholar]
- 14.Funk J.O., Kruse A., Kirchner H. Cytokine production after helium-neon laser irradiation in cultures of human peripheral blood mononuclear cells. J. Photochem. Photobiol., B. 1992;16:347–355. doi: 10.1016/1011-1344(92)80022-n. [DOI] [PubMed] [Google Scholar]
- 15.Ledin A.O., Dobkin V.G., Sadov A.Y., Galichev K.V., Rzeutsky V.S. Vol. 3829. ALT; 1999. Soft-laser Use in the Preoperative Preparation and Postoperative Treatment of the Patients with Chronic Lung Abscesses. SPIE. [Google Scholar]
- 16.Bamps M., Dok R., Nuyts S. Low-level laser therapy stimulates proliferation in head and neck squamous cell carcinoma cells. Front Oncol. 2018;8:343. doi: 10.3389/fonc.2018.00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gulsoy M., Ozer G., Bozkulak O., Tabakoglu H., Aktas E., Deniz G., Ertan C. LLLT increases lymphocyte proliferation. J. Photochem. Photobiol., B. 2006;82:199–202. doi: 10.1016/j.jphotobiol.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Neimark A.I., Muzalevskaia N.I. [Low-intensity laser radiation in preoperative preparation of patients with benign prostatic hyperplasia] Urologiia. 2000:11–15. [PubMed] [Google Scholar]
- 19.Gasparyan L.V., Makela A. 1998. Intravenous Laser Irradiation of Blood: Current State and Future Perspectives. [Google Scholar]
- 20.Xu L., Zhang C.-b., Xu N., Liu S.-x., Zhou L.-y. Quantum theory analysis on microscopic mechanism of low level laser irradiation mending rheology for blood. Optoelectron. Lett. 2010;6:77–80. [Google Scholar]
- 21.Meesters A.A., Pitassi L.H.U., Campos V., Wolkerstorfer A., Dierickx C.C. Transcutaneous laser treatment of leg veins. Laser Med. Sci. 2014;29:481–492. doi: 10.1007/s10103-013-1483-2. [DOI] [PubMed] [Google Scholar]
- 22.Mikhaylov V.A. The use of Intravenous Laser Blood Irradiation (ILBI) at 630-640 nm to prevent vascular diseases and to increase life expectancy. Laser Ther. 2015;24:15–26. doi: 10.5978/islsm.15-OR-02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dean L. 2005. Blood Groups and Red Cell Antigens.https://www.ncbi.nlm.nih.gov/books/NBK2263/ [Internet] [Google Scholar]
- 24.Wirz-Ridolfi Comparison between intravenous and various types of transcutaneous laser blood irradiation. Internet J. Laserneedle Med. 2013;3:6. [Google Scholar]
- 25.Mi X.Q., Chen J.Y., Cen Y., Liang Z.J., Zhou L.W. A comparative study of 632.8 and 532 nm laser irradiation on some rheological factors in human blood in vitro. J. Photochem. Photobiol., B. 2004;74:7–12. doi: 10.1016/j.jphotobiol.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 26.Patil U.A., Dhami L.D. Overview of lasers. Indian J. Plast. Surg.: Off. Publ. Assoc. Plastic Surg. India. 2008;41:S101–S113. [PMC free article] [PubMed] [Google Scholar]
- 27.Karu T.I. Vol. 4159. EBO; 2000. Mechanisms of Low-Power Laser Light Action on Cellular Level. SPIE. [Google Scholar]
- 28.Osipov A.N., Machneva T.V., Buravlev E.A., Vladimirov Y.A. Effects of laser radiation on mitochondria and mitochondrial proteins subjected to nitric oxide. Front. Med. 2018;5 doi: 10.3389/fmed.2018.00112. 112-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karu T. Photobiology of low-power laser effects. Health Phys. 1989;56:691–704. doi: 10.1097/00004032-198905000-00015. [DOI] [PubMed] [Google Scholar]
- 30.Karu T.I. Multiple roles of cytochrome c oxidase in mammalian cells under action of red and IR-A radiation. IUBMB Life. 2010;62:607–610. doi: 10.1002/iub.359. [DOI] [PubMed] [Google Scholar]
- 31.Association W.M. Ethical principles for medical research involving human subjects. J. Am. Med. Assoc. 1975;301:2191–2194. [Google Scholar]
- 32.Ogedegbe G., Pickering T. Principles and techniques of blood pressure measurement. Cardiol. Clin. 2010;28:571–586. doi: 10.1016/j.ccl.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Madi O. Universidade Nove de Julho; 2015. Análise Da Resposta Hemodinâmica Imediata da Aplicação do Laser de Baixa Intensidade em Gestantes Hipertensas and Normotensas. Masters thesis. [Google Scholar]
- 34.Chavantes M.C., Morais T.L., Pinto N.C., Tomimura S., Silva-Assunção B.P., Canal M., Nakata L.S., Callado I.S., Lopes H., Consolin-Colombo F. Study’s significance from arterial elasticity and variation in arterial blood pressure for normotensive and hypertensive patients applying pre and post lasertherapy: preliminary results. BIOS-Proc. 2014;46:8926–8977. [Google Scholar]
- 35.Makela A.M. Laser Florence; 2004. Theoretical Backgrounds for Light Application in Diabetes. [Google Scholar]
- 36.Kazemi Khoo N. A metabolomic study on the effect of intravascular laser blood irradiation on type 2 diabetic patients. Laser Med. Sci. 2013;28:1527–1532. doi: 10.1007/s10103-012-1247-4. [DOI] [PubMed] [Google Scholar]
- 37.Jackson R.F., Roche G.C., Wilser K. Reduction in cholesterol and triglyceride serum levels following low-level laser irradiation: a noncontrolled, nonrandomized pilot study. Am. J. Cosmet. Surg. 2010;27:8. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.