Abstract
Antibiotics are sprayed on apple and pear orchards to control, among other pathogens, the bacterium Erwinia amylovora, the causative agent of fire blight. As with many other pathogens, we observe the emergence of antibiotic‐resistant strains of E. amylovora. Consequently, growers are looking for alternative solutions to combat fire blight. To find alternatives to antibiotics against this pathogen, we have previously isolated three bacterial strains with antagonistic and extracellular activity against E. amylovora, both in vitro and in planta, corresponding to three different bacterial genera: Here, we identified the inhibitory mode of action of each of the three isolates against E. amylovora. Isolate Bacillus amyloliquefaciens subsp. plantarum (now B. velezensis) FL50S produces several secondary metabolites including surfactins, iturins, and fengycins. Specifically, we identified oxydifficidin as the most active against E. amylovora S435. Pseudomonas poae FL10F produces an active extracellular compound against E. amylovora S435 that can be attributed to white‐line‐inducing principle (WLIP), a cyclic lipopeptide belonging to the viscosin subfamily (massetolide E, F, L, or viscosin). Pantoea agglomerans NY60 has a direct cell‐to‐cell antagonistic effect against E. amylovora S435. By screening mutants of this strain generated by random transposon insertion with decreased antagonist activity against strain S435, we identified several defective transposants. Of particular interest was a mutant in a gene coding for a Major Facilitator Superfamily (MFS) transporter corresponding to a transmembrane protein predicted to be involved in the extracytoplasmic localization of griseoluteic acid, an intermediate in the biosynthesis of the broad‐spectrum phenazine antibiotic d‐alanylgriseoluteic acid.
Keywords: apple, antagonism, Fire blight, lipopeptide, oxydifficidin
Antibiotics are sprayed on apple and pear orchards to control the bacterium Erwinia amylovora, the causative agent of fire blight. Growers are looking for solutions to combat this disease. We have previously isolated three bacterial strains with antagonistic activity against E. amylovora. Here, we identified their inhibitory mode of action. Isolate B acillus velezensis FL50S produces several secondary metabolites including oxydifficidin as the most active against E. amylovora S435. Pseudomonas poae FL10F produces a cyclic lipopeptide belonging to the viscosin subfamily. Pantoea agglomerans NY60 has a direct cell‐to‐cell antagonistic effect against E. amylovora S435. By screening mutants with decreased antagonist activity against strain S435, we identified a gene predicted to be involved in the biosynthesis of the broad‐spectrum phenazine antibiotic D‐alanylgriseoluteic acid.

1. INTRODUCTION
The bacterial plant pathogen responsible for fire blight is Erwinia amylovora (Holtappels et al., 2018; Singh & Khan, 2019). It is a rod‐shaped Gram‐negative bacterium capable of infecting different host plants in the Rosaceae family, including apple and pear trees (Philion, 2018). Annual losses due to fire blight can be significant in many countries (Acimovic et al., 2015). In the United States alone, apple and pear producers’ losses were estimated in millions of dollars during the year 2000 in Michigan (Norelli, Jones, et al., 2003, Norelli, Holleran, et al., 2003) and during 1998 in Washington and northern Oregon due to fire blight (Stockwell, Johnson, Sugar, et al., 2002). Growers have been spraying antibiotics on apple and pear orchards, but are looking for alternatives due to resistance development (Acimovic et al., 2015; McManus & Jones, 1994; McManus et al., 2002). There are several published articles exploring fire blight control and providing insights into the infection and propagation of E. amylovora on flowers and leaves; these form a platform for the development of solutions to control this disease. For example, Wright et al. (2001) experimented on a Pantoea agglomerans isolate (strain Eh318), which acts through antibiosis by inhibiting the growth of E. amylovora. This activity was attributed to the production of two antibiotics, pantocin A and B (Wright et al., 2001). Giddens et al. (2002) characterized a novel phenazine antibiotic gene cluster in P. agglomerans Eh1087 effective in the suppression of the fire blight pathogen (Giddens et al., 2002). Smits et al. (2011) studied a Pantoea vagans and found two main factors contributing to the biological activity against E. amylovora: (a) competition for limiting substrates and (b) production of antibacterial metabolites (Pusey et al., 2011). Niu et al. (2013) showed that polymyxin P is the main active ingredient in the suppression of Erwinia species by Paenibacillus polymyxa M‐1 (Niu et al., 2013). Lee et al. (1987) found that culture filtrates of a strain of Pseudomonas aeruginosa strongly inhibited the growth of E. amylovora (Lee et al., 2013). Zhao et al. (2013) isolated a strain of Bacillus amyloliquefaciens subsp. plantarum with high activity against E. carotovora which infects vegetables in post‐harvest (Zhao et al., 2013). Aureobasidium pullulans is also used as a biocontrol agent for fire blight protection in organic apple and pear production. They are highly efficient microorganisms that block Erwinia amylovora from colonizing the apple blossom. It works through natural competition for space and nutrients between pathogens and antagonists on the blossom (Temple et al., 2020). Bacteriophages, bacteria‐infecting viruses, have been recently reconsidered as a biological control tool for preventing bacterial pathogens. A bacteriophage phiEaP‐8 was isolated from apple orchard soil and was shown to control efficiently and specifically E. amylovora (Park et al., 2018).
Several species belonging to the genera Pseudomonas, Pantoea, Bacillus, and Paenibacillus are used to control several plant infectious diseases (El‐Hendawy et al., 2005; Stockwell, Johnson, Loper, et al., 2002). They have been tested against phytopathogenic bacteria and have shown antagonistic activity against most phytopathogens (Allard et al., 2014; Mansfield et al., 2012; Pusey et al., 2009; Yin et al., 2011). Several effective inhibitory metabolites produced by the above active bacteria have been described, especially in Bacillus and Paenibacillus (Zimmerman et al., 1987). Polyketides are one important class of microbial secondary metabolites with a wide spectrum of antibacterial and antifungal activities in an agricultural context (Chen et al., 2006; Olishevska et al., 2019). They include many active ingredients such as antibiotics. Species belonging to the genera Bacillus and Paenibacillus also synthesize non‐ribosomal peptides (NRPs). Bacillus subtilis, for example, synthesizes surfactin belonging to the class of lipopeptides (Fan et al., 2017). Several species of fluorescent Pseudomonas are recognized for their ability to produce NRP‐type secondary metabolites with antibacterial and antifungal characteristics (Omoboye et al., 2019). Several species of Pseudomonas and Bacillus produce lipopeptides that function not only in antagonism against pathogens but also in surface spreading motility, leading to the colonization of new habitats and the development of highly structured biofilms (Hennessy et al., 2017; Nielsen et al., 2002).
We have previously reported the isolation of three bacterial strains with antagonist activity against E. amylovora both in vitro and in planta: (1) Pantoea agglomerans NY60, (2) Pseudomonas poae FL10F and (3) Bacillus amyloliquefaciens subsp. plantarum FL50S (Dagher et al., 2020). Here, we characterized the inhibitory mode of action of each of these strains against E. amylovora.
2. MATERIALS AND METHODS
2.1. Bacterial strains and culture conditions
The bacterial strains used in this study are as follows: B. amyloliquefaciens subsp. plantarum FL50S was isolated from agricultural field soil in Wimauma, Florida, USA, P. poae FL10F was isolated from strawberry leaves collected in Dover, Florida, USA, P. agglomerans NY60 was isolated from Rome apple leaves collected in Geneva, New York, USA and E. amylovora S435 was originally isolated from an infected apple tree and supplied by the Institut de recherche et développement en agroenvironnement (IRDA), Québec, Canada). We have recently reported the characterization of the three antagonistic strains (Dagher et al., 2020). Unless otherwise specified, the bacteria were routinely grown from frozen glycerol stocks by culturing in 3 ml tryptic soy broth (TSB) (BD) overnight in test tubes incubated at 30℃ and shaking in a TC‐7 roller drum (New Brunswick Scientific Co., New Brunswick, NJ) at 240 rpm. For inoculation, appropriate aliquots of each overnight culture were used to inoculate culture media by adjusting the optical density at 600 nm (OD600) to 0.01, corresponding to about 8 × 106 CFU/ml.
2.2. Whole‐genome sequencing
Genomic DNA was isolated from an overnight culture of each strain using a Qiagen DNeasy blood and tissue kit (Qiagen Inc., Valencia, CA). Genome sequencing was performed using the Illumina MiSeq sequencing system (Illumina, San Diego, CA), achieving >50X average genome coverage. De novo assembly was achieved for each genome using SPAdes 3.0.0 (St. Petersburg genome assembler), and annotated with the NCBI Prokaryotic Genomes Automatic Annotation Pipeline (http://www.ncbi.nlm.nih.gov/genomes/static/Pipeline.html). The taxonomy of each isolate was assigned using Kraken (Wood & Salzberg, 2014).
2.3. PacBio sequencing and assembly
Single‐molecule, real‐time sequencing offers longer read lengths making it well‐suited for unsolved problems in genome, transcriptome, and epigenetics research. The highly contiguous sequencing can close gaps in current reference assemblies and characterize structural variation (SV) in genomes (Ee et al., 2014; Rhoads & Au, 2015).
The resulting assembled sequences were polished with Illumina reads using a combination of BWA version 0.7.17‐r1188 (Chen et al., 2009), SAMtools version 1.9 (Chen et al., 2009), and Pilon version 1.22 (Walker et al., 2014).
2.4. Secondary metabolite gene cluster prediction and analysis
Genome mining of biosynthetic gene clusters included non‐ribosomal peptide synthetases (NRPSs), polyketide synthases (PKSs), hybrid PKS/NRPS, siderophores, and bacteriocins were predicted with antibiotics & Secondary Metabolite Analysis SHell (antiSMASH) (Weber et al., 2015) webserver version 5 (http://antismash.secondarymetabolites.org/).
2.5. Production of secondary metabolites
The production of metabolites by bacterial isolates was carried out in 2 L conical flasks with 500 ml of the respective medium. P. agglomerans NY60 was cultivated in Difco Nutrient Broth (Becton, Dickinson and Co., Franklin Lakes, NJ, USA). King's B medium was used as a production medium for P. poae FL10F isolate (King et al., 1954), while Landy medium was used for cultivation of B. amyloliquefaciens subsp. plantarum FL50S (Ben Ayed et al., 2015; Gordillo et al., 2009). Landy medium contained: 20 g/L dextrose, 5.0 g/L L‐glutamic acid, 1.0 g/L yeast extract, 1.0 g/L K2HPO4, 0.5 g/L MgSO4 (7H2O), 0.5 g/L KCl, 1.6 mg/L CuSO4, 1.2 mg/L Fe2(SO4)3, 0.4 mg/L MnSO4. All cultures were shaken at 30℃ for 48 h at 200–250 rpm.
To obtain cell‐free supernatants, cultures of all three strains were centrifuged at 17,700 × g for 1 h. The pellets were discarded and the supernatants were filtered using the Stericup vacuum filtration system (0.2 µm). Fresh cell‐free supernatants were used for the present research, but they could also be stored at +4°C for 1 week, or at −20°C for 6 months while keeping their activity.
2.6. Bioassay‐guided fractionation and isolation of active metabolites
The cell‐free medium supernatant was applied to 40 g of pre‐equilibrated Amberlite XAD‐16 resin packed in a column. The XAD‐16 column was subsequently washed with 1 L of H2O and then eluted with 1 L of 100% methanol. The methanolic elution was evaporated to dryness by rotary evaporation, and the brown residue was redissolved in 5 ml of purified water (MilliQ system; Millipore). The concentrated solution was then applied onto a 12 gram Biotage® SNAP Ultra C18 column for fractionation using reverse‐phase flash chromatography on a Biotage Isorela One instrument (Stockholm, Sweden). The separation was performed using a linear gradient of acetonitrile from 5% to 100% over 50 min at 15 ml/min. To identify column fractions containing active compounds, each fraction was evaporated to dryness by rotary evaporation and redissolved in 2 ml of MilliQ water. The bioactivity of these fractions was further determined by performing agar disk diffusion assays.
2.7. The activity of cell‐free supernatants and fractions against Erwinia amylovora S435
To estimate the activity of bacterial metabolites and fractions against E. amylovora S435, an agar disk diffusion assay was performed. Blank paper disks (d = 6 mm) were saturated by cell‐free supernatants or fractions (20 µl), then air‐dried in a biosafety cabinet for 30 min. The antimicrobial activity was then tested by placing the disk on a lawn of freshly inoculated E. amylovora S435 TSB agar plate and measuring the growth inhibition area (mm2) after 48 h of cultivation at room temperature (~21°C). This assay was performed in triplicate.
2.8. LC‐ESI‐MS/MS analyses
The cell‐free supernatants and active fractions were further analyzed by high‐performance liquid chromatography (HPLC; Waters 2795) equipped with a 250 × 4.6 mm i.d. Luna Omega Polar C18 reversed‐phase column (particle size 3 μm) using a 1% acetic acid–acetonitrile gradient at a flow rate of 500 µl/min. The detector was a quadrupole mass spectrometer (Quattro Premier XE, Waters). Analyses were carried out in the positive electrospray ionization (ESI) mode with a mass‐to‐charge ratio (m/z) window ranging from 130–1930. Collision‐induced dissociation (CID) MS/MS experiments were performed using argon gas at various collision energies.
2.9. Random transposon mutagenesis and colony selection
Random transposon insertions in the P. agglomerans NY60 chromosome were generated by mating NY60 with DAP‐auxotroph E. coli strain χ7213 carrying the pUT/mini‐Tn5 Sm/Sp plasmid on Lysogeny broth (LB) agar plates supplemented with DAP at 37°C (de Lorenzo et al., 1990). Transposants were selected on TSA plates containing spectinomycin (15 µg/ml). The plates were incubated at room temperature (~21°C) for 2 days. Thereafter, a lawn of lab‐derived streptomycin‐resistant E. amylovora S435 was spread onto TSA plates using sterile cotton‐tipped applicators. After incubation for another 2 days at 21°C, transposants forming smaller or no inhibition zones on the E. amylovora S435 lawns were selected for the determination of the transposon insertion site.
2.10. Identification transposon insertions sites using transposon insertion sequencing (Tn‐seq)
Genomic DNA of eight candidate transposants was extracted from cultures using a DNA extraction kit and pooled together. DNA concentrations were determined using the Quant‐iT™ PicoGreen® dsDNA Assay Kit (Life Technologies). DNA was sequenced at the Génome Québec Innovation Centre, McGill University. DNA samples were generated using the NEB Next Ultra II DNA Library Prep Kit for Illumina (New England BioLabs) as per the manufacture protocol. TruSeq adapters and PCR primers were purchased from IDT. Size selection of libraries containing the desired insert size was obtained using SPRI select beads (Beckman Coulter). Briefly, genomic DNA was fragmented and tagged with adapter sequence via one enzymatic reaction (tagmentation). Thereafter, PCR was used to amplify the region between the end of the insertion (primer TnErwinia‐CS1: (5′ ACACTGACGACATGGTTCTACAGCGGCCGCACTTGTGTATAA 3′ [transposon‐specific sequence is in Bold])), and the Illumina adapter with primer 2 (5′ TACGGTAGCAGAGACTTGGTCTCTAGCATAGAGTGCGTAGCTCTGCT 3′) to enrich for transposon insertion sites and allow multiplex sequencing. The thermocycler program was 94°C for 2 min, 94°C for 30 s, 55°C for 30 s 72°C for 30 s for 33 cycles, and 72°C for 7 min. This region was reamplified to add the Illumina adapters for MiSeq sequencing: PE1‐CS1 (AGATCGGAAGAGCACACGTCTGAACTCCAGTCACACACTGACGACATGGTTCTACA) and primer 2. Thereafter, sequencing was performed on an Illumina MiSeq using the MiSeq Reagent Kit v2 Kit (500‐cycles). To find the transposon insertion sites, 288,889 reads were mapped to P. agglomerans NY60 genome (CP034469.1).
3. RESULTS
3.1. Whole‐genome sequencing
We have previously reported the identification of three bacterial isolates presenting an effective inhibitory activity against the phytopathogen E. amylovora (Dagher et al., 2020). We deposited these bacterial isolates at the Center for Food Safety and Applied Nutrition (CFSAN), United States Food and Drug Administration. Bacillus amyloliquefaciens subsp. plantarum FL50S was reclassified as Bacillus velezensis FL50S, a strain corresponding to CFSAN strain CFSAN034340. Isolate FL10F was classified as Pseudomonas poae FL10F, a strain corresponding to CFSAN strain CFSAN034337. Isolate NY60 was classified as Pantoea agglomerans NY60, a strain corresponding to CFSAN strain CFSAN047153 (Dagher et al., 2020).
3.2. Genome mining studies
We next wanted to identify the mode of action of these potential biocontrol agents.
Genome mining for biosynthetic gene clusters (BGC) was performed, including for NRPSs and other secondary metabolites, using the antiSMASH 5.0 prediction tool. The summary of the predicted secondary metabolites for strains B. velezensis FL50S and P. poae FL10F are shown in Tables 1 and 2, respectively.
TABLE 1.
Identified biosynthetic gene cluster regions in the genome of B. velezensis FL50S
| Region on genome | Biosynthetic gene cluster type | Most similar known cluster from the MiBIG database | Similarity a | MIBiG accession number |
|---|---|---|---|---|
| 302440–377131 | NRPS | Surfactin | 82% | BGC0000433 |
| 4851028–4956922 | NRPS, transAT‐PKS | Fengycin | 80% | BGC0001095 |
| 4851028–4956922 | NRPS, transAT‐PKS | Iturin | 88% | BGC0001098 |
| 2290903–2384088 | transAT‐PKS | Difficidin | 100% | BGC0000176 |
| 1390407–1478530 | transAT‐PKS | Macrolactin | 100% | BGC0000181 |
| 1698501–2006499 | transAT‐PKS, NRPS | Bacillaene | 85% | BGC0001089 |
| 4554904–4605413 | NRPS | Bacillibactin | 100% | BGC0000309 |
| 3598088–3639506 | other | Bacilysin | 100% | BGC0001184 |
Abbreviations: MIBiG, The Minimum Information about a Biosynthetic Gene cluster https://mibig.secondarymetabolites.org/; NRPS, Nonribosomal peptides synthetases.
transAT‐PKS, Polyketide synthase.
Similarity shows the % of sequence similarity of the region on the genome to the entries in the MIBiG database using the ClusterBlast algorithm described by Medema et al. (2011)
TABLE 2.
Identified biosynthetic gene cluster regions in the genome of P. poae FL10F
| Region on genome | Biosynthetic gene cluster type | Most similar known cluster from the MiBIG database | Similarity a | MIBiG accession number |
|---|---|---|---|---|
| 1706151–1809187 | NRPS | Viscosin | 43% | BGC0001312 |
| 1981700–2032473 | NRPS | Pyochelin | 100% | BGC0000412 |
| 3613987–3659622 | NRPS | Bananamide 1 | 50% | BGC0001346 |
| 4133067–4179775 | NRPS | Safracin A / Safracin B | 100% | BGC0000421 |
| 6051250–6104146 | NRPS | Pyoverdin | 10% | BGC0000413 |
Abbreviations: MIBiG, The Minimum Information about a Biosynthetic Gene cluster https://mibig.secondarymetabolites.org/; NRPS, Nonribosomal peptides synthetases.
Similarity shows the % of sequence similarity of the region on the genome to the entries in the MIBiG database using the ClusterBlast algorithm described by Medema et al. (2011)
For strain FL50S, a total of 15 putative BGCs were predicted, of which 8 correspond to known functions, including non‐ribosomal peptide synthetases (NRPSs) attributed to the biosynthesis of surfactin, iturin, and fengycin, and polyketide synthases (PKSs) encoding for difficidin, macrolactin, and bacillaene (Table 1). For Pseudomonas poae FL10F, a total of 5 NRPSs gene clusters were predicted. Among them, two unlinked NRPS gene clusters with 100% similarity to viscosin and bananamide 1 gene clusters coding for NRPS responsible for the biosynthesis of cyclic lipopeptides (CLP) were identified (Table 2).
Since the activity of Pantoea agglomerans NY60 was found to be attributed to a direct cell‐to‐cell antagonistic effect against E. amylovora S435 and not to its cell‐free supernatant (Dagher et al., 2020), the antiSMASH analysis did not reveal many secondary metabolite genes with antibacterial properties.
3.3. Identification of metabolites and their inhibitory activity
The antiSMASH analysis suggests that B. velezensis FL50S has the biosynthetic machinery for the production of several CLPs and polyketides. To verify the antiSMASH results and see if these gene clusters are functional, cell‐free supernatant of B. velezensis FL50S grown in TSB was analyzed by HPLC‐ESI MS/MS. In accordance with antiSMASH results, MS spectra of B. velezensis FL50S indicate that it produces three families of CLP: surfactins, iturins, and fengycins. CLPs were identified by comparing the detected peaks with those from literature and the actual mass of CLP (Deng et al., 2017; Rokni‐Zadeh et al., 2012). Pseudomolecular ion peaks of surfactin were observed at m/z 994.6, 1008.6, 1022.7, and 1036.8, while those of iturin were observed at m/z 1043.6, 1057.6, and 1071.6, and doubly charged pseudomolecular ion peak of fengycins were observed at 718.4, 725.4, 732.4, 739.4, 746.6, 753.6 (Figure 1). Regarding polyketides, only the oxidized form of difficidin, oxydifficidin, could be identified (Figure 2). Chen et al. (2006) reported that difficidin and oxydifficidin can only be detected in their deprotonated forms ([M‐H]– = 543.4 and 559.3) in the negative ionization mode (Chen et al., 2006); in positive mode, we detected oxydifficidin in its dephosphorylated (m/z 463.4) and dimer (m/z 1121.3) species (Figure 2) (Wilson et al., 1987).
FIGURE 1.
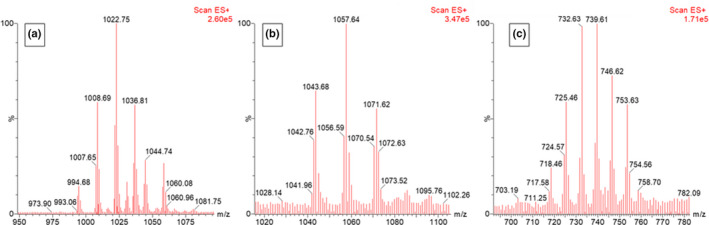
MS spectra of three families of cyclic lipopeptides produced by B. velezensis FL50S. (a) surfactins (b) iturins and (c) fengycins detected in positive ionization mode. In the case of fengycins, peaks correspond to the doubly charged ions
FIGURE 2.
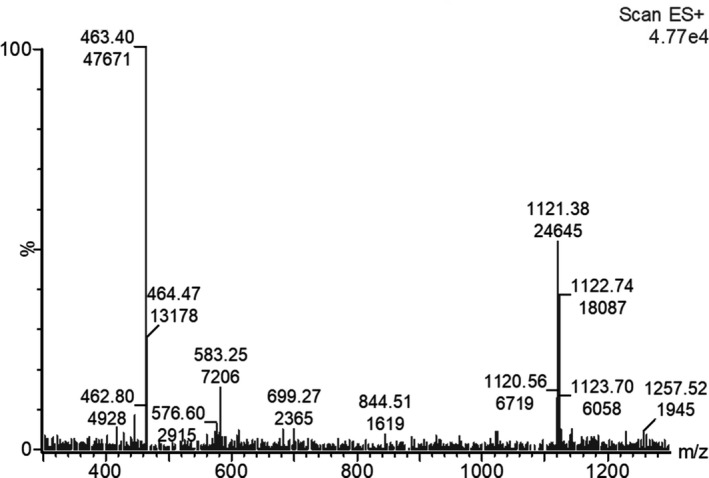
MS spectra of produced Oxydifficidin in the supernatant of B. velezensis FL50S culture detected in positive ionization mode
We performed activity‐guided fractionation of the crude extracellular extract of FL50S to identify the E. amylovora ‐inhibiting metabolites. Out of 30 fractions of B. velezensis FL50S, four displayed activity against E. amylovora S435 (Table A1), with fraction #21 causing the largest inhibition zone. Performing the HPLC‐ESI MS analyses on active fractions revealed that the polyketide oxydifficidin is the main inhibitory metabolite active against E. amylovora S435.
Three out of 30 fractions of P. poae FL10F displayed activity against E. amylovora S435 (Table A2) with fraction #20 causing the largest inhibition zone. Analyzing the MS spectra of the fractions of P. poae FL10F active against E. amylovora S435 showed the presence of a major pseudomolecular ion peak at m/z 1127.1 and some minor peaks including 1113.1 [M + H]+ with 14 unit mass difference with the major peak (Figure 3). Collision‐induced dissociation tandem MS/MS of the major pseudomolecular ion peak at m/z 1127.1, combined with antiSMASH amino acid sequence prediction of a putative product of two unlinked NRPS gene clusters involved in the biosynthesis of cyclic lipopeptides, suggest that the 1127.1 peak corresponds to the white‐line‐inducing principle (WLIP) (Coraiola et al., 2006), a cyclic lipodepsipeptide belonging to the viscosin subfamily (Nguyen et al., 2016) (Figure 4).
FIGURE 3.
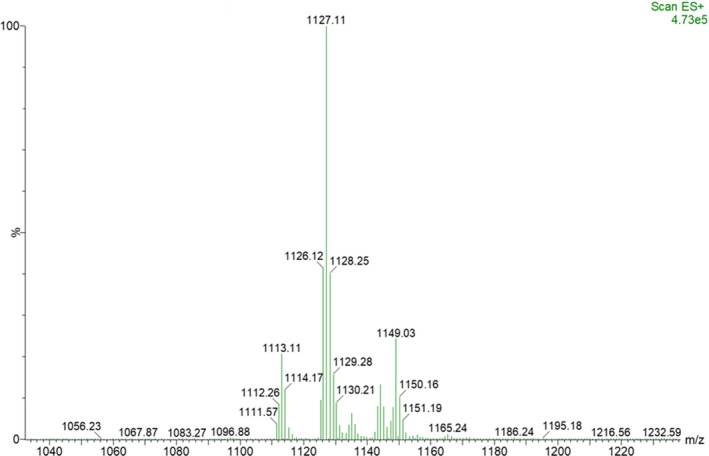
MS spectra of a fraction of P. poae FL10F culture extract active against E. amylovora S435
FIGURE 4.
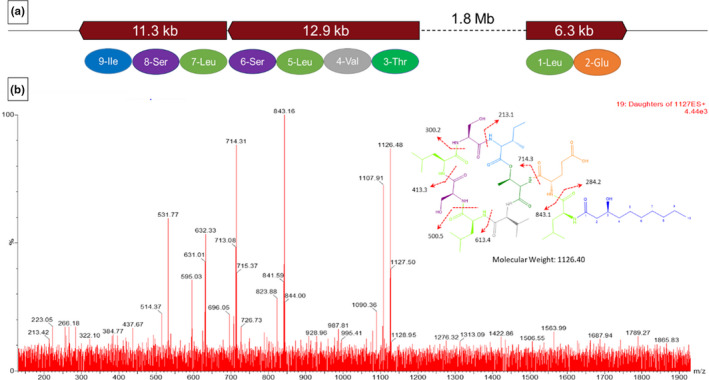
Biosynthetic gene clusters of Pseudomonas poae FL10F corresponding to the viscosin and bananamide 1 by antiSMASH. (a) Organization of CLP biosynthetic gene clusters of Pseudomonas poae FL10F corresponding to the viscosin and bananamide 1 gene clusters (BGC0001312 and BGC0001346 respectively) as identified by antiSMASH. (b) MS/MS fragmentation spectrum of the parent ion peak 1127.3 (m/z) at different collision energies in positive ionization mode. The daughter ion peaks are indicated on the proposed structure
3.4. Screening for transposon mutants of P. agglomerans NY60 with decreased antagonist activity against E. amylovora S435
Regarding the P. agglomerans NY60 strain, while its cell‐free supernatant from nutrient broth culture shows no activity against E. amylovora S435, it is still able to produce an inhibition zone on the lawn of E. amylovora S435 on TSA agar plates (Dagher et al., 2020). The co‐culturing of P. agglomerans NY60 strain in the presence of E. amylovora S435 did not induce the production of free‐cell supernatant active against E. amylovora S435, suggesting that the P. agglomerans NY60 inhibitory activity requires direct contact with live cells (Dagher et al., 2020).
To identify the functions responsible for the antagonistic activity of this strain, random mutagenesis of P. agglomerans NY60 was performed via insertion of a mini‐Tn5 transposon. Five transposants with reduced antagonistic activity were identified (Table A3). Using transposon insertion sequencing (Tn‐seq), the transposon insertion sites of these mutants were mapped, leading to the identification of few genes that altered the production of antagonistic functions (Table 3).
TABLE 3.
Identified transposon insertion sites in the genome of P. agglomerans NY60
| Genome | Transposon insertion site | Locus tag | Putative P. agglomerans ortholog |
|---|---|---|---|
| CP034469 Chromosome | |||
| 474915 | D1628_02140 | TonB‐dependent siderophore receptor | |
| 570285 | D1628_02530 | DNA sulfur modification protein DndD | |
| 1416458 | D1628_06515 | Glycosyltransferase | |
| 1526741 | D1628_07110 | Flagellar hook‐filament junction protein FlgL | |
| CP034471 Plasmid | |||
| 97491 | D1628_22515 | MFS transporter |
Interestingly, one of the transposants has an insertion in a gene predicted to code for an MFS transporter located on the 211 kbp plasmid (CP034471) carried by strain NY60. BLASTing the sequence indicated that it matches the sequence for the EhpJ gene in Pantoea agglomerans (Erwinia herbicola) Eh1087 which is a transmembrane protein predicted to be involved in the extracytoplasmic localization of griseoluteic acid, an intermediate in the biosynthesis of the potent broad‐spectrum phenazine antibiotic d‐alanylgriseoluteic acid (AGA) (Giddens et al., 2002).
4. DISCUSSION
The biocontrol activity of three bacterial isolates presenting an effective inhibitory activity against the phytopathogen E. amylovora was characterized.
For strain B. velezensis FL50S, through the HPLC‐ESI MS analyses on fractions obtained from activity‐guided fractionation of the crude extracellular metabolite, we found that the polyketide oxydifficidin is the major active metabolite against E. amylovora S435. Accordingly, the corresponding biosynthetic genes were identified on the genome of FL50S. Oxydifficidin was previously known to be effective against plant phytopathogens such as Ralstonia solanacearum, a causal agent of tomato bacterial wilt (Im et al., 2020); however, to the best of our knowledge, this study is the first to show the specific activity of oxydifficidin on E. amylovora.
Our results demonstrate that P. poae FL10F produces an extracellular compound active against E. amylovora S435 that can be attributed to white‐line‐inducing principle (WLIP), a lipodepsipeptide belonging to the viscosin subfamily, which includes massetolides E, F, L, and viscosin. WLIP a member of the viscosin group of cyclic lipononadepsipeptides featuring a Glu2 amino acid with both antifungal as well as antibacterial activity (against gram‐positive bacteria) (De Vleeschouwer et al., 2016; Lo Cantore et al., 2006). In addition to linking the known phenotypes of white line production and hemolytic activity of a WLIP producer with WLIP biosynthesis, additional properties of ecological relevance conferred by WLIP production were published, namely, antagonism against Xanthomonas as well as involvement in swarming and biofilm formation (Rokni‐Zadeh et al., 2012). Pseudomonads produce molecules that scavenge nutrients, sense population density, and enhance or inhibit the growth of competing microorganisms (Nguyen et al., 2016). They produce several surface‐active lipopeptides showing antimicrobial properties but that also facilitate surface motility and influence biofilm formation.
In contrast with the other two bacteria, culture supernatants of P. agglomerans NY60 do not inhibit the growth of E. amylovora (Dagher et al., 2020). Therefore, we could not use an approach based on activity‐guided fractionation of culture fluids to identify the mode of action of this isolate. Instead, we performed random mutagenesis coupled with screening to identify mutants with defective inhibitory activities. Among the five transposants with reduced activity against E. amylovora we identified, one mutant has its transposon inserted in the middle of an operon encoding the biosynthesis of broad‐spectrum phenazine antibiotic d‐alanylgriseoluteic acid (AGA).
In phenazine‐producing species like P. agglomerans Eh1087, the phz genes are on a plasmid. Its main phenazine product, AGA, acts in P. agglomerans like a typical antibiotic and is employed in competition with closely related bacterial species in its ecological niche. Interestingly, AGA itself is toxic to P. agglomerans Eh1087, and the phz pathway encodes a special phenazine‐binding protein, EhpR, that prevents self‐poisoning of the producer (Giddens et al., 2003; Mavrodi et al., 2010). Very recently, Mechan et al. (2020) also have pointed to, using a UV‐mutagenesis screen, the AGA synthesis gene cluster as being at the base of the antimicrobial activity of the P. agglomerans isolate against E. amylovora (Mechan Llontop et al., 2020). Our data are also consistent with the literature and the emerging view of the antimicrobial activity of AGA against the phytopathogen E. amylovora (Giddens & Bean, 2007).
More research is needed to better understand the mode of action of our strains against E. amylovora, as well as the factors contributing to the expression of these inhibitory functions.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
Fadi Dagher: Conceptualization (lead); Formal analysis (lead); Investigation (lead); Writing‐original draft (lead); Writing‐review & editing (lead). Arvin Nickzad: Formal analysis (equal); Investigation (equal); Methodology (equal); Validation (equal); Visualization (equal); Writing‐original draft (supporting); Writing‐review & editing (supporting). Jie Zheng: Formal analysis (supporting); Investigation (supporting); Methodology (supporting); Resources (supporting); Writing‐review & editing (supporting). Maria Hoffmann: Formal analysis (supporting); Methodology (supporting); Resources (supporting). Eric Déziel: Conceptualization (equal); Funding acquisition (lead); Methodology (supporting); Project administration (lead); Supervision (lead); Writing‐review & editing (equal).
ETHICS STATEMENT
None required.
ACKNOWLEDGMENTS
The authors would like to thank Marie‐Christine Groleau for her help on this project. This work was supported by a Natural Sciences and Engineering Research Council of Canada (NSERC)‐Collaborative Research and Development (CRD) grant (CRDPJ 488896‐15).
1.
TABLE A1.
HPLC‐ESI MS analyses‐ Activity of fractions of B. velezensis FL50S against E. amylovora S435
| Fraction number | Diameter of inhibition zone of E. amylovora S435 strain (including disk diameter)*, mm |
|---|---|
| #1 | 0.00 ± 0.00 |
| #2 | 0.00 ± 0.00 |
| #3 | 0.00 ± 0.00 |
| #4 | 0.00 ± 0.00 |
| #5 | 0.00 ± 0.00 |
| #6 | 0.00 ± 0.00 |
| #7 | 0.00 ± 0.00 |
| #8 | 0.00 ± 0.00 |
| #9 | 0.00 ± 0.00 |
| #10 | 8.00 ± 0.50 |
| #11 | 0.00 ± 0.00 |
| #12 | 0.00 ± 0.00 |
| #13 | 0.00 ± 0.00 |
| #14 | 0.00 ± 0.00 |
| #15 | 0.00 ± 0.00 |
| #16 | 0.00 ± 0.00 |
| #17 | 9.00 ± 0.50 |
| #18 | 0.00 ± 0.00 |
| #19 | 0.00 ± 0.00 |
| #20 | 0.00 ± 0.00 |
| #21 | 19.00 ± 0.50 |
| #22 | 9.00 ± 0.50 |
| #23 | 0.00 ± 0.00 |
| #24 | 0.00 ± 0.00 |
| #25 | 0.00 ± 0.00 |
| #26 | 0.00 ± 0.00 |
| #27 | 0.00 ± 0.00 |
| #28 | 0.00 ± 0.00 |
| #29 | 0.00 ± 0.00 |
| #30 | 0.00 ± 0.00 |
TABLE A2.
HPLC‐ESI MS analyses‐ Activity of fractions of Pseudomonas poae FL10F against E. amylovora S435
| Fraction number | Diameter of inhibition zone of E. amylovora S435 strain (including disk diameter)*, mm |
|---|---|
| #1 | 0.00 ± 0.00 |
| #2 | 8.00 ± 0.50 |
| #3 | 0.00 ± 0.00 |
| #4 | 0.00 ± 0.00 |
| #5 | 9.00 ± 0.50 |
| #6 | 0.00 ± 0.00 |
| #7 | 0.00 ± 0.00 |
| #8 | 0.00 ± 0.00 |
| #9 | 0.00 ± 0.00 |
| #10 | 0.00 ± 0.00 |
| #11 | 0.00 ± 0.00 |
| #12 | 0.00 ± 0.00 |
| #13 | 0.00 ± 0.00 |
| #14 | 0.00 ± 0.00 |
| #15 | 0.00 ± 0.00 |
| #16 | 0.00 ± 0.00 |
| #17 | 0.00 ± 0.00 |
| #18 | 0.00 ± 0.00 |
| #19 | 0.00 ± 0.00 |
| #20 | 22.00 ± 0.50 |
| #21 | 0.00 ± 0.00 |
| #22 | 0.00 ± 0.00 |
| #23 | 0.00 ± 0.00 |
| #24 | 0.00 ± 0.00 |
| #25 | 0.00 ± 0.00 |
| #26 | 0.00 ± 0.00 |
| #27 | 0.00 ± 0.00 |
| #28 | 0.00 ± 0.00 |
| #29 | 0.00 ± 0.00 |
| #30 | 0.00 ± 0.00 |
±Standard error of the mean (SEM) of three replicates, *Disk diameter = 6 mm
TABLE A3.
Direct antagonistic activity of 5 transposants with reduced antagonistic activity against E. amylovora S435 on TSA plates
| Transposons | Pantoea colony diameter (mm) / inhibition zone on E. amylovora S435 lawn (including Pantoea diameter in mm) |
|---|---|
| Transposon #1 | 10.00 ± 0.50 / 20.0 ± 0.50 |
| Transposon #2 | 11.00 ± 0.50 / 17.0 ± 0.50 |
| Transposon #3 | 10.00 ± 0.50 / 24.0 ± 0.50 |
| Transposon #4 | 9.00 ± 0.50 / 16.0 ± 0.50 |
| Transposon #5 | 12.00 ± 0.50 / 18.0 ± 0.50 |
| P. agglomerans NY60 | 10.00 ± 0.50 / 30.0 ± 0.50 |
Tryptic Soy Agar (TSA) Plate, ±Standard error of the mean (SEM) of three replicates.
Dagher, F. , Nickzad, A. , Zheng, J. , Hoffmann, M. , & Déziel, E. (2021). Characterization of the biocontrol activity of three bacterial isolates against the phytopathogen Erwinia amylovora . MicrobiologyOpen, 10, e1202. 10.1002/mbo3.1202
Fadi Dagher and Arvin Nickzad contributed equally.
DATA AVAILABILITY STATEMENT
The sequences of strain P. agglomerans NY60 and B. velezensis FL50S are available in GenBank under the accession numbers: CP034469 (https://www.ncbi.nlm.nih.gov/nuccore/CP034469) and LYNC00000000 (https://www.ncbi.nlm.nih.gov/nuccore/LYNC00000000), respectively.
REFERENCES
- Acimovic, S. G. , Zeng, Q. , McGhee, G. C. , Sundin, G. W. , & Wise, J. C. (2015). Control of fire blight (Erwinia amylovora) on apple trees with trunk‐ injected plant resistance inducers and antibiotics and assessment of induction of pathogenesis‐related protein genes. Frontiers in Plant Science, 6, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allard, S. , Enurah, A. , Strain, E. , Millner, P. , Rideout, S. L. , Brown, E. W. , & Zheng, J. (2014). In situ evaluation of Paenibacillus alvei in reducing carriage of Salmonella enterica serovar newport on whole tomato plants. Applied and Environment Microbiology, 80(13), 3842–3849. 10.1128/aem.00835-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Ayed, H. , Jemil, N. , Maalej, H. , Bayoudh, A. , Hmidet, N. , & Nasri, M. (2015). Enhancement of solubilization and biodegradation of diesel oil by biosurfactant from Bacillus amyloliquefaciens An6. International Biodeterioration & Biodegradation, 99, 8–14. 10.1016/j.ibiod.2014.12.009 [DOI] [Google Scholar]
- Chen, X. H. , Scholz, R. , Borriss, M. , Junge, H. , Mögel, G. , Kunz, S. , & Borriss, R. (2009). Difficidin and bacilysin produced by plant‐associated Bacillus amyloliquefaciens are efficient in controlling fire blight disease. Journal of Biotechnology, 140(1–2), 38–44. 10.1016/j.jbiotec.2008.10.015 [DOI] [PubMed] [Google Scholar]
- Chen, X.‐H. , Vater, J. , Piel, Jörn , Franke, P. , Scholz, R. , Schneider, K. , Koumoutsi, A. , Hitzeroth, G. , Grammel, N. , Strittmatter, A. W. , Gottschalk, G. , Süssmuth, R. D. , & Borriss, R. (2006). Structural and functional characterization of three polyketide synthase gene clusters in Bacillus amyloliquefaciens FZB 42. Journal of Bacteriology, 188(11), 4024–4036. 10.1128/JB.00052-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coraiola, M. , Lo Cantore, P. , Lazzaroni, S. , Evidente, A. , Iacobellis, N. S. , & Dalla Serra, M. (2006). WLIP and tolaasin I, lipodepsipeptides from Pseudomonas reactans and Pseudomonas tolaasii, permeabilise model membranes. Biochimica et Biophysica Acta, 1758(11), 1713–1722. 10.1016/j.bbamem.2006.06.023 [DOI] [PubMed] [Google Scholar]
- Dagher, F. , Dagher, F. , Olishevska, S. , Philion, V. , Zheng, J. , & Déziel, E. (2020). Development of a novel biological control agent targeting the phytopathogen Erwinia amylovora . Heliyon, 6, e05222. 10.1016/j.heliyon.2020.e05222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lorenzo, V. , Herrero, M. , Jakubzik, U. , & Timmis, K. N. (1990). Mini‐Tn5 transposon derivatives for insertion mutagenesis, promoter probing, and chromosomal insertion of cloned DNA in gram‐negative eubacteria. Journal of Bacteriology, 172(11), 6568–6572. 10.1128/JB.172.11.6568-6572.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vleeschouwer, M. , Martins, J. C. , & Madder, A. (2016). First total synthesis of WLIP: on the importance of correct protecting group choice. Journal of Peptide Science, 22(3), 149–155. 10.1002/psc.2852 [DOI] [PubMed] [Google Scholar]
- Deng, Q. I. , Wang, W. , Sun, L. , Wang, Y. , Liao, J. , Xu, D. , Liu, Y. , Ye, R. , & Gooneratne, R. (2017). A sensitive method for simultaneous quantitative determination of surfactin and iturin by LC‐MS/MS. Analytical and Bioanalytical Chemistry, 409(1), 179–191. 10.1007/s00216-016-9984-z [DOI] [PubMed] [Google Scholar]
- Ee, R. , Lim, Y.‐L. , Yin, W.‐F. , & Chan, K.‐G. (2014). De Novo assembly of the quorum‐sensing pandoraea sp. strain RB‐44 complete genome sequence using PacBio single‐molecule real‐time sequencing technology. Genome Announc, 2(2), e00245‐14. 10.1128/genomeA.00245-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El‐Hendawy, H. H. , Osman, M. E. , & Sorour, N. M. (2005). Biological control of bacterial spot of tomato caused by Xanthomonas campestris pv. vesicatoria by Rahnella aquatilis . Microbiological Research, 160(4), 343–352. 10.1016/j.micres.2005.02.008 [DOI] [PubMed] [Google Scholar]
- Fan, H. , Zhang, Z. , Li, Y. , Zhang, X. , Duan, Y. , & Wang, Q. I. (2017). Biocontrol of bacterial fruit blotch by bacillus subtilis 9407 via surfactin‐mediated antibacterial activity and colonization. Frontiers in Microbiology, 8, 1973. 10.3389/fmicb.2017.01973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giddens, S. R. , & Bean, D. C. (2007). Investigations into the in vitro antimicrobial activity and mode of action of the phenazine antibiotic D‐alanylgriseoluteic acid. International Journal of Antimicrobial Agents, 29(1), 93–97. 10.1016/j.ijantimicag.2006.08.028 [DOI] [PubMed] [Google Scholar]
- Giddens, S. R. , Feng, Y. , & Mahanty, H. K. (2002). Characterization of a novel phenazine antibiotic gene cluster in Erwinia herbicola Eh1087. Molecular Microbiology, 45(3), 769–783. 10.1046/j.1365-2958.2002.03048.x [DOI] [PubMed] [Google Scholar]
- Giddens, S. R. , Houliston, G. J. , & Mahanty, H. K. (2003). The influence of antibiotic production and pre‐emptive colonization on the population dynamics of Pantoea agglomerans (Erwinia herbicola) Eh1087 and Erwinia amylovora in planta. Environmental Microbiology, 5(10), 1016–1021. 10.1046/j.1462-2920.2003.00506.x [DOI] [PubMed] [Google Scholar]
- Gordillo, M. , Navarro, A. R. , Benitez, L. M. , de Plaza,Torres, M. I. , & Cristina, M. (2009). Preliminary study and improve the production of metabolites with antifungal activity by a Bacillus sp. strain IBA 33. Microbiology Insights, 2, 15–24. [Google Scholar]
- Hennessy, R. C. , Phippen, C. B. W. , Nielsen, K. F. , Olsson, S. , & Stougaard, P. (2017). Biosynthesis of the antimicrobial cyclic lipopeptides nunamycin and nunapeptin by Pseudomonas fluorescens strain In5 is regulated by the LuxR‐type transcriptional regulator NunF. Microbiologyopen, 6(6), e00516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtappels, M. , Noben, J.‐P. , Van Dijck, P. , & Valcke, R. (2018). Fire blight host‐pathogen interaction: proteome profiles of Erwinia amylovora infecting apple rootstocks. Scientific Reports, 8(1), 11689. 10.1038/s41598-018-30064-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im, S. M. , Yu, N. H. , Joen, H. W. , Kim, S. O. , Park, H. W. , Park, A. R. , & Kim, J.‐C. (2020). Biological control of tomato bacterial wilt by oxydifficidin and difficidin‐producing Bacillus methylotrophicus DR‐08. Pesticide Biochemistry and Physiology, 163, 130–137. 10.1016/j.pestbp.2019.11.007 [DOI] [PubMed] [Google Scholar]
- Joseph, N. M. , Sistla, S. , Dutta, T. K. , Badhe, A. S. , Rasitha, D. , & Parija, S. C. (2011). Reliability of Kirby‐Bauer disk diffusion method for detecting meropenem resistance among non‐fermenting gram‐negative bacilli. Indian Journal of Pathology and Microbiology, 54(3), 556–560. 10.4103/0377-4929.85092 [DOI] [PubMed] [Google Scholar]
- Justesen, U. S. , Acar, Z. , Olsson, K. , Jensen, T. G. , Kerrn, M. B. , Skov, R. L. , & Gahrn‐Hansen, B. (2013). Comparison of Rosco Neo‐Sensitabs with Oxoid paper disks in EUCAST disk diffusion antimicrobial susceptibility testing on Mueller‐Hinton agar. European Journal of Clinical Microbiology and Infectious Diseases, 32(5), 621–625. 10.1007/s10096-012-1785-5 [DOI] [PubMed] [Google Scholar]
- King, E. O. , Ward, M. K. , & Raney, D. E. (1954). Two simple media for the demonstration of pyocyanin and fluorescin. Journal of Laboratory and Clinical Medicine, 44(2), 301–307. [PubMed] [Google Scholar]
- Lee, X. , Azevedo, M. D. , Armstrong, D. J. , Banowetz, G. M. , & Reimmann, C. (2013). The Pseudomonas aeruginosa antimetabolite L‐2‐amino‐4‐methoxy‐trans‐3‐butenoic acid inhibits growth of Erwinia amylovora and acts as a seed germination‐arrest factor. Environmental Microbiology Reports, 5(1), 83–89. [DOI] [PubMed] [Google Scholar]
- Lo Cantore, P. , Lazzaroni, S. , Coraiola, M. , Serra, M. D. , Cafarchia, C. , Evidente, A. , & Lacobellis, N. S. (2006). Biological characterization of white line‐inducing principle (WLIP) produced by Pseudomonas reactans NCPPB1311. Molecular Plant‐Microbe Interactions, 19(10), 1113–1120. [DOI] [PubMed] [Google Scholar]
- Mansfield, J. , Genin, S. , Magori, S. , Citovsky, V. , Sriariyanum, M. , Ronald, P. , Dow, M. , Verdier, V. , Beer, S. V. , Machado, M. A. , Toth, I. , Salmond, G. , & Foster, G. D. (2012). Top 10 plant pathogenic bacteria in molecular plant pathology. Mol Plant Pathol, 13(6), 614–629. 10.1111/j.1364-3703.2012.00804.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavrodi, D. V. , Peever, T. L. , Mavrodi, O. V. , Parejko, J. A. , Raaijmakers, J. M. , Lemanceau, P. , Mazurier, S. , Heide, L. , Blankenfeldt, W. , Weller, D. M. , & Thomashow, L. S. (2010). Diversity and evolution of the phenazine biosynthesis pathway. Applied and Environment Microbiology, 76(3), 866–879. 10.1128/AEM.02009-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus, P. S. , & Jones, A. L. (1994). Epidemiology and genetic analysis of streptomycin‐resistant Erwinia amylovora from Michigan and evaluation of oxytetracycline for control. Phytopathology, 84, 627–633. [Google Scholar]
- McManus, P. S. , Stockwell, V. O. , Sundin, G. W. , & Jones, A. L. (2002). Antibiotic use in plant agriculture. Annual Review of Phytopathology, 40, 443–465. [DOI] [PubMed] [Google Scholar]
- Mechan Llontop, M. E. , Hurley, K. , Tian, L. , Bernal Galeano, V. A. , Wildschutte, H. K. , Marine, S. C. , Yoder, K. S. , & Vinatzer, B. A. (2020). Exploring rain as source of biological control agents for fire blight on apple. Frontiers in Microbiology, 11, 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medema, M. H. , Blin, K. , Cimermancic, P. , de Jager, V. , Zakrzewski, P. , Fischbach, M. A. , Weber, T. , Takano, E. , & Breitling, R. (2011). antiSMASH: rapid identification, annotation and analysis of secondary metabolite biosynthesis gene clusters in bacterial and fungal genome sequences. Nucleic Acids Research, 39(suppl_2), W339–W346. 10.1093/nar/gkr466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen, D. D. , Melnik, A. V. , Koyama, N. , Xiaowen, L. U. , Schorn, M. , Fang, J. , Aguinaldo, K. , Lincecum Jr, T. L. , Ghequire, M. G. K. , Carrion, V. J. , Cheng, T. L. , Duggan, B. M. , Malone, J. G. , Mauchline, T. H. , Sanchez, L. M. , Marm Kilpatrick, A. , Raaijmakers, J. M. , De Mot, R. , Moore, B. S. , … Dorrestein, P. C. (2016). Indexing the Pseudomonas specialized metabolome enabled the discovery of poaeamide B and the bananamides. Nat Microbiol, 2, 16197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen, T. H. , Nielsen, T. H. , Sørensen, D. , Tobiasen, C. , Andersen, J. B. , Christophersen, C. , Givskov, M. , & Sørensen, J. (2002). Antibiotic and biosurfactant properties of cyclic lipopeptides produced by fluorescent Pseudomonas spp. from the sugar beet rhizosphere. Applied and Environment Microbiology, 68(7), 3416–3423. 10.1128/AEM.68.7.3416-3423.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu, B. , Vater, J. , Rueckert, C. , Blom, J. , Lehmann, M. , Jin‐Jiang, R. U. , Chen, X.‐H. , Wang, Q. I. , & Borriss, R. (2013). Polymyxin P is the active principle in suppressing phytopathogenic Erwinia spp. by the biocontrol rhizobacterium Paenibacillus polymyxa M‐1. BMC Microbiology, 13, 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norelli, J. L. , Holleran, H. T. , Johnson, W. C. , Robinson, T. L. , & Aldwinckle, H. S. (2003). Resistance of Geneva and Other Apple Rootstocks to Erwinia amylovora . Plant Disease, 87(1), 26–32. [DOI] [PubMed] [Google Scholar]
- Norelli, J. L. , Jones, A. L. , & Aldwinckle, H. S. (2003). Fire blight management in the twenty‐first century: using new technologies that enhance host resistance in Apple. Plant Disease, 87(7), 756–765. 10.1094/PDIS.2003.87.7.756 [DOI] [PubMed] [Google Scholar]
- Olishevska, S. , Nickzad, A. , & Deziel, E. (2019). Bacillus and Paenibacillus secreted polyketides and peptides involved in controlling human and plant pathogens. Applied Microbiology and Biotechnology, 103(3), 1189–1215. 10.1007/s00253-018-9541-0 [DOI] [PubMed] [Google Scholar]
- Omoboye, O. O. , Oni, F. E. , Batool, H. , Yimer, H. Z. , De Mot, R. , & Höfte, M. (2019). Pseudomonas cyclic lipopeptides suppress the rice blast fungus magnaporthe oryzae by induced resistance and direct antagonism. Front Plant Sci, 10, 901. 10.3389/fpls.2019.00901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, J. , Lee, G. M. , Kim, D. , Park, D. H. , & Oh, C.‐S. (2018). Characterization of the lytic bacteriophage phiEaP‐8 effective against both Erwinia amylovora and Erwinia pyrifoliae causing severe diseases in apple and pear. Plant Pathol J, 34(5), 445–450. 10.5423/PPJ.NT.06.2018.0100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philion, V. (2018). Description des produits antibiotiques, de lutte biologique et éliciteurs. Guide de référence en production fruitière intégrée à l’intention des producteurs de pommes du Québec. Réseau Pommier. https://reseaupommier.irda.qc.ca/?p=8271 [Google Scholar]
- Pusey, P. L. , Stockwell, V. O. , & Mazzola, M. (2009). Epiphytic bacteria and yeasts on apple blossoms and their potential as antagonists of Erwinia amylovora . Phytopathology, 99(5), 571–581. [DOI] [PubMed] [Google Scholar]
- Pusey, P. L. , Stockwell, V. O. , Reardon, C. L. , Smits, T. H. M. , & Duffy, B. (2011). Antibiosis activity of Pantoea agglomerans biocontrol strain E325 against Erwinia amylovora on apple flower stigmas. Phytopathology, 101(10), 1234–1241. [DOI] [PubMed] [Google Scholar]
- Rhoads, A. , & Au, K. F. (2015). PacBio Sequencing and Its Applications. Genomics Proteomics Bioinformatics, 13(5), 278–289. 10.1016/j.gpb.2015.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokni‐Zadeh, H. , Li, W. , Sanchez‐Rodriguez, A. , Sinnaeve, D. , Rozenski, J. , Martins, J. C. , & De Mot, R. (2012). Genetic and functional characterization of cyclic lipopeptide white‐line‐inducing principle (WLIP) production by rice rhizosphere isolate Pseudomonas putida RW10S2. Applied and Environment Microbiology, 78(14), 4826–4834. 10.1128/AEM.00335-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, J. , & Khan, A. (2019). Distinct patterns of natural selection determine sub‐population structure in the fire blight pathogen, Erwinia amylovora . Scientific Reports, 9(1), 14017. 10.1038/s41598-019-50589-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockwell, V. O. , Johnson, K. B. , Sugar, D. , & Loper, J. E. (2002). Antibiosis contributes to biological control of fire blight by Pantoea agglomerans strain Eh252 in orchards. Phytopathology, 92(11), 1202–1209. [DOI] [PubMed] [Google Scholar]
- Stockwell, V. O. , Jonhson, K. B. , & Loper, J. E. (2002). Biological control of fire blight: understanding interactions among introduced and indigenous microbial communities. In Lindow S. E., Hecht‐Poinar E. I., & Elliot V. J. (Eds.), Phyllosphere microbiology (pp. 225–239). APS Press. [Google Scholar]
- Temple, T. N. , Thompson, E. C. , Uppala, S. , Granatstein, D. , & Johnson, K. B. (2020). Floral colonization dynamics and specificity of aureobasidium pullulans strains used to suppress fire blight of pome fruit. Plant Disease, 104(1), 121–128. 10.1094/pdis-09-18-1512-re [DOI] [PubMed] [Google Scholar]
- Walker, B. J. , Abeel, T. , Shea, T. , Priest, M. , Abouelliel, A. , Sakthikumar, S. , Cuomo, C. A. , Zeng, Q. , Wortman, J. , Young, S. K. , & Earl, A. M. (2014). Pilon: an integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS One, 9(11), e112963. 10.1371/journal.pone.0112963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber, T. , Blin, K. , Duddela, S. , Krug, D. , Kim, H. U. , Bruccoleri, R. , Lee, S. Y. , Fischbach, M. A. , Müller, R. , Wohlleben, W. , Breitling, R. , Takano, E. , & Medema, M. H. (2015). antiSMASH 3.0‐a comprehensive resource for the genome mining of biosynthetic gene clusters. Nucleic Acids Research, 43(W1), W237–W243. 10.1093/nar/gkv437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, K. E. , Flor, J. E. , Schwartz, R. E. , Joshua, H. , Smith, J. L. , Pelak, B. A. , Liesch, J. M. , & Hensens, O. D. (1987). Difficidin and oxydifficidin: novel broad spectrum antibacterial antibiotics produced by Bacillus subtilis. II. Isolation and physico‐chemical characterization. The Journal of Antibiotics (Tokyo), 40(12), 1682–1691. 10.7164/antibiotics.40.1682 [DOI] [PubMed] [Google Scholar]
- Wood, D. E. , & Salzberg, S. L. (2014). Kraken: ultrafast metagenomic sequence classification using exact alignments. Genome Biology, 15(3), R46. 10.1186/gb-2014-15-3-r46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright, S. A. I. , Zumoff, C. H. , Schneider, L. , & Beer, S. V. (2001). Pantoea agglomerans strain EH318 produces two antibiotics that inhibit Erwinia amylovora in vitro. Applied and Environmental Microbiology, 67(1), 284–292. 10.1128/AEM.67.1.284-292.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin, X.‐T. , Li‐Na, X. U. , Liang, X. U. , & Fan, S.‐S. (2011). Evaluation of the efficacy of endophytic Bacillus amyloliquefaciens against Botryosphaeria dothidea and other phytopathogenic microorganisms. African Journal of Micorbiology Research., 5(4), 340‐345. [Google Scholar]
- Zhao, Y. , Li, P. , Huang, K. , Wang, Y. , Hu, H. , & Sun, Y. A. (2013). Control of postharvest soft rot caused by Erwinia carotovora of vegetables by a strain of Bacillus amyloliquefaciens and its potential modes of action. World Journal of Microbiology & Biotechnology, 29(3), 411–420. 10.1007/s11274-012-1193-0 [DOI] [PubMed] [Google Scholar]
- Zimmerman, S. B. , Schwartz, C. D. , Monaghan, R. L. , Pelak, B. A. , Weissberger, B. , Gilfillan, E. C. , Mochales, S. , Hernandez, S. , Currie, S. A. , Tejera, E. , & Stapley, E. O. (1987). Difficidin and oxydifficidin: novel broad spectrum antibacterial antibiotics produced by Bacillus subtilis. I. Production, taxonomy and antibacterial activity. The Journal of Antibiotics (Tokyo), 40(12), 1677–1681. 10.7164/antibiotics.40.1677 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The sequences of strain P. agglomerans NY60 and B. velezensis FL50S are available in GenBank under the accession numbers: CP034469 (https://www.ncbi.nlm.nih.gov/nuccore/CP034469) and LYNC00000000 (https://www.ncbi.nlm.nih.gov/nuccore/LYNC00000000), respectively.


