Abstract
Morphology has a direct influence on animal fitness. Studies addressing the identification of patterns and variations across several guilds are fundamental in ecomorphological research. Wings are the core of ecological morphology in bats; nevertheless, individual bones and structures that support the wing, including metacarpals, phalanges and the length of digits, have rarely been the subject of comprehensive research when studying wing morphology. Here, I analyzed morphological variations of wing structures across 11 bat guilds and how individual bone structures are correlated to diet, foraging mode and habitat use. I obtained wing measurements from 1512 voucher specimens of 97 species. All the specimens analyzed came from the Mammalian Collection at the Museo Javeriano de Historia Natural of Pontificia Universidad Javeriana (MPUJ-MAMM) (Bogotá, Colombia). Positive correlations between size and the length of the third and fifth digit were detected. Bat guilds that capture their preys using aerial strategy in uncluttered habitats had longer third digits but short fifth digits compared to guilds that rely on gleaning strategy and forage in highly cluttered space. Although terminal phalanges were shown to be important structures for guild classification, metacarpals were strongly related to aerial foragers from uncluttered habitats because of their potential role in flight performance and ecological adaptations. Results show that habitat use, as well as foraging mode, are reflected in wing structures. Different wing traits to those evaluated in this study should be considered to better understand the ecological interactions, foraging strategy, wing adaptations, and flight performance in Neotropical bats.
Keywords: Chiroptera, Wing traits, Ecomorphological relationships, Guild, Flight pattern
BACKGROUND
Morphological traits influence the performance— and therefore biological fitness—of animals (Arnold 1983). The interactions between morphology and ecology constitute the primary goal of ecomorphology (Rhodes 2002), a fundamental field for understanding the abilities of species to exploit resources. Within ecomorphological studies, a major focus has become the intersection between locomotory apparatus and characteristics of resource use, including diet, foraging behavior, and habitat use (Kalcounis and Brigham 1995; Giannini and Brenes 2001; Voigt and Holderied 2012). Species that share resources are grouped into guilds (Fauth et al. 1996), usually with high overlapping in morphological dimensions and, at the same time, show a high distinction between other guilds (Rhodes 2002). Analyzing morphological differences between guilds help researchers infer idiosyncratic features in their ecology and behavior (Kalcounis and Brigham 1995). Accordingly, the identification of morphological patterns and variations among several guilds becomes relevant when studying functional morphology in animals (Marinello and Bernard 2014).
The Neotropics harbor one of the richest bat faunas in the world (López-Aguirre et al. 2018), accounting for about 400 species, nine families, and three superfamilies (Arita et al. 2014), and representing a very complex evolutionary history (Peixoto et al. 2014; López-Aguirre et al. 2018) and ecology (Meyer et al. 2008; García-García et al. 2014; Briones-Salas et al. 2019; Castillo-Figueroa 2020). New world bats have an extraordinary morphological diversity related to food habits (Santana et al. 2012), foraging behaviors (Schnitzler et al. 2003), and flight styles (Norberg and Rayner 1987). Particularly, the development of wings has played a central role in the colonization of several ecological niches over bat evolution (Sears et al. 2006), promoting adaptive radiation (Cooper and Sears 2013). Due to this, wing morphology is the basis of ecomorphological correlations in bats (Norberg and Rayner 1987) and wing traits are, therefore, important predictors of resource use (Kalcounis and Brigham 1995).
Traditionally, wing loading, aspect ratio, and wingtip shape index have been the most common measures to assess flight style and aerodynamic abilities of bats (Norberg and Rayner 1987; Thollesson and Norberg 1991; Saunders and Barclay 1992; Rhodes 2002; Marinello and Bernard 2014). Other approaches based on individual bones and structures that support the wing such as metacarpals and phalanges (Stockwell 2001; Castillo-Figueroa and Pérez-Torres 2018; Castillo-Figueroa 2018a) appear to be suitable variables for predicting aerodynamic performance in bats; however, these morphological traits have been largely neglected in ecomorphological studies, especially in New World bats. Indeed, there is little research on wing characterization based on metacarpal and phalanges structures for several Neotropical bat species, especially in the rare ones; filling this knowledge gap may yield new information that allow to elucidate more comprehensively the morphological patterns of bat wings. Importantly, wing measurements that reflect body size (forearm length), wing width (length of the fifth digit) and hand-wing length (length of the third digit) can correlate to ecological adaptations of bats (Findley et al. 1972; Dietz et al. 2006) and may be useful in disentangling morphological patterns across different bat guilds.
Since Neotropical bats vary greatly in morpho-logical features, a representatively large group is necessary to capture the higher variation both within and among guilds. In this paper, I analyzed the variations in wing structures across 11 bat guilds and correlated individual bone structures to diet, foraging mode and habitat use. To do this, I aimed to (1) quantitatively characterize wing digits of 97 New World bat species; (2) explore correlations between body size, wing width, and hand-wing length; (3) assess any differences in wing ratios among bat guilds; (4) determine whether the guilds corresponded to morphologically distinct groups based on individual bone structures (i.e., metacarpals and phalanges), thus identifying which characters best differentiate these groups. A concomitant goal of this study was to discuss the importance of wing structures in ecomorphology for each bat guild.
MATERIALS AND METHODS
Bat species
I obtained wing measurements from 1512 bat vouchers in the Mammalian Collection at the Museo Javeriano de Historia Natural of Pontificia Universidad Javeriana (MPUJ-MAMM) (Bogotá, Colombia). All were dry specimens and corresponded to adults only. On average, the number of individuals measured from each species was 16 (ranging from 1 to 243). The specimens belonged to 97 New world bat species, 43 genera, and 7 families. I excluded bat specimens in a bad state of preservation (i.e., broken wings). Identification of each specimen was corroborated with the keys of Gardner (2007) and, for the genus Platyrrhinus, the classifications of Velazco (2005) and Velazco et al. (2010) were followed. The current nomenclature was revised from the Integrated Taxonomic Information System (ITIS 2020).
Wing morphometry
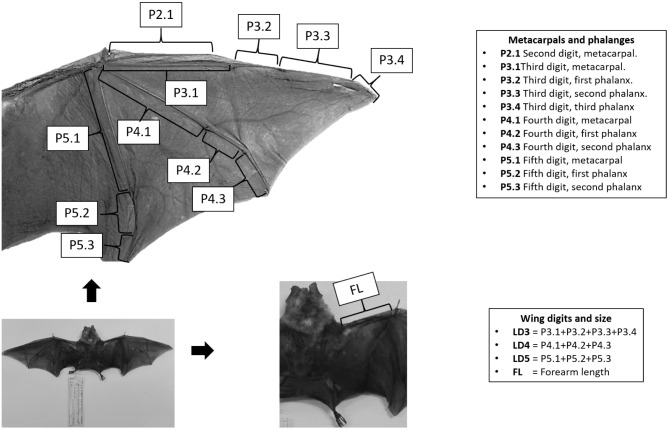
I measured 11 wing traits, including metacarpals and phalanges in the dorsal side of the right wing (Fig. 1). I also measured forearm length (FL) and the length of the third (LD3), fourth (LD4) and fifth digits (LD5) (Fig. 1). Measurements were taken with a digital caliper (Mitutoyo Calibrador Vernier Mod Cd6 -csx 150 Mm) with an accuracy of 0.01 mm to ensure high precision; all measurements were performed by the same researcher (DCF) to avoid researcher bias.
Fig. 1.
Wing traits measured from bat specimens. Wing structures (metacarpals and phalanges) are represented in the figure. Wing digit length is the sum of metacarpals and phalanges of each digit.
Data analysis
To characterize the morphological traits for each species, I calculated the statistical mean and standard deviation for the length of the digits and forearm. I also described frequencies for these traits using histogram plots. To explore the associations between similarity in wing morphology and ecological similarity in bats, I first classified bat species based on foraging mode, habitat use, and diet into 11 guilds following Kalko et al. (1996), Sampaio et al. (2003), Estrada-Villegas et al. (2010), and Aguirre et al. (2016).
To explore correlations between body size, wing width, and hand-wing length, I made Spearman correlations among FL, LD3, and LD5 since assumptions of normally distributed residuals were not fulfilled. In this way, to assess the correlations between body size and wing width, I correlated LD5 with FL. To examine the correlations between body size to hand-wing length I correlated LD3 with FL. Lastly, to evaluate the correlations between hand-wing length to wing width, I correlated LD3 with LD5 (Dietz et al. 2006).
I compared wing digit ratios of LD3/FA (bat size to hand-wing length), LD5/FA ratio (bat size to wing width), and LD3/LD5 (hand-wing length to wing width) among guilds. Since data followed a non-normal distribution (Kolmogorov-Smirnov test, D = 0.29, P < 0.0001), a permuted ANOVA (n = 99999 permutations) with post-hoc comparisons (Dunn’s method) was used to assess differences among guilds. Probabilities at 0.05 were reported as significant. With the aim to differentiate the guilds in the morphospace, I conducted an ordination analysis using the 11 individual bone structures (i.e., metacarpals and phalanges, Fig. 1) scaled to FL in a Multidimensional Scaling (MDS) based on Bray-Curtis distance. Kruskal’s stress less than 0.2 was considered an adequate representation in reduced dimensions (McCune and Grace 2002). All the analyses were performed in Rwizard 4.3 (Guisande et al. 2014) and PAST 4.03 (Hammer et al. 2001).
RESULTS
Wing morphometry and guild classification
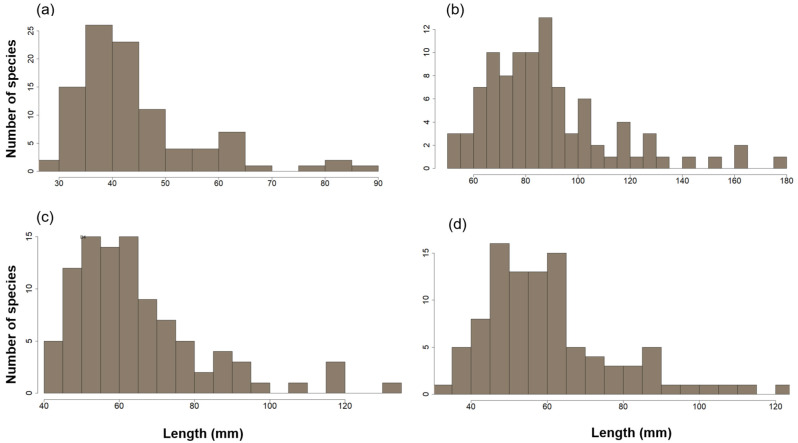
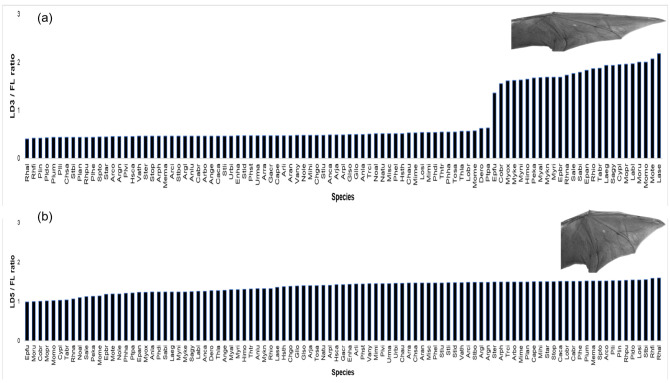
Among the 97 bat species analyzed, the smallest was Rhogessa io (FL mean = 28.64 mm) and the largest was Phyllostomus hastatus (FL mean = 88.52 mm) (Table 1, Fig. 2). Means of LD3 ranged from 50.31 mm (Eptesicus fuscus) to 176.2 mm (Noctilio leporinus), LD4 varied from 41.99 mm (Eptesicus fuscus) to 132.67 mm (Noctilio leporinus), and LD5 were 33.81 mm (Cynomops planirostris) to 120.22 mm (Chrotopterus auritus). However, when scaling wing digits to body size (FL ratio), Lasiurus seminolus displayed the longest wing (LD3/FL), whereas Rhinophylla alethina had the broadest (LD5/FL) (Fig. 3).
Table 1.
Bat species analyzed according to wing digits, including the number of individuals examined (n) and corresponding acronym and guild. Mean and standard deviation are given for: forearm length (FL), length of the third digit (LD3), length of the fourth digit (LD4), and length of the fifth digit (LD5). Measurements are in mm
| FAMILY/Species | Acronym | Guild | n | FL | LD3 | LD4 | LD5 |
| EMBALLONURIDAE | |||||||
| Cormura brevirostris | Cobr | BAI | 1 | 44.58 | 69.48 | 47.16 | 44.74 |
| Peropteryx kappleri | Peka | BAI | 2 | 46.72 ± 0.95 | 78.31 ± 0.59 | 52.68 ± 2.51 | 52.89 ± 0.09 |
| Rhynchonycteris naso | Rhna | BAI | 1 | 36.02 | 62.5 | 42.57 | 38.51 |
| Saccopteryx bilineata | Sabi | BAI | 6 | 47.11 ± 1.67 | 84.41 ± 4.10 | 58.87 ± 2.14 | 58.50 ± 2.27 |
| Saccopteryx gymnura | Sagy | BAI | 1 | 38.44 | 74.62 | 47.89 | 48.12 |
| Saccopteryx leptura | Sale | BAI | 12 | 37.29 ± 2.31 | 66.17 ± 7.73 | 44.55 ± 4.77 | 42.135 ± 4.67 |
| MOLOSSIDAE | |||||||
| Cynomops planirostris | Cypl | UAI | 4 | 32.77 ± 0.52 | 64.1 ± 1.74 | 49.2 ± 1.87 | 33.81 ± 1.10 |
| Molossops temminckii | Mote | UAI | 5 | 30.56 ± 0.95 | 63.37 ± 1.57 | 52.41 ± 1.90 | 52.41 ± 1.42 |
| Molossus molossus | Momo | UAI | 14 | 38.89 ± 2.51 | 78.08 ± 6.84 | 58.37 ± 3.56 | 39.61 ± 2.35 |
| Molossus pretiosus | Mopr | UAI | 1 | 43.56 | 85.34 | 64.82 | 44.08 |
| Molossus rufus | Moru | UAI | 5 | 49.88 ± 0.43 | 100.01 ±7.12 | 71.85 ± 2.07 | 49.98 ± 1.65 |
| Tadarida brasiliensis | Tabr | UAI | 1 | 42.25 | 79.4 | 59.91 | 44.03 |
| MORMOOPIDAE | |||||||
| Mormoops megalophylla | Mome | CAI | 12 | 53.37 ± 1.13 | 93.47 ± 1.62 | 67.86 ± 2.26 | 60.98 ± 1.86 |
| Pteronotus parnellii | Ptpa | CAI | 4 | 60.23 ± 1.47 | 95.4 ± 3.50 | 75.24 ± 0.46 | 73.46 ± 0.59 |
| NATALIDAE | |||||||
| Natalus tumidirostris | atu | BAI | 9 | 39.54 ± 0.89 | 76.52 ± 1.96 | 56.19 ± 1.16 | 55.76 ± 1.54 |
| NOCTILIONIDAE | |||||||
| Noctilio albiventris | Noal | BTP | 6 | 59.68 ± 1.79 | 116.39 ± 3.95 | 86.92 ± 3.43 | 65.52 ± 2.11 |
| Noctilio leporinus | Nole | BTP | 2 | 84.32 ± 1.25 | 176.205 ± 2.80 | 132.67 ± 2.30 | 100.96 ± 2.85 |
| PHYLLOSTOMIDAE | |||||||
| Carollinae | |||||||
| Carollia brevicauda | Cabr | CUF | 72 | 39.47 ± 1.77 | 84.45 ± 4.92 | 60.17 ± 3.71 | 59.80 ± 3.19 |
| Carollia castanea | Caca | CUF | 56 | 36.31 ± 1.44 | 77.59 ± 4.49 | 54.99 ± 2.95 | 54.88 ± 2.54 |
| Carollia perspicillata | Cape | CUF | 243 | 41.90 ± 1.78 | 88.24 ± 4.31 | 63.20 ± 3.41 | 63.00 ± 3.24 |
| Rhynophillinae | |||||||
| Rhinophylla alethina | Rhpu | CUF | 1 | 29.94 | 73.24 | 51.51 | 51.51 |
| Rhinophyllafischerae | Rhfi | CUF | 3 | 31.20 ± 1.32 | 73.37 ± 2.42 | 52.16 ± 1.35 | 49.65 ± 2.89 |
| Rhinophylla pumilio | Rhpu | CUF | 2 | 30.68 ± 0.19 | 69.37 ± 1.78 | 49.69 ± 1.49 | 47.20 ± 1.44 |
| Desmodontinae | |||||||
| Desmodus rotundus | Dero | CGS | 43 | 59.60 ± 3.00 | 96.13 ± 7.26 | 77.74 ± 4.47 | 76.20 ± 4.61 |
| Glossophaginae | |||||||
| Anoura cadenai | Anca | CGN | 1 | 35.23 | 72.2 | 50.49 | 44.53 |
| Anoura geoffroyi | Ange | CGN | 27 | 42.60 ± 1.91 | 90.97 ± 4.35 | 64.11 ± 3.34 | 54.82 ± 3.89 |
| Anoura latidens | Anla | CGN | 3 | 42.31 ± 1.19 | 84.40 ± 1.64 | 60.84 ± 1.85 | 52.48 ± 0.91 |
| Anoura luismanueli | Anlu | CGN | 11 | 35.5 ± 2.26 | 75.95 ± 5.32 | 53.68 ± 3.22 | 47.13 ± 3.29 |
| Choeroniscus godmani | Chgo | CGN | 4 | 32.10 ± 1.46 | 66.78 ± 2.61 | 47.23 ± 1.30 | 44.57 ± 1.76 |
| Glossophaga longirostris | Gllo | CGN | 1 | 37.42 | 75.24 | 54.02 | 52.23 |
| Glossophaga soricina | Glso | CGN | 85 | 35.03 ± 1.15 | 70.77 ± 2.67 | 51.76 ± 1.96 | 49.15 ± 1.91 |
| Lonchophyllinae | |||||||
| Hsunycteris cadenai | Hsca | CGN | 1 | 32.33 | 70.77 | 48.68 | 46.14 |
| Hsunycteris thomasi | Hsth | CGN | 1 | 35.42 | 68.17 | 50.4 | 48.98 |
| Micronycterinae | |||||||
| Micronycteris hirsuta | Mihi | CGI | 1 | 42.17 | 87.61 | 64.37 | 63.57 |
| Micronycteris megalotis | Mige | CGI | 4 | 34.26 ± 1.29 | 63.84 ± 1.82 | 50.19 ± 3.44 | 51.34 ± 2.82 |
| Micronycteris microtis | Mimi | CGI | 2 | 34.36 ± 0.30 | 63.26 ± 0.95 | 48.83 ± 0.05 | 50.04 ± 1.73 |
| Micronycteris schmidtorum | Misc | CGI | 2 | 34.82 ± 0.68 | 67.31 ± 4.49 | 50.47 ± 4.06 | 51.34 ± 3.95 |
| Phyllostominae | |||||||
| Chrotopterus auritus | Chau | CGC | 1 | 82.23 | 153.25 | 116.68 | 120.22 |
| Gardnerycteris crenulatum | Gacr | CGI | 7 | 48.93 | 103.11 | 71.25 | 69.82 |
| Lophostoma brasiliense | Lobr | CGI | 1 | 34.53 | 61.08 | 49.81 | 52.3 |
| Lophostoma silvicolum | Losi | CGI | 13 | 52.22 ± 2.84 | 96.40 ± 6.57 | 78.26 ± 3.39 | 80.72 ± 3.84 |
| Phylloderma stenops | Phst | CUF | 1 | 76.29 | 96.40 | 78.26 | 80.72 |
| Phyllostomus discolor | Phdi | CGN | 23 | 62.23 ± 2.36 | 114.44 ± 4.66 | 83.59 ± 3.85 | 77.32 ± 2.78 |
| Phyllostomus elongatus | Phel | CGO | 1 | 60.71 | 116.98 | 87.65 | 89.47 |
| Phyllostomus hastatus | Phha | CGO | 13 | 88.52 ± 2.14 | 161.07 ± 4.68 | 118.09 ± 4.49 | 106.99 ± 2.52 |
| Tonatia saurophila | Tosa | CGO | 1 | 59.22 | 107.66 | 79.12 | 83.31 |
| Trachops cirrhosus | Trci | CGC | 6 | 60.24 ± 1.84 | 118.73 ± 3.95 | 87.05 ± 2.78 | 90.115 ± 3.07 |
| Stenodermatinae | |||||||
| Artibeus anderseni | Aran | CCF | 7 | 38.52 ± 2.45 | 80.88 ± 5.81 | 59.34 ± 3.79 | 56.82 ± 4.76 |
| Artibeus bogotensis | Arbo | CCF | 5 | 42.09 ± 1.36 | 89.85 ± 3.34 | 66.04 ± 1.56 | 63.05 ± 2.21 |
| Artibeus cinereus | Arci | CCF | 3 | 41.71 ± 1.88 | 89.39 ± 1.29 | 64.65 ± 2.81 | 61.94 ± 2.95 |
| Artibeus concolor | Arco | CCF | 1 | 47.41 | 103.75 | 74.31 | 72.39 |
| Artibeus glaucus | Argl | CCF | 56 | 41.45 ± 1.59 | 88.67 ± 4.91 | 65.66 ± 3.06 | 61.81 ± 2.90 |
| Artibeus gnomus | Argn | CCF | 1 | 38.52 | 84.29 | 60.47 | 57.49 |
| Artibeus jamaicensis | Arja | CCF | 34 | 61.47 ± 3.73 | 125.19 ± 10.48 | 93.44 ± 6.27 | 86.28 ± 5.88 |
| Artibeus lituratus | Arli | CCF | 197 | 68.38 ± 2.92 | 68.38 ± 6.58 | 105.40 ± 4.49 | 98.84 ± 4.54 |
| Artibeus phaeotis | Arph | CCF | 44 | 37.81 ± 1.59 | 81.16 ± 3.73 | 59.24 ± 2.87 | 56.59 ± 2.76 |
| Artibeus planirostris | Arpl | CCF | 58 | 60.80 ± 2.83 | 123.34 ± 7.03 | 91.93 ± 4.99 | 85.86 ± 4.83 |
| Artibeus rava | Arra | CCF | 12 | 38.76 ± 1.06 | 81.68 ± 3.61 | 59.54 ± 1.87 | 56.98 ± 2.16 |
| Chiroderma salvini | Chsa | CCF | 4 | 51.11 ± 2.34 | 115.94 ± 5.21 | 80.07 ± 4.21 | 75.24 ± 4.12 |
| Enchisthenes hartii | Enha | CCF | 6 | 51.11 ± 1.88 | 115.94 ± 3.73 | 80.07 ± 3.05 | 75.24 ± 2.02 |
| Mesophylla macconnelli | Mema | CUF | 2 | 31.93 ± 0.07 | 68.72 ± 4.46 | 50.60 ± 1.40 | 48.66 ± 0.72 |
| Platyrrhinus angustirostris | Plan | CCF | 3 | 37.27 ± 1.61 | 84.43 ± 4.36 | 59.48 ± 2.32 | 55.87 ± 2.20 |
| Platyrrhinus dorsalis | Pldo | CCF | 15 | 45.94 ± 3.26 | 106.56 ± 5.22 | 75.87 ± 3.20 | 70.78 ± 3.26 |
| Platyrrhinus helleri | Plhe | CCF | 42 | 38.66 ± 2.08 | 87.17 ± 3.37 | 61.71 ± 2.28 | 58.55± 2.18 |
| Platyrrhinus infuscus | Plin | CCF | 1 | 54.14 | 126.69 | 87.82 | 83.07 |
| Platyrrhinus lineatus | Plli | CCF | 5 | 44.16 ± 1.35 | 100.40 ± 4.41 | 100.40 ± 3.31 | 67.64 ± 3.52 |
| Platyrrhinus umbratus | Plum | CCF | 4 | 45.75 ± 0.68 | 104.18 ± 3.47 | 74.57 ± 2.10 | 69.61 ± 1.89 |
| Platyrrhinus vittatus | Plvi | CCF | 6 | 60.97 ± 1.45 | 133.44 ± 4.17 | 96.28 ± 2.78 | 88.90 ± 3.69 |
| Sphaeronycteris toxophyllum | Spto | CCF | 1 | 40.95 | 91.95 | 66.52 | 62.54 |
| Sturnira aratathomasi | Star | CUF | 3 | 57.26 ± 1.78 | 128.21 ± 3.40 | 92.77 ± 1.87 | 86.44 ± 1.74 |
| Sturnira bidens | Stbi | CUF | 7 | 41.25 ± 1.11 | 93.61 ± 3.36 | 66.87 ± 2.51 | 64.28 ± 2.47 |
| Sturnira bogotensis | Stbo | CUF | 27 | 43.31 ± 1.86 | 92.90 ± 4.76 | 68.54 ± 3.42 | 64.52 ± 3.37 |
| Sturnira erythromos | Ster | CUF | 25 | 40.42 ± 1.59 | 87.16 ± 3.73 | 63.56 ± 2.54 | 60.36 ± 2.71 |
| Sturnira lilium | Stli | CUF | 65 | 41.18 ± 1.45 | 87.87 ± 4.01 | 64.68 ± 3.61 | 60.82 ± 2.73 |
| Sturnira ludovici | Stld | CUF | 8 | 47.40 ± 1.98 | 100.91 ± 6.01 | 74.87 ± 3.33 | 70.19 ± 2.75 |
| Sturnira luisi | Stlu | CUF | 1 | 41.21 | 85.21 | 64.8 | 60.79 |
| Sturnira oporaphilum | Stop | CUF | 9 | 43.94 ± 1.07 | 94.72 ± 2.46 | 69.52 ± 2.17 | 66.34 ±1.56 |
| Uroderma bilobatum | Urbi | CUF | 56 | 41.38 ± 1.71 | 88.37 ± 3.87 | 63.58 ± 3.07 | 60.44 ± 2.60 |
| Uroderma magnirostrum | Urma | CUF | 16 | 42.32 ± 1.41 | 89.78 ± 3.09 | 64.89 ± 2.49 | 61.68 ± 2.18 |
| Vampyressa thyone | Vath | CCF | 5 | 31.62 ± 1.09 | 68.67 ± 3.23 | 50.56 ± 2.33 | 46.94 ± 2.71 |
| Vampyriscus nymphaea | Vany | CCF | 3 | 37.91 ± 2.01 | 79.41 ± 3.06 | 56.04 ± 1.43 | 55.02 ± 1.94 |
| THYROPTERIDAE | |||||||
| Thyroptera tricolor | Thtr | BAI | 1 | 38.74 | 70.55 | 55.68 | 51.05 |
| Thyroptera lavali | Thla | BAI | 1 | 36.17 | 64.01 | 49.73 | 46.33 |
| VESPERTILIONIDAE | |||||||
| Eptesicus andinus | Epan | BAI | 1 | 46.49 | 85.49 | 68.72 | 57.34 |
| Eptesicus furinalis | Epfu | BAI | 1 | 37.01 | 50.31 | 41.99 | 36.7 |
| Eptesicus brasiliensis | Epbr | BAI | 2 | 42.31 ± 1.78 | 71.73 ± 1.12 | 59.39 ± 2.80 | 50.26 ± 1.68 |
| Histiotus montanus | Himo | BAI | 3 | 48.80 ± 1.30 | 80.56 ± 1.01 | 65.83 ± 0.85 | 63.93 ± 0.81 |
| Lasiurus blossevillii | Labl | BAI | 3 | 38.26 ± 1.07 | 75.55 ± 3.79 | 57.99 ± 1.65 | 48.17 ± 0.92 |
| Lasiurus ega | Laeg | BAI | 2 | 46.66 ± 0.19 | 90.35 ± 1.70 | 71.9 ± 1.90 | 58.04 ± 1.97 |
| Lasiurus seminolus | Lase | BAI | 1 | 36.18 | 78.87 | 57.32 | 49.33 |
| Myotis albescens | Myal | BAI | 2 | 35.03 ± 1.40 | 58.88 ± 1.13 | 49.50 ± 1.23 | 45.54 ± 1.32 |
| Myotis keaysi | Myke | BAI | 9 | 41.46 ± 0.77 | 67.46 ± 1.82 | 56.21 ± 1.56 | 51.69 ± 1.35 |
| Myotis keenii | Mykn | BAI | 1 | 34.92 | 58.95 | 47.82 | 46.36 |
| Myotis nigricans | Myni | BAI | 25 | 33.92 ± 2.47 | 55.57 ± 6.11 | 45.79 ± 4.83 | 42.29 ± 4.37 |
| Myotis oxyotus | Myox | BAI | 6 | 41.03 ± 0.77 | 66.30 ± 2.73 | 54.01 ± 1.42 | 50.62 ± 1.50 |
| Myotis riparius | Myri | BAI | 1 | 32.21 | 54.61 | 43.96 | 42.07 |
| Rhogeessa io | Rhio | BAI | 5 | 28.64 ± 1.48 | 53.36 ± 1.93 | 44.29 ± 2.27 | 38.12 ± 1.48 |
Note: Species were classified into the 11 ecological guilds proposed by Aguirre et al. (2016), Sampaio et al. (2003), Estrada et al. (2010) and Kalko et al. (1996): background cluttered space aerial insectivore (BAI); background cluttered space trawling insectivore/piscivore (BTP); highly cluttered space aerial insectivores (CAI); highly cluttered space gleaning canopy frugivore (CCF); highly cluttered space gleaning understory frugivore (CUF); highly cluttered space gleaning carnivore (CGC); highly cluttered space gleaning insectivores (CGI); highly cluttered space gleaning nectarivore (CGN); highly cluttered space gleaning omnivore (CGO); highly cluttered space gleaning sanguinivores (CGS); uncluttered space aerial insectivore (UAI).
Fig. 2.
Frequency histograms of wing variables for 97 Neotropical bat species. Variables include (a) forearm length (b) length of the third digit (c) length of the fourth digit and (d) length of the fifth digit.
Fig. 3.
Principal wing variables of 97 Neotropical bat species. Variables include (a) wing length (LD3/FL ratio) and (b) wing width (LD5/FL ratio). Acronyms for bat species are defined in table 1.
Bats were classified into 11 guilds: background cluttered space aerial insectivores (BAI, 23 species), background cluttered space trawling insectivore/piscivore (BTP, 2 species), highly cluttered space aerial insectivores (CAI, 2 species), highly cluttered space gleaning insectivores (CGI, 7 species), uncluttered space aerial insectivore (UAI, 6 species), highly cluttered space gleaning canopy frugivore (CCF, 23 species), highly cluttered space gleaning understory frugivore (CUF, 18 species), highly cluttered space gleaning nectarivore (CGN, 10 species), highly cluttered space gleaning carnivore (CGC, 2 species), highly cluttered space gleaning omnivore (CGO, 3 species), and highly cluttered space gleaning sanguinivores (CGS, 1 species) (Table 1).
Correlations among wing digits
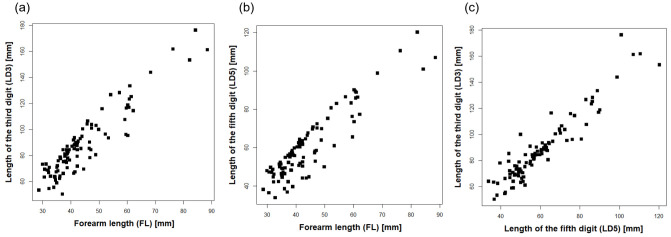
The correlation analyses for all 97 species showed a positive correlation between LD3 and FL (rs = 0.88, P < 0.001, Fig. 4a), as well as LD5 and FL (rs = 0.83, P < 0.001, Fig. 4b) and LD3 and LD5 (rs = 0.91, P < 0.001; Fig. 4c).
Fig. 4.
Scatter plot of wing digits for the 97 Neotropical bat species. Figures include (a) correlation between LD3 and FL, exploring the correlation of hand-wing length and bat size; (b) correlation between LD5 and FL, assessing the correlation of wing width and bat size; and (c) correlation between LD3 and LD5, showing the correlation of hand-wing length and wing width. Acronyms for bat species are defined in table 1.
Wing ratios
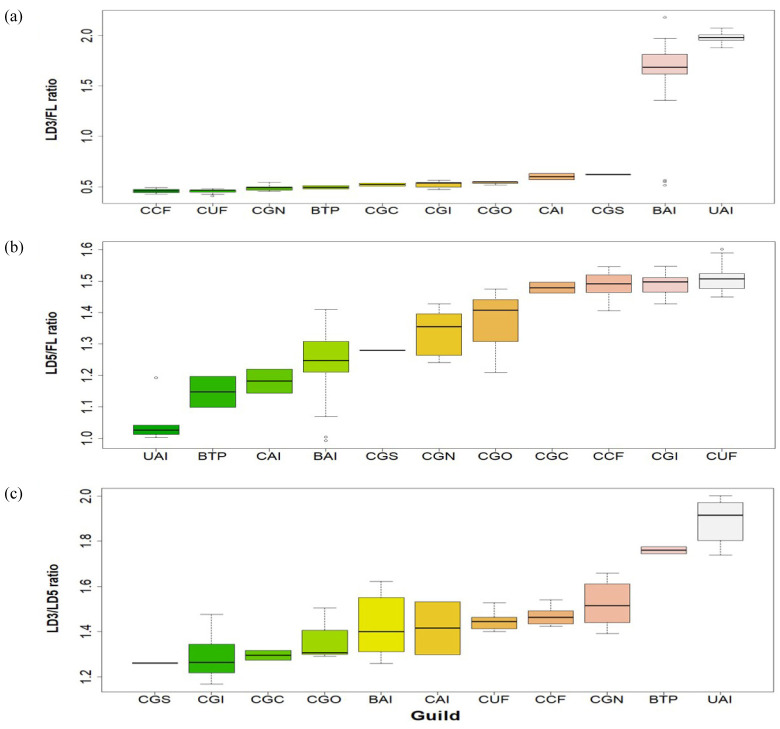
Significant differences were found among bat guilds in terms of the LD3/FL ratio (F = 58.03, d.f. = 10, P < 0.0001, Fig. 5a), LD5/FL ratio (F = 39.13, d.f. = 10, P < 0.0001, Fig. 5b), and LD3/LD5 ratio (F = 25.44, d.f. = 10, P < 0.0001, Fig. 5c). Guilds that capture their prey by employing aerial mode in uncluttered and background habitats (UAI, BAI) differed significantly from the other guilds in the LD3/FL ratio according to post-hoc pairwise comparisons (Dunn P < 0.05). Conversely, guilds that forage in highly cluttered spaces using a gleaning strategy (CUF, CCF, CGN, CGC, CGI, CGO, CGS) presented higher values for the LD5/FL ratio and lower ones for the LD3/LD5 ratio than did aerial and trawling foragers from uncluttered and background habitats (UAI, BTP) (Dunn P < 0.05, Fig. 5b c).
Fig. 5.
Boxplots of wing ratios among 11 Neotropical bat guilds. Comparisons include (a) LD3/FL ratio, (b) LD5/FL ratio, and (c) LD3/LD5 ratio. Acronyms for bat guilds are defined in table 1.
Morphometric ordination
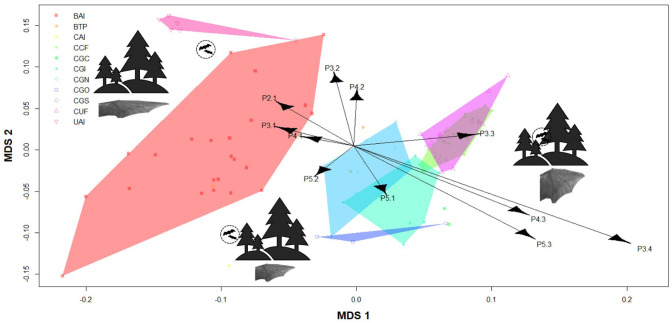
The MDS with individual bone structures resulted in a two-dimensional solution explaining 60.76% of the overall variation (Kruskal’s stress = 0.03, MDS 1 = 37.09%, MDS 2 = 23.67%) and showed a gradient of three disjunct groups that corresponded to different habitat types (cluttered, background, uncluttered) and two foraging modes (gleaning and aerial) (Fig. 6, Table S1). Terminal phalanges were important variables for guild classification (P5.3, P4.3, P3.4, Fig. 6). However, metacarpals were clearly associated with uncluttered and background insectivores (P2.1, P3.1, P4.1, P5.1, Fig. 6).
Fig. 6.
Multidimensional Scaling (MDS) ordination plot showing variation of individual bone structures among bat guilds. Each dot represents a species and the relative distance between two points reflect the relative dissimilarity. Polygons represents each one of the bat guilds. Metacarpals and phalanges are scaled to size (i.e., wing structure /FL ratio) and are found in figure 1. Acronyms for bat guilds are defined in table 1.
DISCUSSION
General findings
Wing structures reflect general patterns of habitat use and foraging mode in Neotropical bats. Since echolocation and foraging behavior are mainly influenced by habitat characteristics and foraging mode, species grouped in the same guild share similar sensory and motor adaptations (Denzinger and Schnitlzer 2013), many of which are reflected in wing structures (Fig. 6). Although diet is not a trait necessarily reflected in wing morphology, guilds may also differ from others depending on ecological features linked to food choice (Denzinger et al. 2016). In their seminal paper, Norberg and Rayner (1987) examined the wing morphology of several guilds based on wing loading, aspect ratio, and wing tip index, showing essential ecomorphological relationships to flight patterns and habitat use (Stockwell 2001; Rhodes 2002; Marinello and Bernard 2014). However, approaches addressing different bat structures that support the wing such as metacarpals, phalanges, and digits have been overlooked. In fact, for several species, data presented in this paper are the first measurements of morphometric wing structures (Table 1). It is particularly important to document individual bone traits when analyzing the relationships among foraging mode, habitat use and wing morphology.
Findley et al. (1972) stressed that the use of FL, LD3, and LD5 may be wing-area proxies that are correlated with wing indices (e.g., tip index and aspect ratio), and thereby reflect aerodynamic performance (Norberg and Rayner 1987) as well as the ecological strategies of the bats (Marinello and Bernard 2014). Species in the same guild share similar wing morphologies, regardless of size (Norberg and Rayner 1987), but some variations among guilds presumably show adaptations to specific habitats and foraging behaviors (Dietz et al. 2006). This agrees with the results presented here. For example, bats that capture prey using an aerial strategy in uncluttered or background spaces (BAI, UAI) presented a higher LD3/FL ratio than did guilds whose food resources are obtained through gleaning strategy in highly cluttered spaces (CAI, CCF, CUF, CGC, CGI, CGN, CGO, CGS; Fig. 5a). By contrast, gleaning guilds showed a higher LD5/FL ratio than did aerial insectivores (Fig. 5b). This is because, on one hand, larger LD3 in relation to FL reflects a longer hand-wing length, which is associated with fast and economic flights, typical of aerial insectivores (Dietz et al. 2006). On the other hand, a larger LD5 in proportion to FL is indicative of wider wings with high maneuverability and hovering ability, easing slow flight in narrow spaces (Dietz et al. 2006; Castillo-Figueroa and Pérez-Torres 2018). Other guilds adapted to foraging on surface dwellings (CGI, CGC, CGS) can localize and hunt prey through a combination of abilities such as echolocation, vision, and detection of prey-generated sounds (Razak 2018). These guilds showed a lower LD3/LD5 ratio, allowing individuals to fly slowly through narrow spaces (Dietz et al. 2006, Fig. 5c).
These results were supported by MDS analysis (Fig. 6), suggesting a clear distinction in wing structures among guilds that display different foraging strategies and habitat types, as was stressed by Marinello and Bernard (2014). Morphological traits may predispose bats to feed on specific habitats and adopt certain foraging strategies (Kalcounis and Brigham 1995). Phalanges were shown to be key traits in guild classification, but metacarpals also presented remarkable associations with bat guilds (Fig. 6). Metacarpals are key for generating lift (Findley et al. 1972; Stevens et al. 2013) and can be a determinant for wing length, which is typical in the morphology of aerial foragers from uncluttered and background habitats. Indeed, these guilds showed the largest metacarpals in the second (P2.1), third (P3.1) and fourth digits (P4.1), thus indicating longer wings. In the case of phalanges, these structures influence wingtip longitude, facilitating agility and propulsion during flights. Thus, larger phalanges substantially improve aerodynamic ability (Findley et al. 1972; Altringham 1996), especially in gleaning bats. Hereby, both metacarpals and phalanges may have a significant role in flight performance and ecological adaptations of guilds. Several factors other than wing morphology, however, may explain the ecological differentiation among guilds; e.g., predator-prey relationships and roosting behavior (Rhodes 2002).
Bat guilds
Although similarities in wing traits does not necessarily restrict species to a foraging mode or habitat use type (Saunders and Barclay 1992), in this study wing structures are likely related to both characteristics. For instance, in canopy frugivores like Stenodermatinae species (CCF), wings are characterized by a large chiropatagium, enabling the reduction of flight speed in obstacle-rich environments (Norberg and Rayner 1987; Stockwell 2001; Marinello and Bernard 2014). Similarly, for understory frugivores such as Carollia, Rhynophilla, Sturnira, and Phylloderma (CUF), broad wings help dodge forest obstacles—i.e., leaves, branches, and trunks—allowing individuals to select the ripe fruits using hovering abilities (Kalko et al. 1996; Marinello and Bernard 2014; Marciente et al. 2015). This is evident in higher values of LD5/FL ratios for both guilds (Fig. 5b), and the intermediate values of aspect ratio and wing loading (Norberg and Rayner 1987; Marinello and Bernard 2014).
I did not find a distinction in wing traits between the two frugivorous guilds according to the strata (Fig. 6). There is probably no clear vertical differentiation in space use and both guilds can forage in the canopy and understory considering that vertical stratum is a flexible characteristic (Meyer et al. 2008; García-García et al. 2014). Bats are not likely specialized to a particular forest stratum, even though some species show differential use to forage in the ground or canopy (Sampaio et al. 2003; Rex et al. 2011; Farneda et al. 2015). Furthermore, it has been suggested that the wing morphology that enables flight performance in cluttered spaces at high strata may also allow for flight in low cluttered strata (Fenton 1990; Rhodes 2002). My results, therefore, suggest that wing structures do not reflect a vertical specialization, reinforcing the idea of resource exploitation from all forest strata by frugivorous bats (Rex et al. 2011).
In the case of gleaning nectarivores (CGN), these bats usually hover over flowers (Glossophaginae and Lonchophyllinae species) or land on them (Phyllostomus discolor) when feeding on nectar. The former involves long, rounded wings reflected in a high tip index and low aspect ratio and wing loading. The latter shows an intermediate body size, aspect ratio and wing loading (Norberg and Rayner 1987). These wing characteristics allow hovering species to feed on flowers in confined spaces, whereas larger species that land on flowers are aerodynamically constrained and may perform direct flights and visit mainly exposed and larger flowers (Giannini and Brenes 2001). In this paper, wing-width (LD5/FL ratio) presented an intermediate-range, whereas wing-length (LD3/LD5 ratio) showed high values (Fig. 5b c), thus reflecting intermediate maneuverability and fast flights in this guild.
Although P. discolor has more maneuverability than other congeneric species like P. hastatus, the former probably belongs to a different guild of nectarivore because of its foraging style (Giannini and Brenes 2001; Marinello and Bernard 2014). In fact, P. discolor exhibits an intermediate behavior between hovering and landing bats (Giannini and Brenes 2001). Notwithstanding, despite it being an important pollinator of many Neotropical plants (Giannini and Brenes 2001; Lobo et al. 2005), P. discolor could also belong to the omnivore guild because of his high feeding plasticity (Marinello and Bernard 2014). Omnivorous species (CGO), such as P. hastatus, P. elongatus, and Tonatia saurophila, are characterized by a variety of diets, including insects, flowers, and fruits, thus performing slow flights around the vegetation to obtain their food (Marciente et al. 2015). This is reflected in higher LD5/LA and lower LD5/LD3 ratios (Fig. 5c).
Gleaning carnivorous bats (CGC), constituting Chrotopterus autitus and Trachops cirrhosus, regularly prey on small terrestrial vertebrates through sit-and-wait behavior using small foraging areas and short commuting distances (Kalko et al. 1999). According to Norberg and Fenton (1988), the combination of large body size and lower aspect ratio and wing loading are idiosyncratic characteristics of carnivorous bats. More broadly, shorter wings and large wing area are key features for better maneuverability in narrow habitats and given that they locate preys through listening to their movements on the foliage or the ground, agile and slow flights allow these bats to capture their prey (Marciente et al. 2015). The results showed a longer metacarpal in the fifth digit (P5.1, Fig. 6), suggesting high maneuverability for catching mobile prey (Fig. 5). Likewise, the gleaning insectivores (CGI)—composed of Mycronicteris, Lophostoma, and Gardnerycteris species—showed the highest values in the LD5/FL ratio (after CUF), because these are gleaning bats specialized to foraging in habitats with a high degree of complexity in understory vegetation, with dense foliage and hollow trees for roosting and feeding sites (Cleary et al. 2016).
Highly cluttered space aerial insectivores (CAI), composed of two mormoopid species, are characterized by high values of phalanges of third (P3.3) and fifth digit (P5.2, Fig. 6), which reflects the ability to fly quickly in several habitats such as terraces, gaps, or forest edges (Schnitzler et al. 2003; Bernard and Fenton 2003; Mancina et al. 2012) and capture a variety of insects such as moths, flies, and earwigs (Boada et al. 2003). Nevertheless, other insectivores from open spaces (UAI), which includes molossid species, perform fast flights reflected in the largest metacarpals and phalanges of the third digit (P3.1, P3.2, Fig. 6), but their mobility is constrained in narrow spaces full of obstacles (Norberg and Rayner 1987; Thollesson and Norberg 1991; Marinello and Bernard 2014). In other words, their foraging is far to be efficient in narrow spaces and some studies have demonstrated the high metabolic cost of flying in cluttered habitats, suggesting an unsuitable site for these insectivores (Voigt and Holderied 2012). In addition, morphological restrictions related to low values in terminal phalanges of the fifth (P5.3) and fourth digits (P4.3, Fig. 6) may restrict the movement of this guild to dense habitats.
Contrary to this, aerial insectivores from back-ground habitats (BAI), composed of emballonurid and vespertilionid bats, showed the second highest values in the third digit but also a high variation in the fifth digit because of their flexibility in different foraging sites ranging from forest edges to forest gaps (Norberg and Rayner 1987). It should be noted that this guild holds more species (23), which can explain the high variation in wing structures (Figs. 5, 6), but, interestingly, in frugivorous guilds, variation was lower, despite the large number of species (23 for CCF and 18 for CUF).
Gleaning sangunivores (CGS), composed only of Desmodus rotundus, showed the lowest LD3/LD5 ratio (Fig. 3c). This ratio confers slow flight but also a secretive displacement in narrow spaces (Dietz et al. 2006), eased by the pollex to allow blood intake from medium and large mammals (Norberg and Rayner 1987). The foraging behavior of D. rotundus relies on movements usually 1 m above the ground and relatively straight flight courses with low speeds of 13.4–13.8 km/h (Sánchez-Hernández et al. 2006). In contrast, trawling piscivores/insectivores (BTP), including the two Noctilionidae species, showed a higher LD3/LD5 ratio (Fig. 5c) and lower values in the phalanges of the fifth digit (P5.3; Fig. 6) and higher ones in the phalanges of the third digit (P3.3; Fig. 6). Piscivores are characterized by plagiopatagium truncated (Fish et al. 1991) and high aspect ratio adapted to fly over open water, close to the ground (Norberg and Rayner 1987), or open pastures (Aranguren et al. 2011).
Bias and limitations
Even though I characterized the wing morphologies of many New World bats by describing the length of their digits (Table 1, Figs. 2, 3) and individual bone structures (Fig. 6), this study analyzed less than 30% of all Neotropical bat species. However, I included the three superfamilies (100%) and eight families (88.8%) of New World bats, thus obtaining good phylogenetic representativeness. Further studies should examine species that I did not include to determine if the composition of bats reflects the same pattern presented in this study.
Some studies have stressed that wing morphology can vary not only at the interspecific level (Marinello and Bernard 2014), but also at the intraspecific level by predicting individual niche specialization (Magalhães de Oliveira et al. 2020) and sexual dimorphism in bats (Camargo and Oliveira 2012). In this paper, however, the guild was the unit of analysis, and therefore intraspecific and interspecific variations were not analyzed, mainly because of the limitations in the specimens available in the Collection (only one individual was available for many species). This is a common issue when working with natural history museums (Pyke and Ehrlich 2010; Castillo-Figueroa 2018b), but it should be noted that these biological repositories provide a tremendous amount of information (Castillo-Figueroa 2018b), as is shown in this study.
CONCLUSIONS
This study demonstrated that wing structure is a reliable indicator of habitat use and foraging mode in Neotropical bats. Overall, bat guilds associated with an aerial strategy in uncluttered or background habitats appear to present a higher LD3/FL ratio, whereas gleaners that obtain their food in highly cluttered spaces display a higher LD5/FL ratio and lower LD3/LD5 ratio than do aerial and trawling foragers from uncluttered and background habitats. Terminal phalanges represent key traits for guild discrimination, but metacarpals were also important for classifying some guilds, probably because of their potential role in flight performance and ecological adaptations. Morphological diversity in bats has been largely focused on wing shape by employing traditional measures (i.e., wing loading, aspect ratio, wingtip index). Nonetheless, in this article, I parsed out another kind of wing traits that can be useful to better understand guild classification in bats. Metacarpals and phalanges are poorly studied in their potential association with flight performance and foraging behavior, and minute variations in those structures could be important to ecological adaptations in bats. I suggest considering individual bone structures that support the wing to assess morphological differentiation between species and guilds. Lastly, I suggest including this approach in physiological, ethological, evolutionary, and ecological studies in an integrative framework that contributes to understanding the functional mechanisms of bats in Neotropical environments.
Supplementary materials
Loadings matrix of wing traits calculated by MDS. Metacarpals and phalanges are scaled to size (i.e., wing structure /FL ratio). Acronyms for wing traits are found in figure 1.
Acknowledgments
I am grateful to Jairo Pérez-Torres, the curator of the Mammalian Collection of Museo Javeriano de Historia Natural from Pontificia Universidad Javeriana (Bogotá, Colombia), for allowing me to access the specimens in the Collection and for the important discussions about bat ecomorphology. The two reviewers and the editor provided insightful comments that substantially improved the paper.
List of abbreviations
- BAI
Background cluttered space aerial insectivore.
- BTP
background cluttered space trawling insectivore/piscivore.
- CAI
highly cluttered space aerial insectivores.
- CCF
highly cluttered space gleaning canopy frugivore.
- CUF
highly cluttered space gleaning understory frugivore.
- CGC
highly cluttered space gleaning carnivore.
- CGI
highly cluttered space gleaning insectivores.
- CGN
highly cluttered space gleaning nectarivore.
- CGO
highly cluttered space gleaning omnivore.
- CGS
highly cluttered space gleaning sanguinivores.
- UAI
uncluttered space aerial insectivore.
- FL
forearm length.
- LD3
length of the third digit.
- LD4
length of the fourth digit.
- LD5
length of the fifth digit.
Footnotes
Authors’ contributions: The study design, data collection, analysis of the information, writing and revision of the manuscript were done entirely by Dennis Castillo-Figueroa.
Competing interests: The author declares no conflict of interest.
Consent for publication: Not applicable.
Ethics approval consent to participate: Not applicable.
References
- Aguirre L, Montaño-Centellas F, Gavilanez M, Stevens R. 2016. Taxonomic and phylogenetic determinantes of functional composition of bolivian bat assamblages. PLoS ONE 11(7):e0158170. doi:10.1371/journal.pone.0158170. [DOI] [PMC free article] [PubMed]
- Altringham JD. 1996. Bats: biology and behavior. Oxford University Press, New York, USA.
- Aranguren C, Gonzalez-Carcacia JA, Martínez H, Nassar J. 2011. Noctilio albiventris (Noctilionidae), a potential seed disperser in disturbed tropical dry forest habitats. Acta Chiropt 13(1):189–194. doi:10.3161/150811011X578732.
- Arita H, Vargas-Barón, J, Villalobos F. 2014. Latitudinal gradients of genus richness and endemism and the diversification of New World bats. Ecography 37(11):1024–1033. doi:10.1111/ecog.00720.
- Arnold SJ. 1983. Morphology, performance and fitness. Am Zool 23(2):347–361. doi:10.1093/icb/23.2.347.
- Bernard E, Fenton MB. 2003. Bat mobility and roosts in a fragmented landscape in Central Amazonia, Brazil. Biotropica 35(2):262–277. doi:10.1646/02156.
- Boada C, Burneo S, Vries T, Tirira S. 2003. Notas ecológicas y reproductivas del murciélago rostro de fantasma Mormoops megalophylla (Chiroptera: Mormoopidae) en San Antonio de Pichincha. Pichincha. Ecuador. Mastozool Neotrop 10(1):21–26.
- Briones-Salas M, Lavariega MC, Moreno CE, Viveros J. 2019. Responses of phyllostomid bats to traditional agriculture in neotropical montane forests of southern Mexico. Zool Stud 58:9. doi:10.6620/ZS.2019.58-09. [DOI] [PMC free article] [PubMed]
- Camargo N, Oliveira H. 2012. Sexual dimorphism in Sturnira lilium (Chiroptera, Phyllostomidae): can pregnancy and pup carrying be responsible for differences in wing shape? PLoS ONE 7(11):e49734. doi:10.1371/journal.pone.0049734. [DOI] [PMC free article] [PubMed]
- Castillo-Figueroa D. 2020. Why bats matters: a critical assessment of bat-mediated ecological processes in the Neotropics. Eur J Ecol 6(1):77–101. doi:10.17161/eurojecol.v6i1.13824.
- Castillo-Figueroa D. 2018a. Fluctuating asymmetry of three bat species in extensive livestock systems of Córdoba department. Colombia. Rev Colomb Cienc Anim 10(2):143–53. doi:10.24188/recia.v10.n2.2018.623.
- Castillo-Figueroa D. 2018b. Beyond specimens: linking biological collections, functional ecology and biodiversity conservation. Rev Peru Biol 25(3):343–348. doi:10.15381/rpb.v25i3.14246.
- Castillo-Figueroa D, Pérez-Torres J. 2018. Respuestas funcionales de murciélagos asociados a fragmentos de bosque seco tropical en Córdoba (Colombia): implicaciones del tipo de manejo en sistemas de ganadería extensiva. Rev Biodivers Neotrop 8(3):197–211.
- Cleary KA, Waits LP, Finegan B. 2016. Agricultural intensification alters bat assemblage composition and abundance in a dynamic Neotropical landscape. Biotropica 48(5):667–676. doi:10.1111/btp.12327.
- Cooper L, Sears K. 2013. How to grow a bat wing. In: Adams RA, Pedersen SC (eds) Bat Evolution. Ecology and Conservation. Springer-Verlag, New York, USA.
- Denzinger A, Kalko EKV, Tschapka M, Grinnell AD, Schnitzler HU. 2016. Guild structure and niche differentiation in echolocating bats. In: Fenton MB, Grinnell AD, Popper AN, Fay RR (eds) Bat bioacoustics. NY: Springer, New York, USA.
- Denzinger A, Schnitzler H. 2013. Bat guilds, a concept to classify the highly diverse foraging and echolocation behaviors of microchiropteran bats. Front Physiol 4:164. doi:10.3389/fphys.2013.00164. [DOI] [PMC free article] [PubMed]
- Dietz C, Dietz I, Siemers BM. 2006. Wing measurement variations in the five European horseshoe bat species (Chiroptera: Rhinolophidae). J Mammal 87(6):1241–1251. doi:10.1644/05-MAMM-A-299R2.1.
- Estrada-Villegas S, Meyer C, Kalko E. 2010. Effects of tropical forest fragmentation on aerial insectivorous bats in a land-bridge island system. Biol Conserv 143(3):597–608. doi:10.1016/j.biocon.2009.11.009.
- Farneda FZ, Rocha R, López-Baucells A, Groenenberg M, Silva I, Palmeirim JM, Bobrowiec PED, Meyer CFJ. 2015. Trait-related responses to habitat fragmentation in Amazonian bats. J Appl Ecol 52(5):1381–1391. doi:10.1111/1365-2664.12490.
- Fauth JE, Bernardo J, Camara M, Resetarits Jr. WJ, van Buskirk J, McCollum SA. 1996. Simplifying the jargon of community ecology: a conceptual approach. American Naturalist 147(2):282–286. doi:10.1086/285850.
- Fenton MB. 1990. The foraging behaviour and ecology of animaleating bats. Can J Zool 68(3):411–422. doi:10.1139/z90-061.
- Findley JS, Studier EH, Wilson DE. 1972. Morphological properties of bat wings. J Mammal 53(3):429–444. doi:10.2307/1379035.
- Fish F, Blood B, Clark B. 1991. Hydrodynamics of the feet of fish-catching bats: influence of the water surface on drag and morphological design. J Exp Zool 258(2):164–173. doi:10.1002/jez.1402580205.
- García-García JL, Santos-Moreno A, Kraker-Castañeda C. 2014. Ecological traits of phyllostomid bats associated with sensitivity to tropical forest fragmentation in Los Chimalapas. Mexico. Trop Conserv Sci 7(3):457–474. doi:10.1177/194008291400700307.
- Gardner AL (ed). 2007. Mammals of South America. Volume 1: Marsupials, Xenarthrans, Shrews, and Bats. The University of Chicago Press, Chicago, USA.
- Giannini NP, Brenes FV. 2001. Flight cage observations of foraging mode in Phyllostomus discolor, P. hastatus, and Glossophaga commissarisi. Biotropica 33(3):546–550. doi:10.1111/j.1744-7429.2001.tb00211.x.
- Guisande C, Heine J, González-DaCosta J, García-Roselló E. 2014. RWizard Software. http://www. ipez.es/rwizard. Accessed on 23 Mar. 2020.
- Hammer O, Harper D, Ryan PD. 2001. PAST: Paleontological Statistics software package for education and data analysis. Palaentol Electron 4(1):1–9.
- Integrated Taxonomic Information System on-line database (ITIS). 2020. Chiroptera. http://www.itis.gov. Accessed 5 Feb. 2020.
- Kalcounis MC, Brigham RM. 1995. Intraspecific variation in wing loading affects habitat use by little brown bats (Myotis lucifugus). Can J Zool 73(1):89–95. doi:10.1139/z95-011.
- Kalko EKV, Friemel D, Handley CO, Schnitzler HU. 1999. Roosting and foraging behavior of two neotropical gleaning bats. Tonatia silvicola and Trachops cirrhosus (Phyllostomidae). Biotropica 31(2):344–353. doi:10.1111/j.1744-7429.1999.tb00146.x.
- Kalko EKV, Handley CO, Handley D. 1996. Organization, diversity and long-term dynamics of a Neotropical bat community. In: Cody ML, Smallwood JA (eds) Long-term studies of vertebrate communities. Academic Press, San Diego, USA.
- Lobo JA, Quesada M, Stoner KE. 2005. Effects of pollination by bats on the mating system of Ceiba pentandra (Bombacaceae) populations in two tropical life zones in Costa Rica. Am J Bot 92(2):370–376. doi:10.3732/ajb.92.2.370. [DOI] [PubMed]
- López-Aguirre C, Hand SJ, Laffan SW, Archer M. 2018. Phylogenetic diversity, types of endemism and the evolutionary history of New World bats. Ecography 41(12):1955–1966. doi:10.1111/ecog.03260.
- Magalhães de Oliveira HF, Camargo NF, Hemprich-Bennett DR, Rodríguez-Herrera B, Rossiter SJ, Clare EL. 2020. Wing morphology predicts individual niche specialization in Pteronotus mesoamericanus (Mammalia: Chiroptera). PLoS ONE 15(5):e0232601. doi:10.1371/journal.pone.0232601. [DOI] [PMC free article] [PubMed]
- Mancina CA, García-Rivera L, Miller BW. 2012. Wing morphology, echolocation, and resource partitioning in syntopic Cuban mormoopid bats. J Mammal 93(5):1308–1317. doi:10.1644/11-MAMM-A-331.1.
- Marciente R, Bobrowiec PED, Magnusson WE. 2015. Ground-vegetation clutter affects phyllostomid bat assemblage structure in lowland amazonian forest. PLoS ONE 10(6):e0129560. doi:10.1371/journal.pone.0129560. [DOI] [PMC free article] [PubMed]
- Marinello MM, Bernard E. 2014. Wing morphology of Neotropical bats: a quantitative and qualitative analysis with implications for habitat use. Can J Zool 92(2):141–147.
- McCune B, Grace JB. 2002. Analysis of Ecological Communities. MjM Software Design: Gleneden Beach, Oregon, USA.
- Meyer CFJ, Fründ. J, Lizano WP, Kalko EKV. 2008. Ecological correlates of vulnerability to fragmentation in Neotropical bats. J Appl Ecol 45(1):381–391. doi:10.1111/j.1365-2664.2007.01389.x.
- Norberg UM, Fenton MB. 1988. Carnivorous bats? Biol J Linn Soc 33(4):383–394. doi:10.1111/j.1095-8312.1988.tb00451.x.
- Norberg UM, Rayner JMV. 1987. Ecological morphology and flight in bats (Mammalia; Chiroptera): wing adaptation. Flight performance. foraging strategy and echolocation. Philos Trans R Soc Lond B Biol Sci 316(1179):335–427. doi:10.1098/rstb.1987.0030.
- Peixoto F, Braga PHP, Cianciaruso MV, Diniz-Filho JAF, Brito D. 2014. Global patterns of phylogenetic beta diversity components in bats. J Biogeogr 41(4):762–772. doi:10.1111/jbi.12241.
- Pyke GH, Ehrlich PR. 2010. Biological collections and ecological/environmental research: a review, some observations and a look to the future. Biol Rev 85(2):247–266. doi:10.1111/j.1469-185X.2009.00098.x. [DOI] [PubMed]
- Razak KA. 2018. Adaptations for substrate gleaning in bats: the pallid bat as a case study. Brain Behav Evol 91:97–108. doi:10.1159/000488873. [DOI] [PubMed]
- Rhodes MP. 2002. Assessment of sources of variance and patterns of overlap in microchiropteran wing morphology in southeast Queensland, Australia. Can J Zool 80(3):450–460. doi:10.1139/z02-029.
- Rex K, Michener R, Kunz TH, Voigt CC. 2011. Vertical stratification of Neotropical leaf-nosed bats (Phyllostomidae: Chiroptera) revealed by stable carbon isotopes. J Trop Ecol 27(3):211–222. doi:10.1017/S0266467411000022.
- Sampaio EM, Kalko EK, Bernard E, Rodríguez-Herrera B, Handley CO. 2003. A biodiversity assessment of bats (Chiroptera) in a tropical lowland rainforest of Central Amazonia, including methodological and conservation considerations. Stud Neotrp Fauna E 38(1):17–31. doi:10.1076/snfe.38.1.17.14035.
- Sánchez-Hernández C, Romero-Almaraz ML, Wooten MC, Schnell GD, Kennedy ML. 2006. Speed in Flight of Common Vampire Bats (Desmodus rotundus). The Southwest Nat 51(3):422–425. doi:10.1894/0038-4909(2006)51[422:SIFOCV]2.0.CO;2.
- Santana S, Grosse I, Dummont E. 2012. Dietary hardness, loading behavior, and the evolution of skull form in bats. Evolution 66(8):2587–2598. doi:10.1111/j.1558-5646.2012.01615.x. [DOI] [PubMed]
- Saunders MB, Barclay RMR. 1992. Ecomorphology of insectivorous bats: a test of predictions using two morphologically similar species. Ecology 73(4):1335–1345. doi:10.2307/1940680.
- Schnitzler HU, Moss CF, Denzinger A. 2003. From spatial orientation to food acquisition in echolocating bats. Trends Ecol Evol 18(8):386–94. doi:10.1016/S0169-5347(03)00185-X.
- Sears K, Behringer R, Rasweiler J, Niswander L. 2006. Development of bat flight: morphologic and molecular evolution of bat wing digits. PNAS 103(17):6581–6586. doi:10.1073/pnas.0509716103. [DOI] [PMC free article] [PubMed]
- Stevens RD, Johnson ME, McCulloch ES. 2013. Absolute and relative secondary-sexual dimorphism in wing morphology: a multivariate test of the ‘Big Mother’ hypothesis. Acta Chiropt 15(1):163–170. doi:10.3161/150811013X667966.
- Stockwell EF. 2001. Morphology and flight manoeuvrability in New World leaf nosed bats (Chiroptera: Phyllostomidae). J Zool (Lond) 254(4):505–514. doi:10.1017/S0952836901001005.
- Thollesson M, Norberg UM. 1991. Moments of inertia of bat wings and body. J Exp Biol 158:19–35.
- Velazco PM. 2005. Morphological phylogeny of the bat genus Platyrrhinus Saussure, 1860 (Chiroptera: Phyllostomidae) with the description of four new species. Fieldiana Zoology, New Series 105:1–53. doi:10.3158/0015-0754(2005)105[1:MPOTBG ]2.0.CO;2.
- Velazco PM, Gardner AL, Patterson B. 2010. Systematics of the Platyrrhinus helleri species complex (Chiroptera: Phyllostomidae), with descriptions of two new species. Zool J Linnean Soc 159(3):785–812. doi:10.1111/j.1096-3642.2009.00610.x.
- Voigt CC, Holderied MW. 2012. High manoeuvring costs force narrow winged molossid bats to forage in open space. J Comp Physiol B 182(3):415–424. doi:10.1007/s00360-011-0627-6. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Loadings matrix of wing traits calculated by MDS. Metacarpals and phalanges are scaled to size (i.e., wing structure /FL ratio). Acronyms for wing traits are found in figure 1.