Abstract
The changes in lipid properties of duck egg yolks during processing may affect the quality of egg yolks. In this paper, various physicochemical and instrumental methods were used to study the changes of lipid characteristics of duck egg yolks under extreme processing conditions such as high salt, high salt-heat synergy and strong alkali. The results showed that both the high salt and high salt-heat treatments resulted in the decrease of the moisture content and the increase of the oil exudation of egg yolks. The iodine value of the lipid extracted from salted egg yolks with or without heat treatment decreased. However, strong alkali treatment increased the moisture content of egg yolks, and the oil exudation increased at first and then decreased. The iodine value of the lipid obtained from preserved egg yolks showed an overall trend with first increase and then decrease, and the saponification value of the lipid got from preserved egg yolks was lower than the lipid got from the raw salted egg yolks. According to the conjugated diene acid value and thiobarbituric acid value, the lipid of egg yolks was oxidized to different degrees under the three processing conditions. At the end of pickling, the fatty acid content of the lipid acquired from egg yolks all increased. Therefore, all three extreme treatments significantly changed the lipid properties of duck egg yolks.
Keywords: salt, salt-heat synergy, strong alkali, egg yolk, lipid
INTRODUCTION
Duck eggs are rich in lipids, which account for 11.40% to 13.52% of the whole egg (Ganesan et al., 2014). The lipids mainly derive from the yolk, which account for about 30% of the fresh weight and 60% of the dry matter of the yolk (El-Bagir et al., 2006). Yolk lipids mainly consist of 62% triglycerides, 33% phospholipids and less than 5% cholesterol. The unsaturated fatty acids in egg yolks commonly possessed such functions: 1) Enhancing antioxidant capacity. 2) Anticancer effect. 3) Forming membrane lipids and regulating membrane-dependent progression (Xiao et al., 2020). For example, monounsaturated fatty acids in egg yolks are beneficial for preventing heart disease; docosahexaenoic acid (DHA) and arachidonic acid in egg yolks are essential fatty acids for growth, brain function and visual development of infants (Cherian and Quezada, 2016; Fraeye et al., 2012).
In order to improve the abundance of egg products, people often use salt and strong alkali to process duck eggs into salted eggs and preserved eggs. Salted egg yolks (SEY) are popular among Chinese consumers for their gritty, aromatic, tender, oily, and nutritious characteristics. Preserved egg yolks (PEY) are also popular in China because of their texture, nutrition, and unique flavor. So the quality and characteristics of egg yolks are main factors that determine the consumer acceptance and market demand.
The microstructure, texture and physicochemical properties of egg yolks during curing (with salt or strong alkali) have been reported in some researches. For instance, (Kaewmanee et al., 2009b) studied the effects of different salt pickling methods on the physical-chemical properties, texture and microstructure of duck eggs. (Ganasen and Benjakul, 2010) studied the physical properties and microstructure of pidan (preserved egg) yolk affected by different divalent and monovalent cations. They concluded that different salt and alkali pickling methods would affect the physical-chemical properties, texture and microstructure of duck eggs. However, there were few studies on the changes of lipid characteristics of egg yolks under different processing conditions. The oxidation of lipid not only affects the human health, but also affects the flavor and nutritional value of egg yolks. Therefore, this paper carried out a comprehensive study on the changes in the oxidation characteristics and other physical-chemical characteristics of yolk lipid under 3 different processing conditions of high salt, high salt-heat synergy and strong alkali. It would provide a theoretical basis for the influence of yolk lipid on the quality and gelation of egg yolks.
MATERIALS AND METHODS
Materials and Reagents
Fresh duck eggs were obtained from Jiangxi Tianyun Agricultural Development Co., Ltd. Salt, sodium hydroxide (NaOH) and copper sulfate (CuSO4) were food grade products, which were purchased from China National Salt Industry Corporation, Tianjin Tianshun Alkali Industry Co., Ltd. and Jiangsu Kolod Food Ingredients Co., Ltd, respectively. The rest of the chemical reagents were of analytical grade and were purchased from Sinopharm Chemical Reagent Co., Ltd.
Sample Preparation
Duck eggs were checked for cracks and graded before being marinated. After checking and grading, the eggs were cleaned with tap water and were dried with air. Then the duck eggs (about 65–70 g) were taken for marinating. The salted eggs were made by completely soaking the duck eggs in the pickling solution containing salt (20%). The formula of preserved eggs pickling solution was: sodium hydroxide (4.5%), copper sulfate (0.4%) and sodium chloride (4.0%) (Zhao et al., 2014b). Eggs were soaked in different pickling solutions at room temperature 25 to 30°C for 6 wk.
During processing, 6 eggs were collected at 0, 7, 14, 21, 28, 35, 42 d from 3 treatment conditions for determination and analysis, respectively. Raw salted egg yolks (RSEY) and PEY were directly separated from egg whites manually. Cooked salted egg yolks (CSEY) were separated from egg whites manually after boiled in boiling water for 10 minutes. Each of the 3 yolks (RSEY, CSEY and PEY) were collected in each beakers for further analyses.
Determination of Moisture Content
The moisture content in egg yolks was determined according to the analytical method AOAC 920.116 (AOAC, 2000). Moisture content was determined by drying the samples in a hot-air oven at 100 ± 5˚C until constant weight.
Determination of Hardening Ratio
The hardening ratio was determined by using the method described by Chi and Tseng (1998). Egg yolks were manually separated by removing residuals from the egg whites. The weight of the whole yolk was recorded as W0. The yolk was cut with a knife and the soft part inner the yolk was scraped out with a medicine spoon. The remaining yolk was recorded as W1. The hardening ratio was calculated as follows: Hardening ratio (%) = W1/W0*100%.
Determination of Oil Exudation
Oil exudation of egg yolks was measured according to the method of Xu et al. (2017) with a slight modification. Yolks (5 g) were homogenized with 25 mL distilled water by using an IK homogenizer (T18, German IKA Company, Germany) at 9000 rpm for about 30 s. The homogenate was centrifuged (TGL-20B, Shanghai Anting Scientific Instrument Factory, China) at 7500 rpm for 30 min. Then 25 mL of organic solution (n-hexane: isopropanol = 3:2, v/v) was added to the supernatant to dissolve the float. The top solvent-lipid layer was separated by using a separating funnel. Most of the solvent of the filtrate evaporated in a water bath at 55°C and then the residue was dried to constant weight in an oven at 105°C. The residue was weighed and then the free lipid content (%) was calculated.
In addition, yolks (3 g) were mixed with 20 mL of organic solvent (n-hexane: isopropanol = 3:2, v/v), then were homogenized at 9000 rpm for 1 min. The homogenate was filtered with a filter paper and the most of solvent evaporated in a 55°C water bath. Then the residue was dried at 105°C to constant weight. The residue was weighed and taken as the total lipid content (%). Oil exudation was calculated based on the following formula: Oil exudation = Free lipid content/Total lipid content*100%.
Extraction of Lipid From Egg Yolk
Lipid was extracted from the egg yolks following the method of Folch et al. (1957) with a slight modification. The egg yolks were crushed using a blender and the sample (20 g) was mixed with 300 mL of CM solution (chloroform: methanol = 2: 1, v/v). The mixture was shaken for 3 h with a shaking table and then was filtered. Sodium chloride solution (0.6 mol/L) was added to the filtrate. The chloroform phase (bottom phase) was sucked by a pipette after stratification. Sodium sulfate anhydrous was added to the chloroform phase and the mixture was shaken thoroughly to remove residual water. Lipid in chloroform was decanted into a round-bottom flask through a filter paper and evaporated at 40°C by using a rotary evaporator (RE-52A, Shanghai Yarong Biochemical Instrument Factory, China). The residual solvent was removed on nitrogen evaporator system (MD200, Hangzhou Aosheng Instrument Co., Ltd., China). The lipid was kept in an amber vial at −20°C until analysis.
Determination of Iodine Value
Iodine value was determined according to International Standard ISO 3961-2018 (ISO, 2018). Yolk lipid (about 0.2 g) was added to 20 mL mixture of cyclohexane and glacial acetic acid (1:1, v/v) and 25 mL Wijs reagent. Then the mixture was shaken thoroughly and placed in the dark for 1 h. After 1 h, KI solution (20 mL, 100 g/L) and water (150 mL) were added into the mixture, then were titrated with the calibrated sodium thiosulfate standard solution (c(Na2S2O3·5H2O ) =0.1 mol/L) until the yellow color of the solution nearly disappeared. Subsequently, a few drops of starch solution were added and continuously titrated until the blue disappeared. The blank test was done without adding sample. The iodine value was calculated according to the following formula:
Where W1 is the iodine value of the sample (g/100 g); c is the concentration of sodium thiosulfate standard solution (mol/L); V1 is the volume of the blank solution consuming sodium thiosulfate standard solution (mL); V2 is the volume of the sample solution consuming sodium thiosulfate standard solution (mL); m is the weight of sample (m).
Determination of Saponification Value
Saponification value was determined according to International Standard ISO 3657-2020 (ISO, 2020). Potassium hydroxide (KOH)-ethanol solution (25 mL, 0.5 mol/L) was added into 1.5 g yolk lipid. Next, the reflux condensing tube was connected with the Erlenmeyer flask and the Erlenmeyer flask was heated to keep the solution boiling for 1 h. Then 0.5 to 1 mL phenolphthalide reagent was added into the hot solution, was titrated with hydrochloric acid standard solution (0.5 mol/L) until the pink of indicator disappeared. Blank test was done without adding sample. The saponification value of the sample was calculated according to the following formula:
Where Is is the saponification value (in KOH) (mg/g), V0 is the volume of standard hydrochloric acid solution consumed in blank test (mL), V1 is the volume of standard hydrochloric acid solution consumed by the sample (mL), c is the actual concentration of hydrochloric acid standard solution (mol/L), m is the weight of sample (g).
Determination of Conjugated Diene Acid Value
According to the method of Coutron-Gambotti et al. (1999) with slight modification, egg yolk lipid (2.5 mg) (Analytical balance, BSA224S, Sartorius, Germany) was dissolved in 20 mL of cyclohexane. The absorbance was measured at 232 nm and 215 nm (NS810, Shenzhen Threenh Technology Co., Ltd., China). Conjugated diene acid (CDA) value was the ratio of absorbance at 232 nm to 215 nm.
Determination of Thiobarbituric Acid Value
Thiobarbituric acid (TBA) value was determined according to the analytic methods specified by AOCS Official Method Cd 19-90 (AOCS, 2009). Egg yolk lipid (200 mg) was dissolved in 1-butanol solution and was diluted to 25 mL. Sample solution (5 mL) was taken to the lab graduated glass test tube with ground-in glass stopper with pipette and 5 mL TBA reagent was added. The mixture was heated for 2 h in 95°C water bath to turn pink color. Then it was cooled to room temperature with running water. The absorbance of sample solution (A) was measured at 530 nm after it was calibrated to spectrophotometer zero point with distilled water. The blank test was done without adding sample. The absorbance of the blank solution was B. The value of TBA was calculated as follows:
Where A is the absorbance of the test solution, B is the absorbance of the reagent blank, m is the mass of the sample (mg), 50 is a valid factor if the volume of the volumetric flask is 25 mL and the cuvette width is 10 mm.
In the case of low blank values (0.05), the absorbance of the test solution can be measured directly against distilled water. The value is then calculated as follows:
Determination of the Type and Content of Fatty Acids in Yolk
Fatty acids were determined according to the analytic methods specified by the Chinese standard GB 5009.168-2016 (China, 2016).
Methylation of fatty acids.
The sodium hydroxide-methanol solution was added to the extracted lipid, and reflux condenser was connected. After the oil droplets disappeared, the boron trifluoride-methanol solution was added to the upper end of the reflux condenser. The extraction solution was poured into the sample bottle for determination after it was extracted by n-heptane (Gas chromatograph, Agilent 6890N, Agilent).
Chromatographic conditions.
Capillary chromatographic column: strong polar stationary phase of polydicyanopropyl siloxane, column length: 100 m, inner diameter: 0.25 mm, film thickness: 0.2 μm; injector temperature: 270°C, detector temperature: 280°C; programmed temperature: initial temperature: 100°C, lasting for 13 min; 100 to 180°C, heating rate: 10°C/min, lasting for 6 min; 180 to 200°C, heating rate: 1°C/min, lasting for 20 min; 200 to 230°C, heating rate: 4°C/min, lasting for 10.5 min. Carrier gas: nitrogen, split ratio: 100:1, injection volume: 1.0 μL.
Statistical Analysis
The experiments were performed in triplicate. All data were expressed as mean values and SD. Statistical analysis was performed using statistical program SPSS 25 for Windows (SPSS Inc., Chicago, IL). One-way analysis of variance (ANOVA) was carried out, and means comparisons were made by Duncan's multiple range tests. Significance differences were determined at P < 0.05. The experiment results were plotted using origin 2018 software.
RESULTS AND DISCUSSION
Changes in Moisture Content of Egg Yolks Under Extreme Processing Conditions
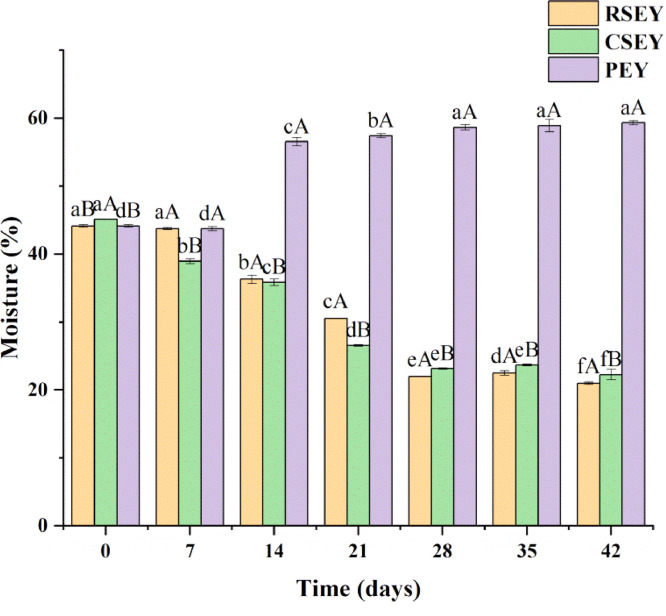
The moisture content of egg yolks under extreme processing conditions were shown in Figure 1. During 7 to 28 days of curing with salt, the moisture content of egg yolks in salted eggs decreased significantly (P < 0.05); after they were pickled for 28 d, the moisture content tended to be stable. From the beginning to the end of pickling, the moisture content of the RSEY and CSEY decreased from 44.15% and 45.13% to 22.00% and 23.18%, respectively. During 7 to 28 d of pickling, the moisture content of CSEY had lower value than that of RSEY (P < 0.05). While the moisture content of CSEY was higher than that of RSEY (P < 0.05) during the period of 28 to 42 d. During 7 to 14 d of strong alkali pickling, the moisture content of PEY increased from 43.73% to 56.57% (P < 0.05) and then remained stable after 14 d of pickling; in the meanwhile, the moisture content was significantly higher in egg yolks after alkali treatment than salt treatment (P < 0.05).
Figure 1.
Moisture content of duck egg yolks under extreme processing conditions during 6 wk of pickling. Bars represent standard deviation from triplicate determinations. Different letters on the bar indicate significant differences (P < 0.05).
The decrease in moisture content of SEY closely related to the water migration. Under high osmotic pressure of salt pickling, the moisture migrated from the egg yolk to egg white and then transfer through the eggshell to the environment (Chi and Tseng, 1998). In addition, the effect of salt can separate the hydrophilic groups and lipophilic groups in the egg yolk, which resulted in that the water in egg yolk to flow more to the egg white (Xu et al., 2017). According to the study of Kaewmanee et al. (2011), the solidification of egg yolk during the pickling process can hinder the migration of NaCl, so the moisture content tended to be stable in the later stage of pickling. During 0 to 28 d of pickling, the difference in moisture content in RSEY and CSEY probably resulted from that heating accelerated the motion of water molecules and promoted the migration of water from egg yolk to egg white. After 28 d of pickling, the egg yolks were dehydrated and hardened. The water fluidity became weak and the effect of heat treatment on water flow was reduced. As a result, the hydrophilic groups separated by salt were trapped in the yolk, resulting in higher moisture content in the CSEY than in the RSEY after 28 d of pickling.
The moisture content of the PEY increased significantly from the 7th day and remained higher than the SEY, which might be assigned to the gradual infiltration of lye through the egg shell to egg white, then to the egg yolk. The concentration of alkali in egg yolks gradually increased (Yang et al., 2019). Under alkaline conditions, the proteins in egg yolks were cross-linked with each other (Chanarat et al., 2012). To some extent, the cross-linked proteins might prevent the yolks from dehydrating (Yang et al., 2019). During the pickling period of 7 to 14 d, the penetration rate of alkali solution was the fastest, and then the alkali concentration stabilized in egg yolk due to that the metal ions blocked the eggshell holes.
Changes in Yolk Hardening Ratio Under Extreme Processing Conditions
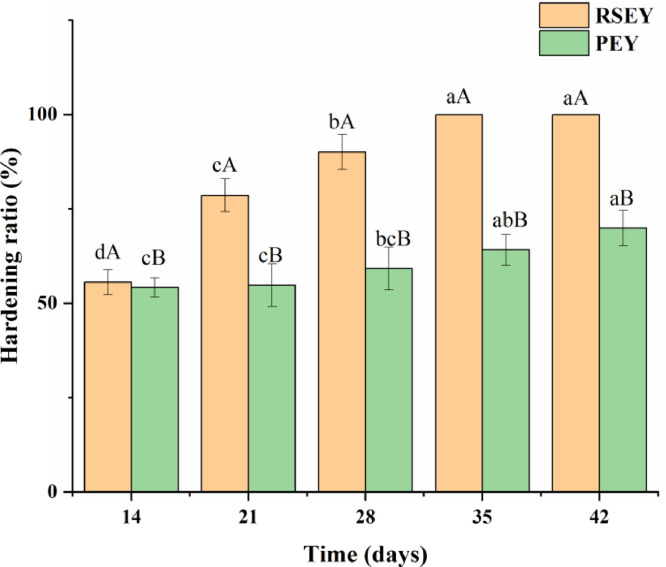
The hardening rate of egg yolk was defined as the weight percentage of hard outer yolk, which was one of the indexes to judge whether the egg yolk was mature (Chi and Tseng, 1998). Since the yolks did not harden during the first 2 weeks of the treatment with strong alkali and high salt, so the data of the hardening rate were shown after 2 wk of the treatments. As shown in Figure 2, the hardening rate of egg yolks gradually increased, irrespective of which kinds of treatments. Under conditions of high salt treatment, the egg yolks gradually hardened and the hardening rate of the SEY rapidly reached to 55.56% after pickling for 2 wk and 100% at the end of pickling. After 7 d of pickling with strong alkali, the outer of the PEY began to form a hard layer and inner of the yolks became viscous. From the 14th day to the end of pickling, the hardening rate of PEY increased from 54.19% to 69.91%. In addition, the hardening rate of SEY was always higher than that of PEY (P < 0.05).
Figure 2.
Hardening ratio of duck egg yolks under extreme processing conditions during 6 wk of pickling. Bars represent standard deviation from triplicate determinations. Different letters on the bar indicate significant differences (P < 0.05).
The hardening rate of the yolks increased gradually after high salt treatment. This indicated that protein aggregation caused by salt penetration in the pickling process was the main cause of the hardening of egg yolk (Ganasen and Benjakul, 2010). Kaewmanee et al. (2013) found that the linear viscoelastic behavior changed significantly by inducing sol-gel transformation when NaCl concentration increased from 0 to 3.0% (w/w). Yang and Baldwin (2017) found that adding salt to the protein suspension can also promote protein aggregation. That was because the dehydration of the egg yolk caused by osmotic pressure due to the continuous diffusion of salt (Kaewmanee et al., 2009b). Dehydration would lead to an increase in the concentration of egg yolk protein. Protein molecules, including lipoproteins, interacted with each other, which might promote the formation of protein gel and increase the hardening rate of egg yolk.
On the one hand, the reason for the increase of hardening ratio of PEY possibly was related to the denaturation and aggregation of protein molecules in egg yolks under the action of strong alkali, which led to the formation of gel network structure (Ji et al., 2013). On the other hand, cations could interact with negative charged protein molecules via a salt bridge to form a polymer, which promoted the formation of gel (Ganasen and Benjakul, 2011). The hardening rate of SEY was always higher than that of PEY, that's because Cu2+ in the pickling solution of preserved eggs could block the egg shell holes and slow down the speed of the pickling solution flowing into the eggs. Furthermore, the moisture in the PEY kept rising, while the moisture in the SEY kept falling (Figure 1), which changed the concentration of the pickling solution in the egg yolks. The hardening of SEY mainly relied on dehydration, while the hardening of PEY was mainly caused by the denaturation and aggregation of protein in yolks by strong alkali, therefore it can be seen that the dehydration effect of SEY was stronger than protein denaturation of the PEY.
Changes in Oil Exudation of Egg Yolk Under Extreme Processing Conditions
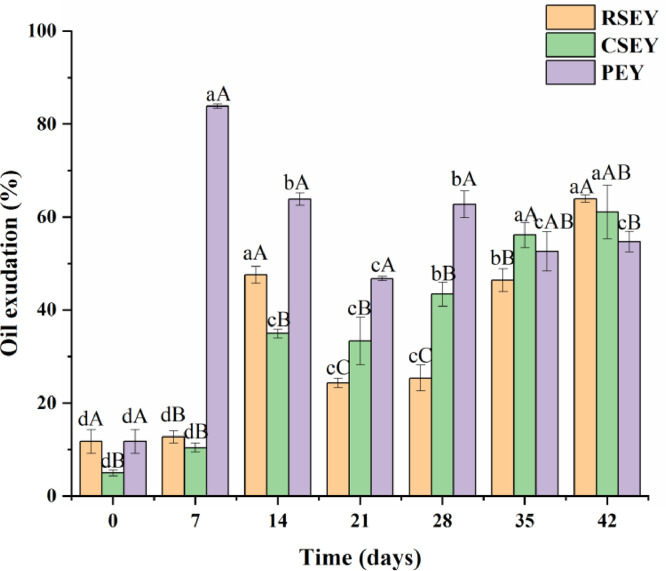
The changes in oil exudation of duck egg yolk under extreme processing conditions were monitored (Figure 3). The oil exudation of RSEY and CSEY was 11.77% and 4.99%, respectively. With the extension of pickling time, the oil exudation of RSEY increased from 11.77% to 61.13%, and the oil exudation of CSEY increased from 4.99% to 51.74%. During 21 to 35 d, the CSEY showed a significant higher oil exudation than the RSEY (P < 0.05). For the PEY, the oil exudation increased sharply in the first week (P < 0.05), reached 83.87% on d 7, and then continuously decreased to 46.78% during 7 to 21 d (P < 0.05). During the pickling period of the first 5 wk, the oil exudation of the egg yolks treated with strong alkali was higher than the egg yolks treated with salt.
Figure 3.
Oil exudation of egg yolk under extreme processing conditions during 6 wk of pickling. Bars represent standard deviation from triplicate determinations. Different letters on the bar indicate significant differences (P < 0.05).
Free lipids referred to lipids that can be extracted by petroleum ether, n-hexane, ethyl ether and other non-polar solvents (Mccann et al., 2009). Plenty of the yolk lipids existed in plasma and were structured in low-density lipoproteins (LDL) (Yang et al., 2020). The increased oil exudation of the SEY probably was related to the dehydration effect of salt (Kaewmanee et al., 2009a). The dehydration effect could lead to the denaturation of egg yolk proteins and the loss of protein emulsification function of LDL, which would release the free lipid. Thus, the increase of salt content in egg yolks gradually increased the amount of oil exudation of egg yolks with the increase of pickling time (Lai et al., 2010). The reason why the CSEY had a higher oil exudation than RSEY after it was pickled for 21 to 35 days could lie in that some lipids in CSEY became free after being heated (Lai et al., 1997). Because heating treatment might destroy more LDL and denature other proteins that stabilized the lipid in egg yolks (Kaewmanee et al., 2011). In the process of pickling, the destruction of lipoproteins might be associated with the contraction of yolk granules, and heating treatment can further facilitate the separation of granules (Xu et al., 2017). Heating treatment would also accelerate the movement of molecules and ions and made lipids more detached from lipoproteins (Yang et al., 2016).
On the 7th day of marinating, the oil exudation of the PEY reached maximum possibly owing to the fact that the eggshell holes had not been completely blocked in the early stage, during which the lye penetrated with the fastest rate. What's more, alkaline conditions can disrupt hydrophobic interactions so that lipids released from LDL could not be recombined and accumulated (Anton, 2013). Then the decrease in oil exudation was probably due to the saponification reaction between free lipids and alkali. During the last weeks of pickling, the dynamic balance between the oil release rate and saponification rate was maintained as the alkali concentration in the yolks did not vary significantly in this period, so the oil exudation tended to be stable gradually (Yang et al., 2019). In the first 5 wk of pickling, the oil exudation of yolks after alkali treatment was higher than salt treatment, which indicated that alkali treatment was more likely to cause lipoprotein destruction and release more free lipids than salt treatment.
Changes in Iodine Value of Egg Yolk Lipid Under Extreme Processing Conditions
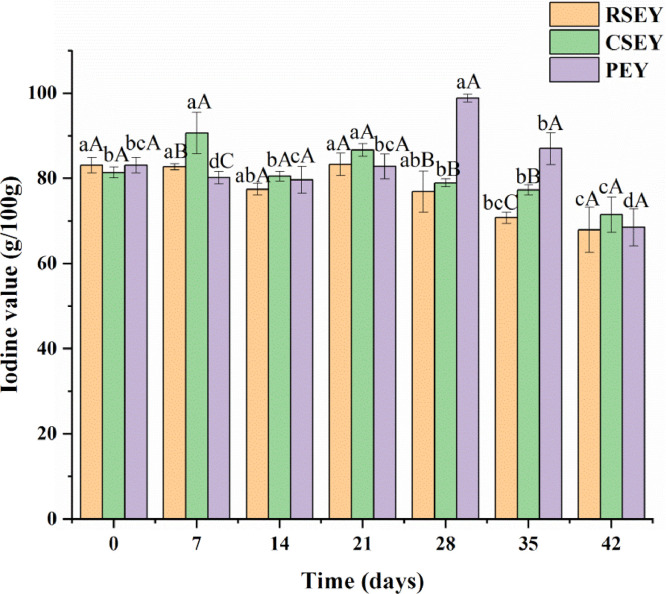
Figure 4 exhibited the variation of iodine value of egg yolk lipid under extreme processing conditions. The iodine value of lipid obtained from RSEY and CSEY generally showed a downward trend, from 83.10 g/100 g and 81.42 g/100 g before pickling to 67.92 g/100 g and 71.48 g/100 g at the end of pickling, respectively. It showed a significant decrease after 21 d (P < 0.05). The lipid extracted from CSEY had a higher iodine value than that extracted from RSEY during the pickling process. As a whole, the iodine value of PEY lipid increased first and then decreased, reaching the maximum value of 98.86 g/100 g at the 28th day of pickling and decreased significantly after 28 days (P < 0.05).
Figure 4.
Iodine value of egg yolk lipid under extreme processing conditions during 6 wk of pickling. Bars represent standard deviation from triplicate determinations. Different letters on the bar indicate significant differences (P < 0.05).
Iodine value was defined as the number of grams of iodine absorbed by 100g oil and was an indicator of the degree of oil unsaturated (Haryati et al., 1998). Low iodine value meant low content of unsaturated fatty acids in lipid. The decrease of iodine value indicated that the lipid was oxidized (Ajith et al., 2015). Oxidation was composed of a series of complex chemical reactions that was characterized by the removal of hydrogen adjacent to the double bond and the formation of free radicals, resulting in the decrease in the total unsaturated content of oil (Naz et al., 2004).
The iodine value of SEY lipid and PEY lipid decreased significantly after 21 d and 28 d of pickling respectively, indicating that the lipids of SEY and PEY were oxidized and degraded at the later stage of pickling, which made the number of double bonds in lipid and the degree of lipid unsaturation keep declining. Study (Haryati et al., 1998) had shown that the increase of saturated triglyceride content would decrease the iodine value. Thus, the decrease of iodine value of three yolk lipids in the later period of pickling might be related with the increase of saturated triglyceride. The higher iodine value of CSEY was probably because heating degraded the lipid to produce some long-chain unsaturated fatty acids. The increase of iodine value of PEY lipid after 7 d of pickling might be in connection with the accumulation of unsaturated fatty acids produced by lipid degradation during this period.
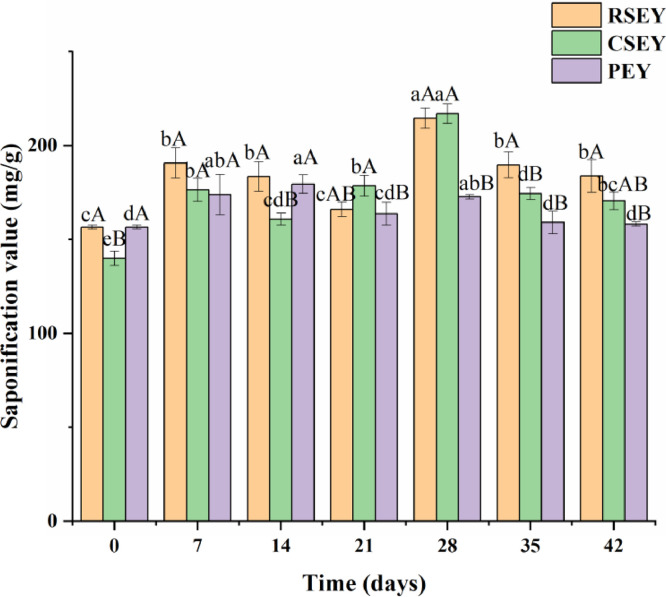
Changes in Saponification Value of Egg Yolk Lipid Under Extreme Processing Conditions
As can be seen in Figure 5, the saponification value of lipid from RSEY and CSEY presented an "M-type" trend, but there was no specific regularity between the saponification value of RSEY and CSEY lipid. The lipid extracted from cooked fresh duck egg yolks showed a lower saponification value than lipid extracted from raw fresh duck egg yolks, which were 131.59 mg/g and 156.57 mg/g, respectively. The variation trend of PEY lipid was similar to that of RSEY and CSEY lipid. A lower saponification value was found in PEY lipid than the RSEY lipid, and the difference was significant after 28 d of pickling (P < 0.05).
Figure 5.
Saponification value of egg yolk lipid under extreme processing conditions during 6 wk of pickling. Bars represent standard deviation from triplicate determinations. Different letters on the bar indicate significant differences (P < 0.05).
Saponification usually referred to the reaction of alkali (usually a strong alkali) and ester to produce alcohol and carboxylate, especially referred to the hydrolysis of lipid. Saponification value was associated with the number of milligrams of KOH needed to saponify 1 g of oil (Odoom and Edusei, 2015). The saponification value can indirectly reflect the fatty acid composition and glycerol content of the lipid. At the same time, the saponification value of oil can be used to calculate the technical parameters of related derivatives based on oil. The saponifiable substances in oil included fatty acid glycerides and free fatty acids. The saponification value was connected with the chemical composition of the glycerides and the molecular weight of the fatty acids that made up the oil. Generally speaking, the higher the saponification value was, the smaller the molecular weight of fatty acids was, and vice versa (Zhang et al., 2012). The saponification value of yolk lipid increased twice under three different processing methods, which was probably caused by the accumulation of free fatty acids produced by hydrolysis in the early stage of pickling. In the later stage of pickling, a large amount of phospholipid was dissolved, resulting in that the average molecular weight of lipid decreased and the saponification value increased (Zhang et al., 2012). However, the saponification value decreased twice, which might be caused by the production of some monoglyceride, diglyceride and unsaponified substances. The saponification value of cooked fresh duck egg yolk lipid was lower than that of raw fresh duck egg yolk lipid, indicating that the yolk fatty acids can be polymerized by heating treatment. The lower saponification value of PEY lipid was perhaps associated with the saponification of PEY lipid during the pickling process with strong alkali.
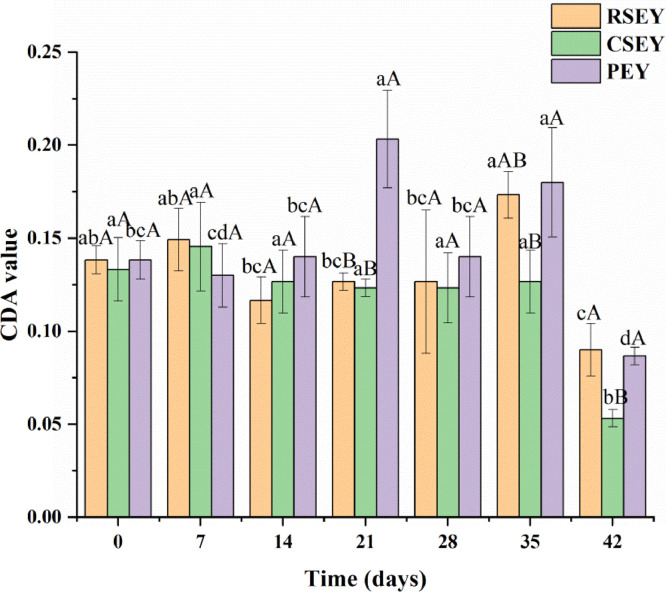
Changes in CDA Value of Egg Yolk Lipid Under Extreme Processing Conditions
As can be seen from Figure 6, the CDA value of RSEY and CSEY lipid did not change significantly before 35 d of pickling, but decreased significantly after 35 days of pickling (P < 0.05). After pickling for 21 d, the CDA value of RSEY lipid was higher than that of CSEY lipid. The CDA value of yolk lipid in preserved eggs showed no significant regularity during pickling, but decreased significantly (P < 0.05) after 35 d. The CDA value of PEY lipid was always higher than that of RSEY lipid from the beginning of 14th day except the 42th day (P > 0.05).
Figure 6.
Conjugated dienoic acid (CDA) value of egg yolk lipid under extreme processing conditions during 6 wk of pickling. Bars represent standard deviation from triplicate determinations. Different letters on the bar indicate significant differences (P < 0.05).
CDA value was an indicator of the degree of hydroperoxides of polyunsaturated fatty acids (PUFA), reflecting the degree of primary oxidation of lipid (Gordon, 2001). In the early stage of pickling by high salt and strong alkali, the CDA value of yolk lipid did not change significantly, which was possibly related to the phospholipids in egg yolks. Kim and Choe (2008) pointed out that the CDA value of fried dough with yolk powder was lower than that without yolk powder, because the phospholipids in the yolks can reduce the oxidation of frying lipid in the frying process to a certain extent. Therefore, the oxidation rate of yolk lipid might be inhibited by the action of phospholipids in the early stage of pickling. After pickling for about 35 d, more lipids were released from the egg yolk. Under the action of high salt and strong alkali, the content of hydroperoxide accumulated by the oxygenolysis of free fatty acids and phospholipids reached the maximum. However, after 35 d, the exudation rate of the egg yolk lipid slowed down and the accumulated lipid oxidation products further achieved secondary oxidation. So after 35 d of pickling, the CDA value of all three kinds of egg yolk lipid showed a downtrend. After pickling for 21 d, the lipid extracted from RSEY exhibited a higher CDA value than that extracted from CSEY, which resulted from the decomposition of some accumulated hydroperoxides caused by heating. The higher CDA value of PEY lipid from d 14 was probably because alkali treatment could cause the isomerization of linoleic acid and linolenic acid, and increase the CDA value (Bachari et al., 2013).
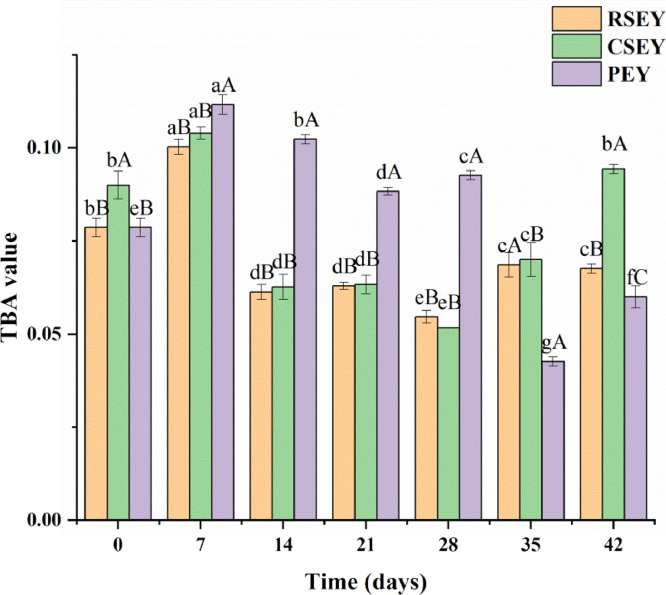
Changes in TBA Value of Egg Yolk Lipid Under Extreme Processing Conditions
The TBA values of yolk lipid with the three processing methods showed a trend of first rising and then falling and finally rising (Figure 7). The TBA value of SEY lipid increased with the passage of the pickling time after the 28th day (P < 0.05). The TBA value of PEY lipid was higher than that of RSEY lipid during the pickling period of 0 to 28 d (P < 0.05). As shown in the results, after 2 weeks of pickling, the variation trend of TBA value was opposite to the trend of CDA value in the most cases (Figure 6). This indicated that the oxidation intermediate products represented by CDA content was decomposed to the final oxidation products, as monitored by TBA value (Kaewmanee et al., 2009b).
Figure 7.
2-thiobarbituric acid (TBA) value of egg yolk lipid under extreme processing conditions during 6 wk of pickling. Bars represent standard deviation from triplicate determinations. Different letters on the bar indicate significant differences (P < 0.05).
Lipid oxidation products such as malondialdehyde can be produced by polyunsaturated fatty acids with at least three double bonds and their concentrations can be determined by reaction with thiobarbituric acid (Gordon, 2001). The variations of TBA values of all three kinds of yolk lipids were consistent with the trend of free lipid released by the yolk during the pickling period (Figure 3). In general, the amount of lipid content was positively correlated with the degree of lipid oxidation (Estévez et al., 2005; Min and Ahn, 2005; Sasaki et al., 2001). Another explanation for the increase of TBA value of lipid extracted from SEY after 28 days pickling was that salt can promote the oxidation of lipid to a certain degree. Salt was regarded as a pro-oxidant, which could accelerate the lipid oxidation in some studies of the lipid oxidation (Coutron-Gambotti et al., 1999; Higgins, 1999; Kanner et al., 1991). Kanner et al. (1991) pointed out that when the addition concentration of NaCl was within 0.1 to 1.0 M, the overall effect was within the range of pro-oxidant. According to our previous study (Xu et al., 2017), salt gradually penetrated into the egg yolk liquid as pickling time went by. In the late stage of pickling, the salt content in the SEY reached nearly 2%, just within the range of promoting oxidation of NaCl. In addition, Kanner's research (Kanner et al., 1991) showed that NaCl could interfere with the ability of iron ions to chelate with proteins, so that more free iron ions could interact with lipids and facilitate lipid peroxidation. In the early stage of pickling, there was no significant change in TBA value, which was perhaps due to the strong binding capacity of the yolk to iron. Moreover, the salt concentration into the yolk was low in the early stage. There was no significant difference between TBA value of CSEY lipid and RSEY lipid, which was because the amount of NaCl in the CSEY was not significantly different from that in RSEY (Xu et al., 2017). The higher TBA value of lipid extracted from PEY during 7 to 28 d of pickling was probably because the free lipid released from PEY was more than that released from SEY during this period.
Changes in the Type and Content of Fatty Acids of Egg Yolk Lipid Under Extreme Processing Conditions
Sixteen kinds of fatty acids were found in egg yolk lipid treated by high salt, high salt-heat synergy and strong alkali respectively, including myristic acid (C14:0), palmitic acid (C16:0), palmitoleic acid (C16:1), stearic acid (C18:0), elaidic acid (C18:1n9t), oleic acid (C18:1n9c), linoleic acid (C18:2n6c), γ-linolenic acid (C18:3n6), cis-11-eicosenoic acid (C20:1n9), α-linolenic acid (C18:3n3), cis-11,14-eicosenoic acid (C20:2), behenic acid (C22:0), cis-8,11,14-eicosenoic acid (C20:3n6), arachidonic acid (C20:4n6), tricosanoic acid (C23:0) and cis-4,7,10,13,16,19-docosahexaenoic acid (C22:6n3).
After pickling, the fatty acid content of RSEY, CSEY and PEY increased from 175.0047 mg/g, 168.8498 mg/g and 175.0047 mg/g to 255.034 mg/g, 262.1796 mg/g and 245.4914 mg/g, respectively. However, the regularity of contents of saturated fatty acids, monounsaturated fatty acids, and PUFA was not obvious. As shown in Table 1, palmitic acid (C16:0) and stearic acid (C18:0) were the most abundant in saturated fatty acids, oleic acid (C18:1n9c) was the most abundant in monounsaturated fatty acids, linoleic acid (C18:2n6c) and arachidonic acid (C20:4n6) were the most abundant in PUFA, no matter what treatments were used. The fatty acids of SEY after heating were slightly higher than those of unheated SEY. In addition, the fatty acids of yolk lipid after pickling with alkali were slightly lower than those of yolk lipid after pickling with salt. The fatty acids in RSEY lipid and CSEY lipid peaked at wk 4, while those in PEY peaked at wk 5 and then decreased slightly.
Table 1.
Changes in the type and content of fatty acids of egg yolk lipid under extreme processing conditions.
| Fatty acid |
Fatty acid content (mg/g) |
|||||||
|---|---|---|---|---|---|---|---|---|
| 0 day | 7 d | 14 d | 21 d | 28 d | 35 d | 42 d | ||
| C14: 0 | RSEY | 0.454±0.042cA | 0.329±0.030dA | 0.446±0.014cA | 0.565±0.083bA | 0.779±0.054aA | 0.619±0.016bB | 0.613±0.064bA |
| CSEY | 0.402±0.004bcdA | 0.403±0.000dA | 0.675±0.000cdA | 0.607±0.021abA | 0.674±0.078aA | 0.586±0.057abcB | 0.552±0.027abcA | |
| PEY | 0.454±0.042cA | 0.259±0.017dA | 0.180±0.006eA | 0.000±0.000fB | 0.000±0.000fB | 0.767±0.000aA | 0.546±0.041bA | |
| C16: 0 | RSEY | 36.033±1.130eA | 33.049±0.627eB | 41.576±1.560dB | 52.929±1.563bcA | 65.117±0.848aA | 51.066±0.829cB | 54.728±2.689bA |
| CSEY | 35.629±1.568fA | 45.333±2.583eA | 48.499±4.083deA | 51.884±0.846cdA | 63.001±1.215aA | 56.653±0.225bB | 56.095±1.862bcA | |
| PEY | 36.033±1.130dA | 24.337±0.831eC | 17.992±0.921fC | 39.278±0.183dB | 44.073±1.836cB | 69.255±4.116aA | 54.525±0.630bA | |
| C18: 0 | RSEY | 10.775±0.122gB | 12.107±0.168fB | 14.226±0.117eB | 17.573±0.225cB | 20.108±0.145aB | 16.615±0.032dC | 18.427±0.206bA |
| CSEY | 11.651±0.079fA | 15.385±0.211eA | 17.501±0.456dA | 19.424±0.223bA | 21.878±0.288aA | 17.434±0.076dB | 18.569±0.127cA | |
| PEY | 10.775±0.122eB | 8.060±0.031fC | 5.679±0.008gC | 14.008±0.143cC | 13.032±0.258dC | 21.088±0.540aA | 17.847±0.180bB | |
| C22: 0 | RSEY | 0.602±0.027dA | 0.489±0.028eB | 0.686±0.043cB | 0.987±0.034aA | 0.974±0.029aA | 0.939±0.009aB | 0.862±0.016bA |
| CSEY | 0.559±0.018eA | 0.639±0.021dA | 0.814±0.056bcA | 0.684±0.007dB | 0.897±0.030aA | 0.774±0.026cC | 0.862±0.033abA | |
| PEY | 0.602±0.027cA | 0.371±0.007dC | 0.238±0.005eC | 0.605±0.009cC | 0.767±0.034bB | 1.054±0.018aA | 0.797±0.011bB | |
| ∑SFA | RSEY | 47.864 | 45.975 | 56.934 | 72.055 | 86.978 | 69.241 | 74.629 |
| CSEY | 48.241 | 61.760 | 67.490 | 72.599 | 86.450 | 75.447 | 76.078 | |
| PEY | 47.864 | 33.027 | 24.088 | 53.892 | 57.872 | 92.164 | 73.715 | |
| C16: 1 | RSEY | 3.292±0.102cA | 2.347±0.046dB | 2.586±0.100dB | 3.670±0.157bA | 5.126±0.078aA | 3.862±0.075bB | 3.667±0.222bA |
| CSEY | 2.962±0.158bB | 3.374±0.215bA | 3.307±0.341bA | 3.149±0.056bB | 4.177±0.115aB | 3.813±0.032aB | 3.997±0.142aA | |
| PEY | 3.292±0.102bcA | 1.605±0.065dC | 1.273±0.069dC | 2.952±0.026cB | 3.490±0.193bC | 5.646±0.396aA | 3.634±0.055bA | |
| C20: 1 | RSEY | 0.684±0.034cA | 0.691±0.049cA | 0.779±0.064cB | 1.160±0.050aA | 1.248±0.029aA | 1.198±0.035aB | 0.970±0.053bA |
| CSEY | 0.550±0.019cB | 0.901±0.037bcA | 0.950±0.062abA | 0.843±0.018abcC | 1.093±0.086aB | 0.902±0.015abcC | 0.975±0.025abA | |
| PEY | 0.684±0.034dA | 0.459±0.010eA | 0.343±0.014fC | 1.021±0.038bB | 0.797±0.043cC | 1.529±0.085aA | 0.987±0.031bA | |
| C18: 1n9t | RSEY | 0.391±0.004abA | 0.290±0.000bcA | 0.000±0.000cC | 0.517±0.000abcA | 0.579±0.019abA | 0.493±0.013aA | 0.447±0.000aB |
| CSEY | 0.000±0.000eB | 0.418±0.000dA | 0.450±0.000cA | 0.451±0.000cB | 0.528±0.010aB | 0.439±0.005dB | 0.486±0.003bA | |
| PEY | 0.391±0.004bA | 0.206±0.006cA | 0.153±0.002dB | 0.000±0.000eC | 0.000±0.000eC | 0.000±0.000eC | 0.446±0.007aB | |
| C18: 1n9c | RSEY | 95.057±1.190eA | 85.661±1.053fB | 94.733±0.812eB | 137.719±1.300bA | 153.085±0.680aA | 133.930±0.240cB | 126.266±0.744dB |
| CSEY | 84.034±0.288fB | 114.206±2.074eA | 117.514±1.702dA | 120.630±0.633cB | 147.721±1.624aB | 122.788±0.836cC | 132.554±0.063bA | |
| PEY | 95.057±1.190eA | 58.989±0.198fC | 43.129±0.340gC | 108.365±1.360cC | 102.358±1.776dC | 186.437±4.378aA | 124.680±1.165bB | |
| ∑MUFA | RSEY | 99.424 | 88.989 | 98.098 | 143.066 | 160.039 | 139.483 | 131.350 |
| CSEY | 87.545 | 118.898 | 122.220 | 125.072 | 153.519 | 127.941 | 138.012 | |
| PEY | 99.424 | 61.260 | 44.899 | 112.338 | 106.645 | 193.612 | 129.747 | |
| C18: 2n6c | RSEY | 19.057±0.184fB | 20.796±0.231eB | 24.997±0.273dB | 35.295±0.282bB | 38.057±0.132aA | 31.573±0.067cB | 31.865±0.090cA |
| CSEY | 21.115±0.086eA | 31.516±0.627cA | 31.408±0.416cA | 36.096±0.106bA | 37.009±0.369aB | 27.899±0.234dC | 31.484±0.006cA | |
| PEY | 19.057±0.184dB | 16.646±0.079eC | 11.735±0.124fC | 30.636±0.399bC | 27.824±0.341cC | 37.101±0.879aA | 28.253±0.258cB | |
| C18: 3n6 | RSEY | 0.398±0.008eB | 0.345±0.007fB | 0.495±0.018dB | 0.759±0.006aA | 0.698±0.009bA | 0.501±0.014dA | 0.585±0.012cB |
| CSEY | 0.469±0.012aA | 0.535±0.015aA | 0.565±0.015aA | 0.662±0.006aB | 0.588±0.011aB | 0.474±0.012aB | 0.624±0.004aA | |
| PEY | 0.398±0.008cB | 0.322±0.002dC | 0.219±0.005eC | 0.525±0.012aC | 0.000±0.000fC | 0.000±0.000fC | 0.480±0.010bC | |
| C18: 3n3 | RSEY | 0.885±0.005dB | 0.654±0.011fB | 0.763±0.002eB | 1.208±0.024bA | 1.248±0.028aA | 1.121±0.009cB | 1.181±0.017bA |
| CSEY | 1.025±0.009abA | 1.078±0.022bA | 1.047±0.008abA | 1.185±0.015aA | 1.231±0.015aA | 0.982±0.015abC | 1.116±0.002abB | |
| PEY | 0.885±0.005dB | 0.550±0.001eC | 0.429±0.002fC | 1.005±0.020bB | 0.929±0.015cB | 1.303±0.024aA | 0.986±0.011bC | |
| C20: 2 | RSEY | 0.517±0.020eA | 0.652±0.023dB | 0.730±0.043cB | 1.113±0.011aA | 1.094±0.023aB | 0.959±0.022bB | 0.956±0.037bA |
| CSEY | 0.508±0.006eA | 0.892±0.027cA | 0.992±0.081bA | 1.018±0.019bB | 1.182±0.008aA | 0.767±0.009dC | 0.867±0.025cB | |
| PEY | 0.517±0.020cA | 0.464±0.015cC | 0.321±0.017dC | 1.055±0.019aB | 0.764±0.036bC | 1.091±0.034aA | 0.814±0.025bB | |
| C20: 3n6 | RSEY | 0.689±0.054eB | 0.836±0.031dB | 1.252±0.056bA | 1.367±0.041aA | 1.390±0.029aA | 0.998±0.010cA | 1.270±0.040bA |
| CSEY | 0.824±0.016eA | 1.088±0.013cA | 1.305±0.069aA | 1.198±0.053bB | 1.355±0.054aA | 0.990±0.011dA | 1.209±0.041bA | |
| PEY | 0.689±0.054dB | 0.648±0.009dC | 0.411±0.016eB | 1.364±0.027aA | 0.940±0.031cB | 0.989±0.032bcA | 1.041±0.015bB | |
| C20:4n6&C23:0 | RSEY | 5.399±0.194fB | 7.057±0.176eA | 10.577±0.391bA | 8.850±0.119cA | 10.110±0.169bB | 8.081±0.124dC | 12.092±0.387aA |
| CSEY | 7.844±0.197cA | 6.619±0.089dB | 8.875±0.561bB | 8.996±0.186bA | 12.061±0.125aA | 9.000±0.129bB | 11.726±0.337aA | |
| PEY | 5.399±0.194eB | 5.455±0.104eC | 3.324±0.084fC | 7.525±0.061dB | 8.108±0.209cC | 10.212±0.441aA | 9.458±0.108bB | |
| C22: 6n3 | RSEY | 0.772±0.082cdB | 0.737±0.042dA | 0.975±0.094abA | 0.814±0.025cdA | 0.893±0.008bcB | 1.070±0.040aA | 1.106±0.067aA |
| CSEY | 1.279±0.072aA | 0.585±0.024eB | 0.728±0.060dB | 0.788±0.042cdA | 1.022±0.070bA | 0.903±0.051cB | 1.064±0.045bA | |
| PEY | 0.772±0.082cB | 0.498±0.020dC | 0.327±0.021eC | 0.555±0.014dB | 0.852±0.043cB | 1.135±0.062aA | 0.998±0.018bA | |
| ∑PUFA | RSEY | 27.717 | 31.076 | 39.789 | 49.407 | 53.489 | 44.304 | 49.055 |
| CSEY | 33.064 | 42.313 | 44.921 | 49.943 | 54.448 | 41.014 | 48.089 | |
| PEY | 27.717 | 24.582 | 16.767 | 42.665 | 39.417 | 51.830 | 42.030 | |
Different letters in a column indicate significant differences (P < 0.05). Fatty acids C20:4n6 and C23:0 are joined in the same line. It is due to the similar retention time of the two fatty acids.
Abbreviations: CSEY, cooked salted egg yolks; PEY, preserved egg yolks; RSEY, raw salted egg yolks.
The increase of fatty acid content in the lipid of RSEY and CSEY after the second week of pickling could be related to the oil exudation from the yolk (Figure 3), which led to the increase of the content of free fatty acid. The more the free lipid was, the more intense the hydrolysis of fat would be. It can be seen from saponification value that the hydrolysis of lipid was the most serious in the pickling time of 28 d (Figure 5). As the pickling time increased, the accumulation of small molecular weight fatty acids increased, so the content of fatty acids increased. Afterward, the hydrolysis rate of lipid became slow as the yolk became hard and the salt penetrated slowly (Kaewmanee et al., 2011), and the content of fatty acids decreased. The decrease of unsaturated fatty acid in PEY lipid in the first few weeks might be owing to the oxidative degradation of lipid in the early stage of pickling (Figure 7) (Zhao et al., 2014a). Endogenous lipase was the main factor that caused the degradation of lipid. Thus, the triglycerides and phospholipids in PEY lipid were hydrolyzed to free fatty acids (Luo et al., 2020). However, with the passage of pickling time, the alkalinity of PEY increased, which inhibited the activity of acid lipase in the yolk. In addition, the accumulation of small molecular weight fatty acids in the early stage would result in an increase of the content of unsaturated fatty acids in PEY in the later stage of pickling. There was no significant difference for the type of fatty acids under the three treatment conditions, indicating that heating treatment, salt treatment and alkali treatment had little effect on the type of fatty acids of egg yolk. Moreover, the type of fatty acids in the lipid of egg yolks was generally related to the type of fatty acids contained in the feed (Sinanoglou et al., 2011).
CONCLUSIONS
After 42 d of treatments under high salt, high salt-heat and strong alkali conditions, it was found that high salt and high salt-heat treatments reduced the moisture content of the yolk, while alkali treatment increased the moisture content of the yolk, indicating that alkali pickling could prevent the loss of water to some extent.
According to oxidation indexes (CDA and TBA value), all three kinds of yolk lipids oxidized during pickling. Under high salt condition, the secondary oxidation degree of yolk lipid was higher in the later stage than in the early stage. The results showed that the salt content could promote the oxidation of yolk lipid. However, the regularity of oxidation of egg yolk lipid under high salt-heat treatment was not obvious. However, under the strong alkali treatment, the yolk lipid in the early stage had a higher oxidation degree than in the later stage, which might be associated with the oil exudation.
In terms of the types and contents of fatty acids, there was no change of the variety of fatty acids, and the content of fatty acids increased during the pickling process under three different treatment conditions. It indicated that high salt and strong alkali pickling could hydrolyze the lipid and lead to the accumulation of small molecular weight fatty acids. Therefore, different processing methods had different effects on the lipid properties of egg yolk. The changes of the characteristics of lipid in duck egg yolks played a pivotal role in the sensory quality of egg yolk.
Acknowledgments
We gratefully acknowledge the financial support provided by the National Natural Science Foundation of China (Grant Nos. 32060551, 31760467, 31871832 and 31760439).
Disclosures
There are no conflicts to declare.
References
- Ajith S., Pramod S., Kumari C.P., Potty V. Effect of storage temperatures and humidity on proximate composition, peroxide value and iodine value of raw cashew nuts. J. Food Sci. Tech. 2015;52:4631–4636. doi: 10.1007/s13197-014-1476-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton M. Egg yolk: structures, functionalities and processes. J. Sci. Food Agr. 2013;93:2871–2880. doi: 10.1002/jsfa.6247. [DOI] [PubMed] [Google Scholar]
- AOAC . 17th ed. Association of Official Analytical Chemists; Washington, DC: 2000. Official Methods of Analysis. [Google Scholar]
- AOCS. 2009. Official method Cd 19-90 in sampling and analysis of commercial fats and oils of 2-thiobarbituric acid value-direct method. AOCS, Champaign, IL.
- Bachari S., Ezzatpanah H., Aminafshar M., Safafar H. The effect of refining process on the conjugated dienes in soybean oil. J. Agric. Sci. Technol. 2013;15:1185–1193. [Google Scholar]
- Chanarat S., Benjakul S., H-Kittikun A. Comparative study on protein cross-linking and gel enhancing effect of microbial transglutaminase on surimi from different fish. J. Sci. Food Agric. 2012;92:844–852. doi: 10.1002/jsfa.4656. [DOI] [PubMed] [Google Scholar]
- Cherian G., Quezada N. Egg quality, fatty acid composition and immunoglobulin Y content in eggs from laying hens fed full fat camelina or flax seed. J. Anim. Sci. Biotechnol. 2016;7:1–8. doi: 10.1186/s40104-016-0075-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi S., Tseng K. Physicochemical properties of salted pickled yolks from duck and chicken eggs. J. Food Sci. 1998;63:27–30. [Google Scholar]
- China . The Standard Press of PR China; Beijing, China: 2016. Chinese standard GB 5009.168-2016 in National Food Safety Standard–Determination of Fatty Acids in Food. (in Chinese) [Google Scholar]
- Coutron-Gambotti C., Gandemer G., Rousset S., Maestrini O., Casabianca F. Reducing salt content of dry-cured ham: effect on lipid composition and sensory attributes. Food Chem. 1999;64:13–19. [Google Scholar]
- El-Bagir N.M., Hama A.Y., Hamed R.M., El Rahim A.A., Beynen A.C. Lipid composition of egg yolk and serum in laying hens fed diets containing black cumin (Nigella sativa) Int. J. Poult. Sci. 2006;5:574–578. [Google Scholar]
- Estévez M., Ventanas S., Cava R. Physicochemical properties and oxidative stability of liver pâté as affected by fat content. Food Chem. 2005;92:449–457. [Google Scholar]
- Folch J., Lees M., Stanley G.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- Fraeye I., Bruneel C., Lemahieu C., Buyse J., Muylaert K., Foubert I. Dietary enrichment of eggs with omega-3 fatty acids: a review. Food Res. Int. 2012;48:961–969. [Google Scholar]
- Ganasen P., Benjakul S. Physical properties and microstructure of pidan yolk as affected by different divalent and monovalent cations. LWT-Food Sci. Technol. 2010;43:77–85. [Google Scholar]
- Ganasen P., Benjakul S. Chemical composition, physical properties and microstructure of pidan white as affected by different divalent and monovalent cations. J. Food Biochem. 2011;35:1528–1537. [Google Scholar]
- Ganesan P., Kaewmanee T., Benjakul S., Baharin B.S. Comparative study on the nutritional value of pidan and salted duck egg. Korean J. Food Sci. An. 2014;34:1–6. doi: 10.5851/kosfa.2014.34.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon, M. H. 2001. Measuring antioxidant activity. Pages 73–82 in Antioxidant in Food: Practical Applications. J. Pokorny, N. Yanishlieva, and M. M. Gordon, eds. CRC Press, Boca Raton, FL.
- Haryati T., Man Y.C., Ghazali H., Asbi B., Buana L. Determination of iodine value of palm oil based on triglyceride composition. J. Am. Oil Chem. Soc. 1998;75:789–792. [Google Scholar]
- Higgins F. Effects of alpha-tocopheryl acetate supplementation and salt addition on the oxidative stability (TBARS) and warmed-over flavour (WOF) of cooked turkey meat. Brit. Poult. Sci. 1999;40:59–64. doi: 10.1080/00071669987845. [DOI] [PubMed] [Google Scholar]
- ISO. 2018. International Organization for Standardization 3961:2018 in animal and vegetable fats and oils-Determination of iodine value. ISO, Geneva, Switzerland.
- ISO. 2020. International Organization for Standardization 3657:2020 in animal and vegetable fats and oils-Determination of saponification value. ISO, Geneva, Switzerland.
- Ji L., Liu H., Cao C., Liu P., Wang H., Wang H. Chemical and structural changes in preserved white egg during pickled by vacuum technology. Food Sci. Technol. Int. 2013;19:123–131. doi: 10.1177/1082013212442186. [DOI] [PubMed] [Google Scholar]
- Kaewmanee T., Benjakul S., Visessanguan W. Changes in chemical composition, physical properties and microstructure of duck egg as influenced by salting. Food Chem. 2009;112:560–569. [Google Scholar]
- Kaewmanee T., Benjakul S., Visessanguan W. Effect of salting processes on chemical composition, textural properties and microstructure of duck egg. J. Sci. Food Agr. 2009;89:625–633. doi: 10.1111/j.1750-3841.2010.01975.x. [DOI] [PubMed] [Google Scholar]
- Kaewmanee T., Benjakul S., Visessanguan W. Effects of salting processes and time on the chemical composition, textural properties, and microstructure of cooked duck egg. J. Food Sci. 2011;76:139–147. doi: 10.1111/j.1750-3841.2010.01975.x. [DOI] [PubMed] [Google Scholar]
- Kaewmanee T., Benjakul S., Visessanguan W., Gamonpilas C. Effect of sodium chloride and osmotic dehydration on viscoelastic properties and thermal-induced transitions of duck egg yolk. Food Bioprocess Tech. 2013;6:367–376. [Google Scholar]
- Kanner J., Harel S., Jaffe R. Lipid peroxidation of muscle food as affected by sodium chloride. J. Agr. Food Chem. 1991;39:1017–1021. [Google Scholar]
- Kim H., Choe E. Effects of egg yolk powder addition to the flour dough on the lipid oxidation development during frying. LWT-Food Sci. Technol. 2008;41:845–853. [Google Scholar]
- Lai K., Chung W., Jao C., Hsu K. Oil exudation and histological structures of duck egg yolks during brining. Poult. Sci. 2010;89:738–744. doi: 10.3382/ps.2009-00334. [DOI] [PubMed] [Google Scholar]
- Lai K., Ko W., Lai T. Effect of NaCl penetration rate on the granulation and oil-off of the yolk of salted duck egg. Food Sci. Technol. Int. 1997;3:269–273. [Google Scholar]
- Luo W., Xue H., Xiong C., Li J., Tu Y., Zhao Y. Effects of temperature on quality of preserved eggs during storage. Poult. Sci. 2020;99:3144–3157. doi: 10.1016/j.psj.2020.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mccann T.H., Small D.M., Batey I.L., Wrigley C.W., Day L. Protein–lipid interactions in gluten elucidated using acetic acid fractionation. Food Chem. 2009;115:105–112. [Google Scholar]
- Min B., Ahn D. Mechanism of lipid peroxidation in meat and meat products-A review. Food Sci. Biotechnol. 2005;14:152–163. [Google Scholar]
- Naz S., Sheikh H., Siddiqi R., Sayeed S.A. Oxidative stability of olive, corn and soybean oil under different conditions. Food Chem. 2004;88:253–259. [Google Scholar]
- Odoom W., Edusei V.O. Evaluation of saponification value, iodine value and insoluble impurities in coconut oils from Jomoro District in the Western Region of Ghana. Asian J. Agr. Food Sci. 2015;3:494–499. [Google Scholar]
- Sasaki K., Mitsumoto M., Kawabata K. Relationship between lipid peroxidation and fat content in Japanese Black beef Longissimus muscle during storage. Meat Sci. 2001;59:407–410. doi: 10.1016/s0309-1740(01)00093-6. [DOI] [PubMed] [Google Scholar]
- Sinanoglou V.J., Strati I.F., Miniadis-Meimaroglou S. Lipid, fatty acid and carotenoid content of edible egg yolks from avian species: a comparative study. Food Chem. 2011;124:971–977. [Google Scholar]
- Xiao N., Zhao Y., Yao Y., Wu N., Xu M., Du H., Tu Y. Biological activities of egg yolk lipids: a review. J. Agr. Food Chem. 2020;68:1948–1957. doi: 10.1021/acs.jafc.9b06616. [DOI] [PubMed] [Google Scholar]
- Xu L., Zhao Y., Xu M., Yao Y., Nie X., Du H., Tu Y. Effects of salting treatment on the physicochemical properties, textural properties, and microstructures of duck eggs. PloS One. 2017;12 doi: 10.1371/journal.pone.0182912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang N., Jin Y., Xu Y., Bin Y., Xu X. Effect of pressure cooking on physicochemical properties of salted eggs. RSC Adv. 2016;6:97089–97095. [Google Scholar]
- Yang S.C., Baldwin R.E. Pages 405–463 in Egg Science and Technology. Macmillan Education; Oxford, UK: 2017. Functional properties of eggs in foods. [Google Scholar]
- Yang Y., Zhao Y., Xu M., Wu N., Yao Y., Du H., Liu H., Tu Y. Changes in physico-chemical properties, microstructure and intermolecular force of preserved egg yolk gels during pickling. Food Hydrocolloid. 2019;89:131–142. [Google Scholar]
- Yang Y., Zhao Y., Xu M., Yao Y., Wu N., Du H., Tu Y. Alkali induced gelation behavior of low-density lipoprotein and high-density lipoprotein isolated from duck eggs. Food Chem. 2020;311 doi: 10.1016/j.foodchem.2019.125952. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Fan S., Liu Y., Ji H., Zhong X. The effect of salting on the quality of yolk lipid in duck eggs. Food Sci. Technol. 2012;37:65–68. (in Chinese) [Google Scholar]
- Zhao Y., Tu Y., Deng W., Li J. Changes of fatty acids during the processing of preserved eggs. Food Sci. 2014;35:69–72. (in Chinese) [Google Scholar]
- Zhao Y., Tu Y., Li J., Xu M., Yang Y., Nie X., Yao Y., Du H. Effects of alkaline concentration, temperature, and additives on the strength of alkaline-induced egg white gel. Poult. Sci. 2014;93:2628–2635. doi: 10.3382/ps.2013-03596. [DOI] [PubMed] [Google Scholar]