Abstract
Hypersensitivity pneumonitis (HP) is an immunologically mediated form of lung disease resulting from inhalational exposure to any of a large variety of antigens. A subgroup of patients with HP develops pulmonary fibrosis (fibrotic HP; FHP), a significant cause of morbidity and mortality. This study will evaluate the safety and efficacy of the antifibrotic pirfenidone in treating FHP.
This single-centre, randomised, double-blind, placebo-controlled trial is enrolling adults with FHP (ClinicalTrials.gov: NCT02958917). Study participants must have fibrotic abnormalities involving ≥5% of the lung parenchyma on high-resolution computed tomography scan, forced vital capacity (FVC) ≥40% and diffusing capacity of the lung for carbon monoxide ≥30% of predicted values. Study participants will be randomised in a 2:1 ratio to receive pirfenidone 2403 mg·day−1 or placebo. The primary efficacy end-point is the mean change in FVC % predicted from baseline to week 52. A number of secondary end-points have been chosen to evaluate the safety and efficacy in different domains.
Short abstract
The design of a phase II study of 52 weeks of pirfenidone or placebo on top of standard of care in patients with fibrotic HP (ClinicalTrials.gov NCT02958917) https://bit.ly/32CfeSF
Introduction
Background and rationale
Hypersensitivity pneumonitis (HP) is an immunologically mediated form of lung disease resulting from inhalational exposure to a large variety of antigens. In the USA, the estimated yearly prevalence of HP ranges from 1.67 to 2.71 cases per 100 000 persons. Between 56% and 68% of HP cases per year are considered chronic disease (prevalence 0.9–1.7 per 100 000 persons) and among chronic cases approximately 36–48% have pulmonary fibrosis [1]. A large proportion of patients with fibrotic HP develop symptomatic, functional and radiographic disease progression [2–4]. Progressive fibrotic HP (FHP) has a mortality rate comparable to idiopathic pulmonary fibrosis (IPF), with an estimated mean survival of 3–5 years from the time of diagnosis [2, 5, 6].
Pirfenidone is an antifibrotic agent that downregulates collagen synthesis, decreases the extracellular matrix, and blocks fibroblast proliferation in vitro [7]. Results from previous clinical trials have shown that pirfenidone is safe and well tolerated, and significantly slows the rate of disease progression as measured by a declining forced vital capacity (FVC) in patients with IPF [8–11]. As IPF is a fibrosing interstitial lung disease (ILD) that shares clinical and pathobiological features with FHP, the use of this drug in progressive FHP has a mechanistic rationale and may be beneficial [12–15].
This report describes the design of a phase 2 randomised, double-blind, placebo-controlled trial looking at safety, tolerability and efficacy of pirfenidone in adults with FHP.
Material and methods
Study design
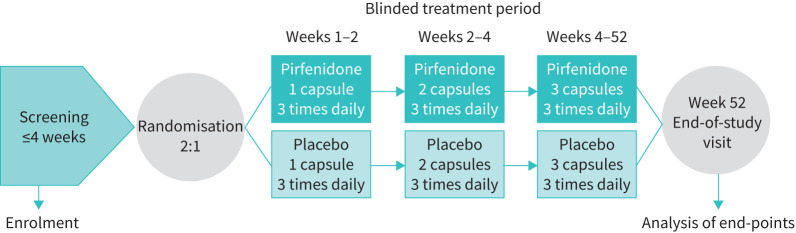
This is a double-blind, randomised, placebo-controlled trial (Clinicaltrials.gov identifier: NCT02958917) being conducted at National Jewish Health (NJH), Denver, CO, USA. Patient enrolment began March 2017. Approximately 40–42 subjects meeting eligibility criteria for the study will be randomized in a 2:1 ratio to receive pirfenidone 2403 mg·day−1 or placebo for 52 weeks (figure 1). The dose of study drug will be titrated over 4 weeks: 2 weeks at a starting dose of 1 capsule (267 mg oral capsule with food) three times daily; then 2 weeks at 2 capsules three times daily; and 48 weeks at a maintenance dose of 2403 mg·day−1 (3 capsules, three times daily).
FIGURE 1.
Trial design.
Subjects will have a telephone assessment at weeks 1 and 3, and an in-clinic assessment at day 1, and weeks 5, 13, 26, 39 and 52. Subjects will be asked to complete an adverse event (AE) and dosing compliance diary between all visits. If subjects discontinue study treatment early for any reason, they will be asked to continue with all scheduled study procedures until week 52.
Study population
Eligible patients aged 18–80 years with HP or with a provisional high-confidence diagnosis will be identified by integration of the clinical, imaging, bronchoscopic and, when available, surgical lung biopsy data during the multidisciplinary discussion performed at the weekly NJH ILD conference (table 1). The final consensus multidisciplinary diagnosis adjudication will be documented on a case report form.
TABLE 1.
Entry criteria
| Inclusion criteria |
| Multidisciplinary consensus diagnosis of FHP |
| Age 18 to 80 years at randomisation |
| Diagnosis of FHP, defined from the first instance in which a patient was informed of having FHP, for at least 3–6 months |
| HRCT according to pre-specified criteria: Typical FHP#: evidence of lung fibrosis (reticular abnormality and/or traction bronchiectasis and/or architectural distortion and/or honeycombing) with either of the following: |
| • Profuse poorly defined centrilobular nodules of ground-glass opacity affecting all lung zones |
| • Inspiratory mosaic attenuation with three-density sign |
| AND |
| • Lack of features suggesting an alternative diagnosis |
| Compatible FHP¶: evidence of lung fibrosis (as above) with any of the following: |
| • Patchy or diffuse ground-glass opacity |
| • Patchy, non-profuse centrilobular nodules of ground-glass attenuation |
| • Mosaic attenuation and lobular air-trapping that do not meet criteria for typical FHP |
| AND |
| • Lack of features suggesting an alternative diagnosis. |
| Indeterminate FHP+: CT signs of fibrosis without other features suggestive of HP and lack of features suggesting an alternative diagnosis |
| FHP disease severity and progression |
| FVC ≥40% pred, DLCO ≥30% pred based on historical pulmonary function tests obtained within the 60 days prior to day 1 |
| Presence of fibrotic abnormalities involving ≥5% of the lung parenchyma, with or without traction bronchiectasis or honeycombing, on HRCT scan |
| Evidence of disease progression: worsening respiratory symptoms and increased extent of fibrosis on HRCT or relative decline in the FVC of at least 5% over 24 months |
| Able to walk ≥100 m during the 6-min walk test at screening |
| Informed consent and protocol adherence |
| Able to understand and sign a written informed consent form |
| Able to understand the importance of adherence to study treatment and the study protocol and willing to follow all study requirements, including the concomitant medication restrictions, throughout the study |
| Exclusion criteria |
| Disease-related exclusions |
| Not a suitable candidate for enrolment or unlikely to comply with the requirements of this study, in the opinion of the investigator |
| Cigarette smoking at screening or unwilling to avoid tobacco products throughout the study |
| Known explanation for the interstitial lung disease, including but not limited to radiation, drug toxicity, sarcoidosis, pneumoconiosis |
| Clinical diagnosis of any connective tissue disease, including but not limited to scleroderma, polymyositis/dermatomyositis, and rheumatoid arthritis |
| Expected to receive a lung transplant within 6–12 months from randomisation or on a lung transplant waiting list at randomisation |
| The Investigator judges that there has been sustained improvement in the severity of FHP during the 6–12 months prior to screening visit 1, based on changes in FVC, DLCO, and/or HRCT scans of the chest |
| Medical exclusions |
| Any condition other than FHP that, in the opinion of the investigator, is likely to result in the death of the patient within 6–12 months |
| Any condition that, in the opinion of the investigator, might be significantly exacerbated by the known side-effects associated with the administration of pirfenidone |
| Pregnancy or lactation Women of childbearing capacity are required to have a negative serum pregnancy test before treatment and must agree to maintain highly effective contraception by practicing abstinence or by using at least two methods of birth control from the date of consent through the end of the study; if abstinence is not practiced, one of the two methods of birth control should be an oral contraceptive (e.g. oral contraceptive and a spermicide) |
| History of ongoing alcohol or substance abuse |
| History of severe hepatic impairment or end-stage liver disease |
| History of end-stage renal disease requiring dialysis |
| Clinical evidence of active infection including, but not limited to, bronchitis, pneumonia, sinusitis, or urinary tract infection |
| Unstable or deteriorating cardiac disease, including but not limited to the following: |
| • Unstable angina pectoris or myocardial infarction |
| • Congestive heart failure requiring hospitalisation |
| • Uncontrolled clinically significant arrhythmias |
| Laboratory exclusions |
| Any of the following liver function test criteria above specified limits: total bilirubin >2.0 mg·dL−1, excluding patients with Gilbert's syndrome; aspartate or alanine aminotransferase (AST/SGOT or ALT/SGPT) >3× ULN; alkaline phosphatase >2.5× ULN within past 30 days |
| Creatinine clearance <30 mL·min−1, calculated using the Cockcroft–Gault formula within past 30 days |
| Electrocardiogram with a QTc interval >500 ms at screening |
| Medication exclusions |
| Prior use of pirfenidone, nintadinib or known hypersensitivity to any of the components of study treatment |
| Introduction, increase or escalation of immunosuppressive pharmacological therapy within 1 month (e.g. prednisone, azathioprine, mycophenolic acid and mycophenolate mofetil)§ |
| Use of any of the following therapies within 28 days before screening: bosentan, ambrisentan, cyclophosphamide, cyclosporine, etanercept, iloprost, infliximab, methotrexate, tacrolimus, tetrathiomolybdate, TNF-α inhibitors, imatinib mesylate, interferon γ-1b, and tyrosine kinase inhibitors, fluvoxamine, Sildenafil (daily use) |
FHP: fibrotic hypersensitivity pneumonitis; HRCT: high-resolution computed tomography; FVC: forced vital capacity; DLCO: diffusing capacity of the lung for carbon monoxide; ULN: upper limit of normal; TNF: tumour necrosis factor. In all subjects with biopsy specimens, data on the overall histological pattern and individual features will be determined and scored by an expert chest pathologist using a standardised data collection form. In the absence of surgical lung histology, a high-confidence provisional diagnosis based on the above computed tomography (CT) confidence pattern and multidisciplinary consensus required. #: these patients are required to have an identifiable antigen exposure, or indeterminate or unidentifiable antigen exposure and bronchoalveolar lavage (BAL) lymphocytosis (≥20%) or transbronchial biopsies demonstrating non-necrotising granuloma(s) or lymphocytosis, or surgical lung histology consistent with HP. ¶: these patients are required to have an identifiable or indeterminate antigen exposure and BAL lymphocytosis (≥20%) or transbronchial biopsies demonstrating non-necrotising granuloma(s) or lymphocytosis, or surgical lung histology consistent with HP. Otherwise, surgical lung histology consistent with HP. +: these patients are required to have a known antigen exposure and BAL lymphocytosis (≥20%) or transbronchial biopsies demonstrating non-necrotising granuloma(s) or lymphocytosis, or surgical lung histology consistent with HP. §: decreasing or tapering off oral corticosteroids is allowed.
High-resolution computed tomography (HRCT) scans must have the presence of fibrotic abnormalities affecting ≥5% of the lung parenchyma (as determined by the trial chest radiologist), with or without traction bronchiectasis or honeycombing.
There must be no evidence of an alternate diagnosis. Patients will be required to have a pre-bronchodilator FVC ≥40% and diffusing capacity of the lung for carbon monoxide (DLCO) ≥30% of predicted values at screening. The screening period may last up to 30 days.
Study participants must also have progressive disease, defined as worsening respiratory symptoms and either an increase in extent of fibrosis on HRCT or relative decline in the FVC % predicted of at least 5% in the 24 months before screening, despite management deemed appropriate in clinical practice. Other inclusion and exclusion criteria for this study are listed in table 1.
Study drug administration and blinding
Neither study personnel nor the subjects will know which study treatment the subject is receiving. Subjects will be randomised by a computer-generated randomisation schedule in a 2:1 ratio to receive either pirfenidone 2403 mg·day−1 or a placebo equivalent on top of standard of care, including antigen avoidance and abatement procedures.
Study treatment will be titrated over 4 weeks to the full maintenance dose of 9 capsules per day (three 267-mg capsules taken orally three times daily with food). Subjects will remain on a stable maintenance dose for the duration of the treatment period unless the dose is reduced to manage an AE or titrated again when restarting study treatment after a 28-day or greater lapse in treatment.
Individual subject treatment assignments will not be unblinded during the study unless a subject safety issue arises in which unblinding is necessary to ensure optimal subject management. Once the study is complete, and the final analysis completed, treatment assignments will be unblinded for all subjects.
Concomitant medications
All of the following are considered concomitant medications, and data regarding their use will be collected and recorded: prescription drugs, over-the-counter drugs, including vitamins, antacids, herbal and dietary supplements. Study participants who are receiving a stable dose of azathioprine (AZA) or mycophenolate mofetil (MMF) for >1 month before screening and who are expected to remain on a stable dose are eligible for inclusion in the trial. For excluded therapies see the exclusion criteria (table 1).
Discontinuation of study treatment
Study treatment will be discontinued for any of the following reasons: unacceptable toxicity (this may include serious AEs related to study treatment), patient request or withdrawal of consent, pregnancy, investigator discretion or lung transplantation. Subjects who discontinue study treatment will be asked to complete all scheduled study assessments and procedures until week 52. If new therapies are approved over the course of the trial, participants will be notified and re-consented both verbally and in writing.
Safety monitoring and ethics
Throughout the study, the safety officer will review individual serious AE reports and laboratory toxicities. In addition, the safety officer will review all safety data after 50% of enrolment is completed as well as serious AEs on an ongoing basis. At all times during the course of the study, the safety officer may request access to unblinded data if needed. Additional ad hoc meetings can be requested at any time by the safety officer or sponsor if necessary.
This trial is being conducted following the International Conference on Harmonisation guideline for Good Clinical Practice and in compliance with the ethical principles of the Declaration of Helsinki and the sponsor standard operating procedures. All documents are initially approved by the NJH institutional review board (HS-3034). Written informed consent will be obtained from all participants before trial participation.
Study end-points
The primary end-point of the study is the mean change from baseline to week 52 in FVC % predicted.
There are a number of secondary end-points listed in table 2. Exploratory outcomes include quantitative HRCT scores, biomarker expression and patient-reported outcomes including the University of California at San Diego Shortness-of-Breath questionnaire, St. George's Respiratory questionnaire, and the Living with IPF questionnaire.
TABLE 2.
End-points
| Primary end-point |
| Mean change from baseline to week 52 in FVC % predicted |
| Secondary end-points |
| Progression-free survival defined as the time from study treatment randomisation to the first occurrence of any of the following events: Relative decline from baseline of ≥10% in FVC and/or DLCO Acute exacerbation of FHP defined as acute respiratory declined leading to hospitalisation or ER or urgent care evaluation; evidence of all of the following criteria within a 4-week period in the outpatient setting: |
| • Increase from baseline FIO2 ≥1 L oxygen |
| • Clinically significant worsening of dyspnoea and/or cough |
| • New, superimposed ground-glass opacities or consolidation or new alveolar opacities on chest radiography or CT |
| • Primary: if all other causes excluded (e.g. acute gastro-oesophageal aspiration, pneumothorax, infection, left heart failure, pulmonary embolism, or identifiable cause of acute lung injury) |
| A decrease from baseline of at least 50 m in 6-min walk distance |
| Change in background therapy (need for a new course of p.o. or i.v. steroids or for the patient receiving maintenance prednisone, as a need to increase the dose by 10 mg or more; and/or addition of cyclophosphamide, azathioprine, mycophenolate mofetil or mycophenolic acid) |
| Death |
| Slope of FVC over 52-week treatment period |
| Mean change in DLCO % predicted at week 52 |
| Proportion of patients with all-cause mortality |
| Proportion of patients with all-cause hospitalisation |
| Proportion of patients with hospitalisation for respiratory cause |
| Proportion of patients with respiratory exacerbations requiring hospitalisations |
| Proportion of patients with evidence of progression of fibrosis on visual comparison of baseline and week 52 HRCT scans |
| Exploratory end-points |
| Mean change from baseline in health-related quality of life, measured by St. George's Respiratory Questionnaire (3 domain scores and total score), at Week 52 |
| Mean change from baseline in health-related quality of life, measured by A Tool to Assess Quality of Life Questionnaire at Week 52 |
| Mean change from Baseline to Week 52 in dyspnoea as measured by the University of California at San Diego Shortness-of-Breath Questionnaire score |
| Proportion of patients with evidence of progression, stability or improvement in fibrosis on texture-based quantitative analysis of CT |
| Candidate biomarker expression in the peripheral blood of patients with HP over the 52-week study follow-up period |
| Safety end-points |
| Proportion of patients with treatment-emergent adverse events |
| Proportion of patients with treatment-emergent serious adverse events |
| Proportion of patients with treatment-emergent adverse drug reaction |
| Proportion of patients with treatment-emergent serious drug reaction |
| Proportion of patients with adverse events leading to early discontinuation of study treatment |
| Proportion of patients with treatment-emergent deaths |
| Proportion of patients with treatment-emergent changes in clinical laboratory findings and ECGs |
FVC: forced vital capacity; DLCO: diffusing capacity of the lung for carbon monoxide; FHP: fibrotic hypersensitivity pneumonitis; ER: emergency room; FIO2: inspiratory oxygen fraction; HRCT: high-resolution computed tomography; HP: hypersensitivity pneumonitis.
Sample size and statistical analysis
This study's sample size and power calculations are based on the primary end-point, mean change in FVC % predicted from baseline to week 52. Estimates were derived from the results of a 52-week NJH pilot study of 30 FHP patients with disease progression defined by lung function decline, independent of prior treatment received. Based on the results of that study, calculations assumed a standard deviation in the FVC % predicted change from baseline to 52 weeks of 2% and are based on a 2-sample means t-test with a 2-sided Type I error probability of 0.05. For the primary efficacy comparison of the change in mean FVC % predicted between the treatment and placebo groups, 13 patients in the placebo and 25 (N=38) in the treatment group will provide at least 85% power to detect a mean difference between groups of at least 2%. Assuming a 10% attrition rate, approximately 42 subjects will be enrolled and randomised in a 2:1 ratio to pirfenidone or placebo.
Primary analyses
The primary efficacy analysis will estimate the mean rank change in FVC % predicted from baseline to week 52. Data will be analysed using a rank linear regression model with the rank change in FVC % predicted from baseline to week 52 as the outcome variable and rank baseline FVC % predicted and HP therapy (placebo or pirfenidone) and concomitant immunosuppressive therapy as covariates. The treatment effect will be tested using the Wald test.
The primary efficacy analysis will be tested at an alpha level of 0.05. Missing data due to reasons other than death will be replaced with imputed values using the multiple imputation via chained equations method [16].
Secondary end-point analyses
Multiplicity adjustment will be performed across all secondary end-points. Progression-free survival will be compared between the pirfenidone and placebo groups using the log-rank test. A proportional hazards model will be used to estimate the hazard ratio. Descriptive analysis of progression-free survival will be presented using Kaplan–Meier curves. Mortality will be analysed using logistic regression with a treatment effect.
The slope for annual rate of FVC decline will be analysed using a random coefficient regression model with treatment and baseline FVC as covariates. DLCO will be analysed using a rank linear regression model with the rank change in DLCO % predicted from baseline to week 52 as the outcome variable and rank baseline DLCO % predicted and HP therapy as covariates. Non-elective hospitalisation from any cause and computed tomography (CT) progression of fibrosis will be compared between the pirfenidone and placebo groups using logistic regression. Adverse event and drug reaction outcomes will be presented using standard summary statistics.
Analysis of the study population
The intent-to-treat population, consisting of all subjects who signed the informed consent form and are randomised, will be used as the primary population for efficacy analyses. The safety analysis population will include all subjects who signed informed consent and received any amount of study drug. The trial results will be reported according to guidelines specified in the Revised Consolidated Standards of Reporting Trials (CONSORT) statement. A flow diagram describing screening, recruitment, randomisation, dropout, and vital status will be included in the primary study report.
Discussion
This study will be the first double-blind, randomised, placebo-controlled trial of pirfenidone conducted exclusively in patients with FHP. The results will provide preliminary information on the safety and dose-exposure of pirfenidone and its effect on several primary, secondary and exploratory end-points in FHP subjects.
A phase III clinical trial has recently investigated the efficacy and safety of the anti-fibrotic nintedanib in patients with different forms of progressive fibrosing ILD, including FHP [17]. Compared with placebo, nintedanib significantly reduced the annual rate of decline in FVC (mL per year) in these patients. However, post hoc analyses of the effect of nintedanib versus placebo on reducing the annual rate of FVC decline among subjects with FHP was not substantially different (difference 73.1, 95% CI –8.6 to 154.8). As of now, there are no randomised controlled trials of pirfenidone in FHP.
The trial design considered several important methodological factors. First, we aimed to ensure the inclusion of a well-phenotyped patient population in whom there was high confidence in the diagnosis of FHP [18]. Because no single test can confidently establish or exclude a diagnosis of HP, and diagnostic variability is common, we elected to enrol only subjects whose diagnosis of FHP came via consensus of a multidisciplinary team discussion. Doing so decreases the likelihood of misclassification. Second, the inclusion criteria enrich for patients who are most likely to progress over a 52-week trial. Third, at the start of the trial in 2017, a 2:1 randomisation was chosen to hasten recruitment, increase the odds of patients receiving active treatment (given the high unmet medical need), and increase the power to gather safety data, particularly on subjects on background immunosuppressive therapy. Finally, limited data are available in the medical literature about concomitant medication use among FHP patients. Concomitant FHP treatment with oral AZA (≤2 mg·kg−1 daily) or MMF (≤1.5 g twice daily) is permitted if patients had received a stable dose for ≥1 month prior to enrolment. Such immunomodulatory therapies are commonly used to treat FHP, so excluding patients on background therapy would limit enrolment and our ability to generalise results to the real-world. The primary end-point will be analysed using a linear regression model with concomitant immunosuppressive therapy as a covariate to minimise the risk of bias. Since MMF and AZA are commonly used in FHP, and it is important that any new FHP therapy can be tolerated in conjunction with these medications, we elected to use a longer pirfenidone titration (over 4 weeks, instead of 2 weeks) to optimise tolerability [19].
The rationale for the chosen primary end-point is that the change in FVC is a universally accepted measure of disease severity, a predictor of progression and associated with mortality risk in patients with pulmonary fibrosis [8, 17, 20]. Secondary end-points were included to assess clinically relevant measures of FHP severity, progression and mortality.
In summary, the high morbidity and mortality risk of FHP underscores the need to explore new pharmacological approaches to managing this devastating disease. Outcomes from this trial will provide valuable data on this antifibrotic agent's role as an add-on treatment in FHP and yield information that will inform future trials. Additionally, the design will allow the collection of biospecimens and collaborative mechanistic studies that will hopefully advance our understanding of the pathobiology of FHP progression.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00054-2021.supplement (593.7KB, pdf)
Footnotes
This article has supplementary material available from openres.ersjournals.com
This study is registered at www.clinicaltrials.gov with identifier number NCT02958917. Clinical study documents (e.g. text, table and figures) and participant clinical study data are available to be shared after the publication of the primary manuscript in a peer-reviewed journal. Before providing access, documents will be examined and, if necessary, redacted. The data will be de-identified to protect the personal data of study participants and personnel and to respect the boundaries of the study participants’ informed consent. Qualified scientific and medical researchers may request access to de-identified, analysable participant clinical study data with corresponding documentation describing the datasets’ structure and content. Upon approval and governed by a Data Sharing Agreement, data are shared in a secured data-access system for a limited period of 1 year, which may be extended upon request.
Conflict of interest: E.R. Fernández Pérez reports grants from the NHLBI and Boehringer Ingelheim outside the submitted work.
Conflict of interest: J.L. Crooks has nothing to disclose.
Conflict of interest: J.J. Swigris has nothing to disclose.
Conflict of interest: J.J. Solomon has nothing to disclose.
Conflict of interest: M.P. Mohning has nothing to disclose.
Conflict of interest: T.J. Huie has nothing to disclose.
Conflict of interest: M. Koslow has nothing to disclose.
Conflict of interest: D.A. Lynch has nothing to disclose.
Conflict of interest: S.D. Groshong has nothing to disclose.
Conflict of interest: K.F. Fier has nothing to disclose.
Support statement: This study is supported by Genentech grant ML29875. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Fernandez Perez ER, Kong AM, Raimundo K, et al. Epidemiology of hypersensitivity pneumonitis among an insured population in the United States: a claims-based cohort analysis. Ann Am Thorac Soc 2018; 15: 460–469. doi: 10.1513/AnnalsATS.201704-288OC [DOI] [PubMed] [Google Scholar]
- 2.Mooney JJ, Elicker BM, Urbania TH, et al. Radiographic fibrosis score predicts survival in hypersensitivity pneumonitis. Chest 2013; 144: 586–592. doi: 10.1378/chest.12-2623 [DOI] [PubMed] [Google Scholar]
- 3.Fernandez Perez ER, Brown KK. Fibrotic hypersensitivity pneumonitis. Curr Respir Care Rep 2014; 3: 170–178. doi: 10.1007/s13665-014-0094-0 [DOI] [Google Scholar]
- 4.Fernandez Perez ER, Sprunger DB, Ratanawatkul P, et al. Increasing hypersensitivity pneumonitis-related mortality in the United States from 1988 to 2016. Am J Respir Crit Care Med 2019; 199: 1284–1287. doi: 10.1164/rccm.201807-1258LE [DOI] [PubMed] [Google Scholar]
- 5.Fernandez Perez ER, Swigris JJ, Forssen AV, et al. Identifying an inciting antigen is associated with improved survival in patients with chronic hypersensitivity pneumonitis. Chest 2013; 144: 1644–1651. doi: 10.1378/chest.12-2685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nathan SD, Shlobin OA, Weir N, et al. Long-term course and prognosis of idiopathic pulmonary fibrosis in the new millennium. Chest 2011; 140: 221–229. doi: 10.1378/chest.10-2572 [DOI] [PubMed] [Google Scholar]
- 7.Schaefer CJ, Ruhrmund DW, Pan L, et al. Antifibrotic activities of pirfenidone in animal models. Eur Respir Rev 2011; 20: 85–97. doi: 10.1183/09059180.00001111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.King TE, Jr., Bradford WZ, Castro-Bernardini S, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med 2014; 370: 2083–2092. doi: 10.1056/NEJMoa1402582 [DOI] [PubMed] [Google Scholar]
- 9.Noble PW, Albera C, Bradford WZ, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet 2011; 377: 1760–1769. doi: 10.1016/S0140-6736(11)60405-4 [DOI] [PubMed] [Google Scholar]
- 10.Taniguchi H, Ebina M, Kondoh Y, et al. Pirfenidone in idiopathic pulmonary fibrosis. Eur Respir J 2010; 35: 821–829. doi: 10.1183/09031936.00005209 [DOI] [PubMed] [Google Scholar]
- 11.Azuma A, Nukiwa T, Tsuboi E, et al. Double-blind, placebo-controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2005; 171: 1040–1047. doi: 10.1164/rccm.200404-571OC [DOI] [PubMed] [Google Scholar]
- 12.Furusawa H, Cardwell JH, Okamoto T, et al. Chronic hypersensitivity pneumonitis, an interstitial lung disease with distinct molecular signatures. Am J Respir Crit Care Med 2020; 202: 1430–1444. doi: 10.1164/rccm.202001-0134OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chung JH, Zhan X, Cao M, et al. Presence of air-trapping and mosaic attenuation on chest CT predicts survival in chronic hypersensitivity pneumonitis. Ann Am Thorac Soc 2017; 14: 1533–1538. doi: 10.1513/AnnalsATS.201701-035OC [DOI] [PubMed] [Google Scholar]
- 14.Ley B, Torgerson DG, Oldham JM, et al. Rare protein-altering telomere-related gene variants in patients with chronic hypersensitivity pneumonitis. Am J Respir Crit Care Med 2019; 200: 1154–1163. doi: 10.1164/rccm.201902-0360OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Willems S, Verleden SE, Vanaudenaerde BM, et al. Multiplex protein profiling of bronchoalveolar lavage in idiopathic pulmonary fibrosis and hypersensitivity pneumonitis. Ann Thorac Med 2013; 8: 38–45. doi: 10.4103/1817-1737.105718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Azur MJ, Stuart EA, Frangakis C, et al. Multiple imputation by chained equations: what is it and how does it work? Int J Methods Psychiatr Res 2011; 20: 40–49. doi: 10.1002/mpr.329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flaherty KR, Wells AU, Cottin V, et al. Nintedanib in progressive fibrosing interstitial lung diseases. N Engl J Med 2019; 381: 1718–1727. doi: 10.1056/NEJMoa1908681 [DOI] [PubMed] [Google Scholar]
- 18.Fernández Pérez Evans R, Travis William D., Lynch David A, et al. Diagnosis and evaluation of hypersensitivity pneumonitis. Chest 2021; in press [ 10.1016/j.chest.2021.03.066]. [DOI] [PubMed] [Google Scholar]
- 19.Khanna D, Albera C, Fischer A, et al. An open-label, phase II study of the safety and tolerability of pirfenidone in patients with scleroderma-associated interstitial lung disease: the LOTUSS trial. J Rheumatol 2016; 43: 1672–1679. doi: 10.3899/jrheum.151322 [DOI] [PubMed] [Google Scholar]
- 20.Collard HR, King TE, Jr., Bartelson BB, et al. Changes in clinical and physiologic variables predict survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2003; 168: 538–542. doi: 10.1164/rccm.200211-1311OC [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00054-2021.supplement (593.7KB, pdf)