Abstract
The aims of studies were to estimate the withdrawal period of antibiotic from milk after the intramammary infusion of cefazolin sodium (CEZ) in cows with difficulties in frequent milk discharge due to disease such as teat injury. The period was compared among cows milked twice a day after 150 or 450 mg of CEZ were administered to all quarters (Study 1, 2) and the cows in which milking of front-right quarter was ceased for five days after administration of these infusions to only that quarter (Study 3). In Studies 1 and 2, the median of 17.66 µg/ml and 83.18 µg/ml of CEZ were detected in the samples of first milking after intramammary administration, respectively; however, there was no residual antibiotic by 72 hr in all cows. In Study 3, the median of 1.96 µg/ml of CEZ was detected in the sample after the resumption of milking at 120 hr, and the residual was eliminated by 174 hr. The withdrawal period may be prolonged by the cessation of milking after administration, and the period is the total time from cessation to 72 hr after the resumption of milking.
Keywords: cefazolin sodium, intramammary administration, residue, temporary cessation of milking, withdrawal period
Intramammary infusion of antibiotics is performed for bovine mastitis and teat injury to treat or prevent bacterial infection [5, 8, 13, 20]. As the infusion remains in the body, the withdrawal period in milk varies among drugs [5, 20]. The residual test is generally performed on cows which are milked twice a day [1, 17]. Immediately after milking, the drug is administered to quarter, serum and milk are taken over time, and then antibiotic levels are measured [1, 17]. The milk contaminated by pathogens and toxins is actively discharged from the affected quarter after treatment of bovine mastitis, which also results in the elimination of antibiotics from the quarter within the determined residual period [1, 17]. On the other hand, it is important in the treatment of teat injury to allow the affected teat to heal; therefore, continued milking of the affected quarter has adverse effects. [3, 6, 12]. Intramammary infusions should be administered to the affected quarter in cows with bacterial infected teat injury, but it is not assumed that milking may not be carried out after administration in the residual test [1, 17]. Thus, the withdrawal period of antibiotics during the treatment of teat injury is considered to differ from that in mastitis. The purpose of this study was to estimate the withdrawal period of cefazolin sodium (CEZ) after intramammary infusion to cows with difficulties in milking due to teat injury.
MATERIALS AND METHODS
All procedures of Studies 1, 2 and 3 were performed at the Central Research Institute of Nippon Zenyaku Kogyo Co., Ltd., Fukushima, Japan in accordance with the Good for the Care and Use of Laboratory Animals and with the standard of Clinical Practice of this Institute (NZ85/02-10C-1, NZ85-10A-1, NZ97/15-01A-1). All the cows were healthy without mastitis and produced approximately 27–30 kg/day on average.
Study 1 was performed using five mid-lactating Holstein cows. One dose of 150 mg of CEZ (Cefamezin QR, Nippon Zenyaku Kogyo Co., Ltd.) was administered to all quarters immediately after milking in the morning (0 hr), and CEZ levels in serum and milk were measured over time while performing milking as usual twice a day. Serum collected before infusion, and after 0.5, 1, 3, 4, 6, 8 and 24 hr, was used to measure CEZ levels of blood. Milk was collected before administration, and after 6, 24, 30, 48, 54 and 72 hr, depending on the time of milking.
CEZ levels in serum and milk were measured using the high-performance liquid chromatography (HPLC method) described in the Japanese Pharmacopoeia 17th edition and the other reports [10, 15, 19]. A stainless-steel column (Intersil Ph, GL Sciences Inc., Tokyo, Japan) temperature was set to 25°C for serum and 30°C for milk. For the mobile phase, the solution prepared by mixing TBA: acetonitol=9:1 was used as solution A and another solution prepared by mixing TBA: acetonitol: methanol=55:30:15 was used as solution B. The mobile phase B was delivered at 1.0 ml/min in the gradient elution mode. The detection limit of CEZ was 0.01 µg/ml.
Study 2 was performed using four mid-lactating Holstein cows. One dose of 450 mg of CEZ (Cefazolin 3L FUJITA, Fujita Pharmaceutical Co., Ltd., Tokyo, Japan) was administered to all quarters immediately after milking in the morning (0 hr), and then CEZ levels of serum and milk were measured over time while performing milking as usual twice a day. Serum collected before infusion, and after 0.5, 1, 2, 4, 6, 8 and 24 hr, was used to measure CEZ levels of blood. Milk was collected before administration, and after 12, 24, 36, 48, 60 and 72 hr.
CEZ levels in serum and milk were measured by the microbiological assay described in The Japanese Pharmacopoeia 17th edition and previous reports [7, 11, 16]. The medium containing Bacillus stearothermophilus var. calidolactis C-953 was used as the test bacterial solution. The test sample was prepared by adding Phosphate Buffered Saline (PBS) (pH 6.0) to the extract obtained from serum or milk with methanol and acetonyl. The concentrations of CEZ standard solutions diluted in PBS (pH 6.0) were 0.16, 0.08, 0.04, 0.02 and 0.01 µg/ml. Four holes with a diameter of 8 mm were made in each plate of medium. Fifty µl of each test sample and standard solution was poured into the holes of test medium, and then incubated at 55 ± 1°C for 16 to 18 hr. The diameter of the inhibition ring observed on the medium was measured within about 2 hr after incubation, and the CEZ levels in the test sample were calculated from the calibration curve prepared by the diameter of the inhibition ring of the standard solution. The detection limit of CEZ was 0.015 µg/ml.
Study 3 was performed using four mid-lactating Holstein cows. One dose of 450 mg of CEZ (Cefamezin S, Nippon Zenyaku Kogyo Co., Ltd.) was administered to the front-right quarter immediately after milking in the morning (0 hr), and then a wax-bougie (Natural teat insert, Eickemeyer, Tuttlingen, Germany) [6, 14] was placed in the teat to stop milking. The bougie was pulled from the teat after 72 hr. Then, 150 mg of CEZ was administered to the teat and the new bougie was placed again. After 120 hr, the bougie was removed from the teat and milking was resumed as usual. The other quarters were milked as usual twice a day by the milking machine during the cessation of milking of the front-right quarter. Serum collected before administering 450 mg of CEZ (−24 hr), and after 72, 120 and 192 hr, was used to measure CEZ levels of blood. Milk was collected at −24, −18, 0, 5, 24, 30, 48, 54, 72, 78, 96, 102, 120, 126, 144, 150, 168, 174, 192 and 198 hr. During the cessation of milking of the front-right quarter (from 0 to 120 hr), the combined milk of three quarters was used as the sample. The serum and milk CEZ levels were measured using the HPLC method employed in Study 1.
Statistical analysis
The sample minimum, first quartile, median, third quartile and sample maximum were calculated for all the data at each time point, and the transition of serum or milk CEZ level was shown by the boxplot. All the data was indicated using median (sample minimum, sample maximum). The statistical software was used for all the statistical analysis (StatLight, version 2.0, Yukms Co., Ltd., Tokyo). First, the data was analyzed by Bartlett’s test to confirmed if variance is equal in each group. Then, Friedman’s test was carried out because significant difference of the variance was confirmed. Finally, Steel’s nonparametric test was used to analyze differences in CEZ levels in serum and milk at each time point. Levels below the detection limit (DL) were assumed to 0 and then significance was calculated. P-values <0.05 were considered significant.
RESULTS
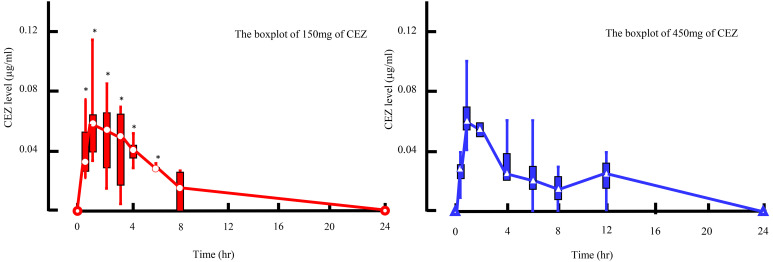
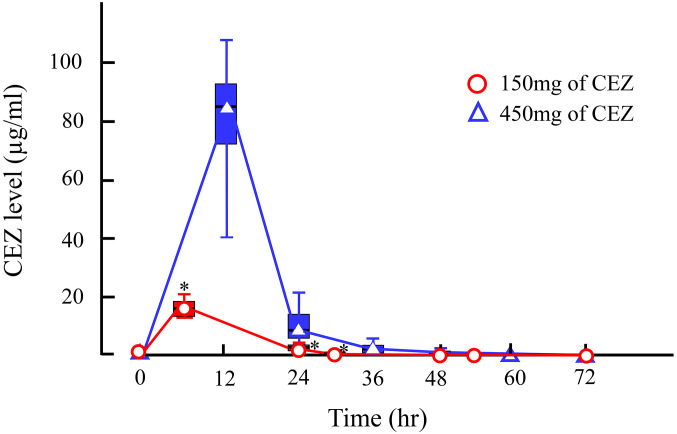
In Study 1, CEZ administered to the quarters was absorbed into the blood stream and the serum CEZ level increased to a maximum of 0.06 (0.04, 0.12) µg/ml at 1 hr (P<0.05). However, it was eliminated from serum by 24 hr (Fig. 1). In milk from the first milking (6 hr) after administration, 17.66 (12.88, 21.53) µg/ml of CEZ was detected (P<0.05); however, the level rapidly decreased to 2.83 (0.35, 4.55) µg/ml by 24 hr at the second milking. Then, the level of CEZ in milk gradually decreased to 0.02 (0.00, 0.04) µg/ml by 54 hr, and was eliminated by 72 hr (Fig. 2).
Fig. 1.
The boxplot of 150 mg of cefazolin sodium (CEZ) is shown in the left figure, and the one of 450 mg of is shown in the right figure. Asterisks (*) indicate the time points when the serum CEZ level was significantly different from the pre-value (Time=0 hr) at each measurement time point after the administration of 150 or 450 mg of CEZ by Steel’s nonparametric test.
Fig. 2.
Time course of milk cefazolin sodium (CEZ) level after intramammary administration of 150 or 450 mg of CEZ. Milk samples were collected when milking was carried out. Asterisks (*) indicate time points when the milk CEZ level was significantly different from the pre-value (Time=0 hr) at each measurement time point after the administration of 150 or 450 mg of CEZ by Steel’s nonparametric test.
In Study 2, the change in serum CEZ level was similar to that in Study 1. The maximum level of 0.06 (0.04, 0.10) µg/ml was observed at 1 hr (P=0.08), and it was eliminated from serum by 24 hr (Fig. 1). In milk from the first milking (12 hr) after administration, 83.18 (37.79, 107.76) µg/ml of CEZ was detected (P=0.06); however, the level rapidly decreased to 7.68 (2.67, 21.14) µg/ml by 24 hr. Then, the level of CEZ gradually decreased to 0.18 (0.00, 1.32) µg/ml by 48 hr, and was eliminated by 60 hr (Fig. 2).
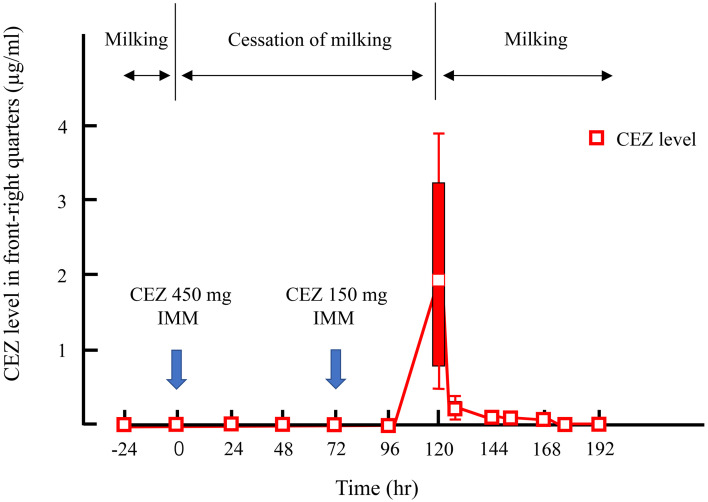
In Study 3, 1.96 (0.44, 3.93) µg/ml of CEZ was detected in milk when milking of the front-right quarter was resumed at 120 hr. The elimination rate from milk differed among cows. The CEZ level in cow No.3 decreased to below the detection limit by the day after the resumption of milking (144 hr), but that in No. 1 became undetectable by 174 hr (Table 1, Fig. 3). CEZ was not detected in milk from the other quarters during the cessation of milking. The level in all serum samples was also below the detection limit.
Table 1. Milk cefazolin sodium (CEZ) level after cessation of milking in the front-right quarter after intramammary administration of 450 and 150 mg of CEZ.
| Milking as usuala
|
Cessation of milkingb
|
Milking as usuala
|
||||||
|---|---|---|---|---|---|---|---|---|
| −24–0 hr | 0–120 hr | 120 hr | 126 hr | 144 hr | 150 hr | 168 hr | 174–198 hr | |
| No. 1 | DL | DL | 0.88 | 0.22 | 0.08 | 0.06 | 0.02 | DL |
| No. 2 | DL | DL | 3.04 | 0.27 | 0.07 | 0.04 | DL | DL |
| No. 3 | DL | DL | 0.44 | 0.12 | DL | DL | DL | DL |
| No. 4 | DL | DL | 3.93 | 0.44 | 0.03 | 0.01 | DL | DL |
|
| ||||||||
| Sample minimum | DL | DL | 0.44 | 0.12 | DL | DL | DL | DL |
| First quartile | DL | DL | 0.77 | 0.20 | 0.02 | 0.01 | DL | DL |
| Median | DL | DL | 1.96 | 0.25 | 0.05 | 0.03 | DL | DL |
| Third quartile | DL | DL | 0.31 | 0.07 | 0.07 | 0.05 | 0.01 | DL |
| Sample maximum | DL | DL | 3.93 | 0.08 | 0.08 | 0.06 | 0.02 | DL |
Milking as usual (µg/ml). a: All quarters were milked twice a day as usual. b: At 0 hr and 72 hr, 450 and 150 mg of CEZ, respectively, was administered to the front-right quarter. The front-right quarter was not milked for 120 hr and the remaining quarters were milked twice a day. DL indicates <the detection limit of 0.01 µg/ml.
Fig. 3.
Time course of milk cefazolin sodium (CEZ) level after cessation of milking following intramammary administration of 450 and 150 mg of CEZ.
DISCUSSION
The withdrawal period after intramammary infusion of 150 or 450 mg of CEZ was 72 hr, because no CEZ was detected in milk at 72 hr after administration in Studies 1 and 2. On the other hand, in Study 3, the drug administered at 72 hr was eliminated by 174 hr instead of 144 hr. This result means that it took 104 hr from administration to elimination. CEZ is thus retained in cows and the withdrawal period is prolonged if milk is not frequently discharged after infusion.
On comparison of 150 and 450 mg of CEZ, the changes in serum level were similar. The amount of CEZ absorbed through the mucosa of the healthy mammary gland and gland cistern is considered to be constant regardless of the dose administered, and most of the infusion remains at the administration site and is eliminated by milking or discharge using teat cannulas. CEZ was eliminated in 52 hr after the resumption of milking in Study 3. The CEZ level in this study was that in the combined milk of all quarters; therefore, that in milk of only the front-right quarter is higher than this result. The withdrawal period was thus 72 hr after the resumption of milking, as specified for the commercial CEZ infusion.
The residual CEZ was measured using the HPLC method and microbiological assay in this study, which are both standard methods for measuring the residual level of antibiotics [15, 16]. Although these methods each have advantages, there is no significant difference between them [2]. As such, the test results were not considered to differ between methods. The hours of sampling were different between Study 1 and Study 2 due to the different milking intervals. It was previously reported that the residual period of antibiotics does not change even if the milking interval changes [18], and the different milking interval in this study had no significant effects on the withdrawal period or pharmacokinetics of CEZ.
The cessation of milking after intramammary infusion prolongs the residual period and results in economic loss due to increased disposal of milk, but it is beneficial for maintaining the level of antibiotics in the mammary gland. The whole milk CEZ level was 1.96 (0.44, 3.93) µg/ml when milking was resumed in Study 3. The milk of front-right quarter was diluted with the milk of other quarters, so it is considered that the CEZ level of the front-right quarter is maintained higher than the level of this whole milk. As the minimum inhibitory concentrations for bacteria detected in bovine mastitis, Ishihara et al. [9] previously reported that CEZ suppressed the growth of 90% of Staphylococcus spp. and Streptococcus spp. at 1 µg/ml, and the growth of 50% of Enterobacteriaceae and E. coli at 2 µg/ml. The purpose of antibiotic therapy for mastitis is to maintain an effective concentration in the affected quarter. As antibiotics are mainly eliminated through milking [17, 18], the antibiotic level cannot be maintained during frequent milking [4, 21]. Therefore, the cessation of milking after intramammary infusion of antibiotics is considered to be useful for bovine mastitis.
In conclusion, withdrawal period after intramammary infusion of CEZ may be prolonged by cessation of milking after administration and is the total time from cessation to 72 hr after the resumption of milking.
CONFLICT OF INTEREST
The authors have no conflicts of interest directly relevant to the content of this article.
REFERENCES
- 1.Burmańczuk A., Grabowski T., Błądek T., Kowalski C., Dębiak P.2017. Withdrawal of cefoperazone with milk after intramammary administration in dairy cows-prospective and retrospective analysis. Pol. J. Vet. Sci. 20: 261–268. doi: 10.1515/pjvs-2017-0031 [DOI] [PubMed] [Google Scholar]
- 2.Christ A. P., Machado M. S., Ribas K. G., Schwarzbold A., Silva C. B., Adams A. I. H.2015. A fully validated microbiological assay for dapotomycin injection and comparison to HPLC method. Braz. J. Pharm. Sci. 51: 775–783. doi: 10.1590/S1984-82502015000400003 [DOI] [Google Scholar]
- 3.Couture Y., Mulon P. Y.2005. Procedures and surgeries of the teat. Vet. Clin. North Am. Food Anim. Pract. 21: 173–204. doi: 10.1016/j.cvfa.2004.12.005 [DOI] [PubMed] [Google Scholar]
- 4.Erskine R. J., Wagner S., DeGraves F. J.2003. Mastitis therapy and pharmacology. Vet. Clin. North Am. Food Anim. Pract. 19: 109–138, vi. doi: 10.1016/S0749-0720(02)00067-1 [DOI] [PubMed] [Google Scholar]
- 5.Fetrow J.2000. Mastitis: an economic consideration. pp. 3–47. In: Proceedings of the 39th Annual Meeting of the National Mastitis Coucil, National Mastitis Council, Madison.
- 6.Geishauser T., Querengässer K., Medl M.1998. Covered teat canal injuries in dairy cows. Compend. Contin. Educ. Pract. Vet. 20: 251–257. [Google Scholar]
- 7.Gilbertson T. J., Mejeur R. L., Yein F. S., Jaglan P. S.1995. Modified microbiological method for the screening of antibiotics in milk. J. Dairy Sci. 78: 1032–1038. doi: 10.3168/jds.S0022-0302(95)76719-4 [DOI] [PubMed] [Google Scholar]
- 8.Hallberg J. W., Wachowski M., Moseley W. M., Dame K. J., Meyer J., Wood S. L.2006. Efficacy of intramammary infusion of ceftiofur hydrochloride at drying off for treatment and prevention of bovine mastitis during the nonlactating period. Vet. Ther. 7: 35–42. [PubMed] [Google Scholar]
- 9.Ishihara K., Sunagawa C., Haneishi T., Miyaguchi N., Endo N., Tanaka T.2020. Comparison of antimicrobial susceptibilities of bacterial isolates between cured and uncured cases of bovine mastitis. J. Vet. Med. Sci. 82: 903–907. doi: 10.1292/jvms.19-0692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Legrand T., Vodovar D., Tournier N., Khoudour N., Hulin A.2016. Simultaneous determination of eight β-lactam antibiotics, amoxicillin, cefazolin, cefepime, cefotaxime, ceftazidime, cloxacillin, oxacillin, and piperacillin, in human plasma by using ultra-high-performance liquid chromatography with ultraviolet detection. Antimicrob. Agents Chemother. 60: 4734–4742. doi: 10.1128/AAC.00176-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peeler J. T., Leslie J. E., Barnett J. E., Houghtby G. A., Messer J. W.1983. Detectability and precision of the AOAC Bacillus stearothermophilus disc assay demonstrated in the 1981 FDA split milk sample testing program. J. Food Prot. 46: 84–86. doi: 10.4315/0362-028X-46.2.84 [DOI] [PubMed] [Google Scholar]
- 12.Querengässer J., Geishauser T., Querengässer K., Bruckmaier R., Fehlings K.2002. Comparative evaluation of SIMPL silicone implants and NIT natural teat inserts to keep the teat canal patent after surgery. J. Dairy Sci. 85: 1732–1737. doi: 10.3168/jds.S0022-0302(02)74247-1 [DOI] [PubMed] [Google Scholar]
- 13.Robert A., Seegers H., Bareille N.2006. Incidence of intramammary infections during the dry period without or with antibiotic treatment in dairy cows-a quantitative analysis of published data. Vet. Res. 37: 25–48. doi: 10.1051/vetres:2005047 [DOI] [PubMed] [Google Scholar]
- 14.Sato K., Suzuki K., Ajito T.2020. Effects of different teat inserts on wound healing of experimentally incised streak canal in non-lactating cattle. J. Vet. Med. Sci. 82: 1708–1713. doi: 10.1292/jvms.20-0325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shiozaki Y.2016. Cefazolin Sodium. pp. 607–610. In: The Japanese Pharmacopoeia 17th ed., Ministry of Health, Labour and Welfare, Tokyo. [Google Scholar]
- 16.Shiozaki Y.2016. Microbial assay for antibiotics. pp. 114–117. In: The Japanese Pharmacopoeia 17th ed., Ministry of Health, Labour and Welfare, Tokyo. [Google Scholar]
- 17.Smith G. W., Gehring R., Riviere J. E., Yeatts J. L., Baynes R. E.2004. Elimination kinetics of ceftiofur hydrochloride after intramammary administration in lactating dairy cows. J. Am. Vet. Med. Assoc. 224: 1827–1830. doi: 10.2460/javma.2004.224.1827 [DOI] [PubMed] [Google Scholar]
- 18.Stockler R. M., Morin D. E., Lantz R. K., Constable P. D.2009. Effect of milking frequency and dosing interval on the pharmacokinetics of cephapirin after intramammary infusion in lactating dairy cows. J. Dairy Sci. 92: 4262–4275. doi: 10.3168/jds.2008-1916 [DOI] [PubMed] [Google Scholar]
- 19.Sørensen L. K., Snor L. K.2000. Determination of cephalosporins in raw bovine milk by high-performance liquid chromatography. J. Chromatogr. A 882: 145–151. doi: 10.1016/S0021-9673(99)01317-5 [DOI] [PubMed] [Google Scholar]
- 20.Tyler J. W., Cullor J. S.2002. Mammary gland health and disorders. pp. 1019–1037. In: Large Animal Internal Medicine, 3rd ed. (Smith, B. P. ed.), Mosby, St. Louis. [Google Scholar]
- 21.Whittem T., Whittem J. H., Constable P. D.2012. Modelling the concentration-time relationship in milk from cattle administered an intramammary drug. J. Vet. Pharmacol. Ther. 35: 460–471. doi: 10.1111/j.1365-2885.2011.01352.x [DOI] [PubMed] [Google Scholar]