Abstract
Background
The magnitude of clinical benefit of solid cancer drugs can be standardly assessed via the Magnitude of Clinical Benefit Scale (MCBS) developed by the European Society for Medical Oncology (ESMO). We applied two ESMO-MCBS versions to the last 12 years of European cancer drug approval and compared two predefined marketing authorisation timeframes to identify potential score changes over time.
Material and methods
Originator solid cancer drugs and indication extensions that were approved between 1 January 2009 and 31 October 2020 by the European Medicines Agency (EMA) were included in our analyses. To evaluate the clinical benefit of these cancer indications, the original ESMO-MCBS (v 1.1) and a locally adapted ESMO-MCBS version were applied to the study sample. Thus, two ESMO-MCBS versions were compared, and an additional analysis was conducted to identify potential score differences between two approval timeframes 2009-2014 versus 2015-2020.
Results
A total of 144 cancer indications intended as curative (n = 9) or non-curative (n = 135) treatment options were eligible for an ESMO-MCBS assessment. Solely a minority of the assessed cancer indications met the meaningful clinical benefit (MCB) criteria independent of the applied version of the scale and treatment intention (original: n = 48/144, 33.3% versus adapted: n = 27/144, 18.8%). Comparing the two EMA approval timeframes, a growing number of approved cancer indications could be observed: 2009-2014: n = 9/year versus 2015-2020: n = 14/year. In addition, almost no difference in the proportion of cancer indications that have met the MCB criteria was detectable when comparing the predefined authorisation timeframes (MCB increase original: +4.1% and adapted: +3.9%).
Conclusion
Applying both versions of the ESMO-MCBS can help to identify potentially beneficial cancer indications, but also those with rather uncertain or low clinical benefit and thus, support the fair allocation of limited health care resources.
Key words: ESMO-MCBS, clinical benefit, health technology assessment, cancer drug approval, evidenced-based decision making
Highlights
-
•
A total of 158 solid cancer indications were approved by the European Medicines Agency (EMA) in the last 12 years.
-
•
A minority of these indications were deemed to be clinically meaningful after applying two versions of the ESMO-MCBS.
-
•
Comparing two EMA approval timeframes, no difference in the highest possible ESMO-MCBS grades could be observed.
-
•
The ESMO-MCBS supports the identification of beneficial as well as rather uncertain or low clinical benefit cancer drugs.
-
•
Evidenced-based decision making and the allocation of limited health care resources can be facilitated by the ESMO-MCBS.
Introduction
Evidence-based decision making and the facilitation of fair allocation of limited health care resources is vitally important for health care systems. To be able to make such decisions, the evaluation of the clinical benefit of medical interventions is necessary.1,2 In recent years, several frameworks have been introduced in the area of pharmaceutical cancer treatments that shall allow the transparent assessment of the meaningful clinical benefit (MCB).3, 4, 5, 6 One of these tools was developed in 2015 by the European Society for Medical Oncology (ESMO), called the Magnitude of Clinical Benefit Scale (MCBS).3,4 The ESMO-MCBS supports a realistic assessment of the true value of novel drugs with providing a tool to guide objective funding decisions.4,7
The misinterpretation of the clinical benefit of a drug can be amplified by the use of surrogate study endpoints.7,8 In contrast to patient-relevant outcomes, such as overall survival (OS), quality of life (QoL) or adverse events (AEs), surrogate parameters fail to directly measure the clinical benefit of cancer drugs.8 The indirectness occurs due to a lack of scientific validation and weak associations with patient-relevant outcomes.7,9 However, the utilisation of surrogate outcomes in approval studies is rising owing to reduced study costs as well as participant numbers and shortened lengths of trials.10
Concurrently, provisional regulatory mechanisms, which allow faster approval of cancer drugs based on uncertain benefit–risk profiles because of less comprehensive data, as well as primary surrogate study endpoints, have increased over the last decades.11,12 In the context of a growing cancer burden due to higher life expectancy, as well as rising cancer drug expenditures for individual therapies and for the quantity of all therapies, it is ever more important to provide funders with objective information for their decisions.13,14 To maintain universal health coverage, stakeholders and policymakers have to thoroughly decide on which medical interventions should be reimbursed, considering the accessible evidence basis. Thus, we aimed to identify the clinical benefit of cancer drugs and their authorised indication extensions at the time of European Medicines Agency (EMA) approval between 2009 and 2020 via two versions of the ESMO-MCBS. Thus, scores resulting from the assessment with the original ESMO-MCBS were contrasted with a locally adapted version of the ESMO-MCBS utilised by the Austrian Institute for Health Technology Assessment (AIHTA). In addition, we compared two authorisation timeframes (2009-2014 versus 2015-2020) to investigate potential changes in ESMO-MCBS scores (original and adapted) over time.
Methods
Identification of the study sample
A preliminary study assessing the clinical benefit of EMA-approved solid cancer indications between 1 January 2011 and 31 December 2016 was used as a data basis.15 For the herein presented analyses, the study sample was further extended to all solid cancer drugs, as well as indication extensions of already approved therapies that were authorised by the EMA between 1 January 2009 and 31 October 2020. To allow better readability, all herein analysed EMA-approved new clinical entities, as well as extensions of existing marketing authorisations, are collectively referred to as cancer indications. Our general focus was on new chemical entities; therefore, biosimilars, generics and hybrid medicines were not considered. Further exclusion criteria were non-solid cancer drugs, pivotal studies without statistically significant results, non-comparative pivotal studies as well as studies with non-amenable endpoints for an ESMO-MCBS assessment (e.g. event-free survival or no assessable endpoint was reached at the time of analysis).3,4 Generally, eligible cancer therapies were identified via the EMA website (https://www.ema.europa.eu/).
Identification of pivotal cancer trials and extraction of study information
To identify pivotal cancer trials the European Public Assessment Reports (EPARs) that are available on the EMA website were used as a main source of information. The clinical registry website, clinictrials.gov, provided additional information on study publications. Thus, only information from the EPARs, the clinicaltrials.gov website and the original publications of the pivotal trials were considered for the analyses, whereby results published solely as conference abstracts were excluded. In addition, ESMO-MCBS assessments were only based on the information available at the time of approval. Therefore, no study updates were considered in our analyses. Results, including efficacy data as well as the toxicity profile and study characteristics, were extracted by one author (NG) and double-checked by two other authors (ER and SW). Disagreements were discussed and dissolved.
ESMO-MCBS assessment
The ESMO-MCBS assessments were conducted via applying version 1.1 of the original scale and a locally adapted version of the ESMO-MCBS that is used by the AIHTA.3,4,16 In both versions of the scale, the MCB threshold that should indicate a high level of proven clinical benefit is represented by scores of A and B in the curative setting (Form 1) as well as in the case of the non-curative setting by scores of 4 and 5 (Form 2).4,16 Original scorings were collated with publicly accessible ESMO-MCBS assessments available on the ESMO website (https://www.esmo.org/guidelines/esmo-mcbs/esmo-mcbs-scorecards).
Both scales solely consider statistically significant study endpoints. Thus, statistically significant OS results were preferentially scored followed by any applicable, as well as statistically significant, primary and secondary outcomes. Furthermore, only accessible and published study results were considered. Therefore, if the median gain of an outcome was not available, it was not calculated based on the respective hazard ratio (HR). Generally, the locally adapted scale used by the AIHTA incorporates some different stringencies: it uses the point estimate of the HR instead of the lower limit of the 95% confidence interval (CI), implicates higher toxicity and QoL adjustments and allows for multiple upgrades or downgrades (Table 1). However, multiple upgrade or downgrades were solely applicable if OS, AEs and QoL were simultaneously improved (+2) or showed negative results (−2). Although the locally adapted ESMO-MCBS version has not undergone structured peer review for reasonableness or statistical validation, it has proven as a highly effective tool for Health Technology Assessment (HTA) use in Austria since its introduction in 2017.15
Table 1.
Differences of the assessment criteria applied in the original versus adapted ESMO-MCBS (based on15)
| Adapted ESMO-MCBS | Original ESMO-MCBS v1.1 |
|---|---|
| Generation of scores | |
| Point estimate of the HR | Lower limit of the 95% CI of the HR |
| Applicable study designs | |
| Comparative studies (Form 1-2c) | Comparative (Form 1-2c) and non-comparative studies (Form 3) |
| Score adjustments | |
| Downgrades due to a negative median OS despite scored endpoint showing a statistically significant, positive difference (Form 2a, b and c) | Not implicated |
| Downgrades OR upgrades due to positive or negative differences, respectively, of at least 10% in any grade ≥3 AEs (Form 2a and b) | Upgrades due to statistically significantly less grade 3-4 toxicities impacting on daily well-being (Form 2a and b) Downgrades due to one or more statistically significantly increased incremental toxicities (Form 2b) |
| Downgrades OR upgrades due to positive or negative differences, respectively, of at least 10% in the discontinuation rates (Form 2a and b) | Not implicated |
| Downgrades due to no difference in QoL OR no QoL assessment carried out AND only PFS showing an improvement (Form 2b) | Downgrades due to no difference in QoL AND only PFS showing an improvement (Form 2b) |
| Downgrades OR upgrades due to statistically significant negative or positive differences (respectively) in QoL (Form 2a and b) | Upgrades due to statistically significant positive differences in QoL (Form 2a and b) |
AE, adverse event; CI, confidence interval; HR, hazard ratio; OS, overall survival; PFS, progression-free survival; QoL, quality of life.
The whole study sample was assessed by one author (NG) using the original (version 1.1) as well as the adapted ESMO-MCBS. The scoring was double-checked by two additional authors (ER and SW) and disagreements were reviewed and discussed by all three assessors (ER, NG and SW) (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2021.100166).
Results
Characteristics of the study sample
In total, 181 solid cancer indications were approved between 1 January 2009 and 31 October 2020 by the EMA (Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2021.100166). Out of those, 37 were ineligible for our analyses due to the lack of evaluable study endpoints, statistically significant results or a non-comparative study design. Thus, the study sample (n = 144) comprised 9 solid cancer indications intended for the curative treatment (Form 1) and 135 for the non-curative setting (Form 2a-c) (Table 2). Thirty-five (n = 35/144, 24.3%) cancer indications underwent regular EMA approval. Hence, the majority of the investigated cancer medicines (n = 109/144, 75.7%) were approved under a conditional authorisation route (n = 13/144, 9.0%) and/or received orphan designation (n = 25/144, 17.4%) and/or were labelled with additional monitoring in the post-approval phase (n = 92/144, 63.9%). Double entries were possible since all of the aforementioned approval pathways, as well as labels, can be granted simultaneously.
Table 2.
Characteristics of the study sample
| Characteristics | EMA-approved cancer indications (n = 144), n (%) |
|---|---|
| Indication (ICD-10 category) | |
| Cancer of lip, oral cavity and pharynx (C00-C14) | 1 (0.7) |
| Gastrointestinal cancer (C15-C26) | 22 (15.3) |
| Lung cancer (C30-C39) | 34 (23.6) |
| Melanoma (C43-C44) | 21 (14.6) |
| Sarcoma (C45-C49) | 2 (1.4) |
| Breast cancer (C50-C50) | 21 (14.6) |
| Cervical carcinoma (C51-C58) | 5 (3.5) |
| Ovarian and peritoneal cancer (C51-C58 and C45-C49) | 5 (3.5) |
| Prostate cancer (C60-C63) | 12 (8.3) |
| Renal cell carcinoma (C64-C68) | 14 (9.7) |
| Thyroid carcinoma and neuroendocrine tumour (C73-C75) | 4 (2.8) |
| Cancer of ill-defined, secondary and unspecified sites (C76-C80) | 3 (2.1) |
| Phase of the study | |
| II | 6 (4.2) |
| II/III | 1 (0.7) |
| III | 137 (95.1) |
| Study participants, n | |
| Mean | 707 |
| Median | 581 |
| Range, n | 50-4805 |
| Statistically significant study endpoints | |
| OS | 65 (45.1) |
| PFS | 111 (77.1) |
| Other | 13 (9.0) |
| ESMO-MCBS forms (original and adapted) | |
| Curative | |
| 1 | 9 (6.3) |
| Non-curative | |
| 2a | 56 (38.9) |
| 2b | 72 (50.0) |
| 2c | 7 (4.9) |
| ESMO-MCBS adjustments | |
| Original (n = 36) | |
| +1 | 31 (86.1) |
| −1 | 3 (8.3) |
| +1/−1 | 2 (5.6) |
| Adapted (n = 95) | |
| +1 | 21 (22.1) |
| +2 | 3 (3.2) |
| −1 | 61 (64.2) |
| −2 | 1 (1.1) |
| +1/−1 | 9 (9.5) |
| Approval year | |
| 2009 | 9 (6.3) |
| 2010 | 9 (6.3) |
| 2011 | 11 (7.6) |
| 2012 | 6 (4.2) |
| 2013 | 11 (7.6) |
| 2014 | 11 (7.6) |
| 2015 | 15 (10.4) |
| 2016 | 18 (12.5) |
| 2017 | 14 (9.7) |
| 2018 | 17 (11.8) |
| 2019 | 16 (11.1) |
| 2020 (until Oct) | 7 (4.9) |
| EMA authorisation route/label | |
| Regular EMA approval | 35 (24.3) |
| Additional monitoring (AM) | 92 (63.9) |
| Conditional approval (CA) | 13 (9.0) |
| Orphan designation (OD) | 25 (17.4) |
| AM and CA | 4 (2.8) |
| AM and OD | 12 (8.3) |
| CA and OD | 3 (2.1) |
| AM and OD and CA | 1 (0.7) |
Deviation of 100% cumulative percentage may be caused by rounding.
AM, additional monitoring; CA, conditional approval; EMA, European Medicines Agency; EMSO, European Society for Medical Oncology; ICD-10, International Classification of Disease (10th Revision); n, number of solid cancer indications; Magnitude of Clinical Benefit Scale; OD, orphan designation; OS, overall survival; PFS, progression-free survival.
The phase of the analysed pivotal studies ranged from II to III, whereby phase III trials were the most commonly assessed (n = 137/144, 95.1%). Considering accessible statistically significant study endpoints of investigated cancer trials, progression-free survival (PFS; n = 111/144, 77.1%) was the predominant outcome followed by OS (n = 65/144, 45.1%) and other endpoints, such as the overall response rate (n = 13/144, 9.0%).
ESMO-MCBS assessment of non-curative and curative cancer indications
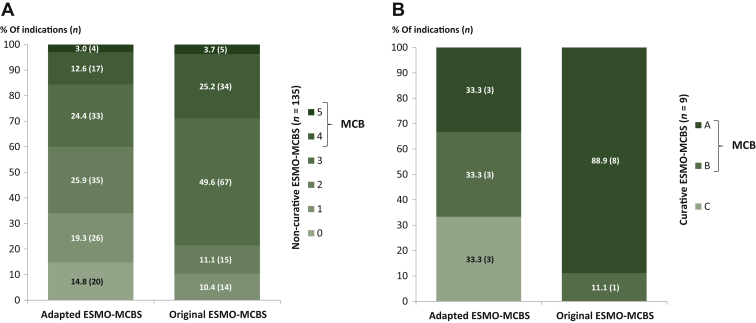
A total of 9 (6.3%) out of 144 authorised cancer indications were intended as a curative treatment option for patients (Form 1, see Figure 1B). All of these indications reached the MCB threshold for substantial clinical benefit (A-B, not C) by applying the original scale (score A: n = 8/9, 88.9%; score B: n = 1/9, 11.1%), compared with six (66.6%) out of nine indications that met the MCB criteria with the adapted ESMO-MCBS (score A: n = 3/9, 33.3%; score B: n = 3/9, 33.3%).
Figure 1.
Adapted and original ESMO-MCBS assessments of (A) non-curative (n = 135) and (B) curative (n = 9) cancer indications.
ESMO, European Society for Medical Oncology; MCB, Magnitude of Clinical Benefit; MCBS, Magnitude of Clinical Benefit Scale; n, number of solid cancer indications.
A total of 135 solid cancer indications for the non-curative setting were analysed (Form 2a-c), out of which 39 (28.9%) and 21 (15.6%) reached the MCB threshold (4-5, not ≤3) by applying the original and the adapted ESMO-MCBS, respectively (Figure 1A). The majority of ESMO-MCBS assessments resulted in grades of 2 or 3 independent of the utilised scale (original: n = 82/135, 60.7%; adapted: n = 68/135, 50.3%). Fourteen cancer indications (n = 14/135, 10.3%) were scored with 1 via applying the original scale, compared with one-third of 0 and 1 assessments (n = 46/135, 34.1%) with the adapted scale.
Comparison of two EMA approval timeframes of non-curative ESMO-MCBS assessments
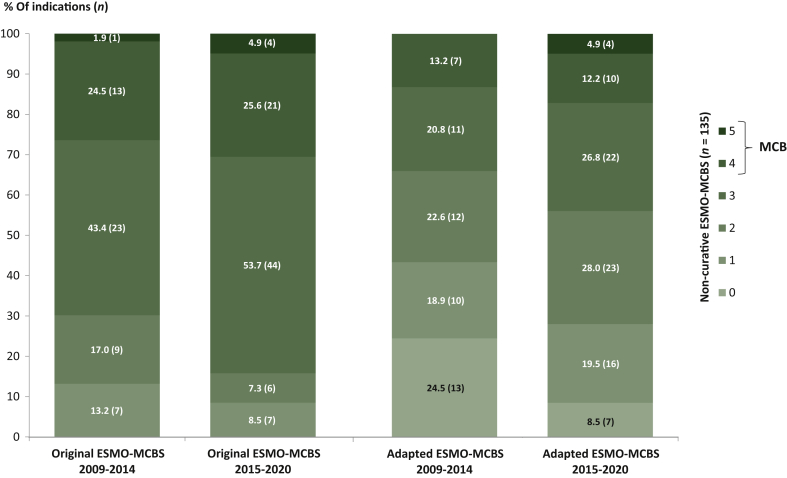
A total of 135 non-curative cancer indications have been approved in Europe in the last 12 years, out of which 53 (39.3%) were authorised during 2009-2014 and 82 (60.7%) during 2015-2020 (Figure 2). Therefore, between 2009 and 2014, on average 9 cancer indications were approved each year compared with 14 indications per year between 2015 and 2020.
Figure 2.
Comparison of the ESMO-MCBS scores (original and adapted scale) of two defined EMA approval timeframes (2009-2014: n = 53 versus 2015-2020: n = 82).
ESMO, European Society for Medical Oncology; MCB, Magnitude of Clinical Benefit; MCBS, Magnitude of Clinical Benefit Scale; n, number of solid cancer indications.
Considering the original ESMO-MCBS assessments, the MCB threshold was met by 26.4% (n = 14/53) and 30.5% (n = 25/82) of cancer indications approved during 2009-2014 and 2015-2020, respectively (see Figure 2). The majority of indications were scored with scores of 2 or 3 independent of the investigated approval timeframe (2009-2014: n = 32/53, 60.4% versus 2015-2020: n = 50/82, 61.0%). In addition, there was a reduction in the lowest possible ESMO-MCBS score (1) in the second timeframe (2015-2020) compared with the first timeframe (2009-2014) (8.5% versus 13.2%; −4.7%).
Applying the adapted ESMO-MCBS scale resulted in around 13%-17% of cancer indications that have reached the MCB criteria independent of the investigated timeframe (2009-2014: n = 7/53, 13.2% versus 2015-2020: n = 14/82, 17.1%) (Figure 2). Considering the lowest possible (0-1) and ‘mid-level’ scores (2-3), a shift of scores could be found over time towards a higher proportion of ‘mid-level’ scores. In fact, the adapted ESMO-MCBS analyses of 2015-2020 showed an 11.4% increase in scores of 2 and 3 (2009-2014: n = 23/53, 43.4% versus 2015-2020: n = 45/82, 54.8%) as well as a 15.4% decrease in lowest scored (0-1) cancer indications (2009-2014: n = 23/53, 43.4% versus 2015-2020: n = 23/82, 28.0%) compared with 2009-2014.
Discussion
In the recently published ‘Europe Beating Cancer Plan’, the European Commission defined cancer of fundamental importance for Europe's future and therefore as a priority area of the new Commission.17 Considering the total health expenditures of €103 billion for cancer care spent in Europe in 2018, 31% accounted for cancer pharmaceuticals.14 Concurrently the number of cancer therapies in development is rising; hence, policymakers are ever more challenged to make evidence-based allocation decisions on limited health care resources at the time of continually faster approval to drugs.18 Therefore, we aimed to assess the clinical benefit of solid cancer drugs that were EMA-approved between January 2009 and October 2020 contrasted by two versions of the ESMO-MCBS (original versus a locally adapted ESMO-MCBS version). In addition, two authorisation periods (2009-2014 versus 2015-2020) were compared to investigate potential changes in ESMO-MCBS scores (original and adapted) over time.
A total of 144 eligible cancer indications were included; out of those 135 were non-curative cancer indications, from which solely 28.9% (n = 39) and 15.6% (n = 21) met the MCB criteria with the original and the adapted ESMO-MCBS framework (scores 4-5), respectively. More than half were scored with ‘mid-level’ scores of 2 or 3 (original: 60.7% versus adapted: 50.3%) and 10.3% were scored with 1 via applying the original scale, compared with 34.1% with the adapted framework (scores: 0-1). Considering curative cancer indications, 100.0% (original, n = 9/9) and 66.6% (adapted, n = 6/9) reached the MCB threshold (scores A-B). Generally, scoring differences between the two ESMO-MCBS frameworks mainly occurred due to the use of the lower limit of the CI of the HR within the adapted scale and its higher weight given to AEs as well as QoL. However, independent of the applied ESMO-MCBS framework, only a minority of the investigated cancer indications (curative as well as non-curative) met ESMO-MCBS criteria of substantial benefit (original: n = 48/144, 33.3% versus adapted: n = 27/144, 18.8%).
Comparing the two EMA approval timeframes by applying both ESMO-MCBS versions, we could identify a trend in growing numbers of approved medicines for solid cancer indications (on average, 2009-2014: n = 9/year versus 2015-2020: n = 14/year). In addition, our results have shown that there is just a slight increase in cancer indications that have met the MCB criteria over time when comparing the two EMA approval timeframes independent of the ESMO-MCBS version (MCB increase original: +4.1% and adapted: +3.9%). However, a shift of scores from the lowest possible scores (0-1) to ‘mid-level’ scores (2-3) could be observed for both scale versions when comparing the two timeframes. Thus, novelty should not automatically be mistaken with greater patient benefits.
To our knowledge, this is the first study that standardly assessed the clinical benefit of EMA-approved indications over a long period of time (12 years) as well as compared assessment results of novel cancer indications (2015-2020) with rather established ones (2009-2014). Nevertheless, our findings on the marginal patient benefit of a high proportion of cancer drugs are in line with recently published studies.1,19, 20, 21, 22 ESMO-MCBS assessments of Vivot et al.22 focusing on solid cancer drugs approved in the USA between 2000 and 2015 could demonstrate that out of 37 drugs only 13 (35%) met the MCB criteria. Another systematic evaluation of EMA-approved cancer drugs (2009-2013) concluded that most therapies enter the European market with lacking benefit on OS and QoL.19 In addition, our observation of increasing numbers of cancer drug approvals is in accordance with Kim and Prasad 2015.23
The herein presented analyses are limited by several factors. On the one hand, external validation of the adapted ESMO-MCBS is currently lacking and should be pursued in the future. However, the current analyses further contribute to the internal validity of the adapted scales and allow a clinical benefit assessment that is more focused on QoL and toxicity outcomes. On the other hand, non-comparative studies were not taken into account. Nevertheless, this does not affect the results of the MCB threshold, since scores of Form 3 for single-arm studies only range from 0 to 3; hence, the MCB threshold cannot be reached with the non-comparative study design. Moreover, additional analyses considering low scored anticancer drugs could generally be of interest especially focusing on the different mechanisms of action (e.g. chemotherapy, targeted agents) of anticancer treatments and their potential effect on ESMO-MCBS scores.
In our analyses, the surrogate outcome metastasis-free survival was assessed by applying the Forms 2a-b, which are normally used to score PFS results. This strategy is based on officially published assessments from the ESMO website: https://www.esmo.org/guidelines/esmo-mcbs/esmo-mcbs-scorecards. However, further validation of the assessment of this surrogate endpoint is needed, and its transparent incorporation into ESMO-MCBS Forms would be beneficial.7 Furthermore, upgrades or downgrades by more than one score were only applicable by using the adapted ESMO-MCBS and were solely carried out if OS, AEs and QoL showed either concurrently positive (n = 3/158) or negative results (n = 1/158), respectively. Since these are the most patient-relevant outcomes, we think that adjustments of two scores are justified and should also be considered in the original tool.8
In practice, the application of the ESMO-MCBS frameworks can support the early identification of cancer drugs with perceived benefit and accordingly accelerate access to cancer patients.24 Concurrently, cancer drugs with low or uncertain patient benefits can be detected and prioritised for further monitoring in the post-approval phase to elicit their actual clinical benefit. Besides, ESMO-MCBS assessments may help to support price negotiations in addition to already established economic as well as clinical evaluations. In conclusion, the ESMO-MCBS framework can support evidence-based reimbursement decision and thereby encourage universal coverage as well as the fair allocation of limited health care resources. Finally, to be able to assess the broad majority of pharmaceutical cancer treatments, the ESMO-MCBS for haematologic cancer therapies is urgently awaited.
Acknowledgments
Funding
None declared.
Disclosure
The authors have declared no conflicts of interest.
Supplementary data
References
- 1.Banzi R., Gerardi C., Bertele V., Garattini S. Approvals of drugs with uncertain benefit-risk profiles in Europe. Eur J Intern Med. 2015;26:572–584. doi: 10.1016/j.ejim.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 2.Salas-Vega S., Iliopoulos O., Mossialos E. Assessment of overall survival, quality of life, and safety benefits associated with new cancer medicines. JAMA Oncol. 2017;3:382–390. doi: 10.1001/jamaoncol.2016.4166. [DOI] [PubMed] [Google Scholar]
- 3.Cherny N.I., Dafni U., Bogaerts J. ESMO-magnitude of clinical benefit scale version 1.1. Ann Oncol. 2017;28:2340–2366. doi: 10.1093/annonc/mdx310. [DOI] [PubMed] [Google Scholar]
- 4.Cherny N.I., Sullivan R., Dafni U. A standardised, generic, validated approach to stratify the magnitude of clinical benefit that can be anticipated from anti-cancer therapies: the European Society for Medical Oncology Magnitude of Clinical Benefit Scale (ESMO-MCBS) Ann Oncol. 2015;26:1547–1573. doi: 10.1093/annonc/mdv249. [DOI] [PubMed] [Google Scholar]
- 5.Schnipper L.E., Davidson N.E., Wollins D.S. Updating the American Society of Clinical Oncology value framework: revisions and reflections in response to comments received. J Clin Oncol. 2016;34:2925–2934. doi: 10.1200/JCO.2016.68.2518. [DOI] [PubMed] [Google Scholar]
- 6.Schnipper L.E., Davidson N.E., Wollins D.S. American Society of Clinical Oncology statement: a conceptual framework to assess the value of cancer treatment options. J Clin Oncol. 2015;33:2563–2577. doi: 10.1200/JCO.2015.61.6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kemp R., Prasad V. Surrogate endpoints in oncology: when are they acceptable for regulatory and clinical decisions, and are they currently overused? BMC Med. 2017;15:134. doi: 10.1186/s12916-017-0902-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson M.K., Karakasis K., Oza A.M. Outcomes and endpoints in trials of cancer treatment: the past, present, and future. Lancet Oncol. 2015;16:e32–e42. doi: 10.1016/S1470-2045(14)70375-4. [DOI] [PubMed] [Google Scholar]
- 9.Schuster Bruce C., Brhlikova P., Heath J., McGettigan P. The use of validated and nonvalidated surrogate endpoints in two European Medicines Agency expedited approval pathways: a cross-sectional study of products authorised 2011–2018. PLoS Med. 2019;16:e1002873. doi: 10.1371/journal.pmed.1002873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Svensson S., Menkes D.B., Lexchin J. Surrogate outcomes in clinical trials: a cautionary tale. JAMA Int Med. 2013;173:611–612. doi: 10.1001/jamainternmed.2013.3037. [DOI] [PubMed] [Google Scholar]
- 11.European Medicines Agency Factsheet: what does the black triangle mean? 2020. https://www.ema.europa.eu/en/documents/other/what-does-black-triangle-mean_en.pdf Available at.
- 12.European Medicines Agency Human regulatory - conditional marketing authorisation. 2020. https://www.ema.europa.eu/en/human-regulatory/marketing-authorisation/conditional-marketing-authorisation Available at.
- 13.Howard D.H., Bach P.B., Berndt E.R., Conti R.M. Pricing in the market for anticancer drugs. J Econ Perspect. 2015;29:139–162. doi: 10.1257/jep.29.1.139. [DOI] [PubMed] [Google Scholar]
- 14.Hofmarcher T., Lindgren P., Wilking N., Jönsson B. The cost of cancer in Europe 2018. Eur J Cancer. 2020;129:41–49. doi: 10.1016/j.ejca.2020.01.011. [DOI] [PubMed] [Google Scholar]
- 15.Grössmann N., Del Paggio J.C., Wolf S. Five years of EMA-approved systemic cancer therapies for solid tumours - a comparison of two thresholds for meaningful clinical benefit. Eur J Cancer. 2017;82:66–71. doi: 10.1016/j.ejca.2017.05.029. [DOI] [PubMed] [Google Scholar]
- 16.Wild C., Grössmann N., Bonanno P.V. Utilisation of the ESMO-MCBS in practice of HTA. Ann Oncol. 2016;27:2134–2136. doi: 10.1093/annonc/mdw297. [DOI] [PubMed] [Google Scholar]
- 17.European Commission . 2020. Europe' Beating Cancer Plan.https://ec.europa.eu/info/law/better-regulation/have-your-say/initiatives/12154-Europe-s-Beating-Cancer-Plan Available at. [Google Scholar]
- 18.Vreman R.A., Naci H., Goettsch W.G. Decision making under uncertainty: comparing regulatory and health technology assessment reviews of medicines in the United States and Europe. Clin Pharm Ther. 2020;108:350–357. doi: 10.1002/cpt.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davis C., Naci H., Gurpinar E. Availability of evidence of benefits on overall survival and quality of life of cancer drugs approved by European Medicines Agency: retrospective cohort study of drug approvals 2009-13. Br Med J. 2017;359:j4530. doi: 10.1136/bmj.j4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang D.M., Chan K.K.W., Jang R.W. Anticancer drugs approved by the Food and Drug Administration for gastrointestinal malignancies: clinical benefit and price considerations. Cancer Med. 2019;8:1584–1593. doi: 10.1002/cam4.2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Hosson L.D., van Veenendaal L.M., Schuller Y. Clinical benefit of systemic treatment in patients with advanced pancreatic and gastrointestinal neuroendocrine tumours according to ESMO-MCBS and ASCO framework. Ann Oncol. 2017;28:3022–3027. doi: 10.1093/annonc/mdx547. [DOI] [PubMed] [Google Scholar]
- 22.Vivot A., Jacot J., Zeitoun J.D. Clinical benefit, price and approval characteristics of -approved new drugs for treating advanced solid cancer, 2000–2015. Ann Oncol. 2017;28:1111–1116. doi: 10.1093/annonc/mdx053. [DOI] [PubMed] [Google Scholar]
- 23.Kim C., Prasad V. Cancer drugs approved on the basis of a surrogate end point and subsequent overall survival: an analysis of 5 years of US Food and Drug Administration Approvals. JAMA Int Med. 2015;175:1992–1994. doi: 10.1001/jamainternmed.2015.5868. [DOI] [PubMed] [Google Scholar]
- 24.Vokinger K.N., Hwang T.J., Grischott T. Prices and clinical benefit of cancer drugs in the USA and Europe: a cost–benefit analysis. Lancet Oncol. 2020;21:664–670. doi: 10.1016/S1470-2045(20)30139-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.