Highlights
-
•
Resting prefrontal cortex (PFC) oxygenation is decreased in adolescents with non-suicidal self-injury (NSSI) compared to healthy controls.
-
•
Lower PFC oxygenation (full sample) is associated with greater adverse childhood experiences and less health-related quality of life (HRQoL).
-
•
On the group-level, patients show no alterations of resting state functional connectivity within the PFC.
-
•
Among other clinical variables, increased PFC connectivity (full sample) is associated with greater borderline personality pathology.
Abbreviations: BMI, body mass index; BOLD, blood oxygenation level dependent; BPD, Borderline Personality Disorder; (dl)PFC, (dorsolateral) prefrontal cortex; (f)NIRS, (functional) Near-infrared spectroscopy; fMRI, functional magnetic resonance imaging; HbR, deoxygenated hemoglobin; HbT, total hemoglobin; HC, healthy controls; HRQoL, health related quality of life; NSSI, non-suicidal self-injury; O2Hb, oxygenated hemoglobin; PIN diode, positive intrinsic negative diode
Keywords: Non-Suicidal Self-Injury, Adolescents, Prefrontal Cortex, Near-Infrared Spectroscopy
Abstract
Introduction
Neural alterations in limbic and prefrontal circuits in association with self-injurious behavior have been studied primarily in adult borderline personality disorder (BPD). In adolescent patients, research is still sparse. Here, we used resting functional near-infrared spectroscopy (NIRS) to examine oxygenation of the prefrontal cortex (PFC) and its association with symptom severity in adolescents engaging in non-suicidal self-injury (NSSI) and matched healthy controls (HC).
Methods
Adolescents (12–17 years) with recurrent episodes of NSSI (n = 170) and healthy controls (n = 43) performed a low-demanding resting-state vanilla baseline task. Mean oxygenation of the PFC and functional connectivity within the PFC, were measured using an 8-channel functional NIRS system (Octamon, Artinis, The Netherlands). Various clinical variables derived from diagnostic interviews and self-reports were included in statistical analyses to explore potential associations with PFC oxygenation and connectivity.
Results
Adolescents with NSSI showed significantly decreased PFC oxygenation compared to HC, as indexed by oxygenated hemoglobin. Lower PFC oxygenation was associated with greater adverse childhood experiences and less health-related quality of life (HRQoL). While there was no evidence for alterations in PFC connectivity in adolescents engaging in NSSI compared to HC, increased PFC connectivity in the full sample was associated with greater adverse childhood experience, greater BPD pathology, greater depression severity and psychological burden in general, as well as lower HRQoL.
Conclusion
This study is the first to examine PFC oxygenation using NIRS technology in adolescents engaging in NSSI. Overall, results indicate small effects not specific to NSSI. Clinical implications of these findings and recommendations for further research are discussed.
1. Introduction
Non-suicidal self-injury (NSSI) is defined by the International Society for the Study of Self-Injury as the deliberate physical damage of own body tissue without suicidal intent (International Society for the Study of Self-injury, 2018). It excludes culturally or spiritually accepted behavior. Most commonly, adolescents injure themselves to regulate intense emotions or to cope with distress (Taylor et al., 2018). NSSI has been associated with greater emotion dysregulation (Haid-Stecher and Sevecke, 2019, In-Albon et al., 2008, Wolff et al., 2019) as well as with higher impulsiveness (Glenn and Klonsky, 2010, Hamza and Willoughby, 2019, Hamza et al., 2015, You et al., 2016). The prevalence of NSSI in clinical samples is reported as high as 50% (Plener et al., 2018). Meta-analytic research on non-clinical samples revealed lifetime prevalence rates for single events of NSSI of 17.2%–22.1% in adolescents, 13.4% for young adults, and 5.5% for adults (Lim et al., 2019, Swannell et al., 2014). The high prevalence of NSSI even in nonclinical populations has made the behavior a major public health concern. In the DSM-5, NSSI has been included as a condition that requires further study and is therefore acknowledged as an entity worth consideration in clinical practice and research (American Psychiatric Association, 2013). Albeit concepts considering NSSI as stand-alone diagnostic entity (also see Ghinea et al., 2020), recurrent NSSI is one of the diagnostic criteria for borderline personality disorder (BPD) in the DSM-5 (American Psychiatric Association, 2013) and the presence, severity and duration of NSSI are important predictors for BPD development (Ghinea et al., 2019, Groschwitz et al., 2015). BPD itself is characterized by instability in affect, identity, and interpersonal relationships, alongside increased impulsivity and a tendency for risk-taking and self-harm behavior (American Psychiatric Association, 2013). The onset of BPD is frequently reported in early adolescence with prevalence rates ranging from 0.9% in 14-year old adolescents to 3.2% in 22-year old young adults among the U.S. population (Johnson, Cohen, Kasen, Skodol, & Oldham, 2008). BPD has been associated with a host of comorbid psychiatric disorders such as mood, anxiety, or substance use disorders (Grant et al., 2008, Kaess et al., 2017a, Lenzenweger et al., 2007), a lower health-related quality of life (HRQoL), and higher distress as a function of the severity of personality pathology (Kaess et al., 2017a). In most cases, emerging BPD during adolescence is strongly associated with NSSI (Kaess et al., 2014). Taken together, while NSSI is one of the major symptoms of BPD, in particular in adolescents, there is ongoing debate whether NSSI disorder should be considered as an independent phenomenon and diagnostic entity, requiring further investigation.
Neurobiological mechanisms underlying various psychiatric entities and phenotypes have been under extensive investigation for the past decade, in the hope that the respective studies may contribute to a better understanding of predictors of treatment outcome, improved diagnostics, and the development of tailored interventions (Ehlis et al., 2014, Oldehinkel et al., 2013). While the neurobiological underpinnings of NSSI have not been extensively investigated, more research has been conducted on the neurobiology of BPD. In a review of existing neuroimaging findings, prefrontal dysfunctions during impulse control tasks in adult BPD patients have been mainly found in the orbitofrontal cortex, the dorsomedial PFC, and the dorsolateral prefrontal cortex (dlPFC) (Sebastian et al., 2014). During emotion regulation, alterations of limbic brain activity in adult BPD have been reported in the amygdala, the ventral striatum, the hippocampus and the posterior cingulate cortex. While the literature is consistent regarding the frontolimbic regions involved, there is mixed evidence regarding increases and decreases of brain activation (Dudas et al., 2017, Schulze et al., 2016). Generally speaking, abnormalities in frontolimbic networks have been found to be characteristic of BPD in adult patients (Chanen and Kaess, 2012, Ruocco et al., 2013). Considering that BPD is associated with high comorbidity and burden, neuroimaging studies in BPD yield limited insight into NSSI per se and neglect a developmental perspective by exclusively focusing on adults. As neuroimaging research in NSSI is sparse, studies on BPD patients still serve as a helpful proxy and direction sign for studies on NSSI in adolescents.
The few existing studies, which examined brain activation in individuals engaging in NSSI, primarily focused on task-dependent alterations in neural activity. For example, one study addressing NSSI specifically found that decreased activation in the PFC and the cingulate cortex of young adults with NSSI (n = 15) during an interference task was associated with poorer emotion regulation abilities and increased impulsivity (Dahlgren et al., 2018). Alongside difficulties in emotion regulation and impulse control, alterations in pain sensitivity in patients engaging in NSSI are well documented (Koenig, Thayer, & Kaess, 2016). Further research showed that pain sensation was associated with brain activation in the posterior insula in participants with NSSI and healthy controls (HC), but only HC showed greater neural activity as a function of increasing pain intensity. However, out of n = 14 participants of the NSSI group, only n = 6 reported actual incidents of NSSI during the past 12 months (Bonenberger, Plener, Groschwitz, Grön, & Abler, 2015). Hence, it might be questionable whether these findings are specifically related to NSSI or are driven by other factors. As emphasized in the definition of the International Society for the Study of Self-Injury (International Society for the Study of Self-injury, 2018), NSSI is often related to interpersonal difficulties. In line with this, adolescents with NSSI showed increased activation of the PFC compared to HC and depressed adolescents in a social exclusion paradigm (Groschwitz, Plener, Groen, Bonenberger, & Abler, 2016). Finally, aberrant amygdala connectivity with various cortical regions was found during resting state and an emotion task (n = 24 females with NSSI and n = 20 HC) (Westlund Schreiner et al., 2017). On a brain structural level, volumetric abnormalities of the insula and the inferior frontal gyrus in female adolescent NSSI patients have been reported to be similar to those observed in adult BPD patients (Beauchaine, Sauder, Derbidge, & Uyeji, 2019). Taken together, neuroimaging studies focusing on NSSI only showed neural alterations in association with impulse control, pain sensation, and social exclusion. Unfortunately, it is unknown whether patterns of altered brain activation are due to task-specific demands or exist during rest. The finding of volumetric abnormalities might implicate that activation patterns at rest might differ between NSSI patients and HC.
Unfortunately, the majority of neuroimaging studies in adolescents engaging in NSSI lack statistically sufficient sample sizes. One alternative method with the potential to overcome difficulties associated with sample size due to its high acceptability in patients and relative ease in application, is near-infrared spectroscopy (NIRS). NIRS recordings are based on light within the near-infrared spectrum (650–950 nm). Human scalp and skull are penetrable for light at this wavelength (Ferrari & Quaresima, 2012) and the greatest light absorbing structure in this area is the hemoglobin in the venous vessels of the cortex. Functional NIRS (fNIRS) devices measure changes of oxygenated (O2Hb) and deoxygenated (HbR) hemoglobin over time. In comparison with (f)MRI, fNIRS is conducted with smaller devices and tolerates body movement to a greater degree, which results in a high acceptance among patients undergoing respective recordings (Lai, Ho, Lim, & Ho, 2017) – especially in adolescents. Additionally, fNIRS has superior time resolution, although struggling with exact spatial resolution and measuring only changes in activation on the cortical surface (Koike, Nishimura, Takizawa, Yahata, & Kasai, 2013). During simultaneous fMRI and fNIRS assessment, it has been shown that fNIRS measurement correlates with blood oxygenation level dependent (BOLD) signals in fMRI, suggesting equivalence of both methods when examining activation on the cortical surface (Alderliesten et al., 2014, Bulgarelli et al., 2018).
To our knowledge, there are no previous studies investigating adolescent NSSI patients using NIRS technology. Even research on BPD using NIRS is sparse. In one of the few studies, adult BPD patients (n = 10) showed increased oxygenation in the left medial PFC during a social exclusion paradigm, related to ratings of rejection and fear of abandonment (Ruocco et al., 2010b). A recent study reported hemodynamic alterations (i.e. decreased O2Hb compared to HC during a verbal fluency task) in the frontal, parietal, and temporal cortices of adult (mean age = 32 years) BPD patients (Husain et al., 2020a, Husain et al., 2020b). Young BPD patients (n = 9) with a mean age of 20 years showed a reduced slope in oxygenation of left prefrontal channels when viewing emotional (sad) pictures (Ehlis et al., 2014, Ruocco et al., 2010a). As BPD emerges during adolescence, investigating young patients seems crucial to disentangle effects of chronicity and long-term illness. To our knowledge, PFC oxygenation has not been investigated in adolescents explicitly engaging in NSSI only.
Unfortunately, current evidence on NSSI is barely existent and research on fMRI in NSSI reports mainly task-dependent alterations in the PFC. When extending the focus to BPD, the reported studies above present mixed findings regarding the question whether activity and/or oxygenation levels in the PFC in BPD patients are decreased or increased compared to HC. While findings on brain functional correlates of BPD from task-based studies yielded somewhat inconsistent results due to the different tasks used, the investigation of alterations in intrinsic brain activation during resting state seems important. Further, to detect cortical changes occurring already during the early course of BPD, it is important to examine adolescents engaging in NSSI across the spectrum of BPD pathology. As pointed out in the DSM-5 (American Psychiatric Association, 2013), NSSI is an entity which should be scrutinized in present research. Hence, this study focuses on brain alterations occurring in adolescents engaging in NSSI. In addition to that, the symptom severity of BPD should also be considered to control for effects solely relying on BPD. The aim of the present study therefore was to investigate resting state oxygenation of the PFC in adolescent NSSI patients compared to HC using NIRS. We hypothesized that (1) the mean oxygenation and deoxygenation is lower in patients compared to HC during a resting-state task and that (2) the relative decrease of oxygenation would be correlated with BPD symptom severity. Furthermore, we aimed to investigate differences in connectivity strength between patients and HC in exploratory analyses.
2. Methods
2.1. Participants
Participants were recruited from the outpatient clinic for risk-taking and self-harming behavior (AtR!Sk; Ambulanz für Risikoverhaltensweisen und Selbstschädigung (Kaess et al., 2017b). The specialized outpatient clinic is part of the Clinic for Child and Adolescent Psychiatry at the Center for Psychosocial Medicine at the University of Heidelberg, Germany. The study was approved by the ethical committee of the University of Heidelberg (study ID S-449/2013; study ID S-514/2015) and consisted of two appointments, a diagnostic interview and a neurobiological assessment, at baseline. Adolescents and their caregivers provided written informed consent before inclusion in the study. The recruitment period for baseline assessments started in August 2016 and ended in January 2020. The general inclusion criteria were (1) presentation at our outpatient clinic, (2) written informed consent of the adolescents and their caregivers, (3) age between 12 and 17 years and (4) fluent German language skills. General exclusion criteria for study participation were: (1) acute psychosis, (2) pregnancy, and (3) neurological, endocrinological, or cardiovascular primary diseases, potentially interfering with the neurobiological assessments. For the present analyses, only patients reporting incidents of NSSI on at least five or more days during the past 12 months were included in the analyses. NSSI incidents were defined according to the definition of the International Society for the Study of Self-Injury (International Society for the Study of Self-injury, 2018) and included the intentional damage of own body tissue (e.g., via cutting, biting, hitting, burning).
HC were recruited via advertisement and matched to the patient sample according to age and sex. Exclusion criteria for HC were the same as for the patient group. Further exclusion criteria for the HC group only were: lifetime self-harming behavior, lifetime psychological or psychiatric treatment, or any current psychiatric disorder. After completing the study, all participants received an allowance of 40€ for study participation.
2.2. Procedures
In a first appointment, participants completed an extensive diagnostic assessment. The presence of NSSI, suicidal thoughts and behavior were assessed using the German version of the Self-Injurious Thoughts and Behaviors interview (SITBI-G; Fischer et al., 2014, Nock et al., 2007). To assess BPD the respective sections of the Structured Clinical Interview for DSM-IV Personality Disorders were queried (SCID-II; Wittchen, Zaudig, & Fydrich, 1997). Additionally, in patients, common axis-I disorders were assessed using the semi-structured Mini International Neuropsychiatric Interview for Children and Adolescents (M.I.N.I.-KID; Sheehan et al., 1998). The interview ended with the investigator’s rating of the patient’s global functioning by means of the Global Assessment of Functioning (GAF; Saß, Wittchen, Zaudig, & Houben, 2003) and rating of the severity of psychiatric symptoms on the basis of the Clinical Global Impression Scale (CGI-S; Busner & Targum, 2007).
HC underwent a shortened clinical interview to make sure they did not meet the criteria for any current mental disorders and were not under psychological or pharmacological treatment. In a first interview via telephone, screening questions from the SITBI-G (Fischer et al., 2014) were used to ensure that there was no history of NSSI or suicidal behavior. The Structured Clinical Interview (non-patient edition) (SCID-N/P) was used to check whether there was any evidence for the presence of any axis-I disorder (First et al., 2002). Whenever HC reported any symptoms indicative of the presence of a psychiatric disorder, the M.I.N.I.-KID was used as an additional diagnostic tool. Those participants meeting the criteria for any psychiatric disorder were compensated for their participation in the diagnostic assessment and excluded from further study appointments.
Furthermore, patients and controls answered questionnaires addressing depressive symptoms using the German version of the Depression Inventory for Children and Adolescents (DIKJ; Stiensmeier-Pelster, Schürmann, & Duda, 1991). HRQoL was assessed with the KIDSCREEN-52 (The KIDSCREEN Group Europe, 2006). Adverse childhood experiences (ACE) were assessed with the Childhood Experiences of Care and Abuse questionnaire (CECA-Q3; Kaess et al., 2011). Lastly, general psychological burden was measured using the Symptom Checklist 90-Revised (SCL-90-R; Franke, 1995).
The second part of the study was designed to investigate a host of neurobiological variables as well as the participantś intelligence. All participants were invited for a second appointment within six weeks after their diagnostic assessment. In the beginning of the second appointment, participantś weight and height were measured to calculate the body mass index (BMI). In addition, information about handedness, allergies, and diseases during the past three months were collected. Subsequently, the intelligence quotient (IQ) was assessed using the Hamburg Wechsler Intelligence Scale for Children IV (HAWIK-IV; Petermann & Petermann, 2007). Afterwards, prefrontal resting oxygenation in patients and HC was assessed using NIRS technology. For this purpose, a NIRS device was attached to the participantś forehead. During a five-minute baseline task, prefrontal resting oxygenation was recorded. A detailed description of the measurement is provided below.
2.3. NIRS measurement
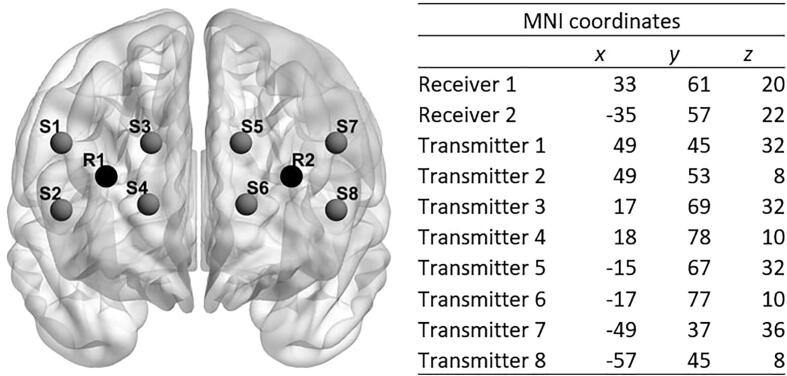
PFC oxygenation was assessed using a portable 8-channel continuous-wave NIRS-system (OctaMon, Artinis, The Netherlands). NIRS is an optical neuroimaging tool consisting of light sources and receivers which are attached to the forehead of the participants with a headband. The light sources emit light in the near-infrared spectrum. The near-infrared light passes the skull cap and the cerebrospinal fluid before encountering the brain. The different tissues have unique light scattering and absorbing properties. The attenuation of light intensity is described by the modified Beer-Lambert law which calculates the absorption of light by different tissues and substances. As oxygenated hemoglobin best absorbs light with a wavelength greater than 800 nm and deoxygenated hemoglobin has its highest sensitivity to light at a wavelength smaller than 800 nm, the eight transmitters of the OctaMon emit light at two wavelengths, 760 nm and 850 nm, to detect both oxygenated and deoxygenated hemoglobin. The emitted light is recorded by two positive intrinsic negative (PIN) diode receivers with ambient light protection. The arrangement of receivers and transmitters (summarized by the term optodes) on the forehead is displayed in Fig. 1. Inter-optode distance was fixed at 35 mm. Optodes were placed onto the forehead of the participants according to the international 10–20 system for EEG electrodes placement (Jaspers, 1958). Estimated coordinates of optodes according to the Montreal Neurological Institute brain template are provided in Fig. 1. When placing the NIRS headband, the investigator made sure that no hair was between optodes and skin impairing signal strength. The data acquisition values for signal strength should be between 3 and 97% of the intensity of the emitted light. If the percentage is close to 0%, light absorption in the tissue is so high that almost no signal reaches the receiver. A percentage close to 100% indicates that environmental light was received and measured. According to the general equation for the differential path length factor and in regard to current study protocols, the differential path length factor was set to six centimeters (Scholkmann & Wolf, 2013). A sampling rate of 50 Hz was set for each channel. The penetration depth of the NIRS light is around 17 mm. All participants were seated in front of a computer screen and performed a five-minute vanilla baseline task. Vanilla tasks are designed to be minimally demanding and have been shown to have consistent within- and baseline stability as well as generalizability between sessions (Jennings, Kamarck, Stewart, Eddy, & Johnson, 1992). The task in our study consisted of a colored rectangle which is presented in the middle of the screen. The rectangle contained one of the following colors: red, green, yellow, blue, purple, white. Every few seconds, it changed its color. Participants were asked to count the incidents of one specific color. After five minutes, they had to report the number of incidents.
Fig. 1.
Optode placement on the forehead; Note. R = receiver, S = source.
2.4. NIRS data preprocessing
Hemoglobin density values were recorded by the NIRS device and sent to a laptop via Bluetooth. There, the raw optical density measures were stored with the Oxysoft software version 3.0.103 (Artinis Medical Systems, 2016). NIRS data were segmented according to the start and end times of the color detection task. For analysis, they were imported to MATLAB (The Math Works Inc., 2015) using the oxysoft2matlab function and preprocessed with the HOMER2 toolbox (Huppert, Diamond, Franceschini, & Boas, 2009). During preprocessing, the raw optical densities were first converted to optical density (hmrIntensity2OD), which is recommended to detect and correct the data for motion artifacts (Cooper et al., 2012). Next, motion artifacts were rectified in a two-step process. First, wavelet-based motion correction with a probability threshold of α = 0.01 was applied (hmrMotionCorrectWavelet). In a second step motion artifacts were corrected (hmrMotionArtifact). Additionally, high-frequent noise was removed with a Bandpass filter which removed frequencies greater than 0.5 Hz. As a final step, optical density rates were converted to hemoglobin concentration for O2Hb, HbR and HbT (HbT = O2Hb + HbR) and exported to Stata/SE software version 16.0 (StataCorp, 2019).
2.5. Statistical analysis
All statistical analyses were conducted either in Stata/SE software version 16.0 (StataCorp, 2019) or in MATLAB (The Math Works Inc., 2015). In line with previous research (Alderliesten et al., 2014, Artemenko et al., 2018, Niu et al., 2013, Niu et al., 2011, Pinti et al., 2020, Seidel et al., 2017, Shi et al., 2014), we assessed the mean of O2Hb, HbR and HbT across the standardized CDT, instead of applying event-related analyses of NIRS data. For each hemoglobin variable (O2Hb, HbR, HbT) mean values per channel and a grand mean value for oxygenated (O2Hb), deoxygenated (HbR), and total hemoglobin (HbT) were calculated. For hypothesis one, t-tests were calculated for each hemoglobin variable to test for general differences between the groups. In a second step, the influence of potential confounding factors on PFC oxygenation was tested via t-tests (handedness, sex) and Pearson product-moment correlations (age, BMI, IQ). In a final step, regression models were calculated with group as predictor for differences in the hemoglobin variables of interest, while adjusting for those factors that showed significant influence on the variable of interest in step two. For hypothesis two, Pearson product-moment correlations were used to detect associations between PFC oxygenation and clinical variables, in case a respective hemoglobin variable showed significant group differences. First, correlations for variables of interest, assessed in both groups (number of BPD criteria, SCL-90-R, KIDSCREEN-52, CECA-Q3, DIKJ), were calculated in the full-sample. Second, analyses were repeated in the NSSI sample only – to avoid inflation of correlation coefficients - further including variables only assessed in patients (e.g., NSSI frequency, number of suicide attempts, CGI-S, GAF). For the exploratory analysis on connectivity within the PFC across all participants, cross-correlations between the n = 8 channels over the time of the CDT were generated with the FC-NIRS toolbox in MATLAB (Xu et al., 2015). Correlation coefficients from connectivity analyses for both groups were compared using Fisheŕs z-transformation. In line with mean oxygenation parameters, associations between cross-correlation coefficients and clinical variables were assessed, following the approach outlined above. For all analyses, a significance level of p = .05 was applied. For visualization of the data, channel locations were provided as MNI coordinates and projected to a brain template using the BrainNet toolbox (see Fig. 1) (Xia et al., 2013). For visualization of results, differences in mean activation were assigned to the corresponding channels. A nifti-data format was generated in xjview (XjView Toolbox, 2019) and visualized using the software SurfIce (Surf Ice, 2019) and BrainNet (Xia et al., 2013). Visualization of the heat maps for the connectivity analyses was done within the FC-NIRS toolbox.
3. Results
3.1. Sample characteristics
For the NSSI group, written informed consent was provided by n = 257 consecutive patients. Of these, n = 242 (94.16%) participated in the baseline assessment. N = 227 (88.33%) completed the NIRS assessment. N = 195 (75.88%) reported five or more events of NSSI during the past twelve months. N = 25 (9.73%) had to be excluded from the present analyses due to problems during the NIRS assessment (e.g., signal loss), resulting in a patient group of n = 170 (66.15%) participants. N = 58 adolescents provided written informed consent for the HC group. Of these, n = 49 (84.48%) completed the baseline assessment. Reasons for not completing the baseline assessment were withdrawal of interest in study participation (n = 7; 12.07%), lifetime history of NSSI (n = 1; 1.72%), and present psychiatric disorder (n = 1; 1.72%). N = 6 (10.34%) adolescents from the HC group were excluded from data analyses due to problems during the NIRS assessment resulting in a HC group of n = 43 (74.14%) adolescents. Eventually, the final study sample consisted of n = 170 NSSI patients and n = 43 HC. For a detailed description of sociodemographic and clinical characteristics of the current sample see Table 1. Groups differed on IQ (t(211) = 2.491; p = .014), with lower IQ in the patient group, and sex (χ2(1) = 5.524; p = .019) due to the fact that the present sample only consisted of a sub sample (only those patients engaging in NSSI) and due to data loss during NIRS assessment. No significant group differences were found for age, school type, handedness, nor BMI.
Table 1.
Sociodemographic and clinical characteristics by group.
| NSSI | HC | P | ||
|---|---|---|---|---|
| N (% female) | 170 (84.12) | 43 (95.55) | 0.019 | |
| Age (SD) | 15.04 (1.47) | 14.74 (1.31) | 0.228 | |
| School type, n (%) | 0.086 | |||
| Hauptschule | 19 (11.18) | 1 (2.33) | ||
| Realschule | 63 (37.06) | 14 (32.56) | ||
| Gymnasium | 61 (35.88) | 27 (62.79) | ||
| Other | 27 (15.88) | 1 (2.33) | ||
| IQ (SD) | 100.83 (1.07) | 106.56 (1.78) | 0.014 | |
| Right-handedness, n (%) | 154 (90.59) | 40 (93.02) | 0.617 | |
| BMI (SD) | 21.58 (0.34) | 20.34 (0.51) | 0.096 | |
| DIKJ (SD) | 28.41 (9.94) | 6.21 (4.03) | <.001 | |
| KIDSCREEN-52 (SD) | 34.95 (6.93) | 56.07 (8.43) | <.001 | |
| CECA-Q3 (SD) | 0.35 (0.30) | 0.02 (0.07) | <.001 | |
| SCL-90-R (SD) | 1.55 (0.74) | 0.21 (0.17) | <.001 | |
| CGI-S (SD) | 4.96 (0.76) | - | ||
| GAF (SD) | 49.76 (8.97) | - | ||
| Comorbidity (ICD-10), n (%) | ||||
| F0X | 0 | - | ||
| F1X | 40 (23.53) | - | ||
| F2X | 0 | - | ||
| F3X | 99 (58.24) | - | ||
| F4X | 69 (40.59) | - | ||
| F5X | 18 (10.59) | - | ||
| F6X | 60 (35.29) | - | ||
| F7X | 0 | - | ||
| F8X | 2 (1.18) | - | ||
| F9X | 54 (31.76) | - | ||
| Number of suicide attempts (SD) | ||||
| lifetime | 10.81 (58.57) | - | ||
| past 12 months | 1.60 (8.37) | - | ||
| past 6 months | 1.71 (4.74) | - | ||
| Number of NSSI events (SD) | ||||
| past 12 months | 75.56 (92.84) | - | ||
| past 6 months | 38.37 (40.21) | - | ||
Note. NSSI = non-suicidal self-injury, HC = healthy controls, SD = standard deviation, IQ = intelligence quotient, BMI = body mass index, DIKJ = Depression Inventory for Children and Adolescents, KIDSCREEN-52 = Health-related quality of life questionnaire, CECA-Q3 = Childhood Experiences of Care and Abuse questionnaire, SCL-90-R = Symptom Checklist 90-Revised, CGI-S = Clinical Global Impression Scale, GAF = Global Assessment of Functioning.
N = 46 (27.06%) patients fulfilled criteria for BPD diagnosis (≥5 criteria). On average, NSSI patients met 3.14 (SD = 2.11) BPD criteria. Mostly, they met the criterion on recurrent suicidality and NSSI behavior (74.7%), followed by emotional instability (54.82%) and chronic feelings of emptiness (33.73%). The mean age of onset of NSSI was 12.81 years (SD = 0.13). N = 73 (42.94%) patients reported at least one suicide attempt. Number of suicide attempts and events of NSSI are also provided in Table 1. NSSI patients and HC differed significantly on all clinical measures of interest (including number of BPD criteria, depression symptoms, ACE, and HRQoL), indicating greater burden of psychopathology in adolescents engaging in NSSI (see Table 1).
3.2. PFC activation
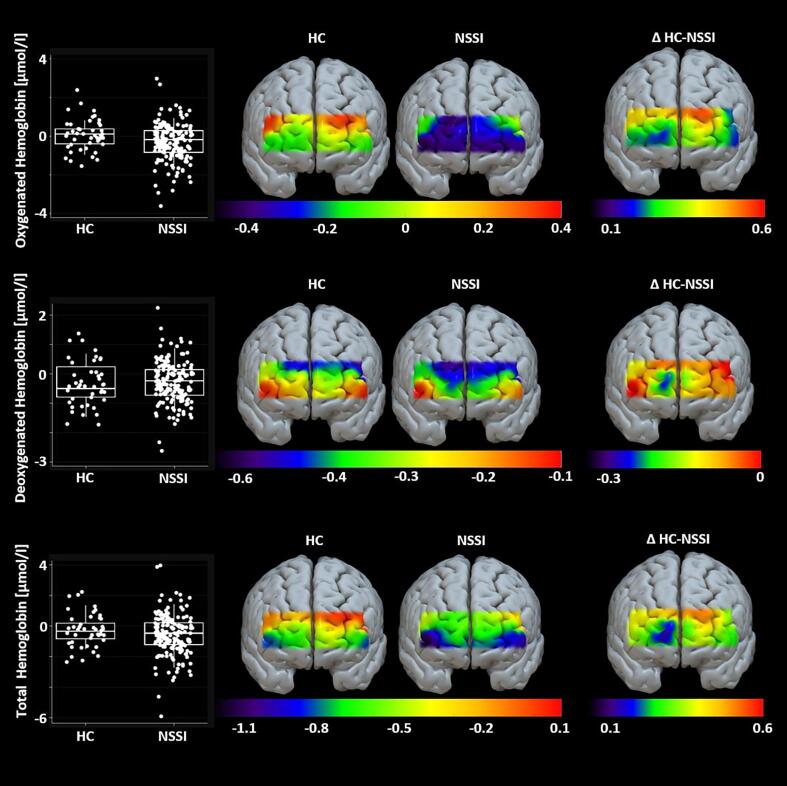
First, paired t-tests were calculated to investigate general group differences in PFC oxygenation. The NSSI group showed significantly decreased O2Hb (t(2 1 1) = 2.333; p = .021) compared with HC. No significant group differences were found for HbR (t(2 1 1) = -0.654; p = .514) nor HbT (t(2 1 1) = 1.281; p = .201; see also SM Table 1). In a second step, we aimed to investigate potential confounders of PFC oxygenation (handedness, sex, age, BMI, IQ). None of the tested variables was significantly related to O2Hb (see SM Table 2, SM Table 3, and SM Table 4). Age and IQ were significantly correlated with HbR (age: r(208) = -0.137, p = .048; IQ: r(211) = 0.141, p = .040) while IQ was also correlated with HbT (r(211) = 0.168, p = .014). None of the remaining variables (handedness, BMI, sex) was significantly related to PFC oxygenation. In a third step, regression models were calculated with group as predictor. Results from model one provided evidence that O2Hb differed between groups (F(1,211) = 5.45, p = .021; NSSI: β = -0.353, t(212) = -2.33, p = .021). Model two yielded no evidence that HbR differed between groups, while adjusting for age and IQ (F(3,204) = 2.90, p = .036; NSSI: β = 0.144, t(207) = -1.21, p = .227; age: β = -0.063, t(207) = -1.91, p = .058; IQ: β = 0.007, t(207) = 1.97, p = .051). Finally, model three did not provide evidence of group differences in HbT, when adjusting for IQ (F(2,209) = 3.40, p = .035; NSSI: β = -0.182, t(211) = -0.84, p = .400; IQ: β = 0.015, t(211) = 2.29, p = .023). Further model characteristics are presented in SM Table 5. Mean PFC oxygenation and group differences for all hemoglobin variables are displayed in Fig. 2.
Fig. 2.
Differences in prefrontal brain activation across groups; Note. Hemoglobin unit = μmol/l, HC = healthy controls, NSSI = non-suicidal self-injury group, ΔHC-NSSI = difference in hemoglobin concentration between HC and NSSI group.
3.3. Association between PFC oxygenation and symptom severity
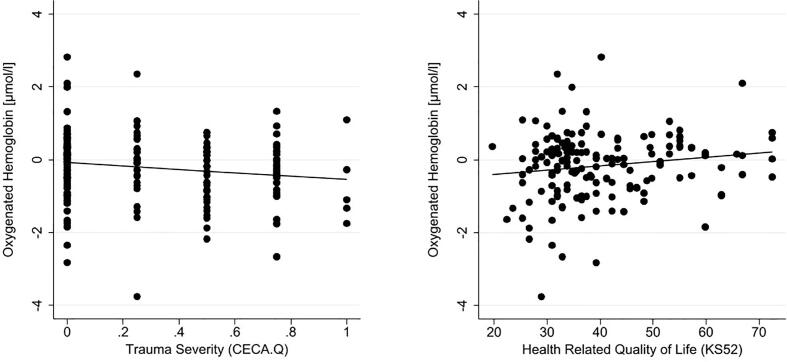
First, Pearson product-moment correlations between O2Hb and clinical variables that were assessed in both groups were calculated. Here, significant correlations were found between O2Hb and ACE (CECA-Q3: r(187) = -0.155; p = .034) and between O2Hb and HRQoL (r(162) = 0.154; p = .049; see also Fig. 3). No significant correlations were found between O2Hb and the number of BPD criteria met (r(207) = -0.105; p = .129), general symptom severity (SCL-90-R; r(188) = -0.100; p = .169), or depression severity (DIKJ; r(186) = -0.100; p = .175). In subsequent calculations, only the patient group was included. In addition to the correlations above, the number of incidents of NSSI during the past 12 months and during the past six months, number of suicide attempts (lifetime), clinical global impression (CGI-S), and level of functioning (GAF) were included to the analysis. None of the Pearson product-moment correlations revealed a significant relationship to O2Hb in the PFC (number of NSSI events during the past 12 months: r(168) = 0.062; p = .420; number of NSSI events during the past six months: r(148) = 0.050; p = .544; number of suicide attempts during lifetime: r(70) = 0.065; p = .589; clinical global impression: r(160) = -0.113; p = .154; GAF: r(160) = 0.118; p = .135; number of BPD criteria: r(164) = -0.022; p = .783; depression severity: r(143) = 0.030; p = .719; general symptom severity: r(146) = 0.014; p = .870; trauma severity: r(145) = -0.109; p = .189; HRQoL: r(122) = 0.024; p = .794).
Fig. 3.
Significant correlation between O2Hb and Adverse Childhood Experiences and Health-Related Quality of Life; Note. CECA = Childhood Experiences of Care and Abuse questionnaire, KIDSCREEN-52 = Health-related quality of life questionnaire.
3.4. Analysis of functional connectivity
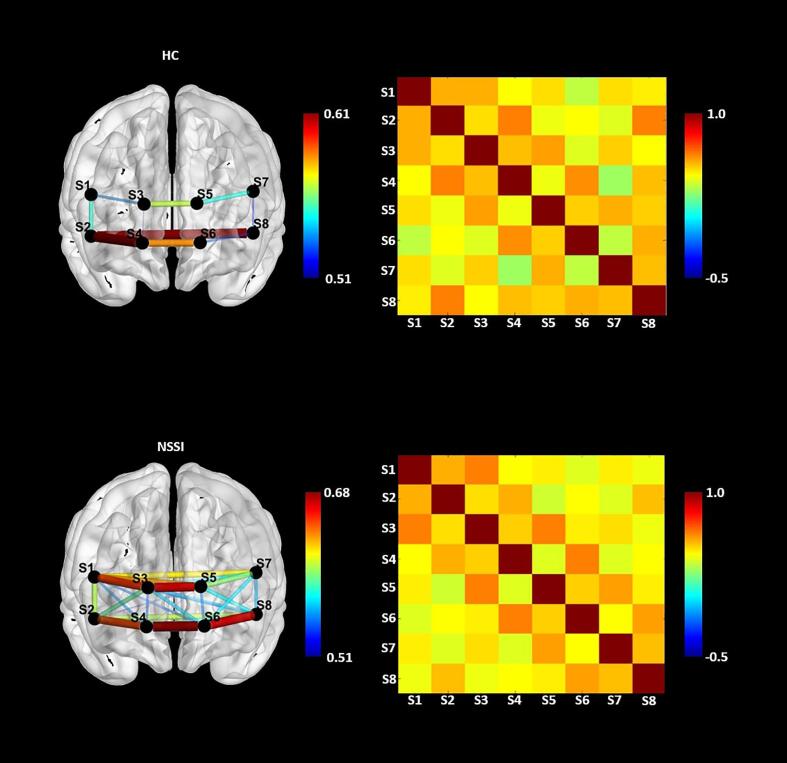
Due to technical problems, n = 2 NSSI patients had to be excluded from the connectivity analyses resulting in n = 168 patients and n = 43 HC that were subject to the analysis. Connectivity analyses within the PFC were calculated only for O2Hb, as this measure differentiated the NSSI and HC group in the preceding analyses. Cross-correlation coefficients were determined between all channels for each group (see SM Table 6 and SM Table 7). Subsequently, Fisheŕs z-transformation was conducted, and PFC connectivity values were compared between groups. Whereas almost all channels revealed a significant connectivity between channels on the within-group level (see Fig. 4), no differences between groups were found (see SM Table 8). As illustrated in Fig. 4, connectivity measures were descriptively higher in NSSI patients (range: r(166) = 0.488 – r(166) = 0.680; all p < .0001) compared to HC (range: r(41) = 0.285 – r(41) = 0.613; p = .064 – p < .001). Both groups showed especially high connectivity between channels covering the orbitofrontal cortex area and slightly lower connectivity in the dorsolateral cortex areas.
Fig. 4.
Brain connectivity pattern of cross correlations between HC and NSSI patients; Note. In the left only the topmost correlations with correlation coefficients greater than r = 0.50 are displayed.
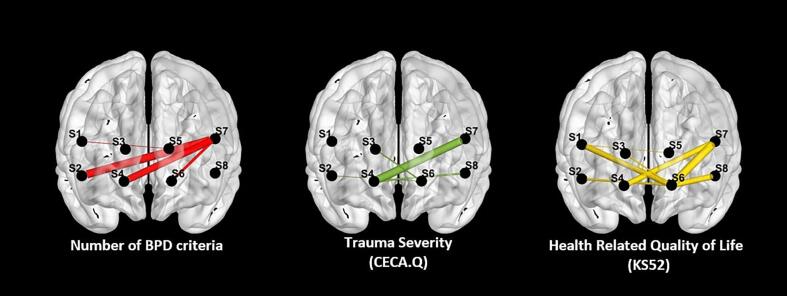
When examining associations between PFC connectivity and clinical variables across all participants, positive correlations were found between the number of BPD criteria and O2Hb connectivity in n = 6 inter- and intrahemispheric channel pairs (range: r(205) = 0.138, p = .047 – r(205) = 0.177, p = .011), depressive symptoms and connectivity in n = 3 mostly left hemispheric channel pairs (range: r(184) = 0.148, p = .045 – r(184) = 0.179), general psychological burden (SCL-90-R) and connectivity in n = 3 channel pairs (range: r(187) = 0.154, p = .035 – r(187) = 0.166, p = .023), and ACE (CECA-Q3) in n = 4 channel pairs (range: r(186) = 0.155, p = .034 – r(186) = 0.184, p = .012). Negative correlations were found between HRQoL (KIDSCREEN-52) and connectivity between n = 8 channel pairs (range: r(161) = -0.157, p = .046 – r(161) = - 0.228, p = .004). We did not find evidence of associations between global clinical impression (CGI-S) and PFC connectivity. Associations are illustrated for the number of BPD criteria, ACE and HRQoL in Fig. 5. All correlations are provided in the Supplemental Material (SM Table 9 to SM Table 14).
Fig. 5.
Significant correlations between Connectivity Measures and Clinical Measures; Note. Only significant correlations with p < .50 are displayed. Positive correlations between number of BPD criteria and adverse childhood experience and negative correlations between health-related quality of life with prefrontal cortex connectivity.
4. Discussion
To our knowledge, this is the first study to investigate resting-state PFC oxygenation in adolescents with NSSI. Analyses revealed significantly decreased O2Hb concentration in the PFC in the NSSI group compared to HC. No group differences were found for HbR and HbT. Thus, hypothesis one was partially confirmed. For hypothesis two, we found decreased O2Hb concentrations associated with ACE as well as lower HRQoL. Unlike hypothesized, there was no association with BPD pathology. Exploratory analyses on PFC connectivity revealed no significant group differences. However, correlations of the connectivity measures with clinical variables revealed that stronger connectivity was associated with greater BPD pathology, more depressive symptoms, higher general psychological burden and ACE as well as a lower HRQoL.
Our finding of reduced PFC oxygenation in adolescents engaging in NSSI adds some clarity to previous findings on brain functional correlates of NSSI and BPD that were somewhat inconsistent. Several studies using task-based fMRI found increased activation of different parts of the PFC, including dlPFC (Dudas et al., 2017), orbitofrontal PFC (Vega et al., 2018), and dmPFC (Malejko et al., 2019) in adults with NSSI and/or BPD. In contrast, meta-analytic research in BPD patients (Schulze et al., 2016) as well as one of the few studies in younger patients with NSSI (n = 15) found decreased activation of the PFC during an interference task (Dahlgren et al., 2018). Our finding is in line with the sparse existing literature in adolescents and potentially illustrates a developmental effect; we suggest that activity of the PFC might switch from under- (adolescence) to over- (adult) activation as a function of age and development. Importantly, however, most of the existing studies – in adults and adolescents - have been conducted in relatively small samples, did not carefully distinguish NSSI and BPD, and group differences in brain activation have been found in task-based designs only. Our study is one of the first to show decreased resting-state PFC oxygenation in a relatively large sample of well-characterized adolescents engaging in NSSI.
As our study examined differences in resting-state prefrontal activation and not of task-dependent alterations, a comparison to findings on structural changes in the PFC seems warranted. For example, studies using structural MRI to characterize brain alterations in patients with NSSI and BPD have shown reduced grey matter volume of the bilateral dlPFC as well as the left orbitofrontal cortex (Brunner et al., 2010) and the right orbitofrontal cortex (Chanen et al., 2008). In the same vein, a meta-analysis found reduced grey matter volume of the right inferior frontal gyrus in BPD patients, with greater alterations in older samples (Schulze et al., 2016). A recent study in adolescents with NSSI, however, found decreased brain volume of the insula and the anterior cingulate cortex but no differences in PFC volume compared to healthy adolescents (Ando et al., 2018). Finally, while not caption by our current investigation using NIRS, alterations of deeper brain structures, indexed e.g., by a decreased volume of the pituitary gland or decreased activity of the limbic system, were shown in adolescent and adult BPD patients (Chanen and Kaess, 2012, Dudas et al., 2017, Groschwitz and Plener, 2012, Jovev et al., 2012, Ruocco et al., 2013, Whittle et al., 2009). Taken together, research consistently shows grey matter volume losses in patients with NSSI and/or BPD. These structural differences might contribute to decreased PFC oxygenation during a rest. Furthermore, it might explain prefrontal overaction during specific tasks, as described in the studies above, to compensate structural deficits. However, further research, integrating structural and functional measures, is needed to support these assumptions.
We have shown that NSSI patients and HC differed significantly on mean O2Hb only. Most studies that investigated alterations in activation using NIRS focused on O2Hb only and did not report values for the other two hemoglobin variables or only in the respective supplemental material (Husain et al., 2020a, Ruocco et al., 2010a, Ruocco et al., 2010b, Ruocco et al., 2016). Generally speaking, differences between groups are easier to detect when focusing on O2Hb (Ferreri et al., 2014). This phenomenon is routed in the physiology of brain activity and reactivity. NIRS technology measures O2Hb and HbR concentration changes in the venous vessels. After O2Hb has arrived in the cells through arterial vessels, the oxygen is partially consumed by the cells resulting in O2Hb and HbR in the venous vessels. Whenever more oxygen is demanded, the cells are overflooded with O2Hb. As the consumption of O2Hb rises, the amount of HbR in the venous vessels rises simultaneously. But because of the overflow of O2Hb, the ratio of O2Hb in the venous vessels increases even more (Obrig, Rossi, Telkemeyer, & Wartenburger, 2010). Hence, it is important to report both O2Hb and HbR and related to this its sum, HbT. But as differences in O2Hb are bigger than in HbR, it is easier to detect significant results. Hence, the reported group differences in O2Hb – also of small effect size - support the assumption that PFC activation is decreased in NSSI patients compared to HC during resting-state. Surprisingly, we did not find evidence of associations between PFC oxygenation and specific NSSI behavior. However, there was some evidence that decreased oxygenation was associated with ACE and HRQoL. Longitudinal treatment studies are warranted, assessing the clinical relevance of these associations. In a very first study, the influence of psychotherapy on cortical activation in BPD patients was examined (Ruocco et al., 2016). An increase in cortical activation in patients (n = 18) that reduced their self-harming behavior the most over the course of dialectical behavioral therapy was found. This finding indicates that changes in prefrontal oxygenation may help to monitor treatment progress and outcome.
Recent efforts in neuroimaging research, aimed at elucidating neural concomitants of psychiatric symptomatology, have been directed at studying not only regional brain activation, but rather the interplay between different regions, i.e., brain connectivity. While we found no group differences in PFC connectivity comparing NSSI and HC, O2Hb connectivity was associated with a range of clinical measures, indicating higher connectivity in case of worse outcome - a seemingly counterintuitive finding. However, the only study examining resting-state connectivity in NSSI, has found greater connectivity between amygdala and the supplementary motor area as well as dorsal anterior cingulate to be associated with NSSI (compared to HC) (Westlund Schreiner 2017). Associations between connectivity and other clinical variables have not been investigated, limiting the integration of the present findings. Potentially, higher connectivity between prefrontal regions might act as a compensatory mechanism in those patients with greater psychopathology in order to counteract overactivation of the limbic system (Niedtfeld et al., 2010, Plener et al., 2012), a speculative hypothesis that remains to be tested in future fMRI studies in NSSI and adolescent BPD.
Albeit the known advantages of NIRS technology, including high feasibility and acceptance by patients, facilitating recruitment of larger samples, this method is limited to the study of cortical brain oxygenation - in the present study even limited to PFC brain oxygenation. While the literature mentioned in the present report, as well as the data, speak for the relevance of the PFC for neurobiological research on NSSI and BPD, findings from MRI studies have also emphasized the importance of limbic structures. In fact, the interplay between the limbic and frontal regions has been demonstrated to be crucial for emotion regulation (e.g., Etkin, Büchel, & Gross, 2015) and as such might be a promising target when investigating neurobiological mechanisms underlying NSSI. Therefore, more research on brain connectivity including prefrontal and limbic regions underlying NSSI as a precursor of BPD, is needed.
Studying resting-state PFC oxygenation may provide insights in basic physiological structures that may determine the phenotype of NSSI and BPD. The current study provides evidence that resting-state NIRS technology is able to differentiate between healthy and psychiatric samples. A critical question in resting state analysis is the required length of the paradigm to be sure that activity levels have stabilized. Research using NIRS technology has shown that signal stabilization occurs within the first minute of application, readily captured by our five minute paradigm (Geng, Liu, Biswal, & Niu, 2017). Further, research has illustrated the general suitability of NIRS devices to index resting-state activation (Niu et al., 2011). Traditionally, connectivity analyses are based on MRI technology or electroencephalogram. When comparing BPD patients either during resting-state or during tasks, altered connectivity was found with fMRI (Das et al., 2014, Zhu et al., 2017). Further, lateral asymmetries were illustrated using electroencephalogram (Beeney et al., 2014, LeBoeuf et al., 2016). In the present study, no significant differences in connectivity between the two groups were found. However, it should be emphasized that this sample did not consist of BPD patients solely but of adolescents engaging in NSSI, across the spectrum of BPD pathology. This might explain why no significant differences were detected although patterns of connectivity differed.
As most research on PFC alterations in association with self-injurious behavior is limited to adult BPD patients, the present research extends our understanding of adolescent NSSI. It shows that findings from adult BPD samples are comparable to adolescent NSSI samples. In both groups, alterations in the PFC were found with decreased activation in patients compared to HC regardless of the imaging modality (MRI or NIRS) (Brunner et al., 2010, Chanen et al., 2008, Dudas et al., 2017, Husain et al., 2020a, Ruocco et al., 2010a, Ruocco et al., 2010b, Ruocco et al., 2016, Schulze et al., 2016). Hence, we suggest that decreased PFC activation is a potential feature of NSSI, independent of BPD. As these alterations are readily evident in adolescents, it can be assumed that prefrontal hypoactivation is not a consequence of the long-term course of comorbid psychopathology (such as BPD) or psychotropic medication intake for years - although an increase of functional aberrations in limbic systems in BPD patients has been found with older age (Schulze et al., 2016). One core feature of BPD and NSSI are difficulties in emotion regulation (Carpenter and Trull, 2012, Dixon-Gordon et al., 2015, Glenn and Klonsky, 2009). Successful emotion regulation requires increased activity of the PFC (Fusar-Poli et al., 2009, Golkar et al., 2012, Kim et al., 2012, Kober et al., 2008, Ochsner et al., 2002). Our finding of reduced PFC oxygenation at rest might be one contributing factor underlying these deficits in executive function. However, difficulties in emotion regulation have been shown in various psychiatric disorders, including depression, anxiety, substance use and eating disorders (Sloan et al., 2017). For example, adult depressive patients showed lower integral values over the course of a verbal fluency task compared to controls (Husain et al., 2020a, Husain et al., 2020b). Studies including clinical controls and explicit measures of emotion regulation are warranted to address the specificity versus generalizability of findings. Hence, the hypoactivation of the PFC found in this study might not represent a specific feature of BPD but supports the assumption that impaired emotion regulation plays a crucial role in NSSI as well as general psychopathology. The finding that self-perceived HRQoL decreased with a decline of PFC activation emphasizes that there might be a relationship between general well-being and prefrontal oxygenation. Further research is required to investigate the relationship between well-being, symptomatology and brain activation in detail utilizing longitudinal designs.
The present study has several limitations that should be considered when interpreting the results. First, although NIRS devices have a great spatial resolution, exact optode placement could only be estimated as we were not able to determine exact positioning with a 3D digitizer. Therefore, exact interpretation of region of interests for connectivity analysis was not possible. Second, as previously mentioned, the focus of this study relied on the PFC surface only. The PFC is known as the neural control center for integrating information from different brain regions. Hence, connectivity and activity analyses covering different regions of interest would be very informative and should be considered in future studies examining BPD and NSSI in adolescents. Furthermore, activation patterns and connectivity analyses could have revealed more distinctive results when using a fNIRS device with a higher density of channels covering the PFC, such as those used in other studies with up to 48 channels covering prefrontal areas (Zhang et al., 2020). Additionally, long-term effects of therapeutic interventions will help to provide more knowledge on the relationship of certain symptoms with neural alterations as well as the durability of these improvements. A further limiting factor of the study sample is that it mainly consisted of female, help-seeking adolescents. Hence, sex differences in PFC oxygenation could not be examined. When considering previous research on sex differences in brain activation, it becomes clear that differences may be related to certain tasks. For example, sex differences in brain activation have been found for working memory tasks (Li et al., 2010, Schmidt et al., 2009), for the processing of emotional stimuli (Stevens & Hamann, 2012) as well as for visuo-spatial, memory and emotion tasks (AlRyalat, 2018). Therefore, it would have been interesting to investigate sex differences in adolescent NSSI patients, especially as self-harming behavior often differs between sexes (Andover, Primack, Gibb, & Pepper, 2010). Lastly, the study sample consisted of a NSSI group and a HC group only. To disentangle whether hypoactivation of the PFC in the NSSI group is specifically related to NSSI or general psychopathology, future studies in the field should include an additional clinical control group.
5. Conclusion
In conclusion, the present results emphasize the importance to investigate neural alterations in young patients with NSSI and provide some insight on underlying etiological factors that may contribute to impaired emotion regulation, associated with NSSI, comorbid psychopathology, and decreased health related quality of life. Future studies are needed to investigate the specificity of the reported neural alterations in patients with NSSI, including samples of clinical controls, and their long-term clinical trajectories in association with comorbid psychiatric diagnoses.
Funding
The outpatient clinic for risk-taking and self-harm behavior (AtR!Sk) and AtR!Sk-Bio are funded by the Dietmar Hopp Stiftung. The authors declare no conflict of interest.
CRediT authorship contribution statement
Julian Koenig: Conceptualization, Formal analysis, Funding acquisition, Project administration, Supervision, Writing - original draft. Saskia Höper: Data curation, Formal analysis, Investigation, Visualization, Writing - original draft. Patrice van der Venne: Project administration, Investigation, Writing - review & editing. Ines Mürner-Lavanchy: Writing - original draft. Franz Resch: Writing - review & editing. Michael Kaess: Conceptualization, Funding acquisition, Project administration, Supervision, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
We wish to thank Felix Birmanns, Nebile Guezel, Anna-Sophia Roesch, Anne Homann, Pelin Kilavuz, Iris Siljak and Henriette Thater for their help in recruiting participants and conducting the neurobiological assessments. We thank Gloria Fischer-Waldschmidt,
Denisa Ghinea, Alexandra Edinger, Sindy Weise, Natascha Schmitt, Ines Baumann, Annika Beckmann and Monika Schwarz for their continuous help in recruiting patients and conducting the clinical interviews.
References
- AlRyalat S.A. Gender similarities and differences in brain activation strategies: voxel-based meta-analysis on fMRI studies. J. Integrative Neurosci. 2018;16(2):227–240. doi: 10.3233/JIN-170015. [DOI] [PubMed] [Google Scholar]
- Alderliesten T., De Vis J.B., Lemmers P.M.A., van Bel F., Benders M.J.N.L., Hendrikse J., Petersen E.T. Simultaneous quantitative assessment of cerebral physiology using respiratory-calibrated MRI and near-infrared spectroscopy in healthy adults. NeuroImage. 2014;85:255–263. doi: 10.1016/j.neuroimage.2013.07.015. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . 5th ed. Author; Arlington, VA: 2013. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- Ando A., Reichl C., Scheu F., Bykova A., Parzer P., Resch F., Brunner R., Kaess M. Regional grey matter volume reduction in adolescents engaging in non-suicidal self-injury. Psychiatry Res.: Neuroimaging. 2018;280:48–55. doi: 10.1016/j.pscychresns.2018.08.005. [DOI] [PubMed] [Google Scholar]
- Andover M.S., Primack J.M., Gibb B.E., Pepper C.M. An examination of non-suicidal self-injury in men: do men differ from women in basic NSSI characteristics? Arch. Suicide Res. 2010;14(1):79–88. doi: 10.1080/13811110903479086. [DOI] [PubMed] [Google Scholar]
- Artemenko C., Soltanlou M., Ehlis A.-C., Nuerk H.-C., Dresler T. The neural correlates of mental arithmetic in adolescents: A longitudinal fNIRS study. Behav. Brain Functions. 2018;14:1–13. doi: 10.1186/s12993-018-0137-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artinis Medical Systems. (2016). Oxysoft (Version 3.0.103). The Netherlands.
- Beauchaine, T.P., Sauder, C.L., Derbidge, C.M., Uyeji, L.L., 2019. Self-injuring adolescent girls exhibit insular cortex volumetric abnormalities that are similar to those seen in adults with borderline personality disorder. Development and Psychopathology, 31, 1203–1212. doi: 10.1017/S0954579418000822. [DOI] [PMC free article] [PubMed]
- Beeney J.E., Levy K.N., Gatzke-Kopp L.M., Hallquist M.N. EEG asymmetry in borderline personality disorder and depression following rejection. Personality Disorders. 2014;5:178–185. doi: 10.1037/per0000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonenberger M., Plener P.L., Groschwitz R.C., Grön G., Abler B. Differential neural processing of unpleasant haptic sensations in somatic and affective partitions of the insula in non-suicidal self-injury (NSSI) Psychiatry Res.: Neuroimaging. 2015;234(3):298–304. doi: 10.1016/j.pscychresns.2015.10.013. [DOI] [PubMed] [Google Scholar]
- Brunner R., Henze R., Parzer P., Kramer J., Feigl N., Lutz K., Essig M., Resch F., Stieltjes B. Reduced prefrontal and orbitofrontal gray matter in female adolescents with borderline personality disorder: Is it disorder specific? NeuroImage. 2010;49(1):114–120. doi: 10.1016/j.neuroimage.2009.07.070. [DOI] [PubMed] [Google Scholar]
- Bulgarelli C., Blasi A., Arridge S., Powell S., de Klerk C.C.J.M., Southgate V., Brigadoi S., Penny W., Tak S., Hamilton A. Dynamic causal modelling on infant fNIRS data: a validation study on a simultaneously recorded fNIRS-fMRI dataset. NeuroImage. 2018;175:413–424. doi: 10.1016/j.neuroimage.2018.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busner J., Targum S.D. The clinical global impressions scale. Psychiatry (Edgmont) 2007;4(7):28–37. [PMC free article] [PubMed] [Google Scholar]
- Carpenter R.W., Trull T.J. Components of emotion dysregulation in borderline personality disorder: a review. Curr. Psychiatry Rep. 2012;15:335. doi: 10.1007/s11920-012-0335-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanen A.M., Kaess M. Developmental pathways to borderline personality disorder. Curr. Psychiatry Rep. 2012;14(1):45–53. doi: 10.1007/s11920-011-0242-y. [DOI] [PubMed] [Google Scholar]
- Chanen A.M., Velakoulis D., Carison K., Gaunson K., Wood S.J., Yuen H.P., Yücel M., Jackson H.J., McGorry P.D., Pantelis C. Orbitofrontal, amygdala and hippocampal volumes in teenagers with first-presentation borderline personality disorder. Psychiatry Res.: Neuroimaging. 2008;163(2):116–125. doi: 10.1016/j.pscychresns.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Cooper R., Selb J., Gagnon L., Phillip D., Schytz H.W., Iversen H.K., Boas D.A. A systematic comparison of motion artifact correction techniques for functional near-infrared spectroscopy. Front. Neurosci. 2012;6:1–10. doi: 10.3389/fnins.2012.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlgren M.K., Hooley J.M., Best S.G., Sagar K.A., Gonenc A., Gruber S.A. Prefrontal cortex activation during cognitive interference in nonsuicidal self-injury. Psychiatry Res.: Neuroimaging. 2018;277:28–38. doi: 10.1016/j.pscychresns.2018.04.006. [DOI] [PubMed] [Google Scholar]
- Das P., Calhoun V., Malhi G.S. Bipolar and borderline patients display differential patterns of functional connectivity among resting state networks. NeuroImage. 2014;98:73–81. doi: 10.1016/j.neuroimage.2014.04.062. [DOI] [PubMed] [Google Scholar]
- Dixon-Gordon K.L., Weiss N.H., Tull M.T., DiLillo D., Messman-Moore T., Gratz K.L. Characterizing emotional dysfunction in borderline personality, major depression, and their co-occurrence. Compr. Psychiatry. 2015;62:187–203. doi: 10.1016/j.comppsych.2015.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudas R.B., Mole T.B., Morris L.S., Denman C., Hill E., Szalma B., Evans D., Dunn B., Fletcher P., Voon V. Amygdala and dlPFC abnormalities, with aberrant connectivity and habituation in response to emotional stimuli in females with BPD. J. Affect. Disord. 2017;208:460–466. doi: 10.1016/j.jad.2016.10.043. [DOI] [PubMed] [Google Scholar]
- Ehlis A.-C., Schneider S., Dresler T., Fallgatter A.J. Application of functional near-infrared spectroscopy in psychiatry. NeuroImage. 2014;85:478–488. doi: 10.1016/j.neuroimage.2013.03.067. [DOI] [PubMed] [Google Scholar]
- Etkin A., Büchel C., Gross J.J. The neural bases of emotion regulation. Nat. Rev. Neurosci. 2015;16(11):693–700. doi: 10.1038/nrn4044. [DOI] [PubMed] [Google Scholar]
- Ferrari M., Quaresima V. A brief review on the history of human functional near-infrared spectroscopy (fNIRS) development and fields of application. NeuroImage. 2012;63(2):921–935. doi: 10.1016/j.neuroimage.2012.03.049. [DOI] [PubMed] [Google Scholar]
- Ferreri L., Bigand E., Perrey S., Bugaiska A. The promise of near-infrared spectroscopy (NIRS) for psychological research: a brief review. LAnnee Psychologique. 2014;114(03):537–569. [Google Scholar]
- First, M.B., Spitzer, R.L., Gibbon, M., Williams, J.B.W., 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition. (SCID-I/NP). New York: Biometrics Research, New York State Psychiatric Institute.
- Fischer G., Ameis N., Parzer P., Plener P.L., Groschwitz R., Vonderlin E., Kölch M., Brunner R., Kaess M. The German version of the self-injurious thoughts and behaviors interview (SITBI-G): A tool to assess non-suicidal self-injury and suicidal behavior disorder. BMC Psychiatry. 2014;14(1) doi: 10.1186/s12888-014-0265-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke G.H. Beltz; Göttingen: 1995. SCL-90_R. Die Symptom-Checkliste von Derogatis—Deutsche Version-Manual. [Google Scholar]
- Fusar-Poli P., Placentino A., Carletti F., Landi P., Allen P., Surguladze S., Politi P. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J. Psychiatry Neurosci.: JPN. 2009;34:418–432. [PMC free article] [PubMed] [Google Scholar]
- Geng S., Liu X., Biswal B.B., Niu H. Effect of resting-state fNIRS scanning duration on functional brain connectivity and graph theory metrics of brain network. Front. Neurosci. 2017;11 doi: 10.3389/fnins.2017.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghinea D., Edinger A., Parzer P., Koenig J., Resch F., Kaess M. Non-suicidal self-injury disorder as a stand-alone diagnosis in a consecutive help-seeking sample of adolescents. J. Affect. Disord. 2020;274:1122–1125. doi: 10.1016/j.jad.2020.06.009. [DOI] [PubMed] [Google Scholar]
- Ghinea D., Koenig J., Parzer P., Brunner R., Carli V., Hoven C.W., Sarchiapone M., Wasserman D., Resch F., Kaess M. Longitudinal development of risk-taking and self-injurious behavior in association with late adolescent borderline personality disorder symptoms. Psychiatry Res. 2019;273:127–133. doi: 10.1016/j.psychres.2019.01.010. [DOI] [PubMed] [Google Scholar]
- Glenn C.R., Klonsky E.D. Emotion dysregulation as a core feature of borderline personality disorder. J. Pers. Disord. 2009;23(1):20–28. doi: 10.1521/pedi.2009.23.1.20. [DOI] [PubMed] [Google Scholar]
- Glenn C., Klonsky E.D. A multimethod analysis of impulsivity in nonsuicidal self-injury. Personality Disorders: Theory, Research, and Treatment. 2010;1:67–75. doi: 10.1037/a0017427. [DOI] [PubMed] [Google Scholar]
- Golkar A., Lonsdorf T.B., Olsson A., Lindstrom K.M., Berrebi J., Fransson P., Schalling M., Ingvar M., Öhman A., Laks J. Distinct contributions of the dorsolateral prefrontal and orbitofrontal cortex during emotion regulation. PLoS ONE. 2012;7(11):e48107. doi: 10.1371/journal.pone.0048107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant B.F., Chou S.P., Goldstein R.B., Huang B., Stinson F.S., Saha T.D., Smith S.M., Dawson D.S., Pulay A.J., Pickering R.P., Ruan W.J. Prevalence, correlates, disability, and comorbidity of DSM-IV borderline personality disorder: results from the wave 2 national epidemiologic survey on alcohol and related conditions. J. Clin. Psychiatry. 2008;69(4):533–545. doi: 10.4088/jcp.v69n0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groschwitz R.C., Kaess M., Fischer G., Ameis N., Schulze U.M.E., Brunner R., Koelch M., Plener P.L. The association of non-suicidal self-injury and suicidal behavior according to DSM-5 in adolescent psychiatric inpatients. Psychiatry Res. 2015;228(3):454–461. doi: 10.1016/j.psychres.2015.06.019. [DOI] [PubMed] [Google Scholar]
- Groschwitz R.C., Plener P.L. The neurobiology of non-suicidal self-injury (NSSI): a review. Suicidology Online. 2012;3:24–32. [Google Scholar]
- Groschwitz R.C., Plener P.L., Groen G., Bonenberger M., Abler B. Differential neural processing of social exclusion in adolescents with non-suicidal self-injury: An fMRI study. Psychiatry Res.: Neuroimaging. 2016;255:43–49. doi: 10.1016/j.pscychresns.2016.08.001. [DOI] [PubMed] [Google Scholar]
- Haid-Stecher N., Sevecke K. Belastende Kindheitserfahrungen und selbstverletzendes Verhalten-die Rolle der Emotionsregulation [Childhood maltreatment and Non-Suicidal Self-Injury-the impact of emotion regulation] Praxis Der Kinderpsychologie Und Kinderpsychiatrie. 2019;68(7):623–638. doi: 10.13109/prkk.2019.68.7.623. [DOI] [PubMed] [Google Scholar]
- Hamza C.A., Willoughby T. Impulsivity and nonsuicidal self-injury: a longitudinal examination among emerging adults. J. Adolescence. 2019;75:37–46. doi: 10.1016/j.adolescence.2019.07.003. [DOI] [PubMed] [Google Scholar]
- Hamza C.A., Willoughby T., Heffer T. Impulsivity and nonsuicidal self-injury: a review and meta-analysis. Clin. Psychol. Rev. 2015;38:13–24. doi: 10.1016/j.cpr.2015.02.010. [DOI] [PubMed] [Google Scholar]
- Huppert T.J., Diamond S.G., Franceschini M.A., Boas D.A. HomER: a review of time-series analysis methods for near-infrared spectroscopy of the brain. Appl. Opt. 2009;48:280–298. doi: 10.1364/AO.48.00D280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain S.F., Tang T.-B., Yu R., Tam W.W., Tran B., Quek T.T., Hwang S.-H., Chang C.W., Ho C.S., Ho R.C. Cortical haemodynamic response measured by functional near infrared spectroscopy during a verbal fluency task in patients with major depression and borderline personality disorder. EBioMedicine. 2020;51:102586. doi: 10.1016/j.ebiom.2019.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain S.F., Yu R., Tang T.-B., Tam W.W., Tran B., Quek T.T., Hwang S.-H., Chang C.W., Ho C.S., Ho R.C. Validating a functional near-infrared spectroscopy diagnostic paradigm for Major Depressive Disorder. Sci. Rep. 2020;10(1) doi: 10.1038/s41598-020-66784-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- In-Albon T., Suppiger A., Schlup B., Wendler S., Margraf J., Schneider S. Validität des Diagnostischen Interviews bei psychischen Störungen (DIPS für DSM-IV-TR) Zeitschrift für Klinische Psychologie und Psychotherapie. 2008;37(1):33–42. doi: 10.1026/1616-3443.37.1.33. [DOI] [Google Scholar]
- International Society for the Study of Self-injury. (2018). What is self-injury? Retrieved from https://itriples.org/about-self-injury/what-is-self-injury.
- Jaspers H.H. Report of the committee on methods of clinical examination in electroencephalography: 1957. Electroencephalogr. Clin. Neurophysiol. 1958;10(2):370–375. doi: 10.1016/0013-4694(58)90053-1. [DOI] [Google Scholar]
- Jennings J.R., Kamarck T., Stewart C., Eddy M., Johnson P. Alternate cardiovascular baseline assessment techniques: Vanilla or resting baseline. Psychophysiology. 1992;29:742–750. doi: 10.1111/j.1469-8986.1992.tb02052.x. [DOI] [PubMed] [Google Scholar]
- Johnson J.G., Cohen P., Kasen S., Skodol A.E., Oldham J.M. Cumulative prevalence of personality disorders between adolescence and adulthood. Acta Psychiatr. Scand. 2008;118:410–413. doi: 10.1111/j.1600-0447.2008.01231.x. [DOI] [PubMed] [Google Scholar]
- Jovev M., Green M., Chanen A., Cotton S., Coltheart M., Jackson H. Attentional processes and responding to affective faces in youth with borderline personality features. Psychiatry Res. 2012;199(1):44–50. doi: 10.1016/j.psychres.2012.03.027. [DOI] [PubMed] [Google Scholar]
- Kaess M., Brunner R., Chanen A. Borderline personality disorder in adolescence. Pediatrics. 2014;134(4):782–793. doi: 10.1542/peds.2013-3677. [DOI] [PubMed] [Google Scholar]
- Kaess M., Fischer-Waldschmidt G., Resch F., Koenig J. Health related quality of life and psychopathological distress in risk taking and self-harming adolescents with full-syndrome, subthreshold and without borderline personality disorder: Rethinking the clinical cut-off? Borderline Personality Disorder and Emotion Dysregulation. 2017;4(1) doi: 10.1186/s40479-017-0058-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaess M., Ghinea D., Fischer-Waldschmidt G., Resch F. Die Ambulanz für Risikoverhalten und Selbstschädigung (AtR!Sk) – ein Pionierkonzept der ambulanten Früherkennung und Frühintervention von Borderline-Persönlichkeitsstörungen [The outpatient clinic for risk-taking and self-harming behavior (AtR!Sk)—A pioneer concept of ambulant early detection and intervention fot borderline personality disorder] Praxis Der Kinderpsychologie Und Kinderpsychiatrie. 2017;66(6):404–422. doi: 10.13109/prkk.2017.66.6.404. [DOI] [PubMed] [Google Scholar]
- Kaess M., Parzer P., Mattern M., Resch F., Bifulco A., Brunner R. Childhood Experiences of Care and Abuse (CECA)—Validation of the German version of the questionnaire and interview, and results of an investigation of correlations between adverse childhood experiences and suicidal behaviour. Zeitschrift Für Kinder- Und Jugendpsychiatrie Und Psychotherapie. 2011;39(4):243–252. doi: 10.1024/1422-4917/a000115. [DOI] [PubMed] [Google Scholar]
- Kim S.H., Cornwell B., Kim S.E. Individual differences in emotion regulation and hemispheric metabolic asymmetry. Biol. Psychol. 2012;89(2):382–386. doi: 10.1016/j.biopsycho.2011.11.013. [DOI] [PubMed] [Google Scholar]
- Kober H., Barrett L.F., Joseph J., Bliss-Moreau E., Lindquist K., Wager T.D. Functional grouping and cortical–subcortical interactions in emotion: a meta-analysis of neuroimaging studies. NeuroImage. 2008;42(2):998–1031. doi: 10.1016/j.neuroimage.2008.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig J., Thayer J.F., Kaess M. A meta-analysis on pain sensitivity in self-injury. Psychol. Med. 2016;46(8):1597–1612. doi: 10.1017/S0033291716000301. [DOI] [PubMed] [Google Scholar]
- Koike S., Nishimura Y., Takizawa R., Yahata N., Kasai K. Near-infrared spectroscopy in schizophrenia: a possible biomarker for predicting clinical outcome and treatment response. Front. Psychiatry. 2013;4 doi: 10.3389/fpsyt.2013.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C.Y.Y., Ho C.S.H., Lim C.R., Ho R.C.M. Functional near-infrared spectroscopy in psychiatry. BJPsych Adv. 2017;23(5):324–330. doi: 10.1192/apt.bp.115.015610. [DOI] [Google Scholar]
- LeBoeuf A., Guilé J.-M., Labelle R., Luck D. Neuroimagerie fonctionnelle chez l’adolescent avec un trouble de personnalité limite. Santé mentale au Québec. 2016;41(1):141–162. doi: 10.7202/1036969ar. [DOI] [PubMed] [Google Scholar]
- Lenzenweger M.F., Lane M.C., Loranger A.W., Kessler R.C. DSM-IV personality disorders in the national comorbidity survey replication. Biol. Psychiatry. 2007;62(6):553–564. doi: 10.1016/j.biopsych.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T., Luo Q., Gong H. Gender-specific hemodynamics in prefrontal cortex during a verbal working memory task by near-infrared spectroscopy. Behav. Brain Res. 2010;209(1):148–153. doi: 10.1016/j.bbr.2010.01.033. [DOI] [PubMed] [Google Scholar]
- Lim K.-S., Wong C.H., McIntyre R.S., Wang J., Zhang Z., Tran B.X. Global lifetime and 12-Month prevalence of suicidal behavior, deliberate self-harm and non-suicidal self-injury in children and adolescents between 1989 and 2018: A meta-analysis. Int. J. Environ. Res. Public Health. 2019;16(22):4581. doi: 10.3390/ijerph16224581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malejko K., Neff D., Brown R.C., Plener P.L., Bonenberger M., Abler B., Graf H. Neural signatures of social inclusion in borderline personality disorder versus non-suicidal self-injury. Brain Topogr. 2019;32(5):753–761. doi: 10.1007/s10548-019-00712-0. [DOI] [PubMed] [Google Scholar]
- Niedtfeld I., Schulze L., Kirsch P., Herpertz S.C., Bohus M., Schmahl C. Affect regulation and pain in borderline personality disorder: a possible link to the understanding of self-injury. Biol. Psychiatry. 2010;68(4):383–391. doi: 10.1016/j.biopsych.2010.04.015. [DOI] [PubMed] [Google Scholar]
- Niu H.-J., Li X., Chen Y.-J., Ma C., Zhang J.-Y., Zhang Z.-J. Reduced frontal activation during a working memory task in mild cognitive impairment: A non-invasive near-infrared spectroscopy study. CNS Neurosci. Ther. 2013;19(2):125–131. doi: 10.1111/cns.2013.19.issue-210.1111/cns.12046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu H., Khadka S., Tian F., Lin Z.-J., Lu C., Zhu C., Liu H. Resting-state functional connectivity assessed with two diffuse optical tomographic systems. J. Biomed. Opt. 2011;16(4):046006. doi: 10.1117/1.3561687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nock M.K., Holmberg E.B., Photos V.I., Michel B.D. Self-injurious thoughts and behaviors interview: development, reliability, and validity in an adolescent sample. Psychol. Assess. 2007;19:309–317. doi: 10.1037/1040-3590.19.3.309. [DOI] [PubMed] [Google Scholar]
- Obrig H., Rossi S., Telkemeyer S., Wartenburger I. From acoustic segmentation to language processing: Evidence from optical imaging. Front. Neuroenerg. 2010;2 doi: 10.3389/fnene.2010.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner K.N., Bunge S.A., Gross J.J., Gabrieli J.D.E. Rethinking feelings: An fMRI study of the cognitive regulation of emotion. J. Cognit. Neurosci. 2002;14(8):1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Oldehinkel M., Francx W., Beckmann C.F., Buitelaar J.K., Mennes M. Resting state FMRI research in child psychiatric disorders. Eur. Child Adolesc. Psychiatry. 2013;22(12):757–770. doi: 10.1007/s00787-013-0480-0. [DOI] [PubMed] [Google Scholar]
- Petermann F., Petermann U. 4th ed. Hogrefe; Bern: 2007. HAWIK-IV Hamburg-Wechsler-Intelligenztest für Kinder IV (Hamburg Wechsler Intelligence Scale for Children IV) [Google Scholar]
- Pinti P., Tachtsidis I., Hamilton A., Hirsch J., Aichelburg C., Gilbert S., Burgess P.W. The present and future use of functional near-infrared spectroscopy (fNIRS) for cognitive neuroscience. Ann. N. Y. Acad. Sci., Special Issue. 2020;1464(1):5–29. doi: 10.1111/nyas.v1464.110.1111/nyas.13948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plener P.L., Kaess M., Schmahl C., Pollak S., Fegert J.M., Brown R.C. Nonsuicidal self-injury in adolescents. Deutsches Aerzteblatt Online. 2018 doi: 10.3238/arztebl.2018.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plener P.L., Kapusta N.D., Kölch M.G., Kaess M., Brunner R. Nicht-suizidale Selbstverletzung als eigenständige Diagnose [Non-suicidal self-injury as autonomous diagnosis – implications for research and clinic of the DSM-5 proposal to establish the diagnosis of Non-Suicidal Self-Injury in adolescents] Zeitschrift Für Kinder- Und Jugendpsychiatrie Und Psychotherapie. 2012;40(2):113–120. doi: 10.1024/1422-4917/a000158. [DOI] [PubMed] [Google Scholar]
- Ruocco A.C., Amirthavasagam S., Choi-Kain L.W., McMain S.F. Neural correlates of negative emotionality in borderline personality disorder: an activation-likelihood-estimation meta-analysis. Biol. Psychiatry. 2013;73(2):153–160. doi: 10.1016/j.biopsych.2012.07.014. [DOI] [PubMed] [Google Scholar]
- Ruocco A.C., Medaglia J.D., Ayaz H., Chute D.L. Abnormal prefrontal cortical response during affective processing in borderline personality disorder. Psychiatry Res.: Neuroimaging. 2010;182(2):117–122. doi: 10.1016/j.pscychresns.2010.01.011. [DOI] [PubMed] [Google Scholar]
- Ruocco A.C., Medaglia J.D., Tinker J.R., Ayaz H., Forman E.M., Newman C.F., Williams J.M., Hillary F.G., Platek S.M., Onaral B., Chute D.L. Medial prefrontal cortex hyperactivation during social exclusion in borderline personality disorder. Psychiatry Res.: Neuroimaging. 2010;181(3):233–236. doi: 10.1016/j.pscychresns.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Ruocco A.C., Rodrigo A.H., McMain S.F., Page-Gould E., Ayaz H., Links P.S. Predicting treatment outcomes from prefrontal cortex activation for self-harming patients with Borderline Personality Disorder: a preliminary study. Front. Hum. Neurosci. 2016;10 doi: 10.3389/fnhum.2016.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saß H., Wittchen H.U., Zaudig M., Houben I. Hogrefe; Göttingen: 2003. Diagnostische Kriterien des Diagnostischen und Statistischen Manuals Psychischer Störungen. DSM-IV-TR. [Google Scholar]
- Schmidt H., Jogia J., Fast K., Christodoulou T., Haldane M., Kumari V., Frangou S. No gender differences in brain activation during the N-back task: An fMRI study in healthy individuals. Hum. Brain Mapp. 2009;30(11):3609–3615. doi: 10.1002/hbm.v30:1110.1002/hbm.20783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholkmann F., Wolf M. General equation for the differential pathlength factor of the frontal human head depending on wavelength and age. J. Biomed. Opt. 2013;18(10):105004. doi: 10.1117/1.JBO.18.10.105004. [DOI] [PubMed] [Google Scholar]
- Schulze L., Schmahl C., Niedtfeld I. Neural correlates of disturbed emotion processing in borderline personality disorder: a multimodal meta-analysis. Biol. Psychiatry. 2016;79(2):97–106. doi: 10.1016/j.biopsych.2015.03.027. [DOI] [PubMed] [Google Scholar]
- Sebastian A., Jung P., Krause-Utz A., Lieb K., Schmahl C., Tüscher O. Frontal dysfunctions of impulse control – a systematic review in borderline personality disorder and attention-deficit/hyperactivity disorder. Front. Hum. Neurosci. 2014;8 doi: 10.3389/fnhum.2014.00698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel O., Carius D., Kenville R., Ragert P. Motor learning in a complex balance task and associated neuroplasticity: a comparison between endurance athletes and nonathletes. J. Neurophysiol. 2017;118(3):1849–1860. doi: 10.1152/jn.00419.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan D.V., Lecrubier Y., Sheehan K.H., Amorim P., Janavs J., Weiller E., Dunbar G.C. The Mini-International Neuropsychiatric Interview (M.I.N.I): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- Shi J., Sakatani K., Okamoto M., Yamaguchi Y., Zuo H.-C. Correlation between LIFG and autonomic activation during stressful tasks: a functional near-infrared spectroscopy (fNIRS) study. J. Huazhong Univ. Sci. Technol. [Med. Sci.] 2014;34(5):663–671. doi: 10.1007/s11596-014-1334-9. [DOI] [PubMed] [Google Scholar]
- Sloan E., Hall K., Moulding R., Bryce S., Mildred H., Staiger P.K. Emotion regulation as a transdiagnostic treatment construct across anxiety, depression, substance, eating and borderline personality disorders: A systematic review. Clin. Psychol. Rev. 2017;57:141–163. doi: 10.1016/j.cpr.2017.09.002. [DOI] [PubMed] [Google Scholar]
- StataCorp. (2019). STATA Statistics/Data Analysis (Version 16.0). College Station, Texas.
- Stevens J.S., Hamann S. Sex differences in brain activation to emotional stimuli: a meta-analysis of neuroimaging studies. Neuropsychologia. 2012;50(7):1578–1593. doi: 10.1016/j.neuropsychologia.2012.03.011. [DOI] [PubMed] [Google Scholar]
- Stiensmeier-Pelster, J., Schürmann, M., & Duda, K. (1991). Das Depressionsinventar für Kinder und Jugendliche (DIKJ): Untersuchungen zu seinen psychometrischen Eigenschaften. Diagnostica, 37(2), 149–159.
- Surf Ice. (2019). Retrieved from https://www.nitrc.org/projects/surfice/.
- Swannell S.V., Martin G.E., Page A., Hasking P., John N.J.S. Prevalence of nonsuicidal self-injury in nonclinical samples: systematic review, meta-analysis and meta-regression. Suicide and Life-Threatening Behavior. 2014;44(3):273–303. doi: 10.1111/sltb.2014.44.issue-310.1111/sltb.12070. [DOI] [PubMed] [Google Scholar]
- Taylor P.J., Jomar K., Dhingra K., Forrester R., Shahmalak U., Dickson J.M. A meta-analysis of the prevalence of different functions of non-suicidal self-injury. J. Affect. Disord. 2018;227:759–769. doi: 10.1016/j.jad.2017.11.073. [DOI] [PubMed] [Google Scholar]
- The KIDSCREEN Group Europe. (2006). The KIDSCREEN Questionnaires—Quality of life questionnaires for children and adolescents. Handbook. Lengerich: Pabst Science Publishers.
- The Math Works Inc. (2015). MATLAB R2015a. Natick, Massachussetts, United States.
- Vega D., Ripolles P., Soto A., Torrubia R., Ribas J., Antonio Monreal J. Orbitofrontal overactivation in reward processing in borderline personality disorder: The role of non-suicidal self-injury. Brain Imaging Behav. 2018;12(1):217–228. doi: 10.1007/s11682-017-9687-x. [DOI] [PubMed] [Google Scholar]
- Westlund Schreiner M., Klimes-Dougan B., Mueller B.A., Eberly L.E., Reigstad K.M., Carstedt P.A. Multi-modal neuroimaging of adolescents with non-suicidal self-injury: Amygdala functional connectivity. J. Affect. Disord. 2017;221:47–55. doi: 10.1016/j.jad.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle S., Chanen A.M., Fornito A., McGorry P.D., Pantelis C., Yücel M. Anterior cingulate volume in adolescents with first-presentation borderline personality disorder. Psychiatry Res.: Neuroimaging. 2009;172(2):155–160. doi: 10.1016/j.pscychresns.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Wittchen, H.-U., Zaudig, M., Fydrich, T., 1997. SKID. Strukturiertes Klinisches Interview für DSM-IV. Achse I und II. Handanweisung. Retrieved from https://pure.mpg.de/pubman/faces/ViewItemOverviewPage.jsp?itemId=item_1646481.
- Wolff J.C., Thompson E., Thomas S.A., Nesi J., Bettis A.H., Ransford B. Emotion dysregulation and non-suicidal self-injury: a systematic review and meta-analysis. Eur. Psychiatry. 2019;59:25–36. doi: 10.1016/j.eurpsy.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia M., Wang J., He Y. BrainNet viewer: a network visualization tool for human brain connectomics. PLoS ONE. 2013;8(7):e68910. doi: 10.1371/journal.pone.0068910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XjView toolbox. (2019). Retrieved from https://www.alivelearn.net/xjview.
- Xu J., Liu X., Zhang J., Li Z., Wang X., Fang F., Niu H. FC-NIRS: a functional connectivity analysis tool for near-infrared spectroscopy data. Biomed. Res. Int. 2015;2015:1–11. doi: 10.1155/2015/248724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You J., Deng B., Lin M.-P., Leung F. The interactive effects of impulsivity and negative emotions on adolescent nonsuicidal self-injury: a latent growth curve analysis. Suicide Life-Threatening Behav. 2016;46(3):266–283. doi: 10.1111/sltb.2016.46.issue-310.1111/sltb.12192. [DOI] [PubMed] [Google Scholar]
- Zhang, Z., Olszewska-Guizzo, A., Husain, S. F., Bose, J., Choi, J., Tan, W., et al. (2020). Brief relaxation practice induces significantly more prefrontal cortex activation during arithmetic tasks comparing to viewing greenery images as revealed by functional near-infrared spectroscopy (fNIRS). International Journal of Environmental Research and Public Health, 17, 8366. doi: 10.3390/ijerph17228366. [DOI] [PMC free article] [PubMed]
- Zhu H., Xu J., Li J., Peng H., Cai T., Li X. Decreased functional connectivity and disrupted neural network in the prefrontal cortex of affective disorders: A resting-state fNIRS study. J. Affect. Disord. 2017;221:132–144. doi: 10.1016/j.jad.2017.06.024. [DOI] [PubMed] [Google Scholar]