Highlights
-
•
Fundamental mechanisms of SACT have yet to be well clarified.
-
•
SACT is effective on bacteria and biofilms.
-
•
SACT has foreseeable medical and environmental applications.
-
•
Further understanding of SACT is needed to progress the field.
Keywords: Sonodynamic therapy, Photodynamic therapy, Bacteria, Sonosensitizer, Ultrasound
Abstract
Sonodynamic antimicrobial chemotherapy (SACT), which relies on a combination of low-intensity ultrasound and chemotherapeutic agents termed sonosensitizers, has been explored as a promising alternative for microbial inactivation. Such treatment has superior penetration ability, high target specificity, and can overcome resistance conferred by the local microenvironment. Taken of these advantages, SACT has been endowed with an extensive application prospect in the past decade and attracted more and more attention. This review focusses on the current understanding of the mechanism of SACT, the interaction of sonodynamic action on different microbes, the factors affecting the efficacy of SACT, discusses the findings of recent works on SACT, and explores further prospects for SACT. Thus, a better understanding of sonodynamic killing facilitates the scientific community and industry personnel to establish a novel strategy to combat microbial burden.
1. Introduction
Over the past decade, the rapid evolution and spread of multidrug-resistant bacteria (MDR) have become a formidable public health concern [1]. These so-called “ESKAPE”-pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter spp.) can provoke antibiotics ineffective within a short time [2]. Researchers strived to provide alternative solutions not only to eliminate infections in medical and veterinary settings, but also to reduce the microbial loads in industrial and environmental applications, since the use of antibiotics in agriculture/environment plays a pivotal role in the spread of resistance, through foodborne microorganisms [3], [4] and sewage [5]. Physical treatments such as irradiation, high pressure, and heat gained most popularity due to their less possibility to induce resistance and the greater potential for application on large scale [6], [7]. Unfortunately, limitations, including less penetration, high-cost investment, and high equipment requirements hamper the industrial applications and commercialization of these treatments.
Ultrasound has been explored as an antimicrobial tool since 1927, when Wood et al. [8] provided the first evidence that ultrasound could induce a lethal effect on microorganisms. Since then, ultrasound, both alone and in combination with other strategies, was found to have the ability to destroy various microorganisms, ranging from bacteria to virus [9], [10], [11], [12], [13]. Because of these characteristics, ultrasound has been applied for diverse antimicrobial strategies, ranging from sterilization in the food industry to the treatment of microbial infections [14]. Sonoantimicrobial chemotherapy (SACT), on the basis of sonodynamic therapy (SDT), has been established and developed as a novel promising antimicrobial approach. Similar to SDT, SACT ultilizes low intensity focused ultrasound (LIFU) to excite sonosensitizers to generate cytotoxic reactive species that are toxic to microbes [15]. Moreover, LIFU with less price can act on deep-seated microbes within the tissue, and can be focused into a small region to active sensitizers [16]. Taken of these advantages, SACT has also exhibited synergistic effects against diversified microorganisms including bacteria, sessile biofilm, and yeasts [9], [17], [18]. Although these synergistic actions following SACT have been investigated in numerous biological models in vitro and in vivo, the precise mechanisms are still unclear and vary. Chen et al. [16] suggested that SACT interferes with the life of these microbes mainly depending on biological models and experimental systems, the type of combined sonosensitizers and the ultrasound parameters such as frequency and intensity. While it is difficult to form a universal mechanism of SACT action, some general traits emerged, such as ROS production by sonoluminescence and pyrolysis, and ROS-independent cytotoxicity [19]. These sonodynamic events result in damage to microbial membranes, proteins, and DNA/RNA, ultimately causing cell death [20]. With the expanding knowledge on the fundamental mechanism of SACT, more and more investigators have realized that sonosensitizer is one of the crucial factors. According to the previous reports, a variety of the sonosensitizers have been employed in SACT, including antibiotics [2], [17], natural product extracts [10], [21], nanoparticles/nanocomposites [22], [23], [24], and xanthene dye [25], [26]. Since Liu et al. [27] first pointed out the antimicrobial potential of SACT, such prediction was continuously confirmed by experimental studies, eventually made its way into medical applications and industrial process sanitizations. To date, the majority of the studies about SACT have focused on its use in medicine through the killing of either pathogens or tumor cells. Only fewer reports have briefly touched upon the potential of SACT for guaranteeing food safety [10], [11] and sewage decontamination [28], [29].
SACT is gaining increasing popularity as a therapeutically useful modality, however, its applications for decontamination and disinfection are still in their infancy. The review aims to give an overview of the available information on the scientific principles of SACT, along with various sonosensitizer-based SACT strategies. Also, the review presents the current state of research and the potential for application of this promising novel treatment to the environment and food industry.
2. Mechanisms of sonodynamic production of cytotoxic species
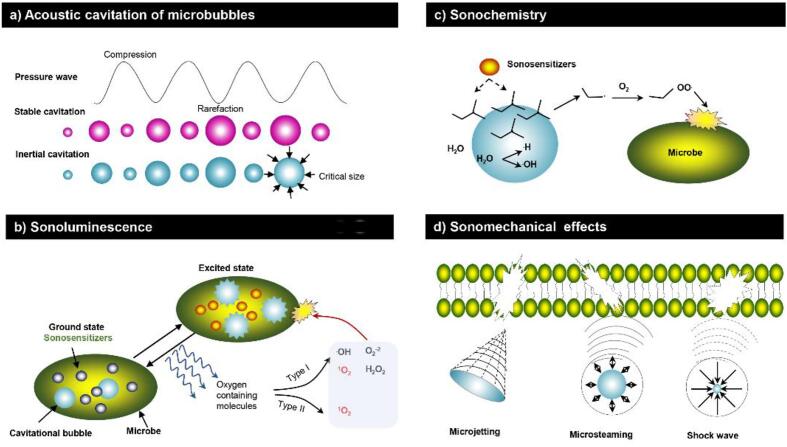
Most of information about the mechanisms of SACT is gained from SDT studies. SDT studies have been extensively investigated in medicine, yet their mechanism is still not comprehensively elucidated. As for SDT, the ultrasound in SACT is thermal-independent. The activation of sensitizer in this treatment involves cavitation [25], [27], [30]. Cavitation produces microbubbles that oscillate and eventually collapse. This dynamic process is usually divided into two types, namely, stable cavitation and inertial cavitation (Fig. 1a) [15]. Stable cavitation occurs in an aqueous medium subjected to low-intensity ultrasound, characterizing by expansion and contraction of the produced bubbles around the same resting radius in the acoustic field [31] (Fig. 1a). These stable cavitation bubbles are forced to oscillate, thus creating mechanical shearing and microsteaming to interfere with the adjacent particles (cells or sensitizers). In contrast, inertial cavitation is a relatively strong bubble dynamic process that occurs in a liquid media exposed to a higher-intensity ultrasound. The inertial cavitation bubbles experience from sharp contraction to collapse, thereby concentrating enormous energy, and then release the energy in the focal area [32]. The energy generated by this phenomenon results in the production of heat (approximately 5000 K), pressures (250 MPa), and extreme conditions that generate mechanical (i.e., liquid jets, shear force, shock wave), thermal (i.e., localized heat generation), and chemical (i.e., free radicals) effects [15]. Both stable cavitation and inertial cavitation can produce mechanical forces, in addition to the chemical effects generated by inertial cavitation [15]. Regardless of the cavitation type, either sonochemical (sonoluminescence, sonochemistry) or sonomechanical (ROS-independent) effects can contribute to the cytotoxicity of sensitizer in SDT.
Fig. 1.
(a) Acoustic cavitation of microbubbles; The activation of sonosensitizers by sonoluminescence (b) or sonochemistry (c); ROS-independent mechanisms of SACT action.
2.1. ROS-dependent cytotoxicity
2.1.1. Sonoluminescence
The most commonly proposed mechanism for the SDT cytotoxic effects is the formation of ROS via the activation of sensitizer by sonoluminescence [30], [33], which are flashes of light that results from the rapid collapse of bubbles during cavitation [34], [35]. Sonoluminescence is generally considered to be caused by inertial cavitation bubbles implosion, whereas Costley et al. [36] attributed this phenomenon to stable cavitation. Sonoluminescence might activate sonosensitizers in SDT through a photochemical pathway similar to that involved in photoantibacterial chemotherapy (PACT) [37], [38]. Upon absorption of sonoluminescent light, the sonosensitizers get excited to a higher energy state (excited state, Fig. 1b). On their way back to the ground state, sonosensitizers could interact with biological substrates to create higher energy, high reactivity molecules known as ROS. The ROS generation can proceed either in a Type I process, leading to the yield of radicals such as hydrogen peroxide, superoxide anion, hydroxyl radical and singlet oxygen (1O2), or in a Type II process, with 1O2. Singlet oxygen, as the main effector, induces the photooxidation of cellular components thereby causing cell death (Fig. 1b) [18]. Evidence supporting the use of this mechanism in SACT is provided by Rahman et al., who suggested that ROS-mediated death of Escherichia coli was attributed to the sonoluminescent activation of TiO2 [39]. Indirect support for the use of sonoluminescence in SACT was revealed from the early studies on ciprofloxacin and levofloxacin [27], [40]. The study proposed that the antimicrobial process was synergized by these compounds and ultrasound involved in the production of ROS. In these studies, the peak absorbance of both ciprofloxacin (λ max at 276, 316, and 328 nm) and levofloxacin (λmax at 288 and 331 nm) [41], [42] correlated well with the maximum emission of sonoluminescence in water (250–600 nm) [36]. Similarly, Nakonechny et al. [25] also attributed the potent bactericidal efficacy of Rose Bengal (RB)-mediated sonodynamic treatment to a good overlap between the peak absorbance of RB and the emission range of sonoluminescence. However, Methylene Blue (MB), an established PACT photosensitizer, exhibited no ultrasound mediated-antimicrobial activity in corresponding SACT experiments. The authors explained the broad emission of sonoluminescence has a minimal overlap with the absorption spectra of MB (500–700, λmax > 650 nm) [5], [25]. Consistent with these observations, some reports also suggested that the limited emission range of sonoluminescence partly elucidate why MB and other molecules can function primarily as either photosensitizer or sonosensitizer [43], while other molecules can serve with both capacities [33].
2.1.2. Sonochemistry
A second mechanism for ROS-mediated SDT has been postulated and known as “pyrolysis”. Under inertial cavitation, the elevated local temperature and pressure promote the pyrolysis of the sensitizer molecules and/or of water molecules occurring in the heated gas–liquid interface or inside collapsing bubbles [44] (Fig. 1c). The radicals formed in this sonodynamic process then react with oxygen to produce peroxyl and alkoxyl radicals, which can act indiscriminately against macromolecules to induce cytotoxic damage [36].
Many studies have demonstrated that the pyrolysis in SDT induced cytotoxicity independent of a sonosensitizer, however, there remains doubt regarding the contribution of hydroxyl radicals (·OH) generated by pyrolytic water to cell destruction [45]. Based on the studies [44], [46], it was suggested that the free radicals formed during this event have extremely high reactivity, short half-life (~1 μs for 1O2, ~1 ns for ·OH), and limited diffusion distances (~20 nm for 1O2, ~5 nm for ·OH), which might limit their involvement in causing cytotoxicity. In contrast, radicals created in the pyrolysis of sonosensitizer, such as peroxyl radicals (H2O2), seem to be more aggressive towards microbial cells. These radicals, which are less reactive, longer-living and hence significant diffusion distances, are capable of attacking critical sites in microbial cells [43]. Thus, the selection of a suitable sonosensitizer is essential to maximize the pyrolytic effect of SDT on microbial cells. In this case, thermolabile molecules (e.g., azo-compounds) have been explored as sonosensitizers since they are capable of decomposing to carbon-centered alkoxyl radicals and ultimately forming H2O2 in the presence of oxygen [47]. Both radical species were successfully identified after ultrasound irradiation through the experimental methods, including spin trapping and electron paramagnetic resonance spectroscopy, suggesting the decomposition of sonosensitizer. Meanwhile, the concentration of radicals formed in this process did not decrease after quenching with sodium azide, potassium iodide and sodium formate. This indicated that a pyrolysis-mediated effect of sonosensitizer was incorporated in SDT instead of an 1O2 or ·OH-mediated response. Besides, some classes of compounds, such as surfactants (e.g., porphyrins) and small molecules with significant vapor pressure (e.g., DMF, DMSO), are also found to be suitable [20].
2.2. ROS-independent cytotoxicity
Many researchers agreed that the dominant mechanism of SDT action is cytotoxic effects through the formation of ROS, however, it has been proposed that SDT also depends on sonomechanical mechanisms, as showcased (Fig. 1d). Ultrasound alone is considered to produce inherent mechanical effects, including microstreaming, microjetting and high shear force, all of which can ultimately lead to cell membrane disruption [5]. An early study conducted by Worthington et al. [48] supported that SDT cytotoxicity resulted from these sonomechanical events as opposed to ROS generation. In their study, the authors assessed ·OH and H· production through Fricke dosimetry after ultrasound irradiation (1.955 MHz, 1.2 W/cm2) with or without hematoporphyrin (Hp). However, the yield of singlet oxygen was too small to explain the notable cell death, and they concluded that the cytotoxicity was closely linked to the sonomechanical forces that disrupt cellular membranes. At all events, these data might loosely provide contradictory evidence toward the ROS-based theory underlying SDT.
Even though comparative studies have demonstrated the mechanical effects of cavitation alone, it still unknown as to how or if ROS-independent cytotoxicity results from the synergy of sonosensitizer and ultrasound. Some studies proposed that shearing stress of cavitation bubbles causes transient alterations in the bacterial cell membrane, by which the sonosensitizer is enhanced to permeate and disrupt microbial cells, causing cell death [49], [50], [51]. Runyan et al. [50] found that ultrasound enhanced the permeability of the cell membrane of Pseudomonas aeruginosa towards macromolecular compounds. They believed that this phenomenon might be caused by the sonoporation created holes in the bacterial membrane. However, some studies thought that the addition of a membrane destabilizing compounds could amplify sonomechanical stresses inducing a synergistic cytolytic effect [36], [52], [53], [54]. Rapoport et al. [53] supported this claim and employed a spin-labeled gentamicin bioreduction kinetics model to explain the synergism against P. aeruginosa and E. coli. They found that the penetration of spin-labeled gentamicin was not affected by ultrasound with an intensity of <2.4 W/cm2. This indicated that the synergism of antibiotics-mediated sonodynamic treatment did not result from the increased penetration of antibiotics through bacterial membrane. The authors speculated that the interaction of the antibiotics with the cell membrane might result in cells being more vulnerable to ultrasound. Similarly, Wang et al. [55] observed that curcumin-sensitized methicillin-resistant Staphylococcus aureus (MRSA) were more susceptible to the following ultrasound compared to the no curcumin-sensitized cells. The potential enhancement of sonomechanical stress might be attributed to the membrane-destabilization of curcumin. Therefore, it is fascinating to characterize the interaction of sonosensitizer with the cell membrane and to elucidate how, or if this reaction augmented sonomechanical effects. Rotenberg et al. [56] adopted a model to reveal the interaction between porphyrins and cell membranes. The hydrophobic porphyrin was able to embed into lipid bilayers of the membrane and destabilize the membrane, which created a potential improvement of mechanical stresses. However, it should be noted that this finding was just obtained from a PDT perspective not from SDT. Overall, different sonosensitizers behave differently in acoustic fields and therefore pass the bacterial membrane through different pathways.
3. Mechanisms of sonodynamic killing of microorganism
Sonosensitizers used in SACT treatment are known as chemicals originally generated for PACT. In SACT treatment, these sonosensitizers are activated by ultrasound irradiation and thus induce cytotoxicity effects. Therefore, it is generally accepted that the interaction of microbes with sonosensitizers in SACT is via a photodynamic pathway in PACT. Sensitizers can interact with target cells through two main routes [57]: formation of a complex with the surface substance of cell wall and /or reaction with cellular components essential for survival. In the first case, the oxidative damage to the cell wall can result in its decomposition and leakage of cytoplasmic materials, thereby causing cell death. Once the sonosensitizer permeates into the cell, it attaches itself to certain cellular components and induces damage after ultrasound irradiation. Such stress may disrupt critical metabolic pathways and thus result in cytotoxicity. These two processes occur in some SACT cases simultaneously. The input of each process into the SACT may vary depending on the cell type, sonosensitizer, and the surrounding environment.
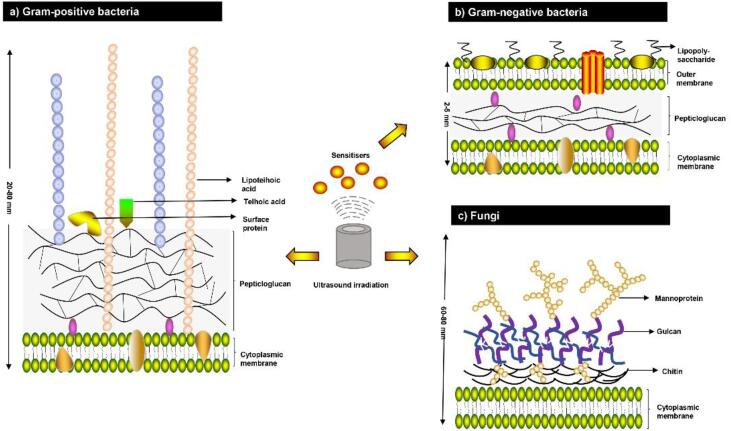
Different types of microorganisms have varying susceptibility to sonosensitizers. Research in PACT has established that, in general, Gram-positive bacteria can be efficiently killed by neutral, cationic, or anionic sensitizer while Gram-negative species often require the use of a cationic sensitizer or the supplementation of PACT with permeabilizing agents for significant inactivation [6]. This difference was attributed to the differences in their physiology, as demonstrated in (Fig. 2).
Fig. 2.
Structures of cell walls of Gram-positive, Gram-negative bacteria and fungi.
3.1. Bacteria
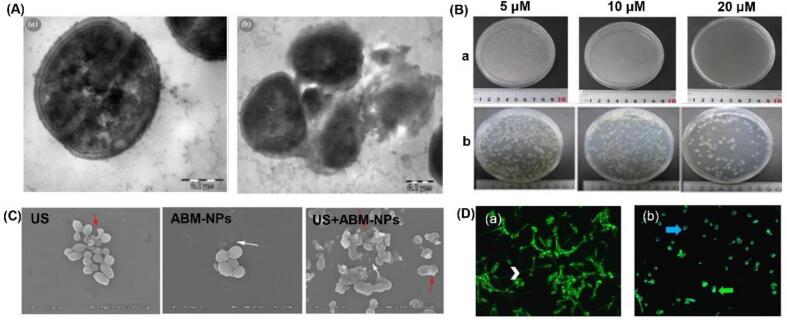
As shown in Fig. 2, Gram-positive bacteria have a thick (20–80 nm) but more porous layer composed of peptidoglycan and lipoteichoic acid around the cytoplasmic membrane. This allows sonosensitizers to interact with cell-wall components and to penetrate the cell wall relatively easy, irrespective of their charge [57]. When sonosensitizer is irradiated by ultrasound, the generated ROS will induce oxidative damage to the cell wall. This in turn leads to perforation of the cell membrane or the total collapse of the cell and subsequent leakage of intracellular materials. Indeed, Ayan et al. [58] observed that the cell wall in SACT-treated samples were partially destroyed or totally collapsed (Fig. 3A). Follow-up studies with flow cytometry and fluorescence microscopy also confirmed that the significant morphological changes on the cellular membrane of S. aureus after SACT. Moreover, no remarkable damage to bacterial DNA was reported following the SACT.
Fig. 3.
(A) Morphologies of control group (a) and S. aureus treated with ultrasound and antibiotics (b). Adapted from [58]; (B) Chlorin e6 with ultrasound treatment inhibited bacterial growth of S. aureus (a) and E. coli (b) in a dose-dependent manner. Adapted from [12]; (C) The morphological changes of C. albicans observed under SEM after distinct treatment methods. US, ultrasound alone. Adapted from [13]; (D) Light and fluorescence microscope images of the control group (a) and biofilms treated with a PDT + SDT PDZ 200 (b). The TB solution stained the nuclei of dead cells (blue arrow) and the Con-A bound to the polysaccharide cell wall with green fluorescence (green arrow). Adapted from [60]. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
In contrast, Gram-negative bacteria have a complex multi-layered structure of the cell wall. It consists of an inner cytoplasmic membrane and an outer membrane comprised of a second lipid bilayer with polysaccharides-lipopolysaccharides (LPS) (Fig. 2A). These two membranes are separated by a periplasm with a thin layer of peptidoglycan. While the thickness of the cell wall is small (2–5 nm) in Gram-negative bacteria, it provides a potent permeability barrier to protect the Gram-negative cell from the harsh surrounding environment [59]. Thus, sonosensitizer is limited to binding the cell wall due to this smaller volume and dense structure. Nevertheless, the negatively charged phosphate groups on the outer membrane of Gram-negative bacteria facilitate the binding of cationic sensitizer [6], [57], and this creates the basis of sonodynamic bactericidal activity of cationic sensitizer on Gram-negative bacteria. Comparative studies have demonstrated the capability of sonodynamic killing against Gram-negative bacteria [9], [10], [11], [12]. In all cases, these SACT treatments resulted in a multilog reduction in the number of microbial cells. When the sonodynamic bactericidal efficiency was compared for Gram-negative and Gram-positive bacteria in similar conditions, the difference in the inactivation efficiency was significant for the different types of bacteria and sonosensitizer. It is generally considered that Gram-positive bacteria are more vulnerable to SACT treatment than Gram-negative ones [10], [12], [55]. For example, Xu et al. [12] compared the effect of the sonodynamic action of chlorin e6 on S. aureus and E. coli. The result showed a 7-log reduction of S. aureus induced by sonodynamic treatment with chlorin e6 at 20 µM. However, E. coli was more resistant to the SACT, with only 2-log bacterial reduction at 80 µM (Fig. 3B). It has also been confirmed in a study [25], reporting that RB-mediated sonodynamic technique was effective for the eradication of S. aureus and E. coli with 3–4 log reductions, however, S. aureus was found to be more sensitive to the same parametric condition than E. coli. Besides, when Pseudomonas aeruginosa was treated with RB-mediated SDT, only a minor reduction in bacterial count was attained (0.5 log), which is considerably lower than that of S. aureus. However, when both bacteria were exposed to the RB-C(KLAKLAK)2 conjugate, the reduction in the P. aeruginosa population decreased more dramatically compared to that of S. aureus, with an additional 2 log reduction [26]. The authors explained that the interaction between the positively charged peptide and the negatively charged bacterial wall enhanced the uptake of RB by the bacteria.
3.2. Fungus and yeast
Fungal cells and yeasts share a relatively thick cell wall (60–80 nm) that comprised of two layers including an inner layer consisting mostly of glucans and outer layer arrayed by mannoproteins (Fig. 2). As for the bacteria, this structure also provides a permeability barrier. However, the electrical charge on the cell wall of yeasts is almost zero [6]. This suggests that there is no electrostatic attraction and subsequent attachment of sonosensitizers to the cell wall. This may explain the significant lower reduction of Saccharomyces cerevisiae by PADT than bacteria species [57]. Nevertheless, under SDT condition, the observed reduction of yeast cells was quite substantial. For instance, when Candida albicans (107 CFU/mL) were treated with chlorin e6 derivative photodithazine (PDZ) (50 or 100 mg/L) or RB (5 or 10 μm), the live cells were totally eradicated from the suspension after 5 min of ultrasound irradiation [60]. The synergistic bactericidal mechanism of ultrasound could be attributed to the cavitation effect, which increased the instantaneous permeability of the cell wall, enhancing the cytotoxicity of sonosensitizers. This result is consistent with a study that reported that the C. albicans suffered significant morphological damages after the simultaneous application of ultrasound and amphotericin B-loaded nanoparticles (AmB-NPs). These damages included thallus damage, distorted cell structures and visible cell debris, as can be seen in Fig. 3C [13]. Moreover, the addition of ultrasound significantly enhanced the release of AmB from nanoparticles. In addition to the effect of cavitation, the enhanced antifungal activity might be due to the increased intracellular activity of ROS.
3.3. Biofilm and spore
Some bacteria have the ability to form a biofilm that is a highly structured community of microorganisms attached to the contact surface. This structure contributed to the maintenance of stable cell-surface, cell–cell and cell-environment interactions [61]. Furthermore, cells in such biofilm exhibit more resistance to detergents and antibiotics due to the protection of the dense extracellular matrix. For this reason, the survival of bacteria in biofilms presents a challenge associated with sanitation processes employed in hospitals or the food industry. However, numerous studies have shown that SACT could be efficient in removing biofilms [1], [60], [61], [62] (Table 1). For example, according to the assessment of biofilm morphology and architecture using fluorescence microscopy (Fig. 3D) [60], the density of the biofilms treated with SDT + aPDT PDZ 200 apparently reduced. Furthermore, the visualized results were consistent with total biomass assays. It was suggested that ultrasound facilitated the generation of transient pores in the biofilm matrix increasing the diffusion of sensitizers into the biofilms, which improved the efficacy of SACT. Whereas Li et al. [63] also observed that SACT was not effective in the inactivation of bacteria within biofilms. This divergent result obtained can be linked to the different SDT parameters and bacterial strains used.
Table 1.
Reports about bacteria inactivation by various SACT.
| Sonosensitizer in SACT | Dose | Ultrasound | Microorganism | Biological model | Microbial reduction (log) | Reference |
|---|---|---|---|---|---|---|
| Antibiotic | ||||||
| Ciprofloxacin/levofloxacin | 0.01 mg/mL | 1 W/cm2, 40 kHz, | E. coli | In vitro | <2 | [27] |
| Gentamicin | 1–2 μg/mL | 100 mW/cm2, 46.5 kHz, 1 : 3 | E. coli | In vitro | 1.01–1.42 | [63] |
| Ciprofloxacin (topical) | 3% | 3 min every other day/every day | P. aeruginosa biofilm | In vivo (rabbit) | No shown | [90] |
| HBD-3 | 100 mg/kg | 200 mW/cm2, 1 : 1 duty cycle, 20 min, 3 times a day |
S. epidermidis biofilm S. aureus biofilm |
In vivo (mouse) | 1 1.25 |
[63] |
| Colistin /Vancomycin | 8 μg/mL | 40 kHz, 600 mW/cm2, 1 : 9 duty cycle, 30 min | A. baumannii biofilm | In vitro | 3.77 | [80] |
| Gentamicin | No shown | 28–48 kHz, 0.5 W/cm2, 48 h | E. coli | In vivo (rabbit) | 2 | [81] |
| Natural product | ||||||
| HMME | 50 μg/mL | 1 MHz, 6 W/ cm2, 30 min. | S. aureus | In vitro | 95% | [82] |
| HMME | 40 μg/mL | 1 MHz, 3 W/cm2, 10 min | P. gingivalis | In vitro | 4.7 | [9] |
| Curcumin | 40 μM | 1 MHz, 1.56 mW/cm2, 5 min | MRSA | In vitro | 5 | [55] |
| Curcumin | 40 μM 2 μM |
1 MHz, 1.56 mW/cm2, 5 min 3 min |
E. coli B. cereus |
In vitro | 2 5–6 |
[10] |
| Curcumin | 100 mM, 50 μM | 50 W/cm2 , 5 min. |
S. aureus E. coli |
0.8 3.02 |
[11] | |
| Curcumin | 40 μM (2 mL) | 100 Hz, 3 W/cm2, 20% of duty cycle, 32 min | S. aureus biofilms | In vitro | 1.7 | [89] |
| Chlorin e6 | 20 µM, 80 µM | 1.0 MHz, 1.56 W/cm2, 15 min |
S. aureus E. coli |
In vitro | 7 2 |
[12] |
| Chlorin e6 derivative Photodithazine ® (PDZ) | 50 μM 200 mg/L |
1 MHz, 5 min, 50% of duty cycle, 2.5 W/cm2 |
C. albicans C. albicans biofilms |
in vitro | 6.38 0 |
[60] |
| Hypocrellin B | 40 µM | 1.38 W/cm2, 5 min | MRSA | In vitro | 5 | [10] |
| Nanoparticle or Nanocomposite | ||||||
| TiO2 (2 mm ø pellets) | 1 mg/mL | 36 kHz, 300 W, 15–60 min | Legionella spp. | In vitro | <2 | [29] |
| TiO2 (21 nm ø nanoparticles) | 5 mg/mL | 24 kHz, 300 W, 15–60 min |
Pseudomonas spp. Total coliformis faecal streptococci C. perfringens |
In vitro | 2 | [28] |
| TiO2 (non-woven fabric) | No shown | 36 kHz, 0.28 W, 0–70 min | E. coli | In vitro | 1 | [39] |
| TiO2 -DVDMS | 50 μg/mL | 3 W, 60 s | S. aureus | In vitro | 92.41% | [74] |
| Silver NP (citrate) Silver NP (miramistin) Gold NP |
10-6 g/mL 10-6 g/mL 10-5/ 10-6 g/mL |
0.88 MHz, 1 W/cm2, 10 min | Enterococus spp. | In vitro | 81% 87% 90% |
[86] |
| SiNPs | 1 mg/mL | 1 MHz, 1 W/cm2 | E. coli | In vitro | 35 /72% | [70] |
| AmB-NPs | 0.5–1 μg/mL. | 42 kHz, 0.30 W/cm2, 15 min. | C. albicans | In vitro | 90%~100% | [13] |
| MLP18 | 20 μM | 1.0 MHz, 0.97 W/cm2, 5 min |
E. coli MRSA |
In vivo (mice) | ~60% ~80% |
[1] |
| CNPs-ICG | 1000 μg/mL | 1 MHz, 1.56 W/cm2, 1 min | Biofilm (P. gingivalis, Prevotella intermedia) | In vitro | 57.9% | [61] |
| Gentamicin (Nano-sized liposomes) |
0.8 mg/mL | 2.25 MHz, 4.4 W/cm2 | Ralstonia insidiosa biofilm | In vitro | 73% | [87] |
| Pd@Pt-T790 | 25 ppm 50 ppm |
1.0 MHz, 0.97 W/cm2, 50% cycle, 8 min | MRSA | In vitro In vivo (mice) |
1 ~ 5 >6 |
[73] |
| Fe@UCNP-HMME | 4.75 µg | 1 W/cm2, 10 min 2 W/cm2, 10 min |
E. coli MRSA |
In vitro | 60% 70% |
[22] |
| UCNP@SiO2 -RB | 125 μg/mL | 2 W/cm2, 10 min |
E. coli MRSA |
In vitro | 70% 70% |
[23] |
| UCNP@mSiO2 (RB)-AgNPs | 45 µg/mL | 2 W/cm2, 10 min | MRSA | In vitro | 5 | [88] |
| NM@Cur | 50 mM | 1.56 W/cm2, 1 min | S. mutans | In vitro | 99.9% (~6 log) | [75] |
| Xanthene dye | ||||||
| RB | 15 μM | 28 kHz, 0.84 W/cm2, 1 h |
S. aureus (109 CFU/mL) E. coli (109 CFU/mL) |
in vitro | 2.1 3.1 |
[25] |
| RB-C(KLAKLAK)2 | 4.5 mg/kg | 1 MHz, 3.0 W/cm2, 50% duty cycle, 10 min |
S. aureus P. aeruginosa |
In vitro | 5 7 |
[26] |
| RB | 5 μM 200 mg/L |
1 MHz, 5 min, 50% duty cycle, 2.5 W/cm2 |
C. albicans C. albicans biofilms |
In vitro | 6.38 0 |
[60] |
a, b The reductions in the viable counts of each bacteria and its biofilm in column, respectively.
The formation of spores is another way in nature to protect microorganisms themselves from environmental stress. Due to their multilayered dense structure, spores are remarkably resistant to chemical and physical treatments commonly used for pathogens [64]. To destroy spores, harsh treatments such as thermal (>121℃), UV radiation, strong hypochlorite solutions, and chlorine dioxide are most commonly used [65]. Unfortunately, these sporicidal conditions are not always compatible with some processing requirements, such as food. PACT as a mild method was recently proposed to achieve effective destruction of bacterial spores [66]. The susceptibility of spores to PDT provides a possibility for the application of SACT to eliminate spores, as SACT not only shares a similar antimicrobial mechanism but can alternatively overcome the limited penetration of light. However, to our knowledge, there are currently no reports that have shown the efficiency of SACT against spores.
3.4. Virus
Unlike in bacteria and fungi, viruses have no protective coat to isolate essential proteins and nucleic acids from adverse conditions. Most viruses consist of nucleic acid polymers (DNA or RNA) and a protein coat (capsid) [57]. They can only naturally grow and reproduce within a host cell. This specific characteristic renders viruses, to some extent, more susceptible to environmental stress than other microorganisms. The photodynamic killing of viruses in blood products has been well established [67], [68]. For example, treatment with MB eradicated hepatitis B, hepatitis C, and human immunodeficiency viruses from plasma products by light [68]. Fortunately, it has been shown that the photosensitizers, such as MB, can also be activated by ultrasound, and therefore the strategy may be useful for the treatment of viral infections. Recently, Zborowska [69]proposed that there is a high probability that SDT could be efficient in the prevention of Coronavirus (COVID-19) pandemic, therapeutic and post-infection complications, even though this therapy has not been clinically tested and approved for this virus.
4. Factors influencing sonodynamic inactivation
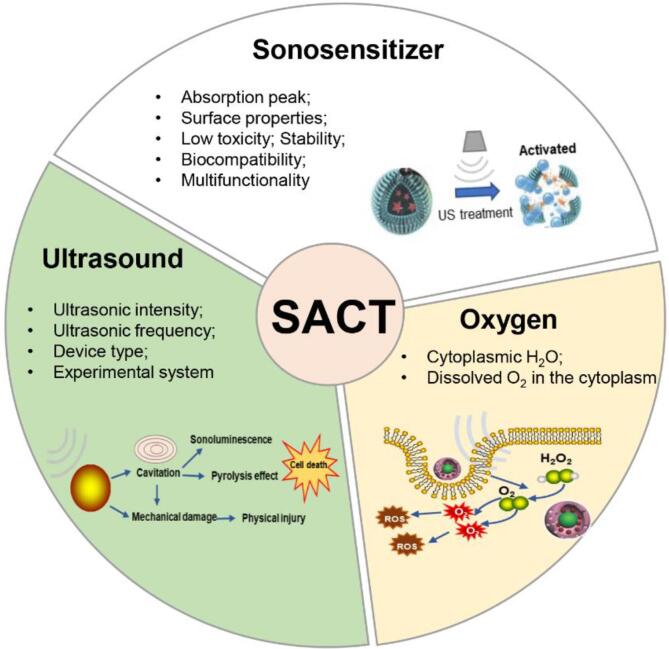
Three essential components, including ultrasound, sonosensitizer, and oxygen, together influence the outcome of the sonodynamic killing process (Fig. 4). Each of these components can be regulated at different degrees, with ultrasound generally being the easiest to controlled and oxygen being the hardest to determine. Meanwhile, sonosensitizer plays a pivotal role in maximizing the success of sonodynamic strategy, as it can enhance the efficacy, selectivity, and safety of the treatment.
Fig. 4.
The three essential components required for SACT to take place- ultrasound, sonosensitizer and oxygen.
4.1. Ultrasound parameter
Ultrasound with intensity below 3 W/cm2 is defined as low-energy ultrasound and that with frequency below 1 MHz as low-frequency ultrasound. According to the summary in Table 1, most of the studies adopted the low-energy (0.3–3 W/cm2) and low-frequency (≤1 MHz) ultrasound to investigate the effectiveness of SDT against microbes. The correlation between SDT effectiveness and the ultrasonic intensity, and frequency has been studied both in vitro and in vivo. Shevchenko et al. [70] found that ultrasonic intensity directly determined the bactericidal effect of Dextran-coated Silicon Nanoparticles (DSiNPs). In this study, SDT treatment with 1 W/cm2 of ultrasound resulted in a drop in the viability of E. coli up to 35–72%. Whereas a significant viability decrease of E. coli was obtained after the combined treatment of higher ultrasound intensity (3 W/cm2) and nanoparticles. Rediske et al. [71] also observed that 300-mW/cm2 ultrasound significantly enhanced the effectiveness of gentamicin against E. coli biofilms in a rabbit model, with a 2.39-log further reduction of biofilms versus treatment with antibiotic alone. However, no significant reduction in the number of E. coli biofilms was obtained due to treatment with 100-mW/cm2 ultrasound and antibiotic. It was believed that the cavitation effect had caused more damage to the cell membrane with an increase in ultrasound energy and mechanical pressure, and the ROS generation increases accordingly [16], [19]. Although the high ultrasonic energy can produce excessive ROS that irreversibly damages the cytomembrane, it may induce side effects to the normal tissue in the in vivo experiments [43], [49], [71]. It was confirmed by the study of Rediske et al. [71], reporting that no damage was induced to rabbit skin at 100 mW/cm2, compared to ultrasound at 300 mW/cm2 that caused apparent skin damage that was attributed to cavitation effect. In contrast, the low-intensity ultrasound temporarily enhanced the permeability of cytomembrane, producing a proper amount of ROS that caused the targeted cell death and avoided the surrounding normal cells being attacked [72].
Despite the fact that SACT has a potent advantage in bacterial decontamination, the ambiguous mechanisms of action have limited the maximizing of disinfection efficacy. Furthermore, this is challenged by the lack of standardization of ultrasound dosimetry and the uniform definition of SACT. Typically, most SACT studies present ultrasound intensity in the form of power density (W/cm2). Acoustic field parameter is dependent largely on ultrasound devices. However, the lack of universal reporting of ultrasound parameters generated uncertainty during the comparison of SACT studies or fail to draw conclusions from the resultant effects [20], [34]. To overcome these challenges, Choi et al. [20] proposed a thorough description of the acoustic intensity in the form of pressures, assessing a series of cavitation effects, and characterizing the beam.
4.2. Sonosensitizer
Sonosensitizers enhanced the rate of the sonodynamic inactivation, propping it up to minutes but not hours. Its distribution and uptake in cells are imperative for congruently generating excessive ROS under ultrasound irradiation and hence establishing excellent SACT efficacy [19]. One of the essential factors affecting the dispersibility of sonosensitizer in cells is its surface properties, such as hydrophobicity and hydrophily. Most of the recently reported sonosensitizers, such as curcumin, RB, hypocrellin B, and some porphyrin-based compounds, are hydrophobic, which caused severe aggregation in the aqueous solution [21], [73]. This aggregation phenomenon can significantly decrease the ROS production by the activated sensitizers inside. Introducing some polar groups onto the molecular surfaces can evidently increase the hydrophilicity of the sensitizers, preventing precipitation in the solution. Wang et al. [74] alleviated the aggregation of TiO2 nanoparticles by properly combing it with DVDMS. The adjunction of DVDMS significantly improved the dispersibility of nanocomposites. Under ultrasound irradiation, the developed nanocomposites produced more singlet oxygen and hydroxyl radical, compared with the other groups. Although axially modification is an efficient way to promote solubility, not all the sonosensitizer can be modified in this way. Encapsulating the sonosensitizers into the nanoparticles is another way to increase the dispersion of the hydrophobic molecules. However, these nanocomposites have inherent shortcomings, for example, the inside sonosensitizers can aggregate in the nanoparticle. To end these problems, a promising way is the chemical conjugation of the sonosensitizers onto the surface of nanoparticles or inside the mesoporous nanoparticles. A similar approach was adopted by Pourhajibagher et al. [75]. The researchers used PEG-PE backbone to bridge with curcumin forming an NM@Cur, which significantly enhanced the aqueous solubility, stability and dissolution of the poorly water-soluble curcumin. Meanwhile, the ROS yield following NM@Cur mediated SACT was 3.5 fold higher than curcumin mediated SACT in bacterial cells, resulting in a significant reduction of S. mutans.
When SACT is applied for microbial disease in in vivo, the sonosensitizer targeting ability is a critical factor for the bactericidal ability. The low specificity of sonosensitizer in the infection area not only decrease the therapeutic effectiveness but also induce unwanted accumulation in normal tissue [76]. Given these gains and losses, Pang et al. [1] developed a bacteria-responsive nanoliposomes, MLP18, as smart sonotheranostics for combating MDR bacterial infections. In this study, the prepared MLP18 specifically targeted the bacteria-infected site of mice via the bacteria-specific maltodextrin transport pathway. Furthermore, the bacteria-responsive characteristics of MLP18 activated the effective release and internalization of high concentration sensitizers into the bacterial cells, thereby effectively eliminating MDR bacteria through acoustic kinetics. Overall, the ideal SACT requires the ROS generation and the specific distribution of sonosensitizers.
4.3. Oxygen supplement
Oxygen is essential to the generation of ROS by an activated sonosensitizer during SACT. In clinical medicine, it is common to observe the hypoxia cells, which are the tumors or deep-seated cells that have been deprived of oxygen [76]. The hypoxic environment of bacteria-infected tissue restrict the bactericidal efficacy of SACT to a high degree. More seriously, the consumption of oxygen in the SDT procedure may aggravate the hypoxic status, further resulting in the poor outcomes of SACT [73]. To overcome these problems, some researchers opted for the addition of a new kind of oxygen microbubble to SACT [77]. The antimicrobial results of the in vitro study demonstrated that microbubble-mediated ultrasound significantly improved the bactericidal activity of antibiotics. The sufficient oxygen supplement obviously improved the generation efficiency of local ROS, increasing the bactericidal ability of SACT. Meanwhile, the microbubble could also enhance acoustic cavitation, assist cell membrane rupture and improve sensitizer absorption. Benefiting from the experience in the molecular design for sonosensitizers, Sun et al. [73] recently overcome this hypoxic microenvironment in another way by adopting the redox reaction between endogenous H2O2 and catalase. An ultrasound-switchable nanozyme system was proposed for alleviating the hypoxia-associated barrier and augmenting SACT efficacy. The results demonstrated this developed nanoenzyme system eradicated the deep-seated bacterial infection controllably and precisely, providing a promising sonodynamic strategy. Instead of developing diverse strategies to overcome hypoxia, some researchers took advantage of the hypoxia environment as favorable conditions, and develop hypoxia-oriented SDT therapy [76], [78]. In these therapies, the hypoxic precursor drugs can be activated to generate high cytotoxicity and improve the hypoxia level of tissues. In turn, the oxygen-dependent SDT could induce a sufficient hypoxia environment to activate silencing drugs. Thus, the combination of SDT and hypoxia-activated drugs was able to remedy their respective deficiencies, thereby achieving a highly effective synergistic disinfection approach.
Hypoxia is also likely to occur in food products due to modified or controlled atmosphere packaging which compromises the levels of oxygen required for SACT. Furthermore, the natural antioxidants present in foods, such as β -carotene and ascorbic acid, which can quench the O2 in the cell’s vicinity and its cytoplasm. This poses a potential challenge for the SACT efficacy. Nevertheless, no studies have reported on the effect of antioxidants in food products following SACT process.
5. SACT for disinfection and decontamination
The antimicrobial ability of SACT has been well established in numerous in vitro studies and a lesser extent in in vivo models in the past decade. This modality was endowed with medical and industrial importance with applications ranging from the sterilization of medical instruments and food processing equipment to the treatment of microbial infections. So far, only four classes of sonosensitizers-mediated SACT have been conducted as a major experimental demonstration, including antibiotics, natural product extracts, nanoparticle/ nanoparticle complexes and xanthene dye (Table 1).
5.1. Antibiotic-mediated SACT
The investigation that ultrasound irradiation possessed the ability to activate sonosensitizers was first conducted by Liu et al. [27], who evaluated the sonodynamic antibacterial effect of two fluoroquinolones (ciprofloxacin and levofloxacin) on E. coli. Irradiation with ultrasound alone for 45 min, only a minor effect on the viability of the E.coli was observed. However, when ultrasound was used simultaneously with two fluoroquinolones, a strong synergistic antimicrobial effect was achieved, with an improvement of <30% on treatment with antibiotics alone (Table 1). These results confirmed the predictions of antimicrobial potential of ultrasound made in 2009 [79]. Moreover, the authors linked the synergistic effect of ultrasound and fluoroquinolones to the involvement of ROS. Similar results were obtained in another in vitro study, which evaluated the ability of a conventional antibiotic, gentamicin, to function as a SACT agent [77]. Its ability was confirmed when the combination of gentamicin and microbubble mediated-ultrasound led to an enhanced bactericidal effect on planktonic E. coli, but separate applications did not reduce the viability of bacteria. They believed that the temporal change in cell membrane permeability was the leading cause of the increased lethal effect of the antibiotic. This change was also visualized in a bacterial cell with an electron microscope. Although ultrasound could apparently enhance the bactericidal effect of gentamicin, the external microbubbles amplified the synergistic effect between ultrasound and gentamicin at 1 and 2 μg/mL. The partial destruction of the bacterial cell wall caused by microbubble mediated-ultrasound was probably an explanation of the amplified effect of the combined treatment. Further, another study [63] demonstrated that a combination low-frequency of ultrasound with antibiotics can augment the bactericidal activity evidently against the formation of biofilms (Table 1). In this study, Human B-defensin 3 (HBD-3), a cationic antimicrobial peptide, was employed to combat MRSA biofilms infections in vivo. Compared with HBD-3 and HBD-3-mediated ultrasound alone, US-targeted microbubble destruction (UTMD) could increase HBD-3 activity, and significantly enhanced the ability of HBD-3 to decrease the biofilm density and the viable count of two tested Staphylococcus biofilms on the titanium surface in mice. The authors believed that UTMD promoted the drug delivery in bacterial cells via the “sonoporation” phenomenon. However, the application of ultrasound at 40 kHz and 600 mW/cm2 failed to improve the bactericidal activity of colistin or vancomycin significantly, even though these combinations did function against biofilms [80]. In contrast, ultrasound apparently enhanced the antibacterial efficacy of combinations of these agents. The antimicrobial ability increased with the concentrations of combined colistin. Although, the authors did not rationalize these data on the basis of the sonodynamic action, their findings have shown that ultrasound in combination with colistin and vancomycin could be promising in removing pan-resistant Acinetobacter baumannii infections. This same phenomenon was also reported elsewhere [81], where pulsed ultrasound significantly prevented the formation of E. coli biofilms in bone cement in vivo (rabbit model) in the presence of gentamycin.
To date, antibiotics-mediated SACT studies were being investigated using both in vitro and animal modal experiments against planktonic bacteria and bacterial biofilms. In the majority of studies, ultrasound with intensity lower than 1 W/cm2, and frequency ranging from 40 to 50 kHz was employed to show the synergistic bactericidal effect with antibiotics. While the data in these studies are promising, differences in the critical parameters, such as ultrasound setup, are evident.
5.2. Natural product-mediated SACT
Sonosensitizers derived from natural products have demonstrated a potent sonodynamic activity for the microbial eradication by recent studies. Hematoporphyrin monomethyl ether (HMME), as porphyrin derivatives, have been effective in triggering bacterial cell death upon activation by ultrasound irradiation. A SACT study carried out by Zhuang et al. [82] using HMME and ultrasound at 1 MHz and 6 W/cm2 against S. aureus. HMME-mediated SACT killed>95% of the bacteria, whereas ultrasound alone only removed 38% of the bacteria. This sonodynamic treatment was dose-dependent, as the killing of more bacteria could only be possible using ultrasound at higher intensities and higher HMME concentrations. Nevertheless, using lower condition parameters than those in [82], HMME-mediated SACT in [9] caused more reductions of 4.7 log in the viable counts of Porphyromonas gingivalis. The difference in the efficacy of HMME-mediated SACT against bacteria was associated with the different types of microbes. Additionally, they also found more ROS are generated in SDT groups compared with the control group.
Curcumin, a naturally occurring active agent, has been evaluated for sonodynamic activity. In the case of curcumin-mediated SACT, curcumin exhibited a different sonodynamic effect against various bacteria including MRSA, Bacillus cereus, and E. coli [10], [55]. A 5-log reduction of MRSA was achieved after treatment with curcumin (40 μM) and 5 min of ultrasound irradiation at 1 MHz and 1.6 W/cm2. When treated with 2.0 μM curcumin and ultrasound, B. cereus was reduced by 5–6 log. However, E. coli was more resistant to the combinations of curcumin and ultrasound, and only 2-log reduction was obtained after treatment even with a higher concentration of curcumin [10]. Furthermore, pulsed-field electrophoresis showed that these treated bacteria exhibited no apparent alteration in chromosomal DNA. In contrast, Bhavya and Hebbar [11] observed different combinational effects of ultrasound and curcumin on E. coli and S. aureus in orange juice. The E. coli showed more sensitivity to curcumin-mediated SACT than S. aureus. Ultrasound combined with curcumin caused 3.02-log and 0.8-log reduction of E.coli and S. aureus, respectively. The divergent behavior was associated with the medium type. However, Alves et al. [89] recently found only 1.7 log of S. aureus biofilms were removed after exposure to the sonodynamic action of curcumin. These results indicated that the efficacy of SACT depended on many factors, such as ultrasound parameters, strain types, and experimental medium.
Moreover, a combination of ultrasound and chlorin e6 (Ce6) and its derivative PDZ significantly decreased the viability of S. aureus and C. albicans by 7-log and 6.38-log reductions, respectively [12], [60]. While only 2-log reductions of E. coli were achieved after the sonodynamic action of Ce6 [12]. In the same study, combined ultrasound and PDZ had little impact on C. albicans. The simultaneous application of PDZ-mediated SACT and light irradiation significantly reduced the viability and entire community of biofilms [60].
Hypocrellin B (HB), a monomeric perylenequinone pigment, has been widely applied as a photosensitizer for the treatment of tumor and microbial diseases. Some studies also proposed that HB can be activated by ultrasound [83], [84]. In order to confirm whether HB-mediated ultrasound has the cytotoxic effect on MRSA, Wang et al. [85] designed an in vitro experiment. They found that 5 min of treatment with HB (40 μM) and ultrasound at 1.38 W/cm2 resulted in a 5-log reduction in the viable counts of MRAS. The results also demonstrated that HB could be a promising antibacterial sonosensitizer for damaging the integrity of the bacterial membrane under ultrasound irradiation.
At present, only the in vitro experiments have been conducted in natural product-mediated SACT studies against bacteria and biofilm. Most of these studies applied ultrasound with a frequency lower than 1 MHz and intensity ranging from 1.56 mW/cm2 to 6 W/cm2. Only one study employed higher intensity (50 W/cm2), in which enhanced bactericidal activity with curcumin was observed (Table 1). These results are encouraging and could pave the way for exploring more novel natural products as sonosensitizers and thus offer more excellent SACT strategies.
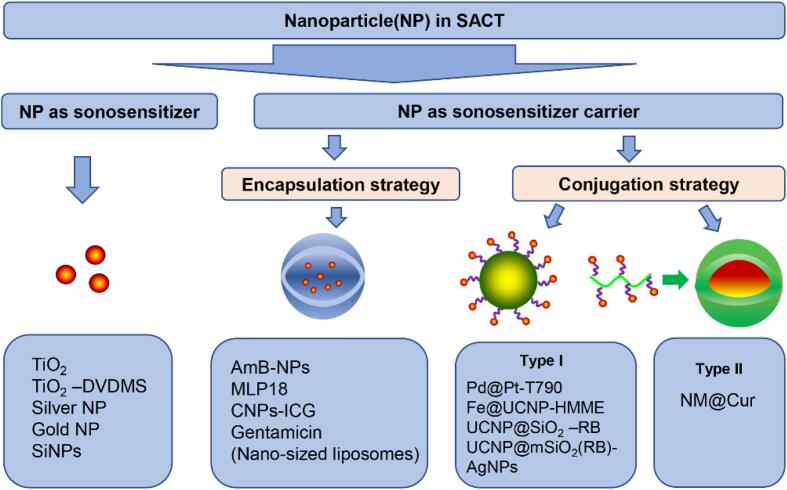
5.3. Nanoparticle-mediated SACT
Recently, more and more novel nanoparticles are being applied to SACT treatment. Functionally, nanoparticles in SACT can be divided into two classes: sonosensitizer nanoparticles and nanoparticle carriers (Fig. 5). Sonosensitizer nanoparticles refer to the sonosensitizer themselves being nanoparticles, which directly involve nanotechnology with sonosensitizing agents. While the latter refer to the use of nanoparticles as carriers to deliver sonosensitizers.
Fig. 5.
Schematic illustration of sonosensitizer with nanoparticles.
Among numerous nanosensitizers, titanium dioxide nanoparticles (Nano-TiO2) are most popular due to their attractive characteristics, such as low toxicity, excellent biocompatibility and outstanding stability in the physiological condition (Table 1). After exposure to ultrasound irradiation, nano-TiO2 inside could generate active hydroxyl ion which in turn resulted in cytotoxicity in vitro and in vivo. An important application of the nano-TiO2 is for the SACT-mediated disinfection of wastewater. Drakopoulou et al. [28] observed a combination of nano-TiO2 and ultrasound irradiation (24 kHz, 300 W) for 60 min resulted in 2 log-reduction in the viability of Gram-negative bacteria (Pseudomonas spp. and coliforms) in wastewater. Meanwhile, the sonodynamic inactivation achieved 103 CFU/100 mL total coliforms, thus meeting USEPA quality standards for wastewater reuse. In another study, the application of ultrasound and TiO2 (1 g/mL) reduced the load of Legionella spp. by 3 log in wastewater. The initial bacterial load of the water did not impact the decontamination capability of the treatment [29]. When wastewater exposed to 36 kHz ultrasound in the presence of non-woven TiO2 for 1 h, one-log reduction in the number of E. coli in wastewater was observed (Table 1). The above studies showed that the sonodynamic action was not affected by the quality of wastewater, providing an evidence for the advantage of SACT over PACT with the same sensitizer. Although nano-TiO2 provides a novel promising platform for SACT, the dispersibility of this particle in the aqueous phase has limited the extensive application. To alleviate such issues, appropriate surface decoration on TiO2 nanoparticles is necessary. For example, Wang et al. [74] synthesized a new nanoparticulate sonosensitizer by bridging nano-TiO2 with a trace amount of DVDMS sensitizer (DFT) for an efficient SACT against S. aureus in vitro. The combined treatment of nano-TiO2 and DFT visibly improved the yield of hydroxyl radicals and single oxygen compared with the simple surface modification of TiO2 and free DFT. The DFT involvement evidently improved the sonocatalytic process of nano-TiO2, achieving 92.4% of eradicating efficiency in S. aureus. Similarly, Gopin et al. [86] designed a method of sonodynamic bactericidal therapy using different nanosensitizers, including silver nanoparticles stabilized by miramistin or citrate ions and gold nanoparticles. In this study, the authors found the combination of ultrasound and gold nanoparticles (10−5) led to superadditive antibacterial activity against Enterococus spp. (Table 1). Furthermore, the antibacterial activity of gold and silver nanoparticles depended on their concentration and the stabilizing nature. These metal nanoparticles-mediated SACT caused an apparent morphological destruction and a significant bacterial killing, nevertheless, a typical disadvantage of using these particles was their high cytotoxicity to normal cells. Silicon nanoparticles (SiNPs) have shown low genotoxicity and teratogenicity in vivo. SiNPs were found to be sonosensitizers for bacterial decontamination. Shevchenko et al. [70] utilized a strategy to destroy E. coli using a combined action of biocompatible SiNPs (1 mg/mL) and ultrasound (1 MHz, 1 W/cm2) (Table 1). The results showed that ultrasound induced the reduction of E. coli up to 35 and 72% in the presence of silicon nanoparticles without (SiNPs) and with polysaccharide (dextran) coating (DSiNPs), respectively. The difference in the reduction of bacterial viability of SiNPs and DSiNPs was attributed to the fact that the polysaccharide coat provided a stronger adhesion of nanoparticles to the bacterial surface.
Comparative researchers developed novel SACT strategies by assembling the sonosensitizer with various nano materials that served as a carrier for sonosensitizer passing through cytomembrane. These sonosensitizers were carried by encapsulating into the core of nanoparticles and covalently conjugating with the nanoparticle substrates (Fig. 5). Investigations into the encapsulation of sonosensitizer by nanoparticles for use in SACT began only a few years with efforts to increase carrier capacity and augmenting antimicrobial efficacy. Yang et al. [13] engineered poly lactic-co-glycolic acid (PLGA) nanoparticles to carry the sonosensitizer of amphotericin B (AmB). Using the developed amphotericin B-loaded nanoparticles (AmB-NPs), the antifungal efficiency of ultrasound irradiation at 42 kHz and 0.30 W/cm2 was significantly enhanced (Table 1). Additionally, the antifungal efficiency increased with increasing the loaded AmB concentration. The authors explained that ultrasound improved the AmB release from nanoparticles, shorten its release time, and achieved synergistic antifungal activity within a short period. Pang et al. [1] used bacteria-responsive nanoliposomes as a SACT strategy for eradicating MDR bacteria. In this case, they developed the bacteria-responsive nanoliposomes by enclosing purpurin 18 (P18) into nanoliposomes (MLP18), which comprise of maltohexaose-modified cholesterol and 1, 2-dioctadecanoylsn-glycero-3-phospho-(1′-racglycerol) (DSPG)-contained lipid compositions (Fig. 6A). The prepared MLP-18 selectively recognized the bacteria-infection site and accumulate there. A high concentration of P18 was effectively released from MLP-18 upon ultrasound irradiation, resulting in potent sonodynamic elimination of MRD bacteria. A study conducted by Ma et al. [87] suggested that ultrasound-driven penetration of gentamicin-loaded liposomes into biofilms is an effective way to kill bacteria. Chitosan could interact with negatively charged sonosensitizers, self-assembled forming complex nanoparticles. These established samples were used to enhance sonodynamic bactericidal activity. Recently, Pourhajibagher et al. [61] used chitosan nanoparticles-indocyanine green (CNPs-ICG) to inhibit the biofilms of polymicrobial periopathogenes (Table 1). They obtained a 6.6-log reduction in the CFU/mL of periopathogens after treatment with ultrasound and CNPs-ICG, while the individual treatment has little impact on the periopathogens biofilms
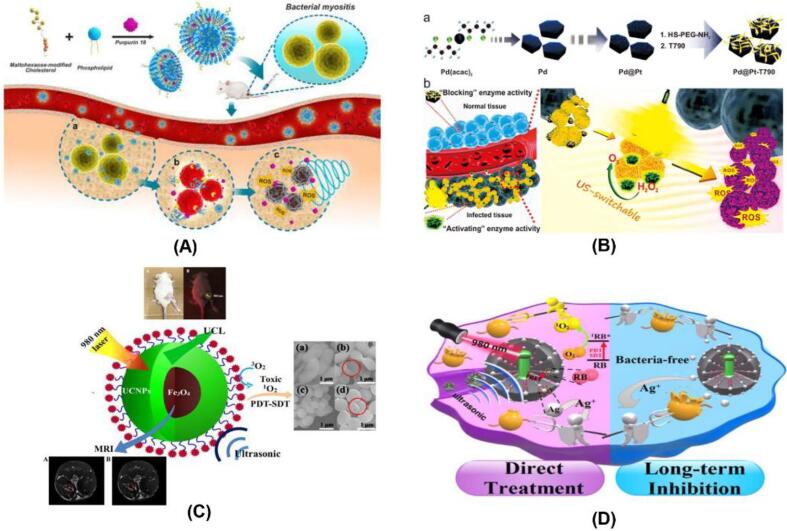
Fig. 6.
(A) Scheme illustration of MLP18 nanoliposomes for diagnosis and eradiation of MDR bacterial infection. Adapted from [1]; (B) Schematic illustration of the main synthesis procedure of the Pd@Pt-T790 nanoplatform and its US-switchable nanozyme catalytic oxygen-generation-enhanced SDT of bacterial infection. Adapted from [73]; (C) Schematic illustration of the sonodynamic action of Fe3O4@NaGdF 4 : Yb : Er-HMME. Adapted from [22]; (D) Schematic illustration of multifunctional therapeutic strategy of UCNP@mSiO2 (RB)-AgNPs. Adapted from [88].
Besides, numerous studies applied sonosensitizer-conjugated nanoparticles to amplify sonodynamic therapeutic activity. These nanoparticles are generally constructed by chemical binding the sonosensitizers to the surface of nanoparticles (Fig. 5). Sun et al. [73] designed an ultrasound-switchable nanoenzyme system which is prepared by conjugating enzyme-catalytic Pd@Pt nanoplates with a sonosensitizer meso-tetra (4-carboxyphenyl) porphine (T790) to eradicate MRSA-induced myositis (Table 1). In this case, the prepared Pd@Pt-T790 effectively accumulated in the deep-seated infection sites where ultrasound irradiation activated the catalase-like activity of the nanoenzyme, thereby alleviating the hypoxia-associated barrier and amplifying the SDA efficacy (Fig. 6B). More importantly, such “blocking and activating” enzyme activity was able to decrease the side effects of nanoenzymes on normal tissues. Also, the developed Pd@Pt-T790 completely killed MRSA in vitro and in vivo upon ultrasound irradiation, which provides a potent strategy to combat deep-seated bacterial infection. Xu et al. [23] developed a novel nanoparticle by encapsulating HMME in its yolk-structured up-conversion core and covalently linked RB on its silica (SiO2) shell. This new nanoparticle is a multifunctional nanoparticle which combines photodynamic and sonodynamic features for combating bacteria. Using this developed nanoparticle, synergistic PDT and SDT caused a higher inhibition rate (100%) of antibiotic-resistant bacteria compared to PDT (74.2%) or SDT (70%) (Table 1). These results stressed the advantage of multifunctional nanoparticles in the disinfection of bacterial diseases. Similarly, Wang et al. [22] also constructed multifunctional nanoparticles with a core–shell structure of Fe3O4 and upconversion nanoparticles (UCNPs), and HMME on its surface (Fig. 6C). When applied to Gram-negative and Gram-positive bacteria, this prepared multifunctional nanoparticles based on SDT and PDT completely killed these pathogens. These inactivation mechanisms involved singlet oxygen generation. Subsequently, another multifunctional nanoparticles, UCNP@mSiO2(RB)-AgNPs, were developed by Zhao et al. [88]. The nanoscale entity integrated PDT, SDT, and silver nanoparticles (AgNPs), offering potent ability of inducing a rapid bactericidal effect by a dualmodality therapy (PDT and SDT) (Fig. 6D). On the other hand, these nanoparticles were generally engineered initially via a covalent combination of the sonosensitizers and polymer backbones, and the obtained conjugates self-assembled into nanoparticles (Fig. 5). For example, nanomicelle curcumin (NM@Cur) is prepared by chemically combining curcumin with polyethylene glycol- L -α phosphatidyl ethanolamine (PEG-PE) backbone. The NM@Cur was used by Pourhajibagher et al. [75] as sonosensitizer for the eradication of S. mutans under SACT. The findings showed that when treated by NM@Cur-mediated SACT, the number of S. mutans was decreased by 99.9%, while Cur-mediated SACT reduced only 90.8% of the viable counts.
Recent researches support that nanoparticle-based SACT is well on its way to improving the accumulation and selectivity of the sonosensitizers. In this way, the antimicrobial efficacy in vivo and in vitro is increased and at the same time reduce the unwanted side effects. However, other issues that still need to be considered under this combination include the efficient release of sonosensitizers and the degradation rate of nanoparticle carriers.
5.4. Xanthene dye
Nakonechny et al. [25] studied the sonodynamic action of RB on E.coli and S. aureus. After simultaneous treatment with RB (15 μM) and ultrasound, E. coli was inhibited by 4–4.7 log compared to the untreated samples (P < 0.05). The inactivation rate of E. coli was associated with its initial concentration in vitro, and the highest eradication rate was achieved for samples at initial bacterial concentration of 106 CFU/mL treated by 15 μM RB. S. aureus was more sensitive to the sonodynamic treatment than E. coli. The concentration of S. aureus decreased by 3.5–6 log compared to the untreated samples (P < 0.05), and the bacteria was mostly eradicated by the sonodynamic treatment of RB (5 μM). Consistently, the results obtained by Alves et al. [60] also demonstrated that RB-mediated SACT could significantly reduce the viability of C. albicans in planktonic cultures, however, this combination had little impacts on biofilms. Costley et al. [26] conjugated RB with antimicrobial peptide to further enhance the efficacy of RB-mediated SACT. Treatment with the conjugate and subsequently ultrasound irradiation resulted in 5-log and 7-log reductions in the number of S. aureus and P. aeruginosa planktonic cultures, respectively. Moreover, the diffusion of this conjugate into P. aeruginosa biofilm was significantly improved after pre-treatment (P < 0.01). In vivo results showed that ultrasound irradiation of conjugate-treated P. aeruginosa-infected wounds in mice induced a substantial bacterial eradication. These findings highlighted a targeted broad-spectrum of novel SACT treatments and stressed its potential for clearing deep-seated bacterial infections.
6. Conclusion and future perspectives
SACT is a relatively new antimicrobial modality, but that seems to have great potential as a therapeutically useful strategy. This potential has already been conclusively demonstrated by numerous studies, while the experimental conditions examined differ between SACT studies makes it uncertain for comparison and rationalization of the data. Meanwhile, lack of standardization in ultrasound dosimetry and a unifying definition of SDT limits the establishment of universal mechanisms of SACT. Ambiguous mechanisms of action at play hinders the maximization of antimicrobial efficacy of SACT. These mechanisms include ROS-independent cytotoxicity such as cell membrane shearing, and ROS-dependent cytotoxicity from sonoluminesence or pyrolysis. Moreover, the prevailing proposed mechanism of action currently rely on the ultrasound-triggered, sonosensitizer-enabled ROS generation. The predominant mechanisms involved in SACT likely depend on the ultrasound parameters, the biological system, and the physiochemical properties of sonosensitizers. Moreover, a large number of experiments should be carried out under the same conditions, to identify the optimal parameters, such as frequency and energy, establishing the optimum SACT strategy. So far, the majority of SACT studies conducted is about the killing of bacteria and bacterial biofilm in in vitro and in vivo, while only two studies have been reported about the inactivation of fungi and yeasts by SACT treatment. Also, there are no SACT treatments on viruses performed yet. The sonodynamic process shares a similar feature with the photodynamic, and it is reasonable to expect that more SACT studies be conducted on these microorganisms in the future. Additionally, the current sonosensitizers limit the efficacy of SACT due to low specificity, rapid aggregation, and potential toxicity. Although considerable efforts are being made to develop new nano-sonosensitizers with excellent target specificity and biocompatibility, several challenges need to be addressed, including side effects on normal tissues, complicated preparation approaches, and sub-optimal accumulation in the target site. The focus, therefore, is on developing sonosensitizers with improved specificity, biocompatibility, biosafety, and therapeutic efficacy. Meanwhile, a natural environment with the vast chemical and biological diversity can provide an excellent source of novel sonosensitizers. Future studies on enlarging the scope of sonosensitizer sources are warranted. Combination treatment offers a new weapon in the fight against MDR microbial infection. Given some classes of sonosensitizers with dual ability to be activated by ultrasound and light, it seems possible to form the basis of a novel antimicrobial modality known as sonophotodynamic antimicrobial chemotherapy (SPACT). SPACT can overcome deficiencies of PACT and present advantages over SACT, and sonophotodynamic therapy (SPDT) have been shown to be efficacious as an anticancer strategy in in vivo studies, while it is rarely investigated for antimicrobial ability. Simultaneously using two or more nano-sensitizers, co-delivering multiple sonosensitizers by a single nanoparticle carrier have also been proved to improve the disinfection capability, it is therefore of particular interest to undertake stimuli-responsive sensitizer delivery systems.
The published findings of SACT may be speculative and remain to be tested experimentally before transfer to clinical antimicrobial therapy, while they do underline the vast scope and potentials offered by SACT. In particular, it is believed that SACT is emerging as a promising alternative for removing the microbial contamination in water and other liquids and surfaces such as food and beverages, medical instrumentation, and food processing equipment.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This work was financially supported by the National Key Research and Development Program of China (grant 2016YFD0400301).
References
- 1.Pang X., Xiao Q., Cheng Y., Ren E., Lian L., Zhang Y., Gao H., Wang X., Leung W., Chen X. Bacteria-responsive nanoliposomes as smart sonotheranostics for multidrug resistant bacterial infections. ACS Nano. 2019;13:2427–2438. doi: 10.1021/acsnano.8b09336. [DOI] [PubMed] [Google Scholar]
- 2.Yu H., Chen S., Cao P. Synergistic bactericidal effects and mechanisms of low intensity ultrasound and antibiotics against bacteria: A review. Ultrason. Sonochem. 2012;19(3):377–382. doi: 10.1016/j.ultsonch.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 3.Allen H.K., Levine U.Y., Looft T., Bandrick M., Casey T.A. Treatment, promotion, commotion: antibiotic alternatives in food-producing animals. Trends Microbiol. 2013;21:114–119. doi: 10.1016/j.tim.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Koluman A., Dikici A. Antimicrobial resistance of emerging foodborne pathogens: status quo and global trends. Crit. Rev. Microbiol. 2013;39(1):57–69. doi: 10.3109/1040841X.2012.691458. [DOI] [PubMed] [Google Scholar]
- 5.Serpe L., Giuntini F.J., Biology P.B. Sonodynamic antimicrobial chemotherapy: First steps towards a sound approach for microbe inactivation. J. Photochem. Photobiol. B, Biol. 2015;150:44–49. doi: 10.1016/j.jphotobiol.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 6.Ghate V.S., Zhou W., Yuk H.-G. Perspectives and trends in the application of photodynamic inactivation for microbiological food safety. Compr. Rev. Food Sci. Food Saf. 2019;18(2):402–424. doi: 10.1111/1541-4337.12418. [DOI] [PubMed] [Google Scholar]
- 7.Magaraggia M., Coppellotti O., Fabris C., Guidolin L., Jori G. Inactivation of microbial pathogens by photosensitized processes: environmental applications. photodynamic inactivation of microbial pathogens. 2011:403–423. doi: 10.1615/jenvironpatholtoxicoloncol.v30.i3.90. [DOI] [PubMed] [Google Scholar]
- 8.Wood R.W., Loomis A.L. The physical and biological effects of high-frequency sound-waves of great intensity. London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science. 1927;4:417–436. [Google Scholar]
- 9.Zhang Y.i., Zhang H., Zhuang D., Bi L., Hu Z., Cao W. Hematoporphyrin monomethyl ether mediated sonodynamic antimicrobial chemotherapy on porphyromonas gingivalis in vitro. Microb. Pathog. 2020;144:104192. doi: 10.1016/j.micpath.2020.104192. [DOI] [PubMed] [Google Scholar]
- 10.Wang X., Ip M., Leung A.W., Yang Z., Wang P., Zhang B., Ip S., Xu C. Sonodynamic action of curcumin on foodborne bacteria Bacillus cereus and Escherichia coli. Ultrasonics. 2015;62:75–79. doi: 10.1016/j.ultras.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Bhavya M.L., Hebbar H.U. Sono-Photodynamic inactivation of Escherichia coli and Staphylococcus aureus in Orange juice. Ultrason. Sonochem. 2019;57:108–115. doi: 10.1016/j.ultsonch.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Xu C., Dong J., Ip M., Wang X., Leung A.W. Sonodynamic action of chlorin e6 on Staphylococcus aureus and Escherichia coli. Ultrason. Sonochem. 2016;64:54–57. doi: 10.1016/j.ultras.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 13.Yang M., Xie S., Adhikari V.P., Dong Y.u., Du Y., Li D. The synergistic fungicidal effect of low-frequency and low-intensity ultrasound with amphotericin B-loaded nanoparticles on C. albicans in vitro. Int. J. Pharm. 2018;542(1-2):232–241. doi: 10.1016/j.ijpharm.2018.03.033. [DOI] [PubMed] [Google Scholar]
- 14.F. Harris, S.R. Dennison, D.A. Phoenix, Frederick Harris, S.R. Dennison, The antimicrobial effects of ultrasound, (2015).
- 15.Rengeng L., Qianyu Z., Yuehong L., Zhongzhong P., Libo L. Sonodynamic therapy, a treatment developing from photodynamic therapy. Photodiagnosis Photodyn. Ther. 2017;19:159–166. doi: 10.1016/j.pdpdt.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Chen H., Zhou X., Gao Y.u., Zheng B., Tang F., Huang J. Recent progress in development of new sonosensitizers for sonodynamic cancer therapy. Drug Discov. Today. 2014;19(4):502–509. doi: 10.1016/j.drudis.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 17.Cai Y., Wang J., Liu X.u., Wang R., Xia L. A review of the combination therapy of low frequency ultrasound with antibiotics. BioMed Res. Int. 2017;2017:1–14. doi: 10.1155/2017/2317846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lovell J.F., Liu T.W., Chen J., Zheng G. Activatable photosensitizers for imaging and therapy. Chem. Rev. 2010;110:2839–2857. doi: 10.1021/cr900236h. [DOI] [PubMed] [Google Scholar]
- 19.Lin X., Song J., Chen X., Yang H. Ultrasound-Activated sensitizers and applications. Angew. Chem. Int. Ed Engl. 2020;59:2–24. doi: 10.1002/anie.201906823. [DOI] [PubMed] [Google Scholar]
- 20.Choi V., Rajora M.A., Zheng G. Activating drugs with sound: mechanisms behind sonodynamic therapy and the role of nanomedicine. Bioconjug. Chem. 2020;31:967–989. doi: 10.1021/acs.bioconjchem.0c00029. [DOI] [PubMed] [Google Scholar]
- 21.Pang X., Xu C., Jiang Y., Xiao Q., Leung A.W. Natural products in the discovery of novel sonosensitizers. Pharmacol. Ther. 2016;162:144–151. doi: 10.1016/j.pharmthera.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 22.Wang Z., Liu C., Zhao Y., Hu M., Ma D., Zhang P.u., Xue Y., Li X. Photomagnetic nanoparticles in dual-modality imaging and photo-sonodynamic activity against bacteria. Chem. Eng. J. 2019;356:811–818. [Google Scholar]
- 23.Xu F., Hu M., Liu C., Choi S.K. Yolk-structured multifunctional up-conversion nanoparticles for synergistic photodynamic–sonodynamic antibacterial resistance therapy. Biomater. Sci. 2017;5(4):678–685. doi: 10.1039/c7bm00030h. [DOI] [PubMed] [Google Scholar]
- 24.Xu H., Zhang X., Han R., Yang P., Ma H., Song Y., Lu Z., Yin W., Wu X., Wang H. Nanoparticles in sonodynamic therapy: state of the art review. Ultrasonics. 2016;6:50697–50705. [Google Scholar]
- 25.Nakonechny F., Nisnevitch M., Nitzan Y., Nisnevitch M. Sonodynamic excitation of rose bengal for eradication of gram-positive and gram-negative bacteria. BioMed. Res. Int. 2013;2013:1–7. doi: 10.1155/2013/684930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Costley D., Nesbitt H., Ternan N., Dooley J. Sonodynamic inactivation of gram-positive and gram-negative bacteria using a rose bengal-antimicrobial peptide conjugate. Int. J. Antimicrob. Agents. 2017;49:31–36. doi: 10.1016/j.ijantimicag.2016.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu B., Wang D.-J., Liu B.-M., Wang X., He L.-L., Wang J., Xu S.-K. The influence of ultrasound on the fluoroquinolones antibacterial activity. Ultrason. Sonochem. 2011;18(5):1052–1056. doi: 10.1016/j.ultsonch.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 28.Drakopoulou S., Terzakis S., Fountoulakis M.S., Mantzavinos D., Manios T. Ultrasound-induced inactivation of gram-negative and gram-positive bacteria in secondary treated municipal wastewater. Ultrason. Sonochem. 2009;16(5):629–634. doi: 10.1016/j.ultsonch.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 29.Farshbaf Dadjour M., Ogino C., Matsumura S., Nakamura S., Shimizu N. Disinfection of Legionella pneumophila by ultrasonic treatment with TiO2. Water Res. 2006;40(6):1137–1142. doi: 10.1016/j.watres.2005.12.047. [DOI] [PubMed] [Google Scholar]
- 30.Kuroki M., Hachimine K., Abe H., Shibaguchi H., Kuroki M., Maekawa S.I., Yanagisawa J., Kinugasa T., Tanaka T., Yamashita Y. Sonodynamic therapy of cancer using novel sonosensitizers. Anticancer Res. 2007;27:3673–3677. [PubMed] [Google Scholar]
- 31.Gogate P.R., Kabadi A.M. A review of applications of cavitation in biochemical engineering/biotechnology. Biochem. Eng. J. 2009;44(1):60–72. [Google Scholar]
- 32.Hiller R.A., Putterman S.J., Weninger K.R. Time-resolved spectra of sonoluminescence. Phys. Rev. Lett. 1998;80(5):1090–1093. [Google Scholar]
- 33.Shibaguchi H., Tsuru H., Kuroki M., Kuroki M. Sonodynamic cancer therapy: A non-invasive and repeatable approach using low-intensity ultrasound with a sonosensitizer. Anticancer Res. 2011;31:2425–2429. [PubMed] [Google Scholar]
- 34.T. Leong, M. Ashokkumar, S. Kentish, The fundamentals of power ultrasound-a review, (2011).
- 35.Rooze J., Rebrov E.V., Schouten J.C., Keurentjes J.T.F. Dissolved gas and ultrasonic cavitation-a review. Ultrason. Sonochem. 2013;20(1):1–11. doi: 10.1016/j.ultsonch.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 36.Costley D., Mc Ewan C., Fowley C., McHale A.P., Atchison J., Nomikou N., Callan J.F. Treating cancer with sonodynamic therapy: A review. Int J Hyperthermia. 2015;31(2):107–117. doi: 10.3109/02656736.2014.992484. [DOI] [PubMed] [Google Scholar]
- 37.Castano A.P., Mroz P., Hamblin M.R. Photodynamic therapy and anti-tumour immunity, Int J Hyperthermia. Nat. Rev. Cancer. 2006;6(7):535–545. doi: 10.1038/nrc1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Di Ianni T., Bose R.J.C., Sukumar U.K., Bachawal S., Wang H., Telichko A., Herickhoff C., Robinson E., Baker S., Vilches-Moure J.G., Felt S.A., Gambhir S.S., Paulmurugan R., Dahl J.D. Ultrasound/microbubble-mediated targeted delivery of anticancer micro RNA-loaded nanoparticles to deep tissues in pigs. J Control Release. 2019;309:1–10. doi: 10.1016/j.jconrel.2019.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rahman M.M., Ninomiya K., Ogino C., Shimizu N. Ultrasound-induced membrane lipid peroxidation and cell damage of Escherichia coli in the presence of non-woven TiO2 fabrics. Ultrason. Sonochem. 2010;17(4):738–743. doi: 10.1016/j.ultsonch.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 40.Liu B., Wang J., Wang X., Liu B.-M., Kong Y.-M., Wang D., Xu S.-K. Spectrometric studies on the sonodynamic damage of protein in the presence of levofloxacin. J Fluoresc. 2010;20(5):985–992. doi: 10.1007/s10895-010-0645-x. [DOI] [PubMed] [Google Scholar]
- 41.Agrawal N., Ray R.S., Farooq M., Pant A.B., Hans R.K. Photosensitizing potential of ciprofloxacin at ambient level of UV radiation. Photochem. Photobiol. 2007;83:1226–1236. doi: 10.1562/2006-10-12-RA-1059. [DOI] [PubMed] [Google Scholar]
- 42.A. Dwivedi, S.F. Mujtaba, H.N. Kushwaha, D. Ali, N. Yadav, S. Singh, R.S. Ray, Photosensitizing mechanism and identification of levofloxacin photoproducts at ambient UV radiation, Photochem. Photobiol. 88 (2012) 344-355. [DOI] [PubMed]
- 43.Harris F., Dennison S.R., Phoenix D.A. Sounding the death knell for microbes. Trends Mol. Med. 2014;20(7):363–367. doi: 10.1016/j.molmed.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 44.V. Mišík, P. Riesz, Free radical intermediates in sonodynamic therapy, Ann. N. Y. Acad. Sci. 899 (2000) 335-348. [DOI] [PubMed]
- 45.Krasovitski B., Frenkel V., Shoham S., Kimmel E. Intramembrane cavitation as a unifying mechanism for ultrasound-induced bioeffects. Proc Natl Acad Sci. 2011;108(8):3258–3263. doi: 10.1073/pnas.1015771108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moan J., Berg K. The photodegradation of porphyrins in cells can be used to estimate the lifetime of singlet oxygen. Photochem. Photobiol. 1991;53(4):549–553. doi: 10.1111/j.1751-1097.1991.tb03669.x. [DOI] [PubMed] [Google Scholar]
- 47.Mišík V., Miyoshi N., Riesz P. EPR spin trapping study of the decomposition of azo compounds in aqueous solutions by ultrasound: potential for use as sonodynamic sensitizers for cell killing. Free Radic. Res. 1996;25:13–22. doi: 10.3109/10715769609145652. [DOI] [PubMed] [Google Scholar]
- 48.Worthington A., Thompson J., Rauth A., Hunt J. Mechanism of ultrasound enhanced porphyrin cytotoxicity. Part I: A search for free radical effects. Ultrasound Med. Biol. 1997;23:1095–1105. doi: 10.1016/s0301-5629(97)00019-7. [DOI] [PubMed] [Google Scholar]
- 49.Hiraoka W., Honda H., Feril L.B., Kudo N., Kondo T. Comparison between sonodynamic effect and photodynamic effect with photosensitizers on free radical formation and cell killing. Ultrason. Sonochem. 2006;13(6):535–542. doi: 10.1016/j.ultsonch.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 50.Runyan C.M., Carmen J.C., Beckstead B.L., Nelson J.L., Robison R.A., Pitt W.G. Low-frequency ultrasound increases outer membrane permeability of Pseudomonas aeruginosa. J. Gen. Appl. Microbiol. 2006;52(5):295–301. doi: 10.2323/jgam.52.295. [DOI] [PubMed] [Google Scholar]
- 51.Vatansever F., de Melo W.C., Avci P., Vecchio D., Sadasivam M., Gupta A., Chandran R., Karimi M., Parizotto N.A., Yin R. Antimicrobial strategies centered around reactive oxygen species-bactericidal antibiotics, photodynamic therapy, and beyond. FEMS Microbiol. Rev. 2013;37:955–989. doi: 10.1111/1574-6976.12026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miller M.W., Miller W.M., Battaglia L.F. Biological and environmental factors affecting ultrasound-induced hemolysis in vitro: 3. antioxidant (Trolox®) inclusion. Ultrasound Med. Biol. 2003;29(1):103–112. doi: 10.1016/s0301-5629(02)00661-0. [DOI] [PubMed] [Google Scholar]
- 53.Rapoport N., Smirnov A.I., Pitt W.G., Timoshin A.A. Bioreduction of tempone and spin-labeled gentamicin by gram-negative bacteria: kinetics and effect of ultrasound. Arch. Biochem. Biophys. 1999;362(2):233–241. doi: 10.1006/abbi.1998.1020. [DOI] [PubMed] [Google Scholar]
- 54.Reitman L.W., Char D.H., Bernstein E.F. Effect of α-tocopherol on lipid peroxide production and hemolysis following mechanical trauma to blood. J. Surg. Res. 1970;10(10):471–476. doi: 10.1016/0022-4804(70)90072-7. [DOI] [PubMed] [Google Scholar]
- 55.Wang X., Ip M., Leung A.W., Xu C. Xu, Sonodynamic inactivation of methicillin-resistant Staphylococcus aureus in planktonic condition by curcumin under ultrasound sonication. Ultrason. Sonochem. 2014;54(8):2109–2114. doi: 10.1016/j.ultras.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 56.Rotenberg M., Margalit R. Porphyrin-membrane interactions: Binding or partition. Biochim Biophys Acta Biomembr. 1987;905(1):173–180. doi: 10.1016/0005-2736(87)90021-6. [DOI] [PubMed] [Google Scholar]
- 57.Brovko L. Elsevier; 2010. Photodynamic treatment: a new efficient alternative for surface sanitation, Advances in food and nutrition research; pp. 119–147. [DOI] [PubMed] [Google Scholar]
- 58.Ayan I., Aslan G., Comelekoglu U., Yilmaz N., Colak M. The effect of low-intensity pulsed sound waves delivered by the Exogen device on Staphylococcus aureus morphology and genetics. Acta Orthop Traumatol Turc. 2008;42:272–277. doi: 10.3944/aott.2008.272. [DOI] [PubMed] [Google Scholar]
- 59.Zupanc M., Pandur Ž., Perdih T.S., Stopar D., Petkovšek M., Dular M. Effects of cavitation on different microorganisms: The current understanding of the mechanisms taking place behind the phenomenon, A review and proposals for further research. Ultrason. Sonochem. 2019;57:147–165. doi: 10.1016/j.ultsonch.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 60.Alves F., Pavarina A.C., Mima E.G.d.O., McHale A.P., Callan J.F. Antimicrobial sonodynamic and photodynamic therapies against Candida albicans. Biofouling. 2018;34(4):357–367. doi: 10.1080/08927014.2018.1439935. [DOI] [PubMed] [Google Scholar]
- 61.Pourhajibagher M., Rokn A.R., Barikani H.R., Bahador A. Photo-sonodynamic antimicrobial chemotherapy via chitosan nanoparticles-indocyanine green against polymicrobial periopathogenic biofilms: Ex vivo study on dental implants. Photodiagnosis Photodyn Ther. 2020;31:101834. doi: 10.1016/j.pdpdt.2020.101834. [DOI] [PubMed] [Google Scholar]
- 62.Carmen J., Roeder B., Nelson J., Robisonogilvie R., Robison R., Schaalje G., Pitt W. Treatment of biofilm infections on implants with low-frequency ultrasound and antibiotics. Am. J. Infect. Control. 2005;33(2):78–82. doi: 10.1016/j.ajic.2004.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li S., Zhu C., Fang S., Zhang W., He N., Xu W., Kong R., Shang X. Ultrasound microbubbles enhance human β-defensin 3 against biofilms. J. Surg. Res. 2015;199(2):458–469. doi: 10.1016/j.jss.2015.05.030. [DOI] [PubMed] [Google Scholar]
- 64.Fan L., Hou F., Muhammad A.I., Ruiling L.V., Watharkar R.B., Guo M., Ding T., Liu D. Synergistic inactivation and mechanism of thermal and ultrasound treatments against Bacillus subtilis spores. Food Res. Int. 2019;116:1094–1102. doi: 10.1016/j.foodres.2018.09.052. [DOI] [PubMed] [Google Scholar]
- 65.Fan L., Hou F., Muhammad A.I., Ismail B.B., Ding T., Liu D. Proteomic responses of spores of Bacillus subtilis to thermosonication involve large-scale alterations in metabolic pathways. Ultrason. Sonochem. 2020;64 doi: 10.1016/j.ultsonch.2020.104992. [DOI] [PubMed] [Google Scholar]
- 66.Preuß A., Saltsman I., Mahammed A., Pfitzner M., Goldberg I., Gross Z., Röder B.J. P.B. Photodynamic inactivation of mold fungi spores by newly developed charged corroles. J. Photochem. Photobiol. B, Biol. 2014;133:39–46. doi: 10.1016/j.jphotobiol.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 67.Konopka K., Goslinski T. Photodynamic therapy in dentistry. J. Dent. Res. 2007;86(8):694–707. doi: 10.1177/154405910708600803. [DOI] [PubMed] [Google Scholar]
- 68.Mohr H., Bachmann B., Klein-Struckmeier A., Lambrecht B. Virus inactivation of blood products by phenothiazine dyes and light. J. Photochem. Photobiol. C. 1997;65(3):441–445. doi: 10.1111/j.1751-1097.1997.tb08586.x. [DOI] [PubMed] [Google Scholar]
- 69.A. Zborowska, Rationale of photodynamic inactivation (PDI), sonodynamic therapy (SDT) and photodynamic therapy (PDT) in Coronavirus (COVID-19). DOI: 10.13140/RG.2.2.23171.04648.
- 70.Shevchenko S.N., Burkhardt M., Sheval E.V., Natashina U.A., Grosse C., Nikolaev A.L., Gopin A.V., Neugebauer U., Kudryavtsev A.A., Sivakov V. Antimicrobial effect of biocompatible silicon nanoparticles activated using therapeutic ultrasound. Langmuir. 2017;33(10):2603–2609. doi: 10.1021/acs.langmuir.6b04303. [DOI] [PubMed] [Google Scholar]
- 71.Rediske A.M., Roeder B.L., Brown M.K., Nelson J.L., Robison R.L., Draper D.O., Schaalje G.B., Robison R.A., Pitt W.G. Ultrasonic enhancement of antibiotic action on Escherichia coli biofilms: an in vivo model. Antimicrob. Agents Chemother. 1999;43(5):1211–1214. doi: 10.1128/aac.43.5.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hao D., Song Y., Che Z., Liu Q. biophysics, Calcium overload and in vitro apoptosis of the C6 glioma cells mediated by sonodynamic therapy (hematoporphyrin monomethyl ether and ultrasound. Cell Biochem. Biophys. 2014;70(2):1445–1452. doi: 10.1007/s12013-014-0081-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sun D., Pang X., Cheng Y., Ming J., Xiang S., Zhang C., Lv P., Chu C., Chen X., Liu G., Zheng N. Ultrasound-switchable nanozyme augments sonodynamic therapy against multidrug-resistant bacterial infection. ACS nano. 2020;14(2):2063–2076. doi: 10.1021/acsnano.9b08667. [DOI] [PubMed] [Google Scholar]
- 74.Wang Y., Sun Y., Liu S., Zhi L., Wang X. Preparation of sonoactivated TiO2-DVDMS nanocomposite for enhanced antibacterial activity. Ultrason. Sonochem. 2020;63 doi: 10.1016/j.ultsonch.2020.104968. [DOI] [PubMed] [Google Scholar]
- 75.Pourhajibagher M., Rahimi esboei B., Hodjat M., Bahador A. Sonodynamic excitation of nanomicelle curcumin for eradication of Streptococcus mutans under sonodynamic antimicrobial chemotherapy: Enhanced anti-caries activity of nanomicelle curcumin. Photodiagnosis Photodyn Ther. 2020;30:101780. doi: 10.1016/j.pdpdt.2020.101780. [DOI] [PubMed] [Google Scholar]
- 76.Wang X., Zhong X., Gong F., Chao Y., Cheng L. Cheng, Newly developed strategies for improving sonodynamic therapy. Mater Horiz. 2020;7(8):2028–2046. [Google Scholar]
- 77.Zhu H.-X., Cai X.-Z., Shi Z.-L., Hu B., Yan S.-G. Microbubble-mediated ultrasound enhances the lethal effect of gentamicin on planktonic Escherichia coli. Biomed. Res. Int. 2014;2014:1–7. doi: 10.1155/2014/142168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pan X., Wang W., Huang Z., Liu S., Guo J., Zhang F., Yuan H., Li X., Liu F., Liu H. MOF-derived double-layer hollow nanoparticles with oxygen generation for multimodal imaging-guided sonodynamic therapy. Angew. Chem. Int. Ed. Engl. 2020 doi: 10.1002/anie.202004894. [DOI] [PubMed] [Google Scholar]
- 79.Ma X., Pan H., Wu G., Yang Z., Yi J. Ultrasound may be exploited for the treatment of microbial diseases. Med. Hypotheses. 2009;73(1):18–19. doi: 10.1016/j.mehy.2009.01.033. [DOI] [PubMed] [Google Scholar]
- 80.Liu X., Yin H., Weng C.-X., Cai Y. Low-Frequency ultrasound enhances antimicrobial activity of colistin-vancomycin combination against pan-resistant biofilm of Acinetobacter baumannii. Ultrasound Med. Biol. 2016;42(8):1968–1975. doi: 10.1016/j.ultrasmedbio.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 81.Ensing G., Roeder B., Nelson J.L.E., Van Horn J., Van Der Mei H., Busscher H., Pitt W. Effect of pulsed ultrasound in combination with gentamicin on bacterial viability in biofilms on bone cements in vivo. J. Appl. Microbiol. 2005;99:443–448. doi: 10.1111/j.1365-2672.2005.02643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhuang D., Hou C., Bi L., Han J., Hao Y., Cao W., Zhou Q. Sonodynamic effects of hematoporphyrin monomethyl ether on Staphylococcus aureus in vitro. FEMS Microbiol. Lett. 2014;361(2):174–180. doi: 10.1111/1574-6968.12628. [DOI] [PubMed] [Google Scholar]
- 83.Wang P., Xu C., Xia X., Xu J., Wang X., Xiang J., Leung A.W. Mitochondrial damage in nasopharyngeal carcinoma cells induced by ultrasound radiation in the presence of hypocrellin B. J. Ultrasound Med. 2010;29:43–50. doi: 10.7863/jum.2010.29.1.43. [DOI] [PubMed] [Google Scholar]
- 84.Wang P., Xu C.S., Xu J., Wang X., Leung A.W. biology, Hypocrellin B enhances ultrasound-induced cell death of nasopharyngeal carcinoma cells. Ultrasound Med. Biol. 2010;36:336–342. doi: 10.1016/j.ultrasmedbio.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 85.Wang X., Ip M., Leung A.W., Wang P., Zhang H., Hua H., Xu C. Sonodynamic action of hypocrellin B on methicillin-resistant Staphylococcus aureus. Ultrason. Sonochem. 2016;65:137–144. doi: 10.1016/j.ultras.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 86.Gopin A., Mazina S., Nikolaev A. 13th Meeting of the European Society of Sonochemistry. 2012. Efficiency of the combined action of ultrasound and nanoparticles of different nature on bacteria. [Google Scholar]
- 87.Ma D., Green A.M., Willsey G.G., Marshall J.S., Wargo M.J., Wu J. Effects of acoustic streaming from moderate-intensity pulsed ultrasound for enhancing biofilm mitigation effectiveness of drug-loaded liposomes. J. Acoust. Soc. Am. 2015;138(2):1043–1051. doi: 10.1121/1.4927413. [DOI] [PubMed] [Google Scholar]
- 88.Zhao Y., Hu M., Zhang Y., Liu J., Liu C., Choi S.K., Zhang Z., Song L. Multifunctional therapeutic strategy of Ag-synergized dual-modality upconversion nanoparticles to achieve the rapid and sustained cidality of methicillin-resistant Staphylococcus aureus. Chem. Eng. J. 2020;385 [Google Scholar]
- 89.Alves F., Inada N.M., Bagnato V.S., Kurachi C. Paper presented at the 17th International Photodynamic Association World Congress. 2019. Sonophotodynamic Therapy for the inactivation of Staphylococcus aureus biofilm. [Google Scholar]
- 90.A.K. Seth, K.T. Nguyen, M.R. Geringer, S.J. Hong, K.P. Leung, T.A. Mustoe, R.D. Galiano, Noncontact, low‐frequency ultrasound as an effective therapy against P. seudomonas aeruginosa-infected biofilm wounds, Wound Repair and Regeneration, 21(2013) 266-274. [DOI] [PubMed]