Abstract
Cortical long-term potentiation (LTP) serves as a cellular model for chronic pain. As an important subtype of adenylyl cyclases (ACs), adenylyl cyclase subtype 1 (AC1) is critical for the induction of cortical LTP in the anterior cingulate cortex (ACC). Genetic deletion of AC1 or pharmacological inhibition of AC1 blocked behavioral allodynia in animal models of neuropathic and inflammatory pain. Our previous experiments have identified a lead candidate AC1 inhibitor, NB001, which is highly selective for AC1 over other AC isoforms, and found that NB001 is effective in inhibiting behavioral allodynia in animal models of chronic neuropathic and inflammatory pain. However, previous experiments were carried out in adult male animals. Considering the potential gender difference as an important issue in researches of pain and analgesia, we investigated the effect of NB001 in female chronic pain animal models. We found that NB001, when administered orally, has an analgesic effect in female animal models of neuropathic and inflammatory pain without any observable side effect. Genetic deletion of AC1 also reduced allodynia responses in models of neuropathic pain and chronic inflammation pain in adult female mice. In brain slices of adult female mice, bath application of NB001(20 μM) blocked the induction of LTP in ACC. Our results indicate that calcium-stimulated AC1 is required for injury-related cortical LTP and behavioral allodynia in both sexes of adult animals, and NB001 can be used as a potential therapeutic drug for treating neuropathic and inflammatory pain in man and woman.
Keywords: Neuropathic pain, inflammatory pain, AC1, NB001, LTP, female
Introduction
Pain is an unpleasant sensation that serves a vital function for animals and humans to avoid tissue damage. Generally, pain is divided into two different major forms: acute pain and chronic pain. Acute pain is short-lasting, and often serves as a protective and learning signal. Chronic pain, that is long-lasting pain, is caused by injury to tissue or nerve systems. Furthermore, the injury and injury-related areas undergo long-term plastic changes, and either painful sensations are significantly enhanced (hyperalgesia), or non-noxious stimuli can cause pain (allodynia). Previous integrative neuroscience studies have found that chronic pain and acute pain operate through different central mechanisms. 1 Chronic pain is a major medical problem that is resistant to conventional medical intervention. 1
The anterior cingulate cortex (ACC) is a key cortical region in pain research. 2 ACC neurons respond to peripheral noxious stimuli, and show increased responses to a greater intensity of pain.3–5 This key finding is further supported by numerous human imaging studies in both normal individuals and patients with chronic pain. Chronic pain can be thought of as a type of persistent sensory memory, and increasing evidence suggests that long-term potentiation (LTP) in the dorsal horn of the spinal cord and cortical areas, including the ACC, are related to chronic pain.1,6,7 Most forms of LTP reported in the ACC are post-synaptically induced. It is likely that chronic pain is caused to prolong overexcitation of ACC. 1
LTP is a key cellular model for cortical excitation in chronic pain. Peripheral inflammation or nerve injury produces LTP in the ACC.8–10 Investigation of the induction and expression of cortical LTP provide potential new targets for treatment of chronic pain. 2 Among several key messengers that contribute to LTP in the ACC, adenylyl cyclase subtype 1 (AC1) is unique for LTP in this region. Pharmacological inhibition of AC1 by a selective inhibitor, NB001, abolishes LTP induction in the mouse ACC neurons. 11 AC1 is also critical for PKMζ activity in chronic pain. 12 Furthermore, AC1 can be upregulated in chronic pain. 13 AC1 is proposed to be a suitable neuron-specific drug target for treating different forms of chronic pain, including inflammation pain, cancer pain, visceral pain and chronic muscle pain. 14 As expected, NB001 produced a potent analgesic effect in behavioral allodynia without any observable side effect. 15 Genetic deletion of AC1 in mice abolishes or reduces allodynia responses in models of neuropathic pain, chronic inflammation and chronic muscle pain, whereas other physiological and behavioral functions such as acute pain, anxiety-like behaviors, and motor functions remain intact in AC1 knockout mice. 16 However, previous experiments were carried out in male animals, but not female.
Recent studies indicate that gender may act as an important factor in pain and analgesia. It has been known that there may be gender-related difference in chronic pain and the mechanism of drugs.17,18 Although recent study indicated that LTP in the ACC does not show any sex-related difference, 19 it is unknown if the effect of NB001 on cortical synaptic plasticity and injury-related behavioral responses may be gender related. Previous studies of NB001 were mostly carried out in adult male animals. To determine if sex difference may exist in the effect of NB001, we carried out pharmacological and electrophysiological experiments to determine the analgesic effects of NB001 in female chronic models, both in vitro and in vivo. The potential behavioral side effects of NB001 were also examined in female mice.
Materials and methods
Animals
Adult female mice (6 to 8 weeks) were used. C57BL/6 mice were purchased from the Experimental Animal Center of Xi’an Jiaotong University (Xi’an, China). Animals were maintained on a 12 h light/dark cycle with food and water provided ad libitum. AC1 knockout mice were bred for several generations on a C57BL/6 background. All procedures and handling of animals were performed with permission according to the guidelines of Xi’an Jiaotong University.
Animal models for neuropathic and inflammatory pain
A model of neuropathic pain was induced by the ligation of the common peroneal nerve (CPN), as described previously. 20 The CPN was visible between the anterior and posterior groups of muscles, running almost transversely. The left CPN was slowly ligated with chromic gut suture 5-0 until contraction of the dorsiflexor of the foot was visible, signified through twitching of the digits. The skin was then sutured and cleaned. The mice were used for behavior and/or electrophysiological studies on postsurgical day 7. To induce inflammatory pain, 50% Complete Freund’s Adjuvant (CFA) (10 μl) in saline was injected subcutaneously in the dorsum of the left hindpaw. 16 The mice were used for behavior and/or electrophysiological studies 3 days after CFA injection.
Mechanical withdrawal threshold measurement
One week following nerve ligation or 3 days following CFA injection, the mice paw withdrawal threshold was tested with von Frey filaments (Stoelting; Wood Dale, Illinois) applied to the paw. The animals were placed in Lucite cubicles over a wire mesh and acclimated for 30 min before testing. A series of filaments (0.008, 0.02, 0.04, 0.16, 0.4, 0.6, 1, 1.4, 2 g) with various bending forces (according to 0.078, 0.196, 0.392, 1.568, 3.92, 5.88, 9.8, 13.72, 19.6 mN) were applied to the plantar surface of the hindpaw ipsilateral of the nerve injury or CFA injection side until the mice withdrew from the stimulus. Each filament was applied twice. The lowest force at which a withdrawal response was obtained was then taken as the paw withdrawal threshold.
Hot plate test
The mice were placed in the behavior room at least 2 h before the test to allow them to become accustomed to the experimental apparatus. The mouse was placed on the hot (55 °C) plate and the latency to their first reaction (licking, shaking, jumping, or lifting of the hind paw) was recorded manually. If the mouse did not show any response within 20 s, the test was terminated to avoid tissue damage and the latency to the response was recorded as 20 s. Three values were used for average of latency to response.
Tail flick test
The spinal nociceptive tail-flick reflex was evoked by focused, radiant heat (Columbus Instruments) provided by a 50 W projector lamp focused on a 1.5 mm by 10 mm area on the underside of the tail. The latency to reflexive removal of the tail from the heat was measured by a digital photocell timer to the nearest 0.1 s. The cutoff time of 10 s was used to minimize damage to the skin of the tail.
Mechanical allodynia
Mice were individually placed in a round, transparent container 20 cm in diameter and were allowed to acclimate for 30 min before testing. Mechanical sensitivity was assessed with a set of von Frey filaments. Based on preliminary experiments that characterized the threshold stimulus in untreated animals, the innocuous 0.04 g filament was used to detect mechanical allodynia.12,21 The filament was applied to the point of bending six times to the surfaces of the hindpaws. Positive responses consisted of prolonged hindpaw withdrawal followed by licking or scratching. Mechanical threshold was assessed on the basis of the responsiveness of the hindpaw to the application of von Frey filaments to the point of bending. The filament was applied over the dorsum of the paw while the animal was resting.
Elevated plus maze
The elevated plus maze (EPM, Med Associates) consisted of two open arms and two closed arms situated perpendicular to each other. The maze was situated ∼70 cm from the floor. For each test, mice were individually placed in the center square and allowed to move freely for 5 min. The number of entries and time spent in each arm were recorded. The animals were given an oral injection of NB001 (10 mg/kg) or saline 30 min before testing. A video camera tracking system (Ethovision) was used to generate the traces.
Open-field test
To record locomotor activity, we used an open-field activity monitor (43.2 cm by 43.2 cm by 30.5 cm; Med Associates). This system uses paired sets of photo beams to detect movement in the open field, and movement is recorded as beam breaks. The open field is placed inside an isolation chamber with dim illumination and a fan. Each subject was given an oral injection of NB001 (10 mg/kg) or saline 30 min before being placed in the center of the open field. Locomotor activity was then measured for 30 min.
RotaRod test
To test motor function, we used a RotaRod from Med Associates. The RotaRod test was performed by measuring the time each animal was able to maintain its balance while walking on a rotating drum. 1 h before testing, animals were trained on the RotaRod at a constant acceleration of 16 rpm until they could stay on for 30 s. For testing, the RotaRod was set to accelerate from 4 to 40 rpm over a 5 min period. Mice were given three trials with a maximum time of 300 s and a 5 min inter-trial rest interval. The latency to fall was taken as a measure of motor function. Each subject was given an oral injection of NB001 (10 mg/kg) or saline 30 min before testing.
Fear memory
Fear conditioning was performed in an isolated shock chamber (Med Associates) and performed in a blind manner to the treatment. The conditioned stimulus (CS) was an 85 dB sound at 2800 Hz, and the unconditioned stimulus (US) was a continuous scrambled footshock at 0.75 mA. After 2 min of habituation, animals received the CS-US pairing (a 30 s tone (CS) and a 2 s shock (US) starting at 28 s; three shock-tone pairings were delivered at 30 s intervals), and the mice remained in the chamber for an additional 30 s for measurement of immediate freezing. At 1 day after training, each mouse was placed back into the shock chamber and the freezing response was recorded for 3 min (contextual conditioning). We pretreated mice with NB001 (10 mg/kg) or saline. Both groups of mice then received fear conditioning 45 min after the injection. One day after the conditioning, we measured the freezing responses. 22
Homecage behaviors
24 h homecage behaviors were tested using AI homecage system (Shanghai Vanbi Intelligent Technology Co., Ltd.). The digital video cameras were mounted perpendicular to the cages. The cameras input into a Pelco video processor connected to computers. Video data were analyzed by Tracking Master software (Shanghai Vanbi Intelligent Technology Co., Ltd.). During 24 h recording, mice were housed in standard cages on a 12 h light/dark cycle with food and water provided ad libitum. 23
Whole-cell patch-clamp recording
Coronal brain slices (300 μm) at the level of the ACC were prepared using standard methods.9,24 Mice were anesthetized with 1-2% isoflurane. The whole brain was quickly removed from the skull and submerged in the oxygenated (95% O2 and 5% CO2) ice-cold cutting artificial cerebrospinal fluid (ACSF) containing the following (in mM): 252 sucrose, 2.5 KCl, 6 MgSO4, 0.5 CaCl2, 25 NaHCO3, 1.2 NaH2PO4, and 10 glucose (pH 7.3-7.4). After cooling in the ACSF for a short time, the whole brain was trimmed for an appropriate part to glue onto the ice-cold stage of a vibrating tissue slicer (VT1200S, Leica). Slices were transferred to a submerged recovery chamber containing oxygenated (95% O2 and 5% CO2) ACSF containing the following (in mM): 124 NaCl, 4.4 KCl, 2 CaCl2, 1 MgSO4, 25 NaHCO3, 1 NaH2PO4, and 10 glucose at room temperature for at least 1 h before recording. Experiments were performed in a recording chamber on the stage of a BX51W1 microscope equipped with infrared DIC (differential interference contrast) optics for visualization. Excitatory postsynaptic currents (EPSCs) were recorded from layer II/III neurons with an Axon 200B amplifier (Axon Instruments), and the stimulations were delivered by a bipolar tungsten stimulating electrode placed in layer V/VI of the ACC. α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA) receptor-mediated EPSCs were induced by repetitive stimulations at 0.05 Hz, and neurons were voltage-clamped at −70 mV. The N-methyl-D-aspartate (NMDA) receptor-mediated component of EPSCs was pharmacologically isolated in Mg2+-free ACSF containing CNQX (20 μM). Picrotoxin (100 μM) was always present to block g-aminobutyric acid type A (GABAA) receptor-mediated inhibitory synaptic currents in all experiments, and the access resistance was 15 to 30 megohms throughout the experiment. Data were discarded if access resistance changed more than 15% during an experiment. Data were filtered at 1 kHz and digitized at 10 kHz.
Field potential recording
Preparation of the multi-electrode array
The MED64 recording system (Panasonic, Japan) was used for extracellular field potential recordings. There is an array of 64 planar microelectrodes in the MED64 probe (P515A, Panasonic, Japan), arranged in an 8 × 8 pattern, with an interpolar distance of 150 μm. The surface of the MED64 probe was treated with 0.1% polyethyleneimine (Sigma, St. Louis, MO; P-3143) in 25 mmol/L borate buffer (pH 8.4) overnight at room temperature before experiments. Then the surface of probe was rinsed three times with sterile distilled water.11,25
Electrophysiological recordings
After incubation, one slice containing the ACC was transferred to the prepared MED64 probe and perfused with oxygenated (95% O2 and 5% CO2) ACSF at 28-30 °C and maintained at a 2 ml/min flow rate. The slice was positioned on the MED64 probe in such a way that the different layers of the ACC were entirely covered by the whole array of the electrodes, and then a fine-mesh anchor was placed on the slice to ensure its stabilization during the experiments. One of the channels located in the layer V of the ACC was chosen as the stimulation site, from which the best synaptic responses can be induced in the surrounding recording channels. Slices were kept in the recording chamber for at least 1 h before the start of experiments. Bipolar constant current pulse stimulation (1-10 µA, 0.2 ms) was applied to the stimulation channel and the intensity was adjusted so that a half-maximal field excitatory postsynaptic potential (fEPSP) was elicited in the channels closest to the stimulation site. The channels with fEPSPs were considered as active channels and their fEPSPs responses were sampled every 1 min and averaged every 5 min. The parameter of “slope” indicated the averaged slope of each fEPSP recorded by activated channels. Stable baseline responses were first recorded until the baseline response variation is less than 5% in most of the active channels within 0.5 h.11,26
Data analysis
Results were expressed as mean ± SEM. Statistical comparisons were performed with one-way ANOVA or two-way ANOVA and Student’s t test. In all cases, * p < 0.05 was considered statistically significant.
Results
Mechanical allodynia after peroneal nerve ligation
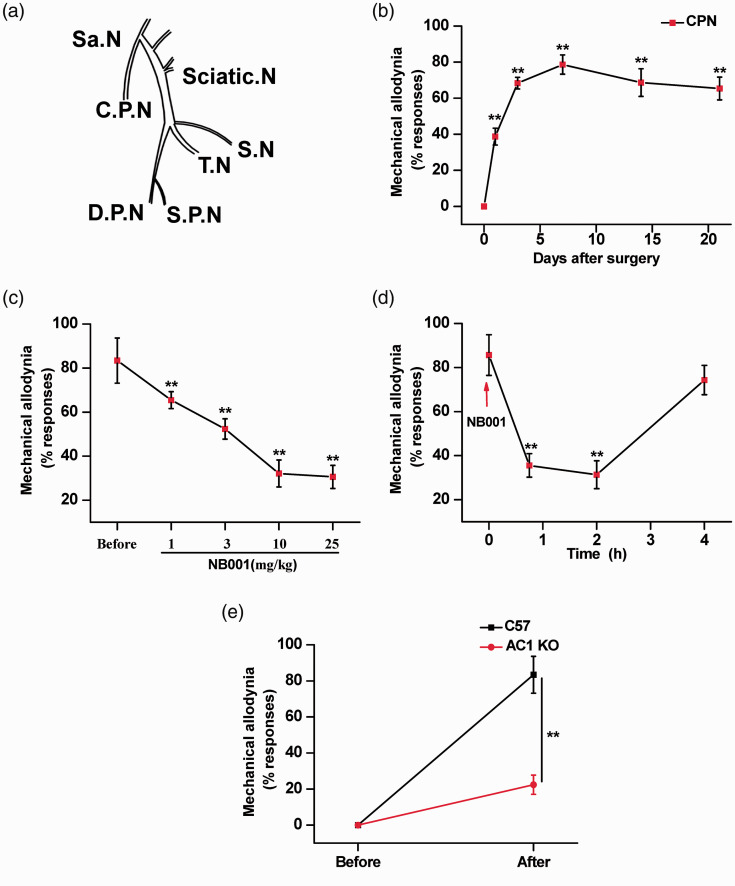
A previous neuropathic pain model common peroneal nerve (CPN) was used to assess neuropathic pain in female mice. We excluded mice (1 out of 9) that showed any response to this pressure, possibly due to some unknown injury during shipment. This mechanical allodynia was persistent significantly until day 21. However, the peak of mechanical allodynia was observed on day 7. The CPN-ligated female animals showed statistically significant allodynia. There was a progressive increase in allodynia up to day 7 that remained statistically significant from the sham-operated animals and was maintained during the following days up to day 21 (n = 8 mice; Figure 1(b)). These data indicate that ligation of the common peroneal nerve can be used as an efficacious mouse model for assessing neuropathic pain in female mice.
Figure 1.
Analgesic effect of NB001 on neuropathic pain in female mice. (a) Schematic diagram of the left lumbar plexus, sciatic nerve, and its branches. Abbreviations: Sa.N, saphenous nerve; C.P.N, common peroneal nerve; D.P.N, deep peroneal nerve; S.N, sural nerve; T.N, tibial nerve; S.P.N, superficial peroneal nerve. (b) Behavioral responses at the ipsilateral hindpaw. (n = 8) (c) Oral administration of NB001 (1, 3, 10, and 25 mg/kg) produced significant analgesic effects. The inhibitory effect is dose-related; greater inhibition was found with a higher dose of NB001 (n = 8 each group). (d) Allodynia was measured at 45 min, 2 h and 4 h after oral application of 10 mg/kg NB001 (n = 10). (e) Elimination of behavioral sensitization (allodynia) nerve injury in AC1 knockout mice (n = 6). *p<0.05, **p<0.01.
Analgesic effects of NB001 in female animal models of neuropathic pain
With the identification of NB001 as an AC1 inhibitor, we examined the effects of NB001 on behavioral allodynia in animal models of neuropathic pain. Oral administration of NB001 (10 mg/kg) given 45 min before behavioral allodynia testing produced a significant analgesic effect. NB001 at higher doses of 10 and 25 mg/kg produced a greater inhibition of behavioral allodynia (Figure 1(c)). And we found that NB001 at 10 mg/kg (orally) produced a significant reduction in behavioral allodynia 45 min after oral ingestion, and the analgesic effect lasted for at least 2 h (Figure 1(d)). The analgesic effect of NB001 is reversible after the oral application NB001 4 h.
We also tested the possible roles of AC1 in allodynia induced by female CPN model in AC1 knockout mice. After CPN surgery, mechanical allodynia of CPN model mice were significantly reduced in female AC1 knockout mice relative to wild-type mice (n = 8 mice for each group; Figure 1(e)), indicating that ablation of AC1 alone is sufficient to affect allodynia in the female animal model of neuropathic pain.
Analgesic effects of NB001 in female animal models of inflammatory pain
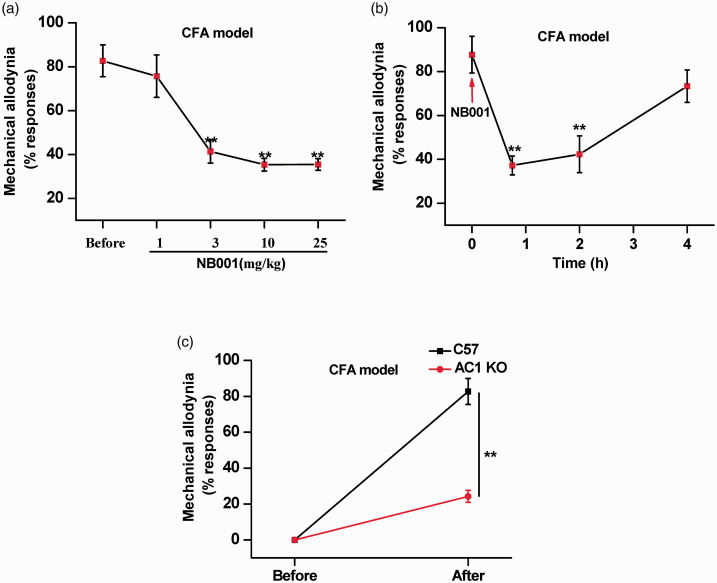
Behavioral allodynia induced by the hindpaw injection of complete Freund’s adjuvant (CFA) (50%) has been commonly used for evaluating a drug’s analgesic effects in chronic inflammatory pain. We used this model to explore whether NB001 would be analgesic in the female animal model of inflammatory pain induced by CFA injection. Application of a 0.04 g Von Frey fiber to the hindpaw elicited no response in control mice (Figure 2(a)). 3 days after the CFA injection, NB001 (3, 10 and 25, but not 1mg/kg, orally) produced significant analgesic effects. The inhibitory effect was dose-related: greater inhibition was found with a higher dose of NB001 (n = 8 mice for each group) (Figure 2(a)).
Figure 2.
Analgesic effect of NB001 on inflammatory pain in female mice. (a) Oral administration of NB001 (3, 10 and 25 mg/kg) produced significant analgesic effects. The inhibitory effect is dose-related; greater inhibition was found with a higher dose of NB001 (n = 8 each group). (b) Allodynia was measured at 45 min, 2 h and 4 h after oral application of 10 mg/kg NB001 (n = 8). (c) Elimination of allodynia before and after CFA injection in C57 and AC1 knockout Mice (n = 7). *p<0.05, **p<0.01.
We also tested the possible roles of AC1 in allodynia induced by a hindpaw injection of female CFA model in AC1 knockout mice. After injection of CFA, mechanical allodynia of CFA model mice was significantly reduced in female AC1 knockout mice relative to wild-type mice (n = 8 mice for each group; Figure 2(c)). These results indicated that AC1 plays an essential role in the female animal model of inflammatory pain.
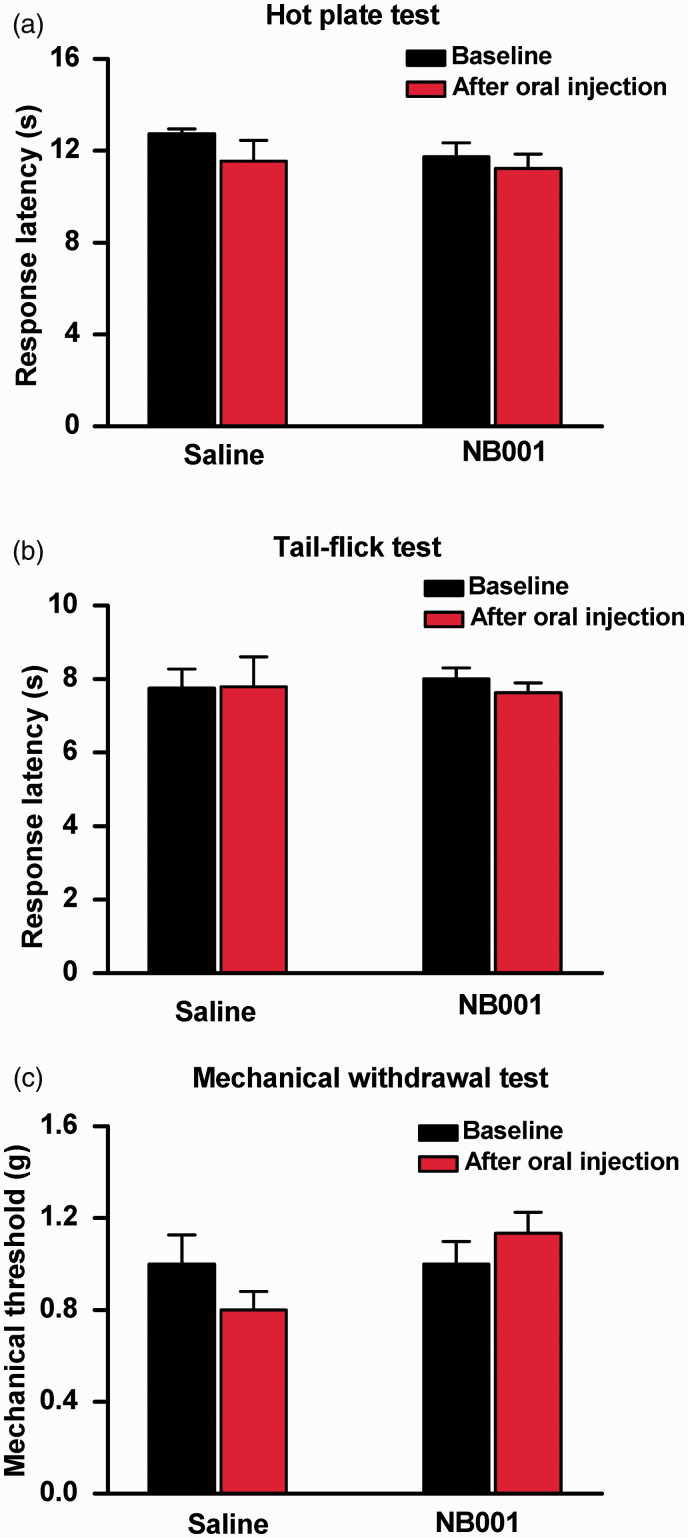
Effect of NB001 on acute pain
We carried out behavioral tests to determine whether NB001 affects physiological nociceptive responses in normal female mice. We tested the effect of orally injected NB001 in different nociceptive tests. For noxious thermal pain, NB001 affected behavior in neither the spinal nociceptive tail-flick reflex (Figure 3(b)) nor the hot-plate test (at 55°C) (Figure 3(a)). For mechanical threshold, we measured hindpaw withdrawal responses to von Frey filaments in normal mice. Again, NB001 did not produce any significant effect (Figure 3(c)). These results suggest that NB001 did not affect acute nociception, in good accord with previous findings of male animals. 15
Figure 3.
No significant effect of NB001 on acute pain. (a) Effect of NB001 in hot plate test at 55 °C. There was no significant difference in response latency before and after oral injection of saline and NB001 (10 mg/kg) (n = 8) in hot plate test set at 55 °C. (b) Effect of NB001 in tail-flick (TF) test. Oral injection of NB001 (10 mg/kg) did not affect spinal nociceptive TF reflex (n = 8). (c) Comparison of behavioral responses to non-noxious mechanical stimulus. There was no significant difference in hindpaw withdrawal to von Frey filaments before and after oral injection of saline and 10 mg/kg NB001 in normal mice (n = 8).
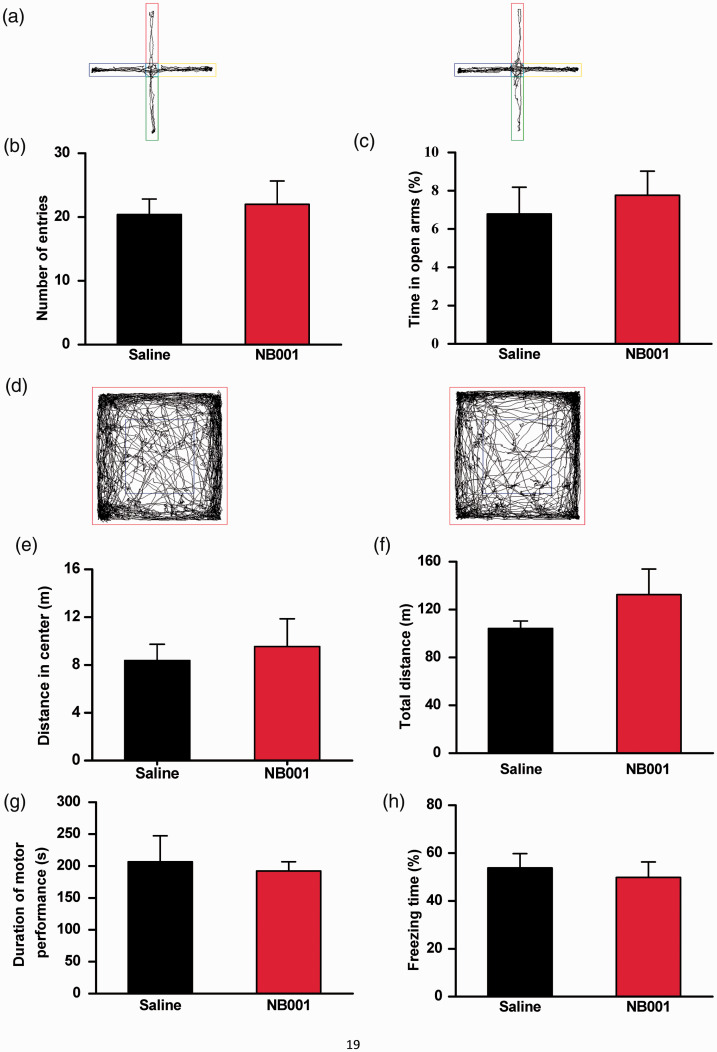
Effect of NB001 on anxiety, motor function, fear memory and homecage behaviors
To test the potential side effects of NB001, we carried out a battery of behavioral anxiety and motor function tests with the same analgesic dose (10 mg/kg) of NB001. First, in two tests of anxiety-like behavior, elevated plus maze (EPM) and open-field test, we found that NB001 did not produce any significant effects (Figure 4(a) to (f)). The NB001 oral injection mice did not make a significantly different in the number of entrances into the arms of the EPM, nor did they spend more time in the open arms when compared to the saline-injected mice (Figure 4(b) and (c)). Also, the time spent in the center of the open-field test did not differ between NB001 and saline injected mice (Figure 4(e)), again demonstrating that NB001 had no effect on anxiety-like behavior. We also tested the motor function of the mice injected with NB001. In the open-field test, we found that oral injection of NB001 didn’t produce significant differences in the total distance traveled between NB001 and saline injected mice (Figure 4(f)). In the RotaRod motor test, NB001 did not cause any significant changes (Figure 4(g)), illustrating that NB001 had no effect on the motor function of the mice.
Figure 4.
No significant effect of NB001 on anxiety-like behavior, locomotor activity, fear memory, and motor function. (a) Representative traces showing the movement of saline- and NB001-injected (10 mg/kg) mice in the EPM for 5 min. (b) There was no significant difference in the number of arm entries (open and closed) between saline-injected (n = 5) and NB001-injected (n = 7) mice. (c) There was no significant difference in the percentage of time spent in the open arms between saline- and NB001-injected mice. (d) Representative traces showing the movement of saline-injected (n = 6) and NB001-injected (n = 6) mice in the open field for 30 min. (e-f) Saline- and NB001-treated mice showed no difference in the open field test. (g) There was no significant difference in latency to fall between saline-injected (n = 5) and NB001-injected (n = 6) mice. In each experiment, mice were given an oral injection of NB001 (10 mg/kg) or saline 30 min before the test. (h) There was no significant difference in fear memory tests (n=6).
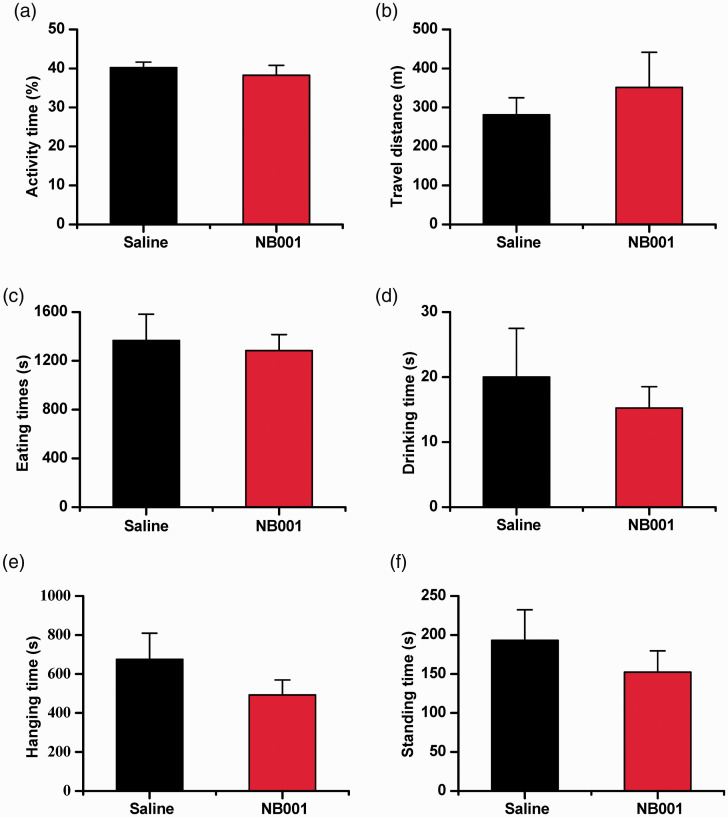
Considering the importance of AC1 in memory function. We tested whether NB001 affects fear memory induced by noxious foot shock. Consistent with previous genetic studies, 16 we found that pretreatment with NB001 (10 mg/kg) did not significantly affect fear memory (Figure 4(h)), indicating that NB001 at the analgesic dosage did not affect the formation of fear memory. By using homecage monitoring, we also proved that drinking, eating, hanging, standing and motor behaviors in homecages were not affected by NB001(Figure 5(a) to (f)).
Figure 5.
No significant effect of NB001 on 24 h homecage behaviors. No significant behavioral alterations in mice in activity time (a), travel distance (b), eating time (c), drinking time (d), hanging time (e) and standing time (f). (n = 8). In each experiment, mice were given an oral injection of NB001 (10 mg/kg) or saline 30 min before the test.
Effect of NB001 on glutamate receptor-mediated currents
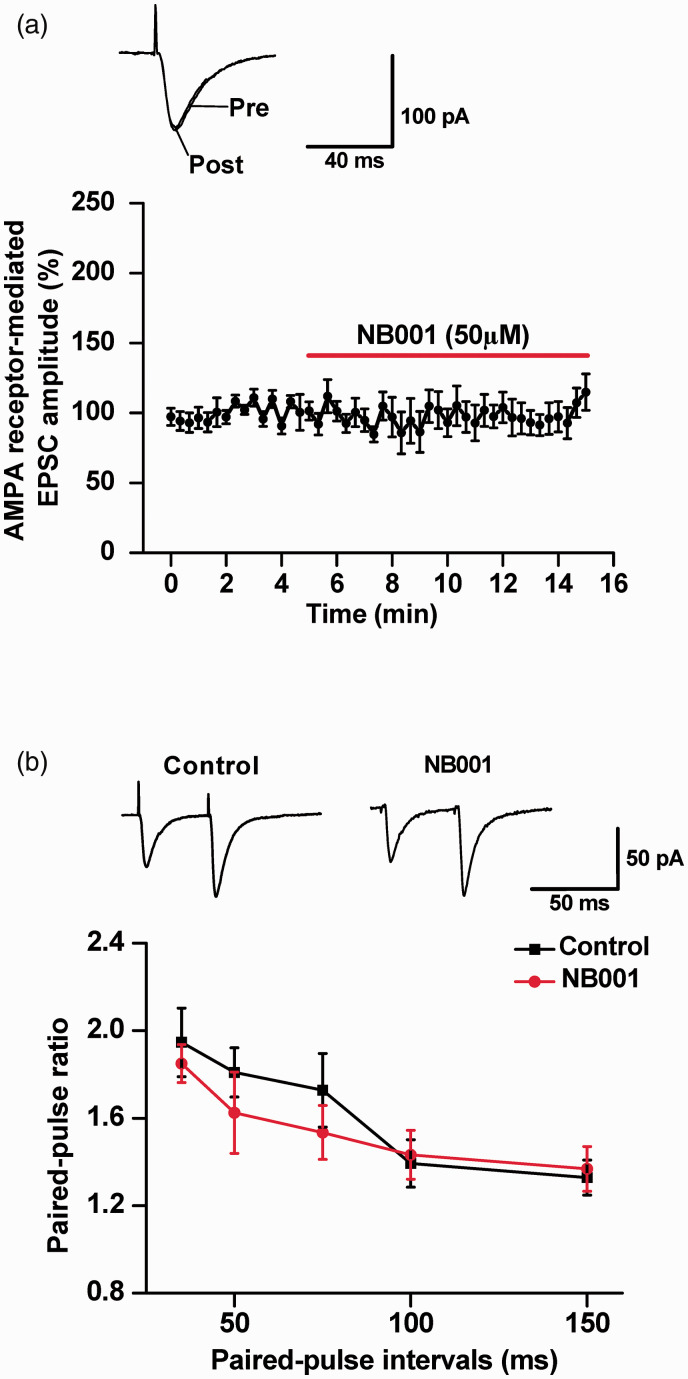
Glutamate AMPA receptors mediate most of the basal synaptic transmission in the spinal dorsal horn and the ACC,27,28 whereas NMDA receptors are important for producing synaptic potentiation.1,24 It is thus important to determine whether NB001 produces analgesic effects by affecting these glutamate receptor-mediated responses in in the ACC of female mice. We performed whole-cell patch-clamp recordings on pyramidal cells from the ACC, a key cortical area for processing pain information in animals and humans. 1 We first measured the effects of NB001 on AMPA receptor-mediated excitatory postsynaptic currents (EPSCs). We did not see any significant change in AMPA receptor-mediated EPSCs after the perfusion with NB001 (50 μM) (n = 8 neurons/6 mice) (Figure 6(a)). Paired-pulse facilitation (PPF), a simple form of synaptic plasticity, was also measured by application of paired stimuli at different intervals. We found that PPF tested at different inter-pulse intervals also did not show any significant change after NB001 application (NB001, n = 8 neurons/4 mice; control, n = 11 neurons/6 mice; two-way ANOVA, P > 0.05) (Figure 6(b)).
Figure 6.
No significant effect of NB001 on AMPA receptor-mediated synaptic responses. (a) Bath application of NB001 (50 μM) did not affect AMPA receptor-mediated basal synaptic responses in the ACC neurons (n = 8 neurons/4 mice). Inset: representative traces and pool data of AMPA receptor-mediated EPSCs in the ACC neurons with the perfusion of NB001 (50 μM) for 10 min. (b) PPF was recorded at intervals of 35, 50, 75, 100, and 150 ms. Black squares: neurons in the absence of NB001 (n = 10 neurons/6 mice); Red circles: neurons in the presence of NB001 (50 μM) (n = 9 neurons/5 mice). Representative traces of PPF with an interval of 50 ms recorded in the ACC are shown in the insets.
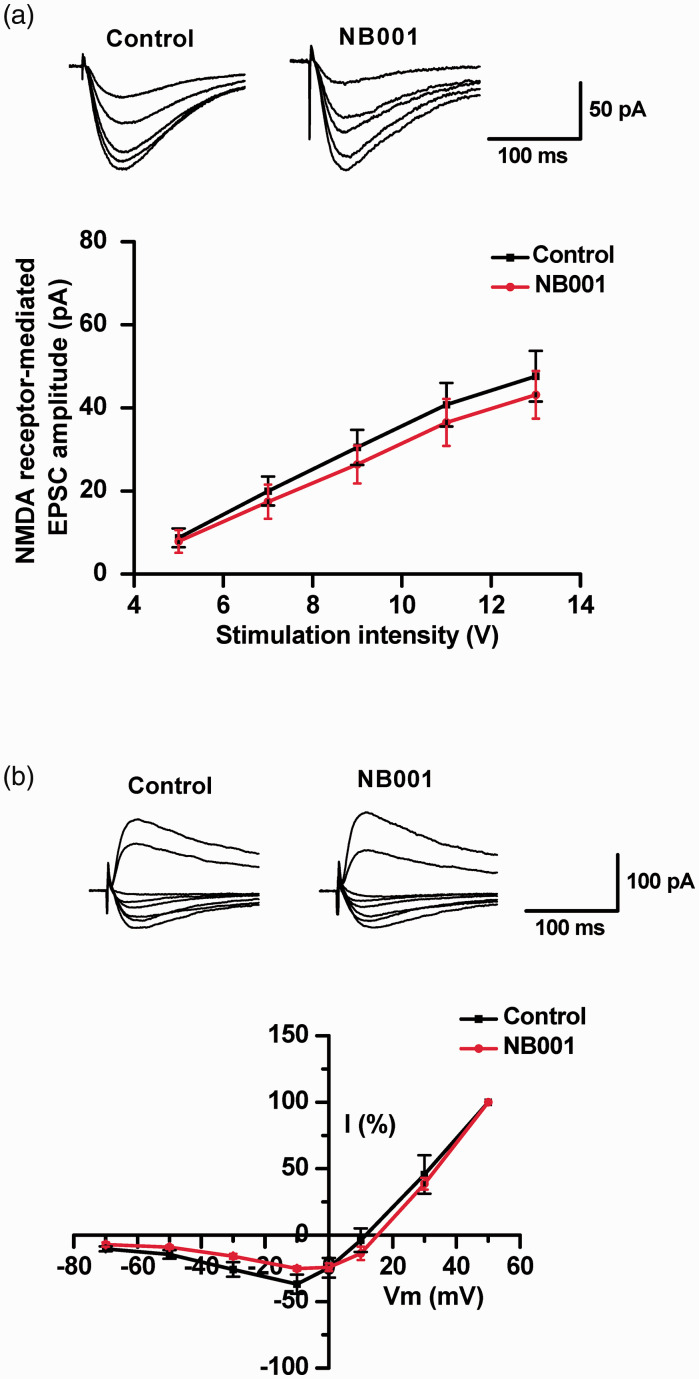
NMDA receptors are important for pain-related plasticity in the spinal cord and cortex.6,29 To rule out the possibility that NB001 may produce analgesic effects through effect on NMDA receptor-mediated responses, we performed two different types of experiments. We first evaluated the effect of NB001 on the input-output curves of NMDA receptor-mediated EPSCs. NB001 (50 μM) application did not produce any reduction of NMDA receptor-mediated input-output curves (Figure 7(a)). Next, we also tested whether NB001 affected the I-V (current-voltage) curve of NMDA receptor-mediated EPSCs. Bath application of NB001 did not significantly affect the functions of NMDA receptor-mediated I-V curves (Figure 7(b)).
Figure 7.
No significant effect of NB001 on NMDA receptor-mediated responses. (a) NB001 application did not affect NMDA receptor-mediated EPSCs recorded from the ACC pyramidal cells. Representative traces and pooled data of synaptic NMDA receptor-mediated input-output curve in ACC neurons in the absence (n = 8 neurons/4 mice) and presence (n = 9 neurons/4 mice) of NB001 (50 μM) in the pipette. (b) NB001 application did not affect the I-V response curves of NMDA receptor-mediated responses. Representative traces and pooled data of synaptic NMDA receptor-mediated I-V curve in ACC neurons in the absence (n = 7 neurons/3 mice) and presence (n = 8 neurons/4 mice) of NB001.
Effect of NB001 on LTP in the ACC in the female mice
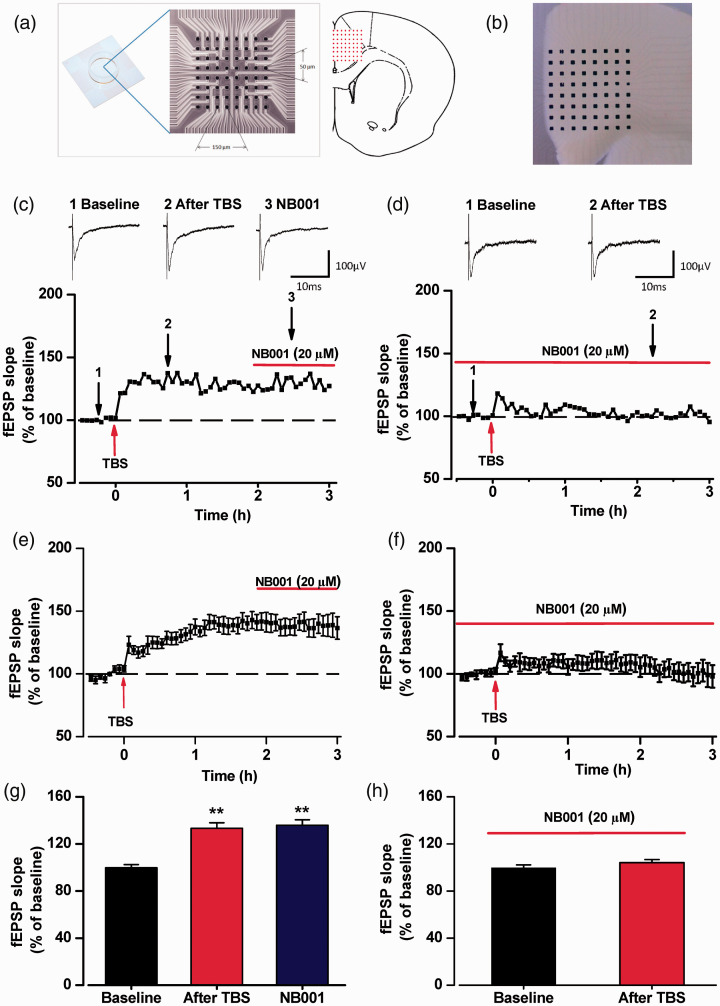
Previous studies has shown that an AC1 inhibitor NB001 blocks LTP in the ACC of male mice. 11 Here we tested the effects of bath application of NB001 to the induction and maintenance of LTP in female ACC slices by using MED64 recording system. In the presence of NB001 (20 μM), we found that LTP induction was totally blocked in ACC (Figure 8(f) and (h)). However, the stable LTP induced in female ACC slices were not affected by the application of NB001(20 μM). Our results showed that presence of NB001(20 μM) could block the induction of LTP, but didn’t affect the maintenance in female mice ACC slices (Figure 8(e) and (g)).
Figure 8.
Effect of NB001 on induction and expression of postsynaptic LTP in ACC. (a) Schematic diagram and the microphotograph showed the scale of the MED-64 probe (left), location of MED-64 probe on the ACC slice (right). (b) One example microscopy photograph of the location of ACC slice and MED-64 probe. (c) The sample temporal change of the fEPSP slopes to illustrate the effect of NB001 on the maintenance of LTP from the female ACC slice. The averaged temporal changes of fEPSP slopes (e) and the final averaged slope (g) showed that the maintenance of LTP were not affected by application of NB001 (20 μM) (n = 9 slices/8 mice). (d) The sample temporal change of the fEPSP slopes to illustrate the effect of NB001 on the induction of LTP from the female ACC slice. The averaged temporal changes of fEPSP slopes (e) and the final averaged slope (g) showed that the induction of LTP were blocked by application of NB001 (20 μM) (n = 13 slices/9 mice). The NB001 were applied before and after TBS. *p<0.05, **p<0.01.
Discussion
Sex-related differences in pain and analgesia are reported in humans and animals. Women tend to have a lower pain threshold than men and suffer longer and more severe pain. 18 As noted in a previous review, at least 79% of animal studies published in Pain over the preceding 10 years included male subjects only, with a mere 8% of studies on females only, and another 4% explicitly designed to test for sex differences. 30 As an AC1 inhibitor, NB001 is known to produce analgesic effects in different pain models.15,17,31,32 However, there is limited information about female. In the present study, we confirmed similar analgesic effects of NB001 on behavioral allodynia induced by neuropathic and inflammatory pain in adult female mice without any observable side effect.
Previous studies have showed that male and female animals may exhibit different responses in same pain animal model. For example, a fibromyalgia model induced by intermittent cold stress showed sex-specificity in male mice, but not female. 33 Recently, the model of neuropathic pain induced by ligation of the common peroneal nerve were only tested in male mice. The present results showed that ligation of the common peroneal nerve in female mice also induced progressive increase in allodynia up to day 7 and maintained to at least day 21, which is similar with male mice.
Our previous studies in adult male mice found that NB001 did not affect basal behavioral anxiety. 15 In consistent with this finding, we found that NB001 also did not affect behavioral anxiety in adult female mice as tested by EPM and open filed tests. In addition, by using homecage behaviors including drinking, eating, hanging, and standing, we found that NB001 did not significantly affect any of these behaviors in female mice. After administration of NB001, female mice did not show marked memory defects, in accord with male mice. 15 One explanation for this lack of effect may be that the function of AC1 may be compensated for by other AC isoforms such as AC8 and other calcium/calmodulin - dependent protein kinases, including CaMKII and CaMKIV. Previous studies using systemic administration of NB001 (intraperitoneally or orally) showed that NB001 produced powerful analgesic effects in mechanical allodynia. 15 In this study, we used oral administration of NB001 and demonstrate that NB001 produced similar potent analgesic effects in adult female mice.
LTP in the ACC is a synaptic model for cortical excitation in chronic pain. 7 AC1 is required for LTP in the ACC. Genetic deletion of AC1 abolished LTP in the ACC pyramidal cell. 34 By using NB001, pharmacological inhibition of AC1 in the ACC neuron abolished LTP in male mice. 15 In a recent report, we found that there was no sex difference in LTP in the ACC slices of adult male and female mice. 19 Previous studies of the effect of NB001 on ACC LTP is mainly collected from adult male mice. In the present studies, we found that LTP in the ACC was also blocked by NB001 in adult female mice. The inhibitory effect of NB001 is selective for induction, not the expression of LTP. These results suggest that NB001 has the same inhibitory effect on ACC LTP in both adult male and female mice. Furthermore, we found that NB001 did not change the AMPA or NMDA receptor-mediated synaptic transmissions in the ACC of female mice.
Potential gender-related difference has also been investigated in emotional fear. In adult mice and rats, our previous study found that fear induced increases in thermal nociceptive thresholds were similar between male and female animals during fear retrieval. 22 These results suggest that there is no gender-related difference in fear-related nociceptive changes. Furthermore, Lu et al. reported that ACC cytokines are not involved in chronic neuropathic pain induced by nerve injury in both adult male and female mice. 35 Our present findings add additional evidence that AC1 is critical for injury-related cortical LTP and behavioral allodynia in both male and female mice. In summary, we provide strong evidence that NB001 is a potential drug for the treatment of chronic pain in both male and females.14,36
Acknowledgments
The authors would like to thank Yuxiang Zhang, Yanan Li, and Yongmin Liu for their contributions to this study.
Footnotes
Authors’ Contributions: ZZ, KF, and WS performed behavioral experiments and analyzed the data. ZZ and QYC performed whole-cell patch clamp recording. MX and SBZ performed MED64 experiments and analyzed the data. ZZ, JSL, QYC, and XHL drafted the manuscript and finished the final version of the manuscript. MZ designed the experiments, discussed, and finished the final version of the paper. All authors read and approved the final manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: MZ is in part supported by grants from the Canadian Institute for Health Research (CIHR) project grants (PJT-148648 and 419286). XHL is supported by grants from the China Postdoctoral Science Foundation (2019M663669) and Basic Research Program of Natural Science in Shaanxi Province (2020JQ-085).
ORCID iDs: Qi-Yu Chen https://orcid.org/0000-0002-5707-6220
Xu-Hui Li https://orcid.org/0000-0003-4376-6252
References
- 1.Zhuo M. Cortical excitation and chronic pain. Trends Neurosci 2008; 31: 199–207. [DOI] [PubMed] [Google Scholar]
- 2.Bliss TV, Collingridge GL, Kaang BK, Zhuo M. Synaptic plasticity in the anterior cingulate cortex in acute and chronic pain. Nature Rev Neurosci 2016; 17: 485–496. [DOI] [PubMed] [Google Scholar]
- 3.Koga K, Li X, Chen T, Steenland H, Descalzi G, Zhuo M. In vivo whole-cell patch-clamp recording of sensory synaptic responses of cingulate pyramidal neurons to noxious mechanical stimuli in adult mice. Mol Pain 2010; 6: 62. [DOI] [PMC free article] [PubMed]
- 4.Yamamura H, Iwata K, Tsuboi Y, Toda K, Sumino R. Morphological and electrophysiological properties of ACCx nociceptive neurons in rats. Brain Res 1996; 735: 83. [DOI] [PubMed] [Google Scholar]
- 5.Hutchison WD, Davis KD, Lozano AM, Tasker RR, Dostrovsky JO. Pain-related neurons in the human cingulate cortex. Nature Neurosci 1999; 2: 403–405. [DOI] [PubMed] [Google Scholar]
- 6.Sandkuhler J. Understanding LTP in pain pathways. Mol Pain 2007; 3: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhuo M. Long-term potentiation in the anterior cingulate cortex and chronic pain. Philos Trans R Soc Lond Ser B Biol Sci 2014; 369: 20130146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu LJ, Toyoda H, Zhao MG, Lee YS, Tang J, Ko SW, Jia YH, Shum FW, Zerbinatti CV, Bu G, Wei F, Xu TL, Muglia LJ, Chen ZF, Auberson YP, Kaang BK, Zhuo M. Upregulation of forebrain NMDA NR2B receptors contributes to behavioral sensitization after inflammation. J Neurosci 2005; 25: 11107–11116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu H, Wu LJ, Wang H, Zhang X, Vadakkan KI, Kim SS, Steenland HW, Zhuo M. Presynaptic and postsynaptic amplifications of neuropathic pain in the anterior cingulate cortex. J Neurosci 2008; 28: 7445–7453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao MG, Ko SW, Wu LJ, Toyoda H, Xu H, Quan J, Li J, Jia Y, Ren M, Xu ZC, Zhuo M. Enhanced presynaptic neurotransmitter release in the anterior cingulate cortex of mice with chronic pain. J Neurosci 2006; 26: 8923–8930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen T, O’Den G, Song Q, Koga K, Zhang MM, Zhuo M. Adenylyl cyclase subtype 1 is essential for late-phase long term potentiation and spatial propagation of synaptic responses in the anterior cingulate cortex of adult mice. Mol Pain 2014; 10: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li XY, Ko HG, Chen T, Descalzi G, Koga K, Wang H, Kim SS, Shang Y, Kwak C, Park SW, Shim J, Lee K, Collingridge GL, Kaang BK, Zhuo M. Alleviating neuropathic pain hypersensitivity by inhibiting PKM in the anterior cingulate cortex. Science 2010; 330: 1400–1404. [DOI] [PubMed] [Google Scholar]
- 13.Liu SB, Wang XS, Yue J, Yang L, Li XH, Hu LN, Lu JS, Song Q, Zhang K, Yang Q, Zhang MM, Bernabucci M, Zhao MG, Zhuo M. Cyclic AMP-dependent positive feedback signaling pathways in the cortex contributes to visceral pain. J Neurochem 2020; 153: 252–263. [DOI] [PubMed] [Google Scholar]
- 14.Zhuo M. Targeting neuronal adenylyl cyclase for the treatment of chronic pain. Drug Discov Today 2012; 17: 573–582. [DOI] [PubMed] [Google Scholar]
- 15.Wang H, Xu H, Wu LJ, Kim SS, Chen T, Koga K, Descalzi G, Gong B, Vadakkan KI, Zhang X, Kaang BK, Zhuo M. Identification of an adenylyl cyclase inhibitor for treating neuropathic and inflammatory pain. Sci Transl Med 2011; 3: 65ra63. [DOI] [PubMed] [Google Scholar]
- 16.Wei F, Qiu CS, Kim SJ, Muglia L, Zhuo M. Genetic elimination of behavioral sensitization in mice lacking calmodulin-stimulated adenylyl cyclases. Neuron 2002; 36: 713–726. [DOI] [PubMed] [Google Scholar]
- 17.Zhang MM, Liu SB, Chen T, Koga K, Zhang T, Li YQ, Zhuo M. Effects of NB001 and gabapentin on irritable bowel syndrome-induced behavioral anxiety and spontaneous pain. Mol Brain 2014; 7: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vallerand AH. Gender differences in pain. Image J Nurs Scholarship 2010; 27: 235–237. [DOI] [PubMed] [Google Scholar]
- 19.Liu RH, Xue M, Li XH, Zhuo M. Sex difference in synaptic plasticity in the anterior cingulate cortex of adult mice. Mol Brain 2020; 13: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vadakkan KI, Jia YH, Zhuo M. A behavioral model of neuropathic pain induced by ligation of the common peroneal nerve in mice. J Pain 2005; 6: 747–756. [DOI] [PubMed] [Google Scholar]
- 21.Koga K, Descalzi G, Chen T, Ko HG, Min Z. Coexistence of two forms of LTP in ACC provides a synaptic mechanism for the interactions between anxiety and chronic pain. Neuron 2014; 85: 377–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou Z, Fan K, Shi W, Chen Q, Zhuo M, Lu J. Reduced behavioral withdrawal responses during fear retrieval in adult mice and rats. Mol Pain 2019; 15: 1744806919876157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steele AD, Jackson WS, King OD, Lindquist S. The power of automated high-resolution behavior analysis revealed by its application to mouse models of Huntington's and prion diseases. Proc Natl Acad Sci U S A 2007; 104: 1983–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao MG, Toyoda H, Lee YS, Wu LJ, Ko SW, Zhang XH, Jia Y, Shum F, Xu H, Li BM, Kaang BK, Zhuo M. Roles of NMDA NR2B subtype receptor in prefrontal long-term potentiation and contextual fear memory. Neuron 2005; 47: 859–872. [DOI] [PubMed] [Google Scholar]
- 25.Kang SJ, Liu MG, Chen T, Ko HG, Baek GC, Lee HR, Lee K, Collingridge GL, Kaang BK, Zhuo M. Plasticity of metabotropic glutamate receptor-dependent long-term depression in the anterior cingulate cortex after amputation. J Neurosci 2012; 32: 11318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song Q, Zheng HW, Li XH, Huganir RL, Kuner T, Zhuo M, Chen T. Selective phosphorylation of AMPA receptor contributes to the network of long-term potentiation in the anterior cingulate cortex. J Neurosci 2017; 37: 8534–8548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toyoda H, Zhao MG, Ulzhofer B, Wu LJ, Xu H, Seeburg PH, Sprengel R, Kuner R, Zhuo M. Roles of the AMPA receptor subunit GluA1 but not GluA2 in synaptic potentiation and activation of ERK in the anterior cingulate cortex. Mol Pain 2009; 5: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Toyoda H, Wu LJ, Zhao MG, Xu H, Zhuo M. Time-dependent postsynaptic AMPA GluR1 receptor recruitment in the cingulate synaptic potentiation. Dev Neurobiol 2007; 67: 498–509. [DOI] [PubMed] [Google Scholar]
- 29.Wu LJ, Zhuo M. Targeting the NMDA receptor subunit NR2B for the treatment of neuropathic pain. Neurotherapeutics 2009; 6: 693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mogil JS, Chanda ML. The case for the inclusion of female subjects in basic science studies of pain. Pain 2005; 117: 1–5. [DOI] [PubMed] [Google Scholar]
- 31.Tian Z, Wang DS, Wang XS, Tian J, Han J, Guo YY, Feng B, Zhang N, Zhao MG, Liu SB. Analgesic effects of NB001 on mouse models of arthralgia. Mol Brain 2015; 8: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kang WB, Yang Q, Guo YY, Wang L, Wang DS, Cheng Q, Li XM, Tang J, Zhao JN, Liu G, Zhuo M, Zhao MG. Analgesic effects of adenylyl cyclase inhibitor NB001 on bone cancer pain in a mouse model. Mol Pain 2016; 12: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bonaterra GA, Then H, Oezel L, Schwarzbach H, Ocker M, Thieme K, Di Fazio P, Kinscherf R. Morphological alterations in gastrocnemius and soleus muscles in male and female mice in a fibromyalgia model. PLoS One 2016; 11: e0151116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liauw J, Wu L-J, Zhuo M. Calcium-stimulated adenylyl cyclases required for long-term potentiation in the anterior cingulate cortex. J Neurophysiol 2005; 94: 878–882. [DOI] [PubMed] [Google Scholar]
- 35.Lu JS, Song Q, Zhang MM, Zhuo M. No requirement of interlukine-1 for long-term potentiation in the anterior cingulate cortex of adult mice. Mol Pain 2018; 14: 1744806918765799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li XH, Chen QY, Zhuo M. Neuronal adenylyl cyclase targeting central plasticity for the treatment of chronic pain. Neurotherapeutics 2020; 17: 861–873. [DOI] [PMC free article] [PubMed] [Google Scholar]