Abstract
Salmonella Heidelberg (S. Heidelberg) is a major pathogen implicated in foodborne outbreaks for which poultry products can serve as an epidemiological source. This study determined the efficacy of GRAS-status lemongrass essential oil (LGEO) against S. Heidelberg in vitro and on the pathogen's attachment to skin and meat. At first, employing in vitro assays, the effect of LGEO on multidrug-resistant S. Heidelberg multiplication and motility was examined. Biofilm inhibition and inactivation assays were also performed. The quorum-sensing modulating effect of LGEO was determined. In follow-up experiments, chicken skin or meat samples inoculated with S. Heidelberg were treated with various concentrations of LGEO at different time points at simulated scalding (54°C) and chilling (4°C) temperatures. The samples were incubated, and the surviving populations of S. Heidelberg were enumerated to determine if LGEO could be a potential processing aid in poultry operations. Duplicate samples were included in each treatment, and the experiments were repeated at least 3 times. Significant reductions of S. Heidelberg of at least 4.0 log10 CFU/mL after 24 h in nutrient broth and poultry cecal contents was observed with 0.5% LGEO. Complete inhibition of motility, biofilm formation, and inactivation of pre-formed biofilms was observed with 0.15% LGEO (P ≤ 0.05). Concentrations of LGEO at 0.5% and 1% affected violacein production (P ≤ 0.05). On skin samples, all concentrations significantly reduced S. Heidelberg by 1.2 to 3.9 log10 CFU/sample after 2 min at 54°C. We obtained a significant reduction of the pathogen in meat samples at 54°C and skin samples at 4°C with 2% LGEO. All concentrations significantly reduced S. Heidelberg from the treatment water kept at 4°C and 54°C (P ≤ 0.05). In conclusion, LGEO could potentially serve as a natural antimicrobial strategy in scalding and chilling waters to reduce S. Heidelberg during processing. However, additional studies are warranted before recommending its commercial use.
Key words: lemongrass essential oil, Salmonella Heidelberg, broiler chicken meat, skin
INTRODUCTION
Salmonella enterica serovar Heidelberg (S. Heidelberg) has emerged as a leading human and poultry serovar as evidenced by its role in large multistate outbreaks associated with the consumption of contaminated poultry products (Foley et al., 2011; CDC, 2013; Gieraltowski et al., 2017). Several multidrug-resistant (MDR) isolates of S. Heidelberg have been implicated in these outbreaks, including the one which occurred at a Tennessee correctional facility involving mechanically separated chicken (CDC, 2014). The invasiveness and high potential for multidrug resistance in S. Heidelberg are of great concern, and infections caused by this serovar may warrant the use of antibiotic therapy to treat complications such as septicemia and myocarditis (Vugia et al., 2004; Dutil et al., 2010).
Salmonella often colonizes poultry without exhibiting any clinical symptoms, and S. Heidelberg has been reported to establish a commensal-like condition in poultry (Bearson et al., 2017). Asymptomatic carriage of Salmonella in live birds increases the risk of pathogen dissemination throughout the farm and their transmission to consumers through poultry products. Although the initial load of pathogens colonizing birds is a critical control point in ensuring the microbiological safety of poultry products, control measures are also be applied at the postharvest stages as poor hygiene and inadequate processing steps could exacerbate transmission of the pathogen. Additional intervention strategies are being investigated for use in the processing stages, particularly at the various critical control points. These include the scalding, plucking, evisceration, and chilling steps.
In broiler chicken processing, the soft scalding stage involves immersion of carcasses in the water at 54°C for up to 2 min to loosen feather follicles and facilitate feather removal (FSIS, 2015). Although more reduction of Salmonella during scalding could be obtained with higher temperatures, the heat may also result in changes to the skin microtopography that would facilitate Salmonella attachment (Kim et al., 1993). Previous investigations on the impact of scalding temperature on Salmonella showed that chicken carcasses scalded at 52°C and 56°C had 50 to 80% fewer Salmonella than carcasses that were scalded at 60°C (Slavik et al., 1995). Another critical control point is the chilling stage, where the carcasses are immersed in cold water to rapidly lower their temperature to approximately 4°C to inhibit microbial growth (FSIS, 2014). Thus, there has been an increase in the number of processing facilities in the United States that employ post-chiller antimicrobial interventions that were found successful in reducing Salmonella and other pathogens when used in combination with the dip systems (Nagel et al., 2013). Several approved antimicrobials are used to prevent bacterial cross-contamination in immersion chilling systems across the United States, and one antimicrobial prominently utilized in these systems is chlorine. However, the efficacy of chlorine for bacterial reduction decreases with an increase in organic load and the alterations in the pH of the water (Byrd and McKee, 2005).
The growing interest in natural antimicrobials as an alternative to synthetic chemicals for food production has propelled research on the antibacterial properties of essential oils (EO) and their potential applications in food production systems. The EO extracted from plants of the Cymbopogon species, commonly known as lemongrass essential oil (LGEO), are generally recognized as safe by the United States Food and Drug Administration (FDA 21 CFR§182.20) and follow European regulations (1334/2008 & 178/2002). The antibacterial properties of LGEO have been demonstrated against major foodborne pathogens in various mediums, including broths, fruit juices, and minced meat (Barbosa et al., 2009; Raybaudi-Massilia et al., 2006). However, there is limited information on the effects of LGEO on S. Heidelberg's survival and several critical virulence factors. The objectives of this study were to assess the effect of LGEO on MDR S. Heidelberg multiplication, motility, biofilm formation, and inactivation as well as LGEO's effect on S. Heidelberg attachment to broiler skin and meat at scalding (54°C) and chilling (4°C) temperatures independently and in sequence.
MATERIALS AND METHODS
In Vitro Experiments
Bacterial Strains and Growth Conditions
Two S. Heidelberg strains, S. Heidelberg 466 (N13F0000466) and S. Heidelberg 1904 (13×001904), obtained from mechanically separated chicken implicated in the 2014 Tennessee correctional facility outbreak (Dr. James Gibson, Division of Laboratory Services, Tennessee Department of Health), were used in the study. Each strain was taken from a -80°C frozen stock and grown individually in 10 mL trypticase soy broth (TSB; catalog no. C7141, Criterion, Hardy Diagnostics, Santa Maria, CA) at 37°C for 24 h. The culture obtained after 3 successive propagations was pelleted by centrifugation (3,600 x g for 15 min at 4°C; Allegra X-14R, Beckman Coulter, South Kraemer Boulevard, Brea, CA) and washed twice with sterile phosphate-buffered saline (PBS, pH 7.2). The bacterial pellet was subsequently resuspended in 10 mL PBS for the inoculum. Salmonella's growth was determined by serial dilution and plating appropriate culture dilutions on xylose lysine deoxycholate agar (XLD; catalog no. C7322, Criterion, Hardy Diagnostics, Santa Maria, CA) at 37°C for 24 h.
Screening the Effective Concentrations of LGEO against S. Heidelberg
Lemongrass essential oil (LGEO; East Indian Origin, Natural, Food Grade; PCode: 1002104017; Product: W262404-SAMPLE-K; Lot # MKBV3916V) was purchased from Sigma–Aldrich (Milwaukee, WI, United States). A 24-well tissue culture plate assay was used to determine effective concentrations of LGEO (Kollanoor Johny et al., 2010). Briefly, each well containing 2 mL of TSB was inoculated with ∼5.0 log10 CFU/mL of either S. Heidelberg 466 or 1904. Then, LGEO diluted with appropriate proportions of ethanol (Reagent Alcohol/ethanol; Catalog no. BDH11560-4LP; BDH Chemicals, VWR, Mississauga, ON, Canada) was added to the inoculated medium at LGEO concentrations of 0.0075%, 0.015%, 0.15%, 0.5%, 1%, and 1.5% (vol/vol). The effect of ethanol as a diluent on the growth of S. Heidelberg was tested using the highest concentration used as a diluent (0.75%). Negative controls (without Salmonella or LGEO), positive controls (with Salmonella without LGEO), and diluent controls (with Salmonella and ethanol, without LGEO) were also kept alongside the treatments. After mixing, the plates were then incubated at 37°C (optimal temperature for Salmonella growth), and bacterial growth monitored by optical density (OD600) using an ELISA plate reader (Dynex Revelation 4.22 Software, Dynex MRX Revelation; Dynex Technologies, Denkendorf, Germany) before incubation and after 8 and 24 h of incubation. Duplicate samples were included, and the experiment was repeated 4 times.
Effect of Selected LGEO Concentrations on S. Heidelberg Growth
The results of the former assay were validated using a macro-broth dilution assay. Separate macro-broth dilution assays were conducted for each strain. Briefly, 10 mL of TSB was inoculated with ∼5.0 log10 CFU/mL of either isolate, supplemented with either 0.0075%, 0.15%, 0.5%, or 1% (vol/vol) of LGEO, and incubated at 37°C. Negative controls (without Salmonella or LGEO) and positive controls (with Salmonella without LGEO) were also included. S. Heidelberg was enumerated by serial dilution in PBS and surface plating 0.1 mL on XLD before incubation, and after 8 and 24 h of incubation. Duplicate samples were kept for all treatments, and the experiment was repeated 3 times.
Effect of LGEO on S. Heidelberg Multiplication in Cecal Contents
The effects of LGEO on S. Heidelberg multiplication in cecal contents were examined as described previously (Kollanoor Johny et al., 2010). Poultry cecal contents were transported to the lab in a frozen state from a Minnesota processor. For studies, the contents were thawed, pooled, diluted with PBS (1:4.5), and autoclaved at 121°C for 15 min to inactivate background microflora. Sterilizing the cecal contents was necessary to observe the reduction obtained by LGEO alone and minimize the potential antagonistic effect of the natural microflora (Vasudevan et al., 2005; Kollanoor Johny et al., 2010; Nair and Kollanoor Johny, 2017a). A 0.1 mL sample of prepared cecal content was plated on XLD before the experiment to ensure the inactivation of indigenous microorganisms. Sterile 15 mL polypropylene tubes containing 5 mL of the cecal contents were inoculated with ∼5.0 log10 CFU/mL of either isolates and treated with either 0.0075%, 0.15%, 0.5%, or 1% (vol/vol) of LGEO. The tubes and their appropriate controls (negative and positive controls) were incubated at 41°C based on the average poultry body temperature. Before incubation and 24 h after incubations, 1 mL was drawn from each tube, serially diluted (1:10) with PBS, and plated on XLD for Salmonella enumeration. Duplicate samples were kept for all treatments, and the experiment was repeated 3 times.
Effect of LGEO on the Motility of S. Heidelberg
The effect of LGEO on S. Heidelberg motility was examined using a motility assay (Niu and Gilbert, 2004). The semi-solid agar was made using 20 mL of Luria-Bertani broth (LB; catalog no. C6001, CulGenex, Hardy Diagnostics, Santa Maria, CA), 0.3% (w/v) agarose (catalog no. C5001, Criterion, Hardy Diagnostics, Santa Maria, CA) and poured into Petri dishes (100 by 15mm; VWR). Treatment groups were supplemented with either 0.0075%, 0.015%, or 0.15% (vol/vol) LGEO into the agar mix before pouring onto plates. Then, 10 µL of either S. Heidelberg isolates (∼5.0 log10 CFU) were inoculated at the center of the plates and incubated for 8 h at 37°C before the zone of motility diameter was measured. Appropriate controls were kept alongside treatments, and at least 8 replicates were included for each strain.
Effect of LGEO on S. Heidelberg Biofilm Formation
The effect of LGEO in inhibiting the formation of biofilms was examined on microtiter plates as described in the published literature with modifications (Mireles et al., 2001; Djordjevic et al., 2002). An overnight culture of the S. Heidelberg isolates grown in LB were centrifuged (3,600 x g for 15 min at 4°C), washed with PBS, resuspended, and diluted in LB. Then, 200 µL of the diluted culture (∼6.0 log10 CFU) was added to sterile 96-well polystyrene microplate wells (Catalog no. 89089-578; Ref. 655184; Greiner Bio-One, VWR) along with either 0.0075%, 0.15%, or 1% (vol/vol) LGEO. Positive controls with only the pathogen and negative controls with only the broth were also included. The outer rows and columns of the well plate were filled with sterile PBS to prevent evaporation from the central wells; the plates were then sealed with Parafilm (American National Can, Greenwich, CT, United States) and incubated for 96 h at 41°C. Biofilm formation and planktonic S. Heidelberg were quantified spectrophotometrically using a 96-well microplate reader with absorption measured at 600 nm. After incubation, the remaining medium was removed from the wells to a separate microtiter plate, and absorbance was measured to quantify planktonic or biofilm-unassociated S. Heidelberg. Wells of the original plate was gently rinsed with sterile deionized water 3 times and allowed to dry at room temperature for 15 min before the addition of 200 µL of 1% crystal violet (Item no. ES802E, Catalog no. 89133-283; Azer Scientific, Morgantown, PA) for 20 min. The stained wells were then rinsed 3 times with sterile deionized water, allowed to dry for 15 minutes, and destained with 200 µL of ethanol (99%). The destaining solution was transferred to a new microtiter plate, and the level of crystal violet present quantified by optical density (OD600) measurement. The experiment was replicated 6 times for each strain.
Effect of LGEO on Pre-Formed S. Heidelberg Biofilm
The effect of LGEO against pre-formed biofilms was investigated on 48-well polystyrene cell culture plates with a modified procedure (Upadhyay et al., 2013). Bacterial culture was prepared similar to that for the biofilm inhibition assay, except the inoculum was diluted with sterile PBS instead of LB. The wells containing 1 mL LB were then inoculated with 100 µL of the S. Heidelberg strains (∼6.0 log10 CFU). The outer rows and columns of the well plate were filled with sterile PBS, and the lid was secured to the plate using Parafilm before incubation for 96 h at 41°C. After biofilm formation, the remaining medium was removed and treated with either 0.0075%, 0.15%, or 1% (vol/vol) LGEO in the wells for 2 h. The wells were subsequently scraped with sterile pipet tips for 5 min per well, and surviving S. Heidelberg were enumerated on XLD plates. Appropriate controls were kept along with the treatments; the experiment was conducted in duplicates and replicated 4 times.
Effect of LGEO on Bacterial Quorum Sensing (QS Inhibition Assay)
The quorum sensing (QS) modulating activities of LGEO was investigated by disk diffusion assay performed with Chromobacterium violaceum (Item no. 154931A; MicroKwik Culture Vial, Carolina Biological Supply Company, Burlington, NC, United States) as described (Adonizio et al., 2006). C. violaceum was grown in LB broth incubated at 30°C for 18 h before 100 µL of the broth was spread on LB agar plates. Then, 100 µL aliquots of either 0.0075%, 0.15%, 0.5%, or 1% (vol/vol) LGEO diluted with ethanol (99%) was added onto sterile 6 mm disks. After 2 min of drying, the disks with treatments, along with their appropriate controls, were placed on the same LB plate and incubated at 30°C for 24 h. The ring of colorless but viable cells around the disk was then measured, and the experiment was repeated 8 times.
Meat and Skin Studies
Bacterial Strain and Growth Condition
Since similar findings were observed with both strains in the in vitro studies, S. Heidelberg 1904 was used for further investigations. To avoid potential competition from the skin and meat microflora, the strain was induced for resistance to nalidixic acid at 50 µl/mL nalidixic acid sodium salt (NA; CAS No. 3374-05-8, Alfa Aesar, Haverhill, MA) for selective enumeration as previously described (Nair and Kollanoor Johny, 2017b). After the growth of culture to 9 log10 CFU/mL was confirmed by plating, an overnight culture was pelleted by centrifugation (3,600 x g for 15 min at 4°C) and suspended in sterile PBS to obtain a final concentration of 4 log10 CFU/50 µl.
Sample Preparation and Inoculation
Samples were prepared from 100% all-natural (non-enhanced) chicken drumsticks and breast fillets purchased from a retail store. The skin samples from drumsticks were separated from the underlying muscle and cut into 1 square inch pieces using a sterile scalpel (Nair and Kollanoor Johny, 2017b). Breast fillets were also aseptically cut into 2 g pieces using a sterile scalpel (Wagle et al., 2017). Both skin and meat samples were first sterilized by UV light for 5 min to eliminate background microorganisms before inoculation. Each skin or meat sample was spot inoculated with 50 µl of the inoculum containing 4 log10 CFU S. Heidelberg 1904. The samples were subsequently kept under the biosafety cabinet at room temperature for 30 min to facilitate Salmonella attachment.
Individual Scalding or Chilling Treatments
The dip treatments were prepared by adding LGEO to sterile deionized (DI) water at concentrations of 0, 0.5, 1, or 2% (v/v). The temperature of treatment solutions was maintained at 4°C using a refrigerator and 54°C using a water bath to simulate the chilling and scalding stages of poultry processing, respectively. The treatment water was vortexed for 30 s before dipping the samples. Each skin or meat sample was immersed in separate 20 mL of the dip solution containing one of the 4 concentrations of LGEO for either 2, 3, or 5 min before microbiological analysis.
Scalding and Chilling Treatments in Sequence
Preparation of dip treatments and temperature of treatment solutions was similar to that of the individual scalding and chilling experiments. For this experiment, each skin or meat sample was first dipped in a separate 20 mL dip solution containing one of the 4 concentrations of LGEO for 2 min at the scalding temperature. The samples were then aseptically moved to a dip treatment containing their corresponding LGEO concentrations at chilling temperature for 2 min before microbiological analysis. Additionally, dip treatments at both temperatures were also analyzed for surviving S. Heidelberg.
Microbiological Analysis
Each skin and meat sample was transferred to a sterile Whirl-Pak bag containing 10 mL PBS to enumerate surviving S. Heidelberg attached to skin or meat after LGEO dip treatments. The samples were subsequently homogenized for 2 min at 200 rpm using a stomacher (100/125V, 50/60Hz; Neutec Group Inc., Farmingdale, NY) followed by 10-fold serial dilution with PBS before plating 100 µl from appropriate dilutions on XLD+NA plates. Additionally, 100 µl from dipping solutions used in the sequential scalding and chilling treatments were also plated on XLD+NA to enumerate surviving pathogens in the treatment water. All XLD+NA plates were incubated at 37°C for 24 h before bacterial enumeration. Dipping solutions and sample homogenates were enriched with 10 mL of Selenite Cysteine Broth (SCB; Hardy Diagnostics, Santa Maria, CA, United States) and streaked on XLD+NA plates after 8 h of incubation to detect any surviving Salmonella that was not observed with initial plating.
Statistical Analysis
Each tube or well served as an experimental unit in the broth dilution, multiplication, and biofilm assays with every experiment repeated at least 3 times in duplicates. For motility and QS inhibition assays, the plates were the experimental unit and were replicated eight times. Each skin or meat sample served as an experimental unit, and each experiment followed a completely randomized design with 4 treatment groups (0, 0.5, 1, and 2% LGEO). All skin and meat experiments were conducted in duplicates and replicated 3 times. S. Heidelberg numbers enumerated on XLD plates were logarithmically transformed (log10 CFU/mL) for statistical analysis (Byrd and McKee, 2005). Samples that were negative for S. Heidelberg's growth after initial spread plating but positive after enrichment with SCB were assumed value of 0.92 log10 CFU/mL (Eeckhaut et al., 2018). Analysis of variance was conducted using the lmerTest package in R (R, version 3.6.1, R Core Team). The difference between treatment means was separated using the least-square means analysis, and a significant difference was established at P < 0.05.
RESULTS
Screening the effect of LGEO against Salmonella Heidelberg
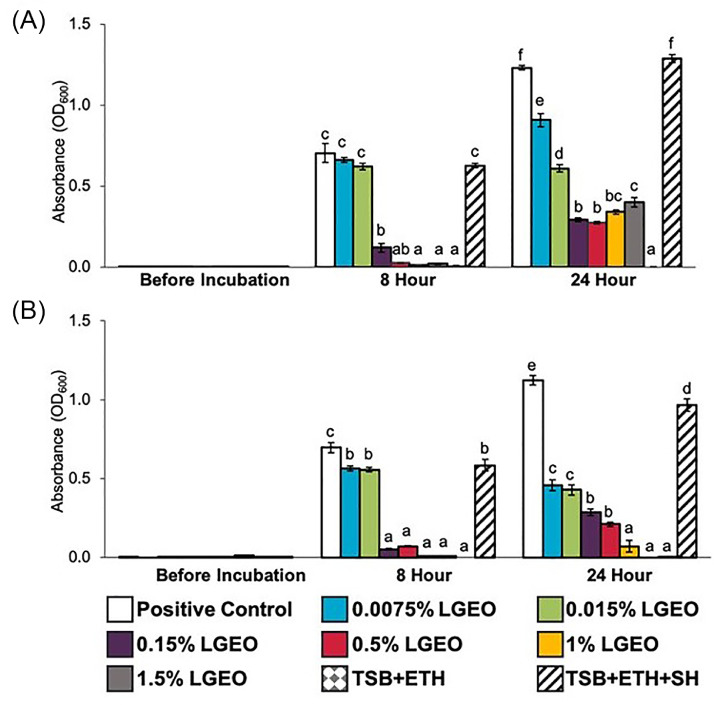
The effect of LGEO at various concentrations on S. Heidelberg 466 and 1904 growth in nutrient broth over time is illustrated in Figure 1. The reductions in optical density (OD) reading at 600 nm from respective controls indicate a decrease in pathogen populations. The highest concentration of ethanol used as a diluent in the study was 0.75%, did not result in changes to the growth of S. Heidelberg compared to the positive control. Concentrations of LGEO at 0.15% (vol/vol) and higher significantly reduced pathogen populations at 8 h of incubation compared to their corresponding controls for both S. Heidelberg 466 (Figure 1A) and S. Heidelberg 1904 (Figure 1B) (P ≤ 0.05). After 24 h of incubation, all concentrations of LGEO significantly reduced pathogen populations compared to the positive controls (P ≤ 0.05).
Figure 1.
Effect of LGEO on Salmonella Heidelberg [(A) S. Heidelberg 466 and (B) S. Heidelberg 1904] growth in TSB at 0, 8, and 24 h after incubation at 37°C (means* ± SEM; n = 8/treatment). a – f Treatments within each sampling time that lack common superscripts differ significantly from one another (P≤ 0.05). * least square means. Abbreviations: LG, lemongrass essential oil; ETH, ethanol control; SH, Salmonella Heidelberg; TSB, Tryptic Soy Broth.
Effect of LGEO on Salmonella Heidelberg Growth (Macro-Dilution Assay)
The effect of LGEO on S. Heidelberg 466 and 1904 multiplication was examined in TSB over time (Figure 2). In positive control groups where no LGEO was added, both isolates of S. Heidelberg grew from an initial concentration of ∼5 log10 CFU/mL to ∼9 log10 CFU/mL. A reduction of ∼1.1 – 2.2 log10 CFU/mL in bacterial numbers compared to their respective positive controls was observed with 0.5% and 1% LGEO for both isolates of S. Heidelberg even before incubation (P ≤ 0.05). After 8 h of incubation, 0.5% and 1% LGEO were able to reduce bacterial numbers by at least 3.4 and 5.4 log10 CFU/mL (P ≤ 0.05), respectively. After 24 h of incubation, at least 4.0 and 4.4 log10 CFU/mL reduction was observed with 0.5% and 1% LGEO (P ≤ 0.05), respectively. The complete elimination of S. Heidelberg 1904 was observed with 1% LGEO after 24 h of incubation (Figure 2B). Whereas for 0.15% LGEO, a significant reduction of 2.6 log10 CFU/mL was only observed for S. Heidelberg 1904 (Figure 2B) after 24 h of incubation. Bacterial counts obtained with 0.0075% LGEO supplementation did not significantly differ from the positive control (P > 0.05).
Figure 2.
Effect of LGEO on Salmonella Heidelberg [(A) S. Heidelberg 466 and (B) S. Heidelberg 1904] growth in TSB at 0, 8, and 24 h after incubation at 37°C (means* ± SEM; n = 6/treatment). a – d Treatments within each sampling time that lack common superscripts differ significantly from one another (P≤ 0.05). * least square means. Abbreviation: LG, lemongrass essential oil.
Effect of LGEO on Salmonella Heidelberg Multiplication in Cecal Contents
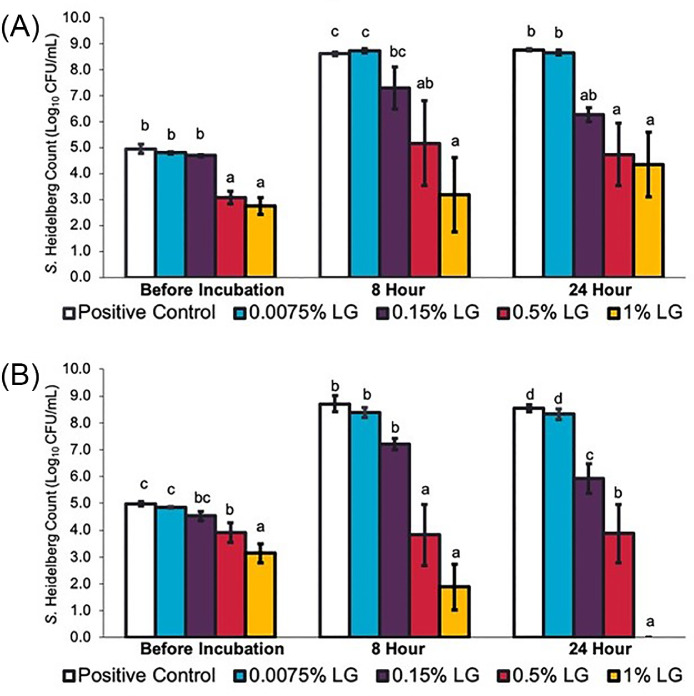
The effects of LGEO on S. Heidelberg 466 and 1904 multiplication in poultry cecal contents are illustrated in Figure 3. In positive control groups where LGEO was not supplemented in the cecal contents, both isolates of S. Heidelberg grew from an initial concentration of ∼5 log10 CFU/mL to ∼9 log10 CFU/mL. Similar to results using TSB, a significant reduction in bacterial numbers compared to the positive control was observed with 0.5% and 1% LGEO for both isolates even before incubation (P ≤ 0.05). After 24 h of incubation, 1% LGEO reduced bacterial numbers by 7.9 and 4.8 log10 CFU/mL for S. Heidelberg 466 and 1904, respectively (P ≤ 0.05). No significant difference in bacterial numbers was found for 0.0075% and 0.15% LGEO compared to the positive control.
Figure 3.
Effect of LGEO on Salmonella Heidelberg [(A) S. Heidelberg 466 and (B) S. Heidelberg 1904] multiplication in turkey cecal contents at 0 and 24 h after incubation at 41°C (means* ± SEM; n = 6/treatment). a – c Treatments within each sampling time without common superscripts differ significantly from one another (P≤ 0.05). * least square means. Abbreviation: LG, lemongrass essential oil.
Effect of LGEO on Salmonella Heidelberg Motility
The results of the motility assay are summarized in Table 1. A similar response to the 3 concentrations of LGEO was observed for both isolates of S. Heidelberg. No difference in motility was observed with 0.0075% and 0.015% LGEO compared to the positive control, as indicated by the similar halo diameter for the treatment and control (P > 0.05). LGEO at 0.15% completely reduced the zone of motility for both isolates of S. Heidelberg (P < 0.05).
Table 1.
Effect of LGEO on Salmonella Heidelberg (Isolates 466 and 1904) motility on semi-solid LB agar after 8 h of incubation at 37°C (means⁎ ± SE; n = 6/treatment).
| Zone of Motility (mm) |
||
|---|---|---|
| Treatment | S. Heidelberg 466 | S. Heidelberg 1904 |
| Positive Control | 41 ± 3.5a | 44 ± 4.0a |
| 0.0075% LGEO | 41 ± 2.8a | 45 ± 3.9a |
| 0.015% LGEO | 41 ± 2.9a | 45 ± 3.2a |
| 0.15% LGEO | 0 ± 0.0b | 0 ± 0.0b |
| Negative Control | 0 ± 0.0b | 0 ± 0.0b |
Values with different superscripts differ significantly for each isolate of Salmonella Heidelberg listed along the column (P ≤ 0.05).
least square means.
Effect of LGEO on Salmonella Heidelberg Biofilm Formation
The effect of LGEO on S. Heidelberg biofilm formation on polystyrene microtiter plates was quantified by determining crystal violet absorbance. The results are summarized in Table 2. For S. Heidelberg 466, all 3 concentrations of LGEO (0.0075%, 0.15%, and 1%) resulted in lower retention of crystal violet stain compared to the untreated group, indicating a potential decrease in biofilm-associated bacterial cells (P ≤ 0.05). However, the medium's turbidity in 0.0075% LGEO treated wells did not significantly differ from that of the positive control (P > 0.05). This result suggests no difference in the number of bacteria that grew in the broth though they were unassociated with the biofilm (planktonic cells). Both 0.15% and 1% LGEO treatment significantly reduced the turbidity of both the nutrient broth in which S. Heidelberg 1904 was cultured and the amount of crystal violet retained by biofilm-associated cells compared to the positive control group (P ≤ 0.05). The amount of crystal violet retained in the wells treated with 0.0075% LGEO did not significantly differ from that of the positive control (P > 0.05). Although a significant difference in turbidity was observed between the S. Heidelberg 1904 positive controls and 0.0075% LGEO treated wells (P ≤ 0.05), the absorbance reading for the S. Heidelberg 1904 positive control group was higher than that of S. Heidelberg 466.
Table 2.
Effect of LGEO on Salmonella Heidelberg (Isolates 466 and 1904) biofilm formation on microtiter plates incubated at 41°C for 96 h (means⁎ ± SE; n = 6/treatment).
| Absorbance (OD600 nm) |
||||
|---|---|---|---|---|
|
S. Heidelberg 466 |
S. Heidelberg 1904 |
|||
| Treatment | Biofilm-associated | Planktonic | Biofilm-associated | Planktonic |
| Positive Control | 0.131 ± 0.008d | 0.198 ± 0.048b | 0.156 ± 0.008b | 0.465 ± 0.030c |
| 0.0075% LGEO | 0.090 ± 0.007c | 0.167 ± 0.021b | 0.170 ± 0.013b | 0.177 ± 0.039b |
| 0.15% LGEO | 0.027 ± 0.007b | 0.016 ± 0.001a | 0.027 ± 0.001a | 0.012 ± 0.002a |
| 1% LGEO | 0.059 ± 0.004a | 0.040 ± 0.003a | 0.045 ± 0.007a | 0.027 ± 0.002a |
Biofilm-associated cells were measured indirectly through absorbance of crystal violet and planktonic cells were measured through absorbance of nutrient broth (LB).
Treatments within each sampling time without common superscripts differ significantly from one another (P≤ 0.05).
least square means.
Effect of LGEO on Pre-Formed Salmonella Heidelberg Biofilm
The effect of LGEO on pre-formed S. Heidelberg biofilms is illustrated in Table 3. No bacterial growth for either strain was observed on XLD plates following exposure of S. Heidelberg biofilms to 0.15% and 1% LGEO for 2 h (P ≤ 0.05). Although the 0.5 log10 CFU/mL difference between S. Heidelberg numbers in wells treated with 0.0075% LGEO and the positive controls were statistically significant for both isolates (P ≤ 0.05), the magnitude of reduction was minuscule compared to that observed with higher concentrations of LGEO.
Table 3.
Effect of LGEO on pre-formed Salmonella Heidelberg (Isolates 466 and 1904) biofilms with 2-h exposure time to the treatment (means⁎ ± SE; n = 8/treatment).
| Biofilm-Associated S. Heidelberg (Log10 CFU/mL) |
||
|---|---|---|
| Treatments | S. Heidelberg 466 | S. Heidelberg 1904 |
| Positive Control | 7.5 ± 0.14c | 7.3 ± 0.11c |
| 0.0075% LGEO | 7.1 ± 0.13b | 6.9 ± 0.17b |
| 0.15% LGEO | NGa | NGa |
| 1% LGEO | NGa | NGa |
NG = No growth observed by surface plating
Treatments with different superscripts differ significantly for each isolate of Salmonella Heidelberg listed along the column (P≤ 0.05).
least square means.
Effect of LGEO on Bacterial QS (QS Inhibition Assay)
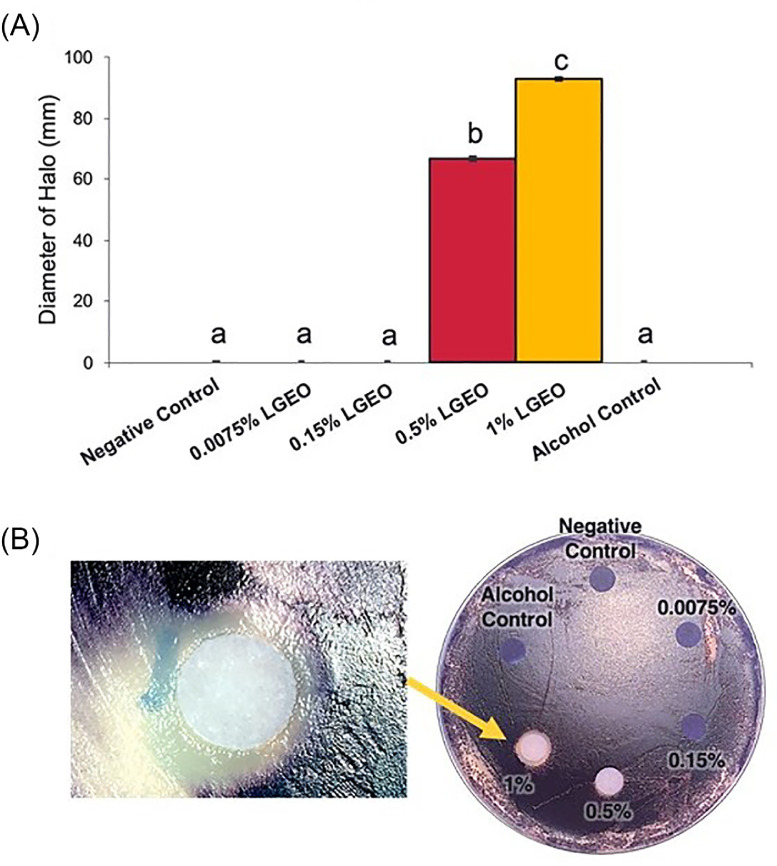
The effect of LGEO on QS of Chromobacterium violaceum is illustrated in Figure 4; this was performed to assess LGEO's potential effect against S. Heidelberg QS. Production of the violacein pigment by C. violaceum involves QS signaling, and loss of its distinct violet pigmentation potentially indicate impairment of the QS signaling. Disks with 0.0075% and 0.15% LGEO was similar to the negative and ethanol control in that a lawn of violet-colored C. violaceum surrounded the disk (Figure 4B). By contrast, disks containing 0.5% and 1% LGEO were surrounded by a halo of colorless C. violaceum with an average diameter of 67 mm and 93 mm (P ≤ 0.05), respectively.
Figure 4.
Effect of LGEO on Chromobacterium violaceum production of violacein on LB agar plates. (A) The diameter of colorless halos on lawns of C. violaceum surrounding the treatment disk was measured in millimeters (means* ± SEM; n = 8/treatment). (B) Illustrates the colorless halo on the plates. * least square means. a – c Treatments with different superscripts are significantly different (P≤ 0.05).
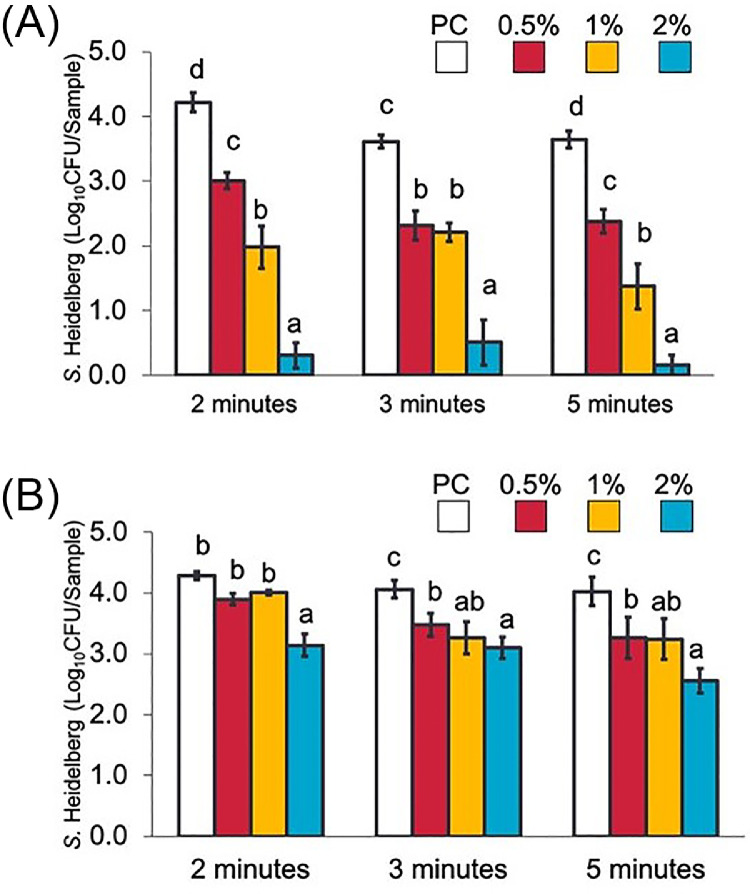
Effect of LGEO dip Treatments on S Heidelberg Attachment to Skin and Meat at a Scalding Temperature
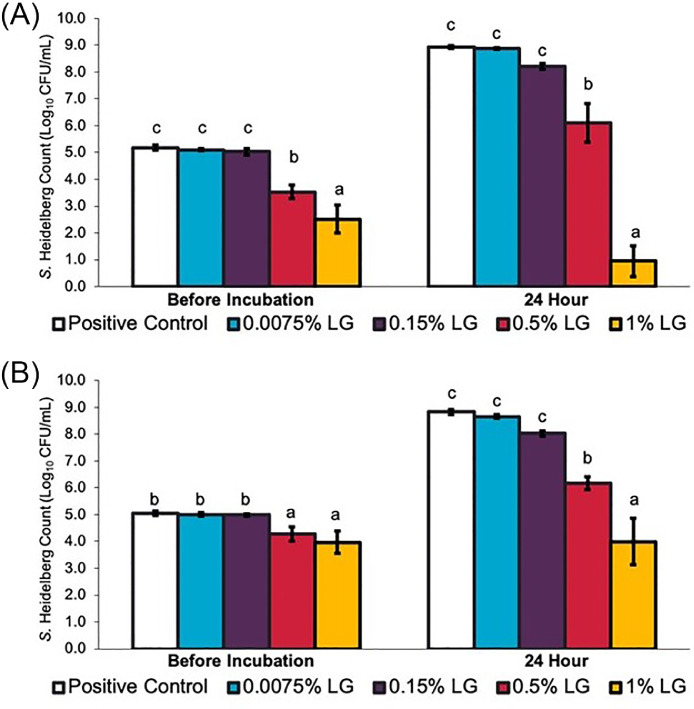
Figures 5A and B illustrate the effect of LGEO dip treatments on S. Heidelberg's attachment on broiler chicken skin and meat, respectively. At scalding (54°C) temperatures, 0.5, 1, and 2% LGEO (vol/vol) rapidly reduced S. Heidelberg numbers on the skin by 1.2, 2.2, and 3.9 log10 CFU/sample (P < 0.05), respectively, in 2 min compared to the S. Heidelberg control (PC). Similar to that observed on skin samples, 2% LGEO (vol/vol) also significantly reduced S. Heidelberg numbers compared to the PC group at all dipping times (P < 0.05). A 1.5 log10 CFU/sample reduction was observed with 2% LGEO after 5 min of treating meat samples. The magnitude of reduction was lower in meat samples compared to those obtained on skin samples.
Figure 5.
Effect of 2, 3, or 5-minute dip treatments in 0 (PC), 0.5, 1, or 2% LGEO (vol/vol) against S. Heidelberg attachment to broiler chicken skin (A) or meat (B) at a scalding (54°C) temperature. S. Heidelberg counts are expressed in log10 CFU/sample (means* ± SE; n = 6 samples per treatment). a – d Treatments within each sampling time without common superscripts differ significantly from one another (P< 0.05). * least square means.
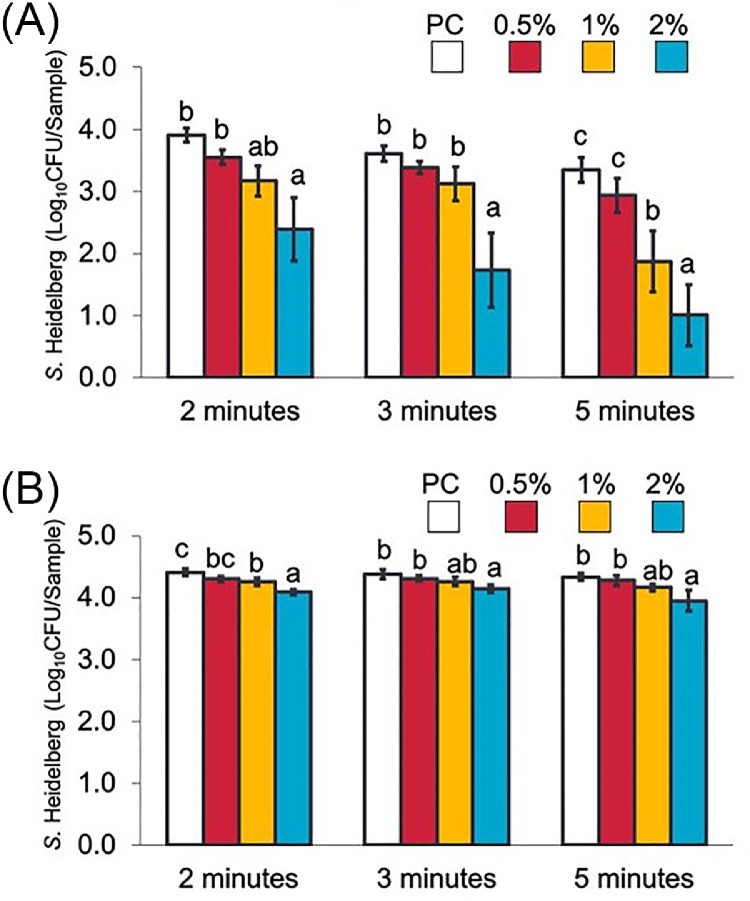
Effect of LGEO dip Treatments on S Heidelberg Attachment to Skin and Meat at Chilling Temperature
The effects of LGEO dip treatments on S. Heidelberg's attachment to skin and meat samples at chilling (4°C) temperatures are illustrated in Figures 6A and B, respectively. On skin samples, 2% LGEO (vol/vol) significantly reduced S. Heidelberg numbers by 1.5, 1.9, and 2.3 log10 CFU/sample (P < 0.05) compared to the PC groups after 2, 3, and 5, min, respectively. Similar to what was previously observed in scalding temperatures, S. Heidelberg's reduction in meat samples was lower compared to that observed with skin samples. The highest reduction in S. Heidelberg on meat samples was observed with 2% LGEO (vol/vol) after 5 min, which resulted in a 0.4 log10 CFU/sample reduction compared to the PC group (P < 0.05).
Figure 6.
Effect of 2, 3, or 5-minute dip treatments in 0 (PC), 0.5, 1, or 2% LGEO (vol/vol) against S. Heidelberg attachment to broiler chicken skin (A) or meat (B) at chilling (4°C) temperature. S. Heidelberg counts are expressed in log10 CFU/sample (means* ± SE; n = 6 samples per treatment). a – c Treatments within each sampling time without common superscripts differ significantly from one another (P< 0.05). * least square means.
Effect of Sequential LGEO Dip Treatments at Scalding and Chilling Temperatures on S. Heidelberg Attachment to Skin and Meat
Tables 4 and 5 summarize the effect of sequential LGEO dip treatments on S. Heidelberg's attachment to broiler chicken skin and meat, respectively. Sequential 2-minute dip treatments at 54°C followed by a dip at 4°C yielded similar reductions as those observed with individual treatments at scalding temperatures (54°C) for skin samples. Reductions of 1.6, 2.3, and 3.3 log10 CFU/sample were observed with 0.5, 1, and 2% LGEO (vol/vol) sequential dip treatments compared to untreated controls (P < 0.05). Subsequent dip treatments of meat samples were not as effective as on the skin, with 2% LGEO resulting in a reduction of 0.7 log10 CFU/sample (P < 0.05).
Table 4.
Effect of sequential 2-minute dip treatments in 0 (Positive Control), 0.5, 1, or 2% LGEO (vol/vol) against S. Heidelberg attachment to broiler chicken skin and in treatment waters at scalding (54°C) then chilling (4°C) temperatures.
|
S. Heidelberg (Log10CFU/Sample) |
|||
|---|---|---|---|
| Treatments | Skin | Chilling water | Scalding water |
| Positive Control | 4.2 ± 0.06c | 3.1 ± 0.24c | 3.5 ± 0.21b |
| 0.5% LGEO | 2.6 ± 0.36b | 0.4 ± 0.25b | NDa |
| 1% LGEO | 1.9 ± 0.39ab | NDa | NDa |
| 2% LGEO | 0.9 ± 0.29a | NDa | NDa |
Abbreviation: ND, non-detectable by surface plating and enrichment.
Values with different superscripts differed significantly at P < 0.05 column-wise within each sample.
⁎least square means.
S. Heidelberg counts are expressed in log10 CFU/sample (means* ± SE; n = 6 skin samples and n = 4 for water samples per treatment).
Table 5.
Effect of sequential 2-minute dip treatments in 0 (Positive Control), 0.5, 1, or 2% LGEO (vol/vol) against S. Heidelberg attachment to broiler chicken meat and in treatment waters at scalding (54°C) then chilling (4°C) temperatures.
|
S. Heidelberg (Log10CFU/Sample) |
|||
|---|---|---|---|
| Treatments | Meat | Chilling Water | Scalding Water |
| Positive Control | 4.3 ± 0.13c | 3.1 ± 0.24b | 3.6 ± 0.12b |
| 0.5% LGEO | 3.9 ± 0.11b | NDa | NDa |
| 1% LGEO | 3.8 ± 0.11b | NDa | NDa |
| 2% LGEO | 3.6 ± 0.04a | NDa | NDa |
Abbreviation: ND, non-detectable by surface plating and enrichment.
Values with different superscripts differed significantly at P < 0.05 column-wise within each sample.
* least square means.
S. Heidelberg counts are expressed in log10 CFU/sample (means* ± SE; n = 6 meat and n = 4 for water samples per treatment).
However, all concentrations of LGEO effectively inactivated S. Heidelberg from the scalding treatment waters for both skin and meat samples. Aside from 0.4 log10 CFU/mL S. Heidelberg recovered from chilling treatment waters containing 0.5% LGEO, no S. Heidelberg was recovered from any other LGEO treated waters where the skin samples were dipped (Table 4). Similarly, no S. Heidelberg was recovered from LGEO treatment waters dipped with inoculated meat samples, and 3.6 and 3.1 log10 CFU/mL S. Heidelberg were recovered from the scalding and chilling treatment waters of meat samples, respectively (Table 5).
DISCUSSION
Immersion of Salmonella-positive carcasses into scalding and chilling tanks provides chances for the pathogen to attach to the skin of other carcasses. It becomes a greater concern when these contaminated carcass parts may be further ground for retail. Salmonellae that are firmly attached to carcasses are found to persist through all processing stages and are less susceptible to the antimicrobial treatments (Lillard, 1989; Nagel et al., 2013; Salehi et al., 2016).
Raising the water temperature in scalding tanks or increasing the concentrations of antimicrobials such as chlorine in chilling tanks have been found to mitigate cross-contamination through the water. However, it had limited effects on bacteria attached to the skin (Yang et al., 2001). These interventions often yield reductions between 1 to 2 log CFU and raise concerns regarding potential residues, hazardous waste, and discoloration of the carcass that may affect consumer acceptance (Cox et al., 1974; Loretz et al., 2010; Wagle et al., 2017). In that regard, research has intensified on the use of natural compounds, including EOs in processing plants to reduce pathogen load and reduce cross-contamination (Wagle et al., 2017; Nair and Kollanoor Johny, 2017b).
Our screening studies in nutrient broth found a significant reduction in S. Heidelberg numbers with different concentrations of LGEO (Figures 1 and 2). We found similar results when sterile cecal contents were used instead of the nutrient broth. Although slight strain variations against LGEO could be observed in growth assays, higher concentrations of the EO always inhibited the growth of both strains significantly, highlighting its antibacterial potential against S. Heidelberg. Similar to our findings, Raybaudi-Massilia et al. (2006) have also reported inhibition of another common Salmonella serovar, S. Enteritidis (a common Salmonella serovar in poultry) with 0.5% LGEO.
Bacterial chemotaxis and motility play an important role in the pathogenicity of Salmonella as it propels pathogens toward their niches in both external environments and inside a host. Zoonotic Salmonella serovars of poultry are often motile (Barua et al., 2012). In the current study, complete inhibition of S. Heidelberg motility was observed with 0.15% LGEO (Table 1) without a reduction in the bacterial numbers (Figure 2). Motility is instrumental for bacterial adherence to surfaces, subsequent formation of biofilms, and colonization of their hosts. Adukwu et al. (2012) found the addition of LGEO at concentrations of 0.125% to 4% (vol/vol) was effective in inactivating biofilm viability of 5 strain of Staphylococcus aureus, a Gram-positive bacterium. Citral, an active component of LGEO, was reported to reduce motility and flagella numbers of a Gram-negative, rod-shaped, foodborne pathogen, Cronobacter sakazakii (Shi et al., 2017). As citral comprises up to 85% of LGEO, the effect observed on S. Heidelberg motility may be associated with its effect on the flagellar structure and/or function, as noted with C. sakazakii. However, this needs more investigation.
Biofilms of Salmonella species are also encountered in both biotic and abiotic surfaces. Their formation on abiotic surfaces like glass, rubber, cement, plastic, and stainless steel also presents a problem as these materials are commonly encountered in farms and poultry processing facilities (Joseph et al., 2001; Stepanovic et al., 2004; Hurrell et al., 2009). The introduction of a Salmonella-positive carcass into a scalding or chilling tank may transfer the pathogen into the water and allow it to form biofilms on the tank surfaces, which could protect the pathogen from environmental stressors and antibacterial agents. The continuous presence of the pathogen in the scalding or chilling tanks would exacerbate the rate of Salmonella transmission and could result in higher numbers of contaminated products. With regards to the effect of LGEO on biofilm formation, a significant reduction in both biofilm-associated and planktonic S. Heidelberg was observed with 0.15% and 1% LGEO compared to the control groups (Figure 4). The reduction in biofilm-associated Salmonella indicated that LGEO inhibits the formation of S. Heidelberg biofilms. This finding is in agreement with the anti-biofilm formation properties of LGEO reported against Staphylococcus aureus (Aiemsaard et al., 2011; Adukwu et al., 2012). Similar to the observed effects on S. Heidelberg biofilm formation, 0.15% and 1% LGEO completely inactivated S. Heidelberg in established biofilms (Figure 5). However, other studies found LGEO ineffective in eradicating established S. aureus biofilms or were found effective only at higher concentrations (Aiemsaard et al., 2011; Adukwu et al., 2012). The disparity could be attributed to the difference between biofilms produced by S. Heidelberg and S. aureus. The latter are known for their biofilm-forming capabilities, which are an essential virulence factor that makes S. aureus challenging to control in the medical field. Overall, it could be suggested that LGEO potentially disrupts S. Heidelberg biofilms by eliminating structures that are integral to their formation.
The effects of LGEO on the QS of S. Heidelberg was evaluated indirectly using C. violaceum, whose unique violacein synthesis is mediated by QS signaling mechanisms. QS is a mechanism by which bacterial populations coordinate community behavior and is an essential regulator of virulence factors in Gram-negative bacteria because they mediate pathogenic processes such as swarming behavior and biofilm formation (Jaramillo-Colorado et al., 2012). The results of the present study (Figure 6) illustrate the potential inhibitory effect of LGEO against C. violaceum QS mechanisms as a loss in pigment synthesis was observed with 0.5% and 1% LGEO (P ≤ 0.05). Other studies also reported similar findings that have demonstrated the QS modulating properties of citral on violacein production by C. violaceum (Jaramillo-Colorado et al., 2012; Shi et al., 2017). This property of LGEO and its constituents may contribute to their observed efficacy against S. Heidelberg's motility and biofilm formation as well.
The LGEO dip treatments at scalding temperatures were effective in reducing attached MDR S. Heidelberg. The addition of LGEO at 2% reduced attached bacteria on both skin and meat samples, although to a smaller magnitude for the latter. The reduction observed in MDR S. Heidelberg numbers may not be due to the water temperature as there was minimal reduction in the untreated controls across the 3 different time points. Studies conducted on S. Typhimurium found that a temperature of 55°C or higher was necessary to observe an antibacterial effect. Furthermore, the study noted minimal impact on bacteria attached to the skin with an increase in water temperature (Yang et al., 2001). At chilling temperatures, LGEO treatment significantly reduced S. Heidelberg on the skin, albeit to a lesser extent compared to that observed at scalding temperatures. By contrast, it was not as effective on meat samples at chilling temperatures. The addition of 2% LGEO in chilling waters yielded a 2.3 log10 CFU reduction in MDR S. Heidelberg numbers attached to the skin after 5 min. This reduction, though smaller than what was obtained at scalding temperatures, is considerably higher than the reduction achieved after a 5-minute dip treatment with 50 ppm chlorine, which yielded less than 1 log10 CFU reduction in S. Typhimurium on the skin (Yang et al., 2001).
Results of the independent scalding and chilling steps indicate that LGEO may potentially be an effective intervention to reduce MDR S. Heidelberg on broiler skin. The reductions are both meaningful and significant because the load of Salmonella on chicken carcasses are comparatively lower than other organisms and was reported to range between 30 to 210 CFU (Kamat et al., 1991). Although the level of S. Heidelberg inoculum used on both skin and meat samples were far higher than the average level that would be detected on a carcass in commercial processing plants, Yang et al. (2001) reported that the initial bacterial numbers present on the skin samples did not affect the reduction obtained during scalding. Other studies have noted a lesser reduction with a larger inoculum (Nair and Kollanoor Johny, 2017b).
The sequential scalding and chilling treatments yielded comparable results to the independent scalding step for the skin samples but were not as effective on the meat samples. Despite the lack of increased reduction observed on both skin and meat samples, no MDR S. Heidelberg was detected in either the scalding or chilling treatment waters with all concentrations of LGEO, despite its 1000-fold recovery from the untreated control waters. Eliminating the pathogen in the treated water would significantly reduce the possibility of cross-contamination from occurring. Chlorine is considered as a major chemical antimicrobial employed in poultry processing. However, the antimicrobial properties of chlorine are known to be affected by the organic load as well as the age of water (Yang et al., 2001). Unlike chlorine, in vitro studies previously conducted in our lab have found the LGEO remains effective against MDR S. Heidelberg over 7 days even in the presence of contaminants such as droppings (Peichel et al., 2019).
Throughout the study, LGEO was consistently more effective in reducing MDR S. Heidelberg on the skin than it was on meat samples. It is hypothesized that the greater surface area of skin samples allowed LGEO to encounter more pathogens than on the meat samples. Studies conducted by Thomas and McMeekin found that immersion of poultry carcass resulted in swelling of the skin, that exposed its deep channels and crevices (Thomas and McMeekin, 1982). This may have allowed for greater contact between LGEO and S. Heidelberg on the sheet-like skin. Furthermore, Kim et al. (1993) demonstrated that S. Typhimurium attached poorly to the epidermis of the skin, particularly the hydrophobic surface of the stratum corneum. By contrast, the breast meat samples were larger and possessed deeper crevices in which S. Heidelberg could be protected from the LGEO treatments. Lillard (1989) demonstrated that once lodged within these deep crevices, bacteria were not visible by scanning electron microscopy until the meat samples were shredded.
Overall, the results of the present study demonstrated the efficacy of LGEO against S. Heidelberg multiplication, motility, and biofilm formation capabilities and indicated having QS modulating activities. The studies also demonstrated that LGEO could reduce S. Heidelberg attached to the skin surface and from the treatment waters, potentially reducing the possibility of cross-contamination between carcasses in scalding and chilling tanks. Anti-virulence strategies are gaining interest as an emerging alternative method of pathogen control because it potentially imposes less pressure on the bacterium and thus combats the rise of resistance to antimicrobials. Testing the efficacy of LGEO against Salmonella on full carcasses are warranted, including a sensory evaluation of the meat exposed to the EO.
Acknowledgments
ACKNOWLEDGMENTS
We acknowledge the Department of Animal Science Rising Scholar Scholarship and the MnDRIVE Graduate Fellowship at the University of Minnesota awarded to the G. Dewi during the studies and publication of this manuscript. Mr. Jason Langlie, the lab manager during the study, is acknowledged and thanked for his assistance in preparing the essential media and buffers for this project.
This research was supported by the USDA NIFA Hatch projects (Accession# 1004609 and 1016910) through Minnesota Agricultural Experimentation Station (# MIN-16-102 & MIN-16-120).
DISCLOSURES
The authors declare no conflict of interest.
REFERENCES
- Adonizio A.L., Downum K., Bennett B.C., Mathee K. Anti-quorum sensing activity of medicinal plants in southern Florida. J. Ethnopharmacol. 2006;105:427–435. doi: 10.1016/j.jep.2005.11.025. [DOI] [PubMed] [Google Scholar]
- Adukwu E.C., Allen S.C.H., Phillips C.A. The anti-biofilm activity of lemongrass (Cymbopogon flexuosus) and grapefruit (Citrus paradisi) essential oils against five strains of Staphylococcus aureus. J. Appl. Microbiol. 2012;113:1217–1227. doi: 10.1111/j.1365-2672.2012.05418.x. [DOI] [PubMed] [Google Scholar]
- Aiemsaard J., Aiumlamai S., Aromdee C., Taweechaisupapong S., Khunkitti W. The effect of lemongrass oil and its major components on clinical isolate mastitis pathogens and their mechanisms of action on Staphylococcus aureus DMST 4745. Res. Vet. Sci. 2011;91:e31–e37. doi: 10.1016/j.rvsc.2011.01.012. [DOI] [PubMed] [Google Scholar]
- Barbosa L.N., Rall V.L.M., Fernandes A.A.H.A., Ushimaru P.I., da Silva Probst I., Fernandes A.A.H.A. Essential oils against foodborne pathogens and spoilage bacteria in minced meat. Foodborne Pathog. Dis. 2009;6:725–728. doi: 10.1089/fpd.2009.0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barua H., Biswas P.K., Olsen K.E.P., Christensen J.P. Prevalence and characterization of motile Salmonella in commercial layer poultry farms in Bangladesh. PLoS One. 2012;7:e35914. doi: 10.1371/journal.pone.0035914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearson B.L., Bearson S.M.D., Looft T., Cai G., Shippy D.C. Characterization of a multidrug-resistant Salmonella enterica serovar heidelberg outbreak strain in commercial turkeys: colonization, transmission, and host transcriptional response. Front. Vet. Sci. 2017;4:1–7. doi: 10.3389/fvets.2017.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd J.A., McKee. S.R. 2005. Improving Slaughter and Processing Technologies. Pages 310–332 in Food Safety Control in the Poultry Industry. [Google Scholar]
- CDC Outbreak of Salmonella Heidelberg infections linked to a single poultry producer – 13 states, 2012-2013. Morb. Mortal. Wkly. Rep. 2013;62:553–556. [PMC free article] [PubMed] [Google Scholar]
- CDC . 2014. Outbreak of Salmonella Heidelberg Infections Linked to Tyson Brand Mechanically Separated Chicken at a Correctional Facility (Final Update)https://www.cdc.gov/salmonella/heidelberg-01-14/ Accessed Mar. 2018. [Google Scholar]
- Cox N.A., Mercuri A.J., Thomson J.E., Gregory D.W. Quality of broiler carcasses as affected by hot water treatments. Poult. Sci. 1974;53:1566–1571. [Google Scholar]
- Djordjevic D., Wiedmann M., McLandsborough L.A. Microtiter plate assay for assessment of Listeria monocytogenes biofilm formation. Appl. Environ. Microbiol. 2002;68:2950–2958. doi: 10.1128/AEM.68.6.2950-2958.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutil L., Irwin R., Finley R., Ng L.K., Avery B., Boerlin P., Bourgault A.-M.M., Cole L., Daignault D., Desruisseau A., Demczuk W., Hoang L., Horsman G.B., Ismail J., Jamieson F., Maki A., Pacagnella A., Pillai D.R. Ceftiofur resistance in Salmonella enterica serovar Heidelberg from chicken meat and humans. Canada. Emerg. Infect. Dis. 2010;16:48–54. doi: 10.3201/eid1601.090729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eeckhaut V., Haesebrouck F., Ducatelle R., Van Immerseel F. Oral vaccination with a live Salmonella Enteritidis/Typhimurium bivalent vaccine in layers induces cross-protection against caecal and internal organ colonization by a Salmonella Infantis strain. Vet. Microbiol. 2018;218:7–12. doi: 10.1016/j.vetmic.2018.03.022. [DOI] [PubMed] [Google Scholar]
- Foley S.L., Nayak R., Hanning I.B., Johnson T.J., Han J., Ricke S.C. Population dynamics of Salmonella enterica serotypes in commercial egg and poultry production. Appl. Environ. Microbiol. 2011;77:4273–4279. doi: 10.1128/AEM.00598-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FSIS . 2014. FSIS Compliance Guide: Modernization of Poultry Slaughter Inspection: Chilling Requirements. Food Safety and Inspection Service. Washington, DC. [Google Scholar]
- FSIS . 2015. Compliance Guideline for Controlling Salmonella and Campylobacter in Raw Poultry. 4th ed. Food Safety and Inspection Service, Washington, DC. [Google Scholar]
- Gieraltowski, L., J. Higa, V. Peralta, A. Green, C. Schwensohn, H. Rosen, T. Libby, B. Kissler, N. Marsden-Haug, H. Booth, A. Kimura, J. Grass, A. Bicknese, B. Tolar, S. Defibaugh-Chávez, I. Williams, and M. Wise. and Salmonella Heidelberg Investigation Team. 2017. National outbreak of multidrug resistant Salmonella Heidelberg infections linked to a single poultry company. PLoS One. 11:1–14 [DOI] [PMC free article] [PubMed]
- Hurrell E., Kucerova E., Loughlin M., Caubilla-Barron J., Forsythe S.J. Biofilm formation on enteral feeding tubes by Cronobacter sakazakii, Salmonella serovars and other Enterobacteriaceae. Int. J. Food Microbiol. 2009;136:227–231. doi: 10.1016/j.ijfoodmicro.2009.08.007. [DOI] [PubMed] [Google Scholar]
- Jaramillo-Colorado B., Olivero-Verbel J., Stashenko E.E., Wagner-Döbler I., Kunze B. Anti-quorum sensing activity of essential oils from Colombian plants. Nat. Prod. Res. 2012;26:1075–1086. doi: 10.1080/14786419.2011.557376. [DOI] [PubMed] [Google Scholar]
- Joseph B., Otta S.K., Karunasagar I., Karunasagar I. Biofilm formation by Salmonella spp. on food contact surfaces and their sensitivity to sanitizers. Int. J. Food Microbiol. 2001;64:367–372. doi: 10.1016/s0168-1605(00)00466-9. [DOI] [PubMed] [Google Scholar]
- Kamat A.S., Alur M.D., Nerkar D.P., Nair P.M. Hygienization of Indian chicken meat by ionizing radiation. J. Food Saf. 1991;12:59–71. [Google Scholar]
- Kim J.-W., Slavik M.F., Griffis C.L., Walker J.T. Attachment of Salmonella Typhimurium to skins of chicken scalded at various temperatures. J. Food Prot. 1993;56:661–665. doi: 10.4315/0362-028X-56.8.661. [DOI] [PubMed] [Google Scholar]
- Kollanoor Johny A., Darre M.J., Donoghue A.M., Donoghue D.J., Venkitanarayanan K. Antibacterial effect of trans-cinnamaldehyde, eugenol, carvacrol, and thymol on Salmonella Enteritidis and Campylobacter jejuni in chicken cecal contents in vitro. J. Appl. Poult. Res. 2010;19:237–244. [Google Scholar]
- Lillard H.S. Factors affecting the persistence of Salmonella during the processing of poultry. J. Food Prot. 1989;52:829–832. doi: 10.4315/0362-028X-52.11.829. [DOI] [PubMed] [Google Scholar]
- Loretz M., Stephan R., Zweifel C. Antimicrobial activity of decontamination treatments for poultry carcasses: a literature survey. Food Control. 2010;21:791–804. [Google Scholar]
- Mireles J.R., Toguchi A., Harshey R.M. Salmonella enterica serovar Typhimurium swarming mutants with altered biofilm-forming abilities: surfactin inhibits biofilm formation. J. Bacteriol. 2001;183:5848–5854. doi: 10.1128/JB.183.20.5848-5854.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel G.M., Bauermeister L.J., Bratcher C.L., Singh M., McKee S.R. Salmonella and Campylobacter reduction and quality characteristics of poultry carcasses treated with various antimicrobials in a post-chill immersion tank. Int. J. Food Microbiol. 2013;165:281–286. doi: 10.1016/j.ijfoodmicro.2013.05.016. [DOI] [PubMed] [Google Scholar]
- Nair D.V.T., Kollanoor Johny. A. Effect of Propionibacterium freudenreichii on Salmonella multiplication, motility, and association with avian epithelial cells. Poult. Sci. 2017;96:1376–1386. doi: 10.3382/ps/pew367. [DOI] [PubMed] [Google Scholar]
- Nair D.V.T., Kollanoor Johny. A. Food grade pimenta leaf essential oil reduces the attachment of Salmonella enterica Heidelberg (2011 ground turkey outbreak isolate) on to turkey skin. Front. Microbiol. 2017;8:2328. doi: 10.3389/fmicb.2017.02328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu C., Gilbert. E.S. Colorimetric method for identifying plant essential oil components that affect biofilm formation and structure. Appl. Environ. Microbiol. 2004;70:6951–6956. doi: 10.1128/AEM.70.12.6951-6956.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peichel C., Nair D.V.T., Dewi G., Donoghue A.M., Reed K.M., Kollanoor Johny A. Effect of lemongrass (Cymbopogon citratus) essential oil on the survival of multidrug-resistant Salmonella enterica serovar Heidelberg in contaminated poultry drinking water1. J. Appl. Poult. Res. 2019;28:1121–1130. [Google Scholar]
- Raybaudi-Massilia R.M., Mosqueda-Melgar J., Martin-Belloso O. Antimicrobial activity of essential oils on Salmonella Enteritidis, Escherichia coli, and Listeria innocua in fruit juices. J. Food Prot. 2006;69:1579–1586. doi: 10.4315/0362-028x-69.7.1579. [DOI] [PubMed] [Google Scholar]
- Salehi S., Howe K., Lawrence M.L., Brooks J.P., Bailey R.H., Karsi A. Salmonella enterica serovar Kentucky flagella are required for broiler skin adhesion and Caco-2 cell invasion. Appl. Environ. Microbiol. 2017;83:e02115–e02116. doi: 10.1128/AEM.02115-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C., Sun Y., Liu Z., Guo D., Sun H., Sun Z., Chen S., Zhang W., Wen Q., Peng X., Xia X. Inhibition of Cronobacter sakazakii virulence factors by citral. Sci. Rep. 2017;7:1–11. doi: 10.1038/srep43243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavik M.F., Kim J.-W., Walker J.T. Reduction of Salmonella and Campylobacter on chicken carcasses by changing scalding temperature. J. Food Prot. 1995;58:689–691. doi: 10.4315/0362-028X-58.6.689. [DOI] [PubMed] [Google Scholar]
- Stepanovic S., Cirkovic I., Ranin L., Svabic-Vlahovic M. Biofilm formation by Salmonella spp. and Listeria monocytogenes on plastic surface. Lett. Appl. Microbiol. 2004;38:428–432. doi: 10.1111/j.1472-765X.2004.01513.x. [DOI] [PubMed] [Google Scholar]
- Thomas C.J., McMeekin. T.A. Effect of water immersion on the microtopography of the skin of chicken carcasses. J. Sci. Food Agric. 1982;33:549–554. [Google Scholar]
- Upadhyay A., Upadhyaya I., Kollanoor-johny A., Venkitanarayanan K. Antibiofilm effect of plant derived antimicrobials on Listeria monocytogenes. Food Microbiol. 2013;36:79–89. doi: 10.1016/j.fm.2013.04.010. [DOI] [PubMed] [Google Scholar]
- Vasudevan P., Marek P., Nair M.K.M., Annamalai T., Darre M., Khan M., Venkitanarayanan K. In vitro inactivation of Salmonella Enteritidis in autoclaved chicken cecal contents by caprylic acid. J. Appl. Poult. Res. 2005;14:1. [Google Scholar]
- Vugia D.J., Samuel M., Farley M.M., Marcus R., Shiferaw B., Shallow S., Smith K., Angulo F.J. Invasive Salmonella infections in the United States, foodNet, 1996–1999: incidence, serotype distribution, and outcome. Clin. Infect. Dis. 2004;38:S149–S156. doi: 10.1086/381581. [DOI] [PubMed] [Google Scholar]
- Wagle B.R., Arsi K., Upadhyay A., Shrestha S., Venkitanarayanan K., Donoghue A.M., Donoghue D.J. β-Resorcylic acid, a phytophenolic compound, reduces Campylobacter jejuni in postharvest poultry. J. Food Prot. 2017;80:1243–1251. doi: 10.4315/0362-028X.JFP-16-475. [DOI] [PubMed] [Google Scholar]
- Yang H., Li Y., Johnson M.G. Survival and death of Salmonella typhimurium and Campylobacter jejuni in processing water and on chicken skin during poultry scalding and chilling. J. Food Prot. 2001;64:770–776. doi: 10.4315/0362-028x-64.6.770. [DOI] [PubMed] [Google Scholar]