Abstract
The aim of this study was to determine the prevalence, serovar distribution, antimicrobial resistance, and genotypic analyses of the dominating serovars of Salmonella in chickens from a national study in Korea. Between 2017 and 2018, a total of 550 chicken samples were collected from the top 12 integrated broiler chicken operations in Korea. Salmonella was isolated from 117 (32.5%) chicken feces and 19 (10.0%) retail chicken meat sources. Ten serovars were identified, and the most common Salmonella serovar was Salmonella ser. Albany (50 isolates, 36.8%), followed by S. Enteritidis (38 isolates, 27.9%), and S. Montevideo (23 isolates, 16.9%) isolated from 6, 10, and 6 operations, respectively. A total of 35 (25.7%) isolates were with the ACSSuTN (ampicillin, chloramphenicol, streptomycin, sulfisoxazole, tetracycline, and nalidixic acid) resistance pattern, with high prevalence of this resistance pattern in S. Albany (29 isolates, 58.0%). A total of 35 PFGE types were identified among Salmonella isolates of the serovars Albany, Enteritidis, Virchow, Montevideo, and Senftenberg, while 11 distinct types of PFGE patterns were found among S. Albany isolates, which showed an overall homology similarity of higher than 85%. Among these 35 PFGE types, 22 PFGE types corresponded to 32 isolates from samples limited to one operation, and the other 13 PFGE types corresponded to 72 isolates from samples widely distributed among different operations. These results highlighted rapid colony dissemination of multidrug-resistant S. Albany in chicken all over Korea after it first appeared in 2016; furthermore, the spread of Salmonella colonies between various integrated operations was common, and several operations played an important role in Salmonella carriage and transmission in Korea.
Key words: salmonella, antimicrobial resistance, colony dissemination, S. Albany, Integrated chicken operations
INTRODUCTION
Salmonella enterica is distributed worldwide and is one of the most common pathogens causing bacterial foodborne diseases in human. Salmonella infection is a significant public health problem, causing an estimated 93.8 million illnesses and 155,000 deaths each year worldwide (Majowicz et al., 2010). Salmonella gastroenteritis is usually a self-limiting disease, and antibiotics like fluoroqoinolones and third-generation cephalosporins are reserved for patients with severe disease. As antibiotics have been extensively used, the increasing prevalence of antibiotic-resistant and multidrug-resistant Salmonella adds to the public health burden and is associated with high medical costs, prolonged hospital stays, and increased mortality (Broughton et al., 2010).
Salmonella is frequently found in poultry; contaminated broiler chicken and chicken products have been identified as an important source of Salmonella infection in humans (EFSA, 2020). Over 2,600 known serovars are found from a variety of hosts. Predominant serovars of S. Enteritidis, S. Typhimurium, S. Infantis, S. Newport, and S. Derby have repeatedly been recovered from chickens and associated with poultry-related infections or outbreaks in humans in the world (EFSA, 2018; CDC, 2018). On comparing serovar distribution of Salmonella between human and chicken sources, the specific serovar distribution in humans could be predicted on the basis of data of chicken sources (Kang et al., 2009). Salmonella serovars vary in geographic regions and are always limited to specific geographic areas, except those of S. Enteritidis and S. Typhimurium, which are distributed worldwide (Lu et al., 2017). In addition, shifts in predominant serovars in certain hosts occur over time (Cardinale et al., 2005; Davis et al., 1999). Recently, we noticed that S. Albany prevalence dramatically increased in chickens in Korea. Shang et al., (2019) found that S. Albany was the most common serovar in one integrated broiler chicken operation in 2016, surpassing S. Montevideo, S. Enteritidis, and S. Typhimurium. Jeon et al., (2019) reported that S. Albany was isolated from 4 out of 6 sampled chicken operations, with it being the most predominant type in three operations. Due to the high public health threat, a better understanding the epidemiology of Salmonella particularly in chicken-is required. Therefore, the aim of this study was to determine the prevalence, serovar distribution, antimicrobial resistance, and genotypic analyses of the dominating serovars of Salmonella in chicken from a national study in Korea.
MATERIALS AND METHODS
Statement of Ethics
This study was carried out in accordance with the ethical guidelines of Jeonbuk National University (CBNU). Moreover, before the initiation of this study, a formal approval was obtained by the Ethics Committee for Animal Experiments of the Jeonbuk National University. There were no vulnerable populations involved; sampling was performed according to standard protocols, and prior consent of the farmer/manager of the facilities was taken. Individual written informed consent for the use of data was obtained from the companies and animal owners.
Sample Collection
Between 2017 and 2018, a total of 550 chicken samples, which included 360 fresh chicken feces and 190 retail chicken meat samples, were collected from the top 12 integrated broiler chicken operations among the 13 integrated broiler chicken operations in Korea. Each operation had separated broiler chicken production chain, including breeder chicken, hatchery, broiler chicken, and slaughterhouse. The sampling locations included all the provinces of Korea, except Jeju Island and Gangwon-do. Pooled feces samples were collected from 72 broiler chicken farms; 5 samples from each farm were collected and tested in this study. In addition, retail meat was collected from 38 farms, and 5 individually packaged chicken meat from each farm were randomly sampled from their final packaging in the slaughterhouse. Collected samples were immediately stored in an ice box after collection and subjected to further processing upon arrival to the laboratory.
Isolation and Identification of Salmonella
The feces and retail meat samples were examined for the presence of Salmonella as recommended by the US Department of Agriculture (USDA-FSIS, 2014). Briefly, upon arrival to the laboratory, 1 g of each feces sample was separately mixed with 9 mL of buffered peptone water (BPW; BD Difco, Sparks, MD, USA) and incubated at 37°C for 24 h. After incubation, 0.1 mL BPW was transferred to 10 mL of Rappaport–Vassiliadis Broth (RV; Thermo Fisher Scientific, Oxoid Ltd, Basingstoke, UK) and incubated for 24 h at 42°C. A loopful was then plated on xylose–lysine–deoxycholate agar (XLD; BD Difco) and incubated at 37°C for 24 h. Retail meat was aseptically placed into a vacuum bag and rinsed with 400 mL of BPW. After shaking for about 20 times, the suspension of rinse fluid was then cultured at 37°C for 24 h. Then, 0.1 mL of enrichment was further incubated in 10 mL of RV for 24 h at 42°C and subcultured on XLD plate at 37°C for 24 h.
Three to 5 suspected Salmonella colonies from each plate were confirmed by latex polyclonal agglutination test (Thermo Fisher Scientific) and further confirmed through the amplification of the specific Salmonella invasive (invA) gene by PCR (Cha et al., 2013). After identification, all Salmonella isolates were serotyped according to Kauffmann–White scheme by slide agglutination with O and H antigen-specific sera (BD Difco; Denka Seiken Co., Ltd., Japan).
Antimicrobial Susceptibility Testing
The minimum inhibitory concentrations were determined using the KRNV5F Sensititre panel (TREK Diagnostic Systems, Incheon, Korea). The antimicrobials tested were amoxicillin/clavulanic acid (AUG2, 2/1−32/16 μg/mL), ampicillin (AMP, 2−64 μg/mL), cefoxitin (FOX, 1−32 μg/mL), ceftazidime (TAZ, 1−16 μg/mL), ceftiofur (XNL, 0.5−8 μg/mL), cefepime (FEP, 0.25−16 μg/mL), meropenem (MERO, 0.25−4 μg/mL), trimethoprim/sulfamethoxazole (SXT, 0.12/2.38−4/76 μg/mL), sulfisoxazole (FIS, 16−256 μg/mL), chloramphenicol (CHL, 2−64 μg/mL), ciprofloxacin (CIP, 0.12−16 μg/mL), nalicixic acid (NAL, 2−128 μg/mL), streptomycin (STR, 16−128 μg/mL), gentamicin (GEN, 1−64 μg/mL), tetracycline (TET, 2−128 μg/mL), and colistin (COL, 2−16 μg/mL). Escherichia coli ATCC 25922 was used as quality control. The interpretive categories-susceptible, intermediate, or resistant-were used according to the CLSI guidelines, except for colistin, where the MIC value of ≥ 4 μg/mL (resistant) was used (CLSI, 2016; Biswas et al., 2012). Multidrug resistance (MDR) was defined as Salmonella isolates being resistant to as least 3 antimicrobial categories.
Pulsed Field Gel Electrophoresis
Pulsed-field gel electrophoresis (PFGE) was used to establish relatedness and diversity among Salmonella isolates, and PFGE was conducted according to the Centers for Disease Control and Prevention PulseNet standardized procedure. Salmonella genomic DNA was digested with XbaI, and PFGE fingerprinting patterns were analyzed using BioNumerics software (version 5.10 for Windows, Applied Maths, Belgium). The sizes of the fragments were calculated based on the fragments for the Salmonella Braenderup H9812 reference standard.
RESULTS
Prevalence and Serovar Distribution of Salmonella
The prevalence of Salmonella in chicken feces and retail chicken meat samples is shown in Table 1. Among the 550 samples, 136 (24.7%) samples were found positive for Salmonella. Out of 360 feces samples and 190 retail meat samples, 117 (32.5%) feces and 19 (10.0%) retail meat samples were found positive, respectively. The prevalence of Salmonella varied from 2.2% to 48.3% among the 12 chicken production operations, and all 12 operations were positive for Salmonella at the farm level, and 5 (41.7%) operations were positive at the retail meat level.
Table 1.
Prevalence of Salmonella isolates in broiler chicken feces and retail meat from 12 operations in South Korea.
| Operation | Total |
Feces |
Retail meat |
|||
|---|---|---|---|---|---|---|
| No. of samples/farms | Positive No. (%) of samples/farms | No. of samples/farms | Positive No. (%) of samples/farms | No. of samples/farms | Positive No. (%) of samples/farms | |
| C1 | 60/12 | 15 (25.0)/7 (58.3) | 40/8 | 8 (20.0)/4 (50.0) | 20/4 | 7 (35.0)/3 (75.0) |
| C2 | 60/12 | 11 (18.3)/6 (50.0) | 40/8 | 9 (22.5)/5 (62.5) | 20/4 | 2 (10.0)/1 (25.0) |
| C3 | 30/6 | 2 (6.7)/2 (33.3) | 20/4 | 2 (10.0)/2 (50.0) | 10/2 | 0 (0.0)/0 (0.0) |
| C4 | 30/6 | 14 (46.7)/6 (100.0) | 20/4 | 8 (40.0)/4 (100.0) | 10/2 | 6 (60.0)/2 (100.0) |
| C5 | 60/12 | 15 (25.0)/5 (41.7) | 40/8 | 15 (37.5)/5 (62.5) | 20/4 | 0 (0.0)/0 (0.0) |
| C6 | 60/12 | 29 (48.3)/8 (66.7) | 40/8 | 27 (67.5)/7 (87.5) | 20/4 | 2 (10.0)/1 (25.0) |
| C7 | 30/6 | 10 (33.3)/3 (50.0) | 20/4 | 10 (50.0)/3 (75.0) | 10/2 | 0 (0.0)/0 (0.0) |
| C8 | 40/8 | 8 (20.0)/3 (37.5) | 20/4 | 8 (40.0)/3 (75.0) | 20/4 | 0 (0.0)/0 (0.0) |
| C9 | 60/12 | 15 (25.0)/7 (58.3) | 40/8 | 13 (32.5)/5 (62.5) | 20/4 | 2 (10.0)/2 (50.0) |
| C10 | 30/6 | 6 (20.0)/3 (50.0) | 20/4 | 6 (30.0)/3 (75.0) | 10/2 | 0 (0.0)/0 (0.0) |
| C11 | 45/9 | 10 (22.2)/3 (33.3) | 30/6 | 10 (33.3)/3 (50.0) | 15/3 | 0 (0.0)/0 (0.0) |
| C12 | 45/9 | 1 (2.2)/1 (11.1) | 30/6 | 1 (3.3)/1 (16.7) | 15/3 | 0 (0.0)/0 (0.0) |
| Total | 550/110 | 136 (24.7)/54 (49.1) | 360/72 | 117 (32.5)/45 (62.5) | 190/38 | 19 (10.0)/9 (23.7) |
Out of 136 Salmonella isolates, 131 isolates were assigned to 10 serovars and 5 uptyped isolates (Table 2). The most common Salmonella serovars were S. Albany (50 isolates, 36.8%), S. Enteritidis (38 isolates, 27.9%), and S. Montevideo (23 isolates, 16.9%) isolated from 6, 10, and 6 operations, respectively. In addition, S. Virchow, S. Senftenberg, S. Rissen, S. Mbandaka, S. Alminko, S. Typhimurium, and S. Moscow were also found in this study.
Table 2.
Distribution of Salmonella serovars among 12 operations.
| Operation | Serovar (No.) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Albany | Enteritidis | Montevideo | Virchow | Senftenberg | Rissen | Mbandaka | Alminko | Typhimurium | Moscow | S. spp | |
| C1 (n = 15) | 7 | 4 | 1 | 3 | |||||||
| C2 (n = 11) | 6 | 1 | 4 | ||||||||
| C3 (n = 2) | 1 | 1 | |||||||||
| C4 (n = 14) | 6 | 2 | 6 | ||||||||
| C5 (n = 15) | 9 | 5 | 1 | ||||||||
| C6 (n = 29) | 22 | 1 | 3 | 1 | 2 | ||||||
| C7 (n = 10) | 2 | 7 | 1 | ||||||||
| C8 (n = 8) | 8 | ||||||||||
| C9 (n = 15) | 4 | 8 | 2 | 1 | |||||||
| C10 (n = 6) | 3 | 1 | 1 | 1 | |||||||
| C11 (n = 10) | 5 | 4 | 1 | ||||||||
| C12 (n = 1) | 1 | ||||||||||
| Total (n = 136) | 50 | 38 | 23 | 6 | 5 | 3 | 3 | 1 | 1 | 1 | 5 |
Antimicrobial Susceptibility in Salmonella
The antimicrobial susceptibility test result of Salmonella isolates from 12 operations is shown in Table 3. Among the 136 isolates, resistance was most frequently observed to nalidixic acid (94.1%), followed by ampicillin (69.9%), sulfisoxazole (67.6%), tetracycline (60.3%), and streptomycin (55.9%); Salmonella isolates were less resistant to ciprofloxacin (5.1%), gentamicin (5.1%), and colistin (11.8%). In addition, resistant to cefoxitin (0.7%), ceftazidime (11.8%), ceftiofur (12.5%), and cefepime (11.0%) was also been observed. Meropenem resistance was not found. We also observed that Salmonella isolates from operation C4 showed high resistance to third- and fourth-generation cephalosporin and gentamicin.
Table 3.
Antimicrobial resistance of Salmonella isolates among 12 operations (resistance No./%).
| Antimicrobial agent | Operation |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C1 (n = 15) | C2(n = 11) | C3(n = 2) | C4(n = 14) | C5(n = 15) | C6(n = 29) | C7(n = 10) | C8(n = 8) | C9(n = 15) | C10(n = 6) | C11(n = 10) | C12(n = 1) | Total (n = 136) | |
| Amoxicillin/ clavulanic acid | 0 (0.0) |
2 (18.2) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
2 (1.5) |
| Ampicillin | 8 (53.3) |
10 (90.9) |
1 (50.0) |
12 (85.7) |
14 (93.3) |
21 (72.4) |
8 (80.0) |
8 (100.0) |
3 (20.0) |
4 (66.7) |
5 (50.0) |
1 (100.0) |
95 (69.9) |
| Cefoxitin | 0 (0.0) |
1 (9.1) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
1 (0.7) |
| Ceftazidime | 0 (0.0) |
1 (9.1) |
0 (0.0) |
11 (78.6) |
0 (0.0) |
1 (3.4) |
0 (0.0) |
0 (0.0) |
3 (20.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
16 (11.8) |
| Ceftiofur | 0 (0.0) |
1 (9.1) |
0 (0.0) |
12 (85.7) |
0 (0.0) |
1 (3.4) |
0 (0.0) |
0 (0.0) |
3 (20.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
17 (12.5) |
| Cefepime | 0 (0.0) |
0 (0.0) |
0 (0.0) |
11 (78.6) |
0 (0.0) |
1 (3.4) |
0 (0.0) |
0 (0.0) |
3 (20.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
15 (11.0) |
| Meropenem | 0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
| Trimethoprim/ sulfamethoxazole | 0 (0.0) |
6 (54.5) |
0 (0.0) |
4 (28.6) |
9 (60.0) |
26 (89.7) |
2 (20.0) |
8 (100.0) |
0 (0.0) |
4 (66.7) |
0 (0.0) |
0 (0.0) |
59 (43.4) |
| Sulfisoxazole | 8 (53.3) |
9 (81.8) |
1 (50.0) |
7 (50.0) |
14 (93.3) |
26 (89.7) |
8 (80.0) |
8 (100.0) |
1 (6.7) |
4 (66.7) |
5 (50.0) |
1 (100.0) |
92 (67.6) |
| Chloramphenicol | 0 (0.0) |
5 (45.5) |
0 (0.0) |
4 (28.6) |
9 (60.0) |
20 (69.0) |
2 (20.0) |
8 (100.0) |
1 (6.7) |
5 (83.3) |
0 (0.0) |
0 (0.0) |
54 (39.7) |
| Ciprofloxacin | 0 (0.0) |
1 (9.1) |
0 (0.0) |
1 (7.1) |
1 (6.7) |
4 (13.8) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
7 (5.1) |
| Nalicixic acid | 12 (80.0) |
11 (100.0) |
1 (50.0) |
14 (100.0) | 15 (100.0) |
29 (100.0) |
8 (80.0) |
8 (100.0) |
15 (100.0) |
4 (66.7) |
10 (100.0) |
1 (100.0) |
128 (94.1) |
| Streptomycin | 9 (60.0) |
7 (63.6) |
2 (100.0) |
7 (50.0) |
10 (66.7) |
19 (65.5) |
7 (70.0) |
5 (62.5) |
1 (6.7) |
2 (33.3) |
6 (60.0) |
1 (100.0) |
76 (55.9) |
| Gentamicin | 0 (0.0) |
0 (0.0) |
0 (0.0) |
6 (42.9) |
0 (0.0) |
1 (3.4) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
7 (5.1) |
| Tetracycline | 0 (0.0) |
6 (54.5) |
1 (50.0) |
12 (85.7) |
13 (86.7) |
22 (75.9) |
8 (80.0) |
8 (100.0) |
3 (20.0) |
3 (50.0) |
5 (50.0) |
1 (100.0) |
82 (60.3) |
| Colistin | 2 (13.3) |
0 (0.0) |
0 (0.0) |
1 (7.1) |
5 (33.3) |
1 (3.4) |
4 (40.0) |
0 (0.0) |
1 (6.7) |
0 (0.0) |
2 (20.0) |
0 (0.0) |
16 (11.8) |
Diversity of antimicrobial resistance in different Salmonella serovars was found in this study (Table 4). All 50 S. Albany isolates were observed to be resistant to trimethoprim/sulfamethoxazole, sulfisoxazole, and nalicixic acid. Furthermore, they showed high resistance to ampicillin (88.0%), tetracycline (88.0%), chloramphenicol (86.0), and streptomycin (64.0%). All S. Virchow isolates were found to be resistant to third- and fourth-generation cephalosporin, and S. Enteritidis showed similarly high resistance as well. Gentamicin resistance was found only in S. Enteritidis isolates. In addition, S. Montevideo isolates showed low resistance to tested antimicrobials, except nalicixic acid; moreover, they showed no resistance to amoxicillin/clavulanic acid, ampicillin, cefoxitin, ceftazidime, ceftiofur, cefepime, meropenem, chloramphenicol, gentamicin, and tetracycline.
Table 4.
Antimicrobial resistance among different Salmonella serovar.
| Antimicrobial agent | Serovar (No./%) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Albany (n = 50) | Enteritidis (n = 38) | Montevideo (n = 23) | Virchow (n = 6) | Senftenberg (n = 5) | Rissen (n = 3) | Mbandaka (n = 3) | Alminko (n = 1) | Typhimurium (n = 1) | Moscow (n = 1) | S. spp (n = 5) | |
| Amoxicillin/ clavulanic acid | 2 (4.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
| Ampicillin | 44 (88.0) |
34 (89.5) |
3 (13.0) |
6 (100.0) |
1 (20.0) |
2 (66.7) |
0 (0.0) |
1 (100.0) |
1 (100.0) |
1 (100.0) |
2 (50.0) |
| Cefoxitin | 1 (2.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
| Ceftazidime | 1 (2.0) |
8 (21.1) |
0 (0.0) |
6 (100.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
1 (25.0) |
| Ceftiofur | 1 (2.0) |
9 (23.7) |
0 (0.0) |
6 (100.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
1 (25.0) |
| Cefepime | 0 (0.0) |
8 (21.1) |
0 (0.0) |
6 (100.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
1 (25.0) |
| Meropenem | 0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
| Trimethoprim/ sulfamethoxazole | 50 (100.0) |
1 (2.6) |
0 (0.0) |
3 (50.0) |
0 (0.0) |
2 (66.7) |
0 (0.0) |
1 (100.0) |
1 (100.0) |
0 (0.0) |
1 (25.0) |
| Sulfisoxazole | 50 (100.0) |
27 (71.1) |
2 (8.7) |
6 (100.0) |
1 (20.0) |
1 (33.3) |
0 (0.0) |
1 (100.0) |
1 (100.0) |
1 (100.0) |
2 (50.0) |
| Chloramphenicol | 43 (86.0) |
2 (5.3) |
0 (0.0) |
3 (50.0) |
0 (0.0) |
2 (66.7) |
0 (0.0) |
1 (100.0) |
1 (100.0) |
0 (0.0) |
2 (50.0) |
| Ciprofloxacin | 3 (6.0) |
1 (2.6) |
1 (4.3) |
0 (0.0) |
0 (0.0) |
2 (66.7) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
| Nalicixic acid | 50 (100.0) |
36 (94.7) |
23 (100.0) |
6 (100.0) |
5 (100.0) |
3 (100.0) |
0 (0.0) |
1 (100.0) |
0 (0.0) |
1 (100.0) |
3 (75.0) |
| Streptomycin | 32 (64.0) |
27 (71.1) |
3 (13.0) |
6 (100.0) |
1 (20.0) |
2 (66.7) |
1 (33.3) |
0 (0.0) |
0 (0.0) |
1 (100.0) |
3 (75.0) |
| Gentamicin | 0 (0.0) |
7 (18.4) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
| Tetracycline | 44 (88.0) |
25 (65.8) |
0 (0.0) |
6 (100.0) |
0 (0.0) |
3 (100.0) |
0 (0.0) |
1 (100.0) |
0 (0.0) |
1 (100.0) |
2 (50.0) |
| Colistin | 2 (4.0) |
11 (28.9) |
2 (8.7) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
1 (100.0) |
0 (0.0) |
A diversity of antimicrobial resistance phenotypes (n = 30) was observed among the Salmonella isolates from broiler chicken farm and retail meat (Table 5). Except 4 isolates susceptible to all antimicrobials, all Salmonella isolates were resistant to as least one antimicrobial. We also found all S. Albany and S. Virchow isolates were MDR and 35 (92.1%) S. Enteritidis isolates were also MDR. While S. Montevideo isolates showed less resistance to the tested antimicrobial and 13.0% of S. Montevideo isolates were MDR. Meanwhile, a total of 35 (25.7%) isolates were with the ACSSuTN (ampicillin, chloramphenicol, streptomycin, sulfisoxazole, tetracycline and nalidixic acid) resistance pattern. The highest percentage of ACSSuTN resistance was identified in S. Albany (29 isolates, 58.0%), followed by S. Virchow (3 isolates, 50.0%), S. Enteritidis (1 isolate, 2.6%), and other serovars (2 isolates, 14.3%). However, all S. Montevideo and S. Senftenberg isolates were ACSSuTN-susceptible.
Table 5.
Resistance pattern of Salmonella isolates from chicken.
| No. | Antimicrobial resistance pattern | No. of isolates |
||||||
|---|---|---|---|---|---|---|---|---|
| Albany (n = 50) | Enteritidis (n = 38) | Montevideo (n = 23) | Virchow (n = 6) | Senftenberg (n = 5) | Others (n = 14) | Total (n = 136) | ||
| 0 | - | 2 | 2 | 4 | ||||
| 1 | AMP | 1 | 1 | |||||
| 2 | CHL | 1 | 1 | |||||
| 3 | NAL | 1 | 18 | 4 | 1 | 24 | ||
| 4 | STR | 2 | 2 | |||||
| 5 | NAL/COL | 1 | 1 | |||||
| 6 | NAL/TET | 1 | 1 | |||||
| 7 | CHL/NAL/COL | 1 | 1 | |||||
| 8 | NAL/STR/COL | 1 | 1 | |||||
| 9 | SXT/FIS/NAL | 4 | 4 | |||||
| 10 | AMP/FIS/NAL/STR | 7 | 2 | 1 | 10 | |||
| 11 | AMP/SXT/FIS/CHL | 1 | 1 | |||||
| 12 | SXT/FIS/NAL/STR | 2 | 2 | |||||
| 13 | AMP/FIS/NAL/STR/COL | 2 | 2 | |||||
| 14 | AMP/FIS/NAL/STR/TET | 9 | 9 | |||||
| 15 | AMP/FIS/NAL/STR/TET/COL | 7 | 1 | 8 | ||||
| 16 | AMP/SXT/FIS/CHL/NAL/TET | 11 | 1 | 12 | ||||
| 17 | AMP/SXT/FIS/NAL/STR/TET | 1 | 1 | |||||
| 18 | AMP/TAZ/XNL/FEP/NAL/TET | 2 | 2 | |||||
| 19 | AMP/SXT/CHL/CIP/NAL/STR/TET | 1 | 1 | |||||
| 20 | AMP/SXT/FIS/CHL/NAL/STR/TET | 25 | 1 | 26 | ||||
| 21 | AMP/SXT/FIS/CHL/NAL/TET/COL | 1 | 1 | |||||
| 22 | AMP/TAZ/XNL/FEP/NAL/GEN/TET | 5 | 5 | |||||
| 23 | AUG2/AMP/SXT/FIS/CHL/NAL/TET | 1 | 1 | |||||
| 24 | AMP/SXT/FIS/CHL/CIP/NAL/STR/TET | 3 | 1 | 4 | ||||
| 25 | AMP/SXT/FIS/CHL/NAL/STR/TET/COL | 1 | 1 | |||||
| 26 | AMP/TAZ/XNL/FEP/FIS/NAL/STR/TET | 3 | 1 | 4 | ||||
| 27 | AMP/TAZ/XNL/FEP/FIS/NAL/STR/GEN/TET | 1 | 1 | |||||
| 28 | AMP/TAZ/XNL/FEP/SXT/FIS/CHL/NAL/STR/TET | 3 | 3 | |||||
| 29 | AUG2/AMP/FOX/TAZ/XNL/SXT/FIS/CHL/NAL/TET | 1 | 1 | |||||
| 30 | AMP/XNL/SXT/FIS/CHL/CIP/NAL/STR/GEN/TET/COL | 1 | 1 | |||||
Genotypic Determination of Diversity Among Salmonella Isolates
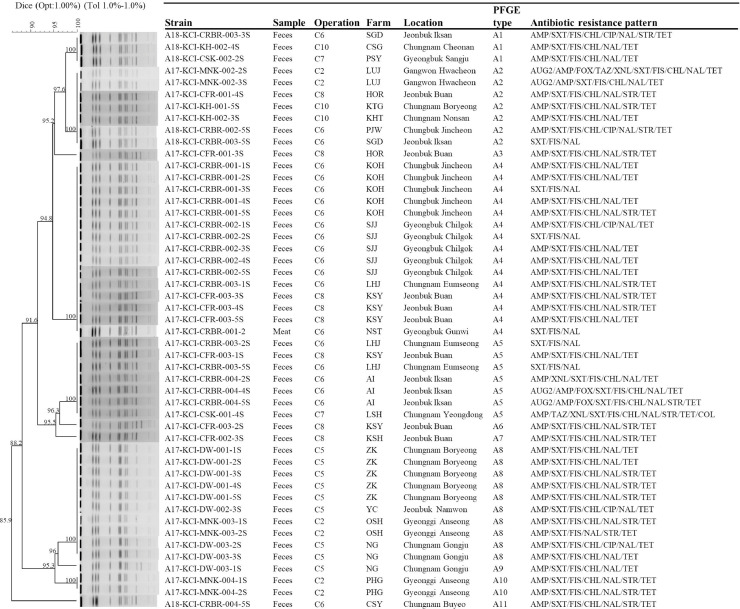
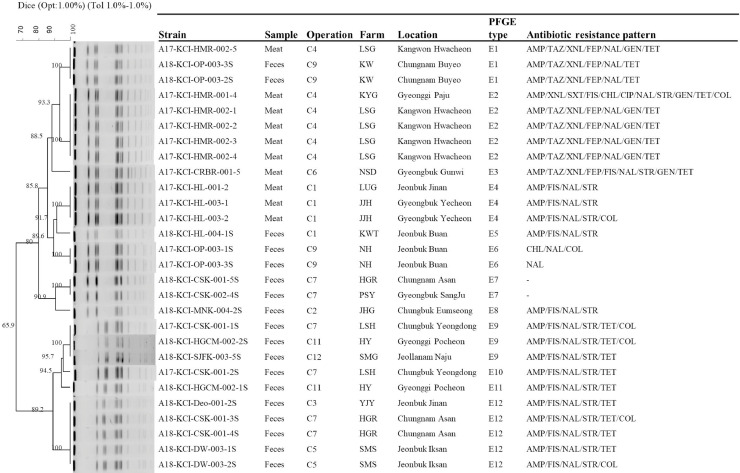
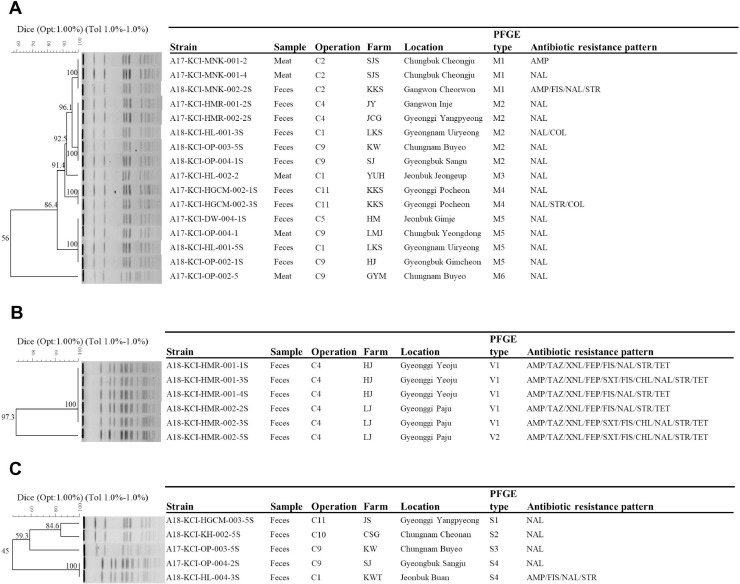
The genetic relatedness of the S. Albany, S. Enteritidis, S. Virchow, S. Montevideo, and S. Senftenberg was evaluated on the basis of the PFGE pattern analysis. Eleven distinct types of PFGE patterns were found among S. Albany isolates which showed an overall homology similarity higher than 85% (Figure 1). Six identical PFGE types (A1, A2, A4, A5, A8, and A10) were found with more than one S. Albany isolate recovered from 6 operations, whereas 5 types (A3, A6, A7, A9, and A11) were found with only one isolate. We found that identical PFGE type A1 of S. Albany isolates was recovered from 3 operations (C6, C7, and C10), type A2 from 4 operations (C2, C6, C8, and C10), type A4 from 2 operations (C6 and C8), type A5 from 3 operations (C6, C7, and C8), and type A8 from 2 operations (C2 and C5). The 12 types of PFGE patterns were found among S. Enteritidis isolates (Figure 2), and 2 clusters of S. Enteristidis isolates were observed using an 80% cut off value. We found that identical PFGE type E1 of S. Enteristidis isolates was recovered from two operations (C4 and C9), type E9 from three operations (C7, C11, and C12), and type E12 from three operations (C3, C5, and C7). A total of 6 types were found in S. Montevideo isolates (Figure 3), except for one isolate of type M6; other S. Montevideo isolates showed a homology similarity higher than 85%. Identical PFGE type M2 of S. Montevideo isolates recovered from three operations (C1, C4, and C9) and type M5 from 3 operations (C1, C5, and C9) were found.
Figure 1.
Dendrograms based on Xba I-pulsed field gel electrophoresis (PFGE) profiles of Salmonella ser. Albany isolates from chicken and the corresponding antimicrobial susceptibility patterns to the 16 indicated antimicrobials. The Dice coefficient was used to perform similarity analysis. The antimicrobials shown in the sequence are amoxicillin/clavulanic acid (AUG2), ampicillin (AMP), cefoxitin (FOX), ceftazidime (TAZ), ceftiofur (XNL), cefepime (FEP), meropenem (MERO), trimethoprim/sulfamethoxazole (SXT), sulfisoxazole (FIS), chloramphenicol (CHL), ciprofloxacin (CIP), nalicixic acid (NAL), streptomycin (STR), gentamicin (GEN), tetracycline (TET), and colistin (COL).
Figure 2.
Dendrograms based on Xba I-pulsed field gel electrophoresis (PFGE) profiles of Salmonella ser. Enteritidis isolates from chicken and the corresponding antimicrobial susceptibility patterns to the 16 indicated antimicrobials.
Figure 3.
Dendrograms based on Xba I-pulsed field gel electrophoresis (PFGE) profiles of Salmonella ser. Montevideo (A), S. Virchow (B), and S. Senftenberg (C) isolates from chicken and the corresponding antimicrobial susceptibility patterns to the 16 indicated antimicrobials.
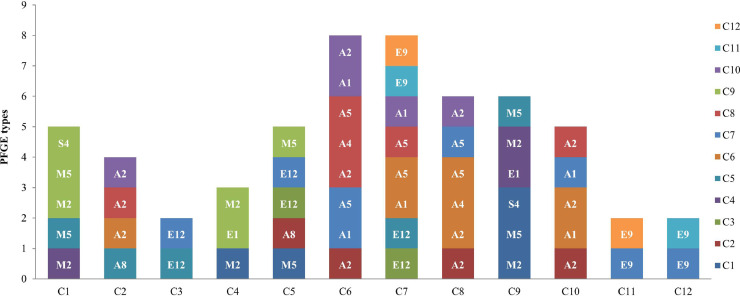
A total of 35 PFGE types were identified among Salmonella isolates of the serovars Albany, Enteritidis, Virchow, Montevideo, and Senftenberg from 12 chicken processing operations. Among these types, 22 PFGE types corresponded to 32 isolates from samples limited to one operation, and the other 13 PFGE types corresponded to 72 isolates from samples widely distributed among different operations. Among these 13 PFGE types, Salmonella isolates from one operation were with the identified PFGE types of Salmonella isolates collected from at least other two operations (Figure 4). Operation C3 of Salmonella isolates with PFGE type E12 was overlapped with the PFGE type of Salmonella isolates from other two operations of C5 and C7; in keeping with operation C4 of Salmonella isolates with PFGE type M2 overlapped with it of Salmonella isolates from operation C1, PFGE types E1 and M2 overlapped with it of Salmonella isolates from operation C9; operation C11 of PFGE type E9 with operations C7 and C12, and operation 12 of type E9 with operations C7 and C11. While operations C1 and C9 of Salmonella isolates with identified PFGE were overlapped with three operations each of C4, C5, and C9 and C1, C4, and C5, respectively, operations C2, C6, C8, and C10 overlapped with four other operations and operation E overlapped with five operations. It is noteworthy that four PFGEs (A1, A5, E9, and E12) of Salmonella isolates from operation C7 were overlapped with seven operations (C3, C5, C6, C8, C10, C11, and C12).
Figure 4.
Overlap each of the PFGE types of Salmonella ser. Albany, S. Enteritidis, S. Montevideo, S. Virchow, and S. Senftenberg from one operation to other operations.
DISCUSSION
In the present study, a national investigation of the prevalence, serovar distribution, antimicrobial resistance, and genetic characterization of Salmonella isolates from 12 integrated broiler chicken operations in South Korea was conducted. The overall prevalence of Salmonella was 24.7%, being 32.5% in chicken feces and 10.0% in retail chicken meat. Although Salmonella prevalence varied among these operations, all 12 operations are confirmed to be Salmonella-positive. This result was in agreement with previous studies stating that Salmonella was widely distributed in broiler chicken population (Antunes et al., 2016).
In this study, S. Albany was the most commonly identified serovar (36.8%), followed by S. Enteritidis (27.9%) and S. Montevideo (16.9%) in broiler chicken. This is in contrast to previous studies reporting S. Enteritidis and S. Montevideo as the most common serovars in chicken, perhaps because S. Albany was not identified in chicken before 2016 in Korea (Shang et al., 2019; Tamang et al., 2011). From previous reports, S. Albany was commonly found in poultry and other domestic animals in Southeast Asia and the Western countries (Fuzihara et al., 2000; Ta et al., 2014), and this serovar has gained significant public attention as this serovar has become among the top five serovars affecting humans in recent times (Kuo et al., 2014). Since serovar diversity of Salmonella has been recorded from different geographical regions and there have always been certain geography-specific serovars, the emergence of S. Albany in Korea suggests that it could have been globally disseminated via international travel or food trade import–export as important vehicles (Huusko et al., 2017; Park et al., 2019). Although imported retail meat has not reported as a possible source for the dissemination of Salmonella in Korea, the introduction of foodborne pathogens through imported retail meat has been reported in Korea recently (Kim et al., 2018; Kim et al., 2015). Therefore, as a potential vehicle for Salmonella transmission in Korea, extensive monitoring and risk assessment of Salmonella in imported chicken meat may be required to estimate the potential public health threat to humans and the environment in the future.
High resistance of Salmonella isolates from chicken to nalidixic acid, ampicillin, sulfonamides, tetracycline, and streptomycin was consistent with the literature from different countries, including Korea (EFSA, 2018; Shang et al., 2019; Tamang et al., 2011). This is not surprising because these antimicrobials have been widely used for infection treatment in poultry since a long time. We also noticed antimicrobial resistance diversity among different serovars of Salmonella isolates. In this study, S. Albany isolates showed high resistance to trimethoprim/sulfamethoxazole, sulfisoxazole, nalicixic acid, ampicillin, tetracycline, chloramphenicol, and streptomycin. In agreement with a previous study in Malaysia, Chuah et al., (2018) reported S. Albany isolates from wet poultry market that had a high frequency of resistance to these antimicrobials. Similarly, in Taiwan, high resistance to nalicixic acid, ampicillin, tetracycline, chloramphenicol, and trimethoprim/sulfamethoxazole was found in S. Albany isolates from humans and pigs (Kuo et al., 2014). Furthermore, it should be noted that all S. Albany isolates were multidrug-resistant, and 29 isolates (58%) showed the ACSSuTN resistance pattern. After the ACSSuT-resistant S. Typhimurium was first identified in United Kingdom in 1984, the ACSSuT resistance pattern has attracted significant attention in the world because of the huge public health threat (Threlfall et al., 1996). Along with the prolonged and excessive use of quinolones in food-producing animals in the past, a dramatic increase in resistance to quinolones was reported in Salmonella (Antunes et al., 2016). Moreover, of major clinical and public health concerns was the observation that three ACSSuTN-resistant S. Albany isolates were co-resistant to ciprofloxacin and one isolate was co-resistant to colistin, concurrently. Currently, ciprofloxacin is commonly used for the treatment of non-typhoidal Salmonella infections, and colistin is considered the last-line of antibiotic defense (Biswas et al., 2012). Co-resistance to these antimicrobials is already a major public health problem because of the possibility of horizontal transmission of the resistant colonies to other humans and horizontal transmission the resistant plasmid between bacterial species. We also identified a multidrug-resistant S. Albany isolate that was co-resistant to third-generation cephalosporins, which were considered an alternative drug for treating Salmonella infection. Therefore, the spread of multidrug-resistant S. Albany isolates co-resistant to these clinically important antibiotics, including fluoroquinolone, third-generation cephalosporins, and colistin, will pose a real threat to global public health resulting in challenges with clinical treatment.
In agreement with previous studies in Korea, the top serovars –S. Enteritidis and S. Montevideo – were commonly found in chickens in this study, whereas the frequencies of S. Virchow, S. Senftenberg, S. Rissen, S. Mbandaka, S. Alminko, S. Typhimurium, and S. Moscow were relatively lower (Jeon et al., 2019; Kang et al., 2009; Shang et al., 2019; Tamang et al., 2011). Notably, a relatively high frequency of multidrug resistance was found in S. Enteritidis (92.1%) and S. Virchow (100.0%) isolates. Of particular interest was high resistance to third-generation cephalosporins in S. Enteritidis (23.7%) and S. Virchow (100.0%) isolates. After the first report of the isolation of third-generation cephalosporin-resistant S. Enteritidis and S. Essen strains from chicken, increased resistance in various Salmonella has been reported in poultry in Korea (Lee et al., 2016; Park et al., 2017; Tamang et al., 2011). We should particularly focus on all S. Virchow isolates with third-generation cephalosporin resistance which were on account of the dramatically increased cefotaxime-resistant S. Virchow in human may source from chicken (Kim et al., 2016). In addition, high resistance (28.9%) to colistin in S. Enteritidis was found in this study. This result was in agreement with a previous study wherein high colistin resistance was limited to specific Salmonella serovars (Chiou et al., 2017). We also noticed that gentamicin resistance was only in S. Enteritidis isolates; this result was in contrast to previous studies which reported that mild gentamicin resistance was always present in Salmonella isolates (EFSA, 2020). High gentamicin resistance in Korea may suggest some fitness benefit of gentamicin resistance in S. Enteritidis; previous studies had showed the persistent distribution of the resistant colonies in Korean poultry industry (Kang et al., 2017). Furthermore, in this study, a multidrug-resistant S. Enteritidis strain with the resistance pattern XNL-CIP-GEN-COL was also identified. The findings of this study corroborate the widely held view that poultry is a major source of multidrug-resistant Salmonella which is resistant to treatment with several antimicrobials. This study underlines the value of an antibiotic susceptibility survey for selecting appropriate treatment options for salmonellosis caused by strains of poultry origin (Antunes et al., 2016). As the emergence of antimicrobial resistant strains has been linked to the use of antimicrobials in the farm, more prudent and appropriate use of antibiotics in food animals is required.
Based on the PFGE results, all S. Albany isolates had a high genetic homology of more than 85% similarity which suggests a colony dissemination of S. Albany in Korea. Furthermore, the high degree of genetic homology may more likely be explained by a common ancestral origin than multiple origins; this is also supported by the fact that most isolates differed by only 1 to 2 bands after XbaI digestion (Okoro et al., 2012). We could also assume that the colony does not allow for acquisition of multiple genetic alterations in such a short period after appearance in chicken in Korea. It is possible that same colony was gained different antibiotics treatment pressure in different chicken operations or farms, and resulted in that the S. Albany isolates from different farms with high degree of genetic homology and different antibiotics resistance patterns. We also noticed that S. Albany was the predominant serovar in 5 among the 6 chicken operations that were positive for S. Albany (Table 2). In addition, the rapid dissemination of S. Albany in all 6 provinces investigated in this study allowed us to hypothesize that this serovar or certain colonies had some growth advantage over other serovars. In addition, the identification of third-generation cephalosporin-resistant MDR S. Albany suggests that MDR S. Albany could also acquire extended-spectrum beta-lactamase resistance genes and that these Albany strains may become a great public health concern in Korea. Since studies on the biological and virulence characteristics of S. Albany are lacking, further studies that are not limited to advanced surveillance are required to prevent the dissemination of the resistance.
We also noticed that each operation shared the same PFGE types of Salmonella isolates with at least 2 other operations (Figure 4). This result suggests that the dissemination of the same Salmonella colonies between different chicken operations is common. The rapid and wide spread of these Salmonella colonies across different chicken operations may indicate an increasing public health concern with increased chance for these colonies to acquire antibiotic resistance and virulence genes in the presence of different environment stresses in different chicken operations (Andino et al., 2015). Our results were contradictory to those of other studies that had demonstrated phenotypic and genetic diversity of Salmonella isolates from different chicken farms and operations (Ha et al., 2018). The dissemination between different operations may indicate a common origin within these broiler chicken operations. This result suggests that external environmental factors play an important role in the dissemination of colonies among these integrated chicken production operations wherein each vertical integrated operation has a separate supply chain that includes broiler breeder, broiler hatchery, broiler, and slaughterhouse, among others. Furthermore, we cannot rule out that the contamination in broiler chicken is vertically infected with Salmonella from broiler breeder chickens. This is because vertical transmission of Salmonella to broiler chicken could result from infected breeder chicken, and it is common for different broiler chicken production operations to share the same breeder chicken company (Davies et al., 2001; Oh et al., 2010). Among these operations, we should specifically focus on operation C7 which shared four PFGE types with seven operations (E12 with operations C3 and C5, A1, and A5 with operation C6, A5 with operation C8, A1 with operation C10, and E9 with operations C11 and C12). This data suggests operation C7 as the original source of Salmonella for these genotypes or the important intermediate route of Salmonella transmission, thus emphasizing the importance to control the spread of Salmonella in operation C7. In addition, attention needs to be paid to multiple interchange activities among operations C1 and C9 as three genotypes in 2 serovars were identified among these two operations. Therefore, to speed up the development of intervention strategies, further epidemiological studies are needed to identify the sources of Salmonella infection for each operation, particularly for the common infection route among these operations.
In conclusion, this nationwide surveillance study presents findings on serovar distribution, antibiotic resistance, and genetic diversity of Salmonella source from 12 integrated broiler chicken operations across Korea. The results obtained the current epidemiological state of Salmonella isolates present in chicken and revealed that the multidrug-resistant serovar S. Albany has distributed all over Korea and suggested that the nationwide occurrence of this serovar during the study period was due to increased circulation of S. Albany colonies and establishment of a specific colony that took place after it first appeared in 2016. In addition, we also noted that the spread of Salmonella colonies between different integrated operations was common, and several operations played a part in Salmonella carriage and transmission in Korea.
ACKNOWLEDGMENTS
This work was supported by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture and Forestry (IPET) through Agriculture, Food and Rural Affairs Convergence Technologies Program for Educating Creative Global Leader (716002-7, 320005-4, 120005-2), funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA).
Disclosures
The authors have no conflicts of interest to declare.
REFERENCES
- Andino A., Hanning I. Salmonella enterica: survival, colonization, and virulence differences among serovars. Sci. World J. 2015;2015 doi: 10.1155/2015/520179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antunes P., Mourao J., Campos J., Peixe L. Salmonellosis: the role of poultry meat. Clin. Microbiol. Infec. 2016;22:110–121. doi: 10.1016/j.cmi.2015.12.004. [DOI] [PubMed] [Google Scholar]
- Biswas S., Brunel J.M., Dubus J.C., Reynaud-Gaubert M., Rolain J.M. Colistin: an update on the antibiotic of the 21st century. Expert Rev. Anti. Infect. Ther. 2012;10:917–934. doi: 10.1586/eri.12.78. [DOI] [PubMed] [Google Scholar]
- Broughton E.I., Ip M., Coles C.L., Walker D.G. Higher hospital costs and lengths of stay associated with quinolone-resistant Salmonella enterica infections in Hong Kong. J. Public Health (Oxf) 2010;32:165–172. doi: 10.1093/pubmed/fdp057. [DOI] [PubMed] [Google Scholar]
- Cardinale E., Gros-Claude J.D.P., Rivoal K., Rose V., Tall F., Mead G.C., Salvat G. Epidemiological analysis of Salmonella enterica ssp. enterica serovars Hadar, Brancaster and Enteritidis from humans and broiler chickens in Senegal using pulsed-field gel electrophoresis and antibiotic susceptibility. J. Appl. Microbiol. 2005;99:968–977. doi: 10.1111/j.1365-2672.2005.02618.x. [DOI] [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention). 2018. National Enteric Disease Surveillance: Salmonella Annual Report, 2016. https://www.cdc.gov/nationalsurveillance/pdfs/2016-Salmonella-report-508.pdf
- Cha S.Y., Kang M., Yoon R.H., Park C.K., Moon O.K., Jang H.K. Prevalence and antimicrobial susceptibility of Salmonella isolates in Pekin ducks from South Korea. Comp. Immunol. Microbiol. Infect. Dis. 2013;36:473–479. doi: 10.1016/j.cimid.2013.03.004. [DOI] [PubMed] [Google Scholar]
- Chiou C.S., Chen Y.T., Wang Y.W., Liu Y.Y., Kuo H.C., Tu Y.H., Lin A.C., Liao Y.S., Hong Y.P. Dissemination of mcr-1-carrying plasmids among colistin-resistant Salmonella strains from humans and food-producing animals in Taiwan. Antimicrob. Agents Chemother. 2017;61 doi: 10.1128/AAC.00338-17. e00338-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuah L.O., Syuhada A.K.S., Suhaimi I.M., Hanim T.F., Rusul G. Genetic relatedness, antimicrobial resistance and biofilm formation of Salmonella isolated from naturally contaminated poultry and their processing environment in northern Malaysia. Food Res. Int. 2018;105:743–751. doi: 10.1016/j.foodres.2017.11.066. [DOI] [PubMed] [Google Scholar]
- CLSI (Clinical and Laboratory Standards Institute) Clinical and Laboratory Standards Institute; Wayne, PA: 2016. Performance standards for antimicrobial susceptibility testing (26th) [Google Scholar]
- Davies R., Breslin M., Corry J.E., Hudson W., Allen V.M. Observations on the distribution and control of Salmonella species in two integrated broiler companies. Vet. Rec. 2001;149:227–232. doi: 10.1136/vr.149.8.227. [DOI] [PubMed] [Google Scholar]
- Davis M.A., Hancock D.D., Besser T.E., Rice D.H., Gay J.M., Gay C., Gearhart L., DiGiacomo R. Changes in antimicrobial resistance among Salmonella enterica serovar typhimurium isolates from humans and cattle in the northwestern United States, 1982-1997. Emerg. Infect. Dis. 1999;5:802–806. doi: 10.3201/eid0506.990610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2017. EFSA J. 2018;16:e05500. doi: 10.2903/j.efsa.2018.5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2017/2018. EFSA J. 2020;18:e06007. doi: 10.2903/j.efsa.2020.6007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuzihara T.O., Fernandes S.A., Franco B.D.G.M. Prevalence and dissemination of Salmonella serotypes along the slaughtering process in Brazilian small poultry slaughterhouses. J. Food Prot. 2000;63:1749–1753. doi: 10.4315/0362-028x-63.12.1749. [DOI] [PubMed] [Google Scholar]
- Ha J.S., Seo K.W., Kim Y.B., Kang M.S., Song C.S., Lee Y.J. Prevalence and characterization of Salmonella in two integrated broiler operations in Korea. Irish Vet. J. 2018;71:3. doi: 10.1186/s13620-018-0114-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huusko S., Pihlajasaari A., Salmenlinna S., Sogel J., Dontsenko I., Lundstrom D.E.P.E, H., Toikkanen S., Rimhanen-Finne R. Outbreak of Salmonella enteritidis phage type 1B associated with frozen pre-cooked chicken cubes, Finland 2012. Epidemiol. Infect. 2017;145:2727–2734. doi: 10.1017/S0950268817001364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon H.Y., Seo K.W., Kim Y.B., Kim D.K., Kim S.W., Lee Y.J. Characteristics of third-generation cephalosporin-resistant Salmonella from retail chicken meat produced by integrated broiler operations. Poult. Sci. 2019;98:1766–1774. doi: 10.3382/ps/pey514. [DOI] [PubMed] [Google Scholar]
- Kang M.S., Oh J.Y., Kwon Y.K., Lee D.Y., Jeong O.M., Choi B.K., Youn S.Y., Jeon B.W., Lee H.J., Lee H.S. Public health significance of major genotypes of Salmonella enterica serovar Enteritidis present in both human and chicken isolates in Korea. Res. Vet. Sci. 2017;112:125–131. doi: 10.1016/j.rvsc.2017.02.010. [DOI] [PubMed] [Google Scholar]
- Kang Z.W., Jung J.H., Kim S.H., Lee B.K., Lee D.Y., Kim Y.J., Lee J.Y., Won H.K., Kim E.H., Hahn T.W. Genotypic and phenotypic diversity of Salmonella Enteritidis isolated from chickens and humans in Korea. J. Vet. Med. Sci. 2009;71:1433–1438. doi: 10.1292/jvms.001433. [DOI] [PubMed] [Google Scholar]
- Kim J.S., Yun Y.S., Kim S.J., Jeon S.E., Lee D.Y., Chung G.T., Yoo C.K., Kim J., PulseNet Korea Working G. Rapid emergence and clonal dissemination of CTX-M-15-producing Salmonella enterica serotype Virchow, South Korea. Emerg. Infect. Dis. 2016;22:68–70. doi: 10.3201/eid2201.151220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.J., Moon J.S., Oh D.H., Chon J.W., Song B.R., Lim J.S., Heo E.J., Park H.J., Wee S.H., Sung K. Genotypic characterization of ESBL-producing E. coli from imported meat in South Korea. Food Res. Int. 2018;107:158–164. doi: 10.1016/j.foodres.2017.12.023. [DOI] [PubMed] [Google Scholar]
- Kim Y.J., Oh D.H., Song B.R., Heo E.J., Lim J.S., Moon J.S., Park H.J., Wee S.H., Sung K. Molecular characterization, antibiotic resistance, and virulence factors of Methicillin-Resistant Staphylococcus aureus strains isolated from imported and domestic meat in Korea. Foodborne Pathog. Dis. 2015;12:390–398. doi: 10.1089/fpd.2014.1885. [DOI] [PubMed] [Google Scholar]
- Kuo H.C., Lauderdale T.L., Lo D.Y., Chen C.L., Chen P.C., Liang S.Y., Kuo J.C., Liao Y.S., Liao C.H., Tsao C.S., Chiou C.S. An association of genotypes and antimicrobial resistance patterns among Salmonella isolates from pigs and humans in Taiwan. PLoS One. 2014;9:e95772. doi: 10.1371/journal.pone.0095772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.K., Choi D., Chon J.W., Seo K.H. Resistance of strains producing Extended-Spectrum beta-Lactamases among Salmonella from duck carcasses at slaughterhouses in three major provinces of South Korea. Foodborne Pathog. Dis. 2016;13:135–141. doi: 10.1089/fpd.2015.2042. [DOI] [PubMed] [Google Scholar]
- Lu X., Li Z.P., Yan M.Y., Pang B., Xu J.L., Kan B. Regional transmission of Salmonella Paratyphi A, China, 1998-2012. Emerg. Infect. Dis. 2017;23:833–836. doi: 10.3201/eid2305.151539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majowicz S.E., Musto J., Scallan E., Angulo F.J., Kirk M., O'Brien S.J., Jones T.F., Fazil A., Hoekstra R.M., Burd I.C.E.D. The global burden of Nontyphoidal Salmonella gastroenteritis. Clin. Infect. Dis. 2010;50:882–889. doi: 10.1086/650733. [DOI] [PubMed] [Google Scholar]
- Oh J.Y., Kang M.S., An B.K., Song E.A., Kwon J.H., Kwon Y.K. Occurrence of purulent arthritis broilers vertically infected with Salmonella enterica serovar Enteritidis in Korea. Poult. Sci. 2010;89:2116–2122. doi: 10.3382/ps.2010-00918. [DOI] [PubMed] [Google Scholar]
- Okoro C.K., Kingsley R.A., Connor T.R., Harris S.R., Parry C.M., Al-Mashhadani M.N., Kariuki S., Msefula C.L., Gordon M.A., de Pinna E., Wain J., Heyderman R.S., Obaro S., Alonso P.L., Mandomando I., MacLennan C.A., Tapia M.D., Levine M.M., Tennant S.M., Parkhill J., Dougan G. Intracontinental spread of human invasive Salmonella Typhimurium pathovariants in sub-Saharan Africa. Nat. Genet. 2012;44:1215–1221. doi: 10.1038/ng.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park A.K., Shin E., Kim S., Park J., Jeong H.J., Chun J.H., Hwang K.J., Kim J. Traveller-associated high-level ciprofloxacin-resistant Salmonella enterica Serovar Kentucky in the Republic of Korea. J. Glob. Antimicrob. Resist. 2019;22:190–194. doi: 10.1016/j.jgar.2019.12.014. [DOI] [PubMed] [Google Scholar]
- Park J.H., Kim H.S., Yim J.H., Kim Y.J., Kim D.H., Chon J.W., Kim H., Om A.S., Seo K.H. Comparison of the isolation rates and characteristics of Salmonella isolated from antibiotic-free and conventional chicken meat samples. Poult. Sci. 2017;96:2831–2838. doi: 10.3382/ps/pex055. [DOI] [PubMed] [Google Scholar]
- Shang K., Wei B., Jang H.K., Kang M. Phenotypic characteristics and genotypic correlation of antimicrobial resistant (AMR) Salmonella isolates from a poultry slaughterhouse and its downstream retail markets. Food Control. 2019;100:35–45. [Google Scholar]
- Ta Y.T., Nguyen T.T., To P.B., Pham D.X., Le H.T.H., Thi G.N., Alali W.Q., Walls I., Doyle M.P. Quantification, serovars, and antibiotic resistance of Salmonella isolated from retail raw chicken meat in Vietnam. J. Food Prot. 2014;77:57–66. doi: 10.4315/0362-028X.JFP-13-221. [DOI] [PubMed] [Google Scholar]
- Tamang M.D., Nam H.M., Kim T.S., Jang G.C., Jung S.C., Lim S.K. Emergence of Extended-Spectrum beta-Lactamase (CTX-M-15 and CTX-M-14)-producing nontyphoid Salmonella with reduced susceptibility to ciprofloxacin among food animals and humans in Korea. J. Clin. Microbiol. 2011;49:2671–2675. doi: 10.1128/JCM.00754-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlfall E.J., Frost J.A., Ward L.R., Rowe B. Increasing spectrum of resistance in multiresistant Salmonella typhimurium. Lancet. 1996;347:1053–1054. doi: 10.1016/s0140-6736(96)90199-3. [DOI] [PubMed] [Google Scholar]
- USDA-FSIS. 2014. Isolation and identification of Salmonella from meat, poultry, pasteurized egg, and catfish products and carcass and environmental sponges.