Abstract
Introduction:
The present study investigated the association between protein intake and cognitive function in older adults.
Methods:
We performed a literature search with no restriction on publication year in MEDLINE, SCOPUS, CINAHL, AgeLine from inception up to October 2020. Observational studies that investigated as a primary or secondary outcome the association of protein intake and cognitive function in older adults aged ⩾60 years were included.
Results:
Nine cross-sectional studies that investigated a total of 4929 older adults were included in the qualitative analysis. Overall cognitive function was examined in 6 studies. Four investigations reported null associations and 2 studies found that older adults with a high protein intake had higher global cognitive function than their counterparts. Results from the meta-analysis suggested that there were no significant associations between protein consumption and global cognitive function in older adults, regardless of gender. Three studies investigated other cognitive domains. Memory and protein intake were significantly and positively correlated in all studies. In addition, visuospatial, verbal fluency, processing speed, and sustained attention were positively associated with protein consumption in 1 study each.
Conclusion:
No significant associations between protein intake and global cognitive function were observed in neither qualitative nor quantitative analyses. The association between protein consumption with multiple other cognitive domains were also tested. As a whole, 3 studies reported a positive and significant association between high protein intake and memory, while 1 study observed a significant and positive association with visuospatial, verbal fluency, processing speed, and sustained attention.
Keywords: Dementia, frailty, nutrition, elderly
Introduction
Cognition might be understood as the expression of brain activity by which the mind interacts with the world. 1 This concept involves many brain functions that allow human beings to create, execute, monitor, adjust, and perform many other tasks with a vital role in the proper accomplishment of dairy activities. 2 Cognitive function is not homogeneous throughout life so that it expands from the gestational period until adulthood, remains constant during adult life, and declines past the age of the sixth decade of life. 2
Such variations on cognition have a direct impact on older adults’ quality of life, given that cognitive decline is significantly associated with depression, falls, vehicle collisions, hospitalization, disability, mild cognitive impairment (MCI), and death.3-8 This scenario has attracted considerable attention of the World Health Organization (WHO), 9 which stated that the maintenance of cognitive function should be prioritized in older adults in an attempt to preserve their autonomy and avoid the genesis of chronic degenerative diseases.
Notably, lifestyle modifications have been recognized by the scientific community as possible tools to counteract age-related cognitive decline. Physical activity and exercise, for example, have been extensively examined and investigations have indicated that older adults engaged in exercise programs might benefit from improvements in cognition,10-12 although these findings were not unanimous. 13 Diet patterns have also been subject to investigation. According to recent systematic reviews,14,15 high adherence to the Mediterranean, Dietary Approach to Stop Hypertension (DASH), and the Mediterranean-DASH diet Intervention for Neurodegenerative Delay (MIND) diets might positively affect cognitive function in older people and likely slow or prevent cognitive decline.
However, a deeper look at the components of the diet, rather than the whole combination, might be necessary, given that the consumption of macronutrients can influence cognitive function. Particularly, the current recommendations for protein intake 16 have been under intense debate.17-19 Most of the criticism is based on the numerous investigations that have identified that a diary protein intake greater than the Recommended Dietary Allowances (RDA) can be required to maintain nitrogen balance and postpone age-related neuromuscular decline.20-23
In the last years, the association between protein intake and cognitive function has also gained considerable attention,24-32 and authors have claimed that a high consumption of proteins might contribute with a better cognitive functioning in older adults.32-34 These premises are based in the fact that increases in peripheral branched-chain amino acids (BCAA) content can contribute to the homeostasis of brain glutamate [GLU]. In addition, substantial evidence has accumulated that protein intake is significantly associated with numerous health-related parameters that might influence cognition, 34 including physical function,20-23 sleep quality,35,36 and microbiota. 37 Nonetheless, there is no consensus about the effects of consuming such amounts of protein on cognition.
Hence, the present systematic review investigated and combined the available evidence on the literature on the association between protein intake and cognitive function in older adults.
Methods
We conducted a systematic review of observational studies to assess the association between protein intake and cognitive function in older adults. The study was fully performed by investigators and no librarians were part of the team. This study complies with the criteria proposed by the Primary Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) Statement, 38 and the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines. 39
Eligibility criteria
The inclusion criteria consisted of: (a) Observational studies, including cross-sectional and case-control studies, which investigated as a primary or secondary outcome the association of protein intake and cognitive function in older adults free of dementia aged ⩾60 years. Longitudinal cohort studies were also included whether crude baseline data were available; (b) assessed at least one cognitive domain via validated questionnaires and tests; and (c) published studies (English, Italian, Portuguese, and Spanish languages). To be included in the meta-analysis, investigations must provide (d) mean and standard deviations (SD) of protein intake in both case (i.e. high cognitive function) and control groups (i.e. low cognitive function). We excluded observational studies that investigated diet patterns, as well as randomized-controlled trials (RCTs), quasi-experimental, cross-over studies, and any kind of investigation that examined the effects of nutritional interventions on cognition. Studies that enrolled people with mild cognitive impairment (MCI), dementia and/or gastrointestinal and/or renal diseases, anorexia, cancer, or any kind of condition that might directly impair protein metabolism (e.g. maple syrup urine disease, tyrosinemia) were also excluded.
Search strategy and selection criteria
Studies published on or before October 2020 were retrieved from the following 4 electronic databases by 2 investigators: (1) MEDLINE (PubMed interface); (2) SCOPUS (Elsevier interface); (3) CINAHL (EBSCO interface); (4) AgeLine (EBSCO interface). Reference lists for reviews and retrieved articles for additional studies were checked and citation searches on key articles were performed in Google Scholar and ResearchGate for additional reports. Initially, a search strategy was designed using keywords, MeSH terms, and free text words, such as protein consumption, cognitive function, older adults. Additionally, keywords and subject headings were exhaustively combined using Boolean operators. The PICO (i.e. Patient, Intervention, Comparison, and Outcome) method was used for literature search and the complete search strategy is shown in Table 1.
Table 1.
PICO strategy used for literature search.
| Patient | Intervention | Outcome |
|---|---|---|
| Aged [MESH] | Protein consumption | Cognition [MESH] |
| Older adults | Protein intake | Cognition |
| Elderly | Animal-protein | Cognitive function |
| Geriatrics# | Animal-based protein | Executive function |
| Seniors# | Plant-based protein | Problem solving |
| Aged 65+# | Plant-protein | Memory |
| Vegetal-protein | Attention | |
| Language | ||
| Visual perception | ||
| Brain function# |
[MESH] terms were only used for MEDLINE search. #These terms were only used for CINAHL and AgeLine searches.
Data extraction and quality assessment
Titles and abstracts of retrieved articles were screened for eligibility by 2 researchers. If an abstract did not provide enough information for evaluation, the full-text was retrieved. Disagreements were solved by a third reviewer. Reviewers were not blinded to authors, institutions, or manuscript journals. Data extraction was independently performed by 2 reviewers using a standardized coding form. Disagreements were solved by a third reviewer. Coded variables involved the characteristics of the studies and included: year, authors, country, study design, setting, sample size, mean age, female prevalence, dietary intake assessment method, protein intake, cognitive scores, cognitive tests, and cognitive functions. The quality of reporting for each study was performed by 2 researchers using the Study Assessment Tool for Observational Cohort and Cross-Sectional Studies developed by the National Heart, Lung, and Blood Institute (NHLBI). 40 The agreement rate between reviewers for the quality assessment was κ = 0.99.
Statistical analysis
The meta-analysis was conducted using Revman 5.4.1 (Cochrane Collaboration, Copenhagen, Denmark). Effect size (ES) was measured using Standard Mean Differences (SMD). When data were not made available by authors as means and SDs, they were calculated according to Cochrane guidelines. 41 Specifically, SDs were calculated from confidence intervals or standard errors and means were converted from medians. A single pairwise comparison was created when multiple studies referred to the same database using the formulas proposed by the Cochrane group. 41 Due to the variability of sample characteristics, a random-effect model was used to calculate the pooled ES. A sensitivity analysis was performed based on the stratification technique according to gender.
Results
Literature search
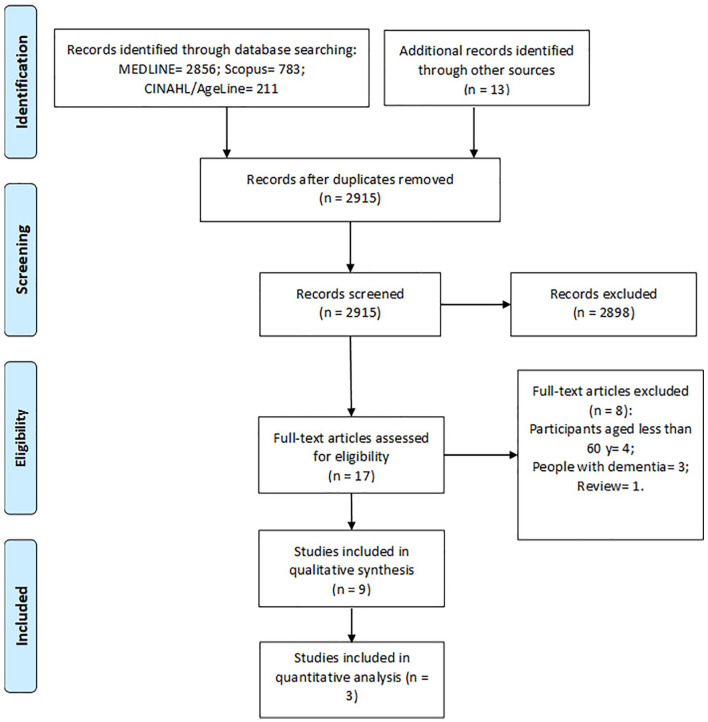
Of the 3863 registers recovered from electronic databases and hand search, 3846 records were excluded based on duplicate data, title, or abstract. Seventeen studies were fully reviewed and assessed for eligibility. Of these, 4 studies included people aged less than 60 years, 3 studies investigated people with dementia, and 1 study was a review of the literature. Finally, 9 studies were included in the qualitative synthesis. Three studies provided data to be included in the meta-analysis (Figure 1).
Figure 1.
Flowchart of the present study.
Characteristics of the included studies
Table 2 provides a general description of the included studies. Eight cross-sectional studies24-32 and 1 prospective24-32 study investigated a total of 4929 older adults from 6 different countries (i.e. France, Greece, Korea, Spain, Portugal, United States of America) between 1983 and 2020. Most studies were performed with community-dwellers,24-29,31,32 whereas 1 study investigated people resident in a nursing home. 30 The mean age of study participants was of approximately 71 years old and the mean prevalence of women in the samples ranged from 48.8% to 70.6%. Four studies reported values to calculate protein intake adjusted for body weight (g/kg of body weight [BW]/d), while the other 4 studies only provided data relative to crude protein intake per day. Mean protein intake values were higher than the RDA 16 and ranged from 0.90 g/kg of BW/d to 1.1 g/kg of BW/d. Dietary intake was assessed using 24-hour dietary recalls, 3-day dietary intake records, Food Frequency Questionnaires (FFQ), and precise weighing methods. Many cognitive tests were performed, including the Mini-Mental State Examination (MMSE), Short Portable Mental State Questionnaire, Word List Learning and Word List Recall Test from the Consortium to Establish a Registry for Alzheimer’s disease (CERAD), Pfeiffer’s Mental Status Questionnaire (PMSQ), Animal Fluency Test, Digit Symbol Substitution Test (DSST), The abstraction scale from the Shipley-Hartford Intelligence Test, The Logical Memory and Visual Reproduction subtests from the Wechsler Memory Scale (WMS), Rey-Osterrieth Complex Figure test, Wechsler Memory Test and Halstead-Reitan Categories Test. These tests were designed to evaluated overall cognitive function, immediate and delayed learning ability, nonverbal learning, verbal and nonverbal memory, short-term memory, working memory, verbal fluency, processing speed, problem-solving ability, sustained attention, and abstract reasoning.
Table 2.
General description of the included studies.
| Authors | Country | Study design | Setting | Sample size | Mean age | Female prevalence (%) | Dietary intake assessment method | Protein intake (g/kg of BW/day) | Cognitive scores | Cognitive tests | Cognitive functions |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Li et al 32 | USA | Cross-sectional | Community-dwelling | 2460 | 69.4 | 52.5 | 24-h dietary recall | 0.92 | Recall Score: 6.36; Delayed Recall Score: 6.01; Animal Fluency Score: 16.79; Digit Symbol Score: 46.83 | (a) Word List Learning Test, (b) Word List Recall Test, (c) Animal Fluency Test, (d) Digit Symbol Substitution Test | Immediate and delayed learning ability, verbal fluency, processing speed, sustained attention and working memory |
| Katsiardanis et al 31 | Greece | Cross-sectional | Community-dwelling | 557 | 65+ | 57.5 | Food Frequency Questionnaire | ~78.4* | MMSE: 22.7 in men; 21.1 in women | Mini-Mental State Examination | Global cognitive function |
| Vizuete et al 30 | Spain | Cross-sectional | Nursing home | 178 | ~81.6 | ~62.3 | Precise weighing methods and food record | ~1.0 | SPMSQ: 1.38 | Short Portable Mental State Questionnaire | Global cognitive function |
| Velho et al 29 | Portugal | Prospective | Community-dwelling | 187 | 69.7 | 70.6 | 3-d dietary intake record | ~75.1* | — | Mini-Mental State Examination | Global cognitive function |
| Lee et al 28 | Korea | Cross-sectional | Community-dwelling | 449 | ~70.9 | 53.3 | 24-h dietary recall | ~0.90 | MMSE: 23.9 in men; 21.65 in women | Mini-Mental State Examination | Global cognitive function |
| Ortega et al 27 | Spain | Cross-sectional | Community-dwelling | 260 | ~71.0 | 58.5 | Weighed-food record | ~1.1 | MMSE: 28.6 in men; 26.2 in women | Mini-Mental State Examination | Global cognitive function |
| La Rue et al 26 | USA | Cross-sectional | Community-dwelling | 137 | 76.9 | 51.1 | 3-d dietary intake record | 72* | Rey-Osterrieth Complex Figure, copy: 31.0; recall: 14.7; Wechsler Memory Scale Visual Reproduction: 6.0; Logical Memory: 7.2; Shipley-Hartford Abstraction: 10.4 | (a) Abstraction scale, (b) Logical memory test, (c) Visual reproduction, (d) Rey-Osterrieth Complex Figure test | Abstract reasoning, verbal and nonverbal memory, nonverbal learning and memory |
| Pradignac et al 25 | France | Cross-sectional | Community-dwelling | 441 | 76.3 | 48.8 | 3-d dietary intake record | 66.6* | — | Mini-Mental State Examination | Global cognitive function |
| Goodwin et al 24 | USA | Cross-sectional | Community-dwelling | 260 | ~71.7 | 54.2 | 3-d dietary intake record | — | — | (a) Wechsler Memry Test and (b) Halstead-Reitan Categories Test | Short-term memory, abstract reasoning and problem solving ability |
Abbreviations: BW, body weight; USA, United States of America.
g/d.
Quality assessment
The qualitative analysis of the included studies is shown in Table 3. The overall quantitative score ranged from 5 to 9 of 12 possible points, while the qualitative score ranged from fair to good. All studies clearly stated their objectives (item 1), specified the studied population (item 2), recruited study participants in the same or similar population (item 4), and described and used valid and reliable instruments to assess protein intake (item 9) and cognitive function (item 11). Items 6 and 7 refer to parameters associated with cohort studies and therefore were not reported in any of the included studies. The participation rate of the eligible person (item 3) and loss to follow-up after baseline (item 13) were only reported in 22% of the studies each. Different levels of exposure (item 8), in this case evaluated according to the sources of protein, were also only investigated in 2 studies (22%). Sample size (item 5) was only justified in one (11.1%) study. Similarly, just 11.1% of the studies described if assessors were blinded to exposure status (item 12). The most heterogeneous result is regarding the adjustment for potential confounding variables (item 14), given that this variable was controlled in 44.4% of the studies. None of the studies assessed exposure more than once (item 10).
Table 3.
Study quality.
| Study | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | Overall score (0/12) | Overall score (qualitative) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Li et al 32 | Y | Y | Y | Y | N | N | N | N | Y | NA | Y | Y | NA | Y | 8 | Good |
| Katsiardanis et al 31 | Y | Y | Y | Y | N | N | N | N | Y | NA | Y | NR | NA | Y | 7 | Good |
| Vizuete et al 30 | Y | Y | NR | Y | N | N | N | N | Y | NA | Y | NR | NA | N | 6 | Fair |
| Velho et al 29 | Y | Y | NR | Y | N | N | N | N | Y | NA | Y | NR | Y | Y | 7 | Good |
| Lee et al 28 | Y | Y | NR | Y | N | N | N | Y | Y | NA | Y | NR | Y | N | 7 | Fair |
| Ortega et al 27 | Y | Y | NR | Y | Y | N | N | N | Y | NA | Y | NR | NR | N | 9 | Fair |
| La Rue et al 26 | Y | Y | NR | Y | N | N | N | N | Y | NA | Y | NR | NR | N | 5 | Fair |
| Pradignac et al 25 | Y | Y | NR | Y | N | N | N | Y | Y | NA | Y | NR | NR | Y | 7 | Good |
| Goodwin et al 24 | Y | Y | NR | Y | N | N | N | N | Y | NA | Y | NR | NR | N | 5 | Fair |
Abbreviations: N, No; NA, not applied; NR, not reported; Y, Yes.
1. Was the research question or objective in this paper clearly stated?; 2. Was the study population clearly specified and defined?; 3. Was the participation rate of eligible persons at least 50%?; 4. Were all the subjects selected or recruited from the same or similar populations (including the same time period)? Were inclusion and exclusion criteria for being in the study prespecified and applied uniformly to all participants?; 5. Was a sample size justification, power description, or variance and effect estimates provided?; 6. For the analyses in this paper, were the exposure(s) of interest measured prior to the outcome(s) being measured?; 7. Was the timeframe sufficient so that one could reasonably expect to see an association between exposure and outcome if it existed?; 8. For exposures that can vary in amount or level, did the study examine different levels of the exposure as related to the outcome (eg, categories of exposure, or exposure measured as continuous variable)?; 9. Were the exposure measures (independent variables) clearly defined, valid, reliable, and implemented consistently across all study participants?; 10. Was the exposure(s) assessed more than once over time?; 11. Were the outcome measures (dependent variables) clearly defined, valid, reliable, and implemented consistently across all study participants?; 12. Were the outcome assessors blinded to the exposure status of participants?; 13. Was loss to follow-up after baseline 20% or less?; 14. Were key potential confounding variables measured and adjusted statistically for their impact on the relationship between exposure(s) and outcome(s)?.
Association between protein intake and overall cognitive function
Six studies25,27-31 investigated the association between protein intake and overall cognitive function in older adults. Study results were highly heterogeneous. Four studies25,27,29,31 observed null associations. Pradignac et al 25 studied apparently healthy community-dwelling French older adults and observed no significant associations between protein intake and global cognitive function. Similar results were found by Velho et al 29 in physically active Portuguese older people. In older adults from a rural Greece area, Katsiardanis et al 31 did not observe significant associations between diary protein intake and global cognitive scores. Ortega et al 27 confirmed and expanded these findings by indicating that protein consumption was not associated with neither MMSE nor PMSQ scores. In contrast, 2 studies28,30 reported positive associations. Lee et al 28 found a significant association between MMSE scores and total and vegetal protein intakes in older women, but not men, who participated of social and physical activities in a welfare center. One study investigated institutionalized older adults without a clinical diagnosis of MCI and/or dementia. In this study, Vizuete et al 30 reported better scores on PMSQ as protein intake increased.
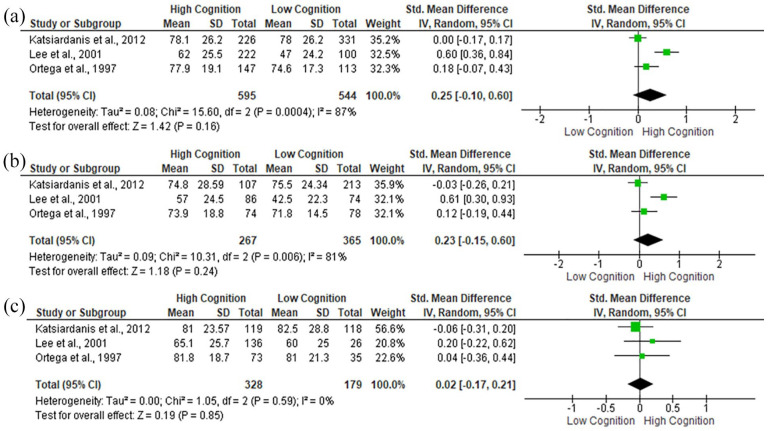
Only 3 studies27,28,31 provided data to be included in the meta-analysis (Figure 2). No significant associations between global cognitive function and protein intake were observed, regardless of gender.
Figure 2.
Standard Mean Differences (SMD) in global cognitive function according to protein intake in: (a) all participants, (b) women, and (c) men.
Association between protein intake and other cognitive domains
Three studies24,26,32 investigated the association between protein intake and immediate and delayed learning ability, nonverbal learning, verbal and nonverbal memory, short-term memory, working memory, verbal fluency, processing speed, problem-solving ability, sustained attention, and abstract reasoning in older adults. Goodwin et al 24 studied older adults who living independently and observed that those subjects in the bottom 5% or 10% of protein intake tended to do poorly on Halstead-Reitan Categories Test, which reflects abstract thinking and problem-solving ability, and WMS, a test designed to measure different subdomains of memory. However, results were only significant for the memory test. La Rue et al 26 confirmed these findings by indicating a positive association between protein intake and Rey-Osterrieth Recall, representing a measurement of visuospatial skill, and WMS logical memory scores in apparently healthy older adults of the New Mexico Aging Process Study. However, no significant correlations were found with Rey-Osterrieth Copy, which assess visuospatial skill and nonverbal memory, and the Shipley-Hartford Intelligence Test, assessing abstract reasoning. Li et al 32 investigated data of the National Health and Nutrition Examination Survey (NHANES), which contained multiple cognitive tests. Authors noted that a protein intake higher than 0.6 g/kg of BW per day was significantly associated with a better performance on the Animal Fluency Test, designed to examines categorical verbal fluency, and Digit Symbol Score, designed to assess processing speed, sustained attention and working memory regardless of gender.
Overall, 3 studies24,26,32 indicated that a high protein intake was positively associated with memory, assessed according to different assessment tools; 1 study 26 observed a significant association with visuospatial skill; and 1 study 32 reported a positive correlation with categorical verbal fluency, processing speed, and sustained attention.
Discussion
The present systematic study investigated the association between protein intake and cognitive function in older adults. After screening 2915 records, 9 cross-sectional studies fulfilled all the requirements to be included in the present investigation. Examined studies varied in many methodological aspects, including sample size, dietary intake assessment method, cognitive tests, adjusting factors, and statistical approach. Results of the included investigations were also highly heterogeneous, with 4 studies25,27,29,31 reporting null associations, whereas 2 investigations28,30 found a significant positive association between protein intake and global cognitive function. No significant associations were observed by pooling data in the meta-analysis. Though, only 3 studies were included. The association between protein consumption with multiple other cognitive domains were also tested. As a whole, 3 studies24,26,32 reported a positive and significant association between high protein intake and memory; 1 study 26 observed a significant and positive association with visuospatial skills; and 1 study 32 reported a positive correlation with categorical verbal fluency, processing speed, and sustained attention. However, no significant associations were observed with many other tests, including Halstead-Reitan Categories Test, Rey-Osterrieth Copy, and the Shipley-Hartford Intelligence Test.
The quality analysis revealed that most investigations failed to report many important methodological aspects, including participation rate of the eligible person, loss to follow-up after baseline, sample size calculation, blindness, different levels of exposure, and the trustworthiness of the exposure.
Particularly, the level of exposure, which was evaluated according to the source of protein, was only investigated in 2 studies. Lee et al 28 examined community-dwelling Korean older adults and reported that both total and vegetal protein, but not animal protein, were significantly associated with global cognitive function in women. In contrast, Pradignac et al 25 did not observe a significant association between protein-related parameters and cognitive function in a sample of community-dwelling French older adults.
This nutritional aspect deserves concern, given that animal protein is expected to provide higher amounts of BCAA than vegetal protein.42-44 BCAAs are directly and indirectly involved in numerous metabolic processes in the neural system.45-49 However, much attention has been paid to their effects on GLU metabolism, given that it might serve as a possible mechanistic explanation for the association between protein intake and cognitive function. 49
GLU is the major excitatory transmitter in the mammalian central nervous system.50-53 This molecule might impact neuronal differentiation, migration, and survival during maturation and cognitive functioning throughout life.50-53 The hypothesis that GLU can affect cognition is based on the fact that its receptors are widely distributed in pre- and post-synaptic neurons, influencing neuronal communication and signal processing.50-53 Particularly, N-methyl-D-aspartate (NMDA), one of the 3 types of ionotropic GLU receptors, is the predominant molecular device for controlling synaptic plasticity and for the proper formation of memory.54,55
GLU concentrations in the brain are tightly controlled. 45 In the intra-neuronal space, GLU supply must be kept constant and at optimum concentrations to maintain neuronal depolarization. On the other hand, high GLU concentrations in the extracellular space might induce excitotoxic injury and even kill susceptible neurons. 56 Hence, the transport of GLU across the blood brain barrier (BBB) and the synaptic space is limited to promote proper cerebral functioning and minimize the risk of harmful effects. 45
GLU concentrations are also regulated by the GLU/glutamine (GLU/GLN) cycle. 45 During neurotransmission, GLU is released in the synapses through a calcium-dependent process that involves the fusion of GLU-containing presynaptic vesicles with the neuronal membrane. 56 Not all GLU is uptake into the postsynaptic compartment and reuptake into presynaptic neurons.45,56 This scenario is problematic to the brain, which must keep GLU levels continuous for new neurotransmission and avoid its traffic to the extracellular fluid. 45 Astrocytes, star-shaped glial cells involved in many neurological functions, such as biochemical homeostasis, sequestrate GLU from the synaptic space by the use of GLU-specific transporters and convert it into a non-neuroactive component GLN via the glutamine synthetase enzyme.45,56
Then, GLN is released to the extracellular space when it cannot cause depolarization or harmful effects. 45 Finally, GLN is reuptake into nerve endings via sodium-dependent and independent mechanisms where it is converted back into GLU via glutaminase enzyme.45,56
However, it is important to note that the current understanding of the GLU/GLA cycle has been believed to be limited, simplified, and incomplete to explain how the brain compensates GLU catabolism. 46 Hence, Yudkoff 46 proposed that peripheral BCAA is required to cross the BBB to provide the necessary amount of nitrogen to keep GLU synthesis at adequate rates. This mechanism involves the neural transamination of BCAA by cytosolic and mitochondrial enzymes, leading to the formation of GLU and α-ketoisocaproate. 46 The role of this model on proper cognitive functioning seems to increase with age, given that age-related cognitive decline can be at least partially explained by changes in GLU concentrations and transportation in central areas dedicated to learning and memory.57-59
These premises suggest that the maintaining of GLU homeostasis in the brain due to an adequate peripheral BCAA content might explain the significant and positive associations between high protein intake and some cognitive domains. Particularly, 3 studies24,26,32 reported that older adults with a high protein consumption had better results in memory tests, and multiple studies have shown that the activation of NMDA receptors is required to induce long-term potentiation (LTP),51,54,60-62 a neuronal event that represents an increased synaptic transmission among neurons argued to be the main mechanism underlying memory encoding. In contrast, LTP in cortical and hippocampal (i.e. CA1 and dental gyrus) areas was blocked by antagonists of the NMDA receptors.60-62
No significant associations between protein intake and global cognitive function were observed in neither qualitative nor quantitative analyses. The main reason why high protein intake might benefit some cognitive domains, but not overall cognitive function is unknown. However, it is important to note that our results were based in a small number of studies and inferences must be made with caution, given the high variability in measuring methods, study outcomes, and participant characteristics, besides the lack of sensitivity analysis in most of the studies.
Indeed, global cognitive function were assessed using MMSE and PMSQ. Although both tests claim to measure the same cognitive domain, only a moderate agreement has been observed among the scales for screening negative events,63,64 which suggests that MMSE and PMSQ capture different dimensions of cognition. Moreover, each test involves recognized limitations. The MMSE, for example, has a ceiling effect and poor content validity in assessing language, visuospatial, and executive functions,65,66 while PMSQ presents a low sensitivity and specificity for screening some cases of dementia, which led some authors to suggest that this tool should not be used alone in certain clinical settings.63,64
Another important aspect involving cognitive assessment is that most cognitive tests do not assess only 1 cognitive domain, but involves 2 or more areas of cognition, limiting conclusions about specific cognitive functions. 67 The DSST, for example, was initially designed to assess associative learning. 68 Currently, it is accept that the performance on the DSST test correlates with memory, motor speed, attention, and visuoperceptual functions. 68
Five different dietary assessment methods (i.e. 3-day dietary intake, 24-hour dietary recall, FFQ, weighed-food record, and precise weighing methods and food record) were used in the studies that investigated global cognitive function. All these methods have strengths and limitations, and specific guidelines for guide their application in older adults are still missing. 69 Hence, experts in the field 69 argued that the reliability of dietary information is also dependent on the approach used to collect data, so that long interviews and questionnaires may be stressful to many older adults, leading to incomplete or unreliable results.
The lack of adjust of the results for potential confounding variables by using complex statistical methods (e.g. multiple regression) is one more potential source of heterogeneity. In fact, most of the included studies based their conclusions on the differences between the means of 2 groups, whereas 1 study 32 provided adjusted odds ratio. Notably, the effects of protein intake on cognition in older adults might be influenced by its relationship with numerous health-related parameters (to review see Glenn et al 34 ). Physical dysfunction70,71 and physical frailty,72,73 for example, are predictors of cognitive decline in older adults, and numerous studies have supported a significant relationship between protein intake and physical performance.20-23 Acute and long-term sleep deprivation impairs memory, language, executive function, and attention domains, 74 and are highly frequent in people with dementia. 75 Besides, cross-sectional studies using nationally representative samples have observed that people with sleep disturbance had a low protein intake.35,36 More recently, the role of microbiota on cognitive impairment 76 and severe mental disorders 77 has received increased attention. These data have important clinical implications since protein intake and microbiota are interconnected so that the structure and function of the microbiome might be impacted by protein quality, while it influences protein catabolism, digestion, and absorption. 37
Taken together, these observations indicate the future studies should investigate global cognition using standard methods with high sensitivity and specificity to predict negative outcomes, utilize validated methods to assess dietary intake and provide a detailed description of the approach used to data collection, and adjust findings by potential confounding factors according to the use of sensitive statistical methods. Preferably, studies should be conducted taken into consideration the current literature and assess the main variables using the same or similar methods to allow comparisons and better inferences. Finally, studies investigating other cognitive domains (e.g. executive function) are still needed.
This study is not free of limitations. First, we only included cross-sectional studies, limiting the establishment of a cause-effect relationship. Second, our sample was composed exclusively of older adults free of dementia and inferences to other populations must be made with caution. Third, most studies provided data of total protein intake. However, numerous studies and experts in the field have suggested that other parameters, including protein intake adjusted by BW, protein distribution across the meals, and protein sources might provide a clearer understanding of protein consumption.20-23,78,79
Conclusion
The association between protein intake and cognition in older adults has attracted considerable attention. However, no specific recommendations are available. In the present study, no significant associations between protein intake and global cognitive function were observed in neither qualitative nor quantitative analyses. The association between protein consumption with multiple other cognitive domains were also tested. As a whole, 3 studies reported a positive and significant association between high protein intake and memory; 1 study observed a significant and positive association with visuospatial skills; and 1 study reported a positive correlation with categorical verbal fluency, processing speed, and sustained attention.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Innovative Medicines Initiative–Joint Undertaking [IMI-JU 115621], the nonprofit research foundation “Centro Studi Achille e Linda Lorenzon”, and by a scholarship to H.J.C.-J. from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior [CAPES; Finance Code 001]. The funders had no role in study design, data collection and analysis, preparation of the manuscript, or decision to publish.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iDs: Hélio José Coelho-Júnior  https://orcid.org/0000-0001-7482-9514
https://orcid.org/0000-0001-7482-9514
Emanuele Marzetti  https://orcid.org/0000-0001-9567-6983
https://orcid.org/0000-0001-9567-6983
References
- 1. American Psychological Association (APA). Dictionary of Psychology. Accessed April 9, 2021. https://dictionary.apa.org/cognition
- 2. Murman DL. The impact of age on cognition. Semin Hear. 2015;36:111-121. doi: 10.1055/s-0035-1555115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lee JH, Sung J, Choi MK. The factors associated with subjective cognitive decline and cognitive function among older adults. J Adv Nurs. 2020;76:555-565. doi: 10.1111/jan.14261 [DOI] [PubMed] [Google Scholar]
- 4. Montero-Odasso M, Speechley M. Falls in cognitively impaired older adults: implications for risk assessment and prevention. J Am Geriatr Soc. 2018;66:367-375. doi: 10.1111/jgs.15219 [DOI] [PubMed] [Google Scholar]
- 5. Fraade-Blanar LA, Ebel BE, Larson EB, et al. Cognitive decline and older driver crash Risk. J Am Geriatr Soc. 2018;66:1075-1081. doi: 10.1111/jgs.15378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brigola AG, Ottaviani AC, Alexandre T, da S, Luchesi BM, Pavarini SCI. Cumulative effects of cognitive impairment and frailty on functional decline, falls and hospitalization: a four-year follow-up study with older adults. Arch Gerontol Geriatr. 2020;87:104005. doi: 10.1016/j.archger.2019.104005 [DOI] [PubMed] [Google Scholar]
- 7. van Harten AC, Mielke MM, Swenson-Dravis DM, et al. Subjective cognitive decline and risk of MCI: the Mayo Clinic Study of Aging. Neurology. 2018;91:e300-e312. doi: 10.1212/WNL.0000000000005863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lv X, Li W, Ma Y, et al. Cognitive decline and mortality among community-dwelling Chinese older people. BMC Med. 2019;17:63. doi: 10.1186/s12916-019-1295-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. World Health Organization (WHO). Risk reduction of cognitive decline and dementia. 2019. Accessed February 2, 2021. https://www.who.int/publications/i/item/risk-reduction-of-cognitive-decline-and-dementia [PubMed]
- 10. Erickson KI, Hillman C, Stillman CM, et al. Physical activity, cognition, and brain outcomes. Med Sci Sports Exerc. 2019;51:1242-1251. doi: 10.1249/MSS.0000000000001936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee J. The relationship between physical activity and dementia: a systematic review and meta-analysis of prospective cohort studies. J Gerontol Nurs. 2018;44:22-29. doi: 10.3928/00989134-20180814-01 [DOI] [PubMed] [Google Scholar]
- 12. Coelho-Júnior HJ, Oliveira Gonçalves I de, Sampaio RAC, et al. Effects of combined resistance and power training on cognitive function in older women: a randomized controlled trial. Int J Environ Res Public Health. 2020;17:3435. doi: 10.3390/ijerph17103435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Coelho-Junior H, Marzetti E, Calvani R, Picca A, Arai H, Uchida M. Resistance training improves cognitive function in older adults with different cognitive status: a systematic review and meta-analysis. Aging Ment Health. Published online December16, 2020. doi: 10.1080/13607863.2020.1857691 [DOI] [PubMed] [Google Scholar]
- 14. Lourida I, Soni M, Thompson-Coon J, et al. Mediterranean diet, cognitive function, and dementia: a systematic review. Epidemiology. 2013;24:479-489. doi: 10.1097/EDE.0b013e3182944410 [DOI] [PubMed] [Google Scholar]
- 15. Chen X, Maguire B, Brodaty H, O’Leary F. Dietary patterns and cognitive health in older adults: a systematic review. J Alzheimers Dis. 2019;67:583-619. doi: 10.3233/JAD-180468 [DOI] [PubMed] [Google Scholar]
- 16. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients) . National Academies Press; 2005. doi: 10.17226/10490 [DOI] [PubMed] [Google Scholar]
- 17. Volpi E, Campbell WW, Dwyer JT, et al. Is the optimal level of protein intake for older adults greater than the recommended dietary allowance? J Gerontol A Biol Sci Med Sci. 2013;68:677-681. doi: 10.1093/gerona/gls229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bauer J, Biolo G, Cederholm T, et al. Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the prot-age study group. J Am Med Dir Assoc. 2013;14:542-559. doi: 10.1016/j.jamda.2013.05.021 [DOI] [PubMed] [Google Scholar]
- 19. Calvani R, Miccheli A, Landi F, et al. Current nutritional recommendations and novel dietary strategies to manage sarcopenia. J Frailty Aging. 2013;2:38-53. [PMC free article] [PubMed] [Google Scholar]
- 20. Coelho-Junior HJ, Calvani R, Gonçalves IO, et al. High relative consumption of vegetable protein is associated with faster walking speed in well-functioning older adults. Aging Clin Exp Res. 2019;31:837-844. doi: 10.1007/s40520-019-01216-4 [DOI] [PubMed] [Google Scholar]
- 21. Coelho-Junior HJ, Calvani R, Picca A, et al. Association between dietary habits and physical function in brazilian and italian older women. Nutrients. 2020;12:1635. doi: 10.3390/nu12061635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Coelho-Júnior H, Rodrigues B, Uchida M, et al. Low protein intake is associated with frailty in older adults: a systematic review and meta-analysis of observational studies. Nutrients. 2018;10:1334. doi: 10.3390/nu10091334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Coelho-Júnior H, Milano-Teixeira L, Rodrigues B, Bacurau R, Marzetti E, Uchida M. Relative protein intake and physical function in older adults: a systematic review and meta-analysis of observational studies. Nutrients. 2018;10:1330. doi: 10.3390/nu10091330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Goodwin JS, Goodwin JM, Garry PJ. Association between nutritional status and cognitive functioning in a healthy elderly population. JAMA. 1983;249:2917-2921. doi: 10.1001/jama.1983.03330450047024 [DOI] [PubMed] [Google Scholar]
- 25. Pradignac A, Schlienger JL, Velten M, Mejean L. Relationships between macronutrient intake, handicaps, and cognitive impairments in free living elderly people. Aging Clin Exp Res. 1995;7:67-74. doi: 10.1007/BF03324295 [DOI] [PubMed] [Google Scholar]
- 26. La Rue A, Koehler KM, Wayne SJ, Chiulli SJ, Haaland KY, Garry PJ. Nutritional status and cognitive functioning in a normally aging sample: a 6-y reassessment. Am J Clin Nutr. 1997;65:20-29. doi: 10.1093/ajcn/65.1.20 [DOI] [PubMed] [Google Scholar]
- 27. Ortega RM, Requejo AM, Andrés P, et al. Dietary intake and cognitive function in a group of elderly people. Am J Clin Nutr. 1997;66:803-809. doi: 10.1093/ajcn/66.4.803 [DOI] [PubMed] [Google Scholar]
- 28. Lee L, Kang S, Lee H, et al. Relationships between dietary intake and cognitive function level in Korean elderly people. Public Health. 2001;115:133-138. doi: 10.1038/sj.ph.1900729 [DOI] [PubMed] [Google Scholar]
- 29. Velho S, Marques-Vidal P, Baptista F, Camilo ME. Dietary intake adequacy and cognitive function in free-living active elderly: a cross-sectional and short-term prospective study. Clin Nutr. 2008;27:77-86. doi: 10.1016/j.clnu.2007.10.011 [DOI] [PubMed] [Google Scholar]
- 30. Vizuete AA, Robles F, Rodríguez-Rodríguez E, López-Sobaler AM, Ortega RM. Association between food and nutrient intakes and cognitive capacity in a group of institutionalized elderly people. Eur J Nutr. 2010;49:293-300. doi: 10.1007/s00394-009-0086-y [DOI] [PubMed] [Google Scholar]
- 31. Katsiardanis K, Diamantaras AA, Dessypris N, et al. Cognitive impairment and dietary habits among elders: the velestino study. J Med Food. 2013;16:343-350. doi: 10.1089/jmf.2012.0225 [DOI] [PubMed] [Google Scholar]
- 32. Li Y, Li S, Wang W, Zhang D. Association between dietary protein intake and cognitive function in adults aged 60 years and older. J Nutr Health Aging. 2020;24:223-229. doi: 10.1007/s12603-020-1317-4 [DOI] [PubMed] [Google Scholar]
- 33. Koh F, Charlton K, Walton K, McMahon A-T. Role of dietary protein and thiamine intakes on cognitive function in healthy older people: a systematic review. Nutrients. 2015;7:2415-2439. doi: 10.3390/nu7042415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Glenn JM, Madero EN, Bott NT. Dietary protein and amino acid intake: links to the maintenance of cognitive health. Nutrients. 2019;11:1315. doi: 10.3390/nu11061315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kant AK, Graubard BI. Association of self-reported sleep duration with eating behaviors of American adults: NHANES 2005–2010. Am J Clin Nutr. 2014;100:938-947. doi: 10.3945/ajcn.114.085191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Grandner MA, Jackson N, Gerstner JR, Knutson KL. Dietary nutrients associated with short and long sleep duration. Data from a nationally representative sample. Appetite. 2013;64:71-80. doi: 10.1016/j.appet.2013.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhao J, Zhang X, Liu H, Brown MA, Qiao S. Dietary protein and gut microbiota composition and function. Curr Protein Pept Sci. 2018;20:145-154. doi: 10.2174/1389203719666180514145437 [DOI] [PubMed] [Google Scholar]
- 38. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. doi: 10.1371/journal.pmed.1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008-2012. doi: 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 40. NHLBI, NIH. Study quality assessment tools. Accessed October 25, 2020. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools
- 41. Green S, Higgins J. Cochrane handbook for systematic reviews of interventions. Published online 2005. Accessed July 21, 2018. https://training.cochrane.org/handbook
- 42. van Vliet S, Burd NA, van Loon LJ. The skeletal muscle anabolic response to plant- versus animal-based protein consumption. J Nutr. 2015;145:1981-1991. doi: 10.3945/jn.114.204305 [DOI] [PubMed] [Google Scholar]
- 43. Landi F, Calvani R, Tosato M, et al. Protein intake and muscle health in old age: from biological plausibility to clinical evidence. Nutrients. 2016;8:295. doi: 10.3390/nu8050295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Coelho-Junior HJ, Marzetti E, Picca A, Cesari M, Uchida MC, Calvani R. Protein intake and frailty: a matter of quantity, quality, and timing. Nutrients. 2020;12:1-20. doi: 10.3390/nu12102915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sperringer JE, Addington A, Hutson SM. Branched-chain amino acids and brain metabolism. Neurochem Res. 2017;42:1697-1709. doi: 10.1007/s11064-017-2261-5 [DOI] [PubMed] [Google Scholar]
- 46. Yudkoff M. Interactions in the metabolism of glutamate and the branched-chain amino acids and ketoacids in the CNS. Neurochem Res. 2017;42:10-18. doi: 10.1007/s11064-016-2057-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Griffin JWD, Bradshaw PC. Amino acid catabolism in Alzheimer’s disease brain: friend or foe? Oxid Med Cell Longev. 2017;2017:5472792. doi: 10.1155/2017/5472792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fernstrom JD. Large neutral amino acids: dietary effects on brain neurochemistry and function. Amino Acids. 2013;45:419-430. doi: 10.1007/s00726-012-1330-y [DOI] [PubMed] [Google Scholar]
- 49. Fernstrom JD. Branched-chain amino acids and brain function. J Nutr. 2005;135:1539S-1546S. doi: 10.1093/jn/135.6.1539s [DOI] [PubMed] [Google Scholar]
- 50. Zhou Y, Danbolt NC. Glutamate as a neurotransmitter in the healthy brain. J Neural Transm. 2014;121:799-817. doi: 10.1007/s00702-014-1180-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Riedel G, Platt B, Micheau J. Glutamate receptor function in learning and memory. Behav Brain Res. 2003;140:1-47. doi: 10.1016/S0166-4328(02)00272-3 [DOI] [PubMed] [Google Scholar]
- 52. McEntee WJ, Crook TH. Glutamate: its role in learning, memory, and the aging brain. Psychopharmacology (Berl). 1993;111:391-401. doi: 10.1007/BF02253527 [DOI] [PubMed] [Google Scholar]
- 53. Meldrum BS. Glutamate as a neurotransmitter in the brain: review of physiology and pathology. J Nutr. 2000;130:1007S-1015S. doi: 10.1093/jn/130.4.1007s [DOI] [PubMed] [Google Scholar]
- 54. Nakazawa K, McHugh TJ, Wilson MA, Tonegawa S. NMDA receptors, place cells and hippocampal spatial memory. Nat Rev Neurosci. 2004;5:361-372. doi: 10.1038/nrn1385 [DOI] [PubMed] [Google Scholar]
- 55. Li F, Tsien JZ. Memory and the NMDA receptors. N Engl J Med. 2009;361:302-303. doi: 10.1056/nejmcibr0902052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Daikhin Y, Yudkoff M. Compartmentation of brain glutamate metabolism in neurons and glia. J Nutr. 2000;130:1026S-1031S. doi: 10.1093/jn/130.4.1026s [DOI] [PubMed] [Google Scholar]
- 57. Potier B, Billard JM, Rivière S, et al. Reduction in glutamate uptake is associated with extrasynaptic NMDA and metabotropic glutamate receptor activation at the hippocampal CA1 synapse of aged rats. Aging Cell. 2010;9:722-735. doi: 10.1111/j.1474-9726.2010.00593.x [DOI] [PubMed] [Google Scholar]
- 58. Price MT, Olney JW, Haft R. Age-related changes in glutamate concentration and synaptosomal glutamate uptake in adult rat striatum. Life Sci. 1981;28:1365-1370. doi: 10.1016/0024-3205(81)90410-0 [DOI] [PubMed] [Google Scholar]
- 59. Dawson R, Wallace DR, Meldrum MJ. Endogenous glutamate release from frontal cortex of adult and aged rats. Neurobiol Aging. 1989;10:665-668. doi: 10.1016/0197-4580(89)90002-X [DOI] [PubMed] [Google Scholar]
- 60. Errington ML, Lynch MA, Bliss TVP. Long-term potentiation in the dentate gyrus: induction and increased glutamate release are blocked by d(-)aminophosphonovalerate. Neuroscience. 1987;20:279-284. doi: 10.1016/0306-4522(87)90019-4 [DOI] [PubMed] [Google Scholar]
- 61. Huerta PT, Sun LD, Wilson MA, Tonegawa S. Formation of temporal memory requires NMDA receptors within CA1 pyramidal neurons. Neuron. 2000;25:473-480. doi: 10.1016/S0896-6273(00)80909-5 [DOI] [PubMed] [Google Scholar]
- 62. Artola A, Singer W. Long-term potentiation and NMDA receptors in rat visual cortex. Nature. 1987;330:649-652. doi: 10.1038/330649a0 [DOI] [PubMed] [Google Scholar]
- 63. Mackenzie DM, Copp P, Shaw RJ, Goodwin GM. Brief cognitive screening of the elderly: a comparison of the Mini-Mental State Examination (MMSE), Abbreviated Mental Test (AMT) and Mental Status Questionnaire (MSQ). Psychol Med. 1996;26:427-430. doi: 10.1017/s0033291700034826 [DOI] [PubMed] [Google Scholar]
- 64. Malhotra C, Chan A, Matchar D, et al. Diagnostic performance of Short Portable Mental Status Questionnaire for screening dementia among patients attending cognitive assessment clinics in Singapore. Ann Acad Med Singapore. 2013;42:315-319. [PubMed] [Google Scholar]
- 65. Mitchell AJ, Shiri-Feshki M. Rate of progression of mild cognitive impairment to dementia - meta-analysis of 41 robust inception cohort studies. Acta Psychiatr Scand. 2009;119:252-265. doi: 10.1111/j.1600-0447.2008.01326.x [DOI] [PubMed] [Google Scholar]
- 66. Spencer RJ, Wendell CR, Giggey PP, et al. Psychometric limitations of the mini-mental state examination among nondemented older adults: an evaluation of neurocognitive and magnetic resonance imaging correlates. Exp Aging Res. 2013;39:382-397. doi: 10.1080/0361073X.2013.808109 [DOI] [PubMed] [Google Scholar]
- 67. Knight A, Bryan J, Murphy K. The Mediterranean diet and age-related cognitive functioning: a systematic review of study findings and neuropsychological assessment methodology. Nutr Neurosci. 2017;20:449-468. doi: 10.1080/1028415X.2016.1183341 [DOI] [PubMed] [Google Scholar]
- 68. Jaeger J. Digit symbol substitution test. J Clin Psychopharmacol. 2018;38:513-519. doi: 10.1097/JCP.0000000000000941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Volkert D, Schrader E. Dietary assessment methods for older persons. Curr Opin Clin Nutr Metab Care. 2013;16:534-540. doi: 10.1097/MCO.0b013e328363c8d1 [DOI] [PubMed] [Google Scholar]
- 70. Auyeung TW, Kwok T, Lee J, Leung PC, Leung J, Woo J. Functional decline in cognitive impairment - the relationship between physical and cognitive function. Neuroepidemiology. 2008;31:167-173. doi: 10.1159/000154929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Clouston SAP, Brewster P, Kuh D, et al. The dynamic relationship between physical function and cognition in longitudinal aging cohorts. Epidemiol Rev. 2013;35:33-50. doi: 10.1093/epirev/mxs004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Fougère B, Daumas M, Lilamand M, et al. Association between frailty and cognitive impairment: cross-sectional data from Toulouse frailty day hospital. J Am Med Dir Assoc. 2017;18:990.e1-990.e5. doi: 10.1016/j.jamda.2017.06.024 [DOI] [PubMed] [Google Scholar]
- 73. Borges MK, Canevelli M, Cesari M, Aprahamian I. Frailty as a predictor of cognitive disorders: a systematic review and meta-analysis. Front Med. 2019;6:26. doi: 10.3389/fmed.2019.00026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Alhola P, Polo-Kantola P. Sleep deprivation: impact on cognitive performance. Neuropsychiatr Dis Treat. 2007;3:553-567. [PMC free article] [PubMed] [Google Scholar]
- 75. McCurry SM, Logsdon RG, Teri L, et al. Characteristics of sleep disturbance in community-dwelling Alzheimer’s disease patients. J Geriatr Psychiatry Neurol. 1999;12:53-59. doi: 10.1177/089198879901200203 [DOI] [PubMed] [Google Scholar]
- 76. Novotný M, Klimova B, Valis M. Microbiome and cognitive impairment: can any diets influence learning processes in a positive way? Front Aging Neurosci. 2019;11:170. doi: 10.3389/fnagi.2019.00170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Sani G, Manchia M, Simonetti A, et al. The role of gut microbiota in the high-risk construct of severe mental disorders: a mini review. Front Psychiatry. 2021;11:585769. doi: 10.3389/fpsyt.2020.585769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Loenneke JP, Loprinzi PD, Murphy CH, Phillips SM. Per meal dose and frequency of protein consumption is associated with lean mass and muscle performance. Clin Nutr. 2016;35:1506-1511. doi: 10.1016/j.clnu.2016.04.002 [DOI] [PubMed] [Google Scholar]
- 79. Ten Haaf DSM, van Dongen EJI, Nuijten MAH, Eijsvogels TMH, de Groot LCPGM, Hopman MTE. Protein intake and distribution in relation to physical functioning and quality of life in community-dwelling elderly people: acknowledging the role of physical activity. Nutrients. 2018;10:506. doi: 10.3390/nu10040506 [DOI] [PMC free article] [PubMed] [Google Scholar]