Abstract
Aim and Background
Covid-19 has been as an important human infectious disease that has affected several countries. Cytokine storm has major role is Covid-19 pathogenesis. The association between inflammation and oxidative stress is well stablished. In this article, we aim to assess oxidative stress markers in Covid-19 patients compare to the healthy subjects.
Method
A total of 48 persons (24 with Covid-19 and 24 controls) were evaluated in this research. Serum oxidative stress markers including Malondialdehyde (MDA), total oxidant status (TOS), activity of catalase (CAT) and super oxide dismutase (SOD) were measured alongside routine laboratory tests.
Results
Patients group were divided into ICU and Non-ICU groups. ESR, CRP and serum level of ferritin were significantly higher in case group. Serum level of albumin was significantly lower in Covid-19 patients. Serum MDA and TOS was significantly increased in Covid-19 patients. Also, Covid-19 patients had higher serum activity of CAT and GPX.
Conclusion
Oxidative stress markers are significantly elevated in Covid-19 patients. This may have significant role in mechanism of disease development. In the fight against Covid-19, as a global struggle, all possible treatments demand more attention. So, Covid-19 patients may benefit from strategies for reducing or preventing oxidative stress.
Key Words: Covid-19, Oxidative stress, Inflammation, Pathogenesis
Introduction
Coronavirus disease 2019 (Covid-19) pandemic has rapidly spread around the world and encountered as a significant threat to global health. This disease identified for the first time in December 2019 in Wuhan, China (1). The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), an enveloped RNA betacoronavirus, is pathogenic agent of Covid-19 disease (2). Easily transmission of SARS-CoV-2 has major role in widely spread of this viral infection (3). The clinical course of Covid-19 may vary from asymptomatic to sever symptoms (acute lung injury), hospitalization for 3–4 d, transfer to intensive care unit (ICU) and even death (4). Upon the past months, Covid-19 crisis has dramatically increased risk of detrimental outcomes. Therefore, it is required to identify complicated pathogenic mechanisms of disease to decrease the risk of hospitalization and mortality. Commonly, respiratory viral infections have close association with inflammation and cytokine production (5) High serum level of cytokines and chemokines are reported in Covid-19 patients (6). In some cases, cytokine storm developed which is regarded as a key factor in acute respiratory distress syndrome development and various organ dysfunctions (7). Oxidative stress has critical function in inflammatory processes; reactive oxygen species (ROS) and H2O2 can activate NF-κB to trigger inflammatory cytokine production (8). It is postulated that oxidative stress is associated with pathogenesis of SARS-CoV-2 infection (9). In this research, we measured oxidative markers including TOS (total oxidant status), MDA (Malondialdehyde) and antioxidant markers including CAT (catalase), SOD (super oxide dismutase) in covid-19 patients compare to healthy control subjects.
Method and Material
Patients and Informed Consent
This case-control study was conducted at the public hospitals of Hamadan province in Iran, which were designated for Covid-19 patients. Twenty-four covid-19 patients (female: male, 17:7) and 24 healthy subjects (female: male, 14:10) were enrolled. The inclusion criteria for the control and case group were as follows: healthy subjects without any underlying condition and patients with laboratory- confirmed Covid-19 without any underlying condition, respectively. The Covid-19 diagnosis was based on World Health Organization interim guidelines (10) infection is suspected: interim guidance 2020. Subjects with a history of diabetes, hypertension, cancers, auto immune disorders, and smokers were excluded both from control and case group. Also, subjects excluded if they had special regimen or take antioxidant supplements such as vitamin C, vitamin E, Coenzyme Q10, selenium, and so on. The protocol of this study was approved by Hamadan University of Medical Sciences with code 9906113797 and ethical code: IR.UMSHA.REC.1399.441. All patients gave their informed consent before being included in the investigation.
Sample Collection
Venous blood samples were collected in any time of the day within 24 h after admission. The samples were centrifuged at 3000g for 10 min; serum was separated, aliquot and stored at –20 C till analyzed.
Biochemical Assays
After blood samples collection, routine laboratory tests, such as CBC, C-reactive protein (CRP), ESR were done. Also, serum Ferritin level was determined by Pishgaman Sanjesh kit. Serum albumin was measured by Man company kit. CRP was measured by a semi-quantitative latex agglutination technique (Bionik slide agglutination test kit). ESR was quantified by a SE 9020 analyzer and CBC was determined by a Sysmex (Kolbe, Japan) SE 9020 analyzer.
Measurement of Oxidative Stress Markers
Serum MDA level measurement was based on Yagi method. Thiobarbituric acid (TBA) reactive substances formation, as a reaction product, was measured at a wavelength of 532 nm using spectrophotometry (11). The serum level of TOS was evaluated according to Erel, s method (12). SOD and CAT serum level were measured using the commercially available ELISA kits according to the instructions of the company (KIAZIST Life Sciences, Iran. Cat. No: KSOD96 and KCAT96, respectively).
Statistical Analysis
The distribution of qualitative data was described by frequencies and percentages and compared between three groups (control, Non-ICU, and ICU patients) by Fisher exact test. The quantitative data were described as the mean ± standard deviation (SD) and their Normal distribution was evaluated by Shapiro-Wilk test. In case of normal distribution, the mean of quantitative data was compared in three groups using one-way Analysis of Variance (ANOVA). Moreover, the pairwise comparisons were done using Tuckey post hoc test. All analyses were performed at 0.05 significance levels using SPSS version 23 (SPSS Inc., USA) and GraphPad Prism version 6 for Windows.
Result
In accordance with clinical features and intensive care unit (ICU) admission, case group were divided into two groups: ICU and Non-ICU patients. The mean ± SD age of the control group (51.5 ± 13.58 years), Non-ICU (57.36 ± 10.87 years) and ICU patients (55.5 ± 15.65 years.) was not statistically significant (p >0.05). The main characteristics of case (ICU and Non-ICU) and control groups are shown in Table 1 . All subjects were comparable for age and sex. The control group was medication free, but all Covid-19 patient received Acetaminophen codeine, enoxaparin, azitromicina, dexamethasone, dextromethorphan, kaletra, naproxen, Vitamin D, and pantoprazole. Ten patients required ICU admission and treatment. The common symptoms on admission were fever (79.6%), cough (62.5%). respiratory distress (79%) and oxygen saturation (Spo2) less than 88 (75%).
Table 1.
Demographic and clinical characteristics of case (ICU and non-ICU patients) and control groups
|
Control |
No ICU |
ICU |
p* | ||||
|---|---|---|---|---|---|---|---|
| Characteristics | Number (N = 24) | % | Number (N = 14) | % | Number (N = 10) | % | |
| Age (year) | 51.5 ± 13.58 | 57.36 ± 10.87 | 55.5 ± 15.65 | 0.402 | |||
| Gender | |||||||
| Male | 7 | 29.2 | 5 | 35.7 | 5 | 50.0 | 0.575 |
| Female | 17 | 70.8 | 9 | 64.3 | 5 | 50.0 | |
| Education Level | |||||||
| Illiterate | 5 | 20.8 | 2 | 14.3 | 2 | 20.0 | 0.799 |
| Primary | 12 | 50.0 | 6 | 42.9 | 3 | 30.0 | |
| Diploma | 5 | 20.8 | 3 | 21.4 | 4 | 40.0 | |
| Academic | 2 | 8.3 | 3 | 21.4 | 1 | 10.0 | |
| Smoke | |||||||
| No | 18 | 75.0 | 13 | 92.9 | 7 | 70.0 | 0.369 |
| Yes | 6 | 25.0 | 1 | 7.1 | 3 | 30.0 | |
| Fever | |||||||
| No | - | - | 5 | 35.7 | 0 | 0.0 | 0.053 |
| Yes | - | - | 9 | 64.3 | 10 | 100.0 | |
| Cough | |||||||
| No | - | - | 6 | 42.9 | 3 | 30.0 | 0.678 |
| Yes | - | - | 8 | 57.1 | 7 | 70.0 | |
| Respiratory distress | |||||||
| No | - | - | 5 | 35.7 | 0 | 0.0 | 0.053 |
| Yes | - | - | 9 | 64.3 | 10 | 100.0 | |
| SPO2 | |||||||
| ≥88 | - | - | 4 | 71.4 | 2 | 20.0 | 0.506 |
| <88 | - | - | 10 | 92.9 | 8 | 80.0 | |
*p-value was assessed by exact Fisher rest.
Some of the biochemical and hematological characteristics are shown in Table 2 . The mean level of ESR, CRP, Ferritin and Neutrophil count were significantly highest in ICU group and the lowest in control group (p <0.001). In addition, a significant difference was observed between the mean level of albumin and Lymphocyte count between these three groups: the lowest level in ICU group and highest level in control group. Moreover, no significant differences were observed in WBC, hemoglobin, and platelet count between these groups. Pairwise comparison of significant characteristics is indicated in Figure 1 .
Table 2.
The baseline characteristics of the case (ICU and non-ICU patients) and control groups
|
Mean (SD) |
p | |||
|---|---|---|---|---|
| Characteristics | Control (n = 24) | NO ICU (n = 14) | ICU (n = 10) | |
| ESR (mm/h) | 9.88 (7.04) | 35.86 (14.10) | 43.20 (17.72) | <0.001 |
| CRP (mg/l) | 6.21 (2.21) | 43.93 (22.80) | 49.80 (19.14) | <0.001 |
| Ferritin (µg/l) | 90.33 (35.50) | 517.57 (270.78) | 544.70 (188.81) | <0.001 |
| Albumin(g/dl) | 5.07 (0.48) | 3.40 (0.38) | 2.86 (0.97) | <0.001 |
| White blood cell count, × 10⁹ per L | 6483.33 (1632.46) | 6907.14 (4695.74) | 8110.00 (4894.99) | 0.480 |
| Neutrophil count × 10⁹ per L | 64.37 (10.62) | 76.14 (13.57) | 80.80 (8.51) | <0.001 |
| Lymphocyte count × 10⁹ per L | 33.46 (10.80) | 21.28 (12.31) | 16.70 (7.53) | <0.001 |
| Haemoglobin, g/l | 12.74 (2.04) | 12.61 (0.89) | 12.73 (2.35) | 0.977 |
| Platelete count × 10⁹ per L | 225.92 (55.59) | 196.71 (50.49) | 217.10 (113.64) | 0.468 |
*p value was determined by ANOVA.
Figure 1.
.
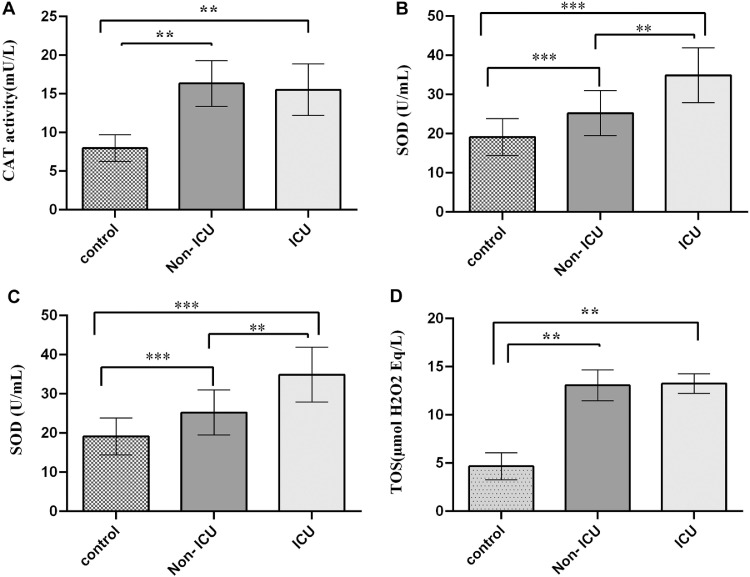
Assessment of TOS, MDA, CAT and SOD in Serum
The serum level of the TOS, MDA, CAT and SOD are reported in Figure 1. Our results showed that CAT activity was significantly higher in case groups (Non-ICU and ICU) compared to control group: 16.34 ± 2.97, 15.54 ± 3.33 and 8.05 ± 1.72 (mU/L), respectively; p <0.001, (Figure 1A). CAT activity showed no significant difference between Non-ICU and ICU group. The serum level of SOD was significantly increased in case group 25.22 ± 5.75 and 34.89 ± 7 (U/mL) in comparison to control group 19.11 ± 4.72 (U/mL); p <0.001. Also, there was a significant difference in serum SOD level between Non-ICU and ICU group; p <0.01(Figure 1B). We observed that oxidative stress increases in Covid-19 patients. MDA serum levels as an indicator of lipid peroxidation, were nearly 3 times higher in case groups in comparison to control group: 1.54 ± 0.24 and 1.78 ± 0.18 (nmol/l) in Non-ICU and ICU, respectively VS 0.55 ± 0.24 (nmol/mL) in control group, p <0.001. Interestingly, MDA serum levels were significantly higher in ICU group compared to Non-ICU, p <0.05 (Figure 1C). TOS is another marker to assess total oxidation state of the body. The mean serum concentration of TOS in Control group was 4.67 ± 1.39 (µmol H2O2 Eq/L) which was significantly less than that of in case groups 13.08 ± 1.6 in Non-ICU and 15.13 ± 2 (µmol H2O2 Eq/L), p <0.001. There was no significant difference in TOS serum level between Non-ICU and ICU groups (Figure 1D)
Discussion
Several studies proposed the association between oxidative stress and Covid-19 pathogenesis (13,14). This study supplies proof that Covid-19 patients showed high serum level of oxidative stress and inflammatory markers and low serum level of antioxidants, in comparison with control group, especially in patients admitted in ICU. Our results presented high serum level of MDA in case groups. MDA is an important indicator of oxidative stress. There is a significant correlation between oxidative stress markers and respiratory viral infection particularly RNA viruses (15). In vitro and In vivo studies indicated that some viruses could change redox balance of cell. The beginning of oxidative stress by virus infection (such as respiratory syncytial virus) is necessary for activation of innate immunity by cytokines production (16). Besides, oxidative stress induced by several viruses involved in facilitation of virus replication inside the cell (17).
It is described the function of macrophage respiratory burst in reaction to Covid-19 infection, which can lead to ROS production (18). Over production of ROS/RNS have role in lung tissue injury and dysfunction of epithelial barrier induced by acute respiratory viral infections (19). NADPH oxidase 2(NOX2) has important role in ROS production, arterial dysfunction, and thrombosis (induced by platelet activation) (20). It is indicated NOX2 overactivation in COVID-19 patients (21).
Viruses can suppress antioxidant systems including super oxide dismutase, glutathione S-transferase, catalase, glutathione peroxidase in human alveolar type 2-like epithelial cells and small airway epithelial cells (22). Although we observed high level of antioxidant enzymes, that may be a remedial mechanism to counteract oxidative stress. CAT and SOD have major role in neutralization of free radicals including ROS and RNS (23). In line with our results, Zhu Z, et al, indicated high level of inflammatory markers such as ESR and CRP (24).
Reduced oxygen transport to the tissues, disseminated intravascular coagulopathy and sepsis are shown in COVID-19 patients (25). Hypoxia can produce reactive species such as superoxide and H2O2 which can up regulate the expression of inflammatory cytokines (13). In turn, inflammatory cytokines can increase oxidative stress markers via activation of macrophages, neutrophils, and endothelium cells (26). These interactions between oxidative stress and inflammatory cytokines can lead to several organ failures in COVID-19 patients who proceed to worsening the condition. “Cytokine storm” in covid-19 patients is well established (27,28). Cytokine storm has prominent role in development of acute respiratory distress syndrome and various organ dysfunctions (13). Inflammasome, as a cytosolic molecular complex, senses inflammatory signals to accelerate cytokines maturation. The NLR protein NLRP3/NALP3 makes well known inflammasme (29). In fact, Inflammasome is a significant component in cytokine storm development (27). ROS as an inflammasome activator (via direct activation of NLRP3/NALP3 inflammasome) has function in inflammation induced by COVID-19 virus and following blood dissemination. As well, it is suggested that adaptive immune response to oxidative stress may be associated with systemic injury (13). Also, ROS can indirectly increase inflammasoe via NF-κB activation (30,31). The results of this study indicated high serum level of antioxidants enzymes; this may be a remedial mechanism to counteract oxidative stress. CAT and SOD have major role in neutralization of free radicals including ROS and RNS (23). According to Table 3 , we observed hematological disorder in COVID-19 patients: increased level of ferritin, ESR and low count of lymphocytes. Lymphopenia is reported in nearly 85% of sever Covid-19 patients (6). In covid-19, uncontrolled inflammatory responses cause low count of lymphocytes. Reduced oxygen transport to the tissues, coagulopathy and sepsis are shown in COVID-19 patients (25). Hypoxia can produce reactive species such as superoxide and H2O2 which can up regulate the expression of inflammatory cytokines (25,26). In turn, inflammatory cytokines can increase oxidative stress markers via activation of macrophages, neutrophils, and endothelium cells (8). This interaction between oxidative stress and inflammatory cytokines can lead to several organ failures in COVID-19 patients who proceed to worsening condition. Ferritin with immune-inhibitory and pro-inflammatory effects, involves in cytokine storm (32). In line with our results, Mehta P, et al, reported the high serum level of ferritin in COVID-19 patients (27). It is hypothesized that high serum ferritin level may be an influencing factor on COVID19 severity, and reduction strategies in ferritin level may decrease exacerbation of this disease (33). In this study, we observed the low level of serum albumin in COVID-19 patients. Albumin has different bioactive roles. This negative acute phase reactant is involved in extracellular antioxidant system via binding metals, scavenging free radicals, and supplying thiol group (34). In consistent with our results, Zhou F, et al. (35) and Zhang Y, et al. (36) reported low level of serum albumin in sever Covid-19 patients. Huang J, et al. (37), in a retrospective study, indicated that serum level of albumin has inverse association with death risk in Covid-19 patients, independent of other markers including lymphocyte count or comorbidities. Low level of serum albumin in COVID-19 patients may be explained by systemic inflammation. The therapeutic role of albumin in inflammatory disorder (such as sepsis) is mediated via controlling effect on inflammation and oxidative stress (38,39). However, the advantage role of albumin in COVID-19 patients should be documented in more studies.
Table 3.
Pairwise comparison of the characteristics of the case (ICU and non-ICU) and control groups using Tuckey post hoc
| Characteristics | Mean Difference | SD |
95% Confidence Interval |
p | |
|---|---|---|---|---|---|
| Lower Bound | Upper Bound | ||||
| ESR (mm/h) | |||||
| (control)-(Non-ICU) | –25.98 | 4.06 | –35.82 | –16.15 | <0.001 |
| (control)-(ICU) | –33.32 | 4.54 | –44.33 | –22.32 | <0.001 |
| (Non-ICU)-(ICU) | –7.34 | 5.00 | –19.45 | 4.77 | 0.315 |
| CRP (mg/l) | |||||
| (control)-(Non-ICU) | –37.72 | 5.05 | –49.97 | –25.47 | <0.001 |
| (control)-(ICU) | –43.59 | 5.66 | –57.30 | –29.88 | <0.001 |
| (Non-ICU)-(ICU) | –5.87 | 6.22 | –20.95 | 9.21 | 0.616 |
| Ferritin (µg/l) | |||||
| (control)-(Non-ICU) | –427.24 | 57.23 | –565.93 | –288.55 | 0.000 |
| (control)-(ICU) | –454.37 | 64.05 | –609.59 | –299.14 | 0.000 |
| (Non-ICU)-(ICU) | –27.13 | 70.45 | –197.88 | 143.63 | 0.922 |
| Albumin(g/dl) | |||||
| (control)-(Non-ICU) | 1.67 | 0.20 | 1.19 | 2.16 | 0.000 |
| (control)-(ICU) | 2.21 | 0.22 | 1.67 | 2.75 | 0.000 |
| (Non-ICU)-(ICU) | 0.54 | 0.24 | –0.06 | 1.13 | 0.085 |
| Neutrophil count × 10⁹ per L | |||||
| (control)-(Non-ICU) | –11.77 | 3.77 | –20.89 | –2.64 | 0.009 |
| (control)-(ICU) | –16.42 | 4.21 | –26.64 | –6.21 | 0.001 |
| (Non-ICU)-(ICU) | –4.66 | 4.64 | –15.89 | 6.58 | 0.578 |
| Lymphocyte count × 10⁹ per L | |||||
| (control)-(Non-ICU) | 12.17 | 3.60 | 3.44 | 20.90 | 0.004 |
| (control)-(ICU) | 16.76 | 4.03 | 6.99 | 26.53 | 0.000 |
| (Non-ICU)-(ICU) | 4.59 | 4.43 | –6.16 | 15.33 | 0.559 |
Conclusion
There is a clear association between oxidative stress and severity of several viral diseases. Our results indicated that Covid-19 patients suffer from oxidative stress which may aggravate patients, condition. One of the advantages of this study is that we exclude any underlying condition which may have effect on oxidative stress. Over production of free radicals and defect in antioxidant system have major role in SARS-COV pathogenesis. On the other hand, the cross talk between oxidative stress and cytokine storm may have significant effect on the severity of Covid-19 patient's symptoms. It seems that strategies for reducing or preventing of oxidative stress may help in Covid-19 managements. It is noted that our study has several limitations: the first and the most important one is small sample size. Secondly, we could not collect data on dietary patterns of subjects. Thirdly, all the participants were middle-aged. More clinical studies are necessary and should address young, aged patients.
Consent to Participate
All participants provided written informed consent.
Conflict Interest
All authors declared no conflict of interest regarding this paper.
Acknowledgments
The authors appreciate the Deputy of Research and Technology, Hamadan University of Medical Sciences for their financial support in this research.
References
- 1.Peng X, Xu X, Li Y, et al. Transmission routes of 2019-nCoV and controls in dental practice. Int J Oral Sci. 2020;12:1–6. doi: 10.1038/s41368-020-0075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wax RS, Christian MD. Practical recommendations for critical care and anesthesiology teams caring for novel coronavirus (2019-nCoV) patients. Can J Anaesth. 2020;67:568–576. doi: 10.1007/s12630-020-01591-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gautret P, Lagier J-C, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;56 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valencia DN. Brief review on COVID-19: the 2020 pandemic caused by SARS-CoV-2. Cureus. 2020;12:e7386. doi: 10.7759/cureus.7386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delgado-Roche L, Mesta F. Oxidative Stress as Key Player in Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) Infection. Arch Med Res. 2020;51:384–387. doi: 10.1016/j.arcmed.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. Lancet (London, England) 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nanduri J, Yuan G, Kumar GK, et al. Transcriptional responses to intermittent hypoxia. Respir Physiol Neurobiol. 2008;164:277–281. doi: 10.1016/j.resp.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cecchini R, Cecchini AL. SARS-CoV-2 infection pathogenesis is related to oxidative stress as a response to aggression. Med Hypotheses. 2020;143 doi: 10.1016/j.mehy.2020.110102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.https://apps.who.int/iris/handle/10665/330893. Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected: interim guidance. (Accessed January 28, 2020).
- 11.Ohkawa H, Ohishi N, Yagi K, et al. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 12.Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Chem. 2005;38:1103–1111. doi: 10.1016/j.clinbiochem.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 13.Cecchini R, Cecchini AL, et al. SARS-CoV-2 infection pathogenesis is related to oxidative stress as a response to aggression. Med Hypotheses. 2020;143 doi: 10.1016/j.mehy.2020.110102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ntyonga-Pono M-P. COVID-19 infection and oxidative stress: an under-explored approach for prevention and treatment? Pan Afr Med J. 2020;35(Suppl 2):12. doi: 10.11604/pamj.2020.35.2.22877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Komaravelli N, Casola A. Respiratory Viral Infections and Subversion of Cellular Antioxidant Defenses. J Pharmacogenomics Pharmacoproteomics. 2014;5 doi: 10.4172/2153-0645.1000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim HJ, Kim CH, Ryu JH, et al. Reactive oxygen species induce antiviral innate immune response through IFN-λ regulation in human nasal epithelial cells. Am J Respir Cell Mol Biol. 2013;49:855–865. doi: 10.1165/rcmb.2013-0003OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gjyshi O, Bottero V, Veettil MV, et al. Kaposi's sarcoma-associated herpesvirus induces Nrf2 during de novo infection of endothelial cells to create a microenvironment conducive to infection. PLoS pathogens. 2014;10 doi: 10.1371/journal.ppat.1004460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jain SK, Parsanathan R, Levine SN, et al. The potential link between inherited G6PD deficiency, oxidative stress, and vitamin D deficiency and the racial inequities in mortality associated with COVID-19. Free Radic Biol Med. 2020;161:84–91. doi: 10.1016/j.freeradbiomed.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ivanov AV, Bartosch B, Isaguliants MG, et al. Oxidative Stress in Infection and Consequent Disease. Oxid Med, Cell Longev. 2017;2017 doi: 10.1155/2017/3496043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fuentes E, Gibbins JM, Holbrook LM, et al. NADPH oxidase 2 (NOX2): A key target of oxidative stress-mediated platelet activation and thrombosis. Trends Cardiovasc Med. 2018;28:429–434. doi: 10.1016/j.tcm.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 21.Violi F, Oliva A, Cangemi R, et al. Nox2 activation in Covid-19. Redox Biology. 2020;36 doi: 10.1016/j.redox.2020.101655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hosakote YM, Liu T, Castro SM, et al. Respiratory syncytial virus induces oxidative stress by modulating antioxidant enzymes. Am J Respir Cell Mol Biol. 2009;41:348–357. doi: 10.1165/rcmb.2008-0330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pham-Huy LA, He H, Pham-Huy C, et al. Free radicals, antioxidants in disease and health. Int J Biomed Sci. 2008;4:89–96. [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu Z, Cai T, Fan L, et al. Clinical value of immune-inflammatory parameters to assess the severity of coronavirus disease 2019. Int J Infect Dis. 2020;95:332–339. doi: 10.1016/j.ijid.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takada Y, Mukhopadhyay A, Kundu GC, et al. Hydrogen peroxide activates NF-kappa B through tyrosine phosphorylation of I kappa B alpha and serine phosphorylation of p65: evidence for the involvement of I kappa B alpha kinase and Syk protein-tyrosine kinase. J Biol Chem. 2003;278:24233–24241. doi: 10.1074/jbc.M212389200. [DOI] [PubMed] [Google Scholar]
- 27.Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet (London, England) 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rana MM. Cytokine storm in COVID-19: Potential therapeutics for immunomodulation. J Res Clin Med. 2020;8 38–38. [Google Scholar]
- 29.Martinon F. Signaling by ROS drives inflammasome activation. Eur J Immunol. 2010;40:616–619. doi: 10.1002/eji.200940168. [DOI] [PubMed] [Google Scholar]
- 30.Braciale TJ, Sun J, Kim TS, et al. Regulating the adaptive immune response to respiratory virus infection. Nat Rev Immunol. 2012;12:295–305. doi: 10.1038/nri3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muralidharan S, Mandrekar P. Cellular stress response and innate immune signaling: integrating pathways in host defense and inflammation. J Leukoc Biol. 2013;94:1167–1184. doi: 10.1189/jlb.0313153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abbaspour N, Hurrell R, Kelishadi R, et al. Review on iron and its importance for human health. J Res Med Sci. 2014;19:164–174. [PMC free article] [PubMed] [Google Scholar]
- 33.Vargas-Vargas M, Cortés-Rojo C. Ferritin levels and COVID-19. Rev Panam Salud Publica. 2020;44:e72. doi: 10.26633/RPSP.2020.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sitar ME, Aydin S, Cakatay U. Human serum albumin and its relation with oxidative stress. Clin Lab. 2013;59:945–952. [PubMed] [Google Scholar]
- 35.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet (London, England) 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y, Zheng L, Liu L, et al. Liver impairment in COVID-19 patients: A retrospective analysis of 115 cases from a single centre in Wuhan city. China. Liver Int. 2020;40:2095–2103. doi: 10.1111/liv.1445. [DOI] [PubMed] [Google Scholar]
- 37.Huang J, Cheng A, Lin S, et al. Individualized prediction nomograms for disease progression in mild COVID-19. J Med Virol Title. 2020;92:2074–2080. doi: 10.1002/jmv.25969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bohl DD, Shen MR, Kayupov E, et al. Is Hypoalbuminemia Associated with Septic Failure and Acute Infection After Revision Total Joint Arthroplasty? A Study of 4517 Patients from the National Surgical Quality Improvement Program. J Arthroplasty. 2016;31:963–967. doi: 10.1016/j.arth.2015.11.025. [DOI] [PubMed] [Google Scholar]
- 39.He Y, Xiao J, Shi Z, et al. Supplementation of enteral nutritional powder decreases surgical site infection, prosthetic joint infection, and readmission after hip arthroplasty in geriatric femoral neck fracture with hypoalbuminemia. J Orthop Surg Res. 2019;14:292. doi: 10.1186/s13018-019-1343-2. [DOI] [PMC free article] [PubMed] [Google Scholar]