Abstract
Intraperitoneal injection of kisspeptin-54 induces a surge-like release of luteinizing hormone that stimulates ovulation in mice.
Keywords: kisspeptin, mouse preovulatory follicle, luteinizing hormone, ovulation
Dear Editor,
The mid-cycle surge of luteinizing hormone (LH) activates the LH/chorionic gonadotropin receptor (LHCGR) in mural granulosa cells of mammalian preovulatory follicles. Luteinizing hormone CGR activation initiates a signaling cascade resulting in meiotic resumption in the oocyte, cumulus cell expansion, ovulation, and differentiation of granulosa and theca cells to form the corpus luteum [1]. This process is tightly regulated to ensure that the ovulated oocyte is capable of being fertilized.
Studies of ovulation in rodents often rely on intraperitoneal injection of human chorionic gonadotropin (hCG) or human or ovine LH [2]. Both hCG and LH activate the rodent LHCGR and induce ovulation; however, there are many questions about their physiological relevance. The kinetics of LHCGR activation after hormone injection is unknown, which is a limitation for studies of LHCGR signaling pathways. Additionally, how the hormone concentration or the biological differences between different sources of LHCGR agonists compare to the endogenous LH concentration and activity in mice is unknown. The extra glycosylation sites on hCG lead to a longer half-life than LH [3], which could impact activity as well. Perhaps most concerning, hCG and LH can activate different signaling pathways via the LHCGR, which suggests that the current standard of using hCG to study LHCGR signaling in rodents may not be optimal [4]. Thus, there is a need for improved methods to induce ovulation for studies of LHCGR signaling in preovulatory follicles in rodents.
Kisspeptins are neuropeptides and potent GnRH secretagogues. Kisspeptin released by neurons in the arcuate nucleus drives pulsatile GnRH and LH secretion. The high levels of estradiol on the afternoon of proestrus promote kisspeptin expression and release by a second group of neurons in the anteroventral periventricular nucleus, which drives the preovulatory LH surge (Figure 1A; [5]). The 145 amino acid kisspeptin precursor is cleaved in vivo into smaller peptides of 54, 14, 13, or 10 amino acids, which can induce ovulation when injected in women and other large mammals by generating surge-like LH release [6]. However, the ability of kisspeptin to induce ovulation in rodents has yet to be investigated. Here, we report that kisspeptin-54, the most potent form [7], induces ovulation in mice by initiating endogenous LH release that is surge-like.
Figure 1.
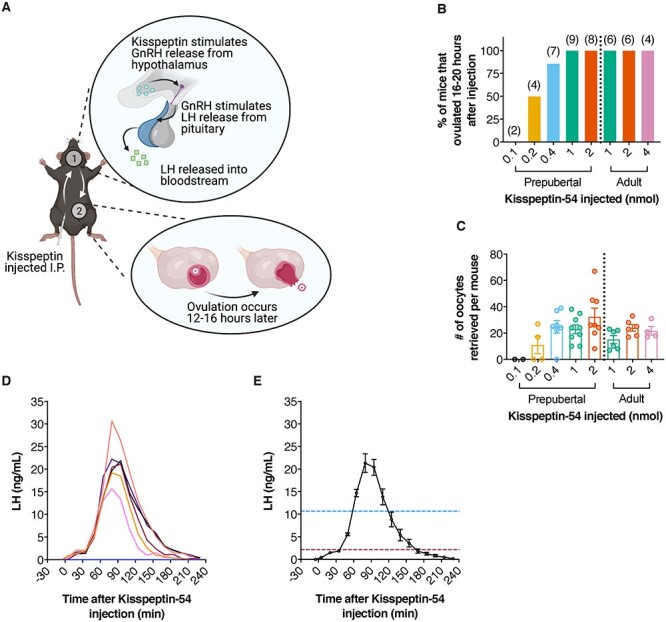
Kisspeptin-54 injected intraperitoneally induces ovulation via stimulation of endogenous surge-like LH release. (A) Schematic of kisspeptin-54 injection to induce ovulation in mice. Created with BioRender.com. (B, C) Ovulation in mice injected with the indicated amounts of kisspeptin-54. Prepubertal (23–25 days old) and adult (8–14 weeks old) mice were injected with eCG to stimulate follicle growth, then injected with kisspeptin-54 44-h later. Oviducts were dissected 16–20-h post-kisspeptin and cumulus-oocyte complexes were collected and counted. (D) Whole blood LH levels in 7 prepubertal mice injected with kisspeptin-54. Blood was collected from the tail vein immediately before injection, 5 min after injection, then every 15 min until 230 min. LH was measured by ELISA [8]. Each trace represents measurements from one mouse. (E) Mean whole blood LH levels in the six responding mice in panel D. The one mouse that did not respond to kisspeptin-54, likely because of a misplaced injection, was excluded from the analysis. Values represent mean whole blood LH ± SEM. The blue and red lines represent 50 and 10% of maximum LH, respectively. All animal experiments were conducted as approved by the University of Connecticut Health Center and McGill University animal care committees.
We first determined the minimal amount of kisspeptin-54 required to induce ovulation. Prepubertal and adult mice (C57BL6/J, 23–25 days old, and 8–14 weeks old, respectively) were injected with 5 I.U, equine chorionic gonadotropin (eCG) to stimulate follicular growth by activating the follicle-stimulating hormone receptor. Equine CG is commonly used for stimulating follicular growth in rodents [2]. Forty-four hours later, we injected kisspeptin-54 (Cayman Chemicals, diluted in PBS to 0.1, 0.2, 0.4, 1, or 2 nmol for prepubertal and 1, 2, or 4 nmol for adults) into the peritoneum. Oviducts were dissected 16–20 h after kisspeptin-54 injection, and ovulation was determined by the presence of cumulus-oocyte complexes in the oviduct. Injection of 1 or 2-nmol kisspeptin-54 induced ovulation in 100% of prepubertal mice, but 0.2 and 0.4 nmol were only partially effective and 0.1 nmol did not induce ovulation (Figure 1B). A similar number of oocytes were ovulated when comparing injections of 0.4, 1, and 2 nmol kisspeptin-54 (Figure 1C). We also examined the response in adult mice and obtained similar results (Figure 1B and C). Since prepubertal mice are most commonly used for studies of LHCGR signaling in the ovary, we focused on the effects of 1-nmol kisspeptin on prepubertal mice for subsequent analyses.
To evaluate the effect of 1-nmol kisspeptin-54 on the timing and amplitude of LH release, we performed a tail tip bleed ELISA as previously described [8]. Briefly, mice were injected with 1-nmol kisspeptin-54 as described previously. Blood was collected from mice immediately before injection, 5 min after injection, and every 15 min after until 230-min postinjection. Kisspeptin-54 induced surge-like LH release in 6/7 mice tested (Figure 1D). Whole blood LH began to increase as soon as 20 min after injection and reached a peak concentration (21.3 ± 2.1 ng/mL) 80–95-min postinjection (Figure 1D and E). The timing of the peak was consistent for the six mice that responded to the hormone. Whole blood LH remained above 10% of the maximum for ~2.1 h and above 50% of the maximum for ~1.0 h (Figure 1E).
The endogenous LH surge has been investigated in adult proestrous mice using the tail tip method used here [8, 9, our unpublished results]. For these adult mice, the peak of the LH surge has a mean amplitude of 12–28 ng/mL, similar to the peak LH of 21 ng/mL induced by kisspeptin-54 injection in prepubertal mice. There is limited information about the duration of the surge in adult proestrous mice, but based on available information, LH levels remain above 50% of the maximum for ~2.3 h. In our study, we find that the surge-like LH release induced by kisspeptin-54 injection in prepubertal mice remains above 50% of the maximum for only ~1 h. Regardless of the cause of the difference in the duration of the natural and kisspeptin-54 driven surges, both are clearly sufficient to drive ovulation. Notably, the amplitude of a typical endogenous LH surge is considerably larger than the minimum needed to cause ovulation in mice [10]. In summary, kisspeptin-54 can be used to induce ovulation in mice by stimulating precisely timed endogenous LH release of consistent amplitude and duration.
Acknowledgments
We thank Aylin Hanyaloglu and Jeremy Egbert for helpful discussions.
Grant support : The Eunice Kennedy Shriver National Institute of Child Health and Human Development (grant no. R37 HD014939 to LAJ) and Canadian Institutes of Health Research Project (grant no. PJT-169184 to DJB).
Contributor Information
Corie M Owen, Department of Cell Biology, University of Connecticut Health Center, Farmington CT, USA.
Xiang Zhou, Department of Pharmacology and Therapeutics, McGill University, Montréal, Canada; Department of Anatomy and Cell Biology, McGill University, Montréal, Canada.
Daniel J Bernard, Department of Pharmacology and Therapeutics, McGill University, Montréal, Canada; Department of Anatomy and Cell Biology, McGill University, Montréal, Canada.
Laurinda A Jaffe, Department of Cell Biology, University of Connecticut Health Center, Farmington CT, USA.
Conflict of interest
The authors have declared no conflict of interest exists.
Authors contributions
All authors contributed to project design, experimental procedures, and manuscript preparation.
Data availability
Data will be made available on request to the corresponding author.
References
- 1. Richards JS, Ascoli M. Endocrine, paracrine, and autocrine signaling pathways that regulate ovulation. Trends Endocrinol Metab 2018; 29:313–325. [DOI] [PubMed] [Google Scholar]
- 2. Martín-Coello J, González R, Crespo C, Gomendio M, Roldan ERS. Superovulation and in vitro oocyte maturation in three species of mice (Mus musculus, Mus spretus and Mus spicilegus). Theriogenology 2008; 70:1004–1013. [DOI] [PubMed] [Google Scholar]
- 3. Ezcurra D, Humaidan P. A review of luteinising hormone and human chorionic gonadotropin when used in assisted reproductive technology. Reprod Biol Endocrinol 2014; 12:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Casarini L, Lispi M, Longobardi S, Milosa F, La Marca A, Tagliasacchi D, Pignatti E, Simoni M. LH and hCG action on the same receptor results in quantitatively and qualitatively different intracellular signalling. PLoS One 2012; 7:e46682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang L, Vanacker C, Burger LL, Barnes T, Shah YM, Myers MG, Moenter SM. Genetic dissection of the different roles of hypothalamic kisspeptin neurons in regulating female reproduction. Elife 2019; 8:e43999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jayasena CN, Nijher GMK, Abbara A, Murphy KG, Lim A, Patel D, Mehta A, Todd C, Donaldson M, Trew GH, Ghatei MA, Bloom SRet al. Twice-weekly administration of kisspeptin-54 for 8 weeks stimulates release of reproductive hormones in women with hypothalamic amenorrhea. Clin Pharmacol Ther 2010; 88:840–847. [DOI] [PubMed] [Google Scholar]
- 7. Tassigny X d’A, Jayasena C, Murphy KG, Dhillo WS, Colledge WH. Mechanistic insights into the more potent effect of KP-54 compared to KP-10 in vivo. PLoS One 2017; 12:e0176821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Toufaily C, Schang G, Zhou X, Wartenberg P, Boehm U, Lydon JP, Roelfsema F, Bernard DJ. Impaired LH surge amplitude in gonadotrope-specific progesterone receptor knockout mice. J Endocrinol 2020; 244:111–122. [DOI] [PubMed] [Google Scholar]
- 9. Czieselsky K, Prescott M, Porteous R, Campos P, Clarkson J, Steyn FJ, Campbell RE, Herbison AE. Pulse and surge profiles of luteinizing hormone secretion in the mouse. Endocrinology 2016; 157:4794–4802. [DOI] [PubMed] [Google Scholar]
- 10. Herbison AE, Porteous R, Pape J-R, Mora JM, Hurst PR. Gonadotropin-releasing hormone neuron requirements for puberty, ovulation, and fertility. Endocrinology 2008; 149:597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request to the corresponding author.


