Abstract
Due to the continuous increase in polystyrene microplastics (PS MPs) incorporation in the environment, growing number of adverse effects on living organisms and ecosystem have become a global concern. Therefore, current study was planned to elucidate the impacts of 5 different concentrations control, 2, 20, 200, and 2000 μgL-1 of PS MPs on testicular tissues of rats. PS MPs significantly reduced the activities of antioxidant enzymes (catalase, superoxide dismutase and peroxidase) as well as total protein contents, while elevated the level of lipid peroxidation and reactive oxygen species. Moreover, expressions of steroidogenic enzymes (3β-hydroxysteroid dehydrogenase, 17β-hydroxysteroid dehydrogenase and steroidogenic acute regulatory protein) as well as the levels of follicle-stimulating hormone (FSH), luteinizing hormone (LH) in plasma, intra-testicular testosterone and plasma testosterone were reduced and a significant (P < 0.05) reduction was noticed in the sperm count, motility and viability. Furthermore, PS MPs significantly up-regulated the expressions of Bax and caspase-3, while down-regulated the Bcl-2 expression. The histomorphological assessment revealed significant damages in the testicles as well as decrease in the number of germ cells (spermatogenic, spermatocytes and spermatids). Collectively, PS MPs generated oxidative stress (OS) and caused potential damage to the testicles of rats in a dose-dependent manner.
Keywords: polystyrene microplastics (PS MPS), oxidative stress, testicular damage, spermatogenesis, male reproductive system
Introduction
Environmental pollution and industrial exposure to various contaminants play a significant role in the induction of male infertility. 1 Global plastic production and its utilization in making various products were increased from 1.7 to 335 million tons during 1950-2016. 2 Plastic manufacturing is relatively convenient and inexpensive. 3 Microplastics (MPs) are the emerging environmental pollutants consisting of plastic particles (<5 mm), which are small fragments of macroplastics. 4 MP pollution also results in other universal threats, such as ocean acidification, climate change and ozone depletion. 5
Microplastics are formed of various materials, such as polyvinyl chloride (PVC), polyethylene (PE) and polystyrene (PS). PS is one of the most abundantly used aromatic polymer amongst these plastics. 4 A reason behind its excessive presence in the ecosystem is its low cost and exceptional physical properties. It is used in the manufacturing of disposable cups, trays, bowls, plates, pegs, toys, office supplies, paper clips, cleansing agents, cosmetics, pharmaceuticals. 6 Moreover, it is reported to induce a number of damages in living organisms. At the biochemical and cellular level, PS exposure leads to stimulation of inflammatory reactions and oxidative stress (OS) in crab liver. 7 In mice, exposure of PS induces the OS, as well as reduces weight of liver and body. 8 PS derived MP is a serious environmental hazard, which is known as a white pollutant of the environment. 9
Few studies have been conducted on the impacts of MPs on male reproductive system of the mice. 10,11 but the effect of MPs and their possible damage inducing mechanisms in the testicular tissues of mammalian species demand a further investigation. It is an established fact that steroidogenic enzymes play an important role in maintaining the normal physiological functions of testis. Thus, by keeping this fact under consideration, the current study was designed to elucidate the dose-dependent effects of PS MPs on rat’s testicular toxicity especially by considering the steroidogenic enzymes.
Materials and Methods
Chemical Reagents
PS MP particles of 10µm diameter were bought from Sigma Aldrich, USA. Other chemicals were of high analytical grade and bought from Merck and Sigma Aldrich, USA.
Animals
Sexually mature male Sprague Dawley rats (n = 60) were collected from breeding and rearing section in the Animal House of the University of Agriculture, Faisalabad. The animals were housed in steel cages at standard temperature (24 ± 2°C) and light conditions (10 h light and 14 h dark). Food pellets (comprising free soy, alfalfa, 40%-50% carbohydrates, 20%-25% protein and 4%-7% adipose tissue) were fed to the rats. Tap water ad libitum was provided in bottles made up of polysulfone. Animals were treated in compliance with the European Union of Animal Care and Experimentation (CEE Council 86/609) approved protocol.
Experimental Design
Sexually mature male Sprague Dawley rats (n = 60) were distributed into 5 groups (n = 12/group). Various concentrations Control (0.9% saline), 2, 20, 200, and 2000 μgL-1 of PS MPs were administered to group 1 to 5 respectively. The animals of the control group were treated with normal saline as a vehicle. Various concentrations of PS MPs were dissolved in culture media and given to rats by oral gavage for 60 days. The dose selection of PS MPs was based on OECD protocol 423 for various complexes with anonymous effect (OECD 2001). On the 61st day, rats were anesthetized with diethyl ether and killed by decapitating. To separate plasma, trunk blood was drawn into heparinized syringes. Blood was stored in the sterile tubes. Testes and epididymis were removed and later on used for the determination of various parameters.
Biochemical Assay
The homogenization of testicular tissues was carried out in PBS. Finally, centrifugation was performed at 3,000 rpm for 10 minutes. The supernatant obtained was used for the biochemical assay, protein evaluation, lipid profile assessment, and hormonal analysis. Biochemical assessment of tissues was performed via supernatant obtained from testicular tissues.
Analysis of catalase (CAT)
The activity of catalase was determined by following the technique of Aebi. 12 50µL of tissues homogenate was diluted using 2 mL of phosphate buffer with 7 pH. Diluted homogenate (2 mL) was added with phosphate buffer (1 mL) of pH 7 comprising 30 mM of H2O2 in the test tube and then, diluted water was added to blanks. After instantaneously mixing, absorbance was noted at 240 nm. CAT activity was expressed as an absorbance alteration of 0.01 Umin-1.
Analysis of superoxide dismutase (SOD)
Superoxide dismutase activity was measured by following technique of Kakkar et al. 13 Reaction solution contained of 1.2 mL of sodium pyrophosphate buffer (0.052 mM; pH 7.0) and 0.1 mL of phenazine methosulphate (186 μM). 0.3 mL of supernatant after centrifugation (1500 × g for 10 min followed by 10000 × g for 15 min) of homogenate was added to the reaction mixture. Then, 0.2 mL of NADH (780 μM) was added to start the enzyme reaction, which was later on ended by adding 1 mL of glacial acetic acid. Finally, chromogen was assessed by noticing the change in color intensity (at 560 nm). The values of SOD activity were presented as unit/mg protein.
Analysis of peroxidase (POD)
In homogenate, the activity of peroxidase was evaluated by following the technique of Chance and Maehly, 14 but with few amendments. First of all, homogenate mixing was carried out with 0.1 mL of guaiacol, 2.5 mL of phosphate buffer (pH 5) and 0.3 mL of hydrogen peroxide. At 470 nm, the variations were detected after 1 min. 1 unit of POD activity was noted at 0.01 absorbance change as U/min.
Analysis of reactive oxygen species (ROS)
ROS were assessed from homogenate as per the process explained by Hayashi et al. 15 Homogenate (5µL) and 0.1 M sodium acetate buffer (140µL) with pH 4.8 were mixed and dispensed in 96 well-plate. After incubating at 37°C for 5 minutes, 100µL of mixed solution of ferrous sulfate and N, N-diethyl-para-phenylenediamine was dispensed to each plate, and then incubated at 37°C for 1 minute. At 505 nm, the absorbance was noticed with the help of a microplate reader for 180 s with a 15 s interval. Finally, the standard curve was plotted. ROS was recorded as Unit/mg tissues and 1 unit of ROS was equivalent to 1.0 mg/L of H2O2 in the sample.
Analysis of lipid peroxidation (LPO) by thiobarbituric acid reactive substances (TBARS)
The analysis of malondialdehyde in the homogenate was performed by reacting it with thiobarbituric acid, as per the procedure described by Iqbal et al. 16 TBARS are produced as a result of lipid peroxidation (LP), therefore, degree of LP was indicated by TBARS level. 1.0 mL total volume of reaction mixture consisted of 0.2 mL homogenate sample, 0.02 mL ferric chloride (100 mM), 0.58 mL phosphate buffer (0.1 M pH 7.4) and 0.2 mL ascorbic acid (100 mM). Incubation was carried out at 37°C for about 1 hour in a shaking water bath. Addition of 1.0 mL 10% trichloroacetic acid terminated the reaction. After adding 1.0 mL 0.67% thiobarbituric acid, tubes were boiled in water bath for about 20 minutes. Then, mixture was transferred to crushed ice-bath prior centrifuging at 2500 × g for about 10 minutes. The TBARS level was determined by measurement of optical density of supernatant at 535 nm with spectrophotometer against reagent blank. Its final values were shown as nM TBARS/min/mg tissue at 37°C using molar extinction coefficient of 1.56 × 105 M-1cm-1.
Analysis of Total Protein Content
Estimation of total protein constituents was carried out by the protein kit (Cat No. BR5202-S, AMEDA Labordiagnostik GmbH, Krenngasse, Graz, Austria). Results were computed by plotting absorbance of standard vs sample absorbance on the graph. Final data was shown in mg/g of tissues.
Real-time Polymerase Chain Reaction (qRT-PCR)
qRT-PCR was used to assess expression levels of 3β-hydroxysteroid dehydrogenase (3β-HSD), 17β-hydroxysteroid dehydrogenase (17β-HSD), steroidogenic acute regulatory protein (StAR), Bax, Bcl-2 and caspase-3. TRIzol reagent (Invitrogen, Carlsbad, CA) was used for isolation of total RNA (Takara Bio, Japan). RNA concentrations were determined by NanoDrop 2000c spectrophotometer (Thermo Fisher, USA). Total RNA was with 260/A280 ratio between 1.8-2.2 was used for reverse transcriptase PCR. Reverse transcription kit (Promega, USA) was employed to transform total RNA into complementary DNA. qRT-PCR was carried out using SYBR@ Premix Ex TaqTMII kit. β-actin was used as internal control and relative expressions were assessed by 2-ΔΔCT. Primer sequences of β-actin and target genes are shown in Table 1 as reported previously in Ijaz et al. 17
Table 1.
Primers Sequences for RT-qPCR.
| Gene | Primers 5’ -> 3’ | Accession number | Product size | Temperature |
|---|---|---|---|---|
| 3β-HSD | Forward: GCATCCTGAAAAATGGTGGC | NM_001007719 | 135 | 57 |
| Reverse: GCCACATTGCCTACATACAC | ||||
| 17β-HSD | Forward: CAGCTTCCAAGGCTTTTGTG | NM_054007 | 161 | 59 |
| Reverse: CAGGTTTCAGCTCCAATCGT | ||||
| StAR | Forward: AAAAGGCCTTGGGCATACTC | NM_031558 | 113 | 58 |
| Reverse: CATAGAGTCTGTCCATGGGC | ||||
| Bax | Forward: GGCCTTTTTGCTACAGGGTT | NM_017059.2 | 119 | 58 |
| Reverse: AGCTCCATGTTGTTGTCCAG | ||||
| Bcl-2 | Forward: ACAACATCGCTCTGTGGAT | NM_016993.1 | 103 | 57 |
| Reverse: TCAGAGACAGCCAGGAGAA | ||||
| Caspase-3 | Forward: ATCCATGGAAGCAAGTCGAT | NM_012922.2 | 233 | 57 |
| Reverse: CCTTTTGCTGTGATCTTCCT | ||||
| β-actin | Forward: TACAGCTTCACCACCACAGC | NM_031144 | 135 | 58 |
| Reverse: GGAACCGCTCATTGCCGATA |
Analysis of Hormones
The levels of LH (serial number-H206), FSH (serial number-H101), and plasma testosterone (serial number-H090) were assessed by ELISA kits (Los Angeles, CA USA) according to manufacturer’s procedures. 50 µL of assay diluent and 10 mL of plasma were added to 96-well ELISA plate and incubated for approximately 2 hours at room temperature. Then, plates were rinsed with the deionized water and before adding 100 mL of peroxidase-conjugated immunoglobulin G (IgG) anti-FSH solution, anti-LH, or anti-testosterone in each well, incubation was carried out for maximum 2 hours. Plates were again rinsed with the deionized water, substrate solution was added in wells and incubated for about 25 minutes at room temperature. 50 µL of stop solution was added into each well to terminate the reaction. Finally, the absorbances of FSH, LH and plasma testosterone were recorded at 450 nm. All samples were run in triplicates and conducted at same time under same conditions to avoid inter-assay variation.
Spermatogenic Analysis
Epididymal spermatozoa were collected by separating the caudal part of the epididymis on both sides. Spermatozoa were separated from epididymal tubules by splitting the caudal part of the epididymis in 5 mL of Hams F10 solution. The solution was incubated for 5 min at 37°C. After pipetting, 1 drop of sperm suspension was placed on a microscope slide and cover slipped. We observed at least 5 microscopic fields at 400X magnification and calculated the % of in situ motile, progressive and immotile spermatozoa as a fraction of the total counted spermatozoa according to the WHO recommendations.
Sperm count
Epididymal sperm count was evaluated with a hemocytometer by following the method described by Ciftci et al 18 but with few amendments. The right epididymal portion was crushed by the help of anatomical scissors in 5 mL saline. After incubation at room temperature, sperms present in the supernatant fluid were counted under a light microscope.
Sperm motility
For evaluation of the percentage of sperm motility, a slide was kept under light microscope equipped with a prewarmed (37°C) stage. A drop of semen was placed on slide and motility was estimated from 3 random fields per sample. Finally, a mean of 3 estimated values was noted as sperm motility. 19
Sperm viability
For determination of sperm viability, eosin/nigrosin stain was dropped on a semen sample on a prewarmed slide and smear was formed. After it dried, the slide was examined under a light microscope. Unstained/white sperms were considered as alive, whereas red sperms were considered dead. 300 spermatozoa were examined, and the percentage of dead sperm was estimated. 20
Tissue Histology
Tissues were preserved in a solution of formalin (10%) for 48 h for fixation. In the next step, tissues were shifted to increasing alcohol grades for dehydration, followed by double washing with xylene. For the formation of blocks, tissues were shifted into paraffin wax for microtomy. 7 µm thick sections of tissues were obtained. Each of the 10th slices of tissues were cut down from the ribbon and transferred to warm water for stretching. Slices were shifted carefully to albumenized slides, which were air-dried for half an hour and placed overnight in paraffin oven at 38 ± 2°C for complete deparaffinization. These deparaffinized slices were then passed through different grades of alcohol for the dehydration and later on, stained with hematoxylin and eosin.
Lastly, these slides were examined under Leica Microscope (DMLB Leica microscope, Leica Microsystems Ltd, UK), and a digital camera (Canon, Japan) was installed with it. After capturing images at 20× and 40×, the histomorphometric assessment was carried out by ImageJ Software. From 20× images, about 30 photographs per rat were chosen and regions of seminiferous tubules, epididymal tubule and interstitial spaces were assessed through the free selection tool of software. Following formula was used to measure area: % As = As× 100/T. Where As known as the seminiferous tubules covered area and T is the total area of the field.
Mean and average area percentages were observed to compare control and treated groups. Count of different germ cell numbers was taken from 50 seminiferous tubules per rat at 100× and the average number of spermatocytes, spermatogonia and spermatid in each seminiferous tubule was computed.
Statistical Analysis
Final data was shown as Mean ± SEM. After applying 1-way analysis of variance (ANOVA), Tukey test was employed using Minitab software. The significance level was P < 0.05.
Results
Effect of PS MPs on Testicular, Epididymis, Seminal Vesicles, and Prostate Gland Weight
There was no significant change in animal weight gain among all groups. PS MPs treatment did not alter the weights of the both testis (Right and Left), epididymis, seminal vesicles, and prostate gland (Table 2).
Table 2.
Effect of Different Concentrations (Control, 2, 20, 200, and 2000 μgL-1) of PS MPs on Body Weight, Testes, Epididymis, Seminal Vesicles, and Prostate Gland Weight in Control and Treated Groups.a
| Groups | Body weight gain (g) | Left testes weight (g) | Right testes weight (g) | Epididymis (g) | Seminal vesicles (g) | Prostate gland (g) |
|---|---|---|---|---|---|---|
| Control | 55.4 ± 1.08 | 1.35 ± 0.03 | 1.40 ± 0.03 | 0.67 ± 0.02 | 0.76 ± 0.02 | 0.63 ± 0.01 |
| 2 μgL-1 | 51.8 ± 0.89 | 1.33 ± 0.02 | 1.38 ± 0.02 | 0.64 ± 0.01 | 0.73 ± 0.01 | 0.63 ± 0.02 |
| 20 μgL-1 | 50.4 ± 0.98 | 1.32 ± 0.03 | 1.38 ± 0.01 | 0.62 ± 0.01 | 0.74 ± 0.04 | 0.63 ± 0.01 |
| 200 μgL-1 | 50.3 ± 1.46 | 1.31 ± 0.04 | 1.34 ± 0.02 | 0.64 ± 0.03 | 0.67 ± 0.07 | 0.60 ± 0.02 |
| 2000 μgL-1 | 50.5 ± 1.80 | 1.30 ± 0.04 | 1.35 ± 0.03 | 0.64 ± 0.03 | 0.70 ± 0.07 | 0.62 ± 0.04 |
a Values are shown as Mean ± SEM (n = 12/group). Means within same row (for each parameter) carrying different superscripts are significantly different at P < 0.05.
Effect of PS MPs on Antioxidant Capacity and Total Protein Content
After PS MPs administration, CAT, SOD and POD activity was significantly (P < 0.05) reduced only at higher doses (200 and 2000 μgL-1) in PS MPs-intoxicated rats compared with control rats (Table 3).
Table 3.
Effect of Different Concentrations (Control, 2, 20, 200, and 2000 μgL-1) of PS MPs on Catalase (CAT), Superoxide Dismutase (SOD), Peroxidase (POD), Reactive Oxygen Species (ROS), Lipid Peroxidation (LPO) and Protein Content in Rat Testicles.*
| Parameters | Control | 2 μgL-1 | 20 μgL-1 | 200 μgL-1 | 2000 μgL-1 |
|---|---|---|---|---|---|
| CAT (U/mg protein) | 9.53 ± 0.35a | 9.02. ± 0.33a | 9.07 ± 0.38a | 5.37 ± 0.21b | 3.62 ± 0.36c |
| SOD (U/mg protein) | 53.23 ± 4.36a | 54.14 ± 6.62a | 53.89 ± 5.94a | 25.25 ± 5.83b | 18.00 ± 4.36b |
| POD (nmole) | 11.48 ± 0.58a | 10.77 ± 0.61a | 12.52 ± 0.35a | 6.70 ± 0.62b | 5.90 ± 0.31b |
| ROS (U/mg tissue) | 0.56 ± 0.05a | 0.55 ± 0.13a | 0.51 ± 0.12a | 2.04 ± 0.09b | 2.30 ± 0.14b |
| LPO (nM TBARS/min/mg tissue) | 31.26 ± 3.30a | 34.56 ± 2.64a | 31.53 ± 3.64a | 52.01 ± 2.60b | 52.00 ± 3.85b |
| Protein (mg/0.5 g) | 260.8 ±19.19a | 247.4 ± 15.20a | 246.48 ± 7.31a | 210.30 ± 6.41ab | 184.76 ± 6.84b |
* Values are shown as Mean ± SEM (n = 12/group). Means within same row (for each parameter) carrying different superscripts are significantly different at P < 0.05.
On the other hand, ROS level was significantly (P < 0.05) increased only in higher doses of PS MPs treated groups compared to control. LPO also showed the similar trend, significant (P < 0.05) elevation in LPO was noticed in groups treated with higher doses (200 and 2000 μgL-1) in PS MPs treated rats compared with control rats. Similarly, protein contents were significantly (P < 0.05) decreased at higher doses (200 and 2000 μgL-1) in PS MPs groups in comparison to control (Table 3).
Effect of PS MPs on Expression of Steroidogenic Enzymes
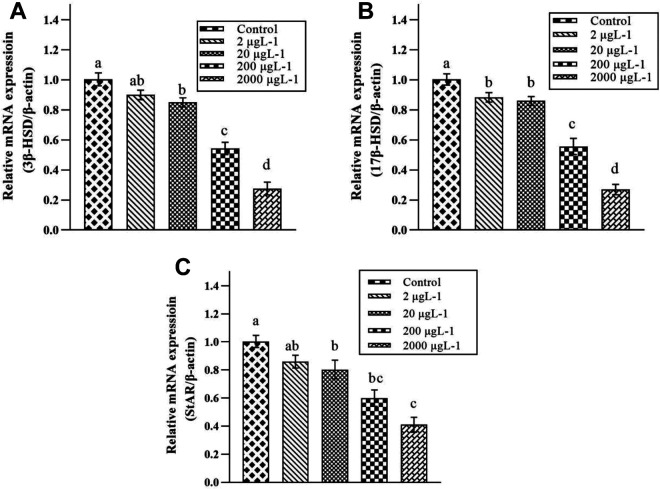
As shown in Figure 1, PS MPs led to a significant down-regulation in expression of 3β-HSD and 17β-HSD at 2 higher-doses (200 and 2000 μgL-1) in PS MPs-intoxicated groups compared to control. While, a significant reduction was seen in expression of StAR at 3 higher-doses (20, 200 and 2000 μgL-1) in PS MPs-intoxicated rats compared with control rats.
Figure 1.
Effect of various doses of PS MPs (Control, 2, 20, 200, and 2000 μgL-1) on the Expression of (A) 3β-Hydroxysteroid dehydrogenase (3β-HSD), (B) 17β-Hydroxysteroid dehydrogenase (17β-HSD), and (C) Steroidogenic acute regulatory protein (StAR). Bars are shown on the basis of mean + SEM values of 3 ndependent experiments (n = 12 rats/group). Different subscripts displaying significant difference at P < 0.05.
Effect of PS MPs on Hormonal Concentrations
Oral exposure to different doses of PS MPs significantly (P < 0.05) suppressed the concentration of plasma LH in all PS MPs-induced groups when compared to control (Table 4). A significant (P < 0.05) suppression was observed in plasma FSH concentration, but only at the highest dose (2000 μgL-1) in PS MPs groups compared to control. In the same manner, the concentration of plasma testosterone was significantly (P < 0.05) suppressed by PS MPs only at highest dose (2000 μgL-1) compared with control group. A significant (P < 0.05) reduction was noticed in intra-testicular testosterone concentration at higher dose (200 and 2000 μgL-1) PS MPs-intoxicated rats compared to control rats (Table 4).
Table 4.
Effect of Different Concentrations (Control, 2, 20, 200, and 2000 μgL-1) of PS MPs on Hormones Luteinizing Hormone (LH), Follicle-Stimulating Hormone (FSH), Testosterone and Sperm Parameters in Rat Testicles.*
| Parameters | Control | 2 μgL-1 | 20 μgL-1 | 200 μgL-1 | 2000 μgL-1 |
|---|---|---|---|---|---|
| LH ((ng/mL) | 2.91 ± 0.02a | 2.70 ± 0.01b | 2.64 ± 0.02b | 2.41 ± 0.01c | 2.18 ± 0.04d |
| FSH (ng/mL) | 3.75 ± 0.04a | 3.72 ± 0.02a | 3.70 ± 0.01a | 3.68 ± 0.02a | 3.37 ± 0.04b |
| Plasma testosterone (ng/mL) | 4.74 ± 0.15a | 4.77 ± 0.13a | 4.67 ± 0.06a | 4.54 ± 0.15a | 4.17 ± 0.12b |
| Intra-testicular testosterone (ng/g tissue) | 45.76 ± 1.94a | 47.14 ± 1.04a | 44.19 ± 1.24ab | 37.52 ± 1.22bc | 36.10 ± 1.57c |
| Sperm count (x 10-6/gm of cauda) | 163.15 ±2.55a | 163.28 ± 1.88a | 163.11 ± 1.59a | 139.92 ± 3.03b | 104.19 ± 1.79c |
| Sperm viability % | 78.78 ± 1.50a | 78.75 ± 0.82a | 78.81 ± 0.87a | 63.51 ± 0.41b | 58.45 ± 0.88c |
| Sperm motility % | 69.93 ± 3.81a | 69.87 ± 2.84a | 69.96 ± 1.11a | 68.75 ± 0.76a | 55.86 ± 0.81b |
* Values are shown as Mean ± SEM (n = 12/group). Means within same row (for each parameter) carrying different superscripts are significantly different at P < 0.05.
Effect of PS MPs on Sperm Indices
The exposure of PS MPs led to a significant (P < 0.05) reduction in quantity of sperms (Table 4). The PS MPs significantly (P < 0.05) reduced the sperm count and viability at higher doses (200 and 2000 μgL-1) in PS MPs groups compared to control. Whereas, sperm motility was significantly (P < 0.05) reduced at highest (2000 μgL-1) PS MPs treated group compared to control (Table 4).
Effect of PS MPs on Apoptotic Markers
As shown in Figure 2, PS MPs significantly increased the gene expression of Bax at all doses (2, 20, 200 and 2000 μgL-1) of PS MPs, while expression of caspase-3 was increased at 3 high-doses (20, 200 and 2000 μgL-1) in PS MPs groups compared to control. On the contrary, the expression of Bcl-2 showed a significant reduction at all concentrations (2, 20, 200 and 2000 μgL-1) in PS MPs-induced group compared to control (Figure 2).
Figure 2.
Effect of various doses of PS MPs (Control, 2, 20, 200, and 2000 μgL-1) on the expression of (A) Bax (Bcl-2 associated X Apoptosis Regulator), (B) B-cell lymphoma 2 (Bcl-2), and (C) cysteine-aspartic acid protease-3 (Caspase-3). Bars are shown on the basis of mean + SEM values of 3 Independent Experiments (n = 12 rats/group). Different subscripts displaying significant difference at P < 0.05.
Effect of PS MPs on Histopathology
In the morphometric analysis of testis, 2 higher-dose groups of PS MPs (200 and 2000 μgL-1) exhibited a significant (P < 0.05) decrease in area and diameter of seminiferous tubules and epithelial height when compared with control rats (Table 5). While, the area of interstitium was significantly (P < 0.05) decreased by PS MPs in 3 groups (20, 200 and 2000 μgL-1) compared to control (Table 5).
Table 5.
Effect of Different Concentrations (Control, 2, 20, 200, and 2000 μgL-1) of PS MPs on Histopathological Parameters of Testes in Rats.*
| Parameters | Control | 2 μgL-1 | 20 μgL-1 | 200 μgL-1 | 2000 μgL-1 |
|---|---|---|---|---|---|
| Area of seminiferous tubules (μm) | 70.18 ± 1.15a | 71.12 ± 1.53a | 69.80 ± 0.92ab | 65.11 ± 0.54bc | 60.49 ± 0.87c |
| Area of interstitium (μm) | 19.21 ± 0.61a | 19.02 ± 0.48a | 16.52 ± 0.35b | 16.32 ± 0.19b | 14.59 ± 0.39b |
| Seminiferous tubules diameter (μm) | 190.01 ± 5.52a | 182.14 ± 2.45a | 177.30 ± 3.91ab | 162.53 ± 3.33bc | 155.22 ± 4.09c |
| Epithelial height (μm) | 64.39 ± 1.58a | 63.90 ± 1.11ab | 63.74 ± 1.185ab | 58.37 ± 1.54ab | 57.70 ± 1.33b |
| Spermatogonia (n) | 55.21 ± 1.07a | 53.41 ± 0.4ab | 52.85 ± 0.52ab | 51.40 ± 0.52ab | 49.79 ± 1.32b |
| Spermatocytes (n) | 69.92 ± 1.44a | 68.55 ± 1.03a | 67.35 ± 0.45a | 62.29 ± 0.61b | 60.92 ± 1.29b |
| Spermatids (n) | 229.99 ± 3.06a | 224.67 ± 2.31ab | 219.52 ± 1.17abc | 216.75 ± 2.06bc | 213.26 ± 2.34c |
* Values are shown as Mean ± SEM (n = 12/group). Means within same row (for each parameter) carrying different superscripts are significantly different at P < 0.05.
A significant (P < 0.05) decrease was observed in number of spermatogonia and spermatocytes in seminiferous tubules by PS MPs in 2 higher-dose groups (200 and 2000 μgL-1) compared to control (Table 5). A similar trend was shown by spermatids. Spermatid number in seminiferous tubules displayed a significant (P < 0.05) decrease by PS MPs administration in 3 higher-dose groups (20, 200 and 2000 μgL-1) in comparison to control (Table 5). Histopathological changes have been shown in Figure 3.
Figure 3.
Seminiferous tubules after the exposure of various concentrations of PS MPs: (A) Control group shows compactly arranged seminiferous tubules having thick epithelial height and luman filled with mature sperms, (B) 2 μgL-1 group displays normal state of tubules with thick epithelial height and lumen filled with spermatids, (C) 20 μgL-1 group also shows thick epithelium and lumen with less spermatids. Interstitial area is slightly decreased, (D) 200 μgL-1 group exhibits loosely arranged tubules with thin epithelial height and a smaller number of all germ cells; and (E) 2000 μgL-1 group shows most disordered condition, i.e. loosely packed tubules with thin epithelium and less germ cells. H&E (x40). TA: Tunica Albuginea; EH: Epithelial Height; TL: Tubular Lumen; IS: Interstitial Spaces; SG: Spermatogonia; ST: Spermatids; and SC: Spermatocyte.
Discussion
In the past 2 decades, MPs have received increased attention due to their wide distribution, persistence and toxic impacts on living beings and ecosystems. 21 The toxic effects of PS MPs are investigated in various animal models due to a possible threat to marine biota as well as human health. 22 A recent study reported that PS MPs exposure led to a reproductive disturbance in oysters and specifically affected their larval stages. 23 Lönnstedt and Eklöv reported that PS derived MPs are ingested by a variety of living beings, which disturb multiple physiological events in the body. 24 Therefore, the current research was planned to evaluate PS MPs-induced reproductive toxicity in male rats by the evaluating the biochemical status, hormonal concentrations, spermatogenic indices, morphometry and histopathological changes was determined.
PS MPs decreased the activities of antioxidant enzymes (CAT, SOD and POD) and total proteins, while elevated the level of ROS and LPO in PS MPs-intoxicated rats compared to control rats. Antioxidant enzymes are the first line of a defense that protects the biological molecules (DNA, lipids, and proteins) from impairment by reducing ROS production. 25,26 CAT, POD and SOD form the antioxidant defense system in semen. 27 CAT is considered as the central enzyme of the antioxidant system as it plays a significant role in H2O2 catabolism. 28 SOD catalyzes superoxide radicals to H2O2 and O2. 29 Normally, antioxidant enzymes nullify the toxicities produced due to ROS. However, when ROS are generated excessively, they overtake the antioxidant defense system of the body, which leads to the generation of OS. 30 The previous study by Xie et al 10 has reported oxidative stress by using 5µm size microplastic in rat testes. Therefore, the current study endorsed the findings of previous study that PS MPs caused toxicity by altering the activities of antioxidant enzymes and generating higher levels of ROS and LPO in testicular tissues of rats, but in the current study these effects were pronounced in the rats treated with only higher doses of 10µm size PS MPs.
In the current investigation, the concentrations of luteinizing hormone (LH), follicle-stimulating hormone (FSH), plasma testosterone as well as intra-testicular testosterone were significantly (P < 0.05) decreased at high doses in PS MPs-intoxicated animals as compared to control. Previously Xie et al 10 and Jin et al 11 also reported the decrease in testosterone level followed by PS MPs exposure, but the current investigation expanded the horizon and studied the complete feedback loop of hypothalamic-pituitary-gonadal axis to reveal the effect of PS MPs exposure on FSH, LH and subsequently on testosterone level. According to Wisniewski et al spermatogenesis is dependent on ratio of FSH, LH and testosterone. 31 Synthesis of testosterone is a vital indicator of male reproductive health since the testosterone is involved in healthy sperm development and regulation of spermatogenesis. 32 The pituitary gonadotropic cells were stimulated by gonadotropin-releasing hormones (GnRH) in the hypothalamus, which releases LH and FSH to regulate spermatogenesis in animals. 33 LH acts on Leydig cells to produce testosterone, while FSH helps in proliferation of Sertoli cells. 34 Karami et al reported the downregulation of GnRH in the brain of African catfishes due to exposure of MPs. 35 Sun et al have also evinced the MPs-induced reproductive disruption due to disturbance in normal functioning of GnRH. 36 As the hypothalamic–pituitary–gonadal (HPG) axis mainly controls the functioning of reproductive system, therefore, PS MPs-induced reduction in level of plasma and intra-testicular testosterone, as well as LH and FSH probably happened due to the disturbance of HPG axis.
To evaluate the mechanism underlying the low concentration of testosterone after PS MPs intoxication, the steroidogenic enzymes expression was determined. PS MPs reduced the expression of steroidogenic enzymes, 3β-HSD, 17β-HSD and StAR, in a dose-dependent manner. 3β-HSD and 17β-HSD mediate steroidogenic activities and play major androgenic role in testes. 37 StAR is a rate-limiting steroidogenic enzyme which regulates the shifting of cholesterol inside mitochondria, to stimulate production of testosterone. 38 As described by Raucci et al 39 testicular steroidogenesis is the vital event for production of testosterone (insert citation number) which is certainly mediated by steroidogenic enzymes and proteins. 40 In current experiment, PS MPs exposure resulted in reduction of expressions of testicular steroidogenic enzymes, 3β-HSD, 17β-HSD and StAR, which eventually decreased the concentration of testosterone. These adverse changes are attributed to the anti-androgenic nature of PS MPs.
Various sperm parameters, such as sperm count, motility and viability are indicators of normal spermatogenesis and male reproductive health. 41 As previously stated, the exposure of PS MPs substantially reduced the concentration of intra-testicular and plasma testosterone. This reduction in concentration of testosterone is considered one of the major factors behind the decrease in sperm count. 42 Male gametes are vulnerable to ROS owing to their continuous divisions. According to Oborna et al OS has been proved to affect the membrane fluidity and spermatogenic motility. 43 As previously described, testicles are specifically susceptible to OS due to the presence of excessive polyunsaturated fatty acids in lipids. 44 Polyunsaturated fatty acids are pivotal to maintain functions and membrane integrity of sperms. 45 However, excess generation of ROS leads to disruption in viscosity and permeability of the spermatozoa membrane, thereby resulting in peroxidation of polyunsaturated fatty acids. 46 OS-generated toxicity decreases the production of ATPs by imparting a direct impact on mitochondria of sperm cells. The reduction in ATPs in spermatozoa impairs the flagellar function, subsequently resulting in sperm immobility and apoptotic death. 47 According to Ko et al, elevated levels of ROS stimulate a cascade of damages in the body and specifically cause toxic effects on spermatological parameters and male fertility. 48 Therefore, these spermatogenic damages are possibly due to the elevated ROS levels and reduced concentration of testosterone. Previously Xie et al 10 and Jin et al 11 also reported similar results, but in the current dose-dependent study no effects were seen with lower doses of PS MPs, only higher doses deteriorated the sperm quality.
PS MPs increased the expression of Bax and caspase-3, while reduced the expression of Bcl-2. Apoptotic processes are particularly mediated by proteins of Bcl-2 and caspase family. 49 Bax and Bcl-2 belong to Bcl-2 family. Bcl-2 is considered as anti-apoptotic protein which mainly prevents apoptotic cell death. 50 While, Bax is a pro-apoptotic protein which performs the antagonist action and promotes cell apoptosis. 51 These proteins affect mitochondrial membrane permeability, thereby releasing cytochrome C in cytoplasm, which subsequently activates caspase 3. 52 Caspase-3 belongs to cysteine proteases family which is essential for cleaving cellular proteins, thus leading to structural alterations in cells which culminates in apoptotic cell death. 53 According to Wang and Luo, caspase-3 over-expression is the key event behind the regulation of mitochondrial apoptotic-pathway. 54 Therefore, PS MPs exposure resulted in apoptosis due to elevation in the expression of apoptotic proteins, Bax and caspase-3, while reduction in the expression of anti-apoptotic protein, Bcl-2.
In the current research, the histopathological assessment revealed a decrease in diameter as well as the epithelial height of seminiferous tubules in the PS MPs-induced rats. In addition, seminiferous tubules having decreased interstitial spaces were noticed in testes. PS MPs significantly (P < 0.05) reduced the number of various germ cells in a dose-dependent manner. Higher doses of PS MPs led to the even excessive generation of ROS and histological damages in testes. Previous investigations have demonstrated that the reduced epithelium height might be due to decreased levels of testosterone, which also reduces the number of germ cells. 55 It was deduced that PS MPs exposure interrupted spermatogenesis, probably by disrupting the differentiation of spermatozoa to its mature stages (spermatocytes and spermatids). These damages may be attributed to disrupted integrity of blood-testis barrier followed by PS MPs exposure.11 Collectively, apoptotic cell death due to elevated levels of ROS as well as alterations in hormonal levels, ultimately affected the number of germ cells and induced morphometric damages within testicular tissues. Therefore, findings of the current study confirmed the endocrine disruptive property and toxic nature of PS MPs especially at higher doses.
Conclusion
Our findings indicated that high doses of PS MPs induced OS not only by elevating the degree of ROS and LPO but also by decreasing the antioxidant enzymes (CAT, SOD, POD). It was observed that the lipid profile was disturbed, and total protein contents were seen to be reduced. PS MPs reduced the expression of steroidogenic enzymes and hormonal concentrations as well as decreased sperm count, motility and viability. In addition to it, the apoptotic profile was changed, and histopathological changes were noticed in testicular tissues. It was observed that higher concentrations of PS MPs caused significant damages as compared to low concentrations. Taken together, PS MPs administration exhibited an anti-androgenic effect, which led to an overall testicular dysfunction in rats.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Haseeb Anwar  https://orcid.org/0000-0001-8438-9700
https://orcid.org/0000-0001-8438-9700
References
- 1. Di Nisio A, Foresta C. Water and soil pollution as determinant of water and food quality/contamination and its impact on male fertility. Reprod Biol Endocrinol. 2019;17(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. PlasticsEurope. Plastics—the facts 2017. Publication 2017. Accessed March, 2020. www.plasticseurope.org/en/resources/publications
- 3. Long M, Paul-Pont I, Hegaret H, et al. Interactions between polystyrene microplastics and marine phytoplankton lead to species-specific hetero-aggregation. Environ Pollut. 2017;228:454–463. [DOI] [PubMed] [Google Scholar]
- 4. Andrady AL. Microplastics in the marine environment. Mar Pollut Bull. 2011;62(8):1596–1605. [DOI] [PubMed] [Google Scholar]
- 5. Amaral-Zettler LA, Zettler ER, Slikas B, et al. The biogeography of the plastisphere: implications for policy. Front Ecol Environ. 2015;13(10):541–546. [Google Scholar]
- 6. Yousif E, Haddad R. Photodegradation and photostabilization of polymers, especially polystyrene. SpringerPlus. 2013;2(1):398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yu P, Liu Z, Wu D, Chen M, Lv W, Zhao Y. Accumulation of polystyrene microplastics in juvenile eriocheir sinensis, and oxidative stress effects in the liver. Aquat Toxicol. 2018;200:28–36. [DOI] [PubMed] [Google Scholar]
- 8. Deng YF, Zhang Y, Lemos B, Ren HQ. Tissue accumulation of microplastics in mice and biomarker responses suggest widespread health risks of exposure. Sci Rep. 2017;7:466–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Duis K, Coors A. Microplastics in the aquatic and terrestrial environment: sources (with a specific focus on personal care products), fate and effects. Environ Sci Eur. 2016;28(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xie X, Deng T, Duan J, Xie J, Yuan J, Chen M. Exposure to polystyrene microplastics causes reproductive toxicity through oxidative stress and activation of the p38 MAPK signaling pathway. Ecotoxicol Environ Saf. 2020;190:110133. [DOI] [PubMed] [Google Scholar]
- 11. Jin H, Ma T, Sha X, et al. Polystyrene microplastics induced male reproductive toxicity in mice. J Hazard Mater. 2021;401:123430. [DOI] [PubMed] [Google Scholar]
- 12. Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. [DOI] [PubMed] [Google Scholar]
- 13. Kakkar P, Das B, Viswanathan P. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 1984;21(2):130–132. [PubMed] [Google Scholar]
- 14. Chance B, Maehly AC. [136] assay of catalases and peroxidases. Meth Enzymol. 1955;2:764–775. [DOI] [PubMed] [Google Scholar]
- 15. Hayashi I, Morishita Y, Imai K, Nakamura M, Nakachi K, Hayashi T. High-throughput spectrophotometric assay of reactive oxygen species in serum. Mutat Res Genet Toxicol Environ Mutagen. 2007;613(1):55–61. [DOI] [PubMed] [Google Scholar]
- 16. Iqbal M, Sharma SD, Zadeh HR, Hasan N, Abdulla M, Athar M. Gluthathione metabolizing enzymes and oxidative stress in ferric nitrioltriacetate mediated hepatic injury. Redox Rep. 1996;2(6):385–391. [DOI] [PubMed] [Google Scholar]
- 17. Ijaz MU, Tahir A, Samad A, Anwar H. Nobiletin ameliorates nonylphenol-induced testicular damage by improving biochemical, steroidogenic, hormonal, spermatogenic, apoptotic and histological profile. Hum Exp Toxicol. 2021;40(3):403–416. [DOI] [PubMed] [Google Scholar]
- 18. Ciftci O, Aydin M, Ozdemir I, Vardi N. Quercetin prevents 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin-induced testicular damage in rats. Andrologia. 2012;44(3):164–173. [DOI] [PubMed] [Google Scholar]
- 19. Momeni HR, Soleimani Mehranjani M, Abnosi MH, Mahmoodi M. Effects of vitamin E on sperm parameters and reproductive hormones in developing rats treated with para-nonylphenol. Int J Reprod Biomed. 2009;7(3):111–116. [Google Scholar]
- 20. Aksu EH, Akman O, Özkaraca M, Ömür A, Uçar Ö. Effect of Maclura pomifera extract on cisplatin-induced damages in reproductive system of male rats. Kafkas Univ Vet Fak Derg. 2015;21:397–403. [Google Scholar]
- 21. Thompson RC. Microplastics in the marine environment: sources, consequences and solutions. In: Bergmann M, Gutow L, Klages M. eds. Marine Anthropogenic Litter. Springer; 2015:185–200. [Google Scholar]
- 22. Wright SL, Kelly FJ. Plastic and human health: a micro issue? Environ Sci Technol. 2017;51(12):6634–6647. [DOI] [PubMed] [Google Scholar]
- 23. Sussarellu R, Suquet M, Thomas Y, et al. Oyster reproduction is affected by exposure to polystyrene microplastics. Proc Natl Acad Sci. 2016;113(9):2430–2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lönnstedt OM, Eklöv P. Environmentally relevant concentrations of microplastic particles influence larval fish ecology. Science. 2016;352(6290):1213–1216. [DOI] [PubMed] [Google Scholar]
- 25. Tremellen K. Oxidative stress and male infertility—a clinical perspective. Hum Reprod Update. 2008;14(3):243–258. [DOI] [PubMed] [Google Scholar]
- 26. Talas ZS, Yilmaz I, Ozdemir I, Ates B, Gok Y, Cetinkaya B. Role of synthesized organoselenium compounds on protection of rat erythrocytes from DMBA-induced oxidative stress. Biol Trace Elem Res. 2009;128(2):167–175. [DOI] [PubMed] [Google Scholar]
- 27. Sposito C, Camargo M, Tibaldi DS, et al. Antioxidant enzyme profile and lipid peroxidation products in semen samples of testicular germ cell tumor patients submitted to orchiectomy. Int Braz J Urol. 2017;43(4):644–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Selamoglu Talas Z. Propolis reduces oxidative stress in l-NAME-induced hypertension rats. Cell Biochem Funct. 2014;32(2):150–154. [DOI] [PubMed] [Google Scholar]
- 29. Liochev SI, Fridovich I. Mechanism of the peroxidase activity of Cu, Zn superoxide dismutase. Free Radic Biol Med. 2010;48(12):1565–1569. [DOI] [PubMed] [Google Scholar]
- 30. Mates JM. Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology. 2000;153(1-3):83–104. [DOI] [PubMed] [Google Scholar]
- 31. Wisniewski P, Romano RM, Kizys MM, et al. Adult exposure to bisphenol A (BPA) in Wistar rats reduces sperm quality with disruption of the hypothalamic–pituitary–testicular axis. Toxicology. 2015;329:1–9. [DOI] [PubMed] [Google Scholar]
- 32. Walker WH. Testosterone signaling and the regulation of spermatogenesis. Spermatogenesis. 2011;1(2):116–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Song YJ, Kim DG, Nam HM, et al. Evaluation of the efficacy of immunocastration vaccine composed of gonadotrophin-releasing hormone conjugated with salmonella typhimurium flagellin in rats. Reprod Domest Anim. 2012;47(4):e47–50. [DOI] [PubMed] [Google Scholar]
- 34. Walker WH, Cheng J. FSH and testosterone signaling in Sertoli cells. Reproduction. 2005;130(1):15–28. [DOI] [PubMed] [Google Scholar]
- 35. Karami A, Romano N, Galloway T, Hamzah H. Virgin microplastics cause toxicity and modulate the impacts of phenanthrene on biomarker responses in African catfish (Clarias gariepinus). Environ Res. 2016;151:58–70. [DOI] [PubMed] [Google Scholar]
- 36. Sun L, Zuo Z, Chen M, Chen Y, Wang C. Reproductive and transgenerational toxicities of phenanthrene on female marine medaka (Oryzias melastigma). Aquat Toxicol. 2015;162:109–116. [DOI] [PubMed] [Google Scholar]
- 37. Aktas C, Kanter M, Erboga M, Ozturk S. Anti-apoptotic effects of curcumin on cadmium-induced apoptosis in rat testes. Toxicol Ind Health. 2012;28(2):122–130. [DOI] [PubMed] [Google Scholar]
- 38. Liu HC, Zhu D, Wang C, et al. Effects of etomidate on the steroidogenesis of rat immature Leydig cells. PLoS One 2015;10(11):1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Raucci F, D’Aniello A, Di Fiore MM. Stimulation of androgen production by D-aspartate through the enhancement of StAR, P450scc and 3β-HSD mRNA levels in vivo rat testis and in culture of immature rat Leydig cells. Steroids. 2014;84:103–110. [DOI] [PubMed] [Google Scholar]
- 40. Rasmussen MK, Ekstrand B, Zamaratskaia G. Regulation of 3beta-hydroxysteroid dehydrogenase/Delta(5)-Delta(4) isomerase: a review. Int J Mol Sci. 2013;14(9):17926–17942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zinaman MJ, Brown CC, Selevan SG, Clegg ED. Semen quality and human fertility: a prospective study with healthy couples. J Androl. 2000;21(1):145–153. [PubMed] [Google Scholar]
- 42. Siu ER, Wong EW, Mruk DD, Porto CS, Cheng CY. Focal adhesion kinase is a blood–testis barrier regulator. Proc Natl Acad Sci. 2009;106(23):9298–9303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Oborna I, Wojewodka G, De Sanctis JB, et al. Increased lipid peroxidation and abnormal fatty acid profiles in seminal and blood plasma of normozoospermic males from infertile couples. Hum Reprod. 2010;25(2):308–316. [DOI] [PubMed] [Google Scholar]
- 44. Nair N. Dose-dependent short-term study of di-n-butyl phthalate on the testicular antioxidant system of Wistar rats. Environ Sci Pollut Res Int. 2015;22(3):2196–2204. [DOI] [PubMed] [Google Scholar]
- 45. Saïd L, Banni M, Kerkeni A, Saïd K, Messaoudi I. Influence of combined treatment with zinc and selenium on cadmium induced testicular pathophysiology in rat. Food Chem Toxicol. 2010;48(10):2759–2765. [DOI] [PubMed] [Google Scholar]
- 46. Bisht S, Faiq M, Tolahunase M, Dada R. Oxidative stress and male infertility. Nat Rev Urol. 2017;14(8):470–485. [DOI] [PubMed] [Google Scholar]
- 47. Yucel C, Arslan FD, Ekmekci S, et al. Protective effect of all-trans retinoic acid in cisplatin-induced testicular damage in rats. World J Mens Health. 2019;37(2):249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ko EYMD, Sabanegh ESJ, Agarwall A. Male infertility testing: reactive oxygen species and antioxidant capacity. Fertil Steril. 2014;102(6):1518–1527. [DOI] [PubMed] [Google Scholar]
- 49. Eno CO, Zhao G, Olberding KE, Li C. The Bcl-2 proteins Noxa and Bcl-xL co-ordinately regulate oxidative stress-induced apoptosis. Biochem J. 2012;444(1):69–78. [DOI] [PubMed] [Google Scholar]
- 50. Desagher S, Martinou JC. Mitochondria as the central control point of apoptosis. Trends Cell Biol. 2000;10(9):369–377. [DOI] [PubMed] [Google Scholar]
- 51. Liu NS, Du X, Lu J, He BP. Diva reduces cell death in response to oxidative stress and cytotoxicity. PLoS One. 2012;7(8):e43180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Siddiqui WA, Ahad A, Ahsan H. The mystery of BCL2 family: Bcl-2 proteins and apoptosis: an update. Arch Toxicol. 2015;89(3):289–317. [DOI] [PubMed] [Google Scholar]
- 53. Li WS, Jiang BH, Cao XL, Xie YJ, Huang T. Protective effect of lycopene on fluoride-induced ameloblasts apoptosis and dental fluorosis through oxidative stress-mediated caspase pathways. Chem Biol Interact. 2017;261:27–34. [DOI] [PubMed] [Google Scholar]
- 54. Wang JY, Luo ZG. Non-apoptotic role of caspase-3 in synapse refinement. Neurosci Bull. 2014;30(4):667–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Karacaoğlu E, Selmanoğlu G. Effects of heat–induced food contaminant furan on reproductive system of male rats from weaning through postpuberty. Food Chem Toxicol. 2010;48(5):1293–1301. [DOI] [PubMed] [Google Scholar]