Highlights
-
•
The optimal sequence of HDR-BT boost and EBRT for prostate cancer is unclear.
-
•
We compared early toxicity based on the timing of HDR-BT boost.
-
•
The timing of HDR-BT was not based on any specific patient or clinical factors.
-
•
We found no difference in early GI/GU toxicity between the two groups.
-
•
Longer follow-up is needed to evaluate late toxicity and long-term disease control.
Keywords: High-dose rate, Brachytherapy, Intensity modulated radiation therapy, Prostate cancer, Androgen deprivation therapy, Toxicity
Abstract
Background
We present the first report comparing early toxicity outcomes with high-dose rate brachytherapy (HDR-BT) boost upfront versus intensity modulated RT (IMRT) upfront combined with androgen deprivation therapy (ADT) as definitive management for intermediate risk or higher prostate cancer.
Methods and Materials
We reviewed all non-metastatic prostate cancer patients who received HDR-BT boost from 2014 to 2019. HDR-BT boost was offered to patients with intermediate-risk disease or higher. ADT use and IMRT target volume was based on NCCN risk group. IMRT dose was typically 45 Gy in 25 fractions to the prostate and seminal vesicles ± pelvic lymph nodes. HDR-BT dose was 15 Gy in 1 fraction, delivered approximately 3 weeks before or after IMRT. The sequence was based on physician preference. Biochemical recurrence was defined per ASTRO definition. Gastrointestinal (GI) and Genitourinary (GU) toxicity was graded per CTCAE v5.0. Pearson Chi-squared test and Wilcoxon tests were used to compare toxicity rates. P-value < 0.05 was significant.
Results
Fifty-eight received HDR-BT upfront (majority 2014–2016) and 57 IMRT upfront (majority 2017–2018). Median follow-up was 26.0 months. The two cohorts were well-balanced for baseline patient/disease characteristics and treatment factors. There were differences in treatment sequence based on the year in which patients received treatment. Overall, rates of grade 3 or higher GI or GU toxicity were <1%. There was no significant difference in acute or late GI or GU toxicity between the two groups.
Conclusion
We found no significant difference in GI/GU toxicity in intermediate-risk or higher prostate cancer patients receiving HDR-BT boost upfront versus IMRT upfront combined with ADT. These findings suggest that either approach may be reasonable. Longer follow-up is needed to evaluate late toxicity and long-term disease control.
1. Introduction
Brachytherapy (BT) is an established method to deliver high-dose, conformal radiotherapy (RT) for prostate cancer. BT results in excellent disease outcomes and acceptable toxicity as monotherapy for low and favorable intermediate-risk disease [1], [2] or as boost therapy in conjunction with external beam radiotherapy (EBRT) with or without androgen-deprivation therapy (ADT) for unfavorable intermediate and high-risk disease [3], [4], [5]. Retrospective series suggest improved biochemical and distant disease control in high-risk patients receiving BT boost + EBRT + ADT compared to those who receive EBRT + ADT or surgery alone [4], [6], [7], [8]. In addition, BT can offer improved patient convenience and cost-effectiveness relative to conventionally fractionated dose-escalated EBRT [9], [10].
Low-dose rate (LDR) and high-dose rate (HDR) BT have both been utilized in prostate cancer [11]. HDR-BT affords several practical, physical, and potential radiobiological advantages over LDR-BT [11], [12], [13]. While prospective trials have helped establish the benefit of HDR-BT boost and optimal dose/fractionation regimens [4], [14], [15], [16], there is no data to rationally inform optimal sequencing of the HDR-BT boost with regards to EBRT. The NCCN, GEC-ESTRO, and ABS guidelines provides no recommendation and sequencing is institution and/or trial-dependent [17], [18], [19]. The Timing of HDR brachytherapy with EBRT in Prostate Cancer (THEPCA) study (NCT02618161) is an ongoing prospective trial designed to compare toxicity, quality of life, (QoL) and disease outcomes in 50 patients with intermediate or high-risk prostate cancer treated with single fraction HDR-BT boost delivered pre-EBRT versus post-EBRT in combination with ADT [20].
We implemented an 192Ir-based HDR-BT program at our institution in 2014 and have treated a similar number of patients with either approach. Here, we present the first report comparing early toxicity outcomes for patients treated with HDR-BT boost upfront versus IMRT upfront combined with ADT.
2. Methods
2.1. Patient selection and treatment information
All patients with non-metastatic prostate cancer who received HDR-BT boost as part of definitive therapy at our institution from 2014 to 2019 were reviewed after IRB approval. HDR-BT boost was offered to patients with histologically confirmed prostate cancer, NCCN intermediate risk or higher, no evidence of distant metastatic disease, no prior pelvic RT, and pre-operative clearance for general anesthesia. Exclusion criteria, considered on a case-by-case basis, included significant seminal vesicle (SV) involvement (extension beyond proximal 0.5 cm) and extraprostatic extension, large transurethral resection defects, and/or conditions where the patient could not follow instructions or control movement including bowel movements. During the initial implementation of the HDR program, we excluded patients with prostate gland volumes >60 cc, American Urologic Association (AUA) scores >20, and/or presence of calcifications obstructing visualization on ultrasound. However, with increased experience, prostate gland volume was no longer a constraint and calcifications rarely excluded a patient except in extreme cases where optimal manipulation of the ultrasound image settings was insufficient to safely guide the needle. Subsequently, strict total AUA symptom score cutoffs were not employed, however particular attention was placed on the presence of weak urinary stream or difficulty initiating urinary stream. If patients were struggling with these symptoms, HDR-BT was not offered unless ADT was clinically indicated and there was subsequent improvement with symptoms at least 2 months later after ADT-mediated prostate cytoreduction.
All patients underwent evaluation with digital rectal exam, transrectal ultrasound guided (TRUS) biopsy for pathologic diagnosis, and serum PSA. Based on NCCN risk category, patients underwent additional staging studies including bone scan, CT abdomen/pelvis with contrast, and/or multiparametric MRI. All patients underwent TRUS-guided volume study prior to the HDR-BT procedure to ensure adequate visualization of prostate. Patients receiving HDR-BT upfront underwent fiducial marker placement at time of HDR procedure. Patients receiving IMRT upfront underwent fiducial marker placement at time of volume study. The interval between HDR-BT and IMRT was typically 2–4 weeks (median 20 days).
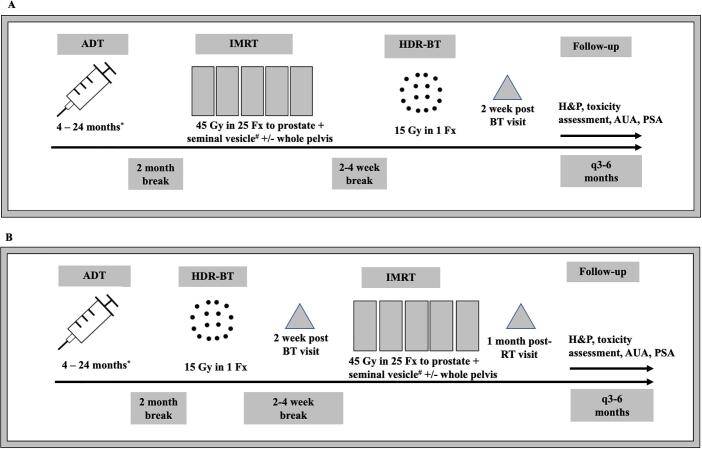
An outline of our treatment schema is shown in Fig. 1 and further details regarding IMRT and ADT are provided in Appendix A1. Ideally, our preference was HDR-BT upfront because the fiducial markers and HDR needle insertion could be done in the same procedure under general anesthesia (2014–2016). As the HDR-BT program grew and with limitations in patient scheduling to one patient per week, we started with IMRT upfront followed by HDR-BT boost (2017–2018). With increased ability to do two cases per week we then were able to choose either sequence based on scheduling availability (2019 onwards). Otherwise, there were no clinical or patient-specific factors that informed the sequence.
Fig. 1.
Treatment Schema for HDR-BT upfront (A) and IMRT upfront (B). * 4–6 months intermediate risk, 24 months high-risk or node positive. ADT initiated neoadjuvantly, given as 6 month leuprolide acetate injections except when delivering a single 4 month injection combined with 1 month of bicalutamide. # proximal seminal vesicle for intermediate risk and entire seminal vesicle for high risk or node positive.
2.2. HDR-BT procedure
All HDR-BT was performed by a single radiation oncologist (H.A.G.). The technique is based on the freehand HDR technique developed by Dr. I-Chow Hsu from UCSF with the following changes: general anesthesia instead of spinal anesthesia due to institutional preference, approximately 10 cc of 0.25% of bupivacaine injected only subcutaneously (never deep into the urogentital diaphragm) on the perineum, and more recently discontinuing the cystoscopy at the end of the procedure [21]. Our HDR-BT technique has been described previously [22] and further details are provided in the Appendix A2. Following HDR-BT procedure, patients underwent a CT simulation scan to verify needle placement, and adjustment of the needles (pushing in for better coverage of the base and/or proximal seminal vesicles) was made as clinically indicated.
The intended HDR boost dose was 15 Gy in one fraction delivered using a Varian VariSource 200 HDR remote afterloading unit (Varian Medical Systems, Inc., Palo Alto, CA) with 192Ir source. HDR treatment was delivered in the afternoon between one and five PM. Institutional dose constraints for CT-based planning used were based on RTOG 0321 [23] and as follows: planning target volume (PTV) receiving prescription dose greater than or equal to 90% (V100% ≥ 90%), urethra V125% < 1 cc, urethra V150% = 0 cc, bladder and rectum V75% < 1 cc, and bladder and rectum V150% = 0 cc. Clinical target volume (CTV) was the prostate. The planning target volume (PTV) was the CTV without additional expansion except in rare cases where there was suspicion or minimal extraprostatic extension on MRI, in which case a 3 mm expansion was added.
The implant was removed after the delivery of RT and the patients were discharged home the same day. The Foley was removed and only re-inserted if clinically significant hematuria occurred or if they failed a voiding trial, at which point re-evaluation for foley removal would occur 1 week from the date of the implant.
2.3. Follow-up
During IMRT, patients were seen once weekly for a standard on-treatment visit. For patients receiving IMRT upfront, there was no planned interval visit between completion of IMRT and HDR-BT and patients were seen ~2 weeks after HDR-BT. For patients receiving HDR-BT upfront, patients were seen ~2 weeks after HDR-BT (at which time CT simulation for IMRT planning was often performed) and ~1 month after completing IMRT. For select patients who developed symptoms or complications from treatment, short interval visits (~1–2 weeks) were planned as needed. After completion of all RT (IMRT and HDR-BT), patients were seen at 3–6 month interval. During each follow-up visit, patients were evaluated for the presence of symptoms or signs of toxicity or recurrence. PSA values and IPSS scores were also obtained.
2.4. Toxicity and recurrence assessment
Biochemical failure was defined per ASTRO criteria [24]. Gastrointestinal (GI) and genitourinary (GU) toxicity evaluation was performed by comprehensive review of the electronic medical record (EMR) for each patient, including follow-up visits with radiation oncology, other relevant physician encounters (such as primary care, urology, urgent care, and emergency room), and any hospitalizations within our large hospital network. We reviewed all available patient records from outside our hospital system as well. Patient-reported symptoms and/or toxicity events were then scored using the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 grading criteria [25]. Any potential event representing GI or GU toxicity was recorded. The relevant CTCAE subcategories for GI toxicity were diarrhea, rectal pain, proctitis. The relevant CTCAE subcategories for GU toxicity were frequency, urgency, dysuria, obstruction, incontinence, hematuria. The timing of any toxicity event was recorded using standard RTOG criteria [26] where acute toxicity was defined as any event occurring during RT or <90 days after completion of RT and late toxicity was defined as any event occurring ≥90 days after completion of RT.
2.5. Statistical analysis
Patient and treatment characteristics were compared between the two cohorts using the Pearson Chi-squared test for categorical variables and Wilcoxon statistical tests for continuous variables. The Kaplan-Meier method was utilized to visualize actuarial biochemical recurrence-free survival (bRFS) probability for patients. Log-rank test was utilized to compare bRFS in the HDR-BT upfront and the IMRT upfront groups. Acute and late toxicities were compared between the two groups using the Pearson chi-squared statistical test. The AUA scores obtained during the first two years of follow-up were compared to baseline for each patient. The mean percentage differences at each time point was compared to baseline for all patients, HDR-BT upfront group, and IMRT upfront groups using the Wilcoxon statistical test. Statistical analysis was performed using SPSS Statistics (Version 26; IBM; Armonk, NY) and R Studio (Version: 1.3.1056). A P value < 0.05 was considered statistically significant.
3. Results
3.1. Patient and treatment characteristics
Patient characteristics are detailed in Table 1 and treatment characteristics are detailed in Table 2. The two cohorts were well balanced. While the median follow-up was significantly longer for the HDR-BT upfront group (37.5 months versus 25.0 months, P = 0.004), there was no other significant difference between the two cohorts with respect to performance status, baseline urinary or sexual dysfunction, initial PSA, clinical T stage, Gleason grade group, or NCCN risk group. There was a significant difference in HDR-BT/IMRT sequence utilization by treatment year. In 2014–2016, most patients (80.9%) were treated with HDR-BT upfront, while in 2017–2018 most patients (80.9%) were treated with IMRT upfront. In 2019, the number treated with either approach was comparable. The time interval between HDR-BT and IMRT was not significantly different (21 days in the HDR-BT upfront group versus 19 days in the IMRT upfront group, P = NS). Additionally, there was no other significant difference between the two cohorts with regards to ADT use, duration of ADT, IMRT target, prostate volume at the time of the volume study or HDR-BT procedure, number of needles implanted, or HDR-BT dosimetry.
Table 1.
Patient Characteristics.
| All Patients | HDR-BT Upfront | IMRT Upfront | p-value* | |
|---|---|---|---|---|
| (n = 115) | (n = 58) | (n = 57) | ||
| Follow-up (months) | ||||
| Median (range) | 26.0 (0.1–71.9) | 37.5 (3.1 – 71.9) | 25.0 (0.1–50.8) | 0.004 |
| Age | ||||
| Median (range) | 67 (47–84) | 65 (47–83) | 69 (55–84) | 0.031 |
| Race | ||||
| Caucasian | 80 (69.6%) | 37 (63.8%) | 43 (75.4%) | NS |
| African American | 35 (30.4%) | 21 (36.2%) | 14 (24.6%) | |
| Body Mass Index | ||||
| Median (range) | 30.14 (19–45.9) | 30.4 (19–45.6) | 30.1 (20.7–45.9) | NS |
| ECOG Performance Status | ||||
| 0 | 86 (74.8%) | 46 (79.3%) | 40 (70.2%) | NS |
| 1 | 28 (24.3%) | 11 (18.0%) | 17 (29.8%) | |
| 2 | 1 (0.9%) | 1 (1.7%) | 0 (0%) | |
| Baseline Erectile Dysfunction | 55 (47.8%) | 28 (48.3%) | 27 (47.4%) | NS |
| Baseline Genitourinary Medication Use | 30 (26.1%) | 13 (22.4%) | 17 (29.8%) | NS |
| AUA Score | ||||
| Median Value (range) | 7 (0–31) | 7.5 (0–25) | 6 (0–31) | NS |
| >20 | 10 (16.5%) | 7 (12.1%) | 3 (5.3%) | NS |
| Initial PSA | ||||
| Median Value (range) | 7.8 (2.3–100.2) | 8.4 (2.3–100.2) | 7.5 (3.1–98.2) | NS |
| <10 | 73 (63.5%) | 34 (58.6%) | 39 (68.4%) | |
| ≥10-<20 | 25 (21.7%) | 10 (17.2%) | 15 (26.3%) | NS |
| ≥ 20 | 17 (14.8%) | 14 (24.1%) | 3 (5.3%) | |
| Clinical T stage | ||||
| T1c | 66 (57.4%) | 33 (56.9%) | 33 (57.9%) | |
| T2a | 17 (14.8%) | 9 (15.5%) | 8 (14.0%) | |
| T2b | 5 (4.3%) | 0 (0%) | 5 (8.8%) | NS |
| T3a | 10 (8.7%) | 5 (8.6%) | 5 (8.8%) | |
| T3b | 15 (13.0%) | 11 (19.0%) | 4 (7.0%) | |
| T4 | 2 (1.7%) | 0 (0%) | 2 (3.5%) | |
| Gleason Grade Group | ||||
| 1 | 3 (2.6%) | 0 (0%) | 3 (5.3%) | |
| 2 | 24 (20.9%) | 16 (27.6%) | 8 (14.0%) | |
| 3 | 33 (28.7%) | 15 (25.9%) | 18 (31.6%) | NS |
| 4 | 46 (18.3%) | 9 (15.5%) | 12 (21.1%) | |
| 5 | 34 (29.6%) | 18 (31.0%) | 16 (28.1%) | |
| NCCN risk group | ||||
| Favorable Intermediate | 13 (11.3%) | 7 (12.1%) | 6 (10.5%) | |
| Unfavorable Intermediate | 35 (30.4%) | 14 (24.1%) | 21 (36.8%) | NS |
| High | 12 (10.4%) | 6 (10.3%) | 6 (10.5%) | |
| Very High | 46 (40.0%) | 28 (48.3%) | 18 (31.6%) | |
| Node Positive | 9 (7.8%) | 3 (5.2%) | 6 (10.5%) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; AUA, American Urologic Association; PSA, prostate specific antigen, NCCN, National Comprehensive Cancer Network; HDR, high dose-rate; IMRT, intensity modulated radiation therapy NS = Not significant.
*Pearson and Wilcoxon statistical tests employed to compare cohorts.
Table 2.
Treatment Characteristics.
| All Patients | HDR-BT Upfront | IMRT Upfront | p-value* | |
|---|---|---|---|---|
| (n = 115) | (n = 58) | (n = 57) | ||
| Treatment Year | ||||
| 2014 | 6 (5.2%) | 6 (10.3%) | 0 (0%) | |
| 2015 | 22 (19.1%) | 20 (34.5%) | 2 (3.5%) | |
| 2016 | 19 (16.5%) | 12 (20.7%) | 7 (12.3%) | <0.001 |
| 2017 | 22 (19.1%) | 4 (6.9%) | 18 (31.6%) | |
| 2018 | 20 (17.4%) | 4 (6.9%) | 16 (28.1%) | |
| 2019 | 26 (22.1%) | 12 (20.7%) | 14 (24.6%) | |
| Interval between HDR-BT and IMRT (days) | ||||
| Median (range) | 20 (3–108) | 21 (10–38) | 19 (3–108) | NS |
| Androgen-deprivation therapy (ADT) | 110 (95.6%) | 53 (91.4%) | 56 (98.2%) | NS |
| Duration of ADT (months) | ||||
| Median (range) | 12 (0–32) | 18 (0–30) | 7 (0–32) | NS |
| IMRT | ||||
| Prostate/Seminal Vesicle | 26 (22.6%) | 15 (25.9%) | 11 (19.3%) | NS |
| Whole Pelvis | 89 (77.4%) | 43 (74.1%) | 46 (80.7%) | NS |
| Prostate volume at volume study | ||||
| Median (range) | 26.5 (9.4 – 84.9) | 26.2 (9.4–58.8) | 27.5 (12.3 – 84.9) | NS |
| 2 (1.7%) | 0 (0%) | 2 (3.5%) | NS | |
| > 60 cc | ||||
| Prostate volume at brachytherapy | ||||
| Median (range) | 28.1 (12.6–82.8) | 28.0 (12.6 – 67.2) | 29.3 (13.8 – 82.8) | NS |
| >60 cc | 6 (5.2%) | 2 (3.4%) | 4 (7.0%) | NS |
| Bladder Perforated | 24 (20.9%) | 12 (20.7%) | 12 (21.1%) | NS |
| Urethra Perforated | 3 (2.6%) | 1 (1.7%) | 2 (3.5%) | NS |
| Number of needles implanted | ||||
| Median (range) | 16 (13–20) | 16 (13–19) | 16 (14–20) | NS |
| Prostate PTV V100 (%) | ||||
| Mean (SD) | 95.93 ± 2.28 | 95.4 ± 2.7 | 96.4 ± 1.6 | NS |
| Bladder V75 (%) | ||||
| Mean (SD) | 0.2 ± 0.3 | 0.2 ± 0.3 | 0.2 ± 0.2 | NS |
| Rectum V75 (%) | ||||
| Mean (SD) | 0.4 ± 0.3 | 0.4 ± 0.3 | 0.3 ± 0.3 | NS |
| Urethra V125 (%) | ||||
| Mean (SD) | 0.1 ± 0.2 | 0.1 ± 0.2 | 0.1 ± 0.2 | NS |
Abbreviations: HDR, high dose rate; BT, brachytherapy; IMRT, intensity modulated radiation therapy; ADT, androgen deprivation therapy; PTV, planning target volume; V100, % volume of organ receiving ≥100% prescription dose; V75, % volume of organ receiving ≥75% prescription dose; V125, % volume of organ receiving 125% prescription dose; SD, standard deviation; NS = not significant.
*Pearson and Wilcoxon statistical tests employed to compare cohorts.
3.2. Disease outcomes
A total of 6 (5.2%) patients developed biochemical recurrence: 3 (5.3%) patients in the HDR-BT upfront cohort and 3 (5.2%) patients in the IMRT upfront cohort. The median time to biochemical failure was 22.8 months (range 3.8 – 52.5). The 2-year bRFS for all patients with favorable intermediate-risk, unfavorable intermediate-risk, high-risk, very high-risk, and node positive disease was 100%, 100%, 96.6%, 86.6%, and 85.7%, respectively (Figure D1). There was no significant difference in 2-year bRFS in the HDR-BT upfront cohort versus IMRT upfront cohort for NCCN high risk (100% versus 80%, P = NS), very high risk (96.3% versus 100%, P = NS), and node positive disease (66.7% versus 100%, P = NS). The 2-year bRFS for all patients in the HDR-BT upfront group compared to IMRT upfront group was similar (95.9% versus 97.9%, P = NS) (Figure E1). Patterns of failure are listed in Appendix Table B1. At last follow-up, 6 (4.3%) patients had died: 5 (6.9%) in the HDR-BT upfront cohort and 1 (1.8%) in the IMRT upfront cohort. No patients died from disease recurrence or treatment-related toxicity.
3.3. Acute toxicity
The acute GU/GI toxicities are detailed in Table 3, Table 4. Rates of Grade 3 or higher toxicity were extremely low in both groups. There was no significant difference in rates of acute toxicity by grade or type between the HDR-BT upfront and IMRT upfront groups. There was 1 (1.7%) acute Grade 3 GU toxicity and 1 (1.7%) acute Grade 3 GI toxicity in the HDR-BT upfront group. There was no Grade 3 or higher acute toxicities in the IMRT upfront cohort. In the HDR-BT upfront cohort, 3 (5.2%) patients required temporary (removed within 1 week of HDR-BT procedure) Foley catheter placement versus 5 (8.8%) patients in the IMRT upfront cohort. In the HDR-BT upfront group, the acute Grade 3 GU toxicity was a patient who required brief hospitalization for hematuria and obstructive symptoms requiring temporary catheterization and continuous bladder irrigation that resolved without need for blood transfusion. The acute Grade 3 GI toxicity occurred in a different patient who experienced severe diarrhea initially refractory to oral anti-diarrheal medication that required outpatient work-up and ultimately resolved without any invasive intervention.
Table 3.
Acute & Late GU/GI Toxicities.
| HDR-BT Upfront (n = 58) |
IMRT Upfront (n = 57) |
|||||||
|---|---|---|---|---|---|---|---|---|
| G1 | G2 | G3 | G4/5 | G1 | G2 | G3 | G4/5 | |
| Acute | ||||||||
| GU | 29 (50%) | 25 (43.1%) | 1 (1.7%) | 0 | 26 (45.6%) | 27 (47.3%) | 0 | 0 |
| GI | 32 (55.2%) | 5 (8.6%) | 1 (1.7%) | 0 | 33 (57.9%) | 2 (3.5%) | 0 | 0 |
| Late | ||||||||
| GU | 34 (58.6%) | 12 (20.7%) | 0 | 0 | 43 (75.4%) | 7 (12.3%) | 0 | 0 |
| GI | 6 (10.3%) | 1 (1.7%) | 0 | 0 | 7 (12.3%) | 1 (1.8%) | 0 | 0 |
Abbreviations: HDR, high dose rate; IMRT, intensity modulated radiation therapy; GU, genitourinary; GI, gastrointestinal.
Toxicity rates by grade for each cohort were compared using Pearson test. No statistically significant differences were found.
Table 4.
Acute Toxicity Details.
| HDR-BT Upfront (n = 58) |
IMRT Upfront (n = 57) |
|||||||
|---|---|---|---|---|---|---|---|---|
| G1 | G2 | G3 | G4/5 | G1 | G2 | G3 | G4/5 | |
| GU | ||||||||
| Frequency | 32 (65.5%) | 9 (10.3%) | 0 | 0 | 32 (56.1%) | 12 (21.1%) | 0 | 0 |
| Urgency | 27 (46.5%) | 5 (8.6%) | 0 | 0 | 36 (63.2%) | 3 (5.3%) | 0 | 0 |
| Dysuria | 26 (44.8%) | 0 | 0 | 0 | 28 (49.1%) | 0 | 0 | 0 |
| Obstruction | 22 (37.9%) | 18 (31.0%) | 1 (1.7%) | 0 | 24 (42.1%) | 19 (33.3%) | 0 | 0 |
| Incontinence | 3 (5.2%) | 1 (1.7%) | 0 | 0 | 10 (17.5%) | 2 (3.5%) | 0 | 0 |
| Hematuria | 24 (41.4%) | 1 (1.7%) | 1 (1.7%) | 0 | 31 (54.4%) | 1 (1.8%) | 0 | 0 |
| Temporary Catheter Placement Required |
0 | 4 (6.9%) | 1 (1.7%) | 0 | 0 | 3 (5.3%) | 0 | 0 |
| GI | ||||||||
| Diarrhea | 28 (50.9%) | 0 | 1 (1.7%) | 0 | 17 (29.8%) | 2 (3.5%) | 0 | 0 |
| Rectal Pain | 8 (13.8%) | 0 | 0 | 0 | 25 (43.9%) | 0 | 0 | 0 |
| Proctitis | 7 (12.1%) | 5 (8.6%) | 0 | 0 | 14 (24.6%) | 4 (7.0%) | 0 | 0 |
3.4. Late toxicity
The late GU/GI toxicities are detailed in Table 3, Table 5. There was no Grade 3 or higher late toxicities in either group. There was no significant difference in rates of GU or GI toxicity between the two groups. In the HDR-BT upfront cohort, 1 (1.7%) patient developed urethral stricture requiring non-urgent, outpatient dilation versus 3 (5.3%) patients in the IMRT upfront cohort.
Table 5.
Late Toxicity Details.
| HDR-BT Upfront (n = 58) |
IMRT Upfront(n = 57) |
|||||||
|---|---|---|---|---|---|---|---|---|
| G1 | G2 | G3 | G4/5 | G1 | G2 | G3 | G4/5 | |
| GU | ||||||||
| Frequency | 38 (65.5%) | 6 (10.3%) | 0 | 0 | 44 (77.2%) | 3 (5.3%) | 0 | 0 |
| Urgency | 31 (53.4%) | 7 (12.1%) | 0 | 0 | 37 (64.9%) | 2 (3.5%) | 0 | 0 |
| Dysuria | 10 (17.2%) | 1 (1.7%) | 0 | 0 | 9 (15.8%) | 1 (1.8%) | 0 | 0 |
| Obstruction | 36 (62.1%) | 3 (5.2%) | 0 | 0 | 35 (61.4%) | 4 (7.0%) | 0 | 0 |
| Incontinence | 12 (20.7%) | 2 (3.5%) | 0 | 0 | 15 (26.3%) | 1 (1.8%) | 0 | 0 |
| Hematuria | 1 (1.7%) | 1 (1.7%) | 0 | 0 | 2 (3.5%) | 1 (1.8%) | 0 | 0 |
| Urethral stricture requiring dilation | 0 | 1 (1.7%) | 0 | 0 | 0 | 3 (5.3%) | 0 | 0 |
| GI | ||||||||
| Diarrhea | 1 (1.7%) | 0 | 0 | 0 | 2 (3.5%) | 1 (1.8%) | 0 | 0 |
| Rectal Pain | 0 | 0 | 0 | 0 | 3 (5.3%) | 0 | 0 | 0 |
| Proctitis | 5 (8.6%) | 1 (1.7%) | 0 | 0 | 2 (3.5%) | 0 | 0 | 0 |
3.5. AUA score trend
The mean percent change in AUA score at each timepoint for the first 24 months compared to baseline is presented in Appendix E1. For all patients, the mean percent change from baseline to 2 weeks, 1 month, 3 months, and 6 months was statistically significant (P < 0.05). For all patients, the mean percent change from baseline to 9 months – 24 months did not reach statistical significance. For the HDR upfront patients, the mean percent change from baseline to 2 weeks, 3 month, 6 months, 9 months, and 12 months was statistically significant (P < 0.05). For the IMRT upfront patients, the mean percent change from baseline to 2 weeks, 1 month, and 3 months was statistically significant (P < 0.05)
4. Discussion
Our study is the first report comparing early toxicity outcomes for prostate cancer patients with intermediate risk disease or higher treated with HDR-BT boost upfront versus IMRT upfront in conjunction with ADT. Our analysis found no significant difference in acute GI/GU toxicity or late GI/GU toxicity two groups. These findings suggest that the sequence of HDR-BT boost and IMRT may not impact early toxicity outcomes in this patient population and either approach may be reasonable. While our results are encouraging, greater follow-up is necessary to better evaluate long-term disease control and late toxicity.
We found a statistically significant change in mean percent change in AUA score from baseline through 6 months for all patients. However, mean percent change in AUA score was not different from baseline to 9–24 months. Overall, our findings suggest no difference in GU patient-reported QoL through 2 years with either approach. Our findings are consistent with a prior report that observed acute worsening of GU symptoms at 1 month post-BT, with gradual improvement over time [27]. Interestingly, on subgroup analysis, there were differences between the HDR-BT upfront and IMRT upfront groups. The HDR-BT had significantly higher mean percent change in AUA scores through 12 months, while the IMRT upfront group had significantly higher mean percent change in AUA scores through 3 months. There was no difference in physician-assessed GU toxicity by CTCAE. This suggests that short-term GU toxicity may have persisted for a longer period in the HDR-BT upfront group. This may have been related to differences in treatment by year. Most patients treated between 2014 and 2016 received HDR-BT upfront. The brachytherapy program began at our institution in 2014 and brachytherapy procedure skill certainly improved with greater experience. Additionally, during this period, the workflow included cystoscopy after each HDR-BT procedure to evaluate implant quality, which was discontinued in more recent years in favor of ultrasound. It is important to note that the number of patients with AUA score data available was variable during the follow-up period and this may have also biased the results. Regardless, as noted above, the mean percent change in AUA scores from baseline was not significantly different with longer follow-up through 24 months in either group.
Currently there is no consensus on the optimal sequence of HDR-BT boost and EBRT in the management of prostate cancer [17], [18]. Sequencing of HDR-BT and EBRT is institution and physician dependent, primarily based on preference and logistical considerations such as scheduling, resource-allocation, and workflow. For example, one particular advantage of utilizing HDR-BT upfront is the ability to place fiducial markers and perform HDR-BT in one procedure, reducing patient burden to one preparation regimen. A similarly designed, prospective randomized trial titled THEPCA is currently underway in England with results pending [20].
Our study has numerous strengths. The comparison groups were well-balanced for nearly every baseline and disease factor. The IMRT and HDR implant were performed by a single radiation oncologist (H.A.G.) limiting treatment heterogeneity. We have maintained a strong follow-up clinic with the goal of scheduled follow up visits for every patient receiving HDR-BT boost at 3–6 month intervals. We performed an exhaustive review of the EMR, including any other visits (such as urgent care, emergency room, urology) within our large hospital system. We also reviewed all available patient records from outside our hospital system. This helps ensure that toxicity or biochemical recurrence events were captured. We evaluated patient-reported symptoms while grading toxicity and included data on patient-reported GU toxicity (AUA scores). We utilized the NCI sponsored CTCAE v5.0 to evaluate toxicity, which is a standardized scoring criteria that has been utilized in many prospective brachytherapy studies [28], [29], [30], [31], [32].
There are several limitations in our study. Most importantly, there were differences in terms of HDR-BT sequence utilized based on treatment year, with patients mostly receiving HDR-BT upfront in 2014–2016, while patients mostly received IMRT upfront in 2017–2018. While this was driven primarily by physician preference and not patient-specific factors, this may have introduced heterogeneity between the treatment delivered to the two groups. Examples of potential confounders include changes to the brachytherapy procedure and workflow over time, improvement in brachytherapy skill with greater experience, or differences in IMRT treatment. Other limitations are related to the retrospective nature of the study. Despite our thorough review of the EMR, there remains a possibility that toxicity or biochemical failure events were not coded for select patients who received follow-up care elsewhere and/or were lost to follow-up. Given that our HDR prostate program was established recently, the length of follow-up was relatively short which limits the ability to evaluate bRFS and late toxicity. The toxicity evaluation in our study was based primarily on physician assessment and grading. Additional patient-reported toxicity and quality of life measures would strengthen the analysis. Despite these limitations, we believe that our study fills a void in the literature and provides data on a practical question for the brachytherapy community.
5. Conclusion
In a single-institution retrospective analysis of patients with intermediate or high-risk prostate cancer treated with IMRT with HDR-BT boost in conjunction with ADT at our institution, we found no significant difference in acute GU/GI toxicity or late GU/GI toxicity at 2-year follow-up in patients treating with HDR-BT upfront versus IMRT upfront. These findings suggest that the sequence of HDT-BT boost and IMRT may not impact early GU/GI toxicity outcomes in this patient population and that either approach may be reasonable. Longer follow-up and prospective validation of our results is warranted.
Declaration of Competing Interest
Dr. Baumann reports other from Mevion Medical Systems, personal fees and other from Regeneron Pharmaceuticals Inc., personal fees and other from Sanofi S.A., outside the submitted work. The remaining authors have no conflicts to report.
Acknowledgments
Acknowledgements
Dr. Gay would like to thank Dr. Joe Hsu for introducing him to HDR and his unwavering HDR guidance and support for more than five years, to Dr. Jeff M. Michalski who gave him the foundation of needle placement through his mentorship with LDR brachytherapy and fiducial marker placement, and to Drs. Alana Desai and Henry Lai who trained him to perform cystoscopies. We would like to thank our physics, dosimetry, brachytherapy, simulation, nurse practitioner, nursing, medical assistant, and nurse coordinator teams especially nurses Melissa Hayden and Michele Lach for their commitment to quality and always rising to the occasion with challenging cases. We would also like to thank Colleen Becker, Sharon Endicott, Barnes Jewish Hospital leadership, our OR staff including nurse Joyce Pedrotti who has since retired, the urology residents for their assistance with cystoscopies, and the enthusiastic support from our urologists since the program’s inception. Most importantly, we would like to thank all our patients who inspire us every day.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data sharing statement
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ctro.2021.05.004.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Krauss D.J., Ye H., Martinez A.A., Mitchell B., Sebastian E., Limbacher A. Favorable preliminary outcomes for men with low- and intermediate-risk prostate cancer treated with 19-Gy single-fraction high-dose-rate brachytherapy. Int J Radiat Oncol Biol Phys. 2017;97(1):98–106. doi: 10.1016/j.ijrobp.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 2.Morton G., McGuffin M., Chung H.T., Tseng C.-L., Helou J., Ravi A. Prostate high dose-rate brachytherapy as monotherapy for low and intermediate risk prostate cancer: Efficacy results from a randomized phase II clinical trial of one fraction of 19 Gy or two fractions of 13.5 Gy. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2020;146:90–96. doi: 10.1016/j.radonc.2020.02.009. [DOI] [PubMed] [Google Scholar]
- 3.Wedde T.B., Småstuen M.C., Brabrand S., Fosså S.D., Kaasa S., Tafjord G. Ten-year survival after high-dose-rate brachytherapy combined with external beam radiation therapy in high-risk prostate cancer: a comparison with the Norwegian SPCG-7 cohort. Radiother Oncol. 2019;132:211–217. doi: 10.1016/j.radonc.2018.10.013. [DOI] [PubMed] [Google Scholar]
- 4.Morris W.J., Tyldesley S., Rodda S., Halperin R., Pai H., McKenzie M. Androgen suppression combined with elective nodal and dose escalated radiation therapy (the ASCENDE-RT Trial): an analysis of survival endpoints for a randomized trial comparing a low-dose-rate brachytherapy boost to a dose-escalated external beam boost for high- and intermediate-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2017;98(2):275–285. doi: 10.1016/j.ijrobp.2016.11.026. [DOI] [PubMed] [Google Scholar]
- 5.Vigneault E., Mbodji K., Magnan S., Després P., Lavallée M.-C., Aubin S. High-dose-rate brachytherapy boost for prostate cancer treatment: different combinations of hypofractionated regimens and clinical outcomes. Radiother Oncol. 2017;124(1):49–55. doi: 10.1016/j.radonc.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 6.Kishan A.U., Cook R.R., Ciezki J.P., Ross A.E., Pomerantz M.M., Nguyen P.L. Radical prostatectomy, external beam radiotherapy, or external beam radiotherapy with brachytherapy boost and disease progression and mortality in patients with gleason score 9–10 prostate cancer. JAMA. 2018;319(9):896. doi: 10.1001/jama.2018.0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson S.B., Lester-Coll N.H., Kelly J.R., Kann B.H., Yu J.B., Nath S.K. Brachytherapy boost utilization and survival in unfavorable-risk prostate cancer. Eur Urol. 2017;72(5):738–744. doi: 10.1016/j.eururo.2017.06.020. [DOI] [PubMed] [Google Scholar]
- 8.Joseph D., Denham J.W., Steigler A., Lamb D.S., Spry N.A., Stanley J. Radiation dose escalation or longer androgen suppression to prevent distant progression in men with locally advanced prostate cancer: 10-year data from the TROG 03.04 RADAR trial. Int J Radiat Oncol Biol Phys. 2020;106(4):693–702. doi: 10.1016/j.ijrobp.2019.11.415. [DOI] [PubMed] [Google Scholar]
- 9.Shah C., Lanni T.B., Ghilezan M.I., Gustafson G.S., Marvin K.S., Ye H. Brachytherapy provides comparable outcomes and improved cost-effectiveness in the treatment of low/intermediate prostate cancer. Brachytherapy. 2012;11(6):441–445. doi: 10.1016/j.brachy.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Vu C.C., Blas K.G., Lanni T.B., Gustafson G.S., Krauss D.J. Cost-effectiveness of prostate boost with high-dose-rate brachytherapy versus intensity-modulated radiation therapy in the treatment of intermediate-high risk prostate cancer. Brachytherapy. 2018;17(6):852–857. doi: 10.1016/j.brachy.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 11.Fischer-Valuck B.W., Gay H.A., Patel S., Baumann B.C., Michalski J.M. A brief review of low-dose rate (LDR) and high-dose rate (HDR) brachytherapy boost for high-risk prostate. Front Oncol. 2019;9 doi: 10.3389/fonc.2019.0137810.3389/fonc.2019.01378.s001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoskin P. High dose rate brachytherapy for prostate cancer. Cancer Radiother J Soc Francaise Radiother Oncol. 2008;12(6-7):512–514. doi: 10.1016/j.canrad.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 13.Zaorsky N.G., Davis B.J., Nguyen P.L., Showalter T.N., Hoskin P.J., Yoshioka Y. The evolution of brachytherapy for prostate cancer. Nat Rev Urol. 2017;14(7):415–439. doi: 10.1038/nrurol.2017.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martell K., Mendez L.C., Chung H.T., Tseng C.L., Alayed Y., Cheung P. Results of 15 Gy HDR-BT boost plus EBRT in intermediate-risk prostate cancer: analysis of over 500 patients. Radiother Oncol. 2019;141:149–155. doi: 10.1016/j.radonc.2019.08.017. [DOI] [PubMed] [Google Scholar]
- 15.Hoskin P.J., Rojas A.M., Bownes P.J., Lowe G.J., Ostler P.J., Bryant L. Randomised trial of external beam radiotherapy alone or combined with high-dose-rate brachytherapy boost for localised prostate cancer. Radiother Oncol. 2012;103(2):217–222. doi: 10.1016/j.radonc.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Sathya J.R., Davis I.R., Julian J.A., Guo Q., Daya D., Dayes I.S. Randomized trial comparing iridium implant plus external-beam radiation therapy with external-beam radiation therapy alone in node-negative locally advanced cancer of the prostate. J Clin Oncol Off J Am Soc Clin Oncol. 2005;23(6):1192–1199. doi: 10.1200/JCO.2005.06.154. [DOI] [PubMed] [Google Scholar]
- 17.Hoskin P.J., Colombo A., Henry A., Niehoff P., Paulsen Hellebust T., Siebert F.-A. GEC/ESTRO recommendations on high dose rate afterloading brachytherapy for localised prostate cancer: an update. Radiother Oncol. 2013;107(3):325–332. doi: 10.1016/j.radonc.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 18.National Comprehensive Cancer Network. Prostate Cancer (Version 1.2020) n.d. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf (accessed August 3, 2020).
- 19.Yamada Y., Rogers L., Demanes D.J., Morton G., Prestidge B.R., Pouliot J. American Brachytherapy Society consensus guidelines for high-dose-rate prostate brachytherapy. Brachytherapy. 2012;11(1):20–32. doi: 10.1016/j.brachy.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 20.Palvai S., Harrison M., Shibu Thomas S., Hayden K., Green J., Anderson O. Timing of high-dose rate brachytherapy with external beam radiotherapy in intermediate and high-risk localized prostate cancer (THEPCA) patients and its effects on toxicity and quality of life: protocol of a randomized feasibility trial. JMIR Res Protoc. 2015;4(2):e49. doi: 10.2196/resprot.4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holloway C, Hsu I-CJ, Albert M, Martin A-G, Suh WW. Prostate Brachytherapy. Brachytherapy Appl. Tech. 1st ed., Philadelphia, PA: Lippincott Williams & Wilkins; 2007, p. 181–222.
- 22.Barnes J., Gabani P., Sanders M., Chundury A., Altman M., Garcia-Ramirez J. Single fraction high-dose-rate brachytherapy as monotherapy for low and intermediate risk prostate cancer: toxicities and early outcomes from a single institutional experience. J Contemp Brachytherapy. 2019;11(5):399–408. doi: 10.5114/jcb.2019.89367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsu I.-C., Bae K., Shinohara K., Pouliot J., Purdy J., Ibbott G. Phase II trial of combined high dose rate brachytherapy and external beam radiotherapy for adenocarcinoma of the prostate: preliminary results of RTOG 0321. Int J Radiat Oncol Biol Phys. 2010;78(3):751–758. doi: 10.1016/j.ijrobp.2009.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roach M., Hanks G., Thames H., Schellhammer P., Shipley W.U., Sokol G.H. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65(4):965–974. doi: 10.1016/j.ijrobp.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 25.Common Terminology Criteria for Adverse Events v5.0 (CTCAE). National Cancer Institute CTEP; n.d.
- 26.Cox J.D., Stetz J., Pajak T.F. Toxicity criteria of the radiation therapy oncology group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) Int J Radiat Oncol Biol Phys. 1995;31(5):1341–1346. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 27.Morton G.C., Loblaw D.A., Sankreacha R., Deabreu A., Zhang L., Mamedov A. Single-fraction high-dose-rate brachytherapy and hypofractionated external beam radiotherapy for men with intermediate-risk prostate cancer: analysis of short- and medium-term toxicity and quality of life. Int J Radiat Oncol Biol Phys. 2010;77(3):811–817. doi: 10.1016/j.ijrobp.2009.05.054. [DOI] [PubMed] [Google Scholar]
- 28.Morton G, Chung HT, McGuffin M, Helou J, D’Alimonte L, Ravi A, et al. Prostate high dose-rate brachytherapy as monotherapy for low and intermediate risk prostate cancer: Early toxicity and quality-of life results from a randomized phase II clinical trial of one fraction of 19Gy or two fractions of 13.5Gy. Radiother Oncol J Eur Soc Ther Radiol Oncol 2017;122:87–92. https://doi.org/10.1016/j.radonc.2016.10.019. [DOI] [PubMed]
- 29.Tsang Y.M., Tharmalingam H., Belessiotis-Richards K., Armstrong S., Ostler P., Hughes R. Ultra-hypofractionated radiotherapy for low- and intermediate risk prostate cancer: high-dose-rate brachytherapy vs stereotactic ablative radiotherapy. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2021;158:184–190. doi: 10.1016/j.radonc.2021.02.028. [DOI] [PubMed] [Google Scholar]
- 30.Corkum M., Loblaw A., Hasan Y., Chung H.T., Tseng C.-L., McGuffin M. Prostate high dose-rate brachytherapy as monotherapy for prostate cancer: late toxicity and patient reported outcomes from a randomized phase II clinical trial. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2021;156:160–165. doi: 10.1016/j.radonc.2020.12.021. [DOI] [PubMed] [Google Scholar]
- 31.Martz N., Bodokh Y., Gautier M., Thamphya B., Schiappa R., Lam Cham Kee D. High-dose rate brachytherapy in localized penile cancer: 5-Year clinical outcome analysis. Clin Transl Radiat Oncol. 2021;27:89–95. doi: 10.1016/j.ctro.2020.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petric P., Lindegaard J.C., Sturdza A., Fokdal L., Kirchheiner K., Tan L.T. Results of image guided brachytherapy for stage IB cervical cancer in the RetroEMBRACE study. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2021;157:24–31. doi: 10.1016/j.radonc.2021.01.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.