Abstract
Medullary thyroid cancer (MTC) represents a rare neuroendocrine neoplasm originating from neoplastic C-cells in the thyroid gland. While localized disease is potentially curable with an optimized surgical approach, the number of relapses is high, and a considerable number of patients present with primary metastatic disease. Multidisciplinary management including standardized surveillance following surgery, but also early involvement of medical oncologists, is therefore important. Several oncogenic pathways are involved in the pathogenesis of MTC including vascular endothelial growth factor receptor, epidermal growth factor receptor, MET, and most importantly RET, and the multi-tyrosine kinase inhibitors vandetanib and cabozantinib have been approved for advanced MTC based on data from phase III studies. As activating RET mutations represent the most important driver, specific RET inhibitors were introduced and suggest high response rates with limited off-target toxicities. The current review provides a practical overview on clinical presentation and management from early to advanced MTC.
Key words: medullary thyroid cancer, neuroendocrine neoplasms, tyrosine kinase inhibitors, RET inhibitors, endocrine surgery
Highlights
-
•
Systemic treatment options in advanced MTC remain limited with particularly immunotherapy being ineffective.
-
•
Multi-tyrosine kinase inhibitors remain the standard of care for advanced MTC.
-
•
Recent approval of selective RET inhibitors is promising.
-
•
Testing of RET mutations should be included routinely into the diagnostic algorithm.
-
•
Multidisciplinary teams should be involved to guarantee the best outcome for our patients.
From pathogenesis to clinical presentation
Medullary thyroid cancer (MTC) accounts for <5% of primary thyroid neoplasms.1 Given its origin in the neuroendocrine parafollicular cells rather than thyrocytes, MTC as a neuroendocrine neoplasm is clearly distinct from differentiated thyroid cancer (DTC) and subject to specific therapeutic algorithms. C-cells are not part of regulatory pathways by thyroid-stimulating hormone (TSH), thus TSH-suppressive therapy is not a premise as opposed to DTC.2, 3, 4 The neuropeptide calcitonin, deriving from the parafollicular cells, and the carcinoembryonic antigen (CEA), originating in the corresponding cell membranes, constitute not only reliable tumor markers measured for post-operative follow-up, but also for monitoring of systemic therapy, with a variable prognostic impact reported.5 Up to 25% of MTCs present with a hereditary background due to an activating germline RET mutation and the most common underlying condition is multiple endocrine neoplasm (MEN) syndrome 2A/B.2,4 In addition, up to 10% of supposedly sporadic cases have a hereditary background detected by respective screening and genetic counseling is required in all patients. Finally, sporadic RET mutations, most commonly M198T, are present in up to 60% of remaining patients with increasing incidence in advanced disease, defining activation of the RET tyrosine kinase and its subsequent pathways as a major hallmark in the pathogenesis of MTC with therapeutic relevance. Clinical presentation depends on the extent of disease, whereas only a minority of patients present with active endocrine symptoms based on calcitonin excess, mainly diarrhea. Overall survival (OS) across all stages is estimated at 75% at 10 years, but decreases to <40% for metastasized disease.4 Adequate imaging, including functional scans with preferably F-DOPA-positron emission tomography (PET) imaging, is important if high(er) tumor load is suspected, i.e. calcitonin levels ≥500 pg/ml.2
Surgical management of MTC
Early diagnosis is crucial for prognosis and allows adequate surgical therapy. Measurement of calcitonin is highly sensitive and specific for C-cell pathologies, and it has been demonstrated that calcitonin screening is efficient and cost-effective for distinction between MTC and other C-cell pathologies, e.g. C-cell neoplasia.6,7 At first, stimulation tests were established for differentiation, while recent studies define clear sex-specific cut-off values of basal unstimulated calcitonin for MTC and even specific values for the occurrence of metastatic disease in the lateral lymph node compartment.8,9 Minimum surgical treatment consists of a total thyroidectomy with lymphadenectomy in the central compartment (level VI and VII). Intraoperative assessment of frozen sections allows one to determine whether the tumor shows a desmoplastic stroma reaction (DSR). DSR-negative MTCs usually do not have lymph node metastases, whereas DSR-positive tumors potentially have lymphatic spread.10 Therefore, in DSR-positive MTCs with calcitonin levels >85 pg/ml (female) or >100 pg/ml (male), respectively, and/or clinically/radiologically positive lymph nodes, a functional lateral neck dissection (level II to IV) is recommended.11 To avoid false positive diagnosis of DSR, preoperative biopsy of the tumor should be omitted as MTC diagnosis is supported by biochemical assessment. In patients with persisting or recurrent disease, surgical resection of metastases (or functional neck dissection in a second step after initial limited surgery) can be considered; a further indication for surgery in the palliative setting is the removal of single painful lymph nodes.
Managment of advanced MTC—systemic therapy
Multi-tyrosine kinase inhibitors
Based on preclinical models, not only RET but also vascular endothelial growth factor receptor (VEGFR), epidermal growth factor receptor (EGFR), and MET are involved in tumorigenesis of MTC and two multi-tyrosine kinase inhibitors (TKIs) targeting these pathways have been approved for the antiproliferative treatment of MTC.4 Vandetanib, an inhibitor of VEGFR, EGFR, and RET was investigated in the placebo-controlled phase III study ZETA and resulted in a significant progression-free survival (PFS) benefit of 30.5 months versus 19.3 months [hazard ratio (HR) 0.46, 95% confidence interval (CI) 0.31-0.69] in 231 randomized patients12 (see Table 1). The objective response rate (ORR) was 45% for vandetanib versus 13% (P < 0.001) for placebo, and the disease control rate 87% versus 71% (P < 0.0001). Cabozantinib, an inhibitor of MET, VEGFR2, and RET, was evaluated in a comparably sized phase III trial (EXAM), including patients with documented progression per RECIST, whilst this was not a prerequisite in the ZETA study.14 Cabozantinib also resulted in a significant PFS improvement from 4 months for placebo to 11.2 months with TKI (HR 0.28, 95% CI 0.19-0.40). The shorter PFS and the lack of objective responses for placebo (0% versus 28%, P < 0.001) highlight a probably more aggressive population in this trial, which might be explained by exclusively including patients with radiologically progressive disease. To date, there is no statistical evidence of OS benefit for one or the other compound; this is, however, hampered by high cross-over rates and secondary efficacy analyses suggest positive trends, particularly for M198T+ patients treated with cabozantinib (44.3 versus 18.3 months, P = 0.03).15 As currently both compounds are approved for upfront treatment but no randomized head-to-head comparison is available, the clinically relevant question of the preferred first-line is unanswered. Potential considerations in our practice include the selected population in each trial, tumor burden, and toxicities. For cabozantinib, all patients were progressive and given the sometimes indolent clinical course of MTC, relevant progression or symptomatic disease is a prerequisite for initiating treatment. However, a post hoc analysis of this distinct cohort of the EXAM study confirmed a PFS benefit also for the symptomatic/progressive cohort if treated with vandetanib (median PFS 8.4 versus 21.4 months, P < 0.001), thus equal efficacy is suggested.13 The ORR was higher for patients treated with vandetanib versus cabozantinib, which is of potential relevance in cases with high disease load, but this finding is again limited by the missing data on radiological disease progression status in the ZETA study.12 Importantly, both compounds present with high efficacy across all relevant subgroups, including RET-mutated patients.12,14 Concerning side-effects, diarrhea and hypertension may occur with both compounds; high-grade skin toxicities, weight loss, and nausea but also VEGFR-specific side-effects such as bleeding and thrombosis were more common with cabozantinib and 80% required dose reduction in the pivotal study. This is underlined by real-world data reporting that only 14% of patients were started at the planned dose of 140 mg cabozantinib.18 If aiming for full dosing according to pivotal studies and objective response, we tend to start treatment with vandetanib; however, there are also patients where cabozantinib might be preferred, especially in view of long-QT interactions, which are frequent with vandetanib. A further unanswered question is the benefit of sequential treatment with both TKIs upon progression following one substance. While the number of patients subject to this will probably decrease due to introduction of new substances (see following text), there is evidence for efficacy following TKI pre-treatment for both compounds, thus we consider this in selected cases.12,14
Table 1.
Overview on approval studies for the treatment of advanced medullary thyroid cancer
| Study | Design | Setting | Number of patients | Primary EP | Outcome primary EP | Overall survival/other EPs |
|---|---|---|---|---|---|---|
| Vandetanib versus placebo (ZETA)12,13 Wells SA, J Clin Oncol. |
Phase III | Locally advanced metastatic MTC, calcitonin ≥500 pg/ml | 231 versus 100 | PFS | Not reached versus 19.3 months (HR 0.46, 95% CI 0.31-0.69) | No significant difference (PFS for progressive/symptomatic HR 0.43, P < 0.0001) |
| Cabozantinib vs. Placebo (EXAM)14,15 Elisei R, J Clin Oncol. 2013 |
Phase III | Locally advanced metastatic MTC, progression per RECIST within 14 months | 219 versus 111 | PFS | 11.2 months versus 4.0 months (HR 0.28, 95% CI 0.19-0.40) | 26.6 months versus 21.1 months (P = 0.24) (post hoc RET M198T 44.3 months versus 18.9 months, P = 0.03) |
| Selpercatinib (LIBRETTO-001)16 Wirth LJ, N Engl J Med. 2020 |
Phase I/II | Locally advanced metastatic MTC with RET mutation | 55 pretreated 88 untreated |
ORR | 69% (95% CI 55% to 81%) 73% (95% CI 62% to 82%) |
PFS NE (24 months-4 NE) PFS 23.6 (NE-NE) |
| Pralsetinib (ARROW)17 Full publication pending |
Phase I/II | Locally advanced metastatic MTC with RET mutation | 55 pretreated 29 untreated |
ORR | 60% (95% CI 46% to 73%) 66% (95% CI 46% to 82%) |
79% DOR >6 months 84% DOR >6 months |
CI, confidence interval; DOR, duration of response; EP, endpoint; HR, hazard ratio; m, months; MTC, medullary thyroid cancer; NE, not estimated; ORR, overall response rate; PFS, progression-free survival.
RET inhibitors
Given the high impact of RET in the pathogenesis of MTC and the toxicities of multi-TKIs mostly attributed to ‘off-RET-target’ effects, selective RET inhibitors are highly attractive for the treatment of MTC.19 In 2020, two compounds were Food and Drug Administration (FDA)-approved: selpercatinib (LOXO-292) and pralsetinib (BLU-667), with the first now also having been approved by the European Medicines Agency (EMA). Results of the phase I/II LIBRETTO-001 study investigating selpercatinib were recently published in the New England Journal of Medicine.16 Treatment of RET-mutated MTC resulted in an ORR of 69% (95% CI 55% to 81%) in 55 TKI pretreated MTC patients with a 1-year PFS of 82% (95% CI 69% to 90%) and comparable outcome in 88 treatment-naive patients (ORR 73%, 95% CI 62% to 82%; 1-year PFS 92%, 95% CI 82% to 97%). As expected, the toxicity profile was favorable compared with multi-TKIs with similar grade III hypertension in about 10%, but grade 3+ diarrhea in only 3% and absence of skin toxicities. Data for pralsetinib suggest comparable efficacy based on efficacy results presented at scientific meetings but a full publication is still lacking to date.17 Central nervous system (CNS) activity is suggested for both compounds, which is already recognized in the NCCN guideline recommending RET-TKI therapy for this cohort of patients.20 RET inhibitors therefore constitute important new players in the treatment of MTC and all patients should be subject to RET testing. However, current approval covers patients progressing on multi-TKIs and in view of the phase I/II evidence only and absence of long-term data, we support this sequence outside of clinical trials. In addition to the compounds discussed here, several other TKIs have been suggested to be partly active in MTC, but no larger positive data are available.21
Treatment beyond TKIs
Classical cytostatic chemotherapy plays a minor role in the treatment of MTC and was not part of the investigated concepts in the last decade. Thus, most exploratory studies are small and underpowered and were assessing response rates rather than more modern endpoints including PFS. Monotherapy with doxorubicin showed modest responses <20%, and combinations with cisplatin or dacarbazine and fluorouracil did not significantly increase efficacy.2,22 In our personal practice, chemotherapy is currently not being used in clinical routine and also the European Society for Medical Oncology (ESMO) guidelines do not further specify its value.
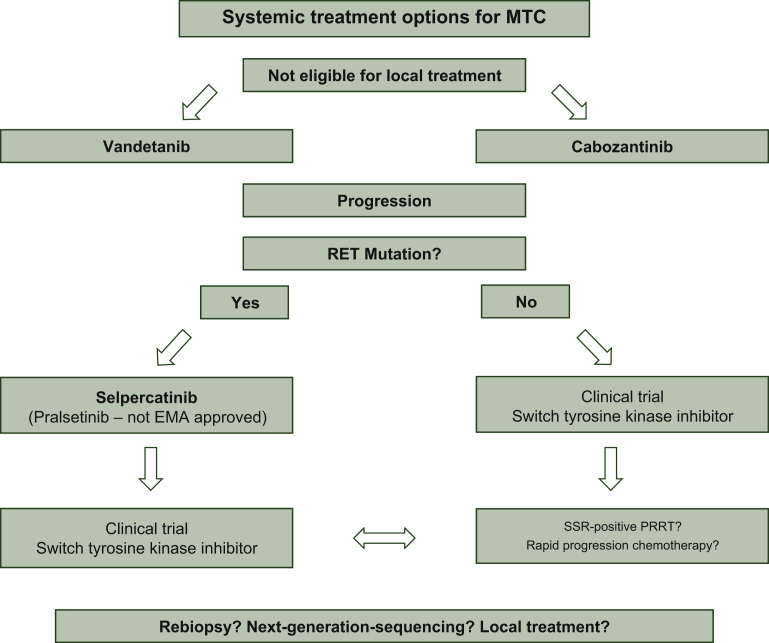
Given the high efficacy in other neuroendocrine neoplasms, somatostatin analogues were investigated, but there is no proof of antiproliferative activity, whereas its use was suggested for salvage treatment of calcitonin-triggered diarrhea.21 Peptide receptor radionuclide therapy (PRRT) with lutetium-177-DOTATATE was explored recently in a retrospective study with 43 patients and median PFS was 24 months (95% CI 15.1-32.9 months).23 Although the ORR was low at 4%, it still might be worth screening patients with progressive disease for somatostatin receptor (SSR) expression by SSR imaging, preferably PET/computed tomography. Finally, personalized medicine in terms of next-generation sequencing (NGS) panels is of academic interest, but NGS analyses have hitherto not resulted in druggable targets beyond RET. Oncogene-RAS activation is a frequent finding but KRAS-G12C mutations are absent and consequently no corresponding drugs are available.24 Nevertheless, we encourage the use of NGS panels in refractory patients not at least due to better characterization of these patients, but even more importantly, patients should be included in clinical trials whenever possible. Immunotherapeutic concepts, and checkpoint inhibitor monotherapy in particular, have so far not resulted in encouraging responses, probably based on the fact the MTC is an ‘immunologically cold’ tumor. See Figure 1 for an overview of our clinical practice algorithm.
Figure 1.
A potential treatment approach for advanced medullary thyroid cancer.
EMA, European Medicines Agency; MTC, medullary thyroid cancer; PRRT, peptide receptor radionuclide therapy; SSR, somatostatin receptor.
Conclusion
Systemic treatment options in advanced MTC remain limited, with immunotherapy in particular being ineffective as opposed to other tumors. Recent approval of selective RET inhibitors is, however, promising and testing of RET mutations should be included routinely into the diagnostic algorithm. The optimal sequencing of the available TKIs has yet to be defined and outside of clinical trials, RET-TKIs are currently reserved for treatment following progression on multi-TKIs. Re-induction in cases of premature discontinuation and subsequent progression, or switch of multi-TKIs, is still of unknown value and may be considered in selected cases. However, it has to be emphasized that MTC usually presents with an indolent course, suggesting watch and wait as an important option in the management of these patients to avoid overtreatment. Monitoring clinical symptoms and calcitonin levels may be supportive for clinical decision making in these cases. Given the rarity of this disease, multidisciplinary teams at tertiary centers including specialized endocrine surgeons, endocrinologists, nuclear medicine physicians, and medical oncologists should be involved to guarantee the best outcome for our patients.
Acknowledgments
Funding
None declared.
Disclosure
The authors have declared no conflicts of interest.
References
- 1.Cabanillas M.E., McFadden D.G., Durante C. Thyroid cancer. Lancet. 2016;388(10061):2783–2795. doi: 10.1016/S0140-6736(16)30172-6. [DOI] [PubMed] [Google Scholar]
- 2.Filetti S., Durante C., Hartl D. Thyroid cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30(12):1856–1883. doi: 10.1093/annonc/mdz400. [DOI] [PubMed] [Google Scholar]
- 3.Roy M., Chen H., Sippel R.S. Current understanding and management of medullary thyroid cancer. Oncologist. 2013;18(10):1093–1100. doi: 10.1634/theoncologist.2013-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ceolin L., Duval M.A.D.S., Benini A.F., Ferreira C.V., Maia A.L. Medullary thyroid carcinoma beyond surgery: advances, challenges, and perspectives. Endocr Relat Cancer. 2019;26(9):R499–R518. doi: 10.1530/ERC-18-0574. [DOI] [PubMed] [Google Scholar]
- 5.Meijer J.A., le Cessie S., van den Hout W.B. Calcitonin and carcinoembryonic antigen doubling times as prognostic factors in medullary thyroid carcinoma: a structured meta-analysis. Clin Endocrinol (Oxf) 2010;72(4):534–542. doi: 10.1111/j.1365-2265.2009.03666.x. [DOI] [PubMed] [Google Scholar]
- 6.Cheung K., Roman S.A., Wang T.S., Walker H.D., Sosa J.A. Calcitonin measurement in the evaluation of thyroid nodules in the United States: a cost-effectiveness and decision analysis. J Clin Endocrinol Metab. 2008;93(6):2173–2180. doi: 10.1210/jc.2007-2496. [DOI] [PubMed] [Google Scholar]
- 7.Vierhapper H., Niederle B., Bieglmayer C., Kaserer K., Baumgartner-Parzer S. Early diagnosis and curative therapy of medullary thyroid carcinoma by routine measurement of serum calcitonin in patients with thyroid disorders. Thyroid. 2005;15(11):1267–1272. doi: 10.1089/thy.2005.15.1267. [DOI] [PubMed] [Google Scholar]
- 8.Niederle M.B., Scheuba C., Gessl A. Calcium-stimulated calcitonin – the “new standard” in the diagnosis of thyroid C-cell disease – clinically relevant gender-specific cut-off levels for an “old test”. Biochem Med (Zagreb) 2018;28(3):030710. doi: 10.11613/BM.2018.030710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niederle M.B., Scheuba C., Riss P., Selberherr A., Niederle B. Early diagnosis of medullary thyroid cancer: are calcitonin stimulation tests still indicated in the era of highly sensitive calcitonin immunoassays? Thyroid. 2020;30(7):974–984. doi: 10.1089/thy.2019.0785. [DOI] [PubMed] [Google Scholar]
- 10.Kaserer K., Scheuba C., Neuhold N. Sporadic versus familial medullary thyroid microcarcinoma: a histopathologic study of 50 consecutive patients. Am J Surg Pathol. 2001;25(10):1245–1251. doi: 10.1097/00000478-200110000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Niederle M.B., Riss P., Selberherr A. Omission of lateral lymph node dissection in medullary thyroid cancer without a desmoplastic stromal reaction. Br J Surg. 2021;108(2):174–181. doi: 10.1093/bjs/znaa047. [DOI] [PubMed] [Google Scholar]
- 12.Wells S.A., Jr., Robinson B.G., Gagel R.F. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol. 2012;30(2):134–141. doi: 10.1200/JCO.2011.35.5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kreissl M.C., Bastholt L., Elisei R. Efficacy and safety of vandetanib in progressive and symptomatic medullary thyroid cancer: post hoc analysis from the ZETA trial. J Clin Oncol. 2020;38(24):2773–2781. doi: 10.1200/JCO.19.02790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elisei R., Schlumberger M.J., Müller S.P. Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol. 2013;31(29):3639–3646. doi: 10.1200/JCO.2012.48.4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schlumberger M., Elisei R., Müller S. Overall survival analysis of EXAM, a phase III trial of cabozantinib in patients with radiographically progressive medullary thyroid carcinoma. Ann Oncol. 2017;28(11):2813–2819. doi: 10.1093/annonc/mdx479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wirth L.J., Sherman E., Robinson B. Efficacy of selpercatinib in RET-altered thyroid cancers. N Engl J Med. 2020;383(9):825–835. doi: 10.1056/NEJMoa2005651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-pralsetinib-ret-altered-thyroid-cancers Available at.
- 18.Koehler V.F., Adam P., Frank-Raue K. Real-world efficacy and safety of cabozantinib and vandetanib in advanced medullary thyroid cancer. Thyroid. 2021;31(3):459–469. doi: 10.1089/thy.2020.0206. [DOI] [PubMed] [Google Scholar]
- 19.Belli C., Penault-Llorca F., Ladanyi M. ESMO recommendations on the standard methods to detect RET fusions and mutations in daily practice and clinical research. Ann Oncol. 2021;32(3):337–350. doi: 10.1016/j.annonc.2020.11.021. [DOI] [PubMed] [Google Scholar]
- 20.https://www.nccn.org Available at.
- 21.Maxwell J.E., Sherman S.K., O’Dorisio T.M., Howe J.R. Medical management of metastatic medullary thyroid cancer. Cancer. 2014;120(21):3287–3301. doi: 10.1002/cncr.28858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hadoux J., Pacini F., Tuttle R.M., Schlumberger M. Management of advanced medullary thyroid cancer. Lancet Diabetes Endocrinol. 2016;4(1):64–71. doi: 10.1016/S2213-8587(15)00337-X. [DOI] [PubMed] [Google Scholar]
- 23.Parghane R.V., Naik C., Talole S. Clinical utility of 177Lu-DOTATATE PRRT in somatostatin receptor-positive metastatic medullary carcinoma of thyroid patients with assessment of efficacy, survival analysis, prognostic variables, and toxicity. Head Neck. 2020;42(3):401–416. doi: 10.1002/hed.26024. [DOI] [PubMed] [Google Scholar]
- 24.Nelkin B. Recent advances in the biology and therapy of medullary thyroid carcinoma. F1000Res. 2017;6:2184. doi: 10.12688/f1000research.12645.1. [DOI] [PMC free article] [PubMed] [Google Scholar]