Abstract
Woody breast (WB) condition has created a variety of challenges for the global poultry industry. To date, there are no effective treatments or preventative measures due to its unknown (undefined) etiology. Several potential mechanisms including oxidative stress, fiber-type switching, cellular damage, and altered intracellular calcium levels have been proposed to play a key role in the progression of the WB myopathy. In a previous study, we have shown that WB is associated with hypoxia-like status and dysregulated oxygen homeostasis. As satellite cells (SC) play a pivotal role in muscle fiber repair and remodeling under stress conditions, we undertook the present study to determine satellite cell fate in WB-affected birds when reared in either normoxic or hypoxic conditions. Modern random bred broilers from 2015 (n = 200) were wing banded and reared under standard brooding practices for the first 2 wk post-hatch. At 15 d, chicks were divided in 2 body weight-matched groups and reared to 6 wk in either control local altitude or hypobaric chambers with simulated altitude of 6,000 ft. Birds were provided ad libitum access to water and feed, according to the Cobb recommendations. At 6 wk of age, birds were processed and scored for WB, and breast samples were collected from WB-affected and unaffected birds for molecular analyses (n = 10/group). SCs were isolated from normal breast muscle, cultured in vitro, and exposed to normoxia or hypoxia for 2 h. The expression of target genes was determined by qPCR using 2−∆∆Ct method. Protein distribution and expression were determined by immunofluorescence staining and immunoblot, respectively. Data were analyzed by the Student's t test with significance set at P < 0.05. Multiple satellite cell markers, myogenic factor (Myf)-5 and paired box (PAX)-7 were significantly decreased at the mRNA and protein levels in the breast muscle from WB-affected birds compared to their unaffected counterparts. Lipogenic-and adipogenic-associated factors (acetyl-CoA carboxylase, ACCα; fatty acid synthase, FASN, malic enzyme, ME; and ATP citrate lyase, ACLY) were activated in WB-affected birds. These data were supported by an in vitro study where hypoxia decreased the expression of Myf5 and Pax7, and increased that of ACCα, FASN, ME, and ACLY. Together, these data indicate that under hypoxic condition, SC change fate by switching from a myogenic to an adipogenic program, which explains at least partly, the etiology of the WB myopathy.
Key words: woody breast, hypoxia, fatty acid metabolism, myogenesis, satellite cell
INTRODUCTION
Genetic selection for high growth rate, body weight, and enhanced breast muscle yield in broiler chickens has enabled the poultry industry to produce more food and support the livelihoods of billions of people worldwide (Vaarst et al., 2015; Lilburn et al., 2019; Mourad et al., 2019). Parallel to these significant progresses, several undesirable changes have occurred including increased incidence of metabolic disorders such as fat deposition, leg problems, and muscle myopathies (Kuttappan et al., 2016; Wideman, 2016).
Woody breast (WB) is a metabolic condition that has a significant impact on modern broilers and is imposing a heavy economic burden on the poultry industry worldwide (Greene et al., 2020a). It is already present in many countries including USA, France, Finland, Italy, Spain, Brazil, UK, and Japan (Sihvo et al., 2013; Mudalal et al., 2015; Russo et al., 2015; Alnahhas et al., 2016; de Brot et al., 2016; Cemin et al., 2018; Huang and Ahn, 2018; Kawasaki et al., 2018), and is emerging on a global scale. Many factors contribute to the adverse economic impact of WB phenotype including (1) on-farm culling and mortality, (2) lower meat yield due to decreased protein content, and increased fat and collagen content, (3) down-grading and condemnation at processing, (4) reduced protein functionality in further processed products, and (5) rejection from human consumption due to negative effects on visual appearance and overall consumer acceptance (Kuttappan et al., 2012; Mudalal et al., 2015; Petracci et al., 2015; Soglia et al., 2016; Tijare et al., 2016; Morey et al., 2020).
Although the exact etiology of WB myopathy is still unknown, omics studies indicated several potential contributing factors including hypoxia and oxidative stress, intracellular calcium, and muscle fiber type switching (Mutryn et al., 2015; Abasht et al., 2016; Greene et al., 2020a). In addition to hardening of the affected muscle area, pale color, surface hemorrhaging and the presence of gelatinous fluid on the muscle surface, WB is histologically and microscopically characterized by infiltration of immune and fat cells (Sihvo et al., 2013). However the cellular origin of the adipocyte-like cells is still unknown. de Almeida Mallmann et al. (2019) have shown abundant infiltration of adipose cells in WB-affected muscle and suggested that they originated from bone marrow adipose tissue. Meloche et al. (2018), on the other hand, have reported an increased population of myogenic stem cell types in WB-affected muscles. Velleman's group indicated that satellite cell-mediated regeneration of muscle is suppressed in WB myopathy (Velleman, 2019).
Muscle satellite cells (SC), stem cells located between the basement membrane and sarcolemma of muscle fiber (Moss and Leblond, 1971), are self-renewing mesenchymal cells that enable hypertrophy, maintenance, and repair of damaged skeletal muscle (Geiger et al., 2018). It has been shown that SC are multipotent and can be induced by various extrinsic factors and environment to follow myogenic, adipogenic, or osteogenic pathways (Asakura et al., 2001; Wang et al., 2015a; Liu et al., 2017).
In continuum of our previous research where we have shown that WB myopathy is associated with dysmetabolism of oxygen homeostasis and activation of hypoxia pathway (Greene et al., 2020a) as well as alteration of fatty acid profile (Cauble et al., 2020), we undertook the present study to determine the fate of SC in WB muscle using both in vivo and in vitro approaches.
MATERIALS AND METHODS
Birds, Housing and Diets
All animal experiments were approved by the University of Arkansas Animal Care and Use Committee (protocol number 18088) and were in accordance with the recommendations in NIH's Guide for the Care and Use of Laboratory Animals. Birds from the 2015 modern random bred (Orlowski et al., 2017; Tabler et al., 2020), which is composed of broiler packages offered by three broiler genetics companies and have been blended homogenously over five generations of random mating, were maintained at the University of Arkansas. At day of hatch, chicks were vent sexed and males were wing banded and reared under standard brooding practices for two weeks at local altitude. At 15 d, chicks were divided in two body weight-matched groups and reared to 6 wk in either control local altitude (LA, 427 m, 1,400 ft) or a simulated high-altitude challenge (HA, 1,829 m, 6,000 ft) in a hypobaric chamber. The hypobaric chamber simulates a set altitude above sea level and creates a hypoxic environment from a decrease in the partial pressure of oxygen by operating under a partial vacuum. An altitude of 6,000 ft was chosen to induce a hypoxic environment without inhibiting growth of the broilers or inducing pulmonary hypertension (ascites) syndrome. Both LA and HA chambers were set up identical to each other with litter shavings floor pens, hanging feed cans and a nipple water line. Ventilation was set to maintain airflow at 17 m3m (600 cfm). Both chambers were warm-room brooded with temperature gradually decreased from 32°C on d 1 to 3, to 31°C on d 4 to 6, 29°C on d 7 to 10, 27°C on d 11 to 14, and 24°C for day 15 through day 42. The photoperiod was maintained at 24 h light. Feed and water were provided ad libitum throughout the study. Birds were individually weighed weekly. All daily and weekly tasks were conducted at altitude. Access to the hypobaric chamber was through an airlock, which allowed for air pressure equilibrium. At 6 wk of age, birds (100 birds/group) were processed in the pilot processing plant at the University of Arkansas and evaluated for the WB myopathy and compression force of the cranial breast lobe as described previously (Orlowski et al., 2018; Greene et al., 2019; Cauble et al., 2020). Breast tissues were collected from WB-affected and unaffected HA birds (n = 10/group), snap frozen in liquid nitrogen, and stored at −80°C for further molecular and biochemical analyses.
SC Culture
Ross 708 broiler SC was previously isolated by Dr. S. G. Velleman (The Ohio State University, Wooster, OH) as described previously (McFarland et al., 1997; Harding et al., 2016; Clark et al., 2018). Briefly, cells were cultured in 6 well-plates containing Dulbecco's Modified Eagle Medium (Life Technologies, Grand Island, NY) with 10% chicken serum (Sigma-Aldrich, St. Louis, MO), 5% horse serum (Sigma-Aldrich, St. Louis, MO), 1% antibiotic/antimycotic (Life Technologies, Grand Island, NY), and 0.1% gentamicin (Life Technologies, Grand Island, NY) at 38°C in a 5% CO2/95% O2 humidified incubator. At exponential phase of growth (~80%), cells were exposed to hypoxia by placing the plates into gas-tight modular hypoxic chamber (1% O2/5% CO2/94% N2; Coy Laboratory Products, Inc, MI) for 2h. The control cells were maintained at normoxic conditions (5% CO2/95% O2). The duration of hypoxia exposures was based on pilot and previous studies (Latil et al., 2012).
RNA Extraction, cDNA Synthesis and Quantitative Real-Time PCR
Total RNA extraction, cDNA synthesis, and quantitative real-time PCR were performed as previously described (Greene et al., 2019). Oligonucleotide primers used for chicken lipogenic and adipogenic genes (acetyl CoA carboxylase alpha, ACC-α; fatty acid synthase, FASN; ATP citrate lyase, ACLY; malic enzyme, ME; stearoyl-CoA desaturase-1, SCD-1; sterol regulatory element-binding protein 1 and 2, SREBP-1/2) and the housekeeping ribosomal 18S were previously described (Nguyen et al., 2015; Flees et al., 2017; Greene et al., 2020b). For satellite cell markers and myogenic transcription factors, pair box protein 3 (PAX-3), PAX-7, myogenic factor (Myf-5), myogenin (MyoG), and myogenic regulatory factor (MRF)-4), the oligonucleotide sequences are summarized in Table 1. Relative expressions of target genes were normalized to the expression of r18S RNA and calculated by the 2−∆∆Ct method (Schmittgen and Livak, 2008). For the in vivo trial, unaffected birds were used as calibrator; while, for the in vitro trial, SC under normoxia were used as calibrator.
Table 1.
Oligonucleotide primers for real-time qPCR.
| Gene | Accession number1 | Primer sequence (5’ → 3’) | Orientation | Product size (bp) |
|---|---|---|---|---|
| PAX7 PAX3 Myf5 MRF4 MyoG |
NM-205065 NM_204269 NM_001030363 D10599 NM_204184 |
AGGCTCCGATGTCGAATCAG GCGGCGCTGCTTCCT GCCTCACCAGCCCCAAA GGCTCCAGACCTCCAGTCAA CCTCATGTGGGCTTGCAAA CCTTCCGCCGGTCCAT GCATGATGATGGACCTTTTCG CCATTCTCCCCGTCCAAGTA GGAGAAGCGGAGGCTGAAG GCAGAGTGCTGCGTTTCAGA |
Forward Reverse Forward Reverse Forward Reverse Forward Reverse Forward Reverse |
55 57 59 61 62 |
Abbreviations: MRF4, myogenic regulated factor 4; Myf5, myogenic factor 5; MyoG, myogenin; PAX, paired box.
Accession number refer to Genbank (NCBI).
Western Blot
Immunoblot for satellite cell cultures, and breast muscle samples were performed according to previously published papers by our group (Flees et al., 2017; Nguyen et al., 2017; Greene et al., 2019). The following primary polyclonal antibodies diluted to 1:1000 were used: rabbit polyclonal anti-HIF-1α (#LS-C287203), rabbit anti-phospho ACCαSer79 (#3661), rabbit anti-ACCα (#3662), rabbit anti-ACLY (#LS-C290517), rabbit anti-ME (#ARP48511_P050), rabbit anti-FASN (#NB400-114), mouse anti-PAX-7 (#sc-81648), and rabbit anti-Myf-5 (#ab139523). All the antibodies were purchased from Cell Signaling (Danvers, MA), except the anti-FASN antibody was from Novus Biologicals (Littleton, CO), the anti-ME from Aviva Systems Biology (San Diego, CA), the anti-ACLY and anti-HIF1α antibodies from LSBio (Seattle, WA), the anti-PAX7 from Santa Cruz Biotechnology (Dallas, TX), and the anti-Myf5 from Abcam (Cambridge, MA). The HRP-conjugated secondary antibodies (dilution 1:5000) were from Santa Cruz Biotechnology (Dallas, TX). A prestained molecular weight marker (Precision Plus Protein Dual Color) was used as a standard (BioRad, Hercules, CA). The signal was visualized by enhanced chemiluminescence (ECL plus; GE Healthcare Bio-Sciences, Buckinghamshire, UK), and captured by FluorChem M MultiFluor System (Proteinsimple, Santa Clara, CA). Image acquisition and analysis were performed by AlphaView software (Version 3.4.0, 1993–2011, Proteinsimple,Santa Clara, CA).
Immunofluorescence
Immunofluorescence staining was performed as previously described (Dridi et al., 2012; Greene et al., 2020b). Briefly, SC were grown in chamber slides and fixed with methanol for 10 min at −20°C, then permeabilized with Triton-X 100. Cells were incubated with serum-free protein block (Dako, Carpinteria, CA) for 1 hour at room temperature, then incubated with anti-HIF1α, anti-PAX7, anti-Myf5, anti-ACLY, anti-FASN, anti-ME, anti-SREBP1 or anti-SREBP2 (1:200, in Antibody Diluent, Dako, Carpinteria, CA, overnight at 4°C) for mono-staining or a combination of anti-PAX7 with anti-Myf5 for double-labeling immunofluorescence. Signal was visualized with DyLight 488- or 594-conjugated secondary antibody (Thermo Fisher Scientific, Grand Island, NY). Slides were cover slipped with Vectashield with 4′,6-diamidino-2-phenylindole (DAPI, Vector Laboratories, Burlingame, CA), and images were obtained and analyzed using Zeiss Imager M2 and AxioVision software (Carl Zeiss Microscopy, Pleasanton, CA).
Statistical Analyses
For body weight, data were analyzed by two-way repeated measure ANOVA with age and altitude as factors. For the rest of growth performances, mRNA abundances, and protein expression, data were analyzed by Student “t” test. Graph Pad Prism software (version 7.0 for Windows, La Jolla, CA) was used. Differences were considered significant at P < 0.05.
RESULTS
High Altitude Induces WB Incidence and Alters CS Fate in Broilers
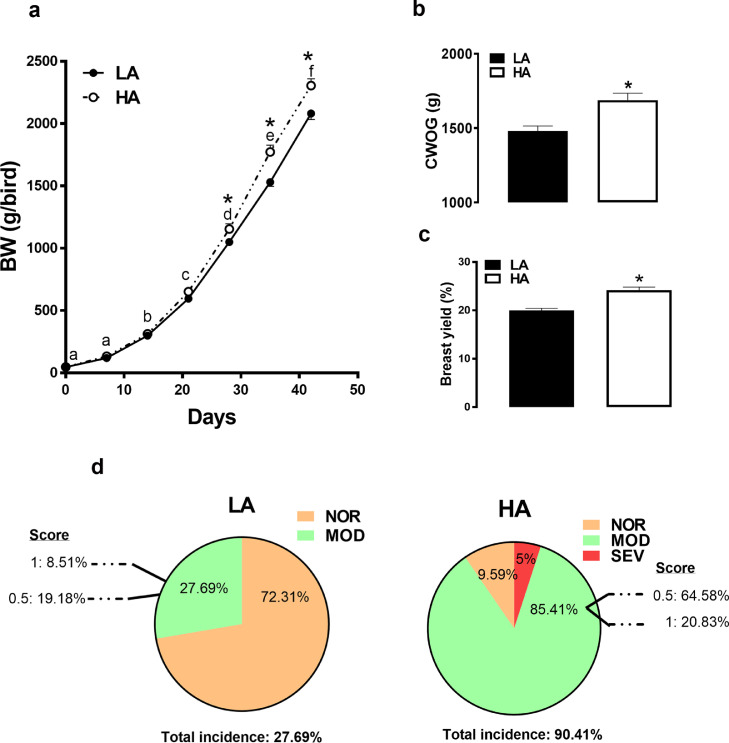
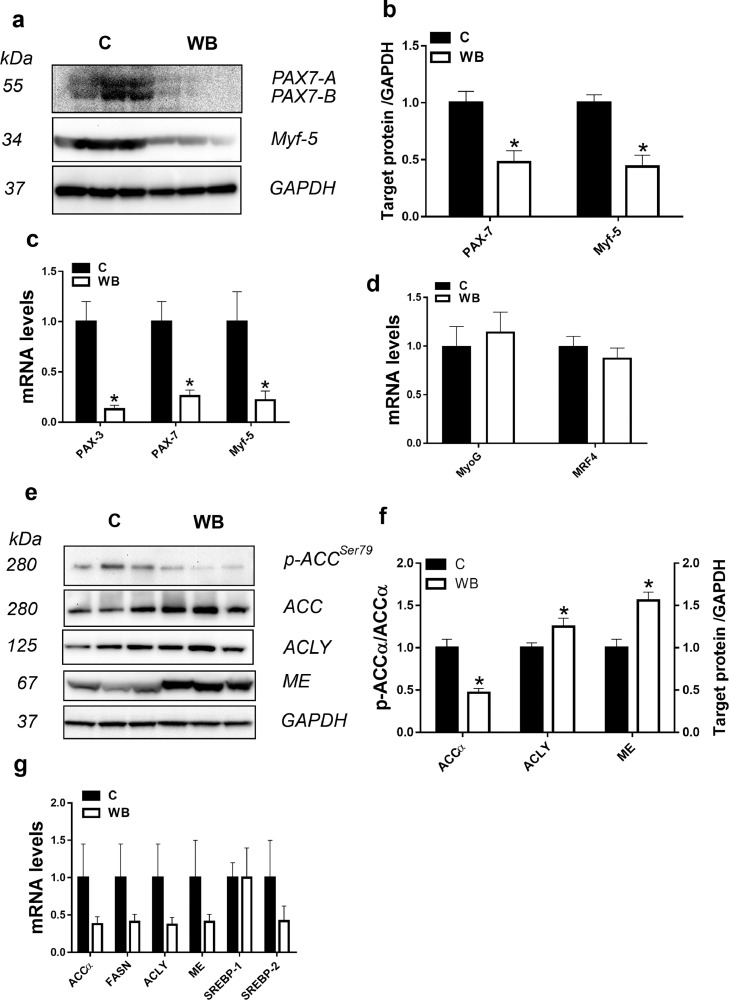
Performance and WB incidence in birds raised in LA vs. birds raised under HA are shown in Figure 1. Body weight of HA birds was significantly higher compared to that of LA counterparts on days 28, 35, and 42 (Figure 1A). Chilled with-out-giblets and breast yield were significantly higher in HA compared to LA birds (Figure 1B, C). Total incidence of WB is higher in the HA (90.41%) compared to LA (27.6%) (Figure 1D). When analysed by category, HA-birds manifest 85.4 % moderate (MOD) and 5% severe (SEV) WB compared to 27.6% MOD and absence of SEV in their LA counterparts. Using SEV WB-affected and unaffected breast tissues from HA birds, molecular and biochemical analyses show that the expression of PAX7 and Myf5 was significantly down regulated at the mRNA and protein levels in WB-affected compared to unaffected-birds (Figure 2A-cC. The mRNA abundances of PAX3 were also significantly decreased in the breast of WB-affected birds compared to their unaffected counterparts (Figure 2C). The expression of myogenic factors (MyoG and MRF4) remained unchanged between the groups (Figure 2D). Concomitantly, the phosphorylated levels of ACCα at Ser79 site were significantly decreased (Figure 2E). The protein, but not the mRNA levels of ACLY and ME were significantly increased in WB-affected muscle compared to unaffected birds (Figure 2E-G). The expression of the transcription factors SREBP-1 and SREBP-2 gene did not differ between the groups (Figure 2G).
Figure 1.
Effects of high altitude (hypoxia) on growth performance and woody breast incidence in broiler chickens. Birds were divided in two body weight-matched groups and reared from 2 to 6 wk in either control local altitude (LA, 427 m, 1400 ft) or high altitude challenge (HA, 1,829 m, 6,000 ft) in hypobaric chambers. Body weight (BW) (A), CWOG (B), and breast yield (C) were recorded. At 6 wk of age, WB was scored as previously described (Greene et al., 2019) (D). Data are presented as mean ± SEM (n = 100/group). *denotes significant difference compared to the LA at the same age and different letters indicate significant difference between age at P < 0.05.
Figure 2.
The expression profile of satellite cell- and adipogenic- markers in woody breast muscle. Protein levels were measured by Western blot (A, B, E, F), and mRNA abundances were determined by qPCR (C, D, G) using 2−∆∆Ct method (Schmittgen and Livak, 2008). Data are presented as mean ± SEM (n= 10/group). *denotes significant difference of treated (affected) compared to the control (unaffected) group at P < 0.05. Abbreviations: ACC, acetyl-CoA carboxylase; ACLY, ATP citrate lyase; C, control; FASN, fatty acid synthase; ME, malic enzyme; Myf5, myogenic factor 5; MyoG, myogenin; MRF4, myogenic regulatory factor 4; PAX, paired box; SREBP, sterol regulatory element binding protein; WB, woody breast.
Hypoxia Exposure Alters the Fate of Chicken Primary SC
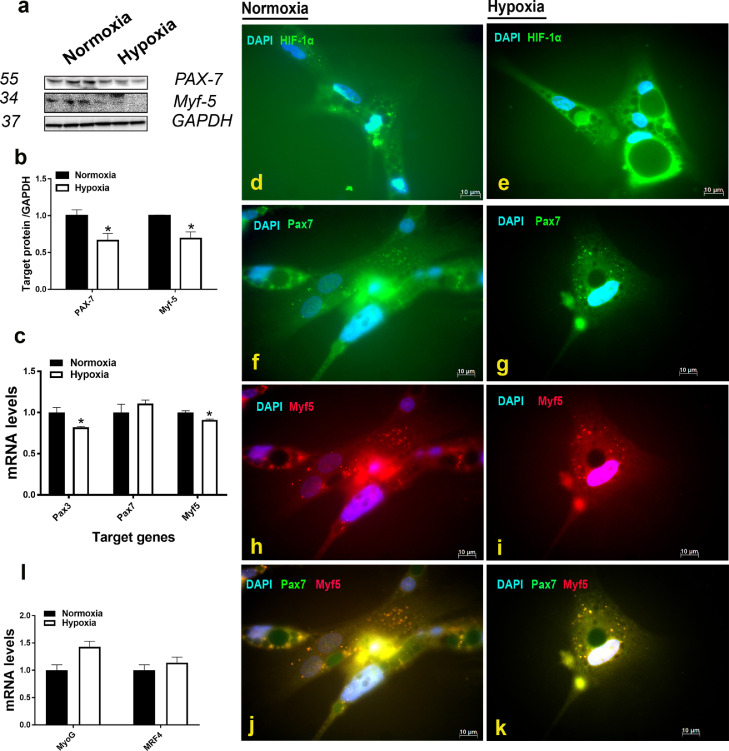
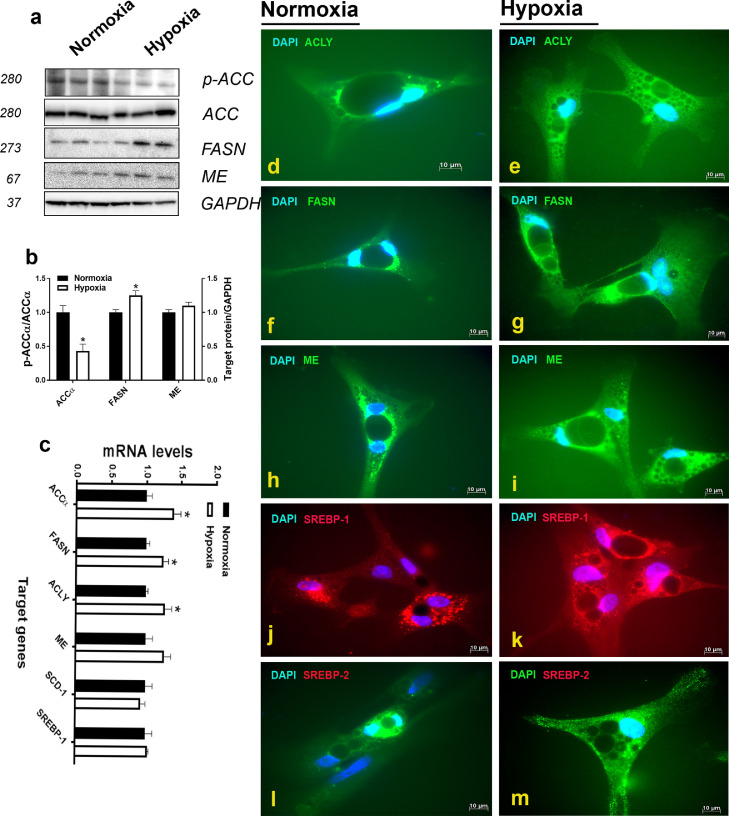
Short exposure (2 h) to hypoxic conditions, as confirmed by HIF-1α activation (Figure 3D, E), down regulates the expression of PAX7 and Myf5 at both the mRNA and protein levels (Figure 3A-C). The expression of the myogenic factors (MyoG and MRF4) did not differ between hypoxic and normoxic SC (Figure 3L). Mono- and double-immunofluorescence staining supports these data and further shows that PAX7- and Myf5-positive cells are decreased in hypoxia compared to normoxia (Figure 3F-K). Phosphorylated levels of ACCα at Ser79 site was significantly reduced in hypoxic compared to normoxic SC (Figure 4A, B). Protein levels of FASN, but not ME, were significantly induced in hypoxic compared to normoxic SC (Figure 4A, B). Immunofluorescence staining support these data and show an increase of lipogenic proteins along with increased levels of both transcription factors SREBP-1 and 2 (Figure 4D-M). As for proteins, mRNA abundances of ACCα, FASN, and ACLY, but not ME were up regulated in hypoxic compared to normoxic cells (Figure 4C). The expression of SCD-1 and SREBP-1 gene was not affected by 2 h-hypoxia exposure (Figure 4C).
Figure 3.
Effects of hypoxia exposure (2 h) on the expression of satellite cell markers in primary satellite cells. Protein levels were measured by Western blot (A, B), and immunofluorescence staining (D-K, scale bars = 10 µm), and mRNA abundances were determined by qPCR (C, L) using 2−∆∆Ct method (Schmittgen and Livak, 2008). Data are presented as mean ±SEM (n= 6/group). *denotes significant difference of treated (hypoxic) compared to the control (normoxic) cells at P < 0.05. Abbreviations: HIF-1α, hypoxia inducible factor 1 alpha; Myf5, myogenic factor 5; MyoG, myogenin; MRF4, myogenic regulatory factor 4; PAX, paired box.
Figure 4.
Effects of hypoxia exposure (2 h) on the expression of adipogenic markers in primary satellite cells. Protein levels were measured by Western blot (A, B), and immunofluorescence staining (D-M, scale bars = 10 µm), and mRNA abundances were determined by qPCR (C) using 2−∆∆Ct method (Schmittgen and Livak, 2008). Data are presented as mean ± SEM (n = 6/group). *denotes significant difference of hypoxic compared to the control (normoxic) cells at P < 0.05. Abbreviations: ACC, acetyl-CoA carboxylase; ACLY, ATP citrate lyase; FASN, fatty acid synthase; ME, malic enzyme.
DISCUSSION
Broiler chickens play a key role in worldwide meat production and support the food security of billions of peoples. However, the relatively new emerging muscle disorder WB has increased drastically on a global scale, and it is negatively impacting worldwide broiler meat production quality, and sustainability (Trocino et al., 2015). It constitutes a major animal health, welfare, and economic concern which are estimated to cost the poultry industry millions of dollars a year due to on-farm culling and mortality, down-grading and condemnation at processing, as well as rejection from human consumption (Petracci et al., 2019). The WB lesions are associated with clinical and histological changes including multifocal degeneration and necrosis, fiber fragmentation, hyalinization, swelling and fibrosis (Kuttappan et al., 2016), which in turn results in palpable severe hardness of the breast muscle. Microscopic analyses showed also infiltration of immune and fat cells (de Almeida Mallmann et al., 2019), with a debatable and unclear origin. Although seminal omics, managerial, and nutritional work has been conducted, the etiology of WB is still not fully defined.
Breast muscles have a low concentration of myoglobin and are poorly vascularized (Lilburn et al., 2019), and our previous research indicated that WB in broiler chickens is associated with hypoxia-like status and dysregulated oxygen homeostasis (Greene et al., 2019; Greene et al., 2020a). Using the hypobaric chambers in the present study, the results clearly support our previous data (Greene et al., 2019) and demonstrate that hypoxia is a cause, not a consequence, of WB myopathy. The WB myopathy in industry broiler flocks has proven to be erratic and unpredictable in both incidence and severity making it hard to understand the mechanism behind it. The hypobaric chambers when run at moderate altitudes could be used as a potential and highly relevant model to consistently trigger high incidences of WB for subsequent identification of mechanism-based strategies to reduce/prevent the myopathy.
Although it is still an area of active research, hypoxia is well known to induce reactive oxygen species production and results in skeletal muscle cell injury and damage (Lundby et al., 2003; Magalhães et al., 2005). In general, responding to injury, skeletal muscle undergoes a highly orchestrated degeneration and regenerative process that takes place at the tissue, cellular, and molecular levels (Yin et al., 2013). SC, which are a heterogeneous population of stem and progenitor cells, are key engines of muscle repair (Wang and Rudnicki, 2011; Chang and Rudnicki, 2014). They proliferate following muscle trauma and form new myofibers through a process similar to embryo muscle development. Many transcription factors including Pax-7, Myf-5, myogenic differentiation, myogenin, and MRF-4 control the myogenic program in SC (Chang and Rudnicki, 2014). Pax-7 is a transcription factor expressed by quiescent SC with myogenic activity, and inactivation of Pax7 results in severe depletion of these muscle stem cells. In adult mice, Pax7 expression appears specific to the satellite cell myogenic lineage (Seale et al., 2000). Myf-5, a member of basic helix-loop-helix transcription factors, is a myogenic regulatory factor and a marker of activated SC (Hernández-Hernández et al., 2017). In quiescent state, Myf-5 gene is transcribed in SC, but Myf-5 mRNA is sequestered in messenger ribonucleoprotein (mRNP) granules. These granules are dissociated upon satellite cell activation; thus, Myf-5 protein is the marker of satellite cell activation (Crist et al., 2012). The down regulation of both PAX7 and Myf5 expression at both the mRNA and protein levels, in our experimental conditions, indicate a loss of myogenic activity in SC in WB muscle and in hypoxic cells.
SC are multipotent cells, and depending on the stimuli and stress type, duration, and severity, they can follow myogenic, adipogenic, or osteogenic pathways (Asakura et al., 2001; Shefer et al., 2004; Wang et al., 2015a; Liu et al., 2017). As WB is characterized by infiltration of fat cells, we sought to evaluate here the potential activation of adipogenic program in SC under hypoxic conditions. Adipogenic/lipogenic program is tightly controlled by the key enzymes ACC, FASN, ME, and ACLY. ACLY is a cytosolic enzyme that catalyzes the cleavage of citrate into acetyl-CoA and oxaloacetate (Verschueren et al., 2019; Wei et al., 2019). ACC is a multi-subunit and biotin-dependent enzyme that catalyzes the irreversible carboxylation of acetyl-CoA to produce malonyl-CoA, the rate limiting step in fatty acid synthesis (Abu-Elheiga et al., 1995; Abu-Elheiga et al., 2001). The main function of FASN is to catalyze the synthesis of long chain fatty acids from malonyl-CoA via a series of decarboxylative Claisen condensation reactions (Smith, 1994; Jayakumar et al., 1995). ME (also known as decarboxylating malate dehydrogenase) catalyzes a reversible oxidative decarboxylation of malate to produce pyruvate (Chang and Tong, 2003; Jiang et al., 2013). The catalytic function of ACC is regulated by phosphorylation (inactive) and dephosphorylation (active) (Witters et al., 1988), and its reduced phosphorylated levels at Ser79 indicate that hypoxia activates ACC in both in vivo and in vitro studies. Concomitant with these changes, the upregulation of ACLY, FASN, and ME proteins in WB and hypoxic cells indicate that hypoxia alters the fate of SC and activates the adipogenic program. Furthermore, the upregulation of ME suggests that hypoxia may alter the fatty acid profile by reducing PUFA and increasing saturated FA as previously reported in WB (Cauble et al., 2020). A previous study reported that a hypoxia state caused by the supplementation of amino acids contributed to the formation of fatty acids, and eventually breast muscle myopathy (Livingston et al., 2019). Additionally, hypoxic conditions have been reported to limit the regenerative capacity of rodent muscle fibers by favoring the replacement of degenerated muscle fibers with lipid and fibrotic tissues (Hoppeler and Vogt, 2001).
Although the upstream mechanisms by which hypoxia activates adipogenic enzyme pathways are not known at this time point, it is possible that HIF-1α is a key mediator. The body's adaptive responses to several stressors including hypoxia is regulated by HIF-1α (Semenza, 2007), which coordinates gene expression relating to the reprogramming required for adaptation to stressors (Mylonis et al., 2019). During hypoxia, it has been shown that fatty acid synthesis is regulated by Akt- and HIF-1α-dependent activation of SREBP-1, which in turn up regulates the expression of FASN (Furuta et al., 2008). Our previous study showed an activation of Akt and HIF-1α in WB (Greene et al., 2020a), and the present data demonstrate, by immunofluorescence staining, an activation of HIF-1α and increased SREBP-1 in hypoxic SC. SREBP-1 is now well established as a key transcription factor for the transactivation of adipogenic genes such as FASN and ACCα (Hansmannel et al., 2006). Once it is activated through a proteolytic process in response to intracellular demands for lipids, the cleaved mature form of SREBP-1 translocate into the nucleus as a homodimer and stimulates the transcription of target genes by binding to the SREs in their promoters (Wang et al., 2015b). The absence of differential expression of SREBP-1 mRNA between WB-affected and unaffected birds and between hypoxic and normoxic SC indicates that SREBP-1 might be regulated at post-transcriptional and/or post-translational levels (Giandomenico et al., 2003; Sundqvist et al., 2005; Bengoechea-Alonso and Ericsson, 2009). Further studies using specific antibodies that cross react with chicken SREBP-1a/c and work in immunoblot technique are warranted. The upregulation of SREBP-2 in hypoxic SC suggests that hypoxia alters the cholesterol metabolism and this needs further in-depth investigation.
In conclusion, results from the present in-vitro and in-vivo studies indicate that:
-
1)
Hypoxia induces and exacerbates WB incidence in broilers and that the hypobaric chamber could be used as a highly relevant model to consistently induce and study the myopathy.
-
2)
Hypoxia alters SC fate by activating the adipogenic rather than the myogenic program, which might explain, at least partially, the progression of WB myopathy and the origin of infiltrated fat cells. However, the potential contribution of bone marrow fat cells and dietary fat are not ruled out, and merit further investigations.
Acknowledgments
ACKNOWLEDGMENTS
This work was supported by grant from AB Vista (to SD). AB Vista had no role in conducting the research, generating the data, interpreting the results, or writing the manuscript.
Authors’ contributions: SD conceptualized and designed the experiment. NE, RC, AD, EG, SO, and NA performed the experiments and analyzed the data. CC and SV provided the satellite cells. SD and MB acquired funding and purchased the reagents. NE and SD wrote the manuscript with inputs from all co-authors.
DISCLOSURES
Author Mike Bedford is employed by the company AB Vista. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
REFERENCES
- Abasht B., Mutryn M.F., Michalek R.D., Lee W.R. Oxidative stress and metabolic perturbations in wooden breast disorder in chickens. PLoS One. 2016;11 doi: 10.1371/journal.pone.0153750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu-Elheiga L., Jayakumar A., Baldini A., Chirala S.S., Wakil S.J. Human acetyl-CoA carboxylase: characterization, molecular cloning, and evidence for two isoforms. PNAS. 1995;92:4011. doi: 10.1073/pnas.92.9.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu-Elheiga L., Matzuk M.M., Abo-Hashema K.A.H., Wakil S.J. Continuous fatty acid oxidation and reduced fat storage in mice lacking acetyl-CoA carboxylase 2. Science. 2001;291:2613. doi: 10.1126/science.1056843. [DOI] [PubMed] [Google Scholar]
- Alnahhas N., Berri C., Chabault M., Chartrin P., Boulay M., Bourin M.C., Le Bihan-Duval E. Genetic parameters of white striping in relation to body weight, carcass composition, and meat quality traits in two broiler lines divergently selected for the ultimate pH of the pectoralis major muscle. BMC Genetics. 2016;17:61. doi: 10.1186/s12863-016-0369-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakura A., Rudnicki M.A., Komaki M. Muscle satellite cells are multipotential stem cells that exhibit myogenic, osteogenic, and adipogenic differentiation. Differentiation. 2001;68:245–253. doi: 10.1046/j.1432-0436.2001.680412.x. [DOI] [PubMed] [Google Scholar]
- Bengoechea-Alonso M.T., Ericsson J. A phosphorylation cascade controls the degradation of active SREBP1. J. Biol. Chem. 2009;284:5885–5895. doi: 10.1074/jbc.M807906200. [DOI] [PubMed] [Google Scholar]
- Cauble R.N., Greene E.S., Orlowski S., Walk C., Bedford M., Apple J., Kidd M.T., Dridi S. Research note: Dietary phytase reduces broiler woody breast severity via potential modulation of breast muscle fatty acid profiles. Poult. Sci. 2020;99:4009–4015. doi: 10.1016/j.psj.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cemin H.S., Vieira S.L., Stefanello C., Kindlein L., Ferreira T.Z., Fireman A.K. Broiler responses to increasing selenium supplementation using Zn-L-selenomethionine with special attention to breast myopathies. Poult. Sci. 2018;97:1832–1840. doi: 10.3382/ps/pey001. [DOI] [PubMed] [Google Scholar]
- Chang G.G., Tong L. Structure and function of malic enzymes, a new class of oxidative decarboxylases. Biochemistry. 2003;42:12721–12733. doi: 10.1021/bi035251+. [DOI] [PubMed] [Google Scholar]
- Chang N.C., Rudnicki M.A. Satellite cells: the architects of skeletal muscle. Curr. Top. Dev. Biol. 2014;107:161–181. doi: 10.1016/B978-0-12-416022-4.00006-8. [DOI] [PubMed] [Google Scholar]
- Clark D.L., McCormick J.L., Velleman S.G. Effect of incubation temperature on neuropeptide Y and neuropeptide Y receptors in turkey and chicken satellite cells. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2018;219-220:58–66. doi: 10.1016/j.cbpa.2018.02.014. [DOI] [PubMed] [Google Scholar]
- Crist C.G., Montarras D., Buckingham M. Muscle satellite cells are primed for myogenesis but maintain quiescence with sequestration of Myf5 mRNA targeted by microRNA-31 in mRNP granules. Cell Stem Cell. 2012;11:118–126. doi: 10.1016/j.stem.2012.03.011. [DOI] [PubMed] [Google Scholar]
- de Almeida Mallmann B., Martin E.M., Soo Kim K., Calderon-Apodaca N.L., Baxter M.F.A., Latorre J.D., Hernandez-Velasco X., Paasch-Martinez L., Owens C.M., Dridi S., Bottje W.G., Greene E.S., Tellez-Isaias G. Evaluation of bone marrow adipose tissue and bone mineralization on broiler chickens affected by wooden breast myopathy. Front. Physiol. 2019;10:674. doi: 10.3389/fphys.2019.00674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Brot S., Perez S., Shivaprasad H.L., Baiker K., Polledo L., Clark M., Grau-Roma L. Wooden breast lesions in broiler chickens in the UK. Vet. Record. 2016;178:141. doi: 10.1136/vr.103561. [DOI] [PubMed] [Google Scholar]
- Dridi S., Hirano Y., Tarallo V., Kim Y., Fowler B.J., Ambati B.K., Bogdanovich S., Chiodo V.A., Hauswirth W.W., Kugel J.F., Goodrich J.A., Ponicsan S.L., Hinton D.R., Kleinman M.E., Baffi J.Z., Gelfand B.D., Ambati J. ERK1/2 activation is a therapeutic target in age-related macular degeneration. PNAS. 2012;109:13781–13786. doi: 10.1073/pnas.1206494109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flees J., Rajaei-Sharifabadi H., Greene E., Beer L., Hargis B.M., Ellestad L., Porter T., Donoghue A., Bottje W.G., Dridi S. Effect of Morinda citrifolia (Noni)-enriched diet on hepatic heat shock protein and lipid metabolism-related genes in heat stressed broiler chickens. Front. Physiol. 2017;8:919. doi: 10.3389/fphys.2017.00919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta E., Pai S.K., Zhan R., Bandyopadhyay S., Watabe M., Mo Y.Y., Hirota S., Hosobe S., Tsukada T., Miura K., Kamada S., Saito K., Iiizumi M., Liu W., Ericsson J., Watabe K. Fatty acid synthase gene is up-regulated by hypoxia via activation of Akt and sterol regulatory element binding protein-1. Cancer Res. 2008;68:1003–1011. doi: 10.1158/0008-5472.CAN-07-2489. [DOI] [PubMed] [Google Scholar]
- Geiger A.E., Daughtry M.R., Gow C.M., Siegel P.B., Shi H., Gerrard D.E. Long-term selection of chickens for body weight alters muscle satellite cell behaviors. Poult. Sci. 2018;97:2557–2567. doi: 10.3382/ps/pey050. [DOI] [PubMed] [Google Scholar]
- Giandomenico V., Simonsson M., Grönroos E., Ericsson J. Coactivator-dependent acetylation stabilizes members of the SREBP family of transcription factors. Mol. Cell. Biol. 2003;23:2587–2599. doi: 10.1128/MCB.23.7.2587-2599.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene E., Cauble R., Dhamad A.E., Kidd M.T., Kong B., Howard S.M., Castro H.F., Campagna S.R., Bedford M., Dridi S. Muscle metabolome profiles in woody breast-(un)affected broilers: Effects of Quantum Blue phytase-enriched diet. Front. Vet. Sci. 2020;7:458. doi: 10.3389/fvets.2020.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene E., Flees J., Dadgar S., Mallmann B., Orlowski S., Dhamad A., Rochell S., Kidd M., Laurendon C., Whitfield H., Brearley C., Rajaram N., Walk C., Dridi S. Quantum Blue reduces the severity of woody breast myopathy via modulation of oxygen homeostasis-related genes in broiler chickens. Front. Physiol. 2019;10:1251. doi: 10.3389/fphys.2019.01251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene E.S., Zampiga M., Sirri F., Ohkubo T., Dridi S. Orexin system is expressed in avian liver and regulates hepatic lipogenesis via ERK1/2 activation. Sci. Rep. 2020;10:19191. doi: 10.1038/s41598-020-76329-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansmannel F., Mordier S., Iynedjian P.B. Insulin induction of glucokinase and fatty acid synthase in hepatocytes: analysis of the roles of sterol-regulatory-element-binding protein-1c and liver X receptor. Biochem. J. 2006;399:275–283. doi: 10.1042/BJ20060811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding R.L., Halevy O., Yahav S., Velleman S.G. The effect of temperature on proliferation and differentiation of chicken skeletal muscle satellite cells isolated from different muscle types. Physiol. Rep. 2016;4:e12770. doi: 10.14814/phy2.12770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Hernández J.M., García-González E.G., Brun C.E., Rudnicki M.A. The myogenic regulatory factors, determinants of muscle development, cell identity and regeneration. Semin. Cell Dev. Biol. 2017;72:10–18. doi: 10.1016/j.semcdb.2017.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppeler H., Vogt M. Muscle tissue adaptations to hypoxia. J. Exp. Biol. 2001;204:3133. doi: 10.1242/jeb.204.18.3133. [DOI] [PubMed] [Google Scholar]
- Huang X., Ahn D.U. The incidence of muscle abnormalities in broiler breast meat - A review. Korean J. Food Sci. Anim. Resour. 2018;38:835–850. doi: 10.5851/kosfa.2018.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayakumar A., Tai M.H., Huang W.Y., al-Feel W., Hsu M., Abu-Elheiga L., Chirala S.S., Wakil S.J. Human fatty acid synthase: properties and molecular cloning. PNAS. 1995;92:8695. doi: 10.1073/pnas.92.19.8695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang P., Du W., Mancuso A., Wellen K.E., Yang X. Reciprocal regulation of p53 and malic enzymes modulates metabolism and senescence. Nature. 2013;493:689–693. doi: 10.1038/nature11776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki T., Iwasaki T., Yamada M., Yoshida T., Watanabe T. Rapid growth rate results in remarkably hardened breast in broilers during the middle stage of rearing: a biochemical and histopathological study. PLoS One. 2018;13 doi: 10.1371/journal.pone.0193307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuttappan V.A., Hargis B.M., Owens C.M. White striping and woody breast myopathies in the modern poultry industry: a review. Poult. Sci. 2016;95:2724–2733. doi: 10.3382/ps/pew216. [DOI] [PubMed] [Google Scholar]
- Kuttappan V.A., Lee Y.S., Erf G.F., Meullenet J.F., McKee S.R., Owens C.M. Consumer acceptance of visual appearance of broiler breast meat with varying degrees of white striping. Poult. Sci. 2012;91:1240–1247. doi: 10.3382/ps.2011-01947. [DOI] [PubMed] [Google Scholar]
- Latil M., Rocheteau P., Châtre L., Sanulli S., Mémet S., Ricchetti M., Tajbakhsh S., Chrétien F. Skeletal muscle stem cells adopt a dormant cell state post mortem and retain regenerative capacity. Nat. Commu. 2012;3:903. doi: 10.1038/ncomms1890. [DOI] [PubMed] [Google Scholar]
- Lilburn M.S., Griffin J.R., Wick M. From muscle to food: oxidative challenges and developmental anomalies in poultry breast muscle. Poult. Sci. 2019;98:4255–4260. doi: 10.3382/ps/pey409. [DOI] [PubMed] [Google Scholar]
- Liu S., Song N., He J., Yu X., Guo J., Jiao X., Ding X., Teng J. Effect of hypoxia on the differentiation and the self-renewal of metanephrogenic mesenchymal stem cells. Stem Cells Int. 2017;2017 doi: 10.1155/2017/7168687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston M.L., Ferket P.R., Brake J., Livingston K.A. Dietary amino acids under hypoxic conditions exacerbates muscle myopathies including wooden breast and white stripping. Poult. Sci. 2019;98:1517–1527. doi: 10.3382/ps/pey463. [DOI] [PubMed] [Google Scholar]
- Lundby C., Pilegaard H., van Hall G., Sander M., Calbet J., Loft S., Møller P. Oxidative DNA damage and repair in skeletal muscle of humans exposed to high-altitude hypoxia. Toxicology. 2003;192:229–236. doi: 10.1016/s0300-483x(03)00328-7. [DOI] [PubMed] [Google Scholar]
- Magalhães J., Ascensão A., Soares J.M.C., Ferreira R., Neuparth M.J., Marques F., Duarte J.A. Acute and severe hypobaric hypoxia increases oxidative stress and impairs mitochondrial function in mouse skeletal muscle. J. Appl. Physiol. 2005;99:1247–1253. doi: 10.1152/japplphysiol.01324.2004. [DOI] [PubMed] [Google Scholar]
- McFarland D.C., Gilkerson K.K., Pesall J.E., Ferrin N.H., Wellenreiter R.H. In vitro characteristics of myogenic satellite cells derived from the pectoralis major and biceps femoris muscles of the chicken. Cytobios. 1997;91:45–52. [PubMed] [Google Scholar]
- Meloche K.J., Dozier W.A., Brandebourg T.D., Starkey J.D. Skeletal muscle growth characteristics and myogenic stem cell activity in broiler chickens affected by wooden breast. Poult. Sci. 2018;97:4401–4414. doi: 10.3382/ps/pey287. [DOI] [PubMed] [Google Scholar]
- Morey A., Smith A.E., Garner L.J., Cox M.K. Application of bioelectrical impedance analysis to detect broiler breast filets affected with woody breast myopathy. Front. Physiol. 2020;11:808. doi: 10.3389/fphys.2020.00808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss F.P., Leblond C.P. Satellite cells as the source of nuclei in muscles of growing rats. Anat. Rec. 1971;170:421–435. doi: 10.1002/ar.1091700405. [DOI] [PubMed] [Google Scholar]
- Mourad R., Jaafar H.H., Daghir N. New estimates of water footprint for animal products in fifteen countries of the Middle East and North Africa (2010–2016) Water Resour. Ind. 2019;22 [Google Scholar]
- Mudalal S., Lorenzi M., Soglia F., Cavani C., Petracci M. Implications of white striping and wooden breast abnormalities on quality traits of raw and marinated chicken meat. Animal. 2015;9:728–734. doi: 10.1017/S175173111400295X. [DOI] [PubMed] [Google Scholar]
- Mutryn M.F., Brannick E.M., Fu W., Lee W.R., Abasht B. Characterization of a novel chicken muscle disorder through differential gene expression and pathway analysis using RNA-sequencing. BMC Genomics. 2015;16:399. doi: 10.1186/s12864-015-1623-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mylonis I., Simos G., Paraskeva E. Hypoxia-inducible factors and the regulation of lipid metabolism. Cells. 2019;8:214. doi: 10.3390/cells8030214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen P., Greene E., Ishola P., Huff G., Donoghue A., Bottje W., Dridi S. Chronic mild cold conditioning modulates the expression of hypothalamic neuropeptide and intermediary metabolic-related genes and improves growth performances in young chicks. PLoS One. 2015;10 doi: 10.1371/journal.pone.0142319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen P.H., Greene E., Kong B.-W., Bottje W., Anthony N., Dridi S. Acute heat stress alters the expression of orexin system in quail muscle. Front. Physiol. 2017;8:1079. doi: 10.3389/fphys.2017.01079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlowski S., Flees J., Anthony N., Dridi S. Differential expression of water channel- and noncoding RNA biogenesis-related genes in three lines of chickens under a short-term water restriction. Poult. Sci. 2017;96:4172–4181. doi: 10.3382/ps/pex263. [DOI] [PubMed] [Google Scholar]
- Orlowski S., Flees J., Greene E.S., Ashley D., Lee S.-O., Yang F.L., Owens C.M., Kidd M., Anthony N., Dridi S. Effects of phytogenic additives on meat quality traits in broiler chickens. J. Anim. Sci. 2018;96:3757–3767. doi: 10.1093/jas/sky238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petracci M., Mudalal S., Soglia F., Cavani C. Meat quality in fast-growing broiler chickens. World's Poult. Sci. J. 2015;71:363–374. [Google Scholar]
- Petracci M., Soglia F., Madruga M., Carvalho L., Ida E., Estévez M. Wooden-breast, white striping, and spaghetti meat: causes, consequences and consumer perception of emerging broiler meat abnormalities. Compr. Rev. Food Sci. Food Saf. 2019;18:565–583. doi: 10.1111/1541-4337.12431. [DOI] [PubMed] [Google Scholar]
- Russo E., Drigo M., Longoni C., Pezzotti R., Fasoli P., Recordati C. Evaluation of white striping prevalence and predisposing factors in broilers at slaughter. Poult. Sci. 2015;94:1843–1848. doi: 10.3382/ps/pev172. [DOI] [PubMed] [Google Scholar]
- Schmittgen T.D., Livak K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Seale P., Sabourin L.A., Girgis-Gabardo A., Mansouri A., Gruss P., Rudnicki M.A. Pax7 is required for the specification of myogenic satellite cells. Cell. 2000;102:777–786. doi: 10.1016/s0092-8674(00)00066-0. [DOI] [PubMed] [Google Scholar]
- Semenza G.L. Hypoxia-inducible factor 1 (HIF-1) pathway. Sci. STKE. 2007;2007:cm8. doi: 10.1126/stke.4072007cm8. [DOI] [PubMed] [Google Scholar]
- Shefer G., Wleklinski-Lee M., Yablonka-Reuveni Z. Skeletal muscle satellite cells can spontaneously enter an alternative mesenchymal pathway. J. Cell Sci. 2004;117:5393. doi: 10.1242/jcs.01419. [DOI] [PubMed] [Google Scholar]
- Sihvo H.K., Immonen K., Puolanne E. Myodegeneration with fibrosis and regeneration in the pectoralis major muscle of broilers. Vet. Pathol. 2013;51:619–623. doi: 10.1177/0300985813497488. [DOI] [PubMed] [Google Scholar]
- Smith S. The animal fatty acid synthase: one gene, one polypeptide, seven enzymes. FASEB J. 1994;8:1248–1259. [PubMed] [Google Scholar]
- Soglia F., Mudalal S., Babini E., Di Nunzio M., Mazzoni M., Sirri F., Cavani C., Petracci M. Histology, composition, and quality traits of chicken Pectoralis major muscle affected by wooden breast abnormality. Poult. Sci. 2016;95:651–659. doi: 10.3382/ps/pev353. [DOI] [PubMed] [Google Scholar]
- Sundqvist A., Bengoechea-Alonso M.T., Ye X., Lukiyanchuk V., Jin J., Harper J.W., Ericsson J. Control of lipid metabolism by phosphorylation-dependent degradation of the SREBP family of transcription factors by SCFFbw7. Cell Metab. 2005;1:379–391. doi: 10.1016/j.cmet.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Tabler T.W., Greene E.S., Orlowski S.K., Hiltz J.Z., Anthony N.B., Dridi S. Intestinal barrier integrity in heat-stressed modern broilers and their ancestor wild jungle fowl. Front. Vet. Sci. 2020;7:249. doi: 10.3389/fvets.2020.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tijare V.V., Yang F.L., Kuttappan V.A., Alvarado C.Z., Coon C.N., Owens C.M. Meat quality of broiler breast fillets with white striping and woody breast muscle myopathies. Poult. Sci. 2016;95:2167–2173. doi: 10.3382/ps/pew129. [DOI] [PubMed] [Google Scholar]
- Trocino A., Piccirillo A., Birolo M., Radaelli G., Bertotto D., Filiou E., Petracci M., Xiccato G. Effect of genotype, gender and feed restriction on growth, meat quality and the occurrence of white striping and wooden breast in broiler chickens. Poult. Sci. 2015;94:2996–3004. doi: 10.3382/ps/pev296. [DOI] [PubMed] [Google Scholar]
- Vaarst M., Steenfeldt S., Horsted K. Sustainable development perspectives of poultry production. World's Poult. Sci. J. 2015;71:609–620. [Google Scholar]
- Velleman S.G. Recent developments in breast muscle myopathies associated with growth in poultry. Annu. Rev. Anim. Biosci. 2019;7:289–308. doi: 10.1146/annurev-animal-020518-115311. [DOI] [PubMed] [Google Scholar]
- Verschueren K.H.G., Blanchet C., Felix J., Dansercoer A., De Vos D., Bloch Y., Van Beeumen J., Svergun D., Gutsche I., Savvides S.N., Verstraete K. Structure of ATP citrate lyase and the origin of citrate synthase in the Krebs cycle. Nature. 2019;568:571–575. doi: 10.1038/s41586-019-1095-5. [DOI] [PubMed] [Google Scholar]
- Wang X., Liu C., Li S., Xu Y., Chen P., Liu Y., Ding Q., Wahafu W., Hong B., Yang M. Hypoxia precondition promotes adipose-derived mesenchymal stem cells based repair of diabetic erectile dysfunction via augmenting angiogenesis and neuroprotection. PLoS One. 2015;10 doi: 10.1371/journal.pone.0118951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Viscarra J., Kim S.J., Sul H.S. Transcriptional regulation of hepatic lipogenesis. Nat. Rev. Mol. Cell Biol. 2015;16:678–689. doi: 10.1038/nrm4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.X., Rudnicki M.A. Satellite cells, the engines of muscle repair. Nat. Rev. Mol. Cell Biol. 2011;13:127–133. doi: 10.1038/nrm3265. [DOI] [PubMed] [Google Scholar]
- Wei J., Leit S., Kuai J., Therrien E., Rafi S., Harwood H.J., Jr., DeLaBarre B., Tong L. An allosteric mechanism for potent inhibition of human ATP-citrate lyase. Nature. 2019;568:566–570. doi: 10.1038/s41586-019-1094-6. [DOI] [PubMed] [Google Scholar]
- Wideman R.F., Jr. Bacterial chondronecrosis with osteomyelitis and lameness in broilers: a review. Poult. Sci. 2016;95:325–344. doi: 10.3382/ps/pev320. [DOI] [PubMed] [Google Scholar]
- Witters L.A., Watts T.D., Daniels D.L., Evans J.L. Insulin stimulates the dephosphorylation and activation of acetyl-CoA carboxylase. PNAS. 1988;85:5473. doi: 10.1073/pnas.85.15.5473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H., Price F., Rudnicki M.A. Satellite cells and the muscle stem cell niche. Physiol. Rev. 2013;93:23–67. doi: 10.1152/physrev.00043.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]