Abstract
Background and Purpose: Drug-induced liver injury is challenging during tuberculosis treatment. There is no epidemiological data investigating the relation between dietary intake and the risk of drug-induced liver injury during tuberculosis treatment. The aim of this study is to investigate the association of food and nutrient intake with the incidence of tuberculosis-drug-induced liver injury.
Methods: A cohort study was conducted in two city-level tuberculosis-specialized hospitals in Linyi City and Qingdao City, China from January 2011 to December 2013. The dietary intake was assessed by a 3-day 24-h food recall survey and a standard food-frequency questionnaire. The liver functions including aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were monitored throughout the 6-month tuberculosis therapy. Liver injury was defined as ALT or AST higher than two times of the upper limit of normal (ULN). Liver dysfunction was defined as ALT or AST higher than the ULN. The ULN for ALT and AST is 40 U/L. Multivariate logistic regression analyses were performed to determine the dietary factors associated with the incidence of liver injury and liver dysfunction.
Results: A total of 605 patients were included in the analysis. During the treatment, 8.1% patients exhibited liver injury and 23.3% patients exhibited liver dysfunction. A lower intake of vegetables was associated with a higher risk of liver injury [OR (95% CI): 3.50 (1.52–8.08), P = 0.003) and liver dysfunction [OR (95% CI): 2.37 (1.31–4.29), P = 0.004], while a lower intake of cooking oil was associated with a lower risk of liver injury [OR (95% CI): 0.44 (0.20–0.96), P = 0.040)] and liver dysfunction [OR (95% CI): 0.51 (0.31–0.85), P = 0.009].
Conclusion: The current study indicated that the higher risks of tuberculosis-drug-induced liver injury and liver dysfunction were statistically associated with decreased vegetable intake and increased cooking oil intake.
Keywords: diet, drug-induced liver injury, tuberculosis, cooking oil, vegetable, cohort study
Introduction
Tuberculosis is a communicable disease caused by Mycobacterium tuberculosis and the infection mainly happens in the lung (about 85% of the cases). In 2018, WHO estimated that the world tuberculosis burden was 10.0 million (1). The standard tuberculosis treatment consists of a 2-month intensive phase and a 4-month continuation phase. In the intensive phase, four antibiotics were used, including isoniazid (INH), rifampin (RIF), pyrazinamide (PZA), and ethambutol (EMB); in the continuation phase, INH and RIF were used (2). This long-term and high-dose antibiotic treatment is effective but hepatotoxic.
Liver injury is defined as aspartate aminotransferase (AST) or alanine aminotransferase (ALT) two times higher than its upper limit of normal (ULN) (3). Liver dysfunction was defined as higher than the ULN. The ULN for both AST and ALT was 40 U/L (4). During tuberculosis treatment, 5–30% patients exhibited different levels of liver injury (5–7). Liver injury is usually accompanied by nausea, vomiting, abdominal pain and asthenia, while more severe liver injury can lead to treatment withdraw, reduced treatment efficacy, drug resistance and acute liver failure (8).
Tuberculosis patients are characterized by malnutrition including low body mass index (BMI), deficiency of protein and multiple vitamins (9–12). Malnutrition also plays an important role in drug-induced liver injury (13–15). Two cohort studies reported an association between a low BMI and an increased incidence of liver injury during tuberculosis treatment (14, 16). It is pivotal to understand the associations between dietary factors and tuberculosis-drug-induced liver injury.
However, to our knowledge, no epidemiological study has investigated the association between dietary intake and the risk of tuberculosis-drug-induced liver injury. For other liver diseases, a meta-analysis including 9 cohort studies concluded that a higher vegetable intake was associated with a 39% reduction in the risk of liver cancer (17). Cohort studies suggested that a higher consumption of fruits and vegetables (18) and nuts (19) was associated with a lower non-alcoholic fatty liver disease (NAFLD) risk. Randomized controlled trial indicated that whole grain consumption alleviated NAFLD (20).
The aim of this study is to investigate the associations between dietary food and nutrient intake and the incidence of tuberculosis-drug-induced liver injury by a cohort study.
Materials and Methods
Ethics
The study was approved by the Ethic Committee of Medicine of Qingdao Center of Disease Control and Prevention (2009-4). The study was conducted in accordance with the Declaration of Helsinki. All participants provided written informed consents. The trial was registered at the Chinese Clinical Trial Registry (No. ChiCTR-OCC-10000994).
Study Design and Population
The study was conducted from January 2011 to December 2013 in two hospitals located in Linyi City and Qingdao City, Shandong Province, China. Qingdao is a coastal city, while Linyi is an inland city. The inclusion criteria were: (1) newly diagnosed as pulmonary tuberculosis. The pulmonary tuberculosis was diagnosed by a combination of sputum smear, computed tomography scan and clinical symptoms (e.g., cough, haemoptysis, weight loss, fever and night sweat etc.); (2) ≥18 years old. The exclusion criteria were: (1) pregnant or lactating; (2) combined with other diseases including gastrointestinal, cardiovascular, respiratory diseases, cancer, liver diseases (such as hepatitis B or C, alcohol hepatitis etc.), HIV or mental diseases; (3) taking nutritional supplements in the previous 2 months; (4) drug-resistant tuberculosis; (5) elevated liver indices (AST or ALT higher than 40 U/L).
Procedure
At entry to the hospital, the weight and height were measured and the demographic information was collected using a standard questionnaire, including sex, age, diabetes, the history of liver diseases, outdoor exercise, education level etc.
Two weeks after starting the treatment, 24-h dietary recalls were conducted by investigators for three consecutive days including two weekdays and one weekend. The participants were asked to recall the food intake and its portion size. The investigators were trained to be familiar with the common local food and used a standard questionnaire to record. The nutrients intake was calculated using the China Food Composition Tables (21). Two months after starting the treatment, a semi-quantitative food frequency questionnaire (FFQ) was conducted to assess the dietary intake in the previous 2 months. The FFQ was modified from a previously validated FFQ for Chinese population (22). The FFQ included 26 items, including white rice, white flour, millet, bran, corn, tofu, beans, soybean milk, egg, chicken, duck, beef, pork, lamb, fish, shrimp, potato, sweet potato, taro, yam, dark vegetable, light vegetable, cooking oil, fruit, tea, dairy products and alcohol. Food consumption frequency was classified as “three times a day,” “twice a day,” “once a day,” “four to five times a week,” “two to three times a week,” “once a week,” “once every 2 weeks,” “once a month,” “once every 2 months,” and “almost never.” The amount of consumed foods was estimated in terms of the Chinese liang (equivalent to 50 grams). The amount of consumed tea was estimated in cups. The 26 items were classified into 15 food categories, including refined grains, whole grains, poultry, meat, vegetables, starchy vegetables, fruits, tofu and beans, egg, fish, cooking oil, dairy, shrimp, tea and alcohol.
All participants received the same standard tuberculosis treatment (2). The treatment consists of a 2-month intensive phase using INH, RIF, PZA and EMB, followed by a 4-month continuation phase using INH and RIF. The dosage for INH, RIF, PZA, and EMB were 0.3, 0.45, 0.75, and 1.5 g/d, respectively. The AST, ALT and albumin (ALB) were routinely checked by the hospital staff before the treatment, at 1, 2, and 6 months after starting the treatment. The liver function results were obtained from the hospital. Liver injury was defined as ALT or AST higher than two times of the ULN (3). Liver dysfunction was defined as ALT or AST higher than the ULN. The ULN for ALT and AST is 40 U/L (4).
Statistical Analysis
The intergroup difference was tested by a Mann–Whitney U-test for non-normal data, a t-test for normal data, and a χ2-test or a Fisher's exact-test for categorical data. Multivariate logistic analysis was conducted to investigate the dietary factors associated with the incidence of liver injury and liver dysfunction. The highest quantile of the intake of each food was used as the reference group. The age, sex, area, energy intake, BMI, diabetes, education level, and outdoor exercise were adjusted as covariates. The statistical analysis was conducted using SPSS 22.0. P < 0.05 was considered statistically significant.
Results
A total of 706 patients were recruited from two hospitals located in Linyi City and Qingdao City, China. Among all the participants, 38 had no or incomplete dietary information (FFQ and 3-day 24-h food recall), 13 reported extreme energy intake, and 50 did not have any follow-up liver function record or changing treatment plan. The median follow-up period was 180 days. During the follow-up, 49 patients exhibited liver injury; 141 patients exhibited liver dysfunction (Figure 1). The characteristics of liver injury and liver dysfunction events were presented in Supplementary Table 1. The median ALT/AST ratios for the liver injury and liver dysfunction group were 1.5 and 1.6, respectively. In comparison, the median ALT/AST ratios for the non-liver-injury and normal liver function group were 1.2 and 1.1, respectively.
Figure 1.
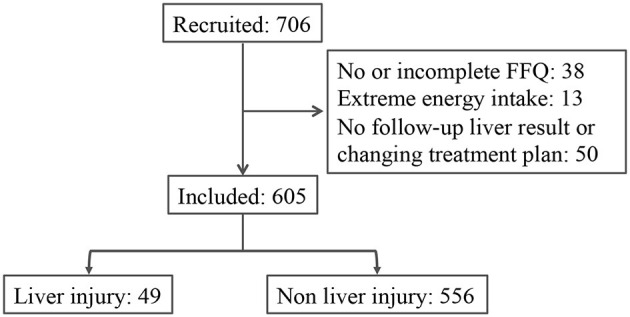
The trial flowchart.
The baseline characteristics were compared between the patients with and without liver injury during tuberculosis treatment (Table 1). The patients with liver injury had significantly higher education levels and more Qingdao residents compared to the patients in the non-liver-injury group. The other demographic parameters including age, gender, BMI, smoking, alcohol consumption, marital status, the history of liver diseases, diabetes and outdoor exercise were similar between the liver injury group and the non-liver-injury group. Compared to the patients in the normal liver function group, the patients in the liver dysfunction group had higher education levels, more Qingdao residents and less outdoor exercise.
Table 1.
The baseline characteristics of the participants classified by liver function.
| Non-liver-injury groupa | Liver injury group | Normal liver function group | Liver dysfunction group | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Valueb | n | Value | Pc | n | Value | n | Value | P | |
| Age | 556 | 50.0 (35.8) | 49 | 43.0 (30.0) | 0.153 | 464 | 50.5 (37.0) | 141 | 46.0 (28.5) | 0.054 |
| Gender | 556 | 49 | 0.429 | 464 | 141 | 0.107 | ||||
| Male | 402 (72.3%) | 38 (77.6%) | 330 (71.1%) | 110 (78.0%) | ||||||
| Female | 154 (27.7%) | 11 (22.4%) | 134 (28.9%) | 31 (22.0%) | ||||||
| Area | 0.000 | 464 | 141 | 0.000 | ||||||
| Qingdao | 179 (32.2%) | 31 (63.3%) | 129 (27.8%) | 81 (57.4%) | ||||||
| Linyi | 377 (67.8%) | 18 (36.7%) | 335 (72.2%) | 60 (42.6%) | ||||||
| BMI, kg/m2 | 551 | 20.8 ± 2.9 | 49 | 21.4 ± 3.0 | 0.133 | 459 | 20.7 ± 2.8 | 141 | 21.1 ± 3.3 | 0.154 |
| Smoking | 556 | 119 (21.4%) | 49 | 10 (20.4%) | 1.000 | 464 | 104 (22.4%) | 141 | 25 (17.7%) | 0.290 |
| Alcohol consumption | 556 | 88 (15.8%) | 49 | 8 (16.3%) | 0.841 | 464 | 69 (14.9%) | 141 | 27 (19.1%) | 0.237 |
| Marital status | 549 | 49 | 0.592 | 457 | 141 | 0.424 | ||||
| Single | 127 (23.1%) | 11 (22.4%) | 110 (24.1%) | 28 (19.9%) | ||||||
| Married | 402 (73.2%) | 38 (77.6%) | 330 (72.2%) | 110 (78.0%) | ||||||
| Widowed | 17 (3.1%) | 0 (0.0%) | 15 (3.3%) | 2 (1.4%) | ||||||
| Divorced | 3 (0.5%) | 0 (0.0%) | 2 (0.4%) | 1 (0.7%) | ||||||
| Education completed | 550 | 48 | 0.001 | 458 | 140 | 0.000 | ||||
| None | 78 (14.2%) | 2 (4.2%) | 76 (16.6%) | 4 (2.9%) | ||||||
| Primary school | 134 (24.4%) | 4 (8.3%) | 112 (24.5%) | 26 (18.6%) | ||||||
| Class VII-IX | 203 (36.9%) | 20 (41.7%) | 164 (35.8%) | 59 (42.1%) | ||||||
| Class X-XII | 92 (16.7%) | 12 (25.0%) | 73 (15.9%) | 31 (22.1%) | ||||||
| Diploma or higher | 43 (7.8%) | 10 (20.8%) | 33 (7.2%) | 20 (14.3%) | ||||||
| History of liver diseasesd | 550 | 6 (1.1%) | 49 | 2 (4.1%) | 0.134 | 458 | 5 (1.1%) | 141 | 3 (2.1%) | 0.400 |
| Diabetes | 554 | 87 (15.7%) | 49 | 13 (26.5%) | 0.051 | 462 | 70 (15.2%) | 141 | 30 (21.3%) | 0.087 |
| Outdoor exercise | 553 | 49 | 0.386 | 461 | 141 | 0.004 | ||||
| Almost none | 24 (4.3%) | 3 (6.1%) | 19 (4.1%) | 8 (5.7%) | ||||||
| <2 h per day | 181 (32.7%) | 20 (40.8%) | 139 (30.2%) | 62 (44.0%) | ||||||
| >2 h per day | 348 (62.9%) | 26 (53.1%) | 303 (65.7%) | 71 (50.4%) | ||||||
Liver injury group had an alanine aminotransferase (ALT) or aspartate aminotransferase (AST) level of higher than two times of the upper limit of normal (ULN); Non-liver-injury group had an ALT and AST level of equal or lower than two times of the ULN. Liver dysfunction had an ALT or AST level of higher than the ULN; Normal liver function group had an ALT and AST level of equal or lower than the ULN. The ULN for ALT and AST is 40 U/L.
Non-normal variables were presented as median (IQR); normal variables were presented as mean ± SD; categorical variables were presented as number of patients in a specific category (percentage).
P was calculated between the non-liver-injury group and liver injury group, and between the normal liver function group and liver dysfunction group. The statistical difference for the non-normal data was tested by a Mann-Whitney U-test; the difference for the normal data was tested by a t-test; the difference for the categorical data was tested by a χ2-test.
The intergroup difference for the history of liver diseases was tested by a Fisher's exact-test. P values were labeled in bold if they were less than 0.05.
The estimated total energy and macronutrients intake were compared between the patients with and without liver injury, and between the patients with and without liver dysfunction (Table 2). The result indicated a similar intake of total energy, carbohydrate and protein, while the intake of fat was significantly higher in the liver injury group compared to the non-liver-injury group (P = 0.028), and significantly higher in the liver dysfunction group compared to the normal liver function group (P = 0.002).
Table 2.
The estimated daily macronutrients intake for the participants.
| Non-liver-injury groupa (n = 556) | Liver injury group (n = 49) | Pc | Normal liver function group (n = 464) | Liver dysfunction group (n = 141) | P | |
|---|---|---|---|---|---|---|
| Total energy, kcal | 1,596.6 (796.1)b | 1,597.0 (637.9) | 0.795 | 1,578.5 (786.6) | 1,625.6 (807.3) | 0.218 |
| Carbohydrates, g | 263.9 (148.5) | 253.2 (99.0) | 0.196 | 265.0 (144.7) | 253.6 (137.6) | 0.798 |
| Protein, g | 65.1 (35.3) | 64.3 (36.5) | 0.540 | 64.4 (34.5) | 65.8 (40.0) | 0.378 |
| Fat, g | 23.3 (27.7) | 30.3 (38.2) | 0.028 | 22.9 (25.3) | 27.6 (38.6) | 0.002 |
Liver injury group had an alanine aminotransferase (ALT) or aspartate aminotransferase (AST) level of higher than two times of the upper limit of normal (ULN); Non-liver-injury group had an ALT and AST level of equal or lower than two times of the ULN. Liver dysfunction had an ALT or AST level of higher than the ULN; Normal liver function group had an ALT and AST level of equal or lower than the ULN. The ULN for ALT and AST is 40 U/L.
Numerical variables are presented as median (IQR).
P was calculated between the non-liver-injury group and liver injury group, and between the normal liver function group and liver dysfunction group. The statistical difference was tested by a Mann-Whitney U-test. P values were labeled in bold if they were less than 0.05.
A binary logistic regression was conducted to explore the association between dietary food intake and the risk of liver injury during tuberculosis treatment (Table 3). The model was adjusted for covariates including sex, age, energy intake, area, diabetes, education level, BMI and outdoor exercise. A lower intake of vegetables was associated with a higher risk of liver injury during tuberculosis treatment. Compared to the highest quantile of vegetable intake, the odds ratio (OR) of the middle quantile was 3.50 [95% confidence interval (CI): 1.52–8.08, P = 0.003]. However, the lowest quantile of vegetable intake was not associated with the risk of liver injury [OR (95% CI): 1.36 (0.44–4.18), P = 0.588]. A lower intake of cooking oil was associated with a lower risk of liver injury during tuberculosis treatment [OR (95% CI): 0.44 (0.20–0.96), P = 0.040)]. The intake of refined grains, whole grains, poultry, meat, starchy vegetables, fruits, tofu and beans, egg, fish, dairy, shrimp, and tea was not associated with the risk of liver injury. No alcohol intake was reported in our participants.
Table 3.
Dietary food intake in relation to the risk of liver injury and liver dysfunction during tuberculosis treatmenta.
| Quantile of food intakeb | n | Liver injury OR (95%CI) | Pc | n | Liver dysfunction OR (95%CI) | P |
|---|---|---|---|---|---|---|
| Refined grain (g/d) | ||||||
| <228.6 | 216 | 1.90 (0.77–4.70) | 0.166 | 216 | 0.77 (0.45–1.30) | 0.320 |
| 228.6–414.3 | 187 | 2.02 (0.81–5.02) | 0.132 | 187 | 1.01 (0.59–1.72) | 0.985 |
| ≥414.3 | 185 | Reference category | 185 | Reference category | ||
| Whole grain (g/d) | ||||||
| <6.7 | 207 | 0.48 (0.21–1.12) | 0.089 | 207 | 1.14 (0.67–1.92) | 0.635 |
| 6.7–57.1 | 165 | 0.61 (0.29–1.28) | 0.187 | 165 | 1.12 (0.68–1.83) | 0.661 |
| ≥57.1 | 216 | Reference category | 216 | Reference category | ||
| Poultry (g/d) | ||||||
| <5.0 | 196 | 0.78 (0.33–1.84) | 0.570 | 196 | 1.13 (0.67–1.91) | 0.657 |
| 5.0–14.3 | 194 | 0.92 (0.45–1.91) | 0.833 | 194 | 0.62 (0.37–1.04) | 0.071 |
| ≥14.3 | 198 | Reference category | 198 | Reference category | ||
| Meat (g/d) | ||||||
| <14.3 | 209 | 1.13 (0.44-2.87) | 0.799 | 209 | 1.15 (0.65–2.04) | 0.631 |
| 14.3–50.0 | 141 | 2.16 (0.99–4.74) | 0.054 | 141 | 1.46 (0.84–2.54) | 0.175 |
| ≥50.0 | 138 | Reference category | 138 | Reference category | ||
| Vegetable (g/d) | ||||||
| <78.6 | 129 | 1.36 (0.44–4.18) | 0.588 | 129 | 2.10 (1.11–3.95) | 0.022 |
| 78.6–200.0 | 113 | 3.50 (1.52–8.08) | 0.003 | 113 | 2.37 (1.31–4.29) | 0.004 |
| ≥200.0 | 346 | Reference category | 346 | Reference category | ||
| Starchy vegetable (g/d) | ||||||
| <13.3 | 182 | 0.82 (0.35–1.94) | 0.654 | 182 | 0.76 (0.44–1.31) | 0.323 |
| 13.3–42.9 | 188 | 0.80 (0.38–1.67) | 0.553 | 188 | 0.72 (0.44–1.17) | 0.187 |
| ≥42.9 | 218 | Reference category | 218 | Reference category | ||
| Fruit (g/d) | ||||||
| <14.3 | 188 | 0.69 (0.29–1.64) | 0.406 | 188 | 0.75 (0.43–1.29) | 0.293 |
| 14.3–71.4 | 182 | 0.90 (0.43–1.92) | 0.788 | 182 | 0.86 (0.52–1.42) | 0.558 |
| ≥71.4 | 218 | Reference category | 218 | Reference category | ||
| Tofu and bean (g/d) | ||||||
| <21.4 | 162 | 1.00 (0.47–2.14) | 0.998 | 162 | 0.87 (0.52–1.47) | 0.606 |
| 21.4–42.9 | 241 | 0.75 (0.36–1.56) | 0.433 | 241 | 0.80 (0.49–1.29) | 0.350 |
| ≥42.9 | 185 | Reference category | 185 | Reference category | ||
| Egg (g/d) | ||||||
| <42.9 | 222 | 1.00 (0.48–2.11) | 0.998 | 222 | 1.18 (0.73–1.93) | 0.502 |
| 42.9–57.1 | 141 | 0.69 (0.32–1.52) | 0.364 | 141 | 0.69 (0.40–1.18) | 0.174 |
| ≥57.1 | 225 | Reference category | 225 | Reference category | ||
| Fish (g/d) | ||||||
| <3.3 | 159 | 1.20 (0.49–2.93) | 0.684 | 159 | 1.64 (0.91–2.94) | 0.098 |
| 3.3–14.3 | 262 | 1.12 (0.54–2.32) | 0.768 | 262 | 1.33 (0.81–2.19) | 0.268 |
| ≥14.3 | 167 | Reference category | 267 | Reference category | ||
| Cooking oil (g/d) | ||||||
| <58.3 | 211 | 0.44 (0.20–0.96) | 0.040 | 211 | 0.51 (0.31–0.85) | 0.009 |
| 58.3–100.0 | 167 | 0.73 (0.35–1.54) | 0.411 | 167 | 0.63 (0.38–1.05) | 0.078 |
| ≥100.0 | 210 | Reference category | 210 | Reference category | ||
| Dairy (g/d) | ||||||
| 0 | 258 | 1.17 (0.53–2.57) | 0.702 | 258 | 1.06 (0.64–1.74) | 0.833 |
| 0.01–64.3 | 137 | 1.74 (0.81–3.74) | 0.158 | 137 | 1.26 (0.74–2.15) | 0.392 |
| >64.3 | 193 | Reference category | 193 | Reference category | ||
| Shrimp | ||||||
| No | 385 | 0.88 (0.42–1.81) | 0.719 | 385 | 0.94 (0.58–1.54) | 0.818 |
| Yes | 203 | Reference category | 203 | Reference category | ||
| Tea | ||||||
| No | 554 | 0.82 (0.26–2.59) | 0.735 | 554 | 0.96 (0.42–2.18) | 0.915 |
| Yes | 34 | Reference category | 34 | Reference category | ||
Multivariate logistic regression models were used to assess the odds ratios (ORs) and 95% confidence intervals (CIs) for tuberculosis-drug-induced liver injury and liver dysfunction. The model was adjusted by age, sex, area, energy intake, diabetes, education level, BMI and outdoor exercise.
The intake of each food category was divided into three quantiles, while the intake of shrimp and tea was divided into two groups. The highest quantile was used as the reference group.
P was calculated with the use of multivariate logistic regression model for liver injury and liver dysfunction. P values were labeled in bold if they were less than 0.05.
A similar regression was conducted to explore the association between dietary food intake and the risk of liver dysfunction during tuberculosis treatment (Table 3). A lower intake of vegetables was associated with a higher risk of liver dysfunction during tuberculosis treatment. Compared to the highest quantile of vegetables intake, the OR of the lowest quantile was 2.10 (95% CI: 1.11–3.95, P = 0.022) and the OR of the middle quantile was 2.37 (95% CI: 1.31–4.29, P = 0.004). The trend was significant (Ptrend = 0.004). A lower intake of cooking oil was associated with a lower risk of liver dysfunction during tuberculosis treatment [OR (95% CI): 0.51 (0.31–0.85), P = 0.009)]. The trend was also significant (Ptrend = 0.007). The intake of other foods was not associated with the risk of liver dysfunction.
A binary logistic regression was also conducted to explore the association between the calculated nutrient (e.g., protein, carbohydrate, dietary fiber, fat, vitamins, and minerals) intake and the risk of liver injury or liver dysfunction during tuberculosis treatment (Table 4). The result indicated that the included nutrient intake was not associated with the risk of liver injury or liver dysfunction during tuberculosis treatment.
Table 4.
Nutrients intake in relation to the risk of liver injury and liver dysfunction during tuberculosis treatmenta.
| Quantile of nutrient intakeb | n | Liver injury OR (95%CI) | Pc | n | Liver dysfunction OR (95%CI) | P |
|---|---|---|---|---|---|---|
| Protein (g/d) | ||||||
| <53.70 | 192 | 1.60 (0.54–4.72) | 0.397 | 192 | 1.29 (0.67–2.48) | 0.444 |
| 53.70–75.90 | 198 | 1.25 (0.48–3.28) | 0.646 | 198 | 1.08 (0.60–1.93) | 0.801 |
| ≥75.90 | 198 | Reference category | 198 | Reference category | ||
| Fat (g/d) | ||||||
| <17.00 | 175 | 0.61 (0.23–1.66) | 0.335 | 175 | 1.04 (0.56–1.96) | 0.895 |
| 17.00–33.40 | 209 | 0.71 (0.33–1.54) | 0.382 | 209 | 0.97 (0.58–1.63) | 0.918 |
| ≥33.40 | 204 | Reference category | 204 | Reference category | ||
| Carbohydrate (g/d) | ||||||
| <223.37 | 195 | 1.68 (0.50–5.68) | 0.402 | 195 | 1.42 (0.72–2.79) | 0.316 |
| 223.37–315.28 | 195 | 2.43 (0.85–6.97) | 0.098 | 195 | 1.39 (0.76–2.54) | 0.292 |
| ≥315.28 | 198 | Reference category | 198 | Reference category | ||
| Dietary fiber (g/d) | ||||||
| <8.80 | 193 | 0.95 (0.37–2.42) | 0.908 | 193 | 1.28 (0.71–2.30) | 0.416 |
| 8.80–15.10 | 196 | 0.96 (0.40–2.27) | 0.921 | 196 | 0.92 (0.53–1.60) | 0.768 |
| ≥15.10 | 199 | Reference category | 199 | Reference category | ||
| Vitamin A (ug RAE/d)d | ||||||
| <207.00 | 193 | 0.95 (0.40–2.28) | 0.913 | 193 | 1.21 (0.69–2.13) | 0.515 |
| 207.00–390.30 | 197 | 0.68 (0.33–1.41) | 0.301 | 197 | 1.30 (0.80–2.10) | 0.294 |
| ≥390.30 | 198 | Reference category | 198 | Reference category | ||
| Thiamine (mg/d) | ||||||
| <0.90 | 188 | 0.83 (0.28–2.48) | 0.739 | 188 | 0.94 (0.49–1.81) | 0.846 |
| 0.90–1.40 | 198 | 0.71 (0.27–1.86) | 0.487 | 198 | 0.78 (0.44–1.40) | 0.407 |
| ≥1.40 | 202 | Reference category | 202 | Reference category | ||
| Nicotinic acid (mg NE/d) | ||||||
| <9.50 | 193 | 1.17 (0.43–3.17) | 0.756 | 193 | 1.19 (0.65–2.19) | 0.574 |
| 9.50–14.20 | 197 | 1.09 (0.45–2.65) | 0.843 | 197 | 0.74 (0.42–1.30) | 0.296 |
| ≥14.20 | 198 | Reference category | 198 | Reference category | ||
| Riboflavin (mg/d) | ||||||
| <0.60 | 169 | 1.76 (0.67–4.63) | 0.249 | 169 | 1.45 (0.78–2.67) | 0.237 |
| 0.60–0.80 | 187 | 1.64 (0.74–3.65) | 0.225 | 187 | 1.18 (0.70–1.99) | 0.539 |
| ≥0.80 | 232 | Reference category | 232 | Reference category | ||
| Vitamin C (mg/d) | ||||||
| <35.50 | 194 | 0.79 (0.33–1.88) | 0.591 | 194 | 1.49 (0.87–2.55) | 0.142 |
| 35.50–69.40 | 197 | 0.98 (0.47–2.03) | 0.958 | 197 | 1.17 (0.71–1.94) | 0.533 |
| ≥69.40 | 197 | Reference category | 197 | Reference category | ||
| Vitamin E (mg α–TE/d) | ||||||
| <8.80 | 189 | 0.86 (0.34–2.13) | 0.739 | 189 | 1.16 (0.63–2.12) | 0.641 |
| 8.80–15.20 | 198 | 0.36 (0.14–0.93) | 0.034 | 198 | 1.00 (0.57–1.75) | 0.998 |
| ≥15.20 | 201 | Reference category | 201 | Reference category | ||
| K (mg/d) | ||||||
| <1,221.00 | 194 | 1.21 (0.46–3.18) | 0.701 | 194 | 1.30 (0.70–2.38) | 0.405 |
| 1,221.00–1,808.50 | 197 | 1.15 (0.48–2.76) | 0.760 | 197 | 1.04 (0.59–1.82) | 0.897 |
| ≥1,808.50 | 197 | Reference category | 197 | Reference category | ||
| Na (mg/d)e | ||||||
| <239.20 | 192 | 0.73 (0.28–1.92) | 0.528 | 192 | 0.71 (0.39–1.29) | 0.257 |
| 239.20–598.40 | 198 | 0.65 (0.31–1.34) | 0.243 | 198 | 0.75 (0.47–1.22) | 0.246 |
| ≥598.40 | 198 | Reference category | 198 | Reference category | ||
| Ca (mg/d) | ||||||
| <230.00 | 192 | 1.25 (0.50–3.10) | 0.634 | 192 | 1.31 (0.73–2.33) | 0.365 |
| 230.00–369.50 | 196 | 1.28 (0.57–2.87) | 0.548 | 196 | 1.10 (0.65–1.86) | 0.734 |
| ≥368.50 | 200 | Reference category | 200 | Reference category | ||
| Mg (mg/d) | ||||||
| <243.00 | 193 | 1.39 (0.49–3.99) | 0.539 | 193 | 1.49 (0.78–2.85) | 0.226 |
| 243.00–355.60 | 195 | 1.21 (0.46–3.18) | 0.702 | 195 | 1.45 (0.80–2.61) | 0.221 |
| ≥355.60 | 200 | Reference category | 200 | Reference category | ||
| P (mg/d) | ||||||
| <839.90 | 194 | 1.93 (0.70–5.34) | 0.207 | 194 | 1.25 (0.66–2.36) | 0.497 |
| 839.90–1,173.90 | 195 | 0.79 (0.29–2.15) | 0.640 | 195 | 0.81 (0.45–1.46) | 0.477 |
| ≥1,173.90 | 199 | Reference category | 199 | Reference category | ||
| Fe (mg/d) | ||||||
| <15.00 | 190 | 0.96 (0.39–2.40) | 0.935 | 190 | 0.89 (0.49–1.60) | 0.693 |
| 15.00–23.40 | 198 | 1.17 (0.50–2.70) | 0.721 | 198 | 1.04 (0.61–1.78) | 0.889 |
| ≥23.40 | 200 | Reference category | 200 | Reference category | ||
| Zn (mg/d) | ||||||
| <7.70 | 191 | 0.56 (0.21–1.54) | 0.261 | 191 | 1.03 (0.55–1.93) | 0.934 |
| 7.70–11.00 | 194 | 0.50 (0.21–1.21) | 0.123 | 194 | 0.82 (0.47–1.44) | 0.485 |
| ≥11.00 | 203 | Reference category | 203 | Reference category | ||
| Cu (mg/d) | ||||||
| <1.40 | 192 | 1.30 (0.44–3.86) | 0.641 | 192 | 0.95 (0.50–1.82) | 0.880 |
| 1.40–2.10 | 184 | 1.02 (0.39–2.71) | 0.964 | 184 | 0.96 (0.54–1.72) | 0.893 |
| ≥2.10 | 212 | Reference category | 212 | Reference category | ||
| Se (ug/d) | ||||||
| <34.50 | 191 | 1.92 (0.74–4.99) | 0.180 | 191 | 1.04 (0.56–1.92) | 0.913 |
| 34.50–49.80 | 196 | 1.25 (0.54–2.86) | 0.600 | 196 | 1.12 (0.66–1.88) | 0.678 |
| ≥49.80 | 201 | Reference category | 201 | Reference category | ||
| Mn (mg/d) | ||||||
| <4.80 | 195 | 1.06 (0.37–3.01) | 0.911 | 195 | 0.98 (0.53–1.83) | 0.951 |
| 4.80–7.20 | 194 | 1.10 (0.43–2.83) | 0.846 | 194 | 0.68 (0.38–1.23) | 0.204 |
| ≥7.20 | 199 | Reference category | 199 | Reference category | ||
Multivariate logistic regression models were used to assess the odds ratios (ORs) and 95% confidence intervals (CIs) for tuberculosis-drug-induced liver injury and liver dysfunction. The model was adjusted by age, sex, area, energy intake, diabetes, education level, BMI and outdoor exercise.
The intake of each nutrient was divided into three quantiles. The highest quantile was used as the reference group.
P was calculated with the use of multivariate logistic regression model for liver injury and liver dysfunction.
1 ug RAE = 1 ug all-trans-retinol + (1/12) ug all-trans-β-carotene + (1/24) ugprovitamin A carotenoid; 1 mg NE = 1 mg niacin + (1/60) mg tryptophan; 1 mg α-TE = 1 mg α-tocopherol + 0.5 mg β-tocopherol + 0.1 mg γ-tocopherol + 0.02 mg δ-tocopherol + 0.3 mg α-tocotrienol.
The intake of Na as salt was not counted. P values were labeled in bold if they were less than 0.05.
Discussions
The current results indicated that a lower vegetable intake and a higher cooking oil intake were associated with an increased risk of liver injury and liver dysfunction during tuberculosis treatment. These findings provided support to recommend high vegetable and low cooking oil intake during tuberculosis treatment.
We first reported here a negative association between vegetable consumption and the incidence of tuberculosis-drug-induced liver injury and liver dysfunction. Similar association has been observed between vegetable consumption and the risk of liver cancer and non-alcoholic liver disease (17, 18). In addition, animal studies indicated that vegetable extract can alleviate xenobiotic-induced hepatic damage (23, 24). Several plant-derived extracts have been shown to relieve tuberculosis-drug-induced liver injury including Spirulina maxima, Crocus sativus, Ficus religiosa, Mucuna pruriens, Cassia auriculata, and Ziziphus oenoplia (25–30).
Vegetables are rich in phytochemicals (including phenolics, flavonoids, and carotenoids), vitamins (vitamin C, folate, and pro-vitamin A), minerals (potassium, calcium, and magnesium) and fibers (31). Our study did not observe an association between the vitamin, mineral and fiber intake and the risk of liver injury. Phytochemicals may play an important role in alleviating tuberculosis-drug-induced liver injury as shown in the current work. Previous in-vitro and animal studies pointed out a protective effect of phytochemicals on liver by increasing hepatic glutathione content, scavenging free radicals, modulating phase II hepatic metabolism, inhibiting the transcription and translocation of nuclear factor-kappa B and reducing pro-inflammatory cytokines (32–34). A 6-month randomized controlled trial indicated that the vegetable and fruit consumption increased the concentration of plasma antioxidants (35). Antioxidant capacity is critical in detoxifying reactive oxygen species and reaction metabolites from tuberculosis drugs (8). Future work should further elucidate the role of common phytochemicals from vegetables on alleviating drug-induced liver injury.
Our study observed a positive association between the cooking oil intake and the risk of liver injury and liver dysfunction during tuberculosis treatment. Vegetable oils, including peanut oil, soybean oil, corn oil and rapeseed oil, are the main cooking oil in China, accounting for 92% dietary oil intake (36). These vegetable oils contain a large amount of unsaturated fatty acids (usually higher than 80% of total fatty acids) with linoleic acid as the major type (37). The consumption of unsaturated fatty acid, especially linoleic acid, increased cytochrome P450 2E1 (CYP2E1) activity, damaged intestinal barrier, and induced liver inflammation (38, 39). CYP2E1 is critical in the hepatotoxicity of tuberculosis drugs which metabolizes isoniazid to reactive oxygen species and diazohydroxide (40). These metabolites are toxic and lead to liver necrosis (40). The activated CYP2E1, increased endotoxemia and liver inflammation by the consumption of unsaturated fatty acids can increase the susceptibility to drug-induced liver injury, and may therefore account for the positive relationship between dietary oil consumption and liver injury during tuberculosis treatment (41, 42).
Our study has several strengths. First, this is the first epidemiological study, which investigates the relationship between the dietary intake and the liver injury during tuberculosis treatment. Second, the adopted FFQ was previously validated for Chinese population. Third, we were able to adjust for common confounding factors for the drug-induced liver injury including age, sex, area, energy intake, diabetes, education level, BMI and outdoor exercise. We also acknowledge a few limitations. First, all participants in this study were Asian, which limited its generalizability. Second, a simplified FFQ was used in this study. We were unable to analyze which specific type of vegetables or cooking oils was associated with the risk of drug-induced liver injury. Third, the sample size was relatively small due to the low incidence of tuberculosis. Fourth, although we used a previously validated FFQ, it is still inherently subject to certain degree of measurement error (22). The measurement error may lead to misclassification in dietary intake, and in turn weaken the association between diet and liver injury.
In conclusion, a high vegetable intake was associated with a low risk of liver injury and liver dysfunction, whereas a low cooking oil intake was associated with a low risk of liver injury and liver dysfunction during tuberculosis treatment. Further studies are required to investigate the mechanism of vegetable and cooking oil consumption in modulating the tuberculosis-drug-induced liver injury.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethic Committee of Medicine of Qingdao Center of Disease Control and Prevention (2009-4). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
AM designed research. LX, CZ, SZ, and YL conducted research. JW and KX analyzed data and wrote the manuscript. AM had primary responsibility for final content. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This study was supported by the National Natural Science Foundation of China (No. 82003446), the Postdoctoral Science Foundation of China (No. 2020M682130), and the World Diabetes Foundation (No. WDF08-380).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2021.652311/full#supplementary-material
References
- 1.World Health Organization . Global Tuberculosis Report 2019. Geneva: World Health Organization; (2019). [Google Scholar]
- 2.Nahid P, Dorman SE, Alipanah N, Barry PM, Brozek JL, Cattamanchi A, et al. Official american thoracic society/centers for disease control and prevention/infectious diseases society of america clinical practice guidelines: treatment of drug-susceptible tuberculosis. Clin Infect Dis. (2016) 63:e147–95. 10.1093/cid/ciw376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chalasani NP, Hayashi PH, Bonkovsky HL, Navarro VJ, Lee WM, Fontana RJ. ACG clinical guideline: the diagnosis and management of idiosyncratic drug-induced liver injury. Am J Gastroenterol. (2014) 109:950–66. 10.1038/ajg.2014.131 [DOI] [PubMed] [Google Scholar]
- 4.Wan X, Lu X. Diagnostics. Beijing, China: People's Medical Publishing House; (2018). [Google Scholar]
- 5.Saukkonen JJ, Cohn DL, Jasmer RM, Schenker S, Jereb JA, Nolan CM, et al. An official ats statement: hepatotoxicity of antituberculosis therapy. Am J Respir Crit Care Med. (2006) 174:935–52. 10.1164/rccm.200510-1666ST [DOI] [PubMed] [Google Scholar]
- 6.Zhou Y, Yang L, Liao Z, He X, Zhou Y, Guo H. Epidemiology of drug-induced liver injury in china: a systematic analysis of the chinese literature including 21 789 patients. Eur J Gastroenterol Hepatol. (2013) 25:825–9. 10.1097/MEG.0b013e32835f6889 [DOI] [PubMed] [Google Scholar]
- 7.Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, et al. Features and outcomes of 899 patients with drug-induced liver injury: the dilin prospective study. Gastroenterology. (2015) 148:1340–52.e1347. 10.1053/j.gastro.2015.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Senousy BE, Belal SI, Draganov PV. Hepatotoxic effects of therapies for tuberculosis. Nat Rev Gastroenterol Hepatol. (2010) 7:543–56. 10.1038/nrgastro.2010.134 [DOI] [PubMed] [Google Scholar]
- 9.Cegielski JP, Arab L, Cornoni-Huntley J. Nutritional risk factors for tuberculosis among adults in the united states, 1971-1992. Am J Epidemiol. (2012) 176:409–22. 10.1093/aje/kws007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kant S, Gupta H, Ahluwalia S. Significance of nutrition in pulmonary tuberculosis. Crit Rev Food Sci Nutr. (2015) 55:955–63. 10.1080/10408398.2012.679500 [DOI] [PubMed] [Google Scholar]
- 11.Oh J, Choi R, Park HD, Lee H, Jeong BH, Park HY, et al. Evaluation of vitamin status in patients with pulmonary tuberculosis. J Infect. (2017) 74:272–80. 10.1016/j.jinf.2016.10.009 [DOI] [PubMed] [Google Scholar]
- 12.Xiong K, Wang J, Ma A. Adjunctive vitamin A and D for glycemic control in patients with concurrent type 2 diabetes and tuberculosis: a randomized controlled trial. Br J Nutr. (2021). 10.1017/S0007114521001185. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki A, Yuen NA, Ilic K, Miller RT, Reese MJ, Brown HR, et al. Comedications alter drug-induced liver injury reporting frequency: data mining in the who vigibase (tm). Regul Toxicol Pharmacol. (2015) 72:481–90. 10.1016/j.yrtph.2015.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ali N, Gupta N, Saravu K. Malnutrition as an important risk factor for drug-induced liver injury in patients on anti-tubercular therapy: an experience from a tertiary care center in south india. Drug Discov Ther. (2020) 14:135–8. 10.5582/ddt.2020.03029 [DOI] [PubMed] [Google Scholar]
- 15.Xiong K, Cai J, Liu P, Wang J, Zhao S, Xu L, et al. Lactobacillus casei alleviated the abnormal increase of cholestasis-related liver indices during tuberculosis treatment: a post hoc analysis of randomized controlled trial. Mol Nutr Food Res. (2021). 10.1002/mnfr.202100108. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 16.Fernandez-Villar A, Sopena B, Fernandez-Villar J, Vazquez-Gallardo R, Ulloa F, Leiro V, et al. The influence of risk factors on the severity of anti-tuberculosis drug-induced hepatotoxicity. Int J Tuberc Lung Dis. (2004) 8:1499–505. [PubMed] [Google Scholar]
- 17.Guo XF, Shao XF, Li JM, Li S, Li KL, Li D. Fruit and vegetable intake and liver cancer risk: a meta-analysis of prospective cohort studies. Food Funct. (2019) 10:4478–85. 10.1039/C9FO00804G [DOI] [PubMed] [Google Scholar]
- 18.Kim SA, Shin S. Fruit and vegetable consumption and non-alcoholic fatty liver disease among korean adults: a prospective cohort study. J Epidemiol Community Health. (2020) 74:1035–42. 10.1136/jech-2020-214568 [DOI] [PubMed] [Google Scholar]
- 19.Zhang S, Fu J, Zhang Q, Liu L, Meng G, Yao Z, et al. Association between nut consumption and non-alcoholic fatty liver disease in adults. Liver Int. (2019) 39:1732–41. 10.1111/liv.14164 [DOI] [PubMed] [Google Scholar]
- 20.Dorosti M, Jafary Heidarloo A, Bakhshimoghaddam F, Alizadeh M. Whole-grain consumption and its effects on hepatic steatosis and liver enzymes in patients with non-alcoholic fatty liver disease: a randomised controlled clinical trial. Br J Nutr. (2020) 123:328–36. 10.1017/S0007114519002769 [DOI] [PubMed] [Google Scholar]
- 21.Yang Y. China Food Composition Tables. Beijing, China: Peking University Medical Press; (2018). [Google Scholar]
- 22.Wen-Hua ZH, Huang ZP, Zhang X, Li HE, Willett W, Jun-Ling WA, et al. Reproducibility and validity of a chinese food frequency questionnaire. Biomed Environ Sci. (2010) 23:1–38. 10.1016/S0895-3988(11)60014-720486429 [DOI] [Google Scholar]
- 23.Salawu SO, Akindahunsi AA. Protective effect of some tropical vegetables against ccl4-induced hepatic damage. J Med Food. (2007) 10:350–5. 10.1089/jmf.2006.212 [DOI] [PubMed] [Google Scholar]
- 24.Ma Y, Li R, Liu Y, Liu M, Liang H. Protective effect of aplysin supplementation on intestinal permeability and microbiota in rats treated with ethanol and iron. Nutrients. (2018) 10:681. 10.3390/nu10060681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rao CV, Rawat AKS, Singh AP, Singh A, Verma N. Hepatoprotective potential of ethanolic extract of ziziphus oenoplia (l.) mill roots against antitubercular drugs induced hepatotoxicity in experimental models. Asian Pac J Trop Med. (2012) 5:283–8. 10.1016/S1995-7645(12)60040-6 [DOI] [PubMed] [Google Scholar]
- 26.Parameswari SA, Chetty CM, Chandrasekhar KB. Hepatoprotective activity of ficus religiosa leaves against isoniazid+rifampicin and paracetamol induced hepatotoxicity. Pharmacognosy Res. (2013) 5:271–6. 10.4103/0974-8490.118828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jatav SK, Kulshrestha A, Zacharia A, Singh N, Tejovathi G, Bisen PS, et al. Spirulina maxima protects liver from isoniazid and rifampicin drug toxicity. J Evid Based Complementary Altern Med. (2014) 19:189–94. 10.1177/2156587214530720 [DOI] [PubMed] [Google Scholar]
- 28.Jaydeokar AV, Bandawane DD, Bibave KH, Patil TV. Hepatoprotective potential of cassia auriculata roots on ethanol and antitubercular drug-induced hepatotoxicity in experimental models. Pharm Biol. (2014) 52:344–55. 10.3109/13880209.2013.837075 [DOI] [PubMed] [Google Scholar]
- 29.Obogwu MB, Akindele AJ, Adeyemi OO. Hepatoprotective and in vivo antioxidant activities of the hydroethanolic leaf extract of mucuna pruriens (fabaceae) in antitubercular drugs and alcohol models. Chin J Nat Med. (2014) 12:273–83. 10.1016/S1875-5364(14)60054-6 [DOI] [PubMed] [Google Scholar]
- 30.Wali AF, Pillai JR, Al Dhaheri Y, Rehman MU, Shoaib A, Sarheed O, et al. Crocus sativus l. Extract containing polyphenols modulates oxidative stress and inflammatory response against anti-tuberculosis drugs-induced liver injury. Plants Basel. (2020) 9:167. 10.3390/plants9020167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu RH. Health-promoting components of fruits and vegetables in the diet. Adv Nutr. (2013) 4:384s−92s. 10.3945/an.112.003517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bishayee A, Darvesh AS, Politis T, McGory R. Resveratrol and liver disease: from bench to bedside and community. Liver Int. (2010) 30:1103–14. 10.1111/j.1478-3231.2010.02295.x [DOI] [PubMed] [Google Scholar]
- 33.Madrigal-Santillan E, Madrigal-Bujaidar E, Alvarez-Gonzalez I, Teresa Sumaya-Martinez M, Gutierrez-Salinas J, Bautista M, et al. Review of natural products with hepatoprotective effects. World J Gastroenterol. (2014) 20:14787–804. 10.3748/wjg.v20.i40.14787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pingili RB, Challa SR, Pawar AK, Toleti V, Kodali T, Koppula S. A systematic review on hepatoprotective activity of quercetin against various drugs and toxic agents: evidence from preclinical studies. Phytother Res. (2020) 34:5–32. 10.1002/ptr.6503 [DOI] [PubMed] [Google Scholar]
- 35.John JH, Ziebland S, Yudkin P, Roe LS, Neil HAW. Oxford Fruit Vegetable Study G. Effects of fruit and vegetable consumption on plasma antioxidant concentrations and blood pressure: a randomised controlled trial. Lancet. (2002) 359:1969–74. 10.1016/S0140-6736(02)98858-6 [DOI] [PubMed] [Google Scholar]
- 36.Jiang H, Zhang J, Su C, Zhang J, Zhang B, Wang H. Cooking oil and salt consumption among chinese adults aged 18-59 years in 2015. Acta Nutrimenta Sinica. (2018) 40:27–31. 10.13325/j.cnki.acta.nutr.sin.2018.01.009 [DOI] [Google Scholar]
- 37.Dubois V, Breton S, Linder M, Fanni J, Parmentier M. Fatty acid profiles of 80 vegetable oils with regard to their nutritional potential. Eur J Lipid Sci Technol. (2007) 109:710–32. 10.1002/ejlt.200700040 [DOI] [Google Scholar]
- 38.Nanji AA, French SW. Dietary factors and alcoholic cirrhosis. Alcohol Clin Exp Res. (1986) 10:271–3. 10.1111/j.1530-0277.1986.tb05088.x [DOI] [PubMed] [Google Scholar]
- 39.Nanji AA. Role of different dietary fatty acids in the pathogenesis of experimental alcoholic liver disease. Alcohol. (2004) 34:21–5. 10.1016/j.alcohol.2004.08.005 [DOI] [PubMed] [Google Scholar]
- 40.Metushi IG, Cai P, Zhu X, Nakagawa T, Uetrecht JP. A fresh look at the mechanism of isoniazid-induced hepatotoxicity. Clin Pharmacol Ther. (2011) 89:911–4. 10.1038/clpt.2010.355 [DOI] [PubMed] [Google Scholar]
- 41.Sheng YJ, Wu G, He HY, Chen W, Zou YS, Li Q, et al. The association between cyp2e1 polymorphisms and hepatotoxicity due to anti-tuberculosis drugs: a meta-analysis. Infect Genet Evol. (2014) 24:34–40. 10.1016/j.meegid.2014.01.034 [DOI] [PubMed] [Google Scholar]
- 42.Hassan HM, Guo H, Yousef BA, Guerram M, Hamdi AM, Zhang L, et al. Role of inflammatory and oxidative stress, cytochrome p450 2e1, and bile acid disturbance in rat liver injury induced by isoniazid and lipopolysaccharide cotreatment. Antimicrob Agents Chemother. (2016) 60:5285–93. 10.1128/AAC.00854-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.


