Abstract
Aquaporins (AQPs) are part of the family of the integral membrane proteins. Their function is dedicated to the transport of water, glycerol, ammonia, urea, H2O2, and other small molecules across the biological membranes. Although for many years they were scarcely considered, AQPs have a relevant role in the development of many diseases. Recent discoveries suggest, that AQPs may play an important role in the process of fat accumulation and regulation of oxidative stress, two crucial aspects of insulin resistance and type-2 diabetes (T2D).
Insulin resistance (IR) and T2D are multi-faceted systemic diseases with multiple connections to obesity and other comorbidities such as hypertension, dyslipidemia and metabolic syndrome. Both IR and T2D transcends different tissues and organs, creating the maze of mutual relationships between adipose fat depots, skeletal muscle, liver and other insulin-sensitive organs. AQPs with their heterogenous properties, distinctive tissue distribution and documented involvement in both the lipid metabolism and regulation of the oxidative stress appear to be feasible candidates in the search for the explanation to this third-millennium plague. A lot of research has been assigned to adipose tissue AQP7 and liver tissue AQP9, clarifying their relationship and coordinated work in the induction of hepatic insulin resistance. Novel research points also to other aquaporins, such as AQP11 which may be associated with the induction of insulin resistance and T2D through its involvement in hydrogen peroxide transport.
In this review we collected recent discoveries in the field of AQP's involvement in the insulin resistance and T2D. Novel paths which connect AQPs with metabolic disorders can give new fuel to the research on obesity, insulin resistance and T2D - one of the most worrying problems of the modern society.
Keywords: Aquaporins, Obesity, Insulin resistance, Type-2 diabetes, Metabolic syndrome
Graphical abstract
Highlights
-
•
Presentation of the current knowledge on the involvement of aquaporins in disease state.
-
•
Overview on latest research regarding insulin resistance and AQPs.
-
•
Conceptualisation of an AQPs network involved regulation of lipid-related metabolism.
1. Aquaporins introduction and overview
One of the most important aspects of the living cells is the ability to establish controlled interface with the external environment. The formulation of the biological membrane, which shields intracellular processes from the extracellular environment, is postulated as one of the milestones achieved by the early life. In multicellular organisms every single cell establishes connections with the environment through integral membrane proteins such as membrane channels, receptors, ion pumps and contact sites. Cells need to obtain the nutrients from the extracellular space to produce energy needed to sustain various biosynthetic mechanisms. In the same way, waste by-products of cellular metabolism need to be transported to the outside. Since just a few small, non-polar molecules can cross the lipid double layer on their own, cells need specialized proteins to efficiently transfer molecules through the biological membranes.
1.1. AQP's - integral membrane proteins involved in solute transport
Integral membrane proteins are usually transmembrane proteins, whose role is to facilitate the movement of ions or polar/charged compounds across the plasma membrane in a regulated way. In many cases they are passive transporters, facilitating the transport which follows the transmembrane concentration gradient. Yet often the transport needs to be carried out against the concentration or electrical gradient. In this case, the transport is an active process which requires energy from ATP to pump molecules or ions in and out of the cells [1]. The transporters use ATP directly or use energy generated by the electrochemical force of another solute which moves across the membrane following its own gradient [2].
There are 2 major categories of transporters: carriers and channels. Carriers can be active or passive, and they behave as described above. Channels allow the compounds to cross the membrane at a much higher speed, getting close to the rate of free diffusion Aquaporins are part of the family of the transmembrane channels: they are integral membrane proteins, dedicated to the water transport, but some of them are selective also for other solutes, such as glycerol, ammonia, urea and hydrogen peroxide [3].
Aquaporin channel stereospecificity is lower compared to the membrane carrier proteins, yet in contrast to the latter ones their transport capacity is hard to saturate [2]. Solute channels are usually oligomers, forming multiunit complexes, with multiple similar subunits among α-helix segments or β-barrel structures. By various triggering mechanisms, they can cycle between open and closed state, thus regulating the influx of water ions or other solutes [4].
1.2. Type of aquaporins and their functions
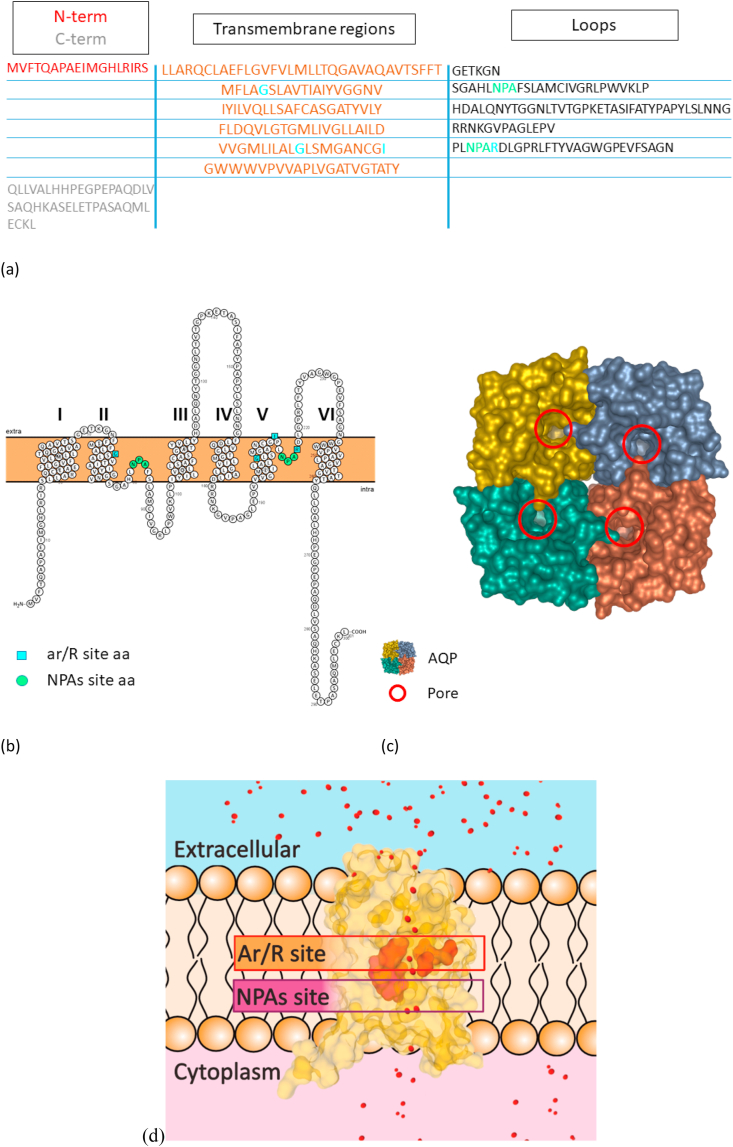
Water homeostasis is an essential process in living cells. Water balance can be obtained through standard diffusion, but faster through aquaporin channels. The osmotic/oncotic pressure first and the hydrostatic pressure second are the powers that lead the water movement [[5], [6], [7]]. Nowadays scientists identified 13 AQPs grouped in 3 different classes in mammals, compared to the decades of members grouped in 7 subfamilies in plants, where their number is species-dependent [[8], [9], [10]]. The 3 typical classes of AQPs are classical AQPs, aquaglyceroporins and superAQPs. AQPs could be categorized also creating 3 functional subgroups based on their main functions: aquaporins, glyceroporins and peroxiporins. Aquaporins reside usually in the plasma membrane. Although some of them are situated in membranes of different cellular compartments such as endoplasmic reticulum in case of AQP11 [11]. Their hydrophobic nature makes protein crystallization troublesome, so the number of 3D structures of various AQP isoforms obtained with the use of X-ray diffraction crystallography is scarce and most of the research has to rely on the bioinformatic predictions [12]. Fully assembled AQP channels usually form tetramers, composed of 4 monomers of approximately 30 kDa. Each of these are usually made of 6 tilted α-helical domains connected by 5 loops, creating a structure similar to a barrel (Fig. 1). Key feature of almost all the aquaporins are the ar/R (aromatic/arginine) and the NPA (asparagine-proline-alanine) sites, essential for the filtering and selectivity properties of the pore (Fig. 1d) [13,14]. An exception to this rule is constituted by AQP11, whose motif is NPC (asparagine-proline-cysteine). NPC motif was shown to be essential for the full expression of the molecular function of AQP11, even before the discovery of its role in hydrogen peroxide flow [11,15].
Fig. 1.
General structure of AQPs on the basis of AQP10. Panel (a) shows the sequence of AQP10 monomer, highlighting the 6 transmembrane regions, loop regions and the N- and C- terms. Panel (b) shows a 2D representation of AQP10 monomer sequence performed with Protter [16]. The transmembrane regions are numbered from 1 to 6. The 2 NPAs, despite their position in the loops, are situated in the plasma membrane. NPAs are essential components of the AQPs funnel, together with the ar/R constriction site. N-term and C-term are usually orientated intracellularly, on the same side of the membrane. In panels (a) and (b) NPAs residues are highlighted in green, ar/R residues are highlighted in light blue. The prediction of the transmembrane regions is based on sequence analysis performed with MPEx tool [17]. Panel (c) is a 3D representation of AQP10 tetramer. The red circles highlight the pore entry in each of the monomers. Panel (d) shows the conformation of a AQP10 monomer located in the plasma membrane. The internal part of the funnel is the most important for the determination of AQPs selectivity. The NPA motifs central position in the pore narrow its diameter and it is believed to have role in the prevention of proton free flow through the channel. On the side of the funnel towards the extracellular entrance there is a second conserved region, the aromatic/R site, which is considered the dimensional and selectivity filter. In the superaquaporins the arginine is substituted by a leucine, yielding NPL motif. Panels (c) and (d) are based on the RCSB PDB [18] crystal structure of AQP10 (PDB ID: 6F7H) published by Gotfryd et al. [19]; the images has been prepared with the use of mol* software [20]. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
The 2 major families of mammalian aquaporins are the classical AQPs and aquaglyceroporins. The partition of AQPs between these 2 groups has been long debated, but nowadays following classification has been widely accepted (Table 1). AQP0, AQP1, AQP2, AQP4 and AQP5 are part of the classical AQPs, whose role is mainly connected to water transport. The aquaglyceroporins family includes AQP3, AQP6, AQP7, AQP8, AQP9, and AQP10. These channels transport water, but are also essential for the movement of small uncharged molecules as glycerol, urea, ammonia and hydrogen peroxide [3,[21], [22], [23], [24], [25], [26], [27], [28]]. Despite being included in the aquaglyceroporins family, AQP8 should be described separately, given its uniqueness from a phylogenetic point of view and its role in cell signaling [22,29]. AQP8 is involved in redox-related signaling with the ability of stopping the flow of hydrogen peroxide under a redox stress [30], with the multi-step mechanism behind the pore closure [12]. The third family – superAQPs – consists of AQP11 and AQP 12 whose sequence has low homology with the other AQPs groups [3]. Even the NPAs sites are modified to NPCs in this group [31]. The function of these aquaporins has been almost unknown until 2020 when it has been first discovered that AQP11 is an ER membrane protein involved in hydrogen peroxide transport [32].
Table 1.
The table summarizes the aquaporins non-controversial information. Classical aquaporins transport mainly water, but some of them also ammonia. Aquaglyceroporins transport a higher variety of solutes. Selectivity of superaquaporins is still under research, yet AQP11 was proven to transport hydrogen peroxide. AQPs tissue localization is heterogeneous and they cover most of the tissues in the human body. Subcellular localization of aquaporins is still object of debate. Most of them are located in the plasma membrane, but AQPs can be found on the membranes of different intracellular compartments. The data regarding transported solutes are mainly taken from the book chapter by Medrano et al. [8,10], the data on the tissue and intracellular localization are taken from “The Human Protein Atlas” database [33].
| Transported solutes | Tissue Localization | Subcellular Localization | ||
|---|---|---|---|---|
| Aquaporins | AQP0 | H2O | Eye | Plasma Membrane, Endoplasmic reticulum |
| AQP1 | H2O, Ammonia | Brain, Lung, Gastrointestinal tract, Liver & gallbladder, Pancreas, Kidney & urinary bladder, Female reproductive tissues, Skin, Bone marrow & lymphoid tissues | Plasma Membrane, Extracellular exosome, Nucleus | |
| AQP2 | H2O | Kidney & urinary bladder, Male reproductive tissues | Plasma Membrane, Extracellular exsome, Golgi apparatus | |
| AQP4 | H2O, Ammonia | Brain, Endocrine tissues, Lung, Gastrointestinal tract, | Plasma membrane, Extracellular exosome, Endosome | |
| AQP5 | H2O | Lung, Proximal digestive tract, Gastrointestinal tract, Male and female reproductive tissues, Pancreas | Plasma membrane, Extracellular exosome | |
| Aquaglyceroporins | AQP3 | Glycerol, Urea, Ammonia, Hydrogen peroxide | Lung, Proximal digestive tract, Gastrointestinal tract, Kidney & urinary bladder, Male and female reproductive tissues, Skin, Bone marrow & lymphoid tissues | Plasma membrane, Nucleus |
| AQP6 | Urea, Glycerol, Anions, Nitrate/Halide Ions, Ammonia | Kidney & urinary bladder | Plasma membrane | |
| AQP7 | Glycerol, Urea, Ammonia, Arsenite | Adipose & soft tissues, Female reproductive tissues, Muscle tissues, Endocrine tissues | Plasma membrane | |
| AQP8 | Ammonia, Hydrogen peroxide | Gastrointestinal tract, Pancreas | Plasma membrane, endoplasmic reticulum | |
| AQP9 | Glycerol, Urea, Small non-charged solutes, Hydrogen peroxide, Arsenite, Monocarboxylates, Ammonia | Liver & gallbladder, Bone marrow & lymphoid tissues | Plasma membrane | |
| AQP10 | Glycerol | Gastrointestinal tract | Plasma membrane | |
| SuperAQPs | AQP11 | Glycerol, Hydrogen peroxide | Gastrointestinal tract, Endocrine tissue, Liver & gallbladder | Endoplasmic reticulum |
| AQP12 A/B | – | Pancreas, Male reproductive tissues | Plasma membrane | |
1.3. Aquaporins and diseases
Although research on the non-canonical functions of aquaporins is still in its early stages, the body of research underline the importance of AQPs in various pathological conditions and disorders. Beginning from the members of the classical AQPs family, it has been shown that mutations in the AQP0 gene bring the onset of genetic cataracts in both the humans and mice [34]. AQP1 seems to be connected with metastasis and angiogenesis processes, since the water passage through AQP1 is associated with cell migration. Maltaneri et al. showed that AQP1 participates in the migration of endothelial cells promoted by Epo [35]. It has also been shown a possible connection between AQP3 and AQP5 overexpression and 5-year disease-free/overall survival in patients with hepatocellular carcinoma [36]. There are also hints of AQP1 participation in polycystic kidney disease, AQP1 and AQP4 connection with Parkinson's disease, AQP1 and AQP5 importance in the aggressiveness of soft tissue sarcoma [[37], [38], [39]]. Mutations in AQP2 are connected with the appearance of the nephrogenic diabetes insipidus [40,41], while the lack of control on the regulation of AQP2 in the renal collecting duct seems to be linked to various water balance disorders in humans and animals [42]. AQP4 is a possible target in neurological disorders, as it has been proposed as a relevant factor in Parkinson's disease and Alzheimer's disease [[43], [44], [45]]. Regarding the metabolic disturbances and classical aquaporins, AQP5 expression is lower in submandibular gland from type-2 diabetes mellitus patients and mice due to excessive autophagy [46]. Classical AQPs role in disease is considered as strictly connected with the water transport, as suggested by their category properties.
Research on aquaglyceroporins shows that they are also connected with disease states. AQP3 is present in basal layer keratinocytes of the epidermis having a relevant function in its biology, especially proliferation. It has been lately addressed as a factor involved in skin tumor development and, consequently, as a possible drug target [47], since mice lacking AQP3 are resistant to the development of skin tumors induced by a tumor initiator and phorbol ester promoter. It is also involved in non-small cell lung cancer (NSCLC) tumor development since the knockdown of this protein inhibits NSCLC proliferation and angiogenesis [48]. Moreover, AQP1, AQP3, and AQP5 overexpression correlates with a lower development of lymph node metastasis in patients with colon cancer [49]. AQP6 has been suggested as a possible protective gene against the infection from Crimean-Congo hemorrhagic fever virus [50]. AQP8 is upregulated in the colon of patients with ulcerative colitis opening the chance that it is involved in the processes of chronic inflammation and ulceration [51]. It is also suggested as a potential drug target for the treatment of diarrheal disorders [52]. From the recent discoveries about its role in cell signaling and oxidative stress homeostasis, AQP8 seems to be involved in tumor cell migration, tumor invasion tumor metastasis, and anti-apoptotic signaling [30,[53], [54], [55], [56]]. AQP9 shows many links with hepatic insulin resistance and type-2 diabetes due to its involvement in adipose tissue triacylglycerol metabolism, but this connection will be explored deeply later on in this review. There are also hints of the involvement of AQP9 involvement in Alzheimer's disease, prion diseases, and Parkinson's disease [[57], [58], [59]] [[57], [58], [59]] [[57], [58], [59]]. Lastly, it has been shown that AQP9 is relevant to the hepatocellular carcinoma proliferation and migration inhibition [60,61]. AQP10 and AQP3 are overexpressed in keratinocytes of people with pompholyx disease [62]. Aquaglyceroporins are emerging as relevant targets in many different diseases: their ability of transporting many solutes involve them in a higher number of processes compared to the classical ones. The connection of hydrogen peroxide transporters with tumors and metastasis development appears as a very interesting study subject.
AQP11, expressed in the kidney proximal tubule, is deeply involved in polycystic kidney disease. Its deletion in mice brings vacuolization, cyst formation, and noteworthy expansion of the endoplasmic reticulum and finally the premature death for renal failure [63,64]. Polycystic kidneys in AQP11−/− mice show enhanced autophagy with NOX2 as a major factor involved in the accumulation of renal oxidative stress [65,66]. AQP12 needs to be further studied, nowadays we know that is involved with pancreatic damage since AQP12−/− mice show severe pancreatitis [31,67]. Despite the partial knowledge on the functions of the AQPs in this group, they already show their relevance in some diseases studied for a long time, such as polycystic kidney disease. New emerging data together with the particularity of this family of aquaporins suggest that more studies on the superAQPs group are needed to fully decipher their molecular role.
2. Type 2 diabetes and insulin resistance - introduction and overview
2.1. Obesity is involved in the onset of liver insulin resistance
Obesity and metabolic disturbances associated with it are a growing health problem at a global level. The ever-increasing percentage of overweight and obese people in 2016 reached 39% in adults and 18% in children and adolescents, as estimated by the WHO [68]. The complex relationship between genetic predisposition, lifestyle, socioeconomic conditions, and even cultural influences lay at the foundation of this third-millennium plague. Obesity usually is not coming alone: obese people have usually a higher comorbidities risk, which can go from hypertension and associated cardiovascular diseases to dyslipidemia, from insulin resistance to type 2 diabetes, and many others [69]. Insulin is an essential hormone in the glucose, lipids, amino acids and energy homeostasis, acting on the insulin-sensitive tissues such as adipose tissue, liver and skeletal muscle. When the response to insulin stimulation decrease in these organs, they develop a metabolic state named insulin resistance [70]. This state of systemic insulin resistance usually stays silent for several years thanks to the increase in the compensating release of insulin by β-cells. To keep insulin-controlled aspects of metabolism in balance, β-cells continuously increase insulin secretion according to blood glucose levels to keep the metabolic homeostasis [71].
Accumulation of adipose tissue in obese people makes them prone to the development of insulin resistance. Due to low-grade inflammation of adipose tissue in IR, fat depots release excess of pro-inflammatory cytokines, increase lipolysis of TAG stores and decrease release of beneficial adipokines. The consequence is an evident increase in plasma free fatty acids (FFA) and free glycerol [72,73] which stimulates induction of insulin resistance in other insulin -sensitive organs such as skeletal muscle and liver. This “rich plasma” brings to the accumulation of ectopic fat in form of TAG and bioactive lipids such as diacylglycerols and ceramides in both the skeletal muscles and liver [74,75]. A high diacylglycerol content in the liver is associated with a PKC isoform activation that brings to diminished phosphorylation of the insulin receptor substrate inhibiting the hepatic insulin signaling [76]. The major role of insulin in the liver regarding carbohydrate metabolism is to inhibit the gluconeogenesis pathway in response to a high glucose concentration in plasma. Insulin also promotes hepatic glycogen storage, so in a hepatic insulin-resistant state, the diminished insulin signaling does not inhibit hepatic gluconeogenesis and glycogenolysis augmenting glucose release in the bloodstream [76]. The final consequence of all these concatenated events is that the insulin concentration to keep the physiological glucose levels will be higher. On the other side, insulin is known also to promote lipogenesis in the liver [77]. The effects of hyperinsulinemia bring to a dysregulation of the lipogenesis, which increases the production of VLDL and reduces the one of the HDL. This brings to an accumulation of plasma triglycerides and to a lower concentration of HDL-cholesterol [78,79].
Liver metabolic disturbances are connected with the excessive flux of lipid-related metabolites. Specific solute channels are responsible for both fatty acids and glycerol import in the hepatocytes. The role of fatty acids transporters (FATPs) and fatty acids binding proteins (FABPs) in the accumulation of bioactive lipids and the induction of insulin resistance in liver has been extensively studied [80,81]. The discovery of aquaporin-mediated glycerol transport in both adipose tissue (mainly AQP7) and liver (mainly AQP9) points to aquaporin 9 as a new candidate to better understand the induction of hepatic insulin resistance [[82], [83], [84], [85]]. The following chapter gathers recent discoveries regarding the AQPs involvement in the induction of insulin resistance or the onset of type-2 diabetes.
3. Possible role of AQPs “metabolic network” in obesity-induced systemic insulin resistance
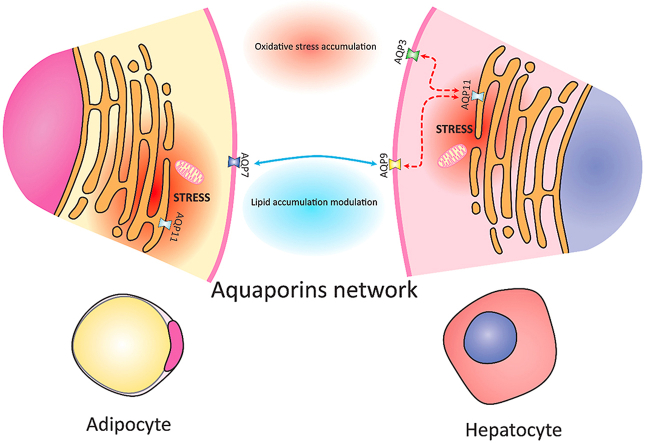
Nowadays the scientific world understood that concentrating just on one protein or on one cell or tissue in the description of a given disease is outdated. A systemic view is needed to find an answer to complex interconnected diseases as diabetes, obesity, and insulin resistance. The period which is beginning can be considered a “golden era” of science where a wealth of data is collected and analyzed. The most important and difficult side is to keep the pace with the data and find connections that can stimulate new hypothesis and researches. Here we are proposing the concept of aquaporin network as a contributor to the regulation of the cellular energy metabolism at the systemic scale.
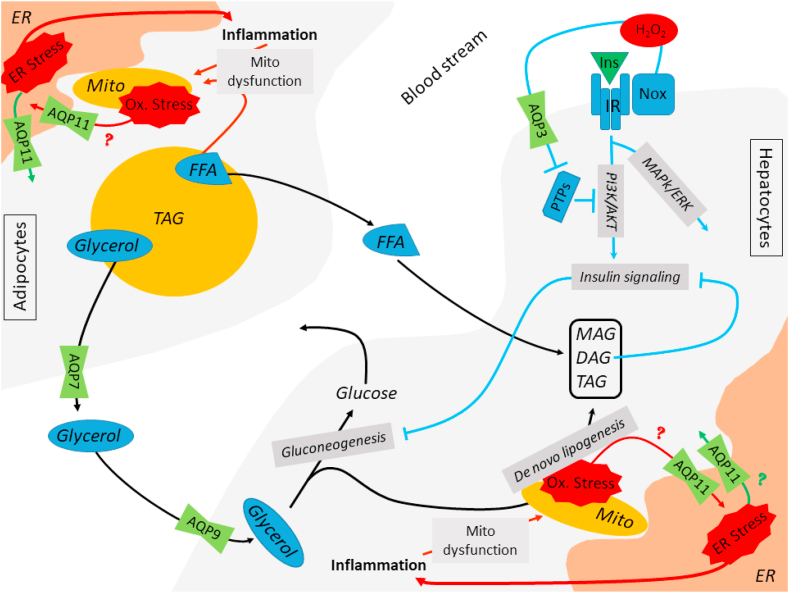
Tissues react to the environmental changes (obesity, insulin resistance, type-2 diabetes), trying to balance the energy metabolism. AQPs intracellular and intercellular/intertissue coordination could have an essential role in this adaptation process. AQP could serve as instruments of communication of the cells and tissues, able to coordinately react to the organism's stimuli depending on the situation or on the insurgence of a disease. The multiple roles covered by the different aquaporins in both adipocytes and hepatocytes highlights their relevance in insulin resistance and type-2 diabetes. It has been shown that AQP7 is a relevant glycerol transporter in adipocytes and AQP9 transports glycerol in hepatocytes [85]. AQP7 allows the glycerol derived from lipolysis of TG stores to reach the bloodstream, which can be assimilated by hepatocytes through its hepatic counterpart, AQP9 (Fig. 2). Both these AQPs are modulated at the level of expression by insulin and leptin. In the physiological state AQP7 and AQP9 are inversely regulated by insulin, leading to an increased AQP7 and AQP9 expression during fasting and a decreased expression during feeding [86,87]. The upregulation of AQP7 and AQP9 was detected also in streptozotocin-induced T1D [83,88]. On the contrary, AQP7 and AQP9 are upregulated by insulin and downregulated by leptin in omental adipocytes and HepG2 hepatocytes [89]. In other words, their modulation in both hepatocytes and adipocytes is driven by the lipogenic or lipolytic state, processes governed by insulin and leptin [90]. As glycerol transporters, aquaporins take part in glycerol efflux from adipocytes and glycerol import into hepatic cells. The previous listed scientific clues are supporting a cooperative role of those AQPs in limiting the accumulation of lipids in the liver, in the hyperglycemic state, diabetes, and obesity, even if strong experimental evidence is still missing [82,90]. The idea of AQPs coordinative action is quite consolidated, and the disturbance of their mutual relationship could bring to faster development of insulin resistance and type-2 diabetes. Other AQPs are also relevant in insulin resistance. Obesity leads to mitochondrial dysfunction in adipocytes and hepatocytes [[91], [92], [93]]. The oxidative stress in form of H2O2 release, triggered by mitochondrial dysfunction, is expected to flow into the ER through AQP11 in both adipocytes and hepatocytes. The further accumulation of oxidative stress in an ER already stressed by obesity is known to lead to an exacerbation of the inflammatory process, promoting mitochondrial dysfunction and increase in ER stress [[91], [92], [93]]. On the other side, in adipocytes from obese patients AQP11 overexpression alleviates ER stress through the compensatory mechanism which allows H2O2 efflux from the ER [94]. It is likely, that similar mechanism could be mimicked in hepatocytes, where an overexpression of AQP11 could lead to a decrease of obesity-induced ER stress. The proposed double function of AQP11 is compatible with its role as H2O2 transporter, and with the physiological adaptations of the cells to the environment in the attempt to balance the redox homeostasis. AQP3 isoform seems to be also involved in the hepatic regulation of redox state, since it is expressed in primary hepatocytes isolated from mice and it is plausibly involved in oxidative stress response in a mouse model of CCL4 acute liver injury [95]. Moreover, it has been shown that AQP3 is involved in the regulation of insulin signaling in McArdle 7777 rat hepatoma cells and primary rat hepatocytes. The hydrogen peroxide channeled by AQP3 contributes to the modulation of the PI3K/AKT side of the insulin cascade [96].
Fig. 2.
Aquaporins network in the induction of metabolic disease. Schematic representation of the likely interaction of AQPs between adipocytes and hepatocytes in the context of insulin resistance and type-2 diabetes. The black arrows highlight the major interaction between adipocytes and hepatocytes during lipolysis of TG stores. Glycerol is trafficked between adipocytes and hepatocytes with the help of AQP7 and AQP9, respectively. Hepatic glycerol of adipose tissue origin can enter gluconeogenesis or de novo lipogenesis together with FFA to yield hepatic MAG, DAG and TAG. Obesity leads to mitochondrial dysfunction with the subsequent oxidative stress accumulation in form of H2O2. This is likely driven into the ER through AQP11, promoting the creation of a stress-promoting-stress circle (Red arrows). Conversely, AQP11 displays beneficial effects in adipocytes, where its overexpression leads to a decrease of the ER stress caused by lipid overload by promoting the efflux of H2O2 from the ER itself. We hypothesize that a similar protective mechanism could be mirrored in hepatocytes (Green arrow). The light blue arrows denote the novel branch of the insulin signaling pathway in hepatocytes, which involves AQP3 for its modulation. The insulin signaling from the PI3K/AKT branch brings to the inhibition of the gluconeogenesis in physiologic conditions. Accumulation of DAG in the cytoplasm of hepatocytes due to increased uptake of glycerol and FFA inhibits the signaling cascade at the level of PI3K/AKT with the subsequent up-regulation of the gluconeogenesis, increasing blood glucose levels. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Mitochondria are the key organelle involved in the development of insulin resistance and type 2 diabetes, especially when fat-driven overload of mitochondrial oxidative capacity leads to mitochondrial dysfunction and accumulation of oxidative stress. Despite the still ongoing discussion regarding the presence of functional AQPs in mitochondrial membranes, we can find a connection between peroxiporins and mitochondria, supporting the relevance of both the mitos and AQPs in the development of insulin resistance. The work by Kruger et al. underlines the essential role of AQP8 in RINm5F rat pancreatic insulinoma cell survival, where the knockdown of AQP8 is lethal to the cells within 10 days. Above work had shown that AQP8 is localized in mitochondria and plasma membrane and is involved in the transport of hydrogen peroxide. More in detail, they show AQP8 participation in the transport of hydrogen peroxide endogenously generated in the mitochondria. This leads to the possibility of AQP8 serving protective role through facilitation of the H2O2 efflux from mitochondria following cytokine attack in T1D, postponing or reducing the mitochondrial dysfunction [97]. Previously, Ikaga et al. proposed a similar role for AQP8 in mitochondrial dysfunction. In their work on the 3T3-L1 preadipocytes cell line, they observed co-localization of AQP8 and mitochondria in mouse adipose tissue and 3T3-L1 preadipocytes. Silencing of AQP8 expression using shRNA, decreased its levels by approx. 75%. Partial AQP8 silencing was not lethal to the cells, yet it impaired mitochondrial functionality through decreasing maximum respiratory capacity. Since the effect of AQP8 silencing is particularly focused on the respiratory chain proteins which release water as a byproduct, Ikaga et al. propose that AQP8 silencing affects the physiological mitochondrial water flux, reducing the electron transport activity and ATP synthesis [98]. Considering that the inhibition of mitochondrial respiration is connected with insulin resistance development, the link between decreased mitochondrial performance and AQPs seems plausible [99]. Another paper from Takashi and colleagues links mitochondrial dysfunction to AQPs in SAS P0 cells. Studying the ferroptosis mechanism they showed that mitochondrial dysfunction is promoting AQPs expression [100]. On the other side, the Verkman group published convincing evidence against functional AQPs presence in mitochondrial membrane from rat brain, liver and kidney, measuring water and glycerol transport. The same measurements repeated on mitochondrial membrane preparations from wild type and knock out cells (lacking AQP1, 4 or 8) suggest that mitochondria are lacking functional aquaporins [101]. Confronting data underlines the importance of further research in the effort to clarify the role of AQPs in mitochondrial physiology and pathology. On the contrary, the involvement of the NFkB signaling pathway in insulin resistance is already well understood, and the connection between NFkB and AQPs was reported several times [102], yet the studies relevant to insulin resistance and T2D are scarce. Chiadak and colleagues performed study on the involvement of JNK and NFkB signaling pathway on AQP3, AQP7 and AQP11 expression in differentiated 3T3-L1 adipocytes. The inflammation observed in the obese state was mimicked through LPS stimulation. The LPS treatment of 3T3-L1 preadipocytes up-regulated AQP3 and AQP7 at the level of mRNA by both JNK and NFkB-dependent manner, while AQP11 was downregulated by the NFkB pathway in the same conditions. The authors propose that the downregulation of AQP7 and AQP11 is likely promoting lipid accumulation, while the upregulation of AQP3 is likely promoting the glycerol efflux [103].
Considering all the above, there is a growing body of evidence supporting the presence of coordinated “AQP network”. Such a network would govern on one side the lipid trafficking between adipose tissue and liver and on the other the modulation of oxidative stress (Fig. 2). The imbalance in the coordination of the AQPs modulation and crosstalk could be one of the important constituents of metabolic diseases. Further studies are needed on this promising subject, but here below are the latest researches on it.
3.1. Aquaporin 7
Aquaporin 7, also known as AQPap, is the main glycerol transporter in adipocytes. Glycerol role in TAG accumulation and stimulation of gluconeogenesis in liver makes the adipose tissue AQP7 important in the development of obesity and comorbidities such as IR and diabetes [90,104]. In support of this consideration, AQP7 knockout mice display higher free fatty acids and lower glycerol levels in plasma as a result of decreased glycerol efflux from adipose tissue. AQP7 KO mice develop also a general insulin resistance. IRS-1-associated PI3K signal is lower in WAT and muscle, whereas IRS-2 associated PI3K signal is lower in liver and muscles of AQP7KO mice. Insulin stimulated pAKT is lower in WAT, liver and muscles. Moreover, upon the β3-adrenergic stimulation the glycerol release from fat tissue is impaired in the AQP7 KO mice as compared to WT. It has also been noted that the AQP7 KO mice develop obesity at the adult stage when housed on standard diet. While until 10 weeks of age the weight was comparable with WT mice, after 12 weeks AQP7 KO mice growth curve is significantly higher [24,105]. This suggests that the inhibition of glycerol efflux due to AQP7 ablation promotes TAG accumulation in adipose tissue. Due to its involvement in the regulation of nutrients flux AQP7 expression is modulated and linked to the organism nutritional status. Fasting and feeding are two of the variables influencing AQP7 expression: it is upregulated by fasting and downregulated by refeeding in both the adipocytes and hepatocytes [89,106]. This suggest that at physiological conditions AQP7 participates in glycerol transport from adipose tissue to liver, supporting hepatic gluconeogenesis in the fasting state. The upregulation of AQP7 in WAT is promoted by the peroxisome proliferator-activated receptor γ (PPARγ), a nuclear receptor acting as a transcription factor and modulating gene expression [107]. On the other side, the downregulation is pushed also by insulin, isoproterenol, dexamethasone, and tumor necrosis factor-α (TNFα), but not by angiotensin 2 or growth hormone. Above data indicate, that the AQP7 expression in 3T3-L1 preadipocytes is selectively regulated by hormones that are connected with insulin resistance and dyslipidemia [108]. AQP7 downregulation by isoproterenol is shown for the first time here and goes against the proposed idea for AQP7 modulation. Inhibition of AQP7 by an adrenergic stimulator would inhibit the glycerol efflux and the lipolytic process in adipocytes. Further studies are needed to understand the particular cases in which this modulation could be valid.
The excess accumulation of white adipose tissue (WAT) is relevant to insulin resistance and type-2 diabetes development [109]. Dysfunctional WAT develops hypertrophy through increase in the size of adipocytes, which is positively correlated with the incidence of the metabolic disease [110,111]. The exact mechanism that drives the WAT hypertrophy is still not well understood. To obtain a clearer image of the mechanisms involved in hypertrophy of WAT, an epigenetic regulation study was performed by Kerr et al. in WAT samples from 126 women. Transcriptome profiling revealed 11 of 638 targeted genes with high degree of differentially methylated CpG sites, which were chosen for the functional evaluation in human mesenchymal stem cells (hMSCs cells) and silenced with siRNA. The functional evaluation revealed that one of the genes selected for the further study – AQP7 – has significant impact on lipid accumulation. AQP7 down-regulation increased the size of lipid droplets per cell and decreased the content of ADIPOQ in hMSCs. Interestingly, despite increased lipogenesis under insulin stimulation – presumably due to decreased efflux of glycerol - the AQP7−/− cells display decreased insulin responsiveness, which mimics obesity-related dysfunctional state of adipose tissue. The authors finally underline the importance of AQP7 regulation in WAT hypertrophy, noting that AQP7 was the only gene whose silencing triggers increase in lipid storage, which is in line with its role as glycerol transporter involved in glycerol efflux from adipocytes [112].
The AQP7 involvement in the regulation of adipose tissue lipogenesis was also noted in the study by Kulyte et al. The microarray-based transcriptome profiling of subcutaneous WAT samples from insulin-sensitive and insulin-resistant obese women revealed 432 differentially expressed genes involved in the possible control of adipose tissue insulin sensitivity. Two insulin resistance associated genes were selected for further functional analysis: KLF15 (Krüppel-like factor 15) and SLC25A10 (mitochondrial dicarboxylate carrier). Silencing of KLF15 gene in the in-vitro model of differentiated adipocytes inhibited insulin-stimulated lipogenesis. Subsequent transcriptome profiling of the KLF15-silenced cells candidates KLF15 to the role of insulin sensitivity controller given its ability to modulate the expression of PPAR γ and its target AQP7 [113].
AQP7 is one of the three targets - together with PKBβ and GLUT4 – selected in the study by Mourelatou et al. aimed at the elucidation of the molecular mechanisms at the basis of insulin resistance and type 2-diabetes development in adipocytes of obese subjects. The in-vitro study was performed on primary adipocytes, derived from different fat depot biopsies from 20 lean and 36 morbidly obese patients (20 without diabetes and 16 with T2D). The study identified AQP7 as one of the genes down-regulated in insulin-resistant adipocytes. Interestingly, down-regulation was observed for typical 34 kDa isoform of AQP7 and also for novel 37kDA variant identified by the authors. Both variants were down-regulated together with GLUT4 glucose transporter in both visceral and subcutaneous adipocytes from insulin-resistant subjects, and up-regulated in lean subjects. Interestingly, AQP7 expression in obese is positively correlated with pAKT phosphorylation which suggest that high AQP7 level is one of the features of adipose tissue insulin sensitivity. Authors propose that the downregulation of 34 and 37 kDa isoforms of AQP7 as responsible for reduced lipolysis, enhancement of adipocyte hypertrophy, and the impairment of insulin signaling. Finally, authors propose that the downregulation of the subcutaneous WAT 34 kDa isoform of AQP7 could be an early marker of insulin resistance [114].
The same group performed another interesting study focused on the role of AQP7 in childhood and adolescence obesity and its comorbidities. The work focused on the AQP7 expression in adipocytes coming from lean and obese children. The adipocytes from healthy young, obese prepubertal children have increased levels of AQP7 mRNA and significant expression of 41 kDa isoform of AQP7 compared to the lean group. Obese adolescents show decreased AQP7 mRNA levels and increased HOMA-IR. Interestingly the obese adolescents expressing the 34 kDa AQP7 isoform had lower triglyceride levels in plasma compared to those who did not express it. Authors suggest that predisposition to metabolic dysfunction in obese adolescents is connected with lower expression of AQP7 mRNA and that the overexpression of 41 kDa AQP7 in young obese prepubertal children acts as protective mechanism against adipocytes hypertrophy [115].
Above studies confirm the participation of AQP7 in modulating adipocytes metabolism, especially lipogenesis and lipolysis. Down-regulation of AQP7 seems to coincidence with increased adipose tissue accumulation and lipogenesis, promoting obesity. The next step in the research is to identify factors which are able to affect AQP7 expression in adipose tissue to modulate its lipogenic response. The recent study by Mehanna et al. identified raspberry ketones (RKs) as the group of compounds able to alter AQP7 expression in WAT of high-fat diet fed rats. Authors divided rats into groups: wild type, high fat diet control, and RKs-treated (250/500 mg/kg). RKs treatment decreased the hyperlipidemia and fat accumulation in both the adipocytes and hepatocytes and the plasma oxidative stress markers. The changes observed in RK-treated animals were connected with improved insulin sensitivity and reduced adipocytes diameter in HFD-fed rats. RK treatment decreased the ratio between fat tissue weight and body weight and improved overall liver function, estimated by the serum levels of ALT and AST enzymes. This again underlines the importance of AQP7-mediated crosstalk between adipose tissue ad liver in the induction of metabolic disorders. Both doses of RKs downregulated leptin and upregulated AQP7 expression, while just the lower dose brought to an upregulation of adiponectin expression. Authors conclude, that beneficial effects of RKs treatment is due -at least in part-to its effect on AQP7 [116].
In recent years, another gene expression analysis study lists AQP7 as one of the signature genes upregulated in obese healthy patients. The study by de Luis et al. compared obese healthy patients with non-obese control ones, analyzing the gene expression profile of PBMCs (peripheral blood mononuclear cells). AQP7 was revealed as one of the overexpressed metabolism-related genes (together with IGF1, ApoA 5, Foxo 4, ADIPOR1) in the obese healthy patients as compared to non-obese healthy ones [117].
AQP7 relevance in insulin resistance goes beyond adipocytes and it seems to be important also in pancreatic β-cells. Da Silva et al. used the in-vitro RIN-m5F β-cell model to understand AQP7 involvement in cellular and obesity-induced inflammatory processes. Tumor necrosis factor-alpha induced inflammation, down-regulated AQP7 expression in RIN-m5F β-cells, with the subsequent marked reduction in insulin secretion. Conversely, induction of inflammation by LPS treatment led to overexpression of AQP7, concluding that inflammation also affects aquaporin expression. Authors suggest, that AQP7 is relevant for the biological functions of pancreatic β cells and that is strictly connected with insulin secretion [118].
Above data can confirm AQP7 relevance in adipocytes glycerol transport and insulin resistance. In detail, we can confirm that AQP7 expression is a constituent of “healthy” insulin-sensitive adipocytes, having an essential role in the efflux of glycerol derived from lipolysis. The downregulation of AQP7 in adipocytes with obesity and adipose tissue insulin resistance shows the key role of AQP7 in the cycle of obesity promoting further obesity through inhibition of the efflux of lipogenic glycerol.
3.2. Aquaporin 9
AQP9 is the major isoform of AQPs expressed in the liver. AQP9 is regarded as the main mediator for glycerol influx in hepatocytes. The liver metabolizes between 70% and 90% of plasma-borne glycerol [90,104]. Unlike adipocytes which in physiological state express glycerol kinase (GK) in small quantities solely for the purpose of TG synthesis, hepatocytes trap glycerol intracellularly through converting glycerol to glycerol-3-phosphate (Gro3P). Gro3P serves as a backbone substrate in hepatic triacylglycerol synthesis or after conversion to dihydroxyacetone-phosphate (DHAP) enters hepatic gluconeogenesis pathway. AQP9 expression appears to be reduced in patients with NAFLD and NASH, and even more reduced in insulin-resistant state. This intriguing down-regulation of AQP9 under lipid overload was suggested as a protective measure to reduce the influx of the glycerol and slow down the hepatic triacylglycerol accumulation [[119], [120], [121]]. Interestingly, despite a similar expression of hepatic AQP9 in both the women and men, women have a lower hepatic glycerol permeability, potentially explaining the lower incidence of NAFLD in women [122]. As aquaporin channel regulation was already confirmed in the case of AQP8 [12], a possible post-transcriptional modification -driven modulation of AQP9 glycerol permeability cannot be excluded. A recent study on the effects of silybin (a compound known for its beneficial lipid-lowering effects) in NAFLD, has once again shown the link between AQP9 and hepatic lipid accumulation. Baldini et al. used a rat hepatoma NAFLD cell model to understand the molecular mechanisms behind the silybin effect. An improvement of both the lipid droplets fatty acids profile and the mitochondrial oxidation was observed under silybin treatment. Most importantly, it is shown that silybin restores the levels of AQP9 and accordingly, also the glycerol permeability [123].
AQP9 is also modulated during fasting, re-feeding and fat accumulation. The changes in the nutritional state and adiposity regulate the plasma levels of insulin and leptin, which have previously been described as aquaporin 9 negative modulators. Fasting and refeeding upregulate and downregulate AQP9 both at the level of mRNA and protein in mice hepatocytes [124,125]. In the context of insulin resistance and obesity-induced NAFLD, the overexpression of AQP3 and AQP7 in adipocytes increases the flux of glycerol available to the hepatocytes through AQP9-dependent mechanism. As glycerol backbones can be used for both the lipid synthesis and gluconeogenesis, down-regulation of AQP9 expression is a likely protective mechanism, which aims to not worsen the metabolic disturbances [88,120,124,126]. On the other hand, the upregulation of AQP9 in the liver of obese and insulin resistant mice has been also observed [88], which could be the outcome of hepatic insulin resistance and lack of inhibitory control of insulin over AQP9 expression. This underlines the difficulties behind understanding the function of AQPs, and confirms that more research is needed to outline the polyhedric face of this channel.
It has been also shown by Portois et al. that AQP9 expression is decreased in n3-PUFA-depleted rats. Surprisingly, AQP9 down-regulation is coupled to an increase of labeled glycerol uptake in rat hepatocytes. The anomaly has been justified proposing the intracellular metabolism as responsible of the glycerol accumulation, since they saw an increase of the glycerol kinase activity [127].
The coordination between AQP7 in adipocytes and AQP9 in hepatocytes could be a possible key element in the explanation of systemic insulin resistance also in men. This in case of human was first shown by Miranda and colleagues in 2009, who studied samples of adipose tissue (subcutaneous –SAT- and visceral –VAT-) and liver taken during bariatric surgery performed on morbidly obese people. The results confirmed a strong positive correlation between SAT AQP7 and liver AQP9, which was more evident in insulin-resistant and type-2 diabetes mellitus subjects [128].
Previously the coordinated regulation of these two channels was shown in mice. In the study by Kuriyama et al. the authors observed up and down -regulation of hepatic AQP9 mRNA after fasting and refeeding, respectively. Plasma glycerol levels were following the same trend. This suggests that the expression of hepatic AQP9 is negatively regulated by insulin in mice. The authors remark how crucial is the coordination of AQP7 and AQP9 regulation for the study of the glucose metabolism [88].
The study by Méndez-Giménez et al. also supports the AQPs cooperative network thesis: AQP9 and AQP7 coordination is deeply involved in hepatic steatosis and the development of insulin resistance. To understand the mechanism behind the success of the sleeve gastrectomy in reducing hepatic steatosis in obese subjects, Méndez-Giménez and colleagues performed bariatric surgery on diet-induced obese mice (DIO rats) and analyzed AQP3, AQP7 and AQP9 levels in the different fat depots and the liver. DIO rats showed an increase in the levels of AQP3 and AQP7 respectively in epididymal and subcutaneous WAT and a decrease in the levels of hepatic AQP9, which suggests that upregulation of adipose tissue glyceroporins and downregulation of hepatic ones is connected with insulin resistance. The protective effects of sleeve gastrectomy such as decrease of body weight, fat mass and hepatic steatosis were accompanied by decrease in AQP7 expression in all of the analyzed fat depots, up-regulation of AQP3 in epididymal WAT and stable AQP9 expression in liver. The interpretation of the results supports the idea that behind the beneficial effects of sleeve gastrectomy there is a better coordination in the aquaporins modulation in the liver and adipose tissue [129].
Despite the multiple reports regarding coordinated regulation of adipose and hepatic aquaporins, it is possible to find countercurrent works. Iena et al. treated mice with a HFD for 12 or 24 weeks and showed a gender-specific effect of HFD on adipose tissue AQPs. After 12 weeks females displayed overexpression of AQP7 in adipocytes compared to the control. Males showed an overexpression of glycerol kinase in adipocytes, a higher weight increment and an increased adipocyte size. Moreover, no effect of HFD on the liver AQP9 and no association between the increasing hepatic steatosis and AQP9 levels were visible. Finally, there was no evidence of coordinated regulation of adipose tissue AQP7 and hepatic AQP9 [122]. Hopefully, this controversy will stimulate the scientific community to deepen the study of AQP7 and AQP9 relationship, also deploying the newest approaches. An omics approach was used by Wang and colleagues for the study of AQP7 and AQP9 gene polymorphisms and T2D in the Chinese population. In the samples collected from 400 T2D patients and 400 controls 5 SNPs were genotyped in each of the AQPs. The results show an association between AQP7 SNP rs2989924 and rs3758269 and T2D risk in Chinese Han population. Any association between the tested AQP9 SNPs and T2D has been found [130].
In the attempt of explaining the insulin sensitivity loss correlation with integrin-linked kinase (ILK), Hatem-Vaquero et al. created transgenic mice with downregulated ILK. Both the control (CTRL) and ILK-low animals were subjected to a low-fat diet (LFD) or high fat diet (HFD) for 6 weeks. The circulating glycerol and the liver AQP9 levels were checked after 2 weeks and at the end of the treatment. The HFD brings to an increase of the circulating glycerol both after 2 and 6 weeks. Similarly, AQP9 overexpression in HFD treated CTRL and ILK-low mice is visible already after 2 weeks and increases even more after 6 weeks. In conclusion they state that ILK could be used to predict metabolic disorders establishment [131].
An interesting manuscript analyzes the expression profiles of AQP9 and MAPK in T1D and T2D rat liver to find the mechanism of AQP9 regulation in gluconeogenesis. AQP9 was overexpressed in both T1D and T2D, together with JNK phosphorylation, while p38 phosphorylation was inhibited and ERK did not change compared to healthy control. The insulin treatment lowered AQP9, probably through the inhibition of p-JNK and activation of phospho-p38. In conclusion, AQP9 and MAPK pathways analysis makes plausible a possible AQP9 regulation through MAPK signaling pathways [132].
AQP9 connections with diabetes are not limited to the liver. Fan and colleagues analyzed the effects of T1D on the recovery of rats from brain injury induced by intracranial hemorrhage (ICH). Interestingly, AQP9 displayed higher expression in diabetic rat hippocampus as compared to non-diabetic ones. AQP9 up-regulation in the hippocampus of diabetic animals was associated with weaker brain angiogenesis, higher neuronal loss in the hippocampus and diminished integrity of the blood-brain barrier after ICH. This discovery suggests that AQP9 overexpression in T1D brain is impairing the recovery from the ICH [133].
Finally, a study about tissue uptake of trivalent inorganic arsenic in mice with T1D involves AQPs, transporters of this compound. Arsenic levels in liver, kidney, and heart of T1D mice were higher than in control, while in the lung not. Tissue arsenic content followed AQP expression, as AQP9 was overexpressed in the and AQP7 was overexpressed in the kidney and heart of T1D mice. AQP9 levels in lungs instead were similar in both CTRL and T1D. The authors propose that T1D alters the expression of transporters of trivalent inorganic arsenic, increasing its uptake [134].
In conclusion AQP9 is a relevant molecule in both the insulin resistance onset and T2D in hepatocytes. Its double regulation by the insulin/leptin and fasting/refeeding show importance of this channel for the metabolic function of hepatocytes. The glycerol transport through AQP9 can influence the accumulation of bioactive lipids, and the recently discovered role of AQP9 as hydrogen peroxide channel expands its influence to the redox homeostasis of the hepatocytes [135].
3.3. Other aquaporins
After having extensively dissected the aquaporins most studied in diabetes, it is important to consider also the novel candidates. Recently published article points at AQP3 as a new member of insulin signaling pathway. It has been shown that insulin stimulates NADPH oxidase 4 (NOX4) to produce O2•– extracellularly in McArdle hepatocytes. NOX4 forms a complex with superoxide dismutase 3 (SOD3) to produce H2O2. AQP3 allows H2O2 to cross the plasma membrane and deactivate PTEN/PTPases phosphatases amplifying insulin receptor signaling (Fig. 2). Wu and colleagues called this pathway NSAPP from the 5 major proteins part of it - NOX4-SOD3-AQP3-PTEN/PTPases – and considering it essential in the modulation of the balanced insulin action through ERK and PI3K-AKT signaling cascade [96].
Recently AQP5 has been connected to diabetes, as it is proposed as a new prognostic biomarker of diabetic nephropathy. It is hard and important to identify diabetic patients who will develop a decline in kidney functions and nowadays it is not possible to predict it. AQP5 is a possible marker of tubular dysfunction and its basal levels were analyzed in two studies: one with a limited number of samples from T2D and T2D with diabetic nephropathy patients and the second with almost 1000 urine samples from patients with T2D with diabetic nephropathy. Both the studies candidate AQP5 as a prognostic marker for diabetic nephropathy progression and suggest an association between AQP5 baseline expression and the estimated glomerular filtration rate (eGFR) [136,137].
AQP5 is also downregulated in the parotid salivary gland of T2D rats that showed decreased salivary flow rate [138]. Rats treatment with bone marrow-derived stem cells improves the salivary flow rate and alters AQP5 levels in the parotid gland [139]. It was also shown that autophagy degrades AQP5 in the submandibular salivary gland [46].
AQP11 variant rs2276415 has been indicated as a potential genetic factor that gives to T2D patients the predisposition of developing chronic kidney disease [140,141].
A new study on AQP11 role in human adipocytes from non-diabetic or T2D patients, candidates it to a future as main character in obesity and T2D. Obesity leads to lipotoxicity and inflammation and subsequently to ER stress, so Frühbeck and colleagues analyzed the possible involvement of AQP11. It has been shown that AQP11 increases in visceral adipose tissue (VAT) from obese and obese-T2D subjects. Reversely, TNF-α and TGF-β1 decrease AQP11 levels in VAT and drive its pooling around the lipid droplets. Finally, the knockdown of AQP11 in visceral adipocytes brings to an increase in ER stress. Data interpretation elevates AQP11 to a double role in adipose tissue. In normal conditions, it allows the movement of glycerol for TAG synthesis in early lipid droplets. In obese conditions, it is overexpressed in VAT to alleviate the ER stress, being a hydrogen peroxide transporter. In conclusion AQP11 is candidate to be a novel target for the therapy of obesity [94].
4. Conclusions
The deep involvement of AQPs in obesity, insulin resistance, and type-2 diabetes is progressively unfolded through continuous research and novel data. Aquaporins have significant influence on human adiposity through AQP7 in adipose tissue. On the other hand, they serve crucial function in the liver, where AQP9 gives its contribution to the gluconeogenesis and lipogenesis processes. Significant is the fact, that both of them are affected by insulin resistance in fat and liver tissue. As AQPs are involved in two most significant aspects of insulin resistance – e.g., tissue lipid accumulation and oxidative stress – they emerge as key players in the obesity-induced IR. The question if AQP imbalance can be a major factor which drives insulin resistance, or is a mere outcome of this state deserves more attention in the future studies. The original concept of aquaporin network has been introduced, emphasizing the relevance of AQP7 and AQP9 inter-tissue coordination. Other aquaporins can be added to the backbone of the network - AQP11 and AQP3 - both known to be hydrogen peroxide transporters. In this way the AQPs located in majority of insulin-sensitive tissues are involved in all the main points connected to the insulin resistance development, from glycerol to gluconeogenesis and bioactive lipids, from hydrogen peroxide to oxidative stress accumulation. Additional space has been reserved to promising novel candidates such as AQP11, with a novel role of intracellular ROS carrier involved in adipocyte mitochondria – mediated ER stress accumulation in obesity and inflammation. Its potential role in hepatocytes should be investigated and clarified. The comprehensive critical analysis of the recent discoveries will contribute to the future findings in insulin resistance and type-2 diabetes.
Author contributions
P.Z. supervised the work. M.G. planned, wrote, and drafted the manuscript. A.H. and A.Z. assisted with the literature search and the interpretation of data in tabular form. All authors have read and agreed to the published version of the manuscript.
Funding
This research was conducted within the project which has received funding from the European Union's Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 754432 and the Polish Ministry of Science and Higher Education, from financial resources for science in 2018–2023 granted for the implementation of an international co-financed project.
This research was supported by the Medical University of Bialystok [SUB/1/DN/19/002/1117].
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Mauro Galli, Email: mauro.galli@umb.edu.pl.
Ahsan Hameed, Email: ahsan.hameed@umb.edu.pl.
Arkadiusz Żbikowski, Email: arkadiusz.zbikowski@umb.edu.pl.
Piotr Zabielski, Email: piotr.zabielski@umb.edu.pl.
References
- 1.Martens C., Politis A. A glimpse into the molecular mechanism of integral membrane proteins through hydrogen–deuterium exchange mass spectrometry. Protein Sci. 2020;29:1285–1301. doi: 10.1002/pro.3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bosshart P.D., Fotiadis D. Subcell. Biochem. Springer; New York: 2019. Secondary active transporters; pp. 275–299. [DOI] [PubMed] [Google Scholar]
- 3.Li C., Wang W. Adv. Exp. Med. Biol. Springer New York LLC; 2017. Molecular biology of aquaporins; pp. 1–34. [DOI] [Google Scholar]
- 4.Kulbacka J., Choromańska A., Rossowska J., Weżgowiec J., Saczko J., Rols M.P. Adv. Anat. Embryol. Cell Biol. Springer Verlag; 2017. Cell membrane transport mechanisms: ion channels and electrical properties of cell membranes; pp. 39–58. [DOI] [PubMed] [Google Scholar]
- 5.Verkman A.S., Yang B., Song Y., Manley G.T., Ma T. Role of water channels in fluid transport studied by phenotype analysis of aquaporin knockout mice. Exp. Physiol. 2000;85 doi: 10.1111/j.1469-445x.2000.tb00028.x. [DOI] [PubMed] [Google Scholar]
- 6.Beall M.H., van den Wijngaard J.P.H.M., van Gemert M.J.C., Ross M.G. Amniotic fluid water dynamics. Placenta. 2007;28:816–823. doi: 10.1016/j.placenta.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen T., Toussaint J., Xue Y., Raval C., Cancel L., Russell S., Shou Y., Sedes O., Sun Y., Yakobov R., Tarbell J.M., Jan K.M., Rumschitzki D.S. Aquaporin-1 facilitates pressure-driven water flow across the aortic endothelium. Am. J. Physiol. Heart Circ. Physiol. 2015;308:H1051–H1064. doi: 10.1152/ajpheart.00499.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laloux T., Junqueira B., Maistriaux L.C., Ahmed J., Jurkiewicz A., Chaumont F. Plant and mammal aquaporins: same but different. Int. J. Mol. Sci. 2018;19 doi: 10.3390/ijms19020521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishimura H., Yang Y. Aquaporins in avian kidneys: function and perspectives. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013;305:1201–1214. doi: 10.1152/ajpregu.00177.2013.-For. [DOI] [PubMed] [Google Scholar]
- 10.Medraño-Fernandez I., Sitia R. Elsevier Inc.; 2019. Aquaporins: Gatekeepers in the Borders of Oxidative Stress and Redox Signaling. [DOI] [Google Scholar]
- 11.Bestetti S., Galli M., Sorrentino I., Pinton P., Rimessi A., Sitia R., Medraño-Fernandez I. Human aquaporin-11 guarantees efficient transport of H2O2 across the endoplasmic reticulum membrane. Redox Biol. 2020;28 doi: 10.1016/j.redox.2019.101326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bestetti S., Medraño-Fernandez I., Galli M., Ghitti M., Bienert G.P., Musco G., Orsi A., Rubartelli A., Sitia R. A persulfidation-based mechanism controls aquaporin-8 conductance. Sci. Adv. 2018;4 doi: 10.1126/sciadv.aar5770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murata K., Mitsuoka K., Hiral T., Walz T., Agre P., Heymann J.B., Engel A., Fujiyoshi Y. Structural determinants of water permeation through aquaporin-1. Nature. 2000;407:599–605. doi: 10.1038/35036519. [DOI] [PubMed] [Google Scholar]
- 14.Engel A., Wspalz T., Fujiyoshi Y. The AQP structure and functional implications. Handb. Exp. Pharmacol. 2009;190:31–56. doi: 10.1007/978-3-540-79885-9_2. [DOI] [PubMed] [Google Scholar]
- 15.Ikeda M., Andoo A., Shimono M., Takamatsu N., Taki A., Muta K., Matsushita W., Uechi T., Matsuzaki T., Kenmochi N., Takata K., Sasaki S., Ito K., Ishibashi K. The NPC motif of aquaporin-11, unlike the NPA motif of known aquaporins, is essential for full expression of molecular function. J. Biol. Chem. 2011;286:3342–3350. doi: 10.1074/jbc.M110.180968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Omasits U., Ahrens C.H., Müller S., Wollscheid B. Protter: interactive protein feature visualization and integration with experimental proteomic data. Bioinformatics. 2014;30:884–886. doi: 10.1093/bioinformatics/btt607. [DOI] [PubMed] [Google Scholar]
- 17.Snider C., Jayasinghe S., Hristova K., White S.H. MPEx: a tool for exploring membrane proteins. Protein Sci. 2009;18:2624–2628. doi: 10.1002/pro.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berman H.M., Westbrook J., Feng Z., Gilliland G., Bhat T.N., Weissig H., Shindyalov I.N., Bourne P.E. The protein data bank. Nucleic Acids Res. 2000;28:235–242. doi: 10.1093/nar/28.1.235. rcsb.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gotfryd K., Mósca A.F., Missel J.W., Truelsen S.F., Wang K., Spulber M., Krabbe S., Hélix-Nielsen C., Laforenza U., Soveral G., Pedersen P.A., Gourdon P. Human adipose glycerol flux is regulated by a pH gate in AQP10. Nat. Commun. 2018;9:1–11. doi: 10.1038/s41467-018-07176-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sehnal D., Rose A.S., Kovca J., Burley S.K., S V. Mol* Towar. A Common Libr. Tools Web Mol. Graph.; 2018. Mol*: towards a common library and tools for web molecular graphics MolVA/EuroVis Proceedings; pp. 29–33. [DOI] [Google Scholar]
- 21.Hara-Chikuma M., Verkman A.S. Aquaporin-3 functions as a glycerol transporter in mammalian skin. Biol. Cell. 2005;97:479–486. doi: 10.1042/bc20040104. [DOI] [PubMed] [Google Scholar]
- 22.Bertolotti M., Bestetti S., García-Manteiga J.M., Medraño-Fernandez I., Dal Mas A., Malosio M.L., Sitia R. Tyrosine Kinase signal modulation: a matter of H2O2 membrane permeability? Antioxidants Redox Signal. 2013;19:1447–1451. doi: 10.1089/ars.2013.5330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ikeda M., Beitz E., Kozono D., Guggino W.B., Agre P., Yasui M. Characterization of aquaporin-6 as a nitrate channel in mammalian cells. Requirement of pore-lining residue threonine 63. J. Biol. Chem. 2002;277:39873–39879. doi: 10.1074/jbc.M207008200. [DOI] [PubMed] [Google Scholar]
- 24.Maeda N., Funahashi T., Hibuse T., Nagasawa A., Kishida K., Kuriyama H., Nakamura T., Kihara S., Shimomura I., Matsuzawa Y. Adaptation to fasting by glycerol transport through aquaporin 7 in adipose tissue. Proc. Natl. Acad. Sci. U.S.A. 2004;101:17801–17806. doi: 10.1073/pnas.0406230101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y., Promeneur D., Rojek A., Kumar N., Frøkiær J., Nielsen S., King L.S., Agre P., Carbrey J.M. Aquaporin 9 is the major pathway for glycerol uptake by mouse erythrocytes, with implications for malarial virulence. Proc. Natl. Acad. Sci. U.S.A. 2007;104:12560–12564. doi: 10.1073/pnas.0705313104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laforenza U., Scaffino M.F., Gastaldi G. Aquaporin-10 represents an alternative pathway for glycerol efflux from human adipocytes. PloS One. 2013;8 doi: 10.1371/journal.pone.0054474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller E.W., Dickinson B.C., Chang C.J. Aquaporin-3 mediates hydrogen peroxide uptake to regulate downstream intracellular signaling. Proc. Natl. Acad. Sci. U.S.A. 2010;107:15681–15686. doi: 10.1073/pnas.1005776107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hara-Chikuma M., Chikuma S., Sugiyama Y., Kabashima K., Verkman A.S., Inoue S., Miyachi Y. Chemokine-dependent T cell migration requires aquaporin-3-mediated hydrogen peroxide uptake. J. Exp. Med. 2012;209:1743–1752. doi: 10.1084/jem.20112398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koyama N., Ishibashi K., Kuwahara M., Inase N., Ichioka M., Sasaki S., Marumo F. Cloning and functional expression of human Aquaporin 8 cDNA and analysis of its gene | elsevier enhanced reader. Genomics. 1998;54:169–172. doi: 10.1006/geno.1998.5552. [DOI] [PubMed] [Google Scholar]
- 30.Medraño-Fernandez I., Bestetti S., Bertolotti M., Bienert G.P., Bottino C., Laforenza U., Rubartelli A., Sitia R. Stress regulates aquaporin-8 permeability to impact cell growth and survival. Antioxidants Redox Signal. 2016;24:1031–1044. doi: 10.1089/ars.2016.6636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ishibashi K., Tanaka Y., Morishita Y. The role of mammalian superaquaporins inside the cell. Biochim. Biophys. Acta Gen. Subj. 2014;1840:1507–1512. doi: 10.1016/j.bbagen.2013.10.039. [DOI] [PubMed] [Google Scholar]
- 32.Bestetti S., Galli M., Sorrentino I., Pinton P., Rimessi A., Sitia R., Medraño-Fernandez I. Human aquaporin-11 guarantees efficient transport of H2O2 across the endoplasmic reticulum membrane. Redox Biol. 2020;28 doi: 10.1016/j.redox.2019.101326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uhlen M., Fagerberg L., Hallstrom B.M., Lindskog C., Oksvold P., Mardinoglu A., Sivertsson A., Kampf C., Sjostedt E., Asplund A., Olsson I., Edlund K., Lundberg E., Navani S., Szigyarto C.A.-K., Odeberg J., Djureinovic D., Takanen J.O., Hober S., Alm T., Edqvist P.-H., Berling H., Tegel H., Mulder J., Rockberg J., Nilsson P., Schwenk J.M., Hamsten M., von Feilitzen K., Forsberg M., Persson L., Johansson F., Zwahlen M., von Heijne G., Nielsen J., Ponten F. Tissue-based map of the human proteome. Science (80-. ) 2015;347 doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 34.Chepelinsky A.B. Structural function of mip/aquaporin 0 in the eye lens; Genetic defects lead to congenital inherited cataracts. Handb. Exp. Pharmacol. 2009;190:265–297. doi: 10.1007/978-3-540-79885-9_14. [DOI] [PubMed] [Google Scholar]
- 35.Maltaneri R.E., Schiappacasse A., Chamorro M.E., Nesse A.B., Vittori D.C. Aquaporin-1 plays a key role in erythropoietin-induced endothelial cell migration. Biochim. Biophys. Acta Mol. Cell Res. 2020;1867 doi: 10.1016/j.bbamcr.2019.118569. [DOI] [PubMed] [Google Scholar]
- 36.Guo X., Sun T., Yang M., Li Z., Li Z., Gao Y. Prognostic value of combined aquaporin 3 and aquaporin 5 overexpression in hepatocellular carcinoma. BioMed Res. Int. 2013 doi: 10.1155/2013/206525. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoshi A., Tsunoda A., Tada M., Nishizawa M., Ugawa Y., Kakita A. Expression of aquaporin 1 and aquaporin 4 in the temporal neocortex of patients with Parkinson's disease. Brain Pathol. 2017;27:160–168. doi: 10.1111/bpa.12369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li D., Shi X., Zhao L., Liang Z., Xie S., Wang G. Overexpression of Aquaporin 1 on cysts of patients with polycystic liver disease. Rev. Esp. Enferm. Dig. 2016;108:71–78. doi: 10.17235/reed.2015.3960/2015. [DOI] [PubMed] [Google Scholar]
- 39.Shimasaki M., Kanazawa Y., Sato K., Tsuchiya H., Ueda Y. Aquaporin-1 and -5 are involved in the invasion and proliferation of soft tissue sarcomas. Pathol. Res. Pract. 2018;214:80–88. doi: 10.1016/j.prp.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 40.Milano S., Carmosino M., Gerbino A., Svelto M., Procino G. Hereditary nephrogenic diabetes insipidus: pathophysiology and possible treatment. An update. Int. J. Mol. Sci. 2017;18 doi: 10.3390/ijms18112385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu H.A.J. Adv. Exp. Med. Biol. Springer New York LLC; 2017. Diabetes insipidus; pp. 213–225. [DOI] [Google Scholar]
- 42.Judith Radin M., Yu M.J., Stoedkilde L., Lance Miller R., Hoffert J.D., Frokiaer J., Pisitkun T., Knepper M.A. Aquaporin-2 regulation in health and disease. Vet. Clin. Pathol. 2012;41:455–470. doi: 10.1111/j.1939-165x.2012.00488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Verkman A.S., Smith A.J., wah Phuan P., Tradtrantip L., Anderson M.O. The aquaporin-4 water channel as a potential drug target in neurological disorders. Expert Opin. Ther. Targets. 2017;21:1161–1170. doi: 10.1080/14728222.2017.1398236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tamtaji O.R., Behnam M., Pourattar M.A., Jafarpour H., Asemi Z. Aquaporin 4: a key player in Parkinson's disease. J. Cell. Physiol. 2019;234:21471–21478. doi: 10.1002/jcp.28871. [DOI] [PubMed] [Google Scholar]
- 45.Yang C., Huang X., Huang X., Mai H., Li J., Jiang T., Wang X., Lü T. Aquaporin-4 and Alzheimer's disease. J. Alzheim. Dis. 2016;52:391–402. doi: 10.3233/JAD-150949. [DOI] [PubMed] [Google Scholar]
- 46.Huang Y., Shi X., Mao Q., Zhang Y., Cong X., Zhang X., Zhang Z., Wu L., Xiang R., Yu G. Aquaporin 5 is degraded by autophagy in diabetic submandibular gland. Sci. China Life Sci. 2018;61:1049–1059. doi: 10.1007/s11427-018-9318-8. [DOI] [PubMed] [Google Scholar]
- 47.Hara-Chikuma M., Verkman A.S. Roles of aquaporin-3 in the epidermis. J. Invest. Dermatol. 2008;128:2145–2151. doi: 10.1038/jid.2008.70. [DOI] [PubMed] [Google Scholar]
- 48.Xia H., Ma Y.-F., Yu C.-H., Li Y.-J., Tang J., Li J.-B., Zhao Y.-N., Liu Y. Aquaporin 3 knockdown suppresses tumour growth and angiogenesis in experimental non-small cell lung cancer. Exp. Physiol. 2014;99:974–984. doi: 10.1113/expphysiol.2014.078527. [DOI] [PubMed] [Google Scholar]
- 49.Kang B.W., Kim J.G., Lee S.J., Chae Y.S., Jeong J.Y., Yoon G.S., Park S.Y., Kim H.J., Park J.S., Choi G.S. Expression of aquaporin-1, aquaporin-3, and aquaporin-5 correlates with nodal metastasis in colon cancer. Oncol. 2015;88:369–376. doi: 10.1159/000369073. [DOI] [PubMed] [Google Scholar]
- 50.Molinas A., Mirazimi A., Holm A., Loitto V.M., Magnusson K.E., Vikström E. Protective role of host aquaporin 6 against Hazara virus, a model for Crimean-Congo hemorrhagic fever virus infection. FEMS Microbiol. Lett. 2016;363 doi: 10.1093/femsle/fnw058. [DOI] [PubMed] [Google Scholar]
- 51.Zahn A., Moehle C., Langmann T., Ehehalt R., Autschbach F., Stremmel W., Schmitz G. Aquaporin-8 expression is reduced in ileum and induced in colon of patients with ulcerative colitis. World J. Gastroenterol. 2007;13:1687–1695. doi: 10.3748/wjg.v13.i11.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Escudero-Hernández C., Münch A., Østvik A.E., Granlund A. van B., Koch S. The water channel aquaporin 8 is a critical regulator of intestinal fluid homeostasis in collagenous colitis. J. Crohns. Colitis. 2020;14:962–973. doi: 10.1093/ecco-jcc/jjaa020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shi Y.H., Tuokan T., Lin C., Chang H. Aquaporin 8 involvement in human cervical cancer siha migration via the EGFR-Erk1/2 pathway. Asian Pac. J. Cancer Prev. APJCP. 2014;15:6391–6395. doi: 10.7314/APJCP.2014.15.15.6391. [DOI] [PubMed] [Google Scholar]
- 54.Chang H., Shi Y.H., Talaf T.K., Lin C. Aquaporin-8 mediates human esophageal cancer Eca-109 cell migration via the EGFR-Erk1/2 pathway. Int. J. Clin. Exp. Pathol. 2014;7:7663–7671. www.ijcep.com/ [PMC free article] [PubMed] [Google Scholar]
- 55.Wang D., Di X., Wang J., Li M., Zhang D., Hou Y., Hu J., Zhang G., Zhang H., Sun M., Meng X., Sun B., Jiang C., Ma T., Su W. Increased formation of follicular antrum in aquaporin-8-deficient mice is due to defective proliferation and migration, and not steroidogenesis of granulosa cells. Front. Physiol. 2018;9 doi: 10.3389/fphys.2018.01193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.S.Y. Yao Jf, Zhou CY, Wei LF, Wang SY, Expression of aquaporin-8 and bcl-2 protein in human cervical carcinoma and their correlations, Zhonghua Fu Chan Ke Za Zhi. 43 (n.d.) 205–8. [PubMed]
- 57.Liu J.Y., Chen X.X., Chen H.Y., Shi J., Leung G.P.H., Tang S.C.W., Lao L.X., Yip H.K.F., Lee K.F., Sze S.C.W., Zhang Z.J., Zhang K.Y. Downregulation of aquaporin 9 exacerbates beta-amyloid-induced neurotoxicity in Alzheimer's disease models in vitro and in vivo. Neuroscience. 2018;394:72–82. doi: 10.1016/j.neuroscience.2018.09.016. [DOI] [PubMed] [Google Scholar]
- 58.Shi Q., Wu Y.Z., Yang X., Xiao K., Maimaitiming A., Gao L.P., Chen C., Gao C., Guo Y., Dong X.P. Significant enhanced expressions of aquaporin-1, -4 and -9 in the brains of various prion diseases. Prion. 2019;13:173–184. doi: 10.1080/19336896.2019.1660487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Avola R., Graziano A.C.E., Pannuzzo G., Albouchi F., Cardile V. New insights on Parkinson's disease from differentiation of SH-SY5Y into dopaminergic neurons: an involvement of aquaporin 4 and 9. Mol. Cell. Neurosci. 2018;88:212–221. doi: 10.1016/j.mcn.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 60.Li C.F., Zhang W.G., Liu M., Qiu L.W., Chen X.F., Lv L., Mei Z.C. Aquaporin 9 inhibits hepatocellular carcinoma through upregulating FOXO1 expression. Oncotarget. 2016;7:44161–44170. doi: 10.18632/oncotarget.10143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.guang Zhang W., fei Li C., Liu M., feng Chen X., Shuai K., Kong X., Lv L., chuan Mei Z. Aquaporin 9 is down-regulated in hepatocellular carcinoma and its over-expression suppresses hepatoma cell invasion through inhibiting epithelial-to-mesenchymal transition. Canc. Lett. 2016;378:111–119. doi: 10.1016/j.canlet.2016.05.021. [DOI] [PubMed] [Google Scholar]
- 62.Soler D.C., Bai X., Ortega L., Pethukova T., Nedorost S.T., Popkin D.L., Cooper K.D., McCormick T.S. The key role of aquaporin 3 and aquaporin 10 in the pathogenesis of pompholyx. Med. Hypotheses. 2015;84:498–503. doi: 10.1016/j.mehy.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Saito T., Tanaka Y., Morishita Y., Ishibashi K. Proteomic analysis of AQP11-null kidney: proximal tubular type polycystic kidney disease. Biochem. Biophys. Reports. 2018;13:17–21. doi: 10.1016/j.bbrep.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rützler M., Rojek A., Damgaard M.V., Andreasen A., Fenton R.A., Nielsen S. Temporal deletion of Aqp11 in mice is linked to the severity of cyst-like disease. Am. J. Physiol. Ren. Physiol. 2017;312:F343–F351. doi: 10.1152/ajprenal.00065.2016. [DOI] [PubMed] [Google Scholar]
- 65.Tanaka Y., Watari M., Saito T., Morishita Y., Ishibashi K. Enhanced autophagy in polycystic kidneys of AQP11 null mice. Int. J. Mol. Sci. 2016;17 doi: 10.3390/ijms17121993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hoshino Y., Sonoda H., Nishimura R., Mori K., Ishibashi K., Ikeda M. Involvement of the NADPH oxidase 2 pathway in renal oxidative stress in Aqp11-/- mice. Biochem. Biophys. Reports. 2019;17:169–176. doi: 10.1016/j.bbrep.2019.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Méndez-Giménez L., Becerril S., Camões S.P., Da Silva I.V., Rodrigues C., Moncada R., Valentí V., Catalán V., Gómez-Ambrosi J., Miranda J.P., Soveral G., Frühbeck G., Rodríguez A. Role of aquaporin-7 in ghrelin-and GLP-1-induced improvement of pancreatic β-cell function after sleeve gastrectomy in obese rats. Int. J. Obes. 2017;41:1394–1402. doi: 10.1038/ijo.2017.135. [DOI] [PubMed] [Google Scholar]
- 68.World Health Organization Obesity and overweight. 2020. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight#:~:text=In 2016%2C more than 1.9 billion adults aged 18 years,women) were obese in 2016
- 69.Lefranc C., Friederich-Persson M., Palacios-Ramirez R., Cat A.N.D. Mitochondrial oxidative stress in obesity: role of the mineralocorticoid receptor. J. Endocrinol. 2018;238:R143–R159. doi: 10.1530/JOE-18-0163. [DOI] [PubMed] [Google Scholar]
- 70.Wang G. Raison d’être of insulin resistance: the adjustable threshold hypothesis. J. R. Soc. Interface. 2014;11 doi: 10.1098/rsif.2014.0892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Khan R.M.M., Chua Z.J.Y., Tan J.C., Yang Y., Liao Z., Zhao Y. From pre-diabetes to diabetes: diagnosis, treatments and translational research. Med. 2019;55 doi: 10.3390/medicina55090546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Arner P., Rydén M. Fatty acids, obesity and insulin resistance. Obes. Facts. 2015;8:147–155. doi: 10.1159/000381224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Robinson C., Tamborlane W.V., Maggs D.G., Enoksson S., Sherwin R.S., Silver D., Shulman G.I., Caprio S. Effect of insulin on glycerol production in obese adolescents. Am. J. Physiol. Endocrinol. Metab. 1998;274 doi: 10.1152/ajpendo.1998.274.4.e737. [DOI] [PubMed] [Google Scholar]
- 74.Blachnio-Zabielska A.U., Chacinska M., Vendelbo M.H., Zabielski P. The crucial role of C18-cer in fat-induced skeletal muscle insulin resistance. Cell. Physiol. Biochem. 2016;40:1207–1220. doi: 10.1159/000453174. [DOI] [PubMed] [Google Scholar]
- 75.Zabielski P., Daniluk J., Hady H.R., Markowski A.R., Imierska M., Górski J., Blachnio-Zabielska A.U. The effect of high-fat diet and inhibition of ceramide production on insulin action in liver. J. Cell. Physiol. 2019;234:1851–1861. doi: 10.1002/jcp.27058. [DOI] [PubMed] [Google Scholar]
- 76.Zabielski P., Hady H.R., Chacinska M., Roszczyc K., Gorski J., Blachnio-Zabielska A.U. The effect of high fat diet and metformin treatment on liver lipids accumulation and their impact on insulin action. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-25397-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Smith G.I., Shankaran M., Yoshino M., Schweitzer G.G., Chondronikola M., Beals J.W., Okunade A.L., Patterson B.W., Nyangau E., Field T., Sirlin C.B., Talukdar S., Hellerstein M.K., Klein S. Insulin resistance drives hepatic de novo lipogenesis in nonalcoholic fatty liver disease. J. Clin. Invest. 2020;130:1453–1460. doi: 10.1172/JCI134165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Najjar S.M. Hepatic Novo Lipogenes. Regul. Metab. Springer International Publishing; 2015. The lipogenic effect of insulin revisited; pp. 285–295. [DOI] [Google Scholar]
- 79.Ighbariya A., Weiss R. Insulin resistance, prediabetes, metabolic syndrome: what should every pediatrician know? J. Clin. Res. Pediatr. Endocrinol. 2017;9:49–57. doi: 10.4274/jcrpe.2017.S005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shi J., Zhang Y., Gu W., Cui B., Xu M., Yan Q., Wang W., Ning G., Hong J. Serum liver fatty acid binding protein levels correlate positively with obesity and insulin resistance in Chinese young adults. PloS One. 2012;7 doi: 10.1371/journal.pone.0048777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fisher R.M., Gertow K. Fatty acid transport proteins and insulin resistance. Curr. Opin. Lipidol. 2005;16:173–178. doi: 10.1097/01.mol.0000162322.39548.b1. [DOI] [PubMed] [Google Scholar]
- 82.Verkman A.S. Aquaporins in clinical medicine. Annu. Rev. Med. 2012;63:303–316. doi: 10.1146/annurev-med-043010-193843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Carbrey J.M., Gorelick-Feldman D.A., Kozono D., Praetorius J., Nielsen S., Agre P. Aquaglyceroporin AqP9: solute permeation and metabolic control of expression in liver. Proc. Natl. Acad. Sci. U.S.A. 2003;100:2945–2950. doi: 10.1073/pnas.0437994100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rojek A.M., Skowronski M.T., Füchtbauer E.M., Füchtbauer A.C., Fenton R.A., Agre P., Frøkiær J., Nielsen S. Defective glycerol metabolism in aquaporin 9 (AQP9) knockout mice. Proc. Natl. Acad. Sci. U.S.A. 2007;104:3609–3614. doi: 10.1073/pnas.0610894104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Maeda N., Hibuse T., Funahashi T. Role of aquaporin-7 and aquaporin-9 in glycerol metabolism; Involvement in obesity. Handb. Exp. Pharmacol. 2009;190:233–249. doi: 10.1007/978-3-540-79885-9_12. [DOI] [PubMed] [Google Scholar]
- 86.Iena F., Lebeck J. Implications of aquaglyceroporin 7 in energy metabolism. Int. J. Mol. Sci. 2018;19:154. doi: 10.3390/ijms19010154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lebeck J. Metabolic impact of the glycerol channels AQP7 and AQP9 in adipose tissue and liver. J. Mol. Endocrinol. 2014;52:R165–R178. doi: 10.1530/JME-13-0268. [DOI] [PubMed] [Google Scholar]
- 88.Kuriyama H., Shimomura I., Kishida K., Kondo H., Furuyama N., Nishizawa H., Maeda N., Matsuda M., Nagaretani H., Kihara S., Nakamura T., Tochino Y., Funahashi T., Matsuzawa Y. Coordinated regulation of fat-specific and liver-specific glycerol channels, aquaporin adipose and aquaporin 9. Diabetes. 2002;51:2915–2921. doi: 10.2337/diabetes.51.10.2915. [DOI] [PubMed] [Google Scholar]
- 89.Rodríguez A., Catalán V., Gómez-Ambrosi J., García-Navarro S., Rotellar F., Valentí V., Silva C., Gil M.J., Salvador J., Burrell M.A., Calamita G., Malagón M.M., Frühbeck G. Insulin- and leptin-mediated control of aquaglyceroporins in human adipocytes and hepatocytes is mediated via the PI3K/Akt/mTOR signaling cascade. J. Clin. Endocrinol. Metab. 2011;96:E586–E597. doi: 10.1210/jc.2010-1408. [DOI] [PubMed] [Google Scholar]
- 90.Rodríguez A., Catalán V., Gómez-Ambrosi J., Frühbeck G. Aquaglyceroporins serve as metabolic gateways in adiposity and insulin resistance control. Cell Cycle. 2011;10:1548–1556. doi: 10.4161/cc.10.10.15672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ozcan U., Cao Q., Yilmaz E., Lee A.-H., Iwakoshi N.N., Ozdelen E., Tuncman G., Gorgun C., Glimcher L.H., Hotamisligil G.S. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2012;1:11–25. doi: 10.1126/science.1103160. (80-. ) [DOI] [PubMed] [Google Scholar]
- 92.Ezquerro S., Becerril S., Tuero C., Méndez-Giménez L., Mocha F., Moncada R., Valentí V., Cienfuegos J.A., Catalán V., Gómez-Ambrosi J., Piper Hanley K., Frühbeck G., Rodríguez A. Role of ghrelin isoforms in the mitigation of hepatic inflammation, mitochondrial dysfunction, and endoplasmic reticulum stress after bariatric surgery in rats. Int. J. Obes. 2020;44:475–487. doi: 10.1038/s41366-019-0420-2. [DOI] [PubMed] [Google Scholar]
- 93.Kawasaki N., Asada R., Saito A., Kanemoto S., Imaizumi K. Obesity-induced endoplasmic reticulum stress causes chronic inflammation in adipose tissue. Sci. Rep. 2012;2:1–7. doi: 10.1038/srep00799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Frühbeck G., Balaguer I., Méndez-Giménez L., Valentí V., Becerril S., Catalán V., Gómez-Ambrosi J., Silva C., Salvador J., Calamita G., Malagón M.M., Rodríguez A. Aquaporin-11 contributes to TGF-β1-induced endoplasmic reticulum stress in human visceral adipocytes: role in obesity-associated inflammation. Cells. 2020;9 doi: 10.3390/cells9061403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hara-Chikuma M., Tanaka M., Verkman A.S., Yasui M. Inhibition of aquaporin-3 in macrophages by a monoclonal antibody as potential therapy for liver injury. Nat. Commun. 2020;11:1–14. doi: 10.1038/s41467-020-19491-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wu X., Chen K., Williams K.J. An oxide transport chain essential for balanced insulin action. Atherosclerosis. 2020;298:42–51. doi: 10.1016/j.atherosclerosis.2020.02.006. [DOI] [PubMed] [Google Scholar]
- 97.Krüger C., Waldeck-Weiermair M., Kaynert J., Pokrant T., Komaragiri Y., Otto O., Michel T., Elsner M. AQP8 is a crucial H2O2 transporter in insulin-producing RINm5F cells. Redox Biol. 2021;43 doi: 10.1016/j.redox.2021.101962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ikaga R., Namekata I., Kotiadis V.N., Ogawa H., Duchen M.R., Tanaka H., Iida-Tanaka N. Knockdown of aquaporin-8 induces mitochondrial dysfunction in 3T3-L1 cells. Biochem. Biophys. Reports. 2015;4:187–195. doi: 10.1016/j.bbrep.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang C.H., Wang C.C., Huang H.C., Wei Y.H. Mitochondrial dysfunction leads to impairment of insulin sensitivity and adiponectin secretion in adipocytes. FEBS J. 2013;280:1039–1050. doi: 10.1111/febs.12096. [DOI] [PubMed] [Google Scholar]
- 100.Takashi Y., Tomita K., Kuwahara Y., Roudkenar M.H., Roushandeh A.M., Igarashi K., Nagasawa T., Nishitani Y., Sato T. Mitochondrial dysfunction promotes aquaporin expression that controls hydrogen peroxide permeability and ferroptosis. Free Radic. Biol. Med. 2020;161:60–70. doi: 10.1016/j.freeradbiomed.2020.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yang B., Zhao D., Verkman A.S. Evidence against functionally significant aquaporin expression in mitochondria. J. Biol. Chem. 2006;281:16202–16206. doi: 10.1074/jbc.M601864200. [DOI] [PubMed] [Google Scholar]
- 102.Lee B.-C., Lee J. Cellular and molecular players in adipose tissue inflammation in the development of obesity-induced insulin resistance. Biochim. Biophys. Acta. 2014;1842:446–462. doi: 10.1016/j.bbadis.2013.05.017.Cellular. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chiadak J.D., Arsenijevic T., Gregoire F., Bolaky N., Delforge V., Perret J., Delporte C. Involvement of JNK/NFκB signaling pathways in the lipopolysaccharide-induced modulation of aquaglyceroporin expression in 3T3-l1 cells differentiated into adipocytes. Int. J. Mol. Sci. 2016;17:1742. doi: 10.3390/ijms17101742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Reshef L., Olswang Y., Cassuto H., Blum B., Croniger C.M., Kalhan S.C., Tilghman S.M., Hanson R.W. Glyceroneogenesis and the triglyceride/fatty acid cycle. J. Biol. Chem. 2003;278:30413–30416. doi: 10.1074/jbc.R300017200. [DOI] [PubMed] [Google Scholar]
- 105.Hibuse T., Maeda N., Funahashi T., Yamamoto K., Nagasawa A., Mizunoya W., Kishida K., Inoue K., Kuriyama H., Nakamura T., Fushiki T., Kihara S., Shimomura I. Aquaporin 7 deficiency is associated with development of obesity through activation of adipose glycerol kinase. Proc. Natl. Acad. Sci. U.S.A. 2005 doi: 10.1073/pnas.0503291102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kishida K., Shimomura I., Kondo H., Kuriyama H., Makino Y., Nishizawa H., Maeda N., Matsuda M., Ouchi N., Kihara S., Kurachi Y., Funahashi T., Matsuzawa Y. Genomic structure and insulin-mediated repression of the aquaporin adipose (AQPap), adipose-specific glycerol channel. J. Biol. Chem. 2001 doi: 10.1074/jbc.M106040200. [DOI] [PubMed] [Google Scholar]
- 107.Kishida K., Shimomura I., Nishizawa H., Maeda N., Kuriyama H., Kondo H., Matsuda M., Nagaretani H., Ouchi N., Hotta K., Kihara S., Kadowaki T., Funahashi T., Matsuzawa Y. Enhancement of the aquaporin adipose gene expression by a peroxisome proliferator-activated receptor γ. J. Biol. Chem. 2001 doi: 10.1074/jbc.M108213200. [DOI] [PubMed] [Google Scholar]
- 108.Fasshauer M., Klein J., Lossner U., Klier M., Kralisch S., Paschke R. Suppression of aquaporin adipose gene expression by isoproterenol, TNFα, and dexamethasone. Horm. Metab. Res. 2003;35:222–227. doi: 10.1055/s-2003-39478. [DOI] [PubMed] [Google Scholar]
- 109.Kahn S.E., Hull R.L., Utzschneider K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006 doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 110.Guilherme A., Virbasius J.V., Puri V., Czech M.P. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat. Rev. Mol. Cell Biol. 2008 doi: 10.1038/nrm2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Weyer C., Foley J.E., Bogardus C., Tataranni P.A., Pratley R.E. Diabetologia; 2000. Enlarged Subcutaneous Abdominal Adipocyte Size, but Not Obesity Itself, Predicts Type II Diabetes Independent of Insulin Resistance. [DOI] [PubMed] [Google Scholar]
- 112.Kerr A.G., Sinha I., Dadvar S., Arner P., Dahlman I. Epigenetic regulation of diabetogenic adipose morphology. Mol. Metab. 2019;25:159–167. doi: 10.1016/j.molmet.2019.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kulyte A., Ehrlund A., Arner P., Dahlman I. Global transcriptome profiling identifies KLF15 and SLC25A10 as modifiers of adipocytes insulin sensitivity in obese women. PloS One. 2017;12 doi: 10.1371/journal.pone.0178485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mourelatou R., Kostopoulou E., Rojas-Gil A.P., Kehagias I., Linos D., Kalfarentzos F.E., Spiliotis B.E. Decreased adipocyte glucose transporter 4 (GLUT4) and aquaglyceroporin-7 (AQP7) in adults with morbid obesity: possible early markers of metabolic dysfunction. Hormones (Basel) 2019;18:297–306. doi: 10.1007/s42000-019-00130-8. [DOI] [PubMed] [Google Scholar]
- 115.Oikonomou E., Kostopoulou E., Rojas-Gil A.P., Georgiou G., Spiliotis B.E. Adipocyte aquaporin 7 (AQP7) expression in lean children and children with obesity. Possible involvement in molecular mechanisms of childhood obesity. J. Pediatr. Endocrinol. Metab. 2018;31:1081–1089. doi: 10.1515/jpem-2018-0281. [DOI] [PubMed] [Google Scholar]
- 116.Mehanna E.T., Barakat B.M., ElSayed M.H., Tawfik M.K. An optimized dose of raspberry ketones controls hyperlipidemia and insulin resistance in male obese rats: effect on adipose tissue expression of adipocytokines and Aquaporin 7. Eur. J. Pharmacol. 2018;832:81–89. doi: 10.1016/j.ejphar.2018.05.028. [DOI] [PubMed] [Google Scholar]
- 117.de Luis D.A., Almansa R., Aller R., Izaola O., Romero E. Gene expression analysis identify a metabolic and cell function alterations as a hallmark of obesity without metabolic syndrome in peripheral blood, a pilot study. Clin. Nutr. 2018;37:1348–1353. doi: 10.1016/j.clnu.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 118.da Silva I.V., Cardoso C., Méndez-Giménez L., Camoes S.P., Frühbeck G., Rodríguez A., Miranda J.P., Soveral G. Aquaporin-7 and aquaporin-12 modulate the inflammatory phenotype of endocrine pancreatic beta-cells. Arch. Biochem. Biophys. 2020;691 doi: 10.1016/j.abb.2020.108481. [DOI] [PubMed] [Google Scholar]
- 119.Rodríguez A., Gena P., Méndez-Giménez L., Rosito A., Valentí V., Rotellar F., Sola I., Moncada R., Silva C., Svelto M., Salvador J., Calamita G., Frühbeck G. Reduced hepatic aquaporin-9 and glycerol permeability are related to insulin resistance in non-alcoholic fatty liver disease. Int. J. Obes. 2014;38:1213–1220. doi: 10.1038/ijo.2013.234. [DOI] [PubMed] [Google Scholar]
- 120.Rodríguez A., Marinelli R.A., Tesse A., Frühbeck G., Calamita G. Sexual dimorphism of adipose and hepatic aquaglyceroporins in health and metabolic disorders. Front. Endocrinol. 2015;6 doi: 10.3389/fendo.2015.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rodríguez A., Catalán V., Gómez-Ambrosi J., Frühbeck G. Aquaglyceroporins serve as metabolic gateways in adiposity and insulin resistance control. Cell Cycle. 2011;10:1548–1556. doi: 10.4161/cc.10.10.15672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Iena F.M., Jul J.B., Vegger J.B., Lodberg A., Thomsen J.S., Brüel A., Lebeck J. Sex-specific effect of high-fat diet on glycerol metabolism in murine adipose tissue and liver. Front. Endocrinol. 2020;11 doi: 10.3389/fendo.2020.577650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Baldini F., Portincasa P., Grasselli E., Damonte G., Salis A., Bonomo M., Florio M., Serale N., Voci A., Gena P., Vergani L., Calamita G. Aquaporin-9 is involved in the lipid-lowering activity of the nutraceutical silybin on hepatocytes through modulation of autophagy and lipid droplets composition. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2020;1865 doi: 10.1016/j.bbalip.2019.158586. [DOI] [PubMed] [Google Scholar]
- 124.Gena P., Mastrodonato M., Portincasa P., Fanelli E., Mentino D., Rodríguez A., Marinelli R.A., Brenner C., Frühbeck G., Svelto M., Calamita G. Liver glycerol permeability and aquaporin-9 are dysregulated in a murine model of non-alcoholic fatty liver disease. PloS One. 2013;8 doi: 10.1371/journal.pone.0078139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kuriyama H., Shimomura I., Kishida K., Kondo H., Furuyama N., Nishizawa H., Maeda N., Matsuda M., Nagaretani H., Kihara S., Nakamura T., Tochino Y., Funahashi T., Matsuzawa Y. Liver-specific glycerol channels, aquaporin adipose. Diabetes. 2002;51:2915–2921. doi: 10.2337/diabetes.51.10.2915. [DOI] [PubMed] [Google Scholar]
- 126.Catalán V., Gómez-Ambrosi J., Pastor C., Rotellar F., Silva C., Rodríguez A., Gil M.J., Cienfuegos J.A., Salvador J., Vendrell J., Frühbeck G. Influence of morbid obesity and insulin resistance on gene expression levels of AQP7 in visceral adipose tissue and AQP9 in liver. Obes. Surg. 2008;18:695–701. doi: 10.1007/s11695-008-9453-7. [DOI] [PubMed] [Google Scholar]
- 127.Portois L., Zhang Y., Ladrière L., Perret J., Louchami K., Gaspard N., Hupkens E., Bolaky N., Delforge V., Beauwens R., Malaisse W.J., Sener A., Carpentier Y.A., Delporte C. Perturbation of glycerol metabolism in hepatocytes from n3-PUFA-depleted rats. Int. J. Mol. Med. 2012;29:1121–1126. doi: 10.3892/ijmm.2012.943. [DOI] [PubMed] [Google Scholar]
- 128.Miranda M., Ceperuelo-Mallafré V., Lecube A., Hernandez C., Chacon M.R., Fort J.M., Gallart L., Baena-Fustegueras J.A., Simó R., Vendrell J. Gene expression of paired abdominal adipose AQP7 and liver AQP9 in patients with morbid obesity. Relationship with glucose abnormalities. Metabolism. 2009;58:1762–1768. doi: 10.1016/j.metabol.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 129.Méndez-Giménez L., Becerril S., Moncada R., Valentí V., Ramírez B., Lancha A., Gurbindo J., Balaguer I., Cienfuegos J.A., Catalán V., Fernández S., Gómez-Ambrosi J., Rodríguez A., Frühbeck G. Sleeve gastrectomy reduces hepatic steatosis by improving the coordinated regulation of aquaglyceroporins in adipose tissue and liver in obese rats. Obes. Surg. 2015;25:1723–1734. doi: 10.1007/s11695-015-1612-z. [DOI] [PubMed] [Google Scholar]
- 130.Wang Y., Chen G., Tu Q., Wu J., Qin Y., Zhu Z., Shen Y., Yan L., Han A., Xiang Q., Wang C. Associations between aquaglyceroporin gene polymorphisms and risk of type 2 diabetes mellitus. BioMed Res. Int. 2018 doi: 10.1155/2018/8167538. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hatem-Vaquero M., Griera M., Garcia-Ayuso D., Campillo S., Bohorquez L., Calleros L., Rodriguez-Puyol D., Rodriguez-Puyol M., de Frutos S. Integrin linked kinase (ILK) downregulation as an early event during the development of metabolic alterations in a short-term high fat diet mice model. Cell. Physiol. Biochem. 2020;54:71–87. doi: 10.33594/000000206. [DOI] [PubMed] [Google Scholar]
- 132.Hou J., Li Y., Ren X., Gao F., Chen Y., Yi Y., Kang L., Luo Y., Yang M. Similar Aquaporin 9 and MAPK expression profiles in the liver of types 1 and 2 diabetes mellitus. Horm. Metab. Res. 2018;50:414–421. doi: 10.1055/s-0043-123470. [DOI] [PubMed] [Google Scholar]
- 133.Fan Z., Yuan Y., Wang F., Qi Y., Han H., Wu J., Zhang G., Yang L. Diabetes mitigates the recovery following intracranial hemorrhage in rats. Behav. Brain Res. 2017;320:412–419. doi: 10.1016/j.bbr.2016.10.047. [DOI] [PubMed] [Google Scholar]
- 134.Wei H., Hu Q., Wu J., Yao C., Xu L., Xing F., Zhao X., Yu S., Wang X., Chen G. Molecular mechanism of the increased tissue uptake of trivalent inorganic arsenic in mice with type 1 diabetes mellitus. Biochem. Biophys. Res. Commun. 2018;504:393–399. doi: 10.1016/j.bbrc.2018.06.029. [DOI] [PubMed] [Google Scholar]
- 135.Watanabe S., Moniaga C.S., Nielsen S., Hara-Chikuma M. Aquaporin-9 facilitates membrane transport of hydrogen peroxide in mammalian cells. Biochem. Biophys. Res. Commun. 2016;471:191–197. doi: 10.1016/j.bbrc.2016.01.153. [DOI] [PubMed] [Google Scholar]
- 136.Gao C., Zhang W. Urinary AQP5 is independently associated with eGFR decline in patients with type 2 diabetes and nephropathy. Diabetes Res. Clin. Pract. 2019;155 doi: 10.1016/j.diabres.2019.107805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lu Y., Chen L., Zhao B., Xiao Z., Meng T., Zhou Q., Zhang W. Urine AQP5 is a potential novel biomarker of diabetic nephropathy. J. Diabet. Complicat. 2016;30:819–825. doi: 10.1016/j.jdiacomp.2016.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chen S., Wang Y., Zhang C., Yang Z. Decreased basal and stimulated salivary parameters by histopathological lesions and secretory dysfunction of parotid and submandibular glands in rats with type 2 diabetes. Exp. Ther. Med. 2020;19 doi: 10.3892/etm.2020.8505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Denewar M., Amin L.E. Role of bone marrow-derived mesenchymal stem cells on the parotid glands of streptozotocin induced diabetes rats. J. Oral Biol. Craniofacial Res. 2020;10:33–37. doi: 10.1016/j.jobcr.2020.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Choma D.P., Vanacore R., Naylor H., Zimmerman I.A., Pavlichenko A., Pavlichenko A., Foye L., Carbone D.P., Harris R.C., Dikov M.M., Tchekneva E.E. Aquaporin 11 variant associates with kidney disease in type 2 diabetic patients. Am. J. Physiol. Ren. Physiol. 2016;310:F416–F425. doi: 10.1152/ajprenal.00295.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Han B., Wu X., Huang P.P., Zhu F.X., Liu S. Aquaporin 11 rs2276415 variant and progression of chronic kidney disease. Nephrol. Dial. Transplant. 2019;34:970–973. doi: 10.1093/ndt/gfy219. [DOI] [PubMed] [Google Scholar]