Abstract
A 2-year, 3-month-old Holstein cow presented with anorexia and enlarged superficial lymph nodes. Fine needle aspiration cytology of the superficial lymph nodes revealed large blast cells. Hematological examination revealed anemia, neutropenia, and blast cells in peripheral blood. Blast cells were the predominant cell type in bone marrow aspirates. Of the non-erythroid cells, 26%, 58%, and 18% were positive for myeloperoxidase, α-naphthyl acetate esterase, and naphthol AS-D chloroacetate esterase, respectively. Pathological examination revealed the proliferation of neoplastic cells, which were positive for monocytic markers, in the affected lymph nodes. The cow was diagnosed with acute myelomonocytic leukemia based on these findings. This report highlights the importance of performing bone marrow aspiration cytology and cytochemical staining when diagnosing bovine myeloid leukemia.
Keywords: acute myelomonocytic leukemia, bone marrow aspiration, cow, cytochemical staining, diagnosis
Lymphoid neoplasms are the most common type of bovine hematological malignancy, and non-lymphocytic leukemia is rare. Acute myeloid leukemia (AML) is characterized by maturation arrest and neoplastic proliferation of myeloid cells in peripheral blood and bone marrow [2]. Several types of AML have been reported in cattle, including acute myelomonocytic leukemia (AMML) [15], acute basophilic leukemia [9, 12], and acute myeloblastic leukemia [13]. In the present report, we describe a clinical case of AMML in a Holstein cow.
A 2-year, 3-month-old Holstein cow was examined by a local veterinarian due to anorexia after a stillbirth. Other cattle in the same facility showed no symptoms similar to the present case. At initial examination, the cow had a body temperature of 40.7°C (reference interval (RI): 38.0–39.17°C) and a heart rate of 90 beats/min (bpm; RI: 60–84 bpm) [5]. Postnatal vaginal discharge and retained placenta were noted. Hematological examination revealed anemia (red blood cell (RBC) count, 453 × 104/µl; RI: 510–760 × 104/µl; hemoglobin, 7.1 g/dl; RI: 8.5–12.2 g/dl; hematocrit, 19.3%; RI: 22–33%), leukopenia (4,500/µl; RI: 4,900–12,000/µl), neutropenia (1,260/µl; RI: 1,800–6,300/µl), and thrombocytopenia (13 × 104/µl; RI: 19.3–63.7 × 104/µl) [5]. The cow was tentatively diagnosed with endometritis and treated with 20 mg/kg ampicillin (Kyoritsu Seiyaku Inc., Tokyo, Japan) and 0.2 mg/kg dexamethasone (Kyoritsu Seiyaku Inc.). Although postnatal vaginal discharge and retained placenta improved, anorexia and hypodynamia persisted, suggesting the presence of other diseases in addition to endometritis. On day 6, the cow was transferred to the Animal Teaching Hospital at the Obihiro University of Agriculture and Veterinary Medicine to confirm the diagnosis. On initial physical examination at the hospital, high rectal temperature (39.5°C; RI: 38.0–39.17°C), tachycardia (96 bpm; RI: 60–84 bpm), polypnea (42 breaths/min; RI: 18–28 breaths/min) [5], and pallor of the mucosal membranes were noted. Mandibular, superficial cervical, and subiliac lymph nodes were enlarged (Fig. 1). Rectal palpation revealed several masses (2–6 cm) in the pelvic cavity. Findings of fine needle aspiration (FNA) cytology of the superficial cervical lymph node included large and round undifferentiated blast cells with a small amount of cytoplasm undergoing mitosis (Supplementary Fig. 1). Based on these findings, the cow was tentatively diagnosed with lymphoma.
Fig. 1.

Left subiliac lymph nodes (arrowheads) were enlarged.
Hematological examination indicated anemia (RBC count, 392 × 104/µl; hemoglobin, 6.2 g/dl; hematocrit, 16.4%) [5]. White blood cell count (5,200/µl) and platelet count (31.8 × 104/µl) were within the normal range [5]. Smear examination of peripheral blood showed that 73% (3,796/µl) of white blood cells were medium or large immature mononuclear cells with round or horse-shoe-shaped nuclei and basophilic cytoplasm (Fig. 2). In addition, lymphopenia (16%; 832/µl; RI: 1,600–5,600/µl) and neutropenia (7%; 364/µl) were noted [5].
Fig. 2.

Medium to large immature mononuclear cells with round (arrow) or horse-shoe-shaped (arrowhead) nuclei in a peripheral blood smear. Wright-Giemsa stain. Bar=10 µm.
Serum biochemical analysis revealed increased total lactate dehydrogenase (LDH; 5,860 U/l; RI: 697–1,450 U/l) and thymidine kinase (49.1 U/l; RI: <5.4 U/l) activities [7, 11]. LDH isozyme analysis showed elevated activities of LDH-2 (2,552 U/l; RI: 137–503 U/l) and LDH-3 (1,055 U/l; RI: 82–262 U/l) [7]. Antibodies against bovine leukemia virus (BLV) were not detected with the BLV Antibody Test ELISA kit (JNC, Tokyo, Japan). Nested polymerase chain reaction (PCR) for the BLV 5′ LTR was performed using genomic DNA extracted from peripheral blood samples and swollen superficial cervical lymph node tissue obtained by FNA [10]; the results were negative. In DNA samples which were used for nested PCR, monoclonal proliferation of B-cells was not detected by the B-cell clonality test [10].
Bone marrow aspiration was performed to further investigate the hematological abnormalities. Bone marrow smears were stained with Wright-Giemsa stain, and more than 500 cells were counted. The marrow was hyperplastic, and percentages of myeloid precursors (10.6%) and erythroid precursors (14.4%) were much lower than those in five control cattle without hematological abnormalities (Table 1). The majority of blast cells had a round to oval nucleus with distinct nucleoli, basophilic cytoplasm without azurophilic granules, and a high nuclear/cytoplasmic ratio (Fig. 3). The percentage of blast cells constituted up to 75.0% of all nucleated cells (ANCs) and 87.6% of all non-erythroid cells (NECs). To further examine these cells, staining for α-naphthyl acetate esterase (ANAE) (ANAE staining kit; Muto Pure Chemicals), myeloperoxidase (MPO) (3,3′-diaminobenzidine (DAB) staining kit; Muto Pure Chemicals, Tokyo, Japan), and naphthol AS-D chloroacetate esterase (NASDCA) (Kishimoto Clinical Laboratory, Hokkaido, Japan) was performed. Of the NECs, 58%, 26%, and 18% were positive for ANAE, MPO, and NASDCA, respectively (Fig. 3). Bone marrow aspiration cytology and cytochemical staining revealed proliferation of blast cells in bone marrow, and proliferation of neoplastic cells in bone marrow showing monocytic and myelocytic differentiation.
Table 1. Myelogram of the present case.
| Results (%) | Reference* (%) |
|||
|---|---|---|---|---|
| Range | Median | |||
| Myeloid cells | ||||
| Myeloblast | 0.0 | 0.2–5.6 | 3.2 | |
| Promyelocyte | 0.0 | 1.6–5.0 | 2.8 | |
| Myelocyte | 0.2 | 4.0–20.8 | 6.4 | |
| Metamyelocyte | 0.8 | 6.0–19.6 | 10.0 | |
| Band neutrophil | 2.2 | 10.2–24.0 | 16.8 | |
| Segment neutrophil | 6.6 | 1.4–19.0 | 11.6 | |
| Eosinophil | 0.4 | 5.8–10.6 | 7.2 | |
| Basophil | 0.4 | 0.0–0.4 | 0.0 | |
| Monocyte | 0.0 | 0.0–0.8 | 0.4 | |
| Erythroid cells | ||||
| Rubriblast | 3.0 | 3.4–6.4 | 4.2 | |
| Prorubricyte | 0.0 | 0.0–2.6 | 0.2 | |
| Rubricyte | 5.0 | 12.6–30.8 | 17.4 | |
| Metarubricyte | 6.4 | 8.8–14.6 | 13.8 | |
| Others | ||||
| Lymphocyte | 0.0 | 0.0–1.6 | 0.4 | |
| Blast cell | 75.0 | N/A | N/A | |
| M/E ratio | 5.94 | 0.97–2.67 | 1.76 | |
*: Range and median of myelogram in 5 control cattle. N/A: not applicable.
Fig. 3.
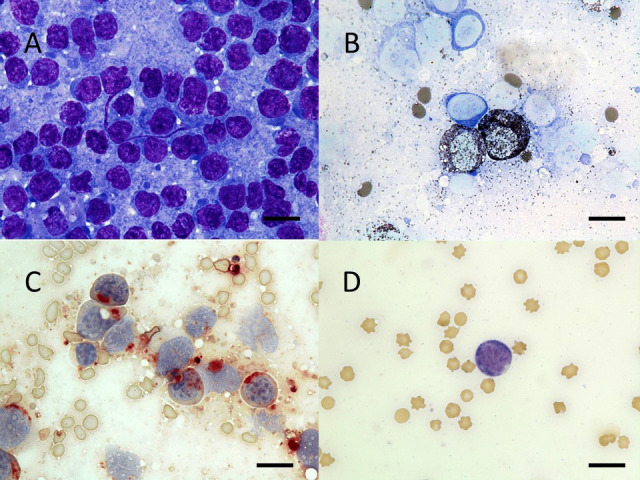
Morphology and cytochemical staining of blast cells in bone marrow. Wright-Giemsa staining revealed that the majority of blast cells in smears had a round to oval nucleus with distinct nucleoli, a basophilic cytoplasm, and a high nuclear/cytoplasmic ratio (A). Of the non-erythroid cells, 26%, 58%, and 18% were positive for myeloperoxidase (B), α-naphthyl acetate esterase (C), and naphthol AS-D chloroacetate esterase (D), respectively. Bar=10 µm.
The cow was euthanized under anesthesia on day 14 for autopsy according to ethical and animal welfare requirements under the guidelines of the Care and Use of Agriculture Animals of Obihiro University (Approval 18-32). At the gross level, swelling of multiple lymph nodes (e.g., parotid, mandibular, superficial cervical, subiliac, mammary, and medial iliac lymph nodes), the spleen, and the liver were noted. The cut surface of affected lymph nodes was firm, homogenous, and white to gray. Similar white foci, 0.5–3 cm in diameter, were disseminated in the heart, liver, kidneys, adrenal glands, and uterus (Supplementary Fig. 2). The sternal bone marrow was pale red.
Histopathologically, neoplastic cells diffusely proliferated, and extensive necrosis was observed in the affected lymph nodes. The normal structure of the lymph nodes was lost. Neoplastic cells were about 1–3 times the size of surrounding red blood cells. These cells had atypical round nuclei with a granular pattern of chromatin, as well as scant cytoplasm (Fig. 4). Anisonucleosis was present and mitoses were common (72 mitotic cells per 10 high-power fields). Neoplastic cells had infiltrated the liver, spleen, heart, adrenal gland, bone marrow, and uterus. Immunohistochemical analysis revealed that neoplastic cells were negative for CD3 (Biogenex Laboratories, Fremont, CA, USA) and CD20 (Biogenex Laboratories), and positive for HLA-DR (Dako Cytomation, Carpinteria, CA, USA) and Iba-1 (Fujifilm, Osaka, Japan) (Fig. 5). CD3 and CD20 negativity indicated that the neoplastic cells were neither T-cells nor B-cells, and HLA-DR and Iba-1 positivity confirmed that the neoplastic cells were of monocytic lineage.
Fig. 4.

Histopathology of white foci in kidneys. Neoplastic cells had a round nucleus with nuclear atypia and granular-patterned chromatin, as well as scant cytoplasm. Hematoxylin and eosin stain. Bar=20 µm.
Fig. 5.
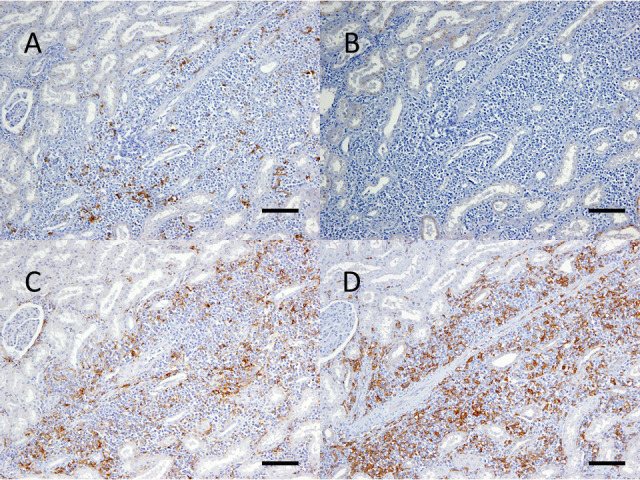
Immunohistochemistry of white foci in kidneys. Neoplastic cells were negative for CD3 (A) and CD20 (B), and positive for HLA-DR (C) and Iba-1 (D). Mayer’s hematoxylin counterstain. Bar=100 µm.
In humans, acute leukemia is classified based on combined morphologic and cytochemical staining patterns of tumor cells (French-American-British (FAB) classification) [2], and the FAB classification has been adapted to the classification of acute leukemia in cats and dogs [8]. According to the FAB classification, acute leukemia is divided into two groups, AML and acute lymphoblastic leukemia (ALL), with AML having eight subgroups (M0-M7) [2]. In the present case, leukemia/lymphoma was suspected based on physical examination. Persistent anemia and neutropenia suggested abnormalities in the bone marrow. In bone marrow aspiration cytology, the percentage of blast cells in ANCs was 75%, and 26% of NECs were positive for MPO. These findings satisfied the diagnostic criteria of AML according to the FAB classification [2]. The M4 type of AML in the FAB classification is diagnosed when the percentages of monocytic lineage cells and granulocytic lineage cells in NECs are ≥20%. In the present case, 58% and 18% of NECs were positive for ANAE and NASDCA, respectively. In the pathological analysis, although a portion of cells in tumor tissues were positive for CD3, almost all cells were negative for lymphocytic markers and positive for monocytic markers, suggesting that they were monocytic cells. Although the percentage of granulocytic lineage cells in NECs was slightly lower than required by the diagnostic criteria, the cow was diagnosed with M4 type AMML based on a combination of the percentage of monocytic lineage cells in NECs and pathological analysis. To the best of our knowledge, this is the second report of bovine M4 type AMML diagnosed using the FAB classification.
Almost all hematopoietic neoplasms reported in cattle are lymphoid malignancies. Enlargement of superficial lymph nodes and pelvic masses are important clinical findings in bovine lymphoma [1], and cytology of enlarged lymph nodes by FNA is used to diagnose the disease [14]. The present case was initially diagnosed as lymphoma based on physical examination and FNA cytology of enlarged lymph nodes. However, cytochemical staining of bone marrow cells suggested that the tumor cells were not lymphocytes, which was somewhat unexpected. In humans, discrimination between AML and ALL is difficult without cytochemical staining [2]. AML is also a possibility in cattle clinically diagnosed with lymphoma. Treatments and diagnostic procedures differ for non-BLV associated leukemia/lymphoma and BLV associated lymphoma, which is an infectious disease. Therefore, bone marrow aspiration cytology and cytochemical staining should be performed in cattle with suspected non-BLV associated leukemia/lymphoma in order to accurately diagnose bovine leukemia/lymphoma.
Proliferation of tumor cells is an important clinical finding in AML [2]. Although tumor cells were observed in peripheral blood in the present case, blood cell counts were within normal ranges. This suggests that neoplastic proliferation of tumor cells in peripheral blood does not necessarily cause hyperleukocytosis, and that smear examination of peripheral blood should be performed, even if the white blood cell count is normal.
Azure granules stain positively for MPO and ANAE. In the present case, some tumor cells were positive for both markers, and tumor cells with clear granules were not observed in Wright-Giemsa-stained smears and paraffin sections. The reason for this is unclear, although it is possible that the granules may have been obscured due to formalin fixation of paraffin embedded tissue.
BLV infects B-cells, T-cells, and monocytes, and causes tumorigenesis of B-cells in cattle [16]. Similar to BLV, feline leukemia virus (FeLV) belongs to the family Retroviridae, and FeLV infection is associated with myeloid neoplasia in cats [3, 6]. The present case was negative for BLV infection, and similar symptoms were not observed among other cattle in the same facility. Therefore, it is unlikely that AMML was caused by an infectious pathogen. However, exposure to potential carcinogens was unknown, and the cause of AMML in the cow was not identified.
In a previous study, a 2-month-old calf was diagnosed with AMML [15]. Similar to this previous case, anemia and neutropenia were observed in the present case. However, the present case differed from the previous case with respect to the presence of enlarged lymph nodes and lack of increased white blood cell count [15]. In humans with AML, enlarged lymph nodes, anemia, thrombocytopenia, lymphopenia, neutropenia, and appearance of blast cells in peripheral blood are commonly observed [4]. Additional studies will be needed to further clarify the characteristics of AML, including AMML, in cattle.
In the present case, we diagnosed a Holstein cow with AMML. This report provides evidence that AMML can occur in Holstein cows, as well as additional information regarding the clinicopathological aspects of AMML. Our findings also highlight the importance of performing bone marrow aspiration cytology and cytochemical staining when diagnosing bovine leukemia.
POTENTIAL CONFLICTS OF INTEREST
The authors declare no conflict of interest.
Supplementary
Acknowledgments
We thank all staff of the Department of Veterinary Medicine in Obihiro University for their technical assistance, and Tokachi Agricultural Mutual Aid Association for introducing this clinical case. This work was supported by JSPS KAKENHI Grant Numbers 20H03142 and 20J10567.
REFERENCES
- 1.Angelos J. A., Thurmond M. C.2015. Bovine lymphoma. pp. 1070–1073. In: Large Animal Internal Medicine, 5th ed. (Smith, B. P. ed.), Elsevier, St. Louis. [Google Scholar]
- 2.Bennett J. M., Catovsky D., Daniel M. T., Flandrin G., Galton D. A., Gralnick H. R., Sultan C.1976. Proposals for the classification of the acute leukaemias. French-American-British (FAB) co-operative group. Br. J. Haematol. 33: 451–458. doi: 10.1111/j.1365-2141.1976.tb03563.x [DOI] [PubMed] [Google Scholar]
- 3.Cristo T. G., Biezus G., Noronha L. F., Gaspar T., Dal Pont T. P., Withoeft J. A., Furlan L. V., Costa L. S., Traverso S. D., Casagrande R. A.2019. Feline leukaemia virus associated with leukaemia in cats in Santa Catarina, Brazil. J. Comp. Pathol. 170: 10–21. doi: 10.1016/j.jcpa.2019.05.002 [DOI] [PubMed] [Google Scholar]
- 4.Deschler B., Lübbert M.2006. Acute myeloid leukemia: epidemiology and etiology. Cancer 107: 2099–2107. doi: 10.1002/cncr.22233 [DOI] [PubMed] [Google Scholar]
- 5.Divers T. J., Peel A. F.2018. The clinical examination. pp. 2–16. In: Diseases of Dairy Cattle, 3rd ed. (Peel, S. F. and Divers, T. J. eds.), Elsevier, St. Louis. [Google Scholar]
- 6.Hartmann K.2012. Clinical aspects of feline retroviruses: a review. Viruses 4: 2684–2710. doi: 10.3390/v4112684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishihara K., Ohtani T., Kitagawa H., Onuma M.1980. Clinical studies on bovine leukemia in Japanese black cattle. III. Serum lactate dehydrogenase activity and its isoenzyme pattern in groups of leukemic cattle and those negative or positive for antibody against bovine leukemia virus. Nippon Juigaku Zasshi 42: 289–295. doi: 10.1292/jvms1939.42.289 [DOI] [PubMed] [Google Scholar]
- 8.Jain N. C., Blue J. T., Grindem C. B., Harvey J. W., Kociba G. J., Krehbiel J. D., Latimer K. S., Raskin R. E., Thrall M. A., Zinkl J. G.1991. Proposed criteria for classification of acute myeloid leukemia in dogs and cats. Vet. Clin. Pathol. 20: 63–82. doi: 10.1111/j.1939-165X.1991.tb00571.x [DOI] [PubMed] [Google Scholar]
- 9.Laabs E. M., Mischke R., Dziallas P., Maiolini A., Tipold A., Raddatz B., Puff C., Rehage J.2015. Acute basophilic leukaemia in a three-month-old calf. Acta Vet. Scand. 57: 48. doi: 10.1186/s13028-015-0141-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maezawa M., Watanabe K., Horiuchi N., Matsumoto K., Kobayashi Y., Inokuma H.2018. A clinical case of enzootic bovine leukosis in a 13-month-old Holstein heifer. Jpn. J. Vet. Res. 66: 209–213. [Google Scholar]
- 11.Sakamoto L., Ohbayashi T., Matsumoto K., Kobayashi Y., Inokuma H.2009. Serum thymidine kinase activity as a useful marker for bovine leukosis. J. Vet. Diagn. Invest. 21: 871–874. doi: 10.1177/104063870902100619 [DOI] [PubMed] [Google Scholar]
- 12.Takahashi Y., Yoneyama S., Yamamoto S., Shibahara T., Kadota K.2006. Acute basophilic leukaemia in a calf. Vet. Rec. 158: 702–703. doi: 10.1136/vr.158.20.702 [DOI] [PubMed] [Google Scholar]
- 13.Takayama H., Gejima S., Honma A., Ishikawa Y., Kadota K.1996. Acute myeloblastic leukaemia in a cow. J. Comp. Pathol. 115: 95–101. doi: 10.1016/S0021-9975(96)80032-6 [DOI] [PubMed] [Google Scholar]
- 14.Washburn K. E., Streeter R. N., Lehenbauer T. W., Snider T. A., Rezabek G. B., Ritchey J. W., Meinkoth J. H., Allison R. W., Rizzi T. E., Boileau M. J.2007. Comparison of core needle biopsy and fine-needle aspiration of enlarged peripheral lymph nodes for antemortem diagnosis of enzootic bovine lymphosarcoma in cattle. J. Am. Vet. Med. Assoc. 230: 228–232. doi: 10.2460/javma.230.2.228 [DOI] [PubMed] [Google Scholar]
- 15.Woods P. R., Gossett R. E., Jain N. C., Smith R., 3rd., Rappaport E. S., Kasari T. R.1993. Acute myelomonocytic leukemia in a calf. J. Am. Vet. Med. Assoc. 203: 1579–1582. [PubMed] [Google Scholar]
- 16.Wu D., Murakami K., Morooka A., Jin H., Inoshima Y., Sentsui H.2003. In vivo transcription of bovine leukemia virus and bovine immunodeficiency-like virus. Virus Res. 97: 81–87. doi: 10.1016/S0168-1702(03)00222-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


