Abstract
We report on the use of systemic heparinization following thrombolysis with intravenous tissue plasminogen activator (t-PA) for acute ischemic large vessel stroke, in the setting of COVID-19-induced hypercoagulability, with partial recanalization of the internal carotid artery. Off-label systemic heparinization was used within 12 hours of t-PA administration, after extensive multidisciplinary collaboration and family discussion, given evidence of severe hypercoagulability. We conclude that thrombolysis should be considered for all eligible patients with suspected or confirmed COVID-19 and acute ischemic stroke, and systemic anticoagulation, although with inherent risks, may be a useful adjunct treatment modality in selected patients who have received intravenous thrombolysis.
Keywords: case report, tissue plasminogen activator, COVID-19, ischemic stroke, anticoagulation
Introduction
Infection with SARS-CoV-2 (hereafter COVID-19) has been associated with hypercoagulability, including disseminated intravascular coagulation, venous thromboembolism, acute myocardial infarction, and acute ischemic stroke. 1 Tissue plasminogen activator (t-PA) remains a mainstay in care in treating acute ischemic stroke with or without large vessel occlusion for those meeting eligibility criteria. In addition, systemic heparinization is being more widely accepted as a potential treatment modality for the hypercoagulability in COVID-19, although prospective randomized controlled trials remain necessary. 2,3 In this case report, we are the first to detail, to our knowledge, the use of t-PA in treating an acute ischemic stroke in this setting, followed by systemic heparinization for severe hypercoagulability.
Case Description
A 69-year-old man with a medical history of multiple vascular risk factors presented to the emergency department with acute right hemiparesis, aphasia, and forced left-gaze deviation. Recent medical history was notable for hospitalization for orthostatic syncope due to dehydration in the setting of COVID-19 with symptoms of fever, chills, cough, and fatigue, beginning 18 days prior to current presentation. COVID-19 positivity was confirmed via nasal swab real time-PCR during a hospitalization for syncope 7 days prior to current presentation. Upon arrival, he was hypertensive with a blood pressure of 181/94, hypoxic with an oxygen saturation of 84% requiring 5 liters/minute of oxygen via nasal cannula, and hyperglycemic with a blood glucose of 360 mg/dl. His neurological exam was notable for a left hemispheric stroke syndrome, consisting of global aphasia, right hemiparesis, leftward gaze deviation, and right homonymous hemianopsia, with a National Institutes of Health Stroke Scale (NIHSS) of 25.
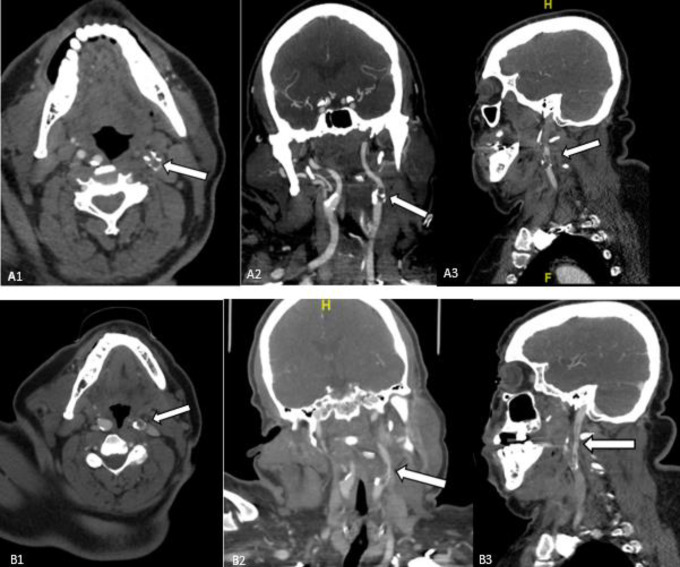
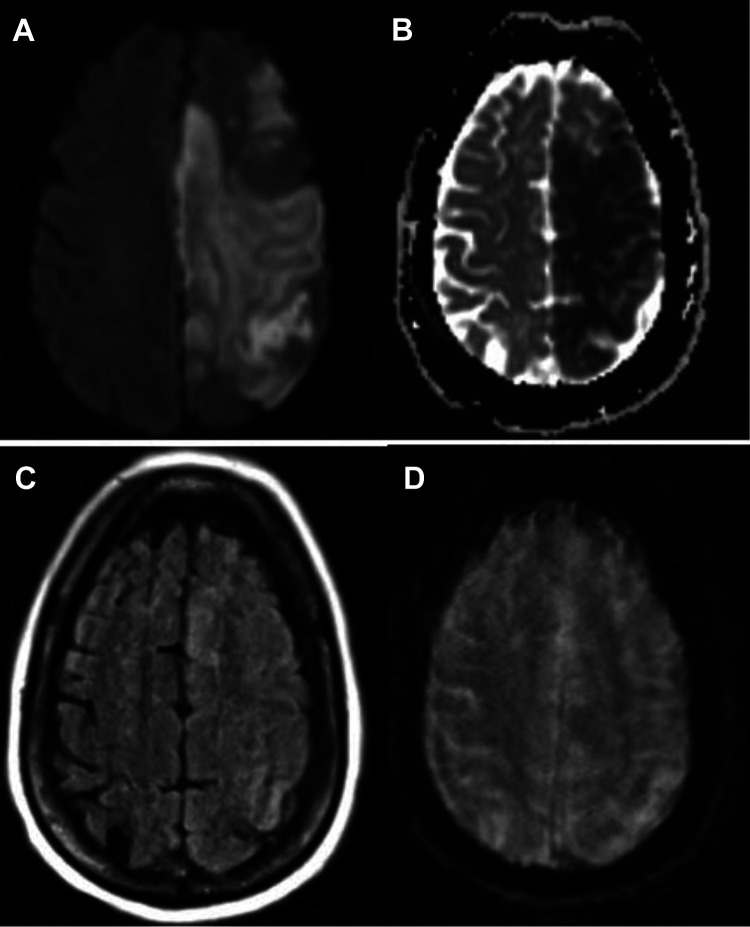
A multiphase CT angiogram (mCTA) of the neck and brain revealed proximal high-grade (85%) left internal carotid artery (ICA) stenosis and in-situ thrombosis, with patent intracranial large arteries (Figure 1a). There was an occlusion of the distal second segment of the left anterior cerebral artery (ACA). There was also observed poor collateral filling within the left ACA territory. Chest CT revealed peripheral ground glass opacities consistent with COVID-19. Despite delays due to newly-instituted protocols surrounding COVID-19 patients and in contacting family, he was administered the maximum weight-limited dose of 90 mg (0.73 mg/kg actual body weight) of tissue plasminogen activator 42 minutes from arrival and 69 minutes from last known normal. After a multidisciplinary discussion, acute endovascular therapy for the proximal thrombus was deferred due to concern for possible embolization to currently patent intracranial vessels. Laboratory workup was notable for D-Dimer of 4238 ng/mL, increased from 555 ng/mL 3 days prior, and fibrinogen >1000 mg/dL. Platelet count was 493 x 109/L. Repeat CTA obtained approximately 1 hour post-tPA bolus, performed due to a transient episode of hypotension to 80/49 mm/Hg, and tachycardia associated with an abrupt decline in level of alertness, revealed partial recanalization of the acute thrombus at the proximal left ICA (Figure 1b). MRI of the brain performed 4 hours after t-PA bolus revealed a large left ACA and middle cerebral artery (MCA)-territory infarction without hemorrhage (Figure 2).
Figure 1.
Panel A1, A2, and A3. Axial, Coronal, and Sagittal views revealing intraluminal thrombus of the left internal carotid artery (white arrow). Panel B1, B2, and B3. Axial, Coronal, and Sagittal views revealing partial recanalization post-thrombolysis (white arrow).
Figure 2.
MRI of the brain performed 4 hours post-thrombolysis. Large anterior and middle cerebellar artery acute infarction seen on Diffusion Weighted Image and Apparent Diffusion Coefficient mapping (panels A and B) without evidence of hemorrhage (panel C, FLAIR image, and panel D, gradient echo image).
In light of his apparent hypercoagulable state from COVID-19, evidenced by his hyperfibrinogenemia, elevated D-dimer, and 6 subsequent sets of clotted serum samples, a multi-disciplinary discussion among neurocritical care, vascular neurology, and neuro-interventional radiology led to initiation of systemic anticoagulation with heparin after extensive discussion with family explaining the potential risks and benefits of this off-label treatment. Although partial thrombolysis was achieved, remnant clot and evidence of severe hypercoagulability provided an indication to initiate systemic heparinization, despite being contraindicated within 24 hours of t-PA administration under normal circumstances. Multidisciplinary analysis deemed the potential for benefit against thromboembolism to outweigh the risk of hemorrhage. Heparinization with an institutional high-risk neuroscience protocol was initiated 9.5 hours after t-PA bolus. This protocol includes no initial bolus of unfractionated heparin, and a moderate infusion initiation at 15 units/kg/hour (with a maximum of 1500 units per hour), and a similar therapeutic target anti-Xa of 0.3-0.7. Six hours after therapeutic anticoagulation was achieved, a surveillance CT of the brain revealed substantial cerebral edema with 5 mm midline shift at the pineal gland and 10 mm midline shift at the septum pellucidum, parenchymal hematoma type 2 (PH2) hemorrhagic transformation, and intraventricular hemorrhage (Figure 3), however with stable clinical examination. Heparinization was stopped and reversed with protamine, per protocol. Neurosurgery was consulted for consideration of cerebrospinal fluid diversion or decompressive hemicraniectomy, though multidisciplinary decision was made to not pursue either of these interventions due to likelihood of minimal benefit and risk of peri-operative thrombosis. Twelve hours after the intracranial hemorrhage was discovered, he developed respiratory failure requiring endotracheal intubation and mechanical ventilation. His course was further complicated by rising D-Dimer (max 14,956 ng/ml) and concern for disseminated intravascular coagulation with rapidly diminishing fibrinogen to 143 mg/dl 2 days after t-PA administration. This figure normalized without need for cryoprecipitate or fresh frozen plasma, although this was considered and ultimately deferred, as, again, thrombosis risk was felt to outweigh hemorrhagic risk. Anti-cardiolipin IgM were elevated (32.8 micrograms); IgG and IgA were normal (<9.4 micrograms). Beta 2 glycoproteins IgG and IgM were normal (<6.4 U/ml and <1.1 U/ml, respectively). Lupus anticoagulant ratio was slightly elevated to 1.28. These were felt not to be significantly abnormal. Two days after the intracerebral hemorrhage was discovered, heparinization was reinitiated with stable hemorrhage on head CT.
Figure 3.
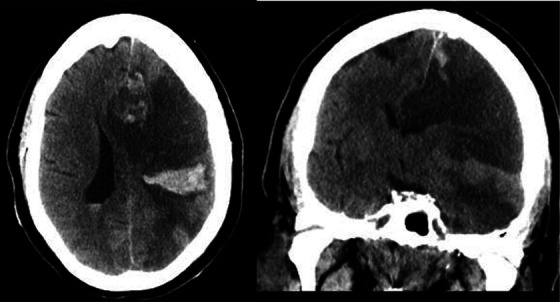
Parenchymal hematoma 2 (PH2) hemorrhagic transformation, approximately 16 hours after thrombolysis and 6 hours after therapeutic anticoagulation achieved, in axial and coronal views.
After several days without neurologic improvement, the patient suffered a clinical herniation syndrome with loss of midbrain reflexes, with radiographic confirmation of brainstem compression without interval increase in hemorrhage size. Family discussion via tele-conference was performed, and the family ultimately decided to transition to comfort measures, followed by terminal extubation and death. Stroke mechanism was adjudicated at time of discharge to be large vessel atherosclerotic disease, with thrombosis exacerbated by COVID-19 related hypercoagulability. This etiology was felt as the most likely mechanism due to the location of thrombus, and underlying calcified plaque.
Discussion
This is the first case report, to our knowledge, that describes t-PA utilization with subsequent systemic anticoagulation for acute ischemic stroke due to large vessel thromboembolism in the setting of COVID-19 associated hypercoagulability. Severe hypercoagulability is a now-recognized manifestation of COVID-19, with ischemic stroke documented as a complication of this hypercoagulability, from 2.8% 4 to 23% of cases. 5 Large vessel strokes have also been documented. 6 IV t-PA alone has been shown to induce successful reperfusion in only about 11% of large vessel occlusions, 7 as was the case in our patient, with evidence of partial recanalization on follow-up vascular imaging. Currently, the safety and efficacy of thrombolysis for acute ischemic stroke in patients with COVID-19 is not well-established, although observational studies have shown the use of alteplase in this population without specific mention of recanalization rates or symptomatic hemorrhage. 8 This population may be at more risk for symptomatic hemorrhage due to presence of disseminated intravascular coagulation, microhemorrhage, or other vasculopathy associated with this virus. Because of collected serological and imaging data indicating persistent hypercoagulability, including remnant clot despite partial thrombolysis, we initiated systemic heparinization within 12 hours after t-PA administration. Although off-label t-PA followed by systemic heparinization demonstrated feasibility and benefit for Acute Respiratory Distress Syndrome (ARDS) pathophysiology in COVID-19 patients, 9 there have been no reports of heparinization in stroke patients with COVID-19, nor of heparinization following t-PA for stroke treatment in those without COVID-19. Current guidelines from the American Heart Association indicate that the usefulness of urgent anticoagulation for non-occlusive intraluminal thrombus is not well-established, although several small observational studies indicate short-term use may be safe. Furthermore, the guidelines do allow for consideration of initiation of anticoagulation if a patient is “found to have abnormal findings on coagulation testing,” as were markedly abnormal in our patient. 10
While parenchymal brain imaging with CT and MRI in the hours following IV t-PA administration did not reveal any hemorrhage, our patient ultimately did have significant hemorrhagic transformation with cerebral edema on subsequent imaging. It is unclear if this was due to heparin administration in the setting of a large infarct core, thrombolytic use, natural evolution, or any combination thereof. He was at risk for hemorrhagic transformation given the size of his infarction, hyperglycemia on admission, as well as his multiple other comorbidities. 11 Although in our patient intracerebral hemorrhage did occur, a retrospective analysis of the decision to initiate systemic heparin early on post-tPA deemed the decision to be concordant with societal guidelines, the best approximation of known pathophysiological ramifications of the disease and treatment, and with the ethical principle of beneficence in mind.
In conclusion, while physicians are increasingly utilizing systemic anticoagulation to prevent thromboembolism in the setting of COVID-19 hypercoagulability, 2,3 it is not well-known what treatment modality is best suited for acute ischemic stroke with comorbid COVID-19. As such, thrombolysis for acute ischemic stroke should be considered for all eligible patients with suspected or confirmed COVID-19. Furthermore, systemic anticoagulation can be considered as a possible adjunct treatment modality in selected (and assented) patients with COVID-19-related hypercoagulability and acute ischemic stroke, although a potentially elevated risk of intracerebral hemorrhage must be taken into account.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the follwing potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Mahesh V. Jayaraman, MD discloses a one time speaking fee for lecture at ISC 2018 from Medtronic, Inc and Linda C. Wendell, MD is the site PI of the CHARM study, funded by Biogen.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Vincent A. LaBarbera  https://orcid.org/0000-0002-1325-0717
https://orcid.org/0000-0002-1325-0717
Daniel C. Sacchetti  https://orcid.org/0000-0001-7207-5640
https://orcid.org/0000-0001-7207-5640
Bradford Thompson  https://orcid.org/0000-0002-4698-7248
https://orcid.org/0000-0002-4698-7248
References
- 1. Lodigiani C, Iapichino G, Carenzo L, et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi:10.1016/j.thromres.2020.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Paranjpe I, Fuster V, Lala A, et al. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol. 2020;76(1):122–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020:77(6);683–696. doi:10.1001/jamaneurol.2020.1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Helms J, Kremer S, Merdji H, et al. Neurologic features in severe SARS-Cov-2 infection. N Engl J Med. 2020;382(24):2268–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Oxley TJ, Mocco J, Majidi S, et al. Large-vessel stroke as a presenting feature of COVID-19 in the young. N Engl J Med. 2020;382(20):e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tsivgoulis G, Katsanos AH, Schellinger PD, et al. Successful reperfusion with intravenous thrombolysis preceding mechanical thrombectomy in large-vessel occlusions. Stroke. 2018;49(1):232–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yaghi S, Ishida K, Torres J, et al. SARS-Cov-2 and stroke in a New York healthcare system [published correction appears in Stroke. 2020;51(8):e179]. Stroke. 2020;51(7):2002–2011. doi:10.1161/STROKEAHA.120.030335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang J, Hajizadeh N, Moore EE, et al. Tissue plasminogen activator (tPA) treatment for COVID-19 associated acute respiratory distress syndrome (ARDS): a case series. J Thromb Haemost. 2020;18(7):1752–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association [published correction appears in Stroke. 2019;50(12):e440-e441]. Stroke. 2019;50(12):e344–e418. [DOI] [PubMed] [Google Scholar]
- 11. Yaghi S, Willey JZ, Cucchiara B, et al. American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; and Council on Quality of Care and Outcomes Research. Treatment and outcome of hemorrhagic transformation after intravenous alteplase in acute ischemic stroke: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2017;48(12):e343–e361. [DOI] [PubMed] [Google Scholar]